Structure-Based In Silico Approaches Reveal IRESSA as a Multitargeted Breast Cancer Regulatory, Signalling, and Receptor Protein Inhibitor
Abstract
:1. Introduction
2. Results
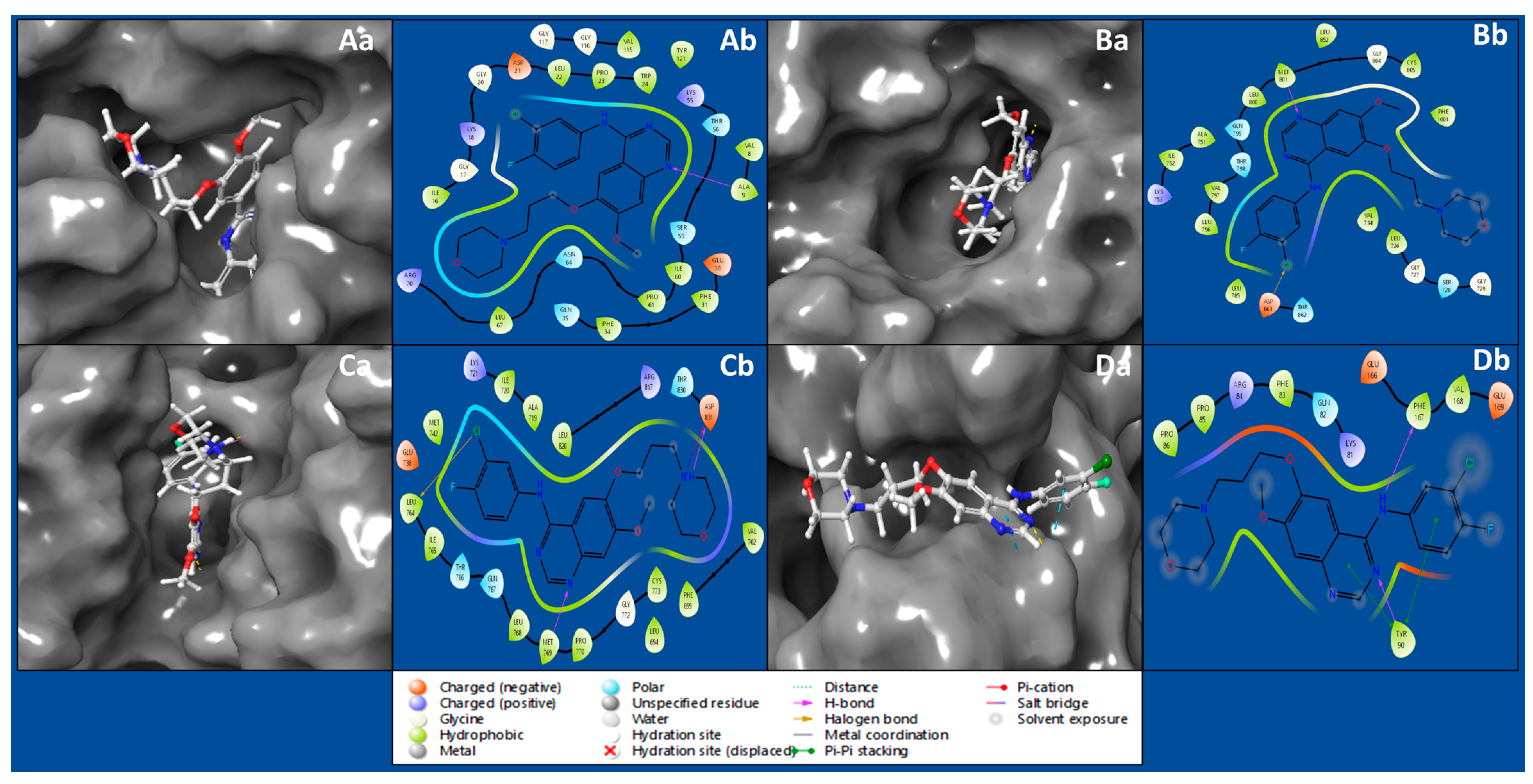
2.1. Protein–Ligand Molecular Interaction Analysis
2.2. Molecular Interaction Fingerprints
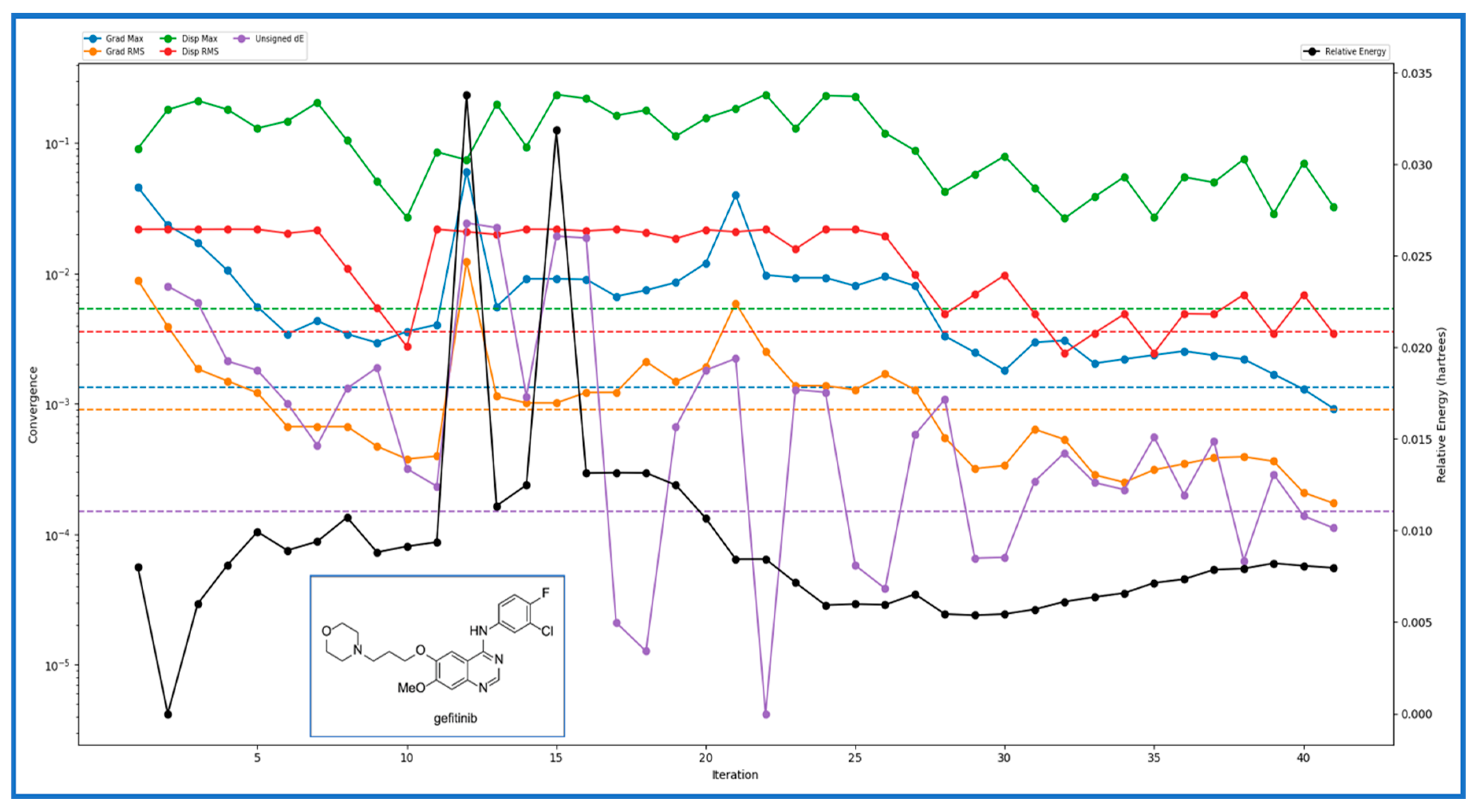
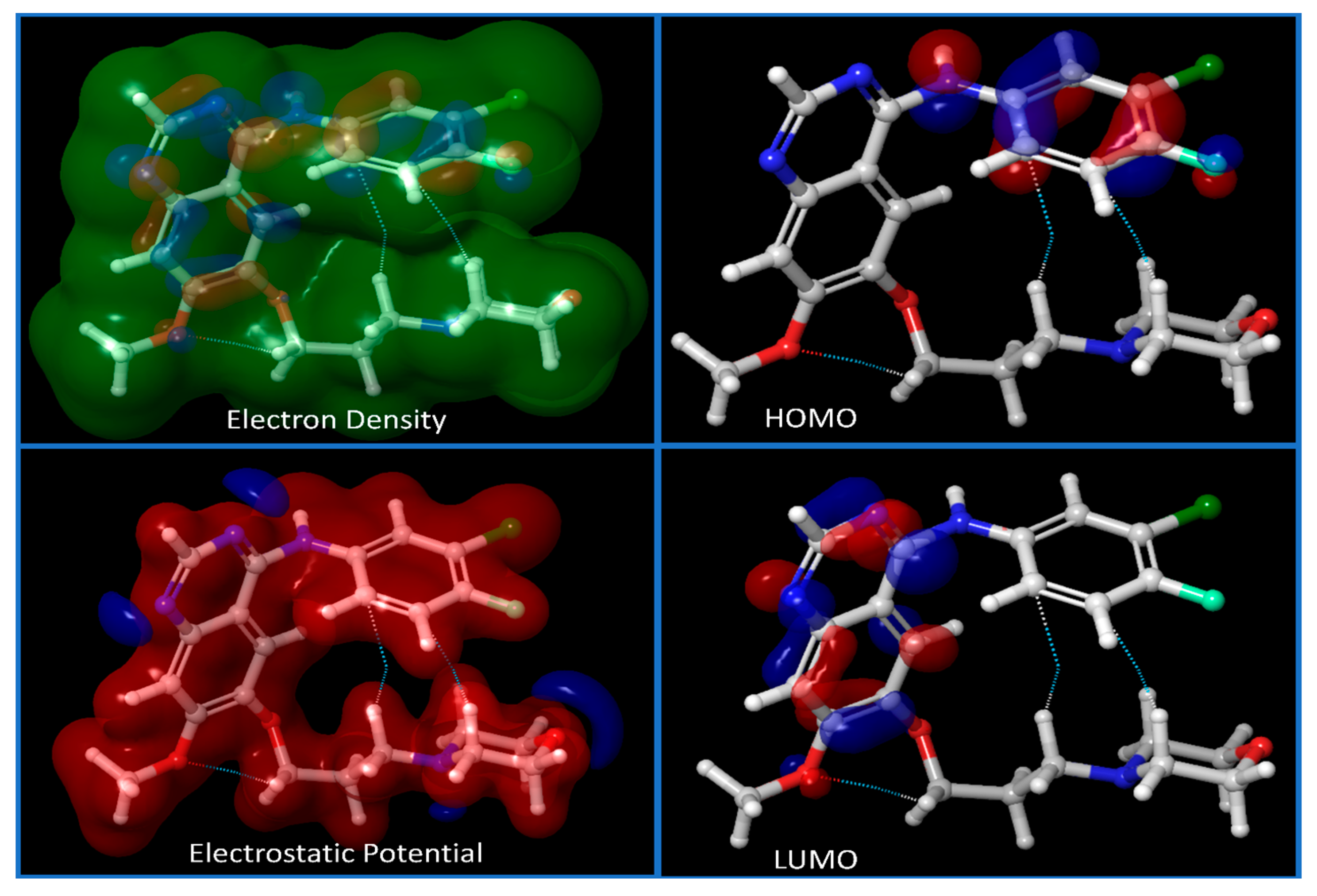
2.3. DFT and Pharmacokinetic Studies
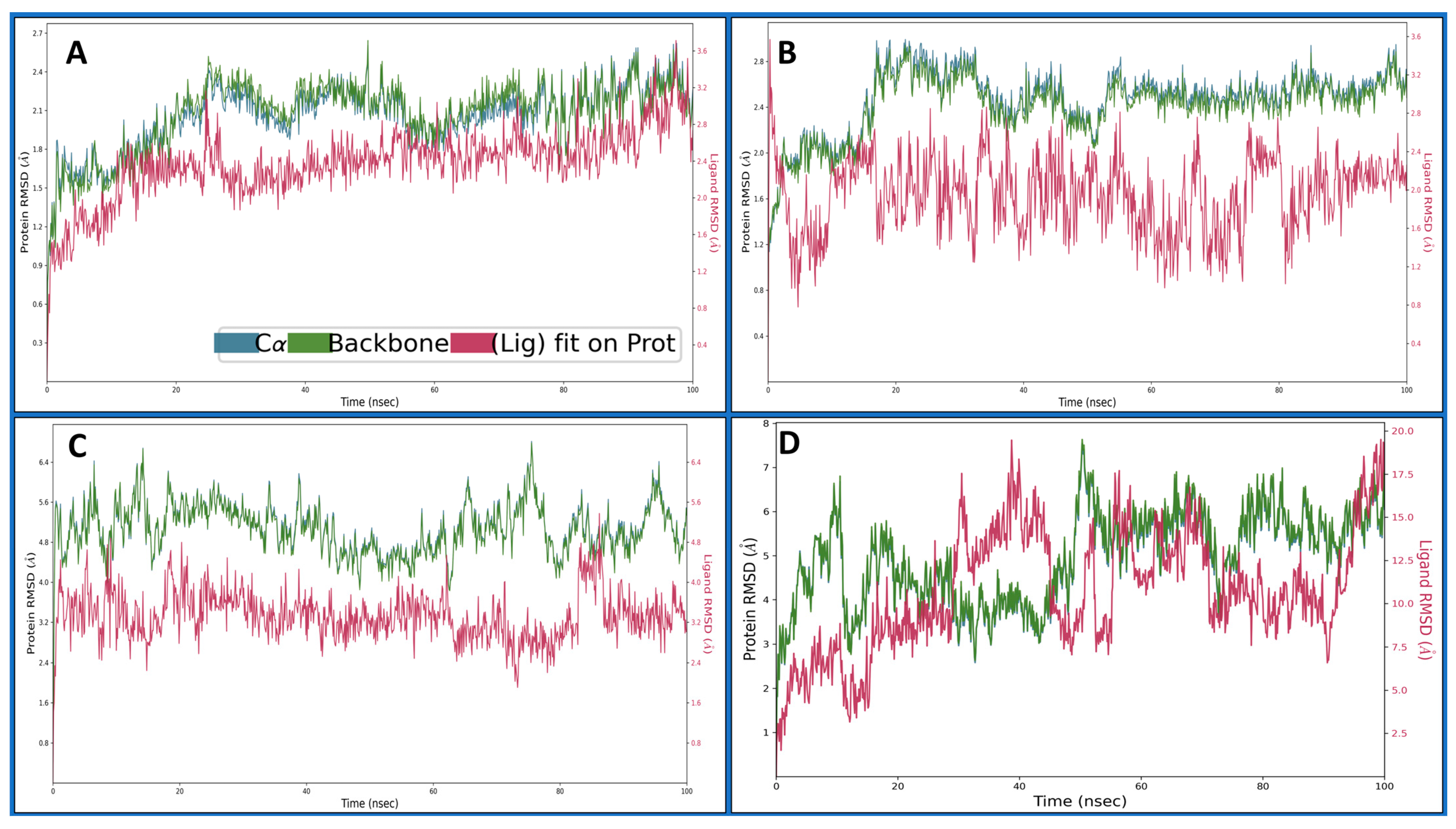
2.4. Molecular Dynamics Simulations
2.4.1. Root Mean Square Deviation
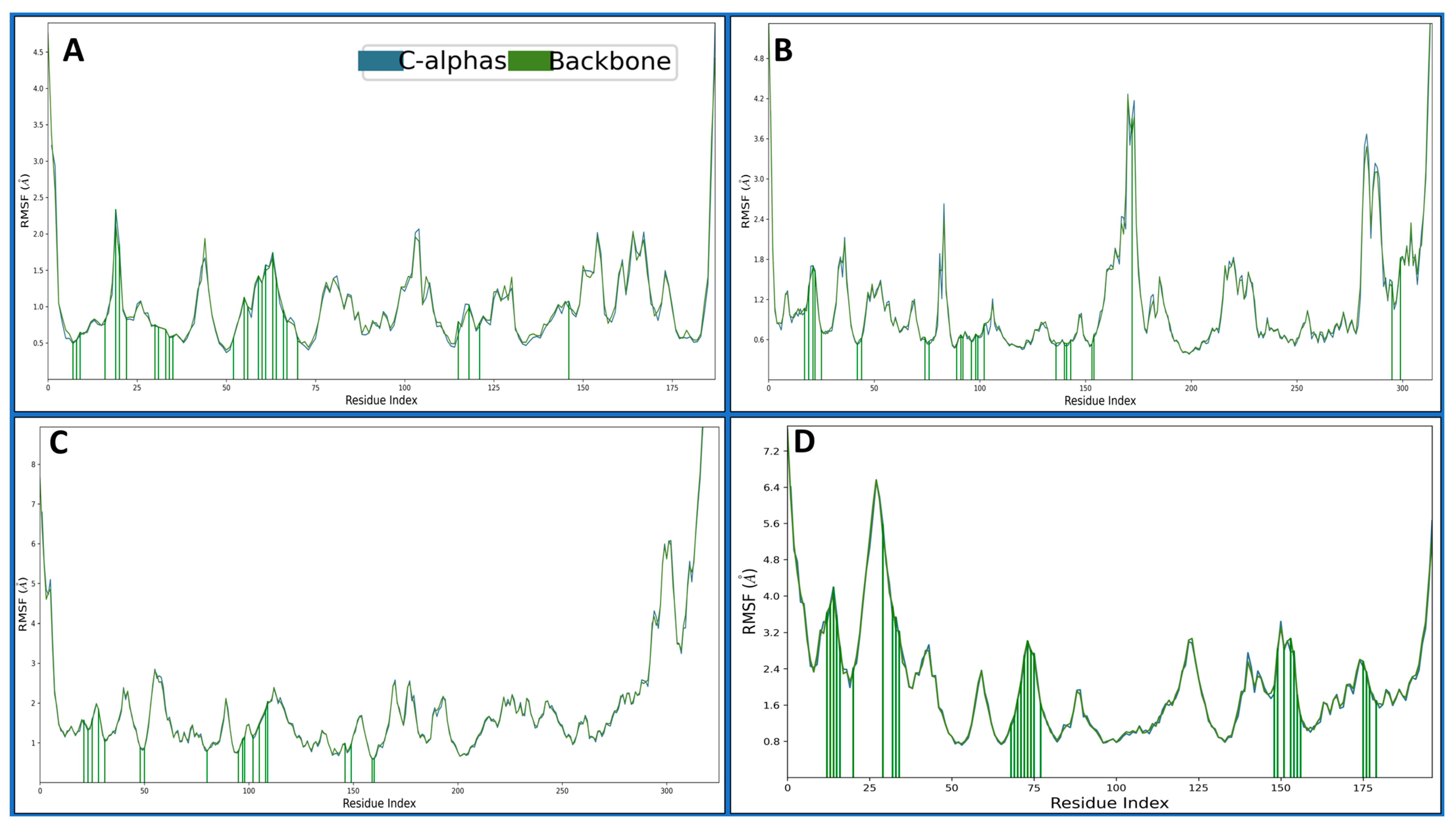
2.4.2. Root Mean Square Fluctuations
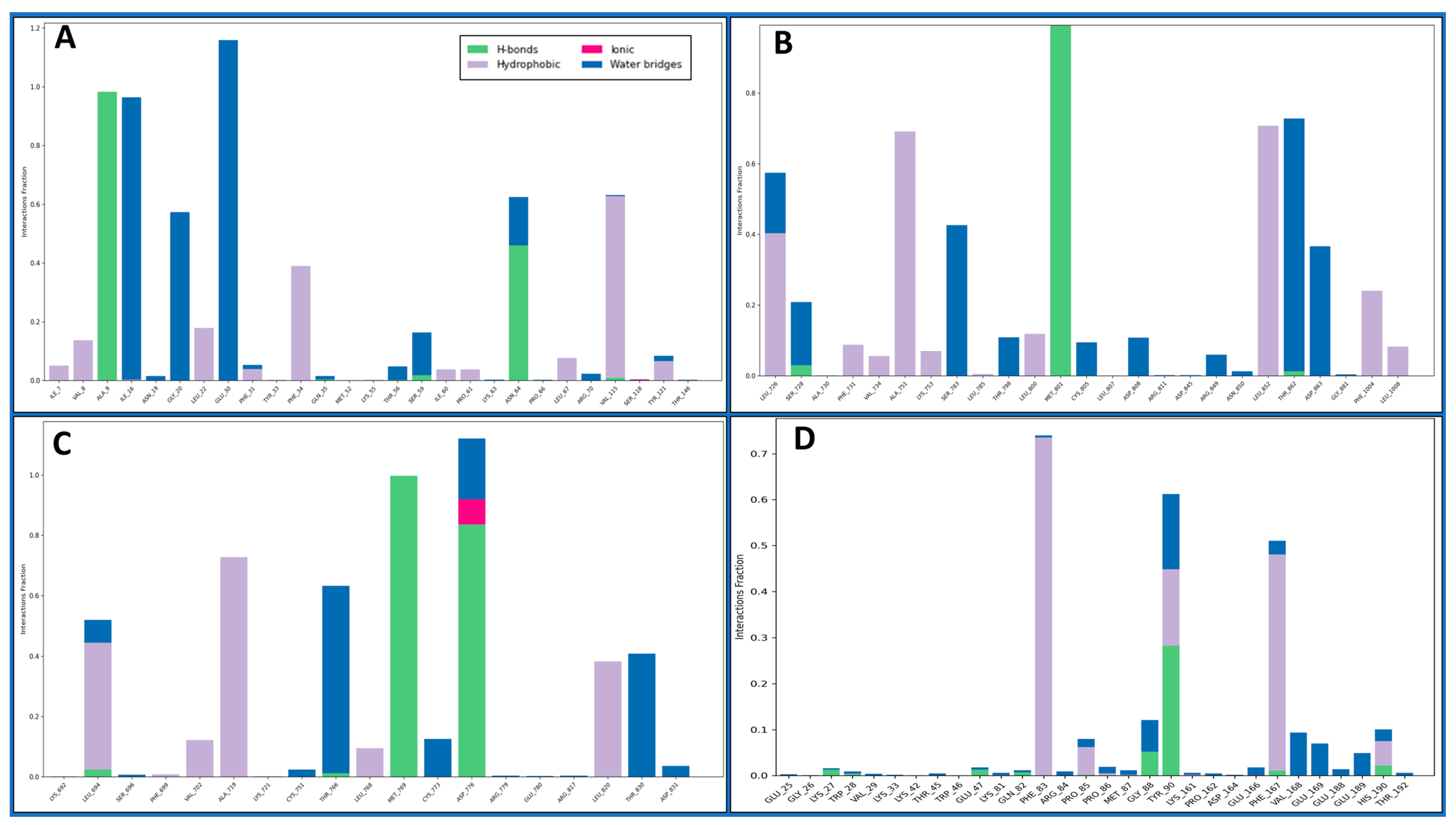
2.4.3. Simulation Interaction Diagrams
3. Discussion
4. Methods
4.1. Protein and Ligand Preparations
4.2. Grid Computation and Multitargeted Molecular Docking
4.3. Molecular Interaction Fingerprints
4.4. Pharmacokinetic and DFT Studies
4.5. Molecular Dynamics Simulation’s System Preparation and Production Run
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Pugalendhi, P. Breast Cancer: An Overview. J. Cell Tissue Res. 2010, 10, 2423–2432. [Google Scholar]
- Russo, J.; Hu, Y.F.; Silva, I.D.; Russo, I.H. Cancer risk related to mammary gland structure and development. Microsc. Res. Tech. 2001, 52, 204–223. [Google Scholar] [CrossRef] [PubMed]
- Karwasra, R.; Ahmad, S.; Bano, N.; Qazi, S.; Raza, K.; Singh, S.; Varma, S. Macrophage-targeted punicalagin nanoengineering to alleviate methotrexate-induced neutropenia: A molecular docking, DFT, and MD simulation analysis. Molecules 2022, 27, 6034. [Google Scholar] [CrossRef] [PubMed]
- Karwasra, R.; Khanna, K.; Singh, S.; Ahmad, S.; Verma, S. Computational Intelligence in Oncology; Springer: Berlin/Heidelberg, Germany, 2022; Volume 1016, pp. 369–384. [Google Scholar]
- Kaul, T.; Eswaran, M.; Ahmad, S.; Thangaraj, A.; Jain, R.; Kaul, R.; Raman, N.M.; Bharti, J. Probing the effect of a plus 1bp frameshift mutation in protein-DNA interface of domestication gene, NAMB1, in wheat. J. Biomol. Struct. Dyn. 2019, 38, 3633–3647. [Google Scholar] [CrossRef] [PubMed]
- Khuntia, B.K.; Sharma, V.; Wadhawan, M.; Chhabra, V.; Kidambi, B.; Rathore, S.; Sharma, G. Antiviral potential of Indian medicinal plants against influenza and SARS-CoV: A systematic review. Nat. Prod. Commun. 2022, 17, 1934578X221086988. [Google Scholar] [CrossRef]
- Rana, M.; Ahmedi, S.; Fatima, A.; Ahmad, S.; Nouman; Siddiqui, N.; Raza, K.; Manzoor, N.; Javed, S. Synthesis, single crystal, TD-DFT, molecular dynamics simulation and DNA binding studies of carbothioamide analog. J. Mol. Struct. 2023, 1287, 135701. [Google Scholar] [CrossRef]
- Hooley, R.J.; Scoutt, L.M.; Philpotts, L.E. Breast ultrasonography: State of the art. Radiology 2013, 268, 642–659. [Google Scholar] [CrossRef]
- Sathish, D.; Kamath, S.; Rajagopal, K.; Prasad, K. Medical imaging techniques and computer aided diagnostic approaches for the detection of breast cancer with an emphasis on thermography-a review. Int. J. Med. Eng. Inform. 2016, 8, 275–299. [Google Scholar] [CrossRef]
- Olopade, O.I.; Grushko, T.A.; Nanda, R.; Huo, D. Advances in breast cancer: Pathways to personalized medicine. Clin. Cancer Res. 2008, 14, 7988–7999. [Google Scholar] [CrossRef]
- Cherny, N.I.; Paluch-Shimon, S.; Berner-Wygoda, Y. Palliative care: Needs of advanced breast cancer patients. Breast Cancer Targets Ther. 2018, 10, 231–243. [Google Scholar] [CrossRef]
- Stamos, J.; Sliwkowski, M.X.; Eigenbrot, C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J. Biol. Chem. 2002, 277, 46265–46272. [Google Scholar] [CrossRef]
- Ishikawa, T.; Seto, M.; Banno, H.; Kawakita, Y.; Oorui, M.; Taniguchi, T.; Kamiyama, K. Design and synthesis of novel human epidermal growth factor receptor 2 (HER2)/epidermal growth factor receptor (EGFR) dual inhibitors bearing a pyrrolo [3, 2-d] pyrimidine scaffold. J. Med. Chem. 2011, 54, 8030–8050. [Google Scholar] [CrossRef]
- Page, B.D.; Valerie, N.C.K.; Wright, R.H.G.; Wallner, O.; Isaksson, R.; Carter, M.; Rudd, S.G.; Loseva, O.; Jemth, A.-S.; Almlöf, I.; et al. Targeted NUDT5 inhibitors block hormone signaling in breast cancer cells. Nat. Commun. 2018, 9, 250. [Google Scholar] [CrossRef]
- Lamb, K.M.; G-Dayanandan, N.; Wright, D.L.; Anderson, A.C. Elucidating features that drive the design of selective antifolates using crystal structures of human dihydrofolate reductase. Biochemistry 2013, 52, 7318–7326. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Hungyo, H.; Parashar, P.; Ahmad, S.; Mehandi, R.; Tandon, V.; El-Bahy, Z.M. Design, synthesis, X-ray crystal structures, anticancer, DNA binding, and molecular modelling studies of pyrazole–pyrazoline hybrid derivatives. RSC Adv. 2023, 13, 26766–26779. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Ahmad, S.; Imtiyaz, K.; Kumaran, A.K.; Islam, M.; Raza, K.; Easwaran, M.; Kunnath, A.K.; Rizvi, M.A.; Verma, S. In-silico and in-vitro study reveals Ziprasidone as a potential aromatase inhibitor against breast carcinoma. Sci. Rep. 2023, 13, 16545. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Ahmad, S.; Yadav, M.K.; Raza, K.; Kamal, M.A.; Akhtar, S. Structure-based virtual screening, molecular docking, molecular dynamics simulation, and metabolic reactivity studies of quinazoline derivatives for their anti-EGFR activity against tumor angiogenesis. Curr. Med. Chem. 2023, 31, 595–619. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Ahmad, S.; Raza, K.; Gautam, H.K. Computational screening and MM/GBSA-based MD simulation studies reveal the high binding potential of FDA-approved drugs against Cutibacterium acnes sialidase. J. Biomol. Struct. Dyn. 2023, 1–11, Online ahead of print. [Google Scholar] [CrossRef]
- Ahmad, S.; Bano, N.; Qazi, S.; Yadav, M.K.; Ahmad, N.; Raza, K. Multitargeted molecular dynamic understanding of butoxypheser against SARS-CoV-2: An in silico study. Nat. Prod. Commun. 2022, 17, 1934578X221115499. [Google Scholar] [CrossRef]
- Ahmad, S.; Bhanu, P.; Kumar, J.; Pathak, R.K.; Mallick, D.; Uttarkar, A.; Mishra, V. Molecular dynamics simulation and docking analysis of NF-κB protein binding with sulindac acid. Bioinformation 2022, 18, 170–179. [Google Scholar] [CrossRef]
- Ahmad, S.; Dahiya, V.; Vibhuti, A.; Pati Pandey, P.; Tripathi, M.K.; Yadav, M.K. Protein-Based Therapeutics. In Therapeutic Protein-Based Vaccines; Springer: Berlin/Heidelberg, Germany, 2023; pp. 355–384. [Google Scholar]
- Ahmad, S.; Pasha Km, M.; Raza, K.; Rafeeq, M.M.; Habib, A.H.; Eswaran, M.; Yadav, M.K. Reporting dinaciclib and theodrenaline as a multitargeted inhibitor against SARS-CoV-2: An in-silico study. J. Biomol. Struct. Dyn. 2023, 41, 4013–4023. [Google Scholar] [CrossRef]
- Ahmad, S.; Chitkara, P.; Khan, F.N.; Kishan, A.; Alok, V.; Ramlal, A.; Mehta, S. Mobile technology solution for COVID-19: Surveillance and prevention. In Computational Intelligence Methods in COVID-19: Surveillance, Prevention, Prediction and Diagnosis; Springer: Berlin/Heidelberg, Germany, 2021; pp. 79–108. [Google Scholar]
- Ahmad, S.; Raza, K. Identification of 5-nitroindazole as a multitargeted inhibitor for CDK and transferase kinase in lung cancer: A multisampling algorithm-based structural study. Mol. Divers. 2023, 1–14. [Google Scholar] [CrossRef]
- Ahmad, S.; Sheikh, K.; Bano, N.; Rafeeq, M.M.; Mohammed, M.R.S.; Yadav, M.K.; Raza, K. Nature-Inspired Intelligent Computing Techniques in Bioinformatics. In Illustrious Implications of Nature-Inspired Computing Methods in Therapeutics and Computer-Aided Drug Design; Springer Nature: Singapore, 2022; pp. 293–308. [Google Scholar]
- Ahmad, S.; Singh, V.; Gautam, H.K.; Raza, K. Multisampling-based docking reveals Imidazolidinyl urea as a multitargeted inhibitor for lung cancer: An optimisation followed multi-simulation and in-vitro study. J. Biomol. Struct. Dyn. 2023, 1–18. [Google Scholar] [CrossRef]
- Rawluk, J.; Waller, C.F. Gefitinib. Small Mol. Oncol. 2018, 211, 235–246. [Google Scholar]
- Herbst, R.S.; Fukuoka, M.; Baselga, J. Gefitinib—A novel targeted approach to treating cancer. Nat. Rev. Cancer 2004, 4, 956–965. [Google Scholar] [CrossRef]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef]
- Rose, P.W.; Beran, B.; Bi, C.; Bluhm, W.F.; Dimitropoulos, D.; Goodsell, D.S.; Prlić, A.; Quesada, M.; Quinn, G.B.; Westbrook, J.D.; et al. The RCSB Protein Data Bank: Redesigned web site and web services. Nucleic Acids Res. 2010, 39, D392–D401. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, L. Maestro; Schrödinger Release 2; Schrödinger, LLC: New York, NY, USA, 2020. [Google Scholar]
- Release, S. Protein Preparation Wizard; Epik, Schrödinger, LLC: New York, NY, USA, 2016. [Google Scholar]
- Tripathi, M.K.; Ahmad, S.; Tyagi, R.; Dahiya, V.; Yadav, M.K. Computer Aided Drug Design (CADD): From Ligand-Based Methods to Structure-Based Approaches. In Chapter 5—Fundamentals of Molecular Modeling in Drug Design; Elsevier: Amsterdam, The Netherlands, 2022; pp. 125–155. [Google Scholar]
- Yadav, M.K.; Ahmad, S.; Raza, K.; Kumar, S.; Eswaran, M.; Pasha Km, M. Predictive modeling and therapeutic repurposing of natural compounds against the receptor-binding domain of SARS-CoV-2. J. Biomol. Struct. Dyn. 2022, 41, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Release, S. Prime; Schrödinger, LLC: New York, NY, USA, 2020. [Google Scholar]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.F.; Honig, B.; Shaw, D.E.; Friesner, R.A. A hierarchical approach to all-atom protein loop prediction. Proteins Struct. Funct. Bioinform. 2004, 55, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Release, S. Epik; Schrödinger Release 1; Schrödinger, LLC: New York, NY, USA, 2020. [Google Scholar]
- Li, H.; Robertson, A.D.; Jensen, J.H. Very fast empirical prediction and rationalization of protein pKa values. Proteins Struct. Funct. Bioinform. 2005, 61, 704–721. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef]
- Huang, R.; Southall, N.; Wang, Y.; Yasgar, A.; Shinn, P.; Jadhav, A.; Nguyen, D.-T.; Austin, C.P. The NCGC pharmaceutical collection: A comprehensive resource of clinically approved drugs enabling repurposing and chemical genomics. Sci. Transl. Med. 2011, 3, 80ps16. [Google Scholar] [CrossRef]
- Release, S. LigPrep; Schrödinger, LLC: New York, NY, USA, 2019. [Google Scholar]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein− ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Release, S. Glide; Schrödinger Release 3; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Release, S. QikProp; Schrödinger, LLC: New York, NY, USA, 2020. [Google Scholar]
- Alghamdi, Y.S.; Mashraqi, M.M.; Alzamami, A.; Alturki, N.A.; Ahmad, S.; Alharthi, A.A.; Alshamrani, S.; Asiri, S.A. Unveiling the multitargeted potential of N-(4-Aminobutanoyl)-S-(4-methoxybenzyl)-L-cysteinylglycine (NSL-CG) against SARS CoV-2: A virtual screening and molecular dynamics simulation study. J. Biomol. Struct. Dyn. 2023, 41, 6633–6642. [Google Scholar] [CrossRef] [PubMed]
- Alturki, N.A.; Mashraqi, M.M.; Alzamami, A.; Alghamdi, Y.S.; Alharthi, A.A.; Asiri, S.A.; Alshamrani, S. In-silico screening and molecular dynamics simulation of drug bank experimental compounds against SARS-CoV-2. Molecules 2022, 27, 4391. [Google Scholar] [CrossRef] [PubMed]
- Alzamami, A.; Alturki, N.A.; Alghamdi, Y.S.; Ahmad, S.; Alshamrani, S.; Asiri, S.A.; Mashraqi, M.M. Hemi-Babim and fenoterol as potential inhibitors of MPro and papain-like protease against SARS-CoV-2: An in-silico study. Medicina 2022, 58, 515. [Google Scholar] [CrossRef] [PubMed]
- Bhati, R.; Nigam, A.; Ahmad, S.; Raza, K.; Singh, R. Structural–functional analysis and molecular characterization of arsenate reductase from Enterobacter cloacae RSC3 for arsenic biotransformation. 3 Biotech 2023, 13, 305. [Google Scholar] [CrossRef]
- Famuyiwa, S.O.; Ahmad, S.; Fakola, E.G.; Olusola, A.J.; Adesida, S.A.; Obagunle, F.O.; Raza, K.; Ugwo, J.P.; Oyelekan, E.I.; Faloye, K.O. Comprehensive computational studies of naturally occurring kuguacins as antidiabetic agents by targeting visfatin. Chem. Afr. 2023, 6, 1415–1427. [Google Scholar] [CrossRef]
- Hou, J.; Bhat, A.M.; Ahmad, S.; Raza, K.; Qazi, S. In silico analysis of ACE2 receptor to find potential herbal drugs in COVID-19 associated neurological dysfunctions. Nat. Prod. Commun. 2022, 17, 1934578X221118549. [Google Scholar] [CrossRef]
- Bochevarov, A.D.; Harder, E.; Hughes, T.F.; Greenwood, J.R.; Braden, D.A.; Philipp, D.M.; Rinaldo, D.; Halls, M.D.; Zhang, J.; Friesner, R.A. Jaguar: A high-performance quantum chemistry software program with strengths in life and materials sciences. Int. J. Quantum Chem. 2013, 113, 2110–2142. [Google Scholar] [CrossRef]
- Schrödinger, L. Jaguar; Schrödinger release 4; Schrödinger, LLC: New York, NY, USA, 2015. [Google Scholar]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, November 2006; p. 84-es. Available online: https://dl.acm.org/doi/abs/10.1145/1188455.1188544 (accessed on 1 February 2024).
- Release, S. Desmond Molecular Dynamics System; DE Shaw Research: New York, NY, USA, 2017. [Google Scholar]
- Mark, P.; Nilsson, L. Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- McDonald, I. NpT-ensemble Monte Carlo calculations for binary liquid mixtures. Mol. Phys. 1972, 23, 41–58. [Google Scholar] [CrossRef]



| S No | PDB ID | Docking Score | MMGBSA | Prime Hbond | Prime vdW | Ligand Efficiency ln | Ligand Efficiency sa |
|---|---|---|---|---|---|---|---|
| 1 | 4KD7 | −8.809 | −59.08 | −91.21 | −897.44 | −1.987 | −0.893 |
| 2 | 3RCD | −8.459 | −60.59 | −152.23 | −1316.69 | −1.908 | −0.857 |
| 3 | 1M17 | −9.021 | −61.74 | −151.3 | −1374.68 | −2.035 | −0.914 |
| 4 | 5NWH | −4.527 | −49.09 | −97.75 | −703.04 | −1.021 | −0.459 |
| Descriptors | Iressa | Standard Values | Descriptors | Iressa | Standard Values |
|---|---|---|---|---|---|
| #acid | 0 | 0–1 | HumanOralAbsorption | 3 | - |
| #amide | 0 | 0–1 | IP(eV) | 8.475 | 7.9–10.5 |
| #amidine | 0 | 0 | Jm | 0.007 | - |
| #amine | 1 | 0–1 | mol MW | 446.908 | 130.0–725.0 |
| #in34 | 0 | - | PercentHumanOralAbsorption | 100 | >80% is high, <25% is poor |
| #in56 | 22 | - | PISA | 242.502 | 0.0–450.0 |
| #metab | 5 | 1–8 | PSA | 61.141 | 7.0–200.0 |
| #NandO | 7 | 2–15 | QPlogBB | 0.312 | −3.0–1.2 |
| #noncon | 4 | - | QPlogHERG | −7.087 | concern below −5 |
| #nonHatm | 31 | - | QPlogKhsa | 0.349 | −1.5–1.5 |
| #ringatoms | 22 | - | QPlogKp | −2.682 | −8.0–−1.0 |
| #rotor | 8 | 0–15 | QPlogPC16 | 13.202 | 4.0–18.0 |
| #rtvFG | 0 | 0–2 | QPlogPo/w | 4.31 | −2.0–6.5 |
| #stars | 0 | 0 – 5 | QPlogPoct | 20.444 | 8.0–35.0 |
| accptHB | 7.7 | 2.0–20.0 | QPlogPw | 10.783 | 4.0–45.0 |
| ACxDN^.5/SA | 0.0101519 | 0.0–0.05 | QPlogS | −5.129 | −6.5–0.5 |
| Category | small | - | QPPCaco | 1049.999 | <25 poor, >500 great |
| CIQPlogS | −5.22 | −6.5–0.5 | QPPMDCK | 2306.642 | <25 poor, >500 great |
| CNS | 1 | −2 (inactive), +2 (active) | QPpolrz | 44.448 | 13.0–70.0 |
| dip^2/V | 0.0220798 | 0.0–0.13 | RuleOfFive | 0 | maximum is 4 |
| dipole | 5.429 | 1.0–12.5 | RuleOfThree | 0 | maximum is 3 |
| donorHB | 1 | 0.0–6.0 | SAamideO | 0 | 0.0–35.0 |
| EA(eV) | 1.279 | −0.9–1.7 | SAfluorine | 41.345 | 0.0–100.0 |
| FISA | 39.187 | 7.0–330.0 | SASA | 758.477 | 300.0–1000.0 |
| FOSA | 366.922 | 0.0–750.0 | volume | 1334.913 | 500.0–2000.0 |
| glob | 0.7730209 | 0.75–0.95 | WPSA | 109.866 | 0.0–175.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almasoudi, H.H.; Mashraqi, M.M.; Alshamrani, S.A.; Alharthi, A.A.; Alsalmi, O.; Nahari, M.H.; Al-Mansour, F.S.H.; Alhazmi, A.Y.M. Structure-Based In Silico Approaches Reveal IRESSA as a Multitargeted Breast Cancer Regulatory, Signalling, and Receptor Protein Inhibitor. Pharmaceuticals 2024, 17, 208. https://doi.org/10.3390/ph17020208
Almasoudi HH, Mashraqi MM, Alshamrani SA, Alharthi AA, Alsalmi O, Nahari MH, Al-Mansour FSH, Alhazmi AYM. Structure-Based In Silico Approaches Reveal IRESSA as a Multitargeted Breast Cancer Regulatory, Signalling, and Receptor Protein Inhibitor. Pharmaceuticals. 2024; 17(2):208. https://doi.org/10.3390/ph17020208
Chicago/Turabian StyleAlmasoudi, Hassan Hussain, Mutaib M. Mashraqi, Saleh A. Alshamrani, Afaf Awwadh Alharthi, Ohud Alsalmi, Mohammed H. Nahari, Fares Saeed H. Al-Mansour, and Abdulfattah Yahya M. Alhazmi. 2024. "Structure-Based In Silico Approaches Reveal IRESSA as a Multitargeted Breast Cancer Regulatory, Signalling, and Receptor Protein Inhibitor" Pharmaceuticals 17, no. 2: 208. https://doi.org/10.3390/ph17020208





