Biological Effects and Biomedical Applications of Areca Nut and Its Extract
Abstract
:1. Introduction
2. Chemical Composition
3. Biological Effects
3.1. The Effects of Areca Nut on the Digestive System
3.2. The Effects of Areca Nut on the Nervous Systems
3.3. The Effects of Areca Nut on Blood Circulatory System
3.4. The Side Effects of Areca Nut
4. Biomedical Applications
4.1. Antidepressant Activity
4.2. Anti-Inflammatory Activity
4.3. Antioxidant Activity
4.4. Antimicrobial Activity

| Pharmacological Effects | Main Bioactive Phytoconstituents | Main Experimental Methods | Dosage | Main Mechanism of Action | Reference |
|---|---|---|---|---|---|
| Antidepressant activity | Ethanol, hexane, ethyl acetate, aqueous extract fractions of the AN | Forced swim test; tail suspension test; locomotion test; Monoamine oxidase assay. | 2.5, 5, 10, 13, 20 and 50 mg/kg | The alkaloids viz arecaine, arecaidine, and arecoline have been shown to be present in the AN with only arecoline having MAO-inhibiting properties. The possibility that the MAO inhibition in the present report may be due to arecoline and some other unknown compounds needs further investigation. | [66] |
| Dichloromethane and aqueous extract of the AN | Tail-suspension test; yohimbine potentiation test; locomotor test; monoamine oxidase assay. | 5 mL/kg and 10 mL/kg for rats and mice | Inhibiting monoamine oxidase type A in rat brain homogenates. | [67] | |
| Ethanol extracts from AN | Acute and sub-chronic forced swimming tests. | AN ethanol extract (50 mg/kg), aqueous extract (20 mg/kg) | Increased serotonin levels by approximately 35% and norepinephrine levels by approximately 30%. | [69] | |
| Areca Thirteen Pill (GY-13) | Sucrose preference tests, open field tests, and body weight measurements. | 0.25 g/kg (low), 0.50 g/kg (medium), 1.00 g/kg (high) | Increases the levels of cAMP and PKA, restores the mRNA levels of CREB and BDNF, and enhances growth activity in the hippocampus. | [72] | |
| Anti-inflammatory activity | Acetone, ethanol extract of ANs | Paw edema in rats; free radical scavenging activity. | 10, 50, and 100 mg/kg | Carrageenan in rats and reduce the levels of prostaglandin E2; the inhibition of cyclooxygenase. | [75] |
| Active components of ANs | Quantitative real-time PCR analysis; detection of IL-6 production; apoptosis analysis; cell cycle analysis. | 2 mg/kg | Lower the levels of interleukin-6 (IL-6); increase the levels of the tumor suppressor factor p53 | [76] | |
| The water extract derived from the pericarpium of AN | Biochemical analysis; Masson’s trichrome staining Immunofluorescence staining; real-time RT-PCR; Western blot. | 50, 100, or 200 mg/kg | Deactivating pancreatic stellate cells; the water extract from ARP exhibited the ability to impede the constituents of the extracellular matrix (ECM), specifically targeting alpha-smooth muscle actin (α-SMA), collagen I, and fibronectin 1 (FN1) in both pancreatic tissue and pancreatic stellate cells (PSCs). | [78] | |
| Anti-inflammatory activity | Gwakhyangjeonggi-san (GHJGS) | LPS-induced phosphorylation of mitogen-activated protein kinases; Western blotting and enzyme-linked immunosorbent assay (ELISA). | 0, 250, 500 or 1000 µg/mL | Increased expression of heme oxygenase-1 and prevention of reactive oxygen species generation. | [81] |
| Anti-inflammatory activity | Arecoline | Bone marrow macrophage (BMM) isolation; cell viability assay; osteoclast differentiation; tartrate-resistant acid phosphatase (TRAP) staining; real-time RT-PCR; Western blot. | 10 mg/kg | The interference of arecoline with signal pathways activated by AKT, MAPK, and NF-κB, leading to the suppression of gene expression and the translocation of genes associated with osteoclast differentiation. | [82] |
| AN polyphenols | Cell viability assays; specific marker protein detection; fluorescence investigations | 0, 25, 50, 100, 150 μg/mL | Areca nut seed polyphenol (ACP) remarkably enhanced the content of ALP in osteoblasts, which promoted the differentiation of osteoblasts. | [84] | |
| Antioxidant activity | AN polyphenol; ethanol extract from AN | Quantitative real-time PCR analysis; Western blot; free radical scavenging activity assays. | Oral administration of ANE (250 mg/kg and 500 mg/kg) | Inhibit vascularization in plasma protein-nitroglycerin venous and local models, as well as inhibit the expression of inducible nitric oxide. | [88] |
| AN polyphenol | Quantitative real-time PCR analysis; Western blot; free radical scavenging activity assays. | IC50 values were 26.9 mg/mL for porcine pancreatic elastase and 60.8 mg/mL for human neutrophil elastase. | The elimination of free radicals and the enhancement of antioxidant enzyme activities, such as catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPX). | [89] | |
| AN polyphenol | Free radical scavenging activity assays. | IC50 = 1483.43 μg DW/mL | Inhibiting elastase, reduction in skin tissue aging and inflammation. | [90] | |
| EtOAc extract from AN | DPPH radical scavenging assay; hydroxyl radical scavenging activity. | 20, 40, 60, 80, 120, and 200 µg/mL | The polyphenols in the ethanol extract of areca nut seed can remove free radicals and exert antioxidant activity. | [94] | |
| Antimicrobial Activity | Methanol, ethanol, and water extract from ANs | Agar disk diffusion assay; microtiter broth dilution method; agar disc diffusion technique. | The agar disc diffusion technique: 100 mg/L | AN is rich in tannins, terpenoids, alkaloids, and flavonoids, and tannin in AN is effective to inhibit gram-positive bacteria. Tannin binds with peptide on the peptidoglycan component from cell walls, which in turn disturb the integrity of bacterial cell walls, which cause bacterial cell damage. Finally, it led to disturbance of the metabolism process and, subsequently, the death of cells. | [98] |
| AN polyphenols | Microwave and Soxlet apparatus; resazurin microtiter assay. | MIC: 0.975 ± 0.02 µg/mL | The bioactivity of the extract was attributed to the nontoxic polyphenols present. This extract also showed selective inhibition of M. tuberculosis over other gram-positive and gram-negative bacteria. | [99] | |
| ANE | High-performance liquid chromatography (HPLC) method; phagocytosis assay; analysis of macrophage activity and capacity; analysis of serum biochemistry. | Oral administration of ANE (500, 1000, or 1500 mg/kg) | Administration of areca nut extract increased the number of WBCs and improved the activity and capacity of macrophages significantly in rats infected with S. aureus. | [102] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, W.; Liu, Y.J.; Wu, N.; Sun, T.; He, X.Y.; Gao, Y.X.; Wu, C.J. Areca catechu L. (Arecaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Ethnopharmacol. 2015, 164, 340–356. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, L.; Xu, C.; Qi, L.; Wu, Z.; Li, J.; Chen, H.; Wu, Y.; Fu, T.; Zhu, H.; et al. Chromosome-scale genome assembly of areca palm (Areca catechu). Mol. Ecol. Resour. 2021, 21, 2504–2519. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-F.; Chang, Y.-F. The Controversial Roles of Areca Nut: Medicine or Toxin? Int. J. Mol. Sci. 2023, 24, 8996. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pang, X.; Gu, Z.; Guo, Z.; Xin, Y.; Zhang, L. Rapidly analyzing of ingredients during chewing and processing of areca nut using feature-based molecular networking. Food Chem. 2023, 410, 135205. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Deng, S.; Ma, L.; Li, Q.; Tan, S.; Zheng, Y.; Xu, A.; Wang, H. Research Progress on Processing Technology of Refined Betel Nut in China: A Review. Processes 2023, 11, 3199. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, P.; He, Q.; Dong, X.; Zhang, S. Is gastrointestinal motility related to alkaloids of Charred Semen Arecae? J. Ethnopharmacol. 2020, 257, 112825. [Google Scholar] [CrossRef] [PubMed]
- Kozlakidis, Z.; Cheong, I.H.; Wang, H. Betel Nut and Arecoline: Past, Present, and Future Trends. Innov. Digit. Health Diagn. Biomark. 2022, 2, 64–72. [Google Scholar] [CrossRef]
- Calogero, A.E.; Kamilaris, T.C.; Gomez, M.T.; Johnson, E.O.; Tartaglia, M.E.; Gold, P.W.; Chrousos, G.P. The muscarinic cholinergic agonist arecoline stimulates the rat hypothalamic-pituitary-adrenal axis through a centrally-mediated corticotropin-releasing hormone-dependent mechanism. Endocrinology 1989, 125, 2445–2453. [Google Scholar] [CrossRef]
- Salehi, B.; Konovalov, D.A.; Fru, P.; Kapewangolo, P.; Peron, G.; Ksenija, M.S.; Cardoso, S.M.; Pereira, O.R.; Nigam, M.; Nicola, S.; et al. Areca catechu-From farm to food and biomedical applications. Phytother. Res. 2020, 34, 2140–2158. [Google Scholar] [CrossRef]
- Hung, C.C.; Lee, C.H.; Chung, C.M.; Nithiyanantham, S.; Lane, H.Y.; Ko, Y.C. Antidepressant-induced reduction in betel-quid use in patients with depression: A pioneer clinical study. Medicine 2020, 99, e18672. [Google Scholar] [CrossRef]
- Bhandare, A.M.; Kshirsagar, A.D.; Vyawahare, N.S.; Hadambar, A.A.; Thorve, V.S. Potential analgesic, anti-inflammatory and antioxidant activities of hydroalcoholic extract of Areca catechu L. nut. Food Chem. Toxicol. 2010, 48, 3412–3417. [Google Scholar] [CrossRef] [PubMed]
- Senevirathna, K.; Pradeep, R.; Jayasinghe, Y.A.; Jayawickrama, S.M.; Illeperuma, R.; Warnakulasuriya, S.; Jayasinghe, R.D. Carcinogenic Effects of Areca Nut and Its Metabolites: A Review of the Experimental Evidence. Clin. Pract. 2023, 13, 326–346. [Google Scholar] [CrossRef] [PubMed]
- Sumithrarachchi, S.R.; Jayasinghe, R.; Warnakulasuriya, S. Betel Quid Addiction: A Review of Its Addiction Mechanisms and Pharmacological Management as an Emerging Modality for Habit Cessation. Subst. Use Misuse 2021, 56, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.-C.; Tsai, M.-H.; Lin, Y.-T.; Chou, M.-H.; Yang, K.-L.; Chien, C.-Y. Systemic and Local Effects Among Patients with Betel Quid-Related Oral Cancer. Technol. Cancer Res. Treat. 2022, 21, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.Y.; Kuo, T.M.; Chen, P.K.; Lin, Y.Z.; Hua, C.H.; Chen, Y.C.; Ko, Y.C. Arecoline N-Oxide Upregulates Caspase-8 Expression in Oral Hyperplastic Lesions of Mice. J. Agric. Food Chem. 2017, 65, 10197–10205. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Cheng, A.J.; Lee, L.Y.; Huang, Y.C.; Chang, J.T. Multifaceted Mechanisms of Areca Nuts in Oral Carcinogenesis: The Molecular Pathology from Precancerous Condition to Malignant Transformation. J. Cancer 2019, 10, 4054–4062. [Google Scholar] [CrossRef] [PubMed]
- Pant, I.; Rao, S.G.; Kondaiah, P. Role of areca nut induced JNK/ATF2/Jun axis in the activation of TGF-β pathway in precancerous Oral Submucous Fibrosis. Sci. Rep. 2016, 6, 34314. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, C.; Huang, Y.; He, X. Betel quid may stimulate oral submucous fibrosis by inducing increased mitochondrial reactive oxygen species generation via copper overload. Med. Hypotheses 2023, 174, 111067. [Google Scholar] [CrossRef]
- Moutasim, K.A.; Jenei, V.; Sapienza, K.; Marsh, D.; Weinreb, P.H.; Violette, S.M.; Lewis, M.P.; Marshall, J.F.; Fortune, F.; Tilakaratne, W.M.; et al. Betel-derived alkaloid up-regulates keratinocyte alphavbeta6 integrin expression and promotes oral submucous fibrosis. J. Pathol. 2011, 223, 366–377. [Google Scholar] [CrossRef]
- Chang, Y.F.; Liu, T.Y.; Liu, S.T. Arecoline inhibits and destabilizes agrin-induced acetylcholine receptor cluster formation in C2C12 myotubes. Food Chem. Toxicol. 2013, 60, 391–396. [Google Scholar] [CrossRef]
- Khasbage, S.B.D.; Bhowate, R.R.; Khatib, N. Risk of liver disease in areca nut habitual: A systematic review. J. Oral Max. Surg. Med. 2022, 26, 128–129. [Google Scholar]
- Holdsworth, D.K.; Jones, R.A.; Self, R. Volatile alkaloids from Areca catechu. Phytochemistry 1998, 48, 581–582. [Google Scholar] [CrossRef]
- Hugar, P.; Belur, P.D.; Raval, K. Fermentative extraction of phenolic compounds from unripe areca nuts by solid-state fermentation using newly isolated Rhizopus orizae MW538932. Int. J. Food Sci. Technol. 2023, 58, 4738–4751. [Google Scholar] [CrossRef]
- Nonaka, G.-i.; Hsu, F.-L.; Nishioka, I. Structures of dimeric, trimeric, and tetrameric procyanidins from Areca catechu L. J. Chem. Soc. Chem. Commun. 1981, 15, 781–783. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, Y.; Simon, J.E. Qualitative and Quantitative HPLC/MS Determination of Proanthocyanidins in Areca Nut (Areca catechu). Chem. Biodivers. 2007, 4, 2817–2826. [Google Scholar] [CrossRef]
- Saeed, S.A.; Farnaz, S.; Simjee, R.U.; Malik, A. Triterpenes and B-sitosterol from piper betle: Isolation, antiplatelet and anti-inflammatory effects. Biochem. Soc. Trans. 1993, 21, 462S. [Google Scholar] [CrossRef]
- Triveni, G.; Girish, C. A phytopharmacological review on therapeutic potential of Areca catechu. Indo Am. J. Pharm. Res. 2020, 7, 262–266. [Google Scholar]
- Huang, J.L.; Lu, H.H.; Lu, Y.N.; Hung, P.S.; Lin, Y.J.; Lin, C.C.; Yang, C.C.; Wong, T.Y.; Lu, S.Y.; Lin, C.S. Enhancement of the genotoxicity of benzo[a]pyrene by arecoline through suppression of DNA repair in HEp-2 cells. Toxicol. In Vitro 2016, 33, 80–87. [Google Scholar] [CrossRef]
- Yeh, C.Y.; Chen, H.M.; Chang, M.C.; Kok, S.H.; Lee, J.J.; Chang, B.E.; Jeng, P.Y.; Chan, C.P.; Jeng, J.H. Cytotoxicity and transformation of C3H10T1/2 cells induced by areca nut components. J. Formos. Med. Assoc. 2016, 115, 108–112. [Google Scholar] [CrossRef]
- Jin, X.; Morsy, N.; Shoeb, F.; Zavzavadjian, J.; Akbarali, H.I. Coupling of M2 muscarinic receptor to L-type Ca2+ channel via c-src kinase in rabbit colonic circular smooth muscle. Gastroenterology 2002, 123, 827–834. [Google Scholar] [CrossRef]
- Xie, D.P.; Chen, L.B.; Liu, C.Y.; Zhang, C.L.; Liu, K.J.; Wang, P.S. Arecoline excites the colonic smooth muscle motility via M3 receptor in rabbits. Chin. J. Physiol. 2004, 47, 89–94. [Google Scholar]
- Li, C.B.; Yang, X.; Tang, W.B.; Liu, C.Y.; Xie, D.P. Arecoline excites the contraction of distal colonic smooth muscle strips in rats via the M3 receptor-extracellular Ca2+ influx–Ca2+ store release pathway. Can. J. Physiol. Pharm. 2010, 88, 439–447. [Google Scholar] [CrossRef]
- Yao, N.; Feng, L.; Jiang, W.; Wu, P.; Ren, H.; Shi, H.; Tang, L.; Li, S.; Wu, C.; Li, H.; et al. An emerging role of arecoline on growth performance, intestinal digestion and absorption capacities and intestinal structural integrity of adult grass carp (Ctenopharyngodon idella). Anim. Nutr. 2023, 15, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Satapathy, T.; Pandey, R.K. Acaricidal activity and clinical safety of arecoline hydrobromide on calves infested with cattle tick Rhipicephalus microplus (Acari: Ixodidae). Vet. Parasitol. 2021, 298, 109490. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Satapathy, T.; Ravindra; Pandey, R. Efficacy of arecoline hydrobromide against cattle tick Rhipicephalus (Boophilus) microplus. Int. J. Acarol. 2020, 46, 268–275. [Google Scholar] [CrossRef]
- Zhou, M.X.; Tian, X.; Wu, Z.Q.; Li, K.; Li, Z.J. Fuzhuan brick tea supplemented with areca nuts: Effects on serum and gut microbiota in mice. J. Food Biochem. 2021, 45, e13737. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ito, A.; Chen, X.; Long, C.; Okamoto, M.; Raoul, F.; Giraudoux, P.; Yanagida, T.; Nakao, M.; Sako, Y.; et al. Usefulness of pumpkin seeds combined with areca nut extract in community-based treatment of human taeniasis in northwest Sichuan Province, China. Acta Trop. 2012, 124, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.L.; Li, C.Y.; Lin, L.W.; Chang, L.C.; Chen, Y.L.; Chang, F.R.; Yang, S.N.; Tsai, C.C. Arecae pericarpium extract induces porcine lower-esophageal-sphincter contraction via muscarinic receptors. BMC Complement. Med. 2021, 21, 275. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Hwang, G.S.; Chen, T.J.; Wang, P.S. Effects of arecoline on testosterone release in rats. Am. J. Physiol. Metab. 2008, 295, E497–E504. [Google Scholar] [CrossRef]
- Lim, D.Y.; Kim, I.S. Arecoline inhibits catecholamine release from perfused rat adrenal gland. Acta Pharmacol. Sin. 2006, 27, 71–79. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Liu, Q.; Luo, J.; Guo, P.; Chen, F.; Lawrence, A.J.; Liang, J.-H. Systemic Administration of Arecoline Reduces Ethanol-Induced Sleeping Through Activation of Central Muscarinic Receptor in Mice. Alcohol. Clin. Exp. Res. 2010, 34, 150–157. [Google Scholar] [CrossRef]
- Molinengo, L.; Fundaró, A.M.; Cassone, M.C. Action of a chronic arecoline administration on mouse motility and on acetylcholine concentrations in the CNS. J. Pharm. Pharmacol. 1988, 40, 821–822. [Google Scholar] [CrossRef]
- Chandra, J.N.; Malviya, M.; Sadashiva, C.T.; Subhash, M.N.; Rangappa, K.S. Effect of novel arecoline thiazolidinones as muscarinic receptor 1 agonist in Alzheimer’s dementia models. Neurochem. Int. 2008, 52, 376–383. [Google Scholar] [CrossRef]
- Maiese, K.; Holloway, H.H.; Larson, D.M.; Soncrant, T.T. Effect of acute and chronic arecoline treatment on cerebral metabolism and blood flow in the conscious rat. Brain Res. 1994, 641, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Tsai, C.C.; Yao, C.H.; Hsu, Y.M.; Chen, Y.S.; Wu, M.C. Effect of arecoline on regeneration of injured peripheral nerves. Am. J. Chin. Med. 2013, 41, 865–885. [Google Scholar] [CrossRef]
- Ono, M.; Minamoto, Y.; Shibata, S.; Watanabe, S. Attenuating effect of arecoline and physostigmine on an impairment of mealtime-associated activity rhythm in old rats. Physiol. Behav. 1995, 57, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Bales, A.; Peterson, M.J.; Ojha, S.; Upadhaya, K.; Adhikari, B.; Barrett, B. Associations between betel nut (Areca catechu) and symptoms of schizophrenia among patients in Nepal: A longitudinal study. Psychiatry Res. 2009, 169, 203–211. [Google Scholar] [CrossRef]
- Bozorgi, M.; Najafi, Z.; Omidpanah, S.; Sadri, A.; Narimani, Z.; Moghadam, F.H.; Edraki, N.; Akbarzadeh, T.; Saeedi, M. Investigation of anti-Alzheimer’s activity of aqueous extract of areca nuts (Areca catechu L.): In vitro and in vivo studies. Bol. Latinoam. Caribe Plantas Med. Aromat. 2021, 20, 406–415. [Google Scholar] [CrossRef]
- Nadig, A.P.R.; Suman; Sahyadri, M.; Mehdi, S.; Krishna, K.L. Aqueous extract of Piper betle L. leaf and Areca catechu L. nut protects against pentylenetetrazole-induced seizures and positively modulates cognitive function in adult Zebrafish. Adv. Tradit. Med. 2023, 23, 1137–1152. [Google Scholar] [CrossRef]
- Woo, S.Y.; Joh, J.H.; Han, S.A.; Park, H.C. Prevalence and risk factors for atherosclerotic carotid stenosis and plaque: A population-based screening study. Medicine 2017, 96, e5999. [Google Scholar] [CrossRef]
- Park, Y.B.; Jeon, S.M.; Byun, S.J.; Kim, H.S.; Choi, M.S. Absorption of intestinal free cholesterol is lowered by supplementation of Areca catechu L. extract in rats. Life Sci. 2002, 70, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.L.; Lin, J.C.; Yang, S.F.; Liu, T.Y.; Hung, S.L. Areca nut extracts reduce the intracellular reactive oxygen species and release of myeloperoxidase by human polymorphonuclear leukocytes. J. Periodontal Res. 2007, 42, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.J.; Kim, H.S.; Jeon, S.M.; Park, Y.B.; Choi, M.S. Supplementation of Areca catechu L. extract alters triglyceride absorption and cholesterol metabolism in rats. Ann. Nutr. Metab. 2001, 45, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Kim, H.S.; Lee, T.G.; Ryu, S.H.; Suh, P.G.; Byun, S.J.; Park, Y.B.; Choi, M.S. Lower absorption of cholesteryl oleate in rats supplemented with Areca catechu L. extract. Ann. Nutr. Metab. 2000, 44, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Pithayanukul, P.; Nithitanakool, S.; Bavovada, R. Hepatoprotective Potential of Extracts from Seeds of Areca catechu and Nutgalls of Quercus infectoria. Molecules 2009, 14, 4987–5000. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, G.; Tang, Y.; Guo, M.; Ul Haq Khan, Z.; Guo, Y.; Gu, W.; Ma, Y.; Sui, M.; Li, J.; et al. Potential health risk of areca nut consumption: Hazardous effect of toxic alkaloids and aflatoxins on human digestive system. Food. Res. Int. 2022, 162, 112012. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Chaturvedi, P.; Gupta, P.C. A review of the systemic adverse effects of areca nut or betel nut. Indian J. Med. Paediatr. Oncol. 2014, 35, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.S.; Mohod, S.; Lohe, V.; Patel, S.; Khubchandani, M.; Kukde, M.M. Role of Betel Nut in Liver Toxicity in Oral Submucous Fibrosis and Oral Cancer Patients: A Case-Control Study. Cureus 2023, 15, e48562. [Google Scholar] [CrossRef]
- Adhikari, A.; De, M. Toxic Effects of Betel Quid. Int. J. Hum. Genet. 2013, 13, 7–14. [Google Scholar] [CrossRef]
- Giri, S.; Idle, J.R.; Chen, C.; Zabriskie, T.M.; Krausz, K.W.; Gonzalez, F.J. A metabolomic approach to the metabolism of the areca nut alkaloids arecoline and arecaidine in the mouse. Chem. Res. Toxicol. 2006, 19, 818–827. [Google Scholar] [CrossRef]
- Mahdavi Mortazavi, S.M.; Ataollahi, M.; Mashhadiagha, A.; Moosavi, S.A.; Moshfeghinia, R.; Soheili, M. Acute toxic effects of areca nut on central nervous system and liver: A case report. Clin. Case Rep. 2023, 11, e7976. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.L. Metabolism of the areca alkaloids–toxic and psychoactive constituents of the areca (betel) nut. Drug Metab. Rev. 2022, 54, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 2018, 72, 3–12. [Google Scholar] [CrossRef]
- Dar, A.; Khatoon, S. Antidepressant Effects of Ethanol Extract of Areca catechu in Rodents. Phytother. Res. 1997, 11, 174–176. [Google Scholar] [CrossRef]
- Dar, A.; Khatoon, S.; Rahman, G.; Atta Ur, R. Anti-depressant activities of Areca catechu fruit extract. Phytomedicine 1997, 4, 41–45. [Google Scholar] [CrossRef]
- Dar, A.; Khatoon, S. Behavioral and Biochemical Studies of Dichloromethane Fraction from the Areca catechu Nut. Pharmacol. Biochem. Behav. 2000, 65, 1–6. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Allen, J.S.; Otto, C.; Tiobech, J.; Nero, K. Effects of chewing betel nut (Areca catechu) on the symptoms of people with schizophrenia in Palau, Micronesia. Braz. J. Psychiatry 2000, 177, 174–178. [Google Scholar] [CrossRef]
- Abbas, G.; Naqvi, S.; Erum, S.; Ahmed, S.; Rahman, A.U.; Dar, A. Potential antidepressant activity of Areca catechu nut via elevation of serotonin and noradrenaline in the hippocampus of rats. Phytother. Res. 2013, 27, 39–45. [Google Scholar] [CrossRef]
- Khan, S.; Abbas, G.; Ahmed, F.S.; Rahman, A.; Dar, A. Effect of dichloromethane fraction of Areca catechu nut on monoamines associated behaviors and tyramine pressor sensitivity in rodents. Pak. J. Pharm. Sci. 2014, 27, 303–307. [Google Scholar]
- Goud, U.K.; Hasanthi, K.; Pandiri, K.; Manipriya, K. Neuropharmacological Evaluation of Areca catechu on Chronic Unpredictable Mild Stress Model of Depression in Mice: Behavioral and Biochemical Evidences. J. Drug Vigil. Altern. Ther. 2021, 1, 37–45. [Google Scholar] [CrossRef]
- Cai, M.Y.; Yang, Z.; Huang, X.J.; Li, J.; Bao, W.Y.; Hurilebagen; Wulanqiqige; Wuyunsiriguleng; Cui, J.W.; Ma, L.Q.; et al. Mongolian Medicine Areca Thirteen Pill (GY-13) Improved Depressive Syndrome via upregulating cAMP/PKA/CREB/BDNF signaling pathway. J. Ethnopharmacol. 2022, 293, 115310. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Cox, S.; Kelly, E.; Moore, M.A.S.; Zoellner, H. Arecoline increases basic fibroblast growth factor but reduces expression of IL-1, IL-6, G-CSF and GM-CSF in human umbilical vein endothelium. J. Oral. Pathol. Med. 2015, 44, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Pithayanukul, P.; Ruenraroengsak, P.; Bavovada, R.; Pakmanee, N.; Suttisri, R.; Saen-oon, S. Inhibition of Naja kaouthia venom activities by plant polyphenols. J. Ethnopharmacol. 2005, 97, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Mehmood, M.H.; Ali, A.N.; Ahmed, F.S.; Dar, A.; Gilani, A.H. Studies on anti-inflammatory and analgesic activities of betel nut in rodents. J. Ethnopharmacol. 2011, 135, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.W.; Hsieh, B.S.; Cheng, H.L.; Hu, Y.C.; Chang, W.T.; Chang, K.L. Arecoline decreases interleukin-6 production and induces apoptosis and cell cycle arrest in human basal cell carcinoma cells. Toxicol. Appl. Pharm. 2012, 258, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Choi, J.D. The effects of areca catechu L extract on anti-inflammation and anti-melanogenesis. Int. J. Cosmet. Sci. 1999, 21, 275–284. [Google Scholar] [CrossRef]
- Kweon, B.; Kim, D.U.; Oh, J.Y.; Oh, H.; Kim, Y.C.; Mun, Y.J.; Bae, G.S.; Park, S.J. Arecae pericarpium water extract alleviates chronic pancreatitis by deactivating pancreatic stellate cells. Front. Pharmacol. 2022, 13, 941955. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Kong, F.-D.; Yang, L.; Ma, Q.-Y.; Xie, Q.-Y.; Yu, J.; Chen, P.-W.; Zhou, L.-M.; Wu, Y.-G.; Dai, H.-F.; et al. Phenethoxy Derivatives with Anti-inflammatory Activities from the Betelnut Endophytic Trichoderma asperellum G10. J. Nat. Prod. 2022, 85, 1193–1200. [Google Scholar] [CrossRef]
- Thangjam, G.S.; Kondaiah, P. Regulation of oxidative-stress responsive genes by arecoline in human keratinocytes. J. Periodontal. Res. 2009, 44, 673–682. [Google Scholar] [CrossRef]
- Jeong, S.J.; Kim, O.S.; Yoo, S.R.; Seo, C.S.; Kim, Y.; Shin, H.K. Anti-inflammatory and antioxidant activity of the traditional herbal formula Gwakhyangjeonggi-san via enhancement of heme oxygenase-1 expression in RAW264.7 macrophages. Mol. Med. Rep. 2016, 13, 4365–4371. [Google Scholar] [CrossRef]
- Liu, F.-L.; Chen, C.-L.; Lai, C.-C.; Lee, C.-C.; Chang, D.-M. Arecoline suppresses RANKL-induced osteoclast differentiation in vitro and attenuates LPS-induced bone loss in vivo. Phytomedicine 2020, 69, 153195. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wei, Y.; Song, W.; Zhang, H.; Liu, G.; Chen, Y.; Li, L.Z.; Alolga, R.N.; Ma, G.; Reiter, R.J.; et al. Melatonin as an inducer of arecoline and their coordinated roles in anti-oxidative activity and immune responses. Food Funct. 2020, 11, 8788–8799. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, J.; Li, J.; Li, P.; Zhao, M.; Xia, G. Optimization of ultrasonic-assisted extraction of polyphenol from Areca nut (Areca catechu L.) seeds using response surface methodology and its effects on osteogenic activity. Ultrason. Sonochem. 2023, 98, 106511. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Meng, K.; Gu, Z.; Yun, Y.; Zhang, W.; Zhang, C.; Zhong, Q.; Pan, F.; Shen, X.; Xia, G.; et al. Arecanut (Areca catechu L.) Seed Polyphenol-Ameliorated Osteoporosis by Altering Gut Microbiome via LYZ and the Immune System in Estrogen-Deficient Rats. J. Agric. Food Chem. 2021, 69, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, R.; Luo, K.; Guo, Y.; Xiao, M.; Du, G. Areca nut extract protects against ovariectomy-induced osteoporosis in mice. Exp. Ther. Med. 2017, 13, 2893–2899. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L.; Chi, C.W.; Liu, T.Y. Effects of Areca catechu L. containing procyanidins on cyclooxygenase-2 expression in vitro and in vivo. Food Chem. Toxicol. 2010, 48, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Bhandare, A.; Kshirsagar, A.; Vyawahare, N.; Sharma, P.; Mohite, R. Evaluation of anti-migraine potential of Areca catechu to prevent nitroglycerin-induced delayed inflammation in rat meninges: Possible involvement of NOS inhibition. J. Ethnopharmacol. 2011, 136, 267–270. [Google Scholar] [CrossRef]
- Lee, K.K.; Cho, J.J.; Park, E.J.; Choi, J.D. Anti-elastase and anti-hyaluronidase of phenolic substance from Areca catechu as a new anti-ageing agent. Int. J. Cosmet. Sci. 2001, 23, 341–346. [Google Scholar] [CrossRef]
- Wang, R.; Pan, F.; He, R.; Kuang, F.; Wang, L.; Lin, X. Arecanut (Areca catechu L.) seed extracts extracted by conventional and eco-friendly solvents: Relation between phytochemical compositions and biological activities by multivariate analysis. J. Appl. Res. Med. Aromat. Plant 2021, 25, 100336. [Google Scholar] [CrossRef]
- A, A.K.; Abuthahir, S.S.; Aboul-Enein, H. Phytochemical extraction and comparative analysis of antioxidant activities of Areca catechu L. nut extracts. Pharmacia 2022, 69, 447–451. [Google Scholar]
- Hu, M.; Peng, W.; Liu, Y.; Wu, N.; Zhao, C.; Xie, D.; Yan, D.; Zhang, X.; Tao, X.; Wu, C.-J. Optimum Extraction of Polysaccharide from Areca catechu Using Response Surface Methodology and its Antioxidant Activity. J. Food Process. Pres. 2017, 41, e12798. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Han, Z.; Mei, W.L.; Dai, H.F. Antioxidant and Cytotoxic Phenolic Compounds of Areca Nut (Areca catechu). Chem. Res. Chin. Univ. 2010, 26, 161–164. [Google Scholar]
- Zhang, W.M.; Huang, W.Y.; Chen, W.X.; Han, L.; Zhang, H.D. Optimization of extraction conditions of areca seed polyphenols and evaluation of their antioxidant activities. Molecules 2014, 19, 16416–16427. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Guo, J.; Pan, F.; Kuang, F.; Chen, H.; Guo, X.; Liu, Y. Structural Elucidation and Antioxidant Activities of a Neutral Polysaccharide from Arecanut (Areca catechu L.). Front. Nutr. 2022, 9, 853115. [Google Scholar] [CrossRef] [PubMed]
- Edison, T.N.J.I.; Sethuraman, M.G. Areca catechu Assisted Synthesis of Silver Nanoparticles and its Electrocatalytic Activity on Glucose Oxidation. J. Clust. Sci. 2017, 28, 3139–3148. [Google Scholar] [CrossRef]
- Hafizah, I.; Aisyah, Y.; Hasni, D. Effect of betel type (Piper sp) and concentration of betel leaf extract on quality and antibacterial activities of glycerine bar soap. IOP Conf. Ser. Earth Environ. Sci. 2021, 667, 012016. [Google Scholar] [CrossRef]
- Jam, N.; Hajimohammadi, R.; Gharbani, P.; Mehrizad, A. Evaluation of Antibacterial Activity of Aqueous, Ethanolic and Methanolic Extracts of Areca Nut Fruit on Selected Bacteria. BioMed Res. Int. 2021, 2021, 6663399. [Google Scholar] [CrossRef]
- Raju, A.; De, S.S.; Ray, M.K.; Degani, M.S. Antituberculosis activity of polyphenols of Areca catechu. Int. J. Mycobact. 2021, 10, 13–18. [Google Scholar]
- Boniface, P.; Verma, S.K.; Cheema, H.S.; Darokar, M.P.; Pal, A. Evaluation of antimalarial and antimicrobial activites of extract and fractions from Areca catechu. Int. J. Infect. Dis. 2014, 21, 228–229. [Google Scholar] [CrossRef]
- Yenjit, P.; Issarakraisila, M.; Intana, W.; Chantrapromma, K. Fungicidal activity of compounds extracted from the pericarp of Areca catechu against Colletotrichum gloeosporioides in vitro and in mango fruit. Postharvest Biol. Technol. 2010, 55, 129–132. [Google Scholar] [CrossRef]
- Sari, L.M.; Hakim, R.F.; Mubarak, Z.; Andriyanto, A. Analysis of phenolic compounds and immunomodulatory activity of areca nut extract from Aceh, Indonesia, against Staphylococcus aureus infection in Sprague-Dawley rats. Vet. World 2020, 13, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Sun, Y.; Zheng, Y.D.; He, W.; Yang, Y.Y.; Xie, Y.J.; Feng, Z.X.; Qiao, K. A biocompatible bacterial cellulose/tannic acid composite with antibacterial and anti-biofilm activities for biomedical applications. Mater. Sci. Eng. C-Mater. 2020, 106, 110249. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Jung, H.C.; Baek, Y.J.; Kim, B.Y.; Lee, M.W.; Kim, H.D.; Kim, S.W. Antibacterial Activity of Green-Synthesized Silver Nanoparticles Using Areca Catechu Extract against Antibiotic-Resistant Bacteria. Nanomaterials 2021, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, W.; Zhang, X.; Song, Z.; Tong, T. An Overview of Stimuli-Responsive Intelligent Antibacterial Nanomaterials. Pharmaceutics 2023, 15, 2113. [Google Scholar] [CrossRef] [PubMed]
- Kasai, D.; Chougale, R.; Masti, S.; Gouripur, G.; Malabadi, R.; Chalannavar, R.; Raghu, A.V.; Radhika, D.; Dhanavant, S. Preparation, characterization and antimicrobial activity of betel-leaf-extract-doped polysaccharide blend films. Green Mater. 2020, 9, 49–68. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, C.; Dai, W.; Jing, H.; Du, X.; Peng, M.; Zhang, Y.; Mo, L.; Wang, L.; Chen, X.; et al. Effects of different extraction on the antibacterial and antioxidant activities of phenolic compounds of areca nut (husks and seeds). J. Food Meas. Charact. 2022, 16, 1502–1515. [Google Scholar] [CrossRef]

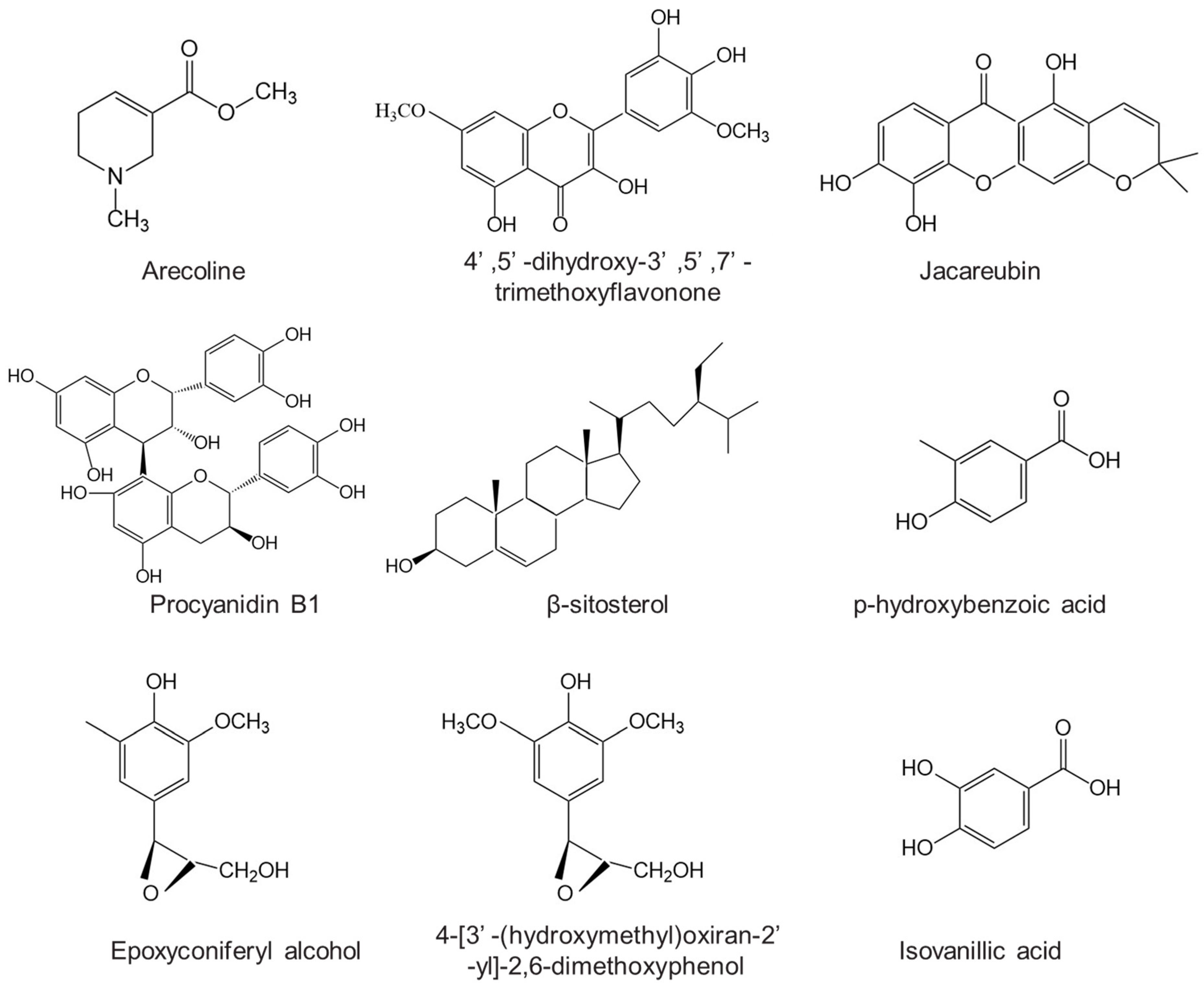




| Classification | Chemical Component | Reference |
|---|---|---|
| Alkaloids | Arecoline, Arecaidine, Guavacoline, Guavacine, Arecolidine, Ethyl N-methyl-l,2,5,6-tetrahydro-pyridine-3-carboxylate, Methyl nicotinate, Ethyl nicotinate, Methyl N-methylpiperidine-3-carboxylate, Ethyl N-methylpiperidine-3-carboxylate, Nicotine, Isoguvacine, Homoarecoline | [22] |
| Flavonoids | Isorhamnetin, Chrysoeriol, Luteolin, Quercetin, 4′,5′-dihydroxy-3′,5′,7′-trimethoxyflavonone, 5,7,4′-trihydroxy-3′,5′-di methoxy flavanone, Liquiritigenin, Jacareubin, | [1,23] |
| Tannins | Catechin, Epicatechin, Procyanidin A1, Procyanidin B1, Procyanidin B2, Arecatannin A1, Arecatannin B1, Arecatannin C1, Arecatannin A2, Arecatannin A3, Arecatannin B2 | [24,25] |
| Triterpenoids and steroidals | Arborinol, Arborinol methyl ether, Ursonic acid, 3β-acetyl ursolic acid, Cycloartenol, Fernenol, Arundoin, 5,8-epidioxiergosta-6,22-dien-3β-ol, Stigmasta-4-en-3-one, β-sitosterol | [26] |
| Fatty acids | Lauric acid, Myristic acid, Palmitic acid, Stearic acid, Oleic acid | [1] |
| others | Chrysophanol, Physcion, Resveratrol, Ferulic acid, p-hydroxybenzoic acid, Vanillic acid, de-O-methyllasiodiplodin, Epoxyconiferyl alcohol, 4-[3′-(hydroxymethyl) oxiran-2′-yl]-2,6-dimethoxyphenol, Protocatechuic acid, Isovanillic acid, Cyclo-(Leu-Tyr) | [1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, T.; Xu, A.; Tan, S.; Jiang, H.; Liu, L.; Deng, S.; Wang, H. Biological Effects and Biomedical Applications of Areca Nut and Its Extract. Pharmaceuticals 2024, 17, 228. https://doi.org/10.3390/ph17020228
Tong T, Xu A, Tan S, Jiang H, Liu L, Deng S, Wang H. Biological Effects and Biomedical Applications of Areca Nut and Its Extract. Pharmaceuticals. 2024; 17(2):228. https://doi.org/10.3390/ph17020228
Chicago/Turabian StyleTong, Ting, Aiqing Xu, Shuhua Tan, Hengzhi Jiang, Lixin Liu, Senwen Deng, and Haihua Wang. 2024. "Biological Effects and Biomedical Applications of Areca Nut and Its Extract" Pharmaceuticals 17, no. 2: 228. https://doi.org/10.3390/ph17020228
APA StyleTong, T., Xu, A., Tan, S., Jiang, H., Liu, L., Deng, S., & Wang, H. (2024). Biological Effects and Biomedical Applications of Areca Nut and Its Extract. Pharmaceuticals, 17(2), 228. https://doi.org/10.3390/ph17020228







