Nanophytosomal Gel of Heydotis corymbosa (L.) Extract against Psoriasis: Characterisation, In Vitro and In Vivo Biological Activity
Abstract
:1. Introduction
2. Results
2.1. Characterisation of Extract
2.2. Evaluation of Prepared HC-NPs
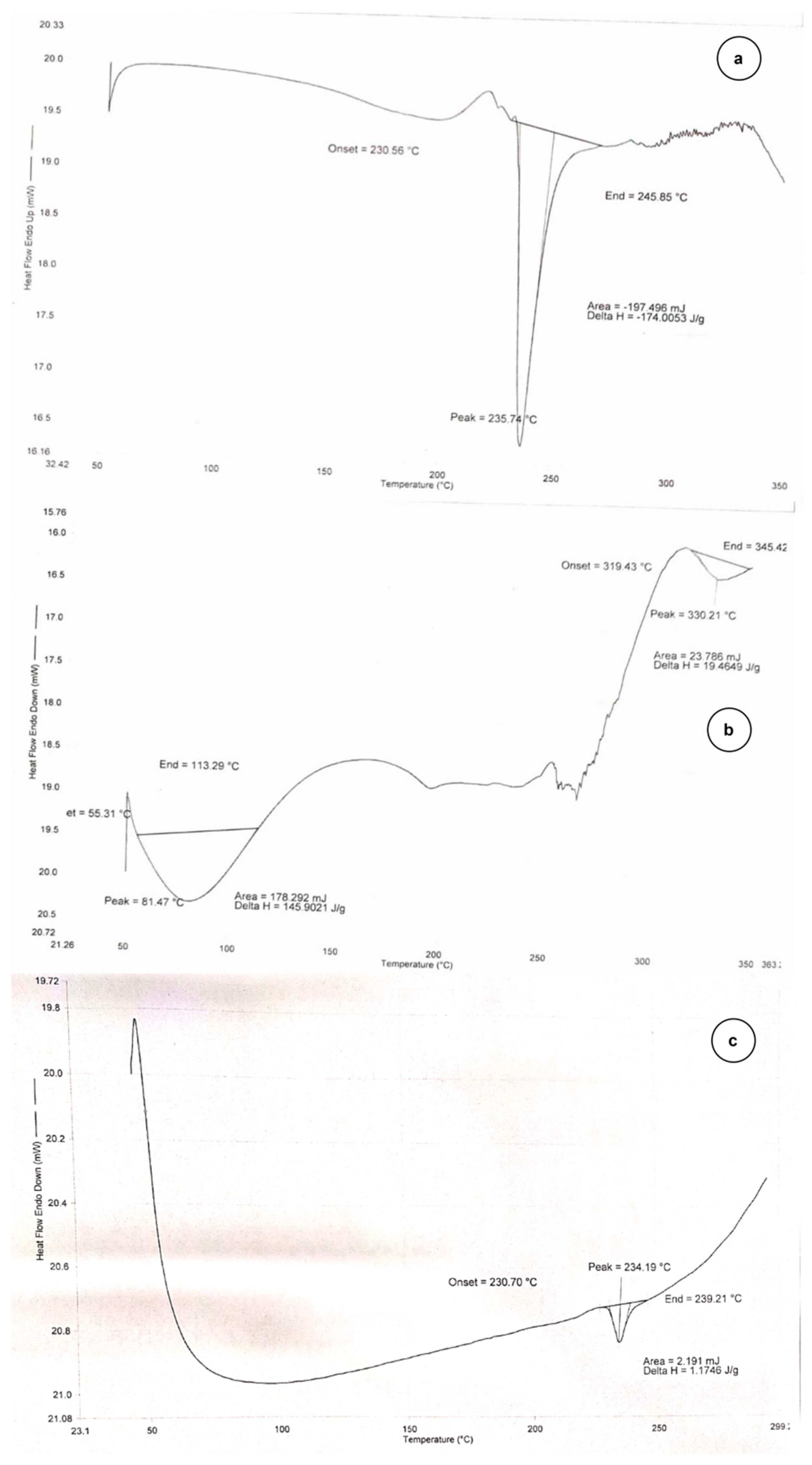
2.2.1. DSC Thermogram
2.2.2. Particle Size and Surface Charge
2.2.3. Morphological Characterisation
2.2.4. Entrapment Efficiency of HC-NPs
2.2.5. Drug Content
2.2.6. In Vitro Drug Release
2.2.7. Storage Stability Study of HC-NPs
2.3. Evaluation of HC-NPs Pluronic Gel (HC-NpsPO)
2.3.1. Texture Analysis and pH
2.3.2. Drug Permeation through Excised Rat Skin
2.3.3. Dermatokinetics Modelling of Topical Gel
2.4. In Vivo Analysis
2.4.1. Acute Dermal Toxicity Study
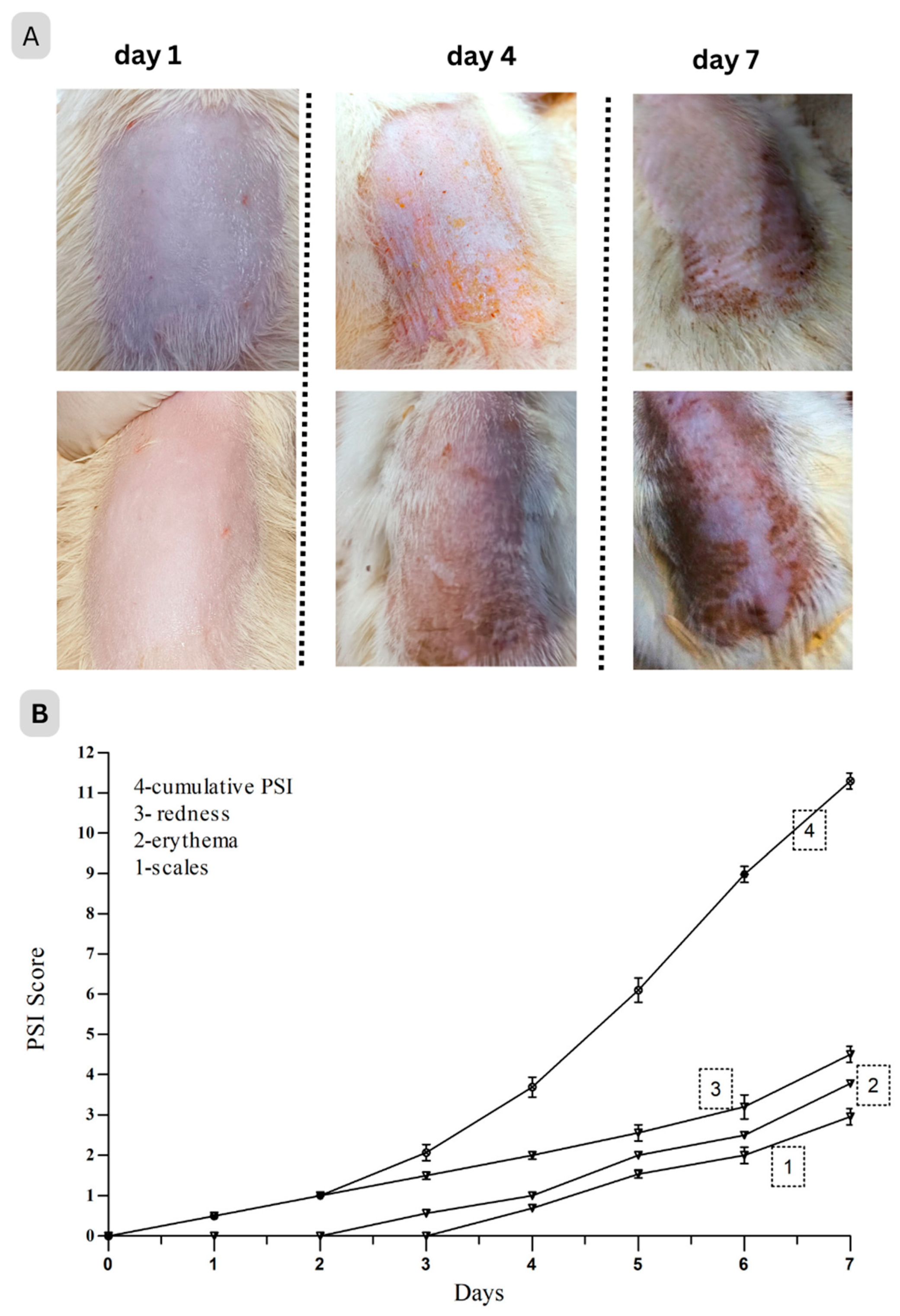
2.4.2. Skin Irritation Study
2.4.3. Anti-Psoriatic Activity
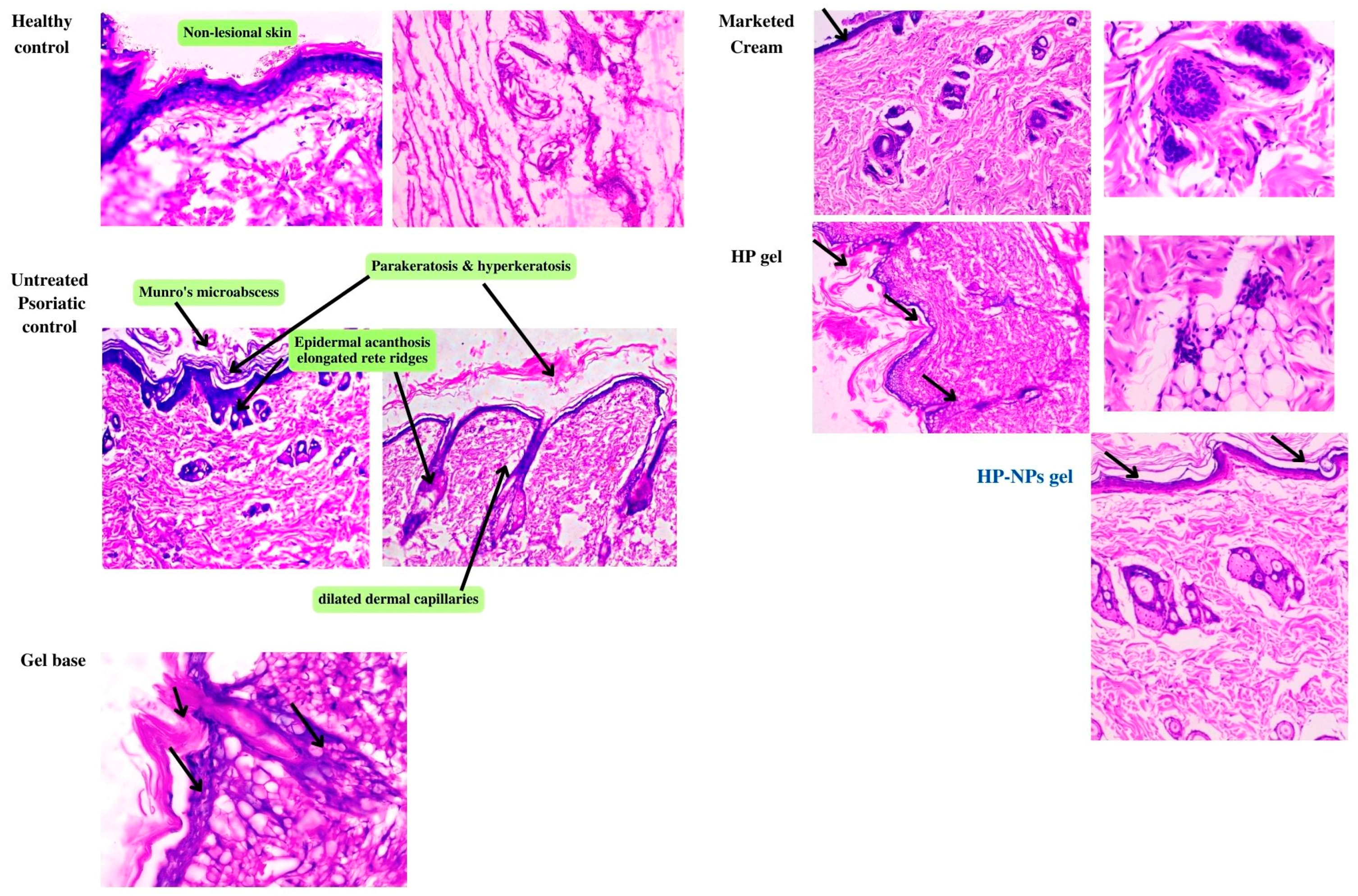
2.4.4. Histopathological Study
2.4.5. Downregulation of Inflammatory Cytokines
3. Discussion
4. Materials and Methods
4.1. Reagents and Drugs
4.2. Characterisation of Hedyotis Corymbosa extract (HCE)
4.2.1. Total Flavonoid and Phenolic Content Estimation of HCE
4.2.2. Polysaccharides Quantification
4.3. Preparation and Optimisation of Nano-Phytosome of HCE
4.3.1. Excipient Screening and Compatibility
4.3.2. Preparation of HC Loaded Nano-Phytosome (HC-NPs)
4.3.3. Experimental Design
4.3.4. Structural Evaluations and Characterisation of HC-NPs
Differential Scanning Calorimetry (DSC)
Particle Size (PS), Polydispersity Index (PDI) and Zeta Potential
Transmission Electron Microscopy (TEM) of HC-NPs
Entrapment Efficiency (% EE)
Drug Content
In Vitro Drug Release
Physical Stability of HC-NPs
4.3.5. Incorporation of HC-NPs in Pluronic Gel and Characterisation
Estimation of pH and Texture Profile Analysis of the HC-NPsPO
Ex Vivo Skin Permeation Study
Ex Vivo Dermatokinetics Study
4.3.6. In Vivo study
Animals and Ethical Statement
Acute Dermal Toxicity
In Vivo Skin Irritation Test
Establishment of Psoriasis-Like Dermatitis Rat Model and Treatment Protocol
Histopathology
Detection of Inflammatory Cytokines in Skin Tissues Lysate
4.3.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| DSC | Differential Scanning Calorimetry |
| EE | Entrapment efficiency |
| ELISA | enzyme-linked immunoassay |
| HC | Hedyotis corymbosa |
| H&E | Hematoxylin and Eosin |
| IMQ | Imiquimod |
| IL | Interleukin |
| MCP-1 | monocyte chemotactic protein 1 |
| NPs | Nano-Phytosome |
| PBS | Phosphate buffer saline |
| PDI | Polydispersity index |
| TEM | Transmission electron microscopy |
| TNF | Tumour Necrosis Factor |
References
- Campanati, A.; Marani, A.; Martina, E.; Diotallevi, F.; Radi, G.; Offidani, A. Psoriasis as an Immune-Mediated and Inflammatory Systemic Disease: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 1511. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Chawla, S.; Kumar, S. Psoriasis: A Review of Existing Therapies and Recent Advances in Treatment. Differentiation 2018, 4, 2018. [Google Scholar]
- Hashizume, H.; Ito, T.; Yagi, H.; Takigawa, M.; Kageyama, H.; Furukawa, F.; Hata, M.; Shirahama, S.; Tanaka, M.; Higashishiba, T.; et al. Efficacy and Safety of Preprandial versus Postprandial Administration of Low-Dose Cyclosporin Microemulsion (Neoral) in Patients with Psoriasis Vulgaris. J. Dermatol. 2007, 34, 430–434. [Google Scholar] [CrossRef]
- Abu Hashim, I.; Abo El-Magd, N.; El-Sheakh, A.; Hamed, M.; Abd El-Gawad, A.E.-G. Pivotal Role of Acitretin Nanovesicular Gel for Effective Treatment of Psoriasis: Ex Vivo-In Vivo Evaluation Study. IJN 2018, 13, 1059–1079. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Qadir, A.; Ali, F.; Mohd, A. Phytoconstituents Based Nanomedicines for the Management of Psoriasis. J. Drug Deliv. Sci. Technol. 2021, 64, 102663. [Google Scholar] [CrossRef]
- Pradhan, M.; Alexander, A.; Singh, M.R.; Singh, D.; Saraf, S.; Saraf, S.; Ajazuddin. Understanding the Prospective of Nano-Formulations towards the Treatment of Psoriasis. Biomed. Pharmacother. 2018, 107, 447–463. [Google Scholar] [CrossRef]
- Clark, R.A. Gone but Not Forgotten: Lesional Memory in Psoriatic Skin. J. Investig. Dermatol. 2011, 131, 283–285. [Google Scholar] [CrossRef]
- Pradhan, M.; Singh, D.; Singh, M.R. Novel Colloidal Carriers for Psoriasis: Current Issues, Mechanistic Insight and Novel Delivery Approaches. J. Control Release 2013, 170, 380–395. [Google Scholar] [CrossRef]
- Heydendael, V.M.R.; Spuls, P.I.; Opmeer, B.C.; de Borgie, C.A.J.M.; Reitsma, J.B.; Goldschmidt, W.F.M.; Bossuyt, P.M.M.; Bos, J.D.; de Rie, M.A. Methotrexate versus Cyclosporine in Moderate-to-Severe Chronic Plaque Psoriasis. N. Engl. J. Med. 2003, 349, 658–665. [Google Scholar] [CrossRef]
- Micha, R.; Imamura, F.; von Ballmoos, M.W.; Solomon, D.H.; Hernán, M.A.; Ridker, P.M.; Mozaffarian, D. Systematic Review and Meta-Analysis of Methotrexate Use and Risk of Cardiovascular Disease. Am. J. Cardiol. 2011, 108, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Richard, E.G.; Hönigsmann, H. Phototherapy, Psoriasis, and the Age of Biologics. Photodermatol. Photoimmunol. Photomed. 2014, 30, 3–7. [Google Scholar] [CrossRef]
- De Alcantara, C.C.; Reiche, E.M.V.; Simão, A.N.C. Cytokines in Psoriasis. Adv. Clin. Chem. 2021, 100, 171–204. [Google Scholar] [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Jacquin-Porretaz, C.; Cordonnier, M.; Nardin, C.; Boullerot, L.; Chanteloup, G.; Vautrot, V.; Adotevi, O.; Garrido, C.; Gobbo, J.; Aubin, F. Increased Levels of Interleukin-17A Exosomes in Psoriasis. Acta Derm. Venereol. 2019, 99, 1143–1147. [Google Scholar] [CrossRef]
- Yang, B.-Y.; Cheng, Y.-G.; Liu, Y.; Liu, Y.; Tan, J.-Y.; Guan, W.; Guo, S.; Kuang, H.-X.; Datura Metel, L. Ameliorates Imiquimod-Induced Psoriasis-Like Dermatitis and Inhibits Inflammatory Cytokines Production through TLR7/8–MyD88–NF-κB–NLRP3 Inflammasome Pathway. Molecules 2019, 24, 2157. [Google Scholar] [CrossRef] [PubMed]
- Behfar, S.; Hassanshahi, G.; Nazari, A.; Khorramdelazad, H. A Brief Look at the Role of Monocyte Chemoattractant Protein-1 (CCL2) in the Pathophysiology of Psoriasis. Cytokine 2018, 110, 226–231. [Google Scholar] [CrossRef]
- Novoszel, P.; Holcmann, M.; Stulnig, G.; De Sa Fernandes, C.; Zyulina, V.; Borek, I.; Linder, M.; Bogusch, A.; Drobits, B.; Bauer, T.; et al. Psoriatic Skin Inflammation Is Promoted by c-Jun/AP-1-dependent CCL2 and IL-23 Expression in Dendritic Cells. EMBO Mol. Med. 2021, 13, e12409. [Google Scholar] [CrossRef]
- Petit, R.G.; Cano, A.; Ortiz, A.; Espina, M.; Prat, J.; Muñoz, M.; Severino, P.; Souto, E.B.; García, M.L.; Pujol, M.; et al. Psoriasis: From Pathogenesis to Pharmacological and Nano-Technological-Based Therapeutics. Int. J. Mol. Sci. 2021, 22, 4983. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Hashemi, S.; Mortazavi, S.A.; Moghimi, H.R.; Darbasizadeh, B. Development and Evaluation of a Novel Methotrexate-Loaded Electrospun Patch to Alleviate Psoriasis Plaques. Drug Dev. Ind. Pharm. 2022, 48, 355–366. [Google Scholar] [CrossRef]
- Ramanunny, A.K.; Wadhwa, S.; Thakur, D.; Singh, S.K.; Kumar, R. Treatment Modalities of Psoriasis: A Focus on Requisite for Topical Nanocarrier. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 418–433. [Google Scholar] [CrossRef]
- Sadasivan, S.; Latha, P.G.; Sasikumar, J.M.; Rajashekaran, S.; Shyamal, S.; Shine, V.J. Hepatoprotective Studies on Hedyotis corymbosa (L.). Lam. J. Ethnopharmacol. 2006, 106, 245–249. [Google Scholar] [CrossRef]
- You, B.-J.; Wu, Y.-C.; Wu, C.-Y.; Bao, B.-Y.; Chen, M.-Y.; Chang, Y.-H.; Lee, H.-Z. Proteomics Displays Cytoskeletal Proteins and Chaperones Involvement in Hedyotis corymbosa—Induced Photokilling in Skin Cancer Cells. Exp. Dermatol. 2011, 20, 653–658. [Google Scholar] [CrossRef]
- Yun, C.; Zheng, T.; Zhang, J.; Shi, Y.; Peng, J.; Liu, L.; Xiao, H.; Han, Y.; Chen, K.; Zhang, X.; et al. Neuroprotective and anti-inflammatory effect of Hedyotis corymbosa extract on chronic stress-induced depression model of rat—A in vivo and ex vivo study. Res. Sq. 2020, in press. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Ferdous, A.; Irin, S. Evaluation of Antinociceptive Effect of Ethanol Extract of Hedyotis corymbosa Linn. Whole Plant in Mice. J. Ethnopharmacol. 2015, 161, 82–85. [Google Scholar] [CrossRef]
- Lina, S.M.M.; Ashab, I.; Bhuiya, M.S.; Shahriar, M. Hepatoprotective Activity of Hedyotis corymbosa (Linn.) Lam. Extract Against Anti-Tubercular Drug Induced Hepatic Damage in Sprague-Dawley Rats. Bangla. Pharma. J. 2018, 21, 131–138. [Google Scholar] [CrossRef]
- Lin, L.; Xiong, S.; Zhao, L.; Tang, J.; Zhang, Z.; Li, Y.; Zheng, T.; Xia, B. Extraction, Characterization, Antioxidant, and Immunostimulatory Activities of Polysaccharides from Hedyotis corymbosa. Evid. Based Complement. Alternat. Med. 2018, 2018, 8617070. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, K.K.; Rao, R. Enhanced Anti-Psoriatic Efficacy and Regulation of Oxidative Stress of a Novel Topical Babchi Oil (Psoralea corylifolia) Cyclodextrin-Based Nanogel in a Mouse Tail Model. J. Microencapsul. 2019, 36, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Nafady, M.M.; Ewees, M.G.E.-D.; Hassan, H.; Khallaf, R.A. Rosuvastatin Calcium-Based Novel Nanocubic Vesicles Capped with Silver Nanoparticles-Loaded Hydrogel for Wound Healing Management: Optimization Employing Box–Behnken Design: In Vitro and in Vivo Assessment. J. Liposome Res. 2022, 32, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Maji, R.; Omolo, C.A.; Jaglal, Y.; Singh, S.; Devnarain, N.; Mocktar, C.; Govender, T. A Transferosome-Loaded Bigel for Enhanced Transdermal Delivery and Antibacterial Activity of Vancomycin Hydrochloride. Int. J. Pharm. 2021, 607, 120990. [Google Scholar] [CrossRef]
- Yu, M.; Yuan, W.; Li, D.; Schwendeman, A.; Schwendeman, S.P. Predicting Drug Release Kinetics from Nanocarriers inside Dialysis Bags. J. Control Release 2019, 315, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Aldawsari, H.M.; Ali, J.; Gupta, D.K.; Warsi, M.H.; Bilgrami, A.L.; Asfour, H.Z.; Noor, A.O.; Md, S. Brucine-Loaded Transliposomes Nanogel for Topical Delivery in Skin Cancer: Statistical Optimization, in Vitro and Dermatokinetic Evaluation. 3 Biotech 2021, 11, 288. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lee, G.-Y.; Bae, G.; Kang, M.-J.; Lim, K.-M. ChemSkin Reference Chemical Database for the Development of an In Vitro Skin Irritation Test. Toxics 2021, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Sohail, T.; Yasmeen, S.; Imran, H.; Ferheen, S.; Rehman, A.U.; Khan, R.A. Standardadization and Skin Irritation Potential of Herbal Analgesic Cream Containing Nigella Sativa Seed Oil. Bangladesh J. Med. Sci. 2020, 19, 163–168. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Y.; Cui, L.; Shi, Y.; Guo, C. Advances in the Pathogenesis of Psoriasis: From Keratinocyte Perspective. Cell Death Dis. 2022, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Deb, S.; Karanth, T. Phytochemical Screening and Metallic Ion Content and Its Impact on the Antipsoriasis Activity of Aqueous Leaf Extracts of Calendula Officinalis and Phlebodium Decumanum in an Animal Experiment Model. Turk. J. Pharm. Sci. 2019, 16, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Romoser, A.; Banerjee, N.; Zebda, R.; Sayes, C.M. The Relationship between pH and Zeta Potential of ∼30 Nm Metal Oxide Nanoparticle Suspensions Relevant to in Vitro Toxicological Evaluations. Nanotoxicology 2009, 3, 276–283. [Google Scholar] [CrossRef]
- Skóra, B.; Piechowiak, T.; Szychowski, K.A.; Gmiński, J. Entrapment of Silver Nanoparticles in L-α-Phosphatidylcholine/Cholesterol-Based Liposomes Mitigates the Oxidative Stress in Human Keratinocyte (HaCaT) Cells. Eur. J. Pharm. Biopharm. 2021, 166, 163–174. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Ahmad, J. Co-Delivery of Imiquimod and Curcumin by Nanoemugel for Improved Topical Delivery and Reduced Psoriasis-Like Skin Lesions. Biomolecules 2020, 10, 968. [Google Scholar] [CrossRef]
- Lee, W.H.; Rho, J.G.; Yang, Y.; Lee, S.; Kweon, S.; Kim, H.-M.; Yoon, J.; Choi, H.; Lee, E.; Kim, S.H.; et al. Hyaluronic Acid Nanoparticles as a Topical Agent for Treating Psoriasis. ACS Nano 2022, 16, 20057–20074. [Google Scholar] [CrossRef]
- Aswar, M.; Bhalekar, M.; Trimukhe, A.; Aswar, U. Self-Microemulsifying Drug Delivery System (SMEDDS) of Curcumin Attenuates Depression in Olfactory Bulbectomized Rats. Heliyon 2020, 6, E04482. [Google Scholar] [CrossRef]
- Zafar, A.; Imam, S.S.; Alruwaili, N.K.; Alsaidan, O.A.; Elkomy, M.H.; Ghoneim, M.M.; Alshehri, S.; Ali, A.M.A.; Alharbi, K.S.; Yasir, M.; et al. Development of Piperine-Loaded Solid Self-Nanoemulsifying Drug Delivery System: Optimization, In-Vitro, Ex-Vivo, and In-Vivo Evaluation. Nanomaterials 2021, 11, 2920. [Google Scholar] [CrossRef]
- Badri, T.; Kumar, P.; Oakley, A.M. Plaque Psoriasis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Qadir, A.; Aqil, M.; Ali, A.; Warsi, M.H.; Mujeeb, M.; Ahmad, F.J.; Ahmad, S.; Beg, S. Nanostructured Lipidic Carriers for Dual Drug Delivery in the Management of Psoriasis: Systematic Optimization, Dermatokinetic and Preclinical Evaluation. J. Drug Deliv. Sci. Technol. 2020, 57, 101775. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Sharma, S.; Roy, A.; Singhvi, G. Design and Dermatokinetic Evaluation of Apremilast Loaded Nanostructured Lipid Carriers Embedded Gel for Topical Delivery: A Potential Approach for Improved Permeation and Prolong Skin Deposition. Colloids Surf. B Biointerfaces 2021, 206, 111945. [Google Scholar] [CrossRef]
- Shao, S.; Chen, J.; Swindell, W.R.; Tsoi, L.C.; Xing, X.; Ma, F.; Uppala, R.; Sarkar, M.K.; Plazyo, O.; Billi, A.C.; et al. Phospholipase A2 Enzymes Represent a Shared Pathogenic Pathway in Psoriasis and Pityriasis Rubra Pilaris. JCI Insight 2021, 6, e151911. [Google Scholar] [CrossRef] [PubMed]
- Tschachler, E. Psoriasis: The Epidermal Component. Clin. Dermatol. 2007, 25, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Parmar, K.M.; Jagtap, C.S.; Katare, N.T.; Dhobi, M.; Prasad, S.K. Development of a Psoriatic-like Skin Inflammation Rat Model Using Imiquimod as an Inducing Agent. Indian J. Pharmacol. 2021, 53, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Purzycka-Bohdan, D.; Nedoszytko, B.; Zabłotna, M.; Gleń, J.; Szczerkowska-Dobosz, A.; Nowicki, R.J. Chemokine Profile in Psoriasis Patients in Correlation with Disease Severity and Pruritus. Int. J. Mol. Sci. 2022, 23, 13330. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, J.; Patil, M. Qualitative Tests for Preliminary Phytochemical Screening: An Overview. Int. J. Chem. Stud. 2020, 8, 603–608. [Google Scholar] [CrossRef]
- Temesgen, S.; Sasikumar, J.M.; Egigu, M.C. Effect of Extraction Solvents on Total Polyphenolic Content and Antioxidant Capacity of Syzygium aromaticum L. Flower Bud from Ethiopia. Biomed. Res. Int. 2022, 2022, 4568944. [Google Scholar] [CrossRef]
- Shousha, W.G.; Aboulthana, W.M.; Salama, A.H.; Saleh, M.H.; Essawy, E.A. Evaluation of the Biological Activity of Moringa oleifera Leaves Extract after Incorporating Silver Nanoparticles, in Vitro Study. Bull. Natl. Res. Cent. 2019, 43, 212. [Google Scholar] [CrossRef]
- Stojilkovski, K.; Uranič, N.; Kolar, D.; Kreft, S. Simple Method for the Determination of Polysaccharides in Herbal Syrup. J. Carbohydr. Chem. 2018, 37, 431–441. [Google Scholar] [CrossRef]
- Moghddam, S.M.M.; Ahad, A.; Mohd, A.; Imam, S.S.; Sultana, Y. Optimization of Nanostructured Lipid Carriers for Topical Delivery of Nimesulide Using Box–Behnken Design Approach. Artif. Cells Nanomed. Biotechnol. 2017, 45, 617–624. [Google Scholar] [CrossRef]
- Danaei, M.; Kalantari, M.; Raji, M.; Samareh Fekri, H.; Saber, R.; Asnani, G.P.; Mortazavi, S.M.; Mozafari, M.R.; Rasti, B.; Taheriazam, A. Probing Nanoliposomes Using Single Particle Analytical Techniques: Effect of Excipients, Solvents, Phase Transition and Zeta Potential. Heliyon 2018, 4, e01088. [Google Scholar] [CrossRef]
- Tamilarasan, N.; Yasmin, B.M.; Anitha, P.; Umme, H.; Cheng, W.H.; Mohan, S.; Ramkanth, S.; Janakiraman, A.K. Box–Behnken Design: Optimization of Proanthocyanidin-Loaded Transferosomes as an Effective Therapeutic Approach for Osteoarthritis. Nanomaterials 2022, 12, 2954. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, N.S.; Shetta, A.; Elhalawani, J.E.; Mamdouh, W. Applying Box–Behnken Design for Formulation and Optimization of PLGA-Coffee Nanoparticles and Detecting Enhanced Antioxidant and Anticancer Activities. Polymers 2022, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Padhye, S.G.; Nagarsenker, M.S. Simvastatin Solid Lipid Nanoparticles for Oral Delivery: Formulation Development and In Vivo Evaluation. Indian J. Pharm. Sci. 2013, 75, 591–598. [Google Scholar] [PubMed]
- Rezk, A.I.; Obiweluozor, F.O.; Choukrani, G.; Park, C.H.; Kim, C.S. Drug Release and Kinetic Models of Anticancer Drug (BTZ) from a pH-Responsive Alginate Polydopamine Hydrogel: Towards Cancer Chemotherapy. Int. J. Biol. Macromol. 2019, 141, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kao, W.J. Drug Release Kinetics and Transport Mechanisms of Non-Degradable and Degradable Polymeric Delivery Systems. Expert. Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Zothanpuii, F.; Ravindran, R.; Kanthiah, S. A Review on Stability Testing Guidelines of Pharmaceutical Products. Asian J. Pharm. Clin. Res. 2020, 13, 2020. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Q1A (R2) Stability Testing of New Drug Substances and Drug Products—Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-and-drug-products-scientific-guideline (accessed on 15 January 2024).
- Chimkode, R.; Patil, M.B.; Jalalpure, S.; Pasha, T.Y.; Sarkar, S. A Study of Hepatoprotective Activity of Hedyotis corymbosa. Linn, in Albino Rats. Anc. Sci. Life 2009, 28, 32. [Google Scholar] [PubMed]
- Kasparaviciene, G.; Kalveniene, Z.; Pavilonis, A.; Marksiene, R.; Dauksiene, J.; Bernatoniene, J. Formulation and Characterization of Potential Antifungal Oleogel with Essential Oil of Thyme. Evid.-Based Complement. Altern. Med. 2018, 2018, 9431819. [Google Scholar] [CrossRef]
- Sainy, J.; Atneriya, U.; Kori, J.l.; Maheshwari, R. Development of an Aloe Vera-Based Emulgel for the Topical Delivery of Desoximetasone. Turk. J. Pharm. Sci. 2021, 18, 465–475. [Google Scholar] [CrossRef]
- Tonchev, M.; Atanasov, T.; Todorova, A.; Atanasova, T.; Shtrankova, N.; Momchilova, M.; Zsivanovits, G. Sensory and Instrumental Texture Analysis of Bulgarian Commercial Pates. Agric. Sci. Technol. 2017, 9, 251–256. [Google Scholar] [CrossRef]
- Guth, K.; Schäfer-Korting, M.; Fabian, E.; Landsiedel, R.; van Ravenzwaay, B. Suitability of Skin Integrity Tests for Dermal Absorption Studies in Vitro. Toxicol. In Vitro 2015, 29, 113–123. [Google Scholar] [CrossRef]
- Thotakura, N.; Kumar, P.; Wadhwa, S.; Raza, K.; Katare, P. Dermatokinetics as an Important Tool to Assess the Bioavailability of Drugs by Topical Nanocarriers. Curr. Drug Metab. 2017, 18, 404–411. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, R.; Singh, B.; Malik, R.; Sharma, G.; Chitkara, D.; Katare, O.P.; Raza, K. Biocompatible Phospholipid-Based Mixed Micelles for Tamoxifen Delivery: Promising Evidences from In-Vitro Anticancer Activity and Dermatokinetic Studies. AAPS PharmSciTech 2017, 18, 2037–2044. [Google Scholar] [CrossRef]
- Schön, M.P.; Manzke, V.; Erpenbeck, L. Animal Models of Psoriasis-Highlights and Drawbacks. J. Allergy Clin. Immunol. 2021, 147, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Pourhoseingholi, M.A.; Baghestani, A.R.; Vahedi, M. How to Control Confounding Effects by Statistical Analysis. Gastroenterol. Hepatol. Bed Bench. 2012, 5, 79–83. [Google Scholar] [PubMed]
- Howard, B. Book Review: Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington DC, United States of America, 1998; Volume 26, ISBN 0309588693. [Google Scholar]
- Dev, S.K.; Choudhury, P.K.; Srivastava, R.; Sharma, M. Antimicrobial, Anti-Inflammatory and Wound Healing Activity of Polyherbal Formulation. Biomed. Pharmacother. 2019, 111, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Reduan, F.H.; Shaari, R.M.; Sayuti, N.S.A.; Mustapha, N.M.; Abu Bakar, M.Z.; Sithambaram, S.; Hamzah, H. Acute and Subacute Dermal Toxicity of Ethanolic Extract of Melastoma Malabathricum Leaves in Sprague-Dawley Rats. Toxicol. Res. 2020, 36, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, C.; Liu, H.; Wang, M.; Zhao, H.; Yan, Y.; Han, L. Quercetin Ameliorates Imiquimod-Induced Psoriasis-like Skin Inflammation in Mice via the NF-κB Pathway. Int. Immunopharmacol. 2017, 48, 110–117. [Google Scholar] [CrossRef]
- Smajlović, A.; Haverić, A.; Alić, A.; Hadžić, M.; Smajlović, A.; Mujezinović, I.; Lojo-Kadrić, N.; Ramić, J.; Elez-Burnjaković, N.; Haverić, S.; et al. Molecular and Histopathological Profiling of Imiquimod Induced Dermatosis in Swiss Wistar Rats: Contribution to the Rat Model for Novel Anti-Psoriasis Treatments. Mol. Biol. Rep. 2021, 48, 4295–4303. [Google Scholar] [CrossRef]




| Storage | Physical Appearance | Particle Size (nm) | Drug Content (%) | EE (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 45 Days | 90 Days | 45 days | 90 Days | 45 Days | 90 Days | 45 Days | 90 Days | |
| 40 ± 2 °C/75 ± 5% | clear | clear | 89.61 | 92.11 | 88.71 | 86.02 | 72.19 | 71.08 |
| Parameters | HCPO | HC-NPsPO | ||
|---|---|---|---|---|
| Epidermis | Dermis | Epidermis | Dermis | |
| Tskin max (h) | 1.5 ± 0.03 | 2 ± 0.02 | 1.6 ± 0.04 | 3 ± 0.03 |
| Cskin max (µg/cm2) | 68 ± 1.32 | 37 ± 1.23 | 196 ± 11.23 | 102 ± 7.25 |
| AUC 0–8 (µg/cm2 h) | 204 ± 12.32 | 241 ± 10.23 | 425 ± 14.29 | 792 ± 21.45 |
| Ke (h − 1) | 1 ± 0.01 | 1.55 ± 0.04 | 1.67 ± 0.04 | 1.8 ± 0.07 |
| MRT | 1.68 ± 0.03 | 2.0 ± 0.02 | 4.21 ± 0.06 | 4.46 ± 0.03 |
| Time (h) | Control | Formalin (0.8%) | HC-NPsPO | |||
|---|---|---|---|---|---|---|
| Erythema | Edema | Erythema | Edema | Erythema | Edema | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 2 | 2 | 1 | 1 |
| 16 | 0 | 0 | 3 | 4 | 0 | 1 |
| 24 | 0 | 0 | 4 | 4 | 0 | 1 |
| Mean Score ± SD | 0 | 0 | 2.22 ± 0.16 | 2.5 ± 0.36 | 0.25 ± 0.012 | 0.69 ± 0.16 |
| PII | 0 | 4.72 | 1.00 | |||
| Days | Group 1 (Untreated Psoriatic Control) | Group 2 (Gel Base Group) | Group 3 (Giosun Psoriasis Cream) | Group 4 (HCPO-Treated) | Group 5 (HC-NPsPO-Treated) |
|---|---|---|---|---|---|
| 7 | 10.89 ± 0.36 | 10.03 ± 0.35 | 6.58 ± 0.87 | 8.35 ± 0.05 | 7.02 ± 0.56 |
| 14 | 10.78 ± 0.47 | 10.45 ± 1.09 | 4.78 ± 0.46 | 7.89 ± 0.057 | 4.03 ± 0.25 |
| 21 | 10.02 ± 0.28 | 9.98 ± 1.21 | 1.24 ± 0.036 | 5.46 ± 0.36 | 1.46 ± 0.048 |
| Independent Variable (Factor) | Factors Level | ||
|---|---|---|---|
| Low | Medium | High | |
| X1 = Concentration of Leciva-S90 (mg) | 50 | 225 | 400 |
| X2 = Concentration Tween 80 (mg) | 60 | 280 | 500 |
| X3 = Hydration time (min) | 30 | 45 | 60 |
| X4 = Sonication time (min) | 5 | 17.5 | 30 |
| Dependent variables (Response) | Desirability | ||
| Y1 = Particle size (nm) and PDI | Minimize | ||
| Y2 = Zeta Potential (mV) | Maximize | ||
| Y3 = Entrapment efficiency (%) | Maximize | ||
| Y4 = Drug release (%) | Maximize | ||
| Optimised Formula Composition | Response, Confidence = 95% | |||
|---|---|---|---|---|
| Type | Desirability | Predicted Mean | Std Dev | |
| X1 = Conc. Leciva-S90 (350 mg) | Y1 = particle size (nm) and PDI (%) | Minimize | 105 nm and 26.9% | 3.12 |
| X2 = Conc. Tween 80 (460 mg) | Y2 = Zeta Potential (mV) | optimize | −15.13 | 4.53 |
| X3 = Hydration time (30 min) | Y3 = Entrapment efficiency (%) | Maximize | 73.89 | 1.17 |
| X4 = Sonication time (10 min) | Y4 = Drug release (%) | Maximize | 85.43 | 2.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, N.; Shaikh, A.M.; Gupta, P.; Kovács, B.; Abuzinadah, M.F.; Ahmad, A.; Goel, R.; Singh, S.; Vinayak, C. Nanophytosomal Gel of Heydotis corymbosa (L.) Extract against Psoriasis: Characterisation, In Vitro and In Vivo Biological Activity. Pharmaceuticals 2024, 17, 213. https://doi.org/10.3390/ph17020213
Singh N, Shaikh AM, Gupta P, Kovács B, Abuzinadah MF, Ahmad A, Goel R, Singh S, Vinayak C. Nanophytosomal Gel of Heydotis corymbosa (L.) Extract against Psoriasis: Characterisation, In Vitro and In Vivo Biological Activity. Pharmaceuticals. 2024; 17(2):213. https://doi.org/10.3390/ph17020213
Chicago/Turabian StyleSingh, Neelam, Ayaz Mukarram Shaikh, Puneet Gupta, Béla Kovács, Mohammed F. Abuzinadah, Aftab Ahmad, Radha Goel, Swapnil Singh, and Chaitanya Vinayak. 2024. "Nanophytosomal Gel of Heydotis corymbosa (L.) Extract against Psoriasis: Characterisation, In Vitro and In Vivo Biological Activity" Pharmaceuticals 17, no. 2: 213. https://doi.org/10.3390/ph17020213
APA StyleSingh, N., Shaikh, A. M., Gupta, P., Kovács, B., Abuzinadah, M. F., Ahmad, A., Goel, R., Singh, S., & Vinayak, C. (2024). Nanophytosomal Gel of Heydotis corymbosa (L.) Extract against Psoriasis: Characterisation, In Vitro and In Vivo Biological Activity. Pharmaceuticals, 17(2), 213. https://doi.org/10.3390/ph17020213











