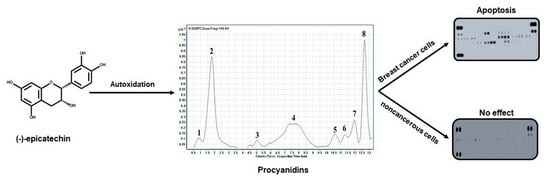
The Autoxidized Mixture of (-)-Epicatechin Contains Procyanidins and Shows Antiproliferative and Apoptotic Activity in Breast Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
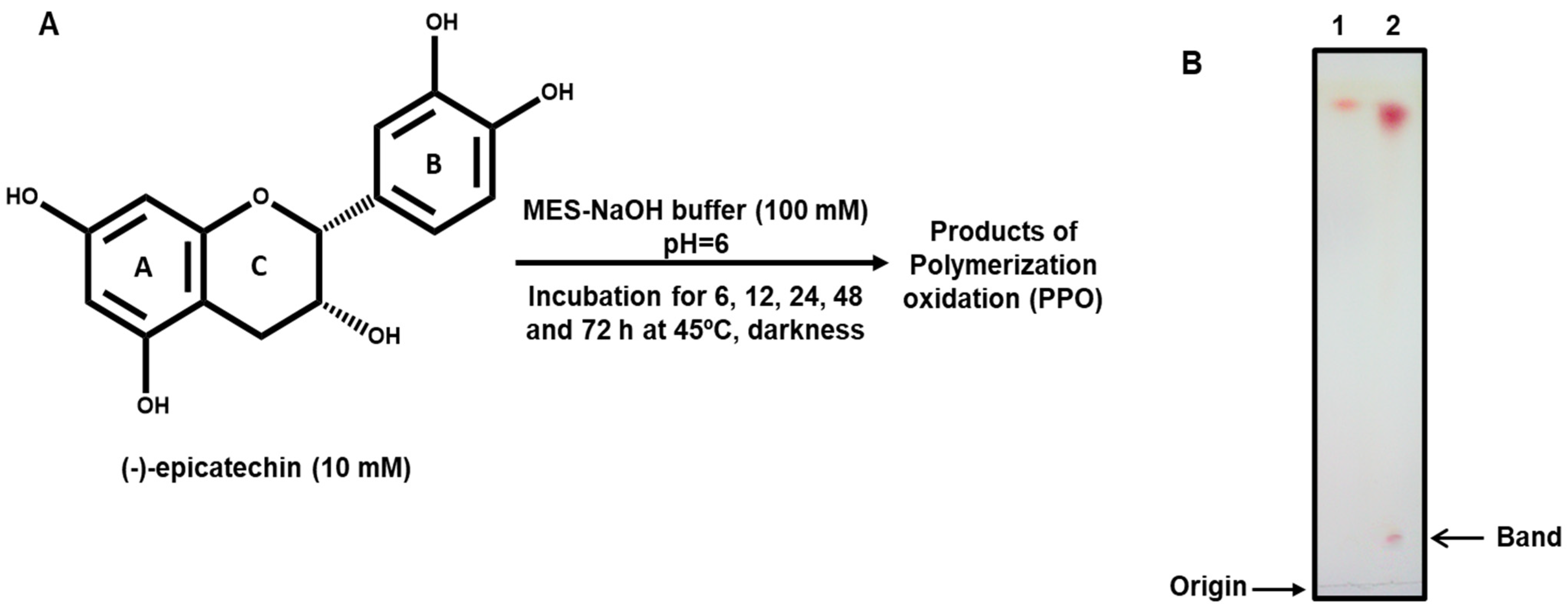
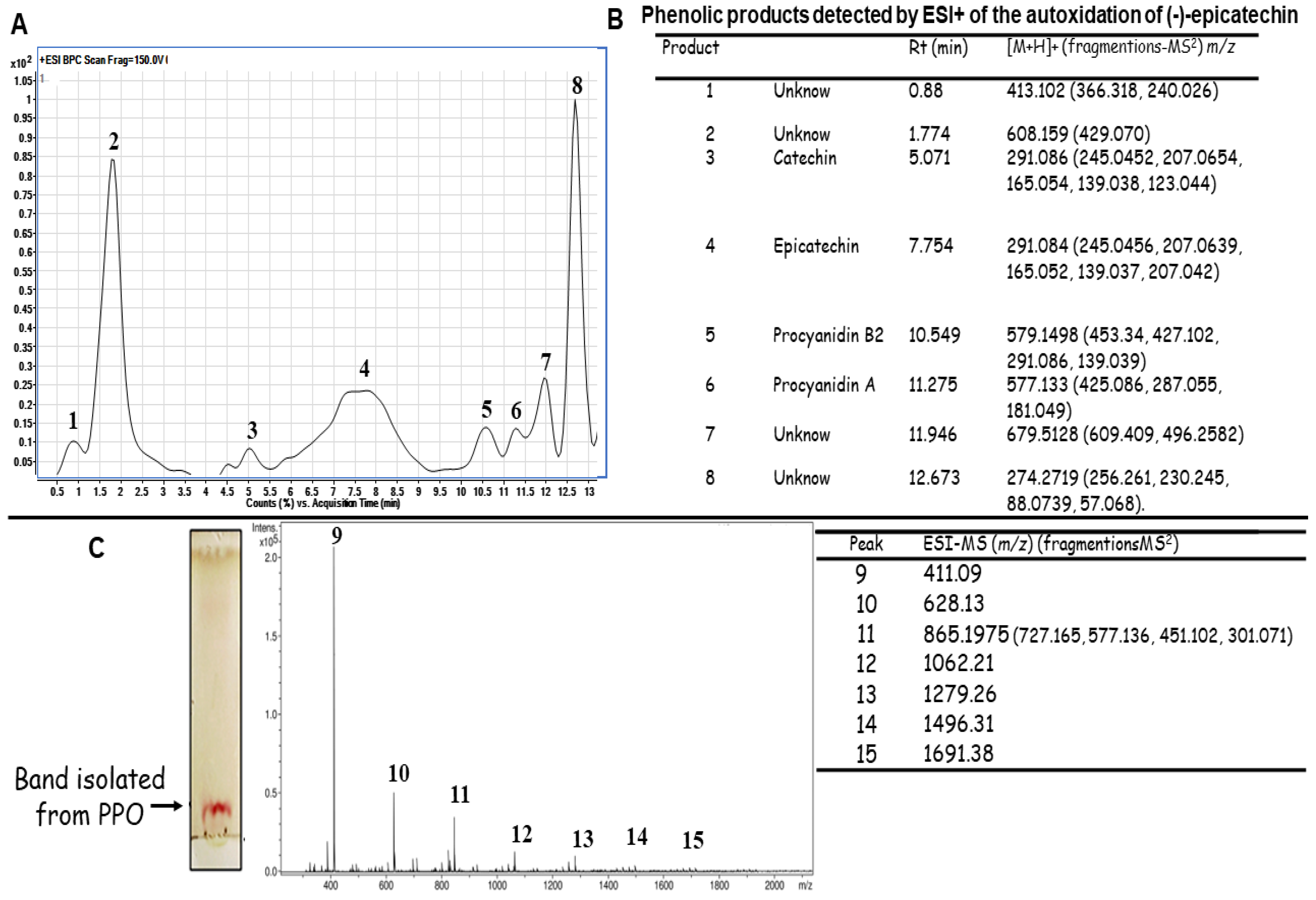
2.1. Autoxidation of (-)-Epicatechin
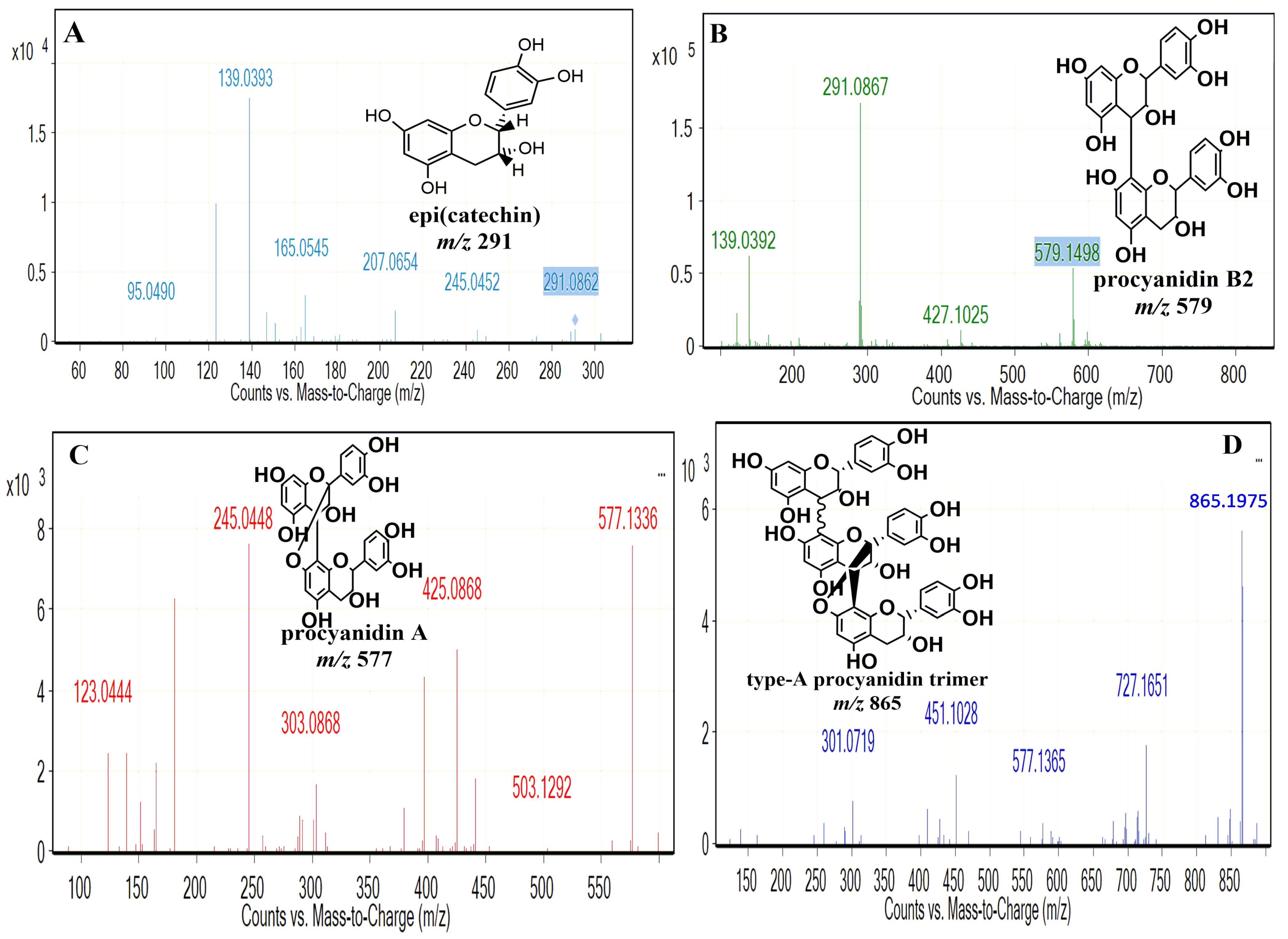
Identification of the Molecules Present in the PPO Mix
2.2. PPO Mixture Inhibited Cell Proliferation of Breast Cancer Cells
2.3. Morphology Changes and DNA Fragmentation Induced by PPO Mix in MDA-MB-231 Breast Cancer Cells
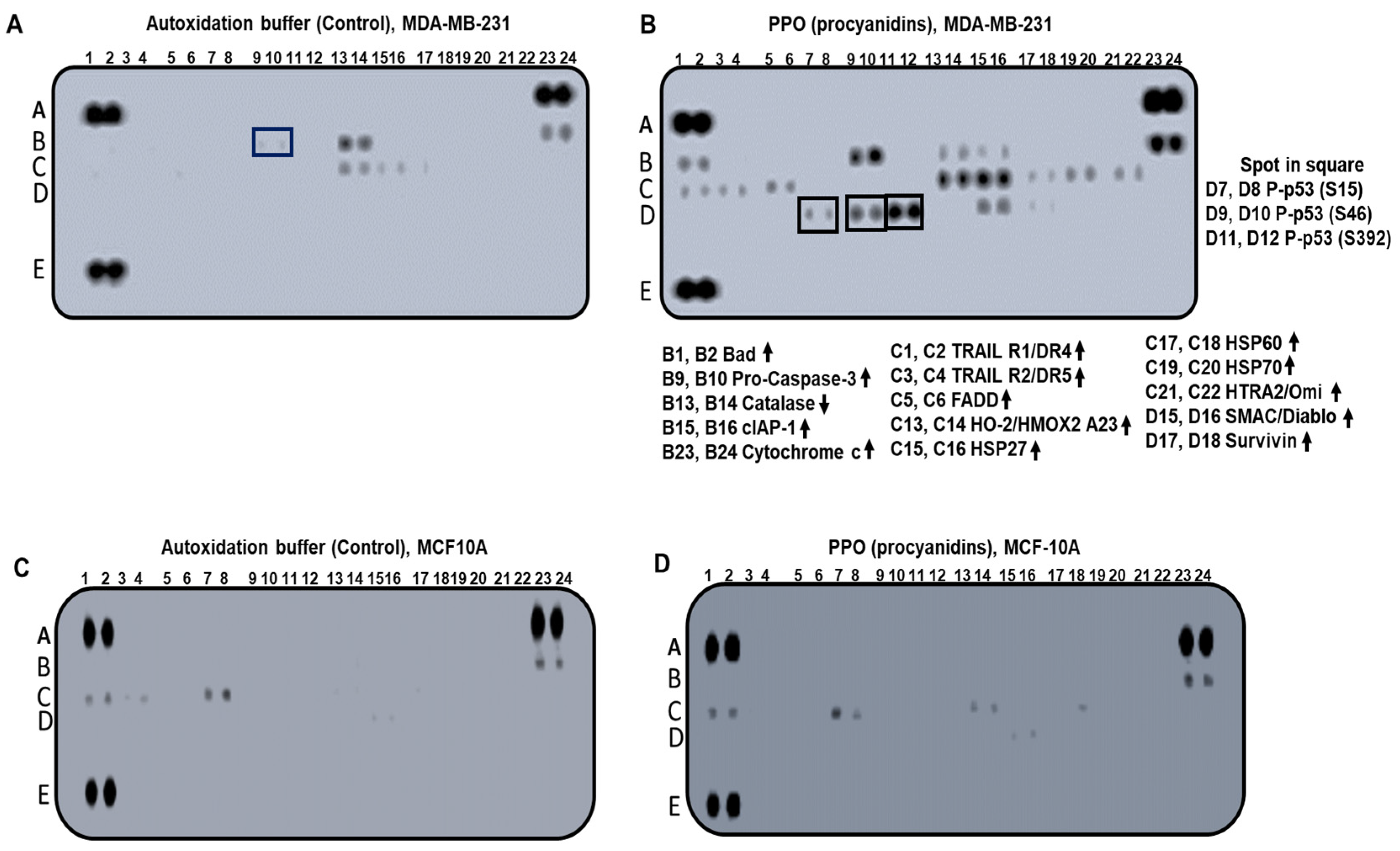
2.4. The Expression Pattern of Apoptosis-Related Proteins Activated by PPO Mixture
3. Materials and Methods
3.1. Materials
3.2. Autoxidation of (-)-Epicatechin
3.3. UHPLC-TOF/MS Analysis of Products of Polymerization Oxidation
3.4. Cell Cultures
3.5. Treatment of Breast Cancer Cells with (-)-Epicatechin and PPO
3.6. Cytotoxicity Assays
3.7. Assessment of Apoptosis Using DNA Fragmentation
3.8. Human Apoptosis Antibody Array
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Aryal, P.; Robinson, S.; Rafiu, R.; Obrenovich, M.; Perry, G. Polyphenols in Alzheimer’s Disease and in the Gut–Brain Axis. Microorganisms 2020, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Rees, A.; Dodd, G.F.; Spencer, J.P.E. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Yuan, Y.; Lin, B.; Miao, Z.; Li, Z.; Guo, Q.; Lu, N. LW-215, a newly synthesized flavonoid, exhibits potent anti-angiogenic activity in vitro and in vivo. Gene 2018, 642, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Kumar, V.; Rahman, M.; Verma, A.; Patel, D.K. New insights into the medicinal importance, physiological functions and bioanalytical aspects of an important bioactive compound of foods ‘Hyperin’: Health benefits of the past, the present, the future. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 31–42. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, X.; Wang, J.; Feng, Y.; Ji, F.; Li, Z.; Bian, J. A review on flavones targeting serine/threonine protein kinases for potential anticancer drugs. Bioorganic Med. Chem. 2019, 27, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Jian, Y.; Liu, Y.; Jiang, S.; Muhammad, D.; Wang, W. Flavanols from Nature: A Phytochemistry and Biological Activity Review. Molecules 2022, 27, 719. [Google Scholar] [CrossRef]
- Mannino, G.; Chinigò, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and Where to Find Them: A Meta-Analytic Approach to Investigate Their Chemistry, Biosynthesis, Distribution, and Effect on Human Health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef]
- Pierini, R.; Kroon, P.A.; Guyot, S.; Ivory, K.; Johnson, I.T.; Belshaw, N.J. Procyanidin effects on oesophageal adenocarcinoma cells strongly depend on flavan-3-ol degree of polymerization. Mol. Nutr. Food Res. 2008, 52, 1399–1407. [Google Scholar] [CrossRef]
- Shoji, T.; Masumoto, S.; Moriichi, N.; Kobori, M.; Kanda, T.; Shinmoto, H.; Tsushida, T. Procyanidin trimers to pentamers fractionated from apple inhibit melanogenesis in B16 mouse melanoma cells. J. Agric. Food Chem. 2005, 53, 6105–6111. [Google Scholar] [CrossRef]
- Desrues, O.; Fryganas, C.; Ropiak, H.M.; Mueller-Harvey, I.; Enemark, H.L.; Thamsborg, S.M. Impact of chemical structure of flavanol monomers and condensed tannins on in vitro anthelmintic activity against bovine nematodes. Parasitology 2016, 143, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.; Xian, D.; Xiong, X.; Yang, L.; Song, J.; Zhong, J. Proanthocyanidins: Novel treatment for psoriasis that reduces oxidative stress and modulates Th17 and Treg cells. Redox Rep. 2018, 23, 130–135. [Google Scholar] [CrossRef]
- Huang, L.-L.; Pan, C.; Wang, L.; Ding, L.; Guo, K.; Wang, H.-Z.; Xu, A.-M.; Gao, S. Protective effects of grape seed proanthocyanidins on cardiovascular remodeling in DOCA-salt hypertension rats. J. Nutr. Biochem. 2015, 26, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, X.; Dai, T.; Liu, C.; Li, T.; McClements, D.J.; Chen, J.; Liu, J. Proanthocyanidins, Isolated from Choerospondias axillaris Fruit Peels, Exhibit Potent Antioxidant Activities In Vitro and a Novel Anti-angiogenic Property In Vitro and In Vivo. J. Agric. Food Chem. 2016, 64, 3546–3556. [Google Scholar] [CrossRef]
- Zhu, W.; Oteiza, P.I. Proanthocyanidins at the gastrointestinal tract: Mechanisms involved in their capacity to mitigate obesity-associated metabolic disorders. Crit. Rev. Food Sci. Nutr. 2024, 64, 220–240. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef] [PubMed]
- Goya, L.; Kongor, J.E.; de Pascual-Teresa, S. From Cocoa to Chocolate: Effect of Processing on Flavanols and Methylxanthines and Their Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 14365. [Google Scholar] [CrossRef]
- Gil, M.; Uribe, D.; Gallego, V.; Bedoya, C.; Arango-Varela, S. Traceability of polyphenols in cocoa during the postharvest and industrialization processes and their biological antioxidant potential. Heliyon 2021, 7, e07738. [Google Scholar] [CrossRef]
- Robbins, R.J.; Leonczak, J.; Li, J.; Johnson, J.C.; Collins, T.; Kwik-Uribe, C.; Schmitz, H.H. Determination of flavanol and procyanidin (by degree of polymerization 1–10) content of chocolate, cocoa liquors, powder(s), and cocoa flavanol extracts by normal phase high-performance liquid chromatography: Collaborative study. J. AOAC Int. 2012, 95, 1153–1160. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Wang, T.K.; Xu, S.; Li, S.; Zhang, Y. Proanthocyanidins Should Be a Candidate in the Treatment of Cancer, Cardiovascular Diseases and Lipid Metabolic Disorder. Molecules 2020, 25, 5971. [Google Scholar] [CrossRef]
- Toden, S.; Ravindranathan, P.; Gu, J.; Cardenas, J.; Yuchang, M.; Goel, A. Oligomeric proanthocyanidins (OPCs) target cancer stem-like cells and suppress tumor organoid formation in colorectal cancer. Sci. Rep. 2018, 8, 3335. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Pang, Y.; Dixon, R.A. The mysteries of proanthocyanidin transport and polymerization. Plant Physiol. 2010, 153, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Xie, D.-Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, R.W.; Foo, L.Y. Condensed tannins: Quinone methide intermediates in procyanidin synthesis. J. Chem. Soc. Chem. Commun. 1983, 1035–1036. [Google Scholar] [CrossRef]
- Hemingway, R.W.; Laks, P.E. Condensed tannins—A proposed route to 2R, 3R-(2,3-Cis)- Proanthocyanidins. J. Chem. Soc. Chem. Comm. 1985, 11, 746–747. [Google Scholar] [CrossRef]
- Yu, K.; Dixon, R.A.; Duan, C. A role for ascorbate conjugates of (+)-catechin in proanthocyanidin polymerization. Nat. Commun. 2022, 13, 3425. [Google Scholar] [CrossRef] [PubMed]
- Di Gennaro, P.; Sabatini, V.; Fallarini, S.; Pagliarin, R.; Sello, G. Polyphenol Polymerization by an Alternative Oxidative Microbial Enzyme and Characterization of the Biological Activity of Oligomers. BioMed Res. Int. 2018, 2018, 3828627. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Pan, Q.; Shi, Y.; Zhang, X.; Duan, C. Identification of autoxidation oligomers of flavan-3-ols in model solutions by HPLC-MS/MS. J. Mass Spectrom. 2009, 44, 633–640. [Google Scholar] [CrossRef]
- Sun, W.; Miller, J.M. Tandem mass spectrometry of the B-type procyanidins in wine and B-type dehydrodicatechins in an autoxidation mixture of (+)-catechin and (−)-epicatechin. J. Mass Spectrom. 2003, 38, 438–446. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.d.M. Extraction Systems and Analytical Techniques for Food Phenolic Compounds: A Review. Foods 2022, 11, 3671. [Google Scholar] [CrossRef] [PubMed]
- Rue, E.A.; Glinski, J.A.; Glinski, V.B.; van Breemen, R.B. Ion mobility-mass spectrometry for the separation and analysis of procyanidins. J. Mass Spectrom. 2020, 55, e4377. [Google Scholar] [CrossRef] [PubMed]
- Merkytė, V.; Longo, E.; Jourdes, M.; Jouin, A.; Teissedre, P.-L.; Boselli, E. High-Performance Liquid Chromatography–Hydrogen/Deuterium Exchange–High-Resolution Mass Spectrometry Partial Identification of a Series of Tetra- and Pentameric Cyclic Procyanidins and Prodelphinidins in Wine Extracts. J. Agric. Food Chem. 2020, 68, 3312–3321. [Google Scholar] [CrossRef] [PubMed]
- Kozikowski, A.P.; Tückmantel, W.; Böttcher, G.; Romanczyk, L.J., Jr. Studies in polyphenol chemistry and bioactivity. 4.1 synthesis of trimeric, tetrameric, pentameric, and higher oligomeric epicatechin-derived procyanidins having all-4β,8-interflavan connectivity and their inhibition of cancer cell growth through cell cycle arrest. J. Org. Chem. 2003, 68, 1641–1658. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-J.; Deinzer, M.L. Tandem mass spectrometry for sequencing proanthocyanidins. Anal. Chem. 2007, 79, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Symma, N.; Hensel, A. Advanced analysis of oligomeric proanthocyanidins: Latest approaches in liquid chromatography and mass spectrometry based analysis. Phytochem. Rev. 2022, 21, 809–833. [Google Scholar] [CrossRef]
- Pereyra-Vergara, F.; Olivares-Corichi, I.M.; Perez-Ruiz, A.G.; Luna-Arias, J.P.; García-Sánchez, J.R. Apoptosis Induced by (−)-Epicatechin in Human Breast Cancer Cells is Mediated by Reactive Oxygen Species. Molecules 2020, 25, 1020. [Google Scholar] [CrossRef]
- Zeng, Y.-X.; Wang, S.; Wei, L.; Cui, Y.-Y.; Chen, Y.-H. Proanthocyanidins: Components, Pharmacokinetics and Biomedical Properties. Am. J. Chin. Med. 2020, 48, 813–869. [Google Scholar] [CrossRef]
- Kari, S.; Subramanian, K.; Altomonte, I.A.; Murugesan, A.; Yli-Harja, O.; Kandhavelu, M. Programmed cell death detection methods: A systematic review and a categorical comparison. Apoptosis 2022, 27, 482–508. [Google Scholar] [CrossRef]
- Boice, A.; Bouchier-Hayes, L. Targeting apoptotic caspases in cancer. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2020, 1867, 118688. [Google Scholar] [CrossRef]
- Dorstyn, L.; Akey, C.W.; Kumar, S. New insights into apoptosome structure and function. Cell Death Differ. 2018, 25, 1194–1208. [Google Scholar] [CrossRef]
- Freilich, R.; Arhar, T.; Abrams, J.L.; Gestwicki, J.E. Protein–Protein Interactions in the Molecular Chaperone Network. Accounts Chem. Res. 2018, 51, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lostao, L.; de Miguel, D.; Anel, A.; Naval, J. APO2L/TRAIL. In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Yamada, K.; Yoshida, K. Mechanical insights into the regulation of programmed cell death by p53 via mitochondria. Biochim. et Biophys. Acta (BBA)—Mol. Cell Res. 2019, 1866, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Dybczynski, I.; Plucienniczak, A. A protocol for DNA fragment extraction from polyacrylamide gels. Biotechniques 1988, 6, 924–926. [Google Scholar] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio-Cruz, Y.; Olivares-Corichi, I.M.; Correa-Basurto, J.; González-Garrido, J.A.; Pereyra-Vergara, F.; Rivera, G.; García-Sánchez, J.R. The Autoxidized Mixture of (-)-Epicatechin Contains Procyanidins and Shows Antiproliferative and Apoptotic Activity in Breast Cancer Cells. Pharmaceuticals 2024, 17, 258. https://doi.org/10.3390/ph17020258
Osorio-Cruz Y, Olivares-Corichi IM, Correa-Basurto J, González-Garrido JA, Pereyra-Vergara F, Rivera G, García-Sánchez JR. The Autoxidized Mixture of (-)-Epicatechin Contains Procyanidins and Shows Antiproliferative and Apoptotic Activity in Breast Cancer Cells. Pharmaceuticals. 2024; 17(2):258. https://doi.org/10.3390/ph17020258
Chicago/Turabian StyleOsorio-Cruz, Yazmin, Ivonne María Olivares-Corichi, José Correa-Basurto, José Arnold González-Garrido, Fernando Pereyra-Vergara, Gildardo Rivera, and José Rubén García-Sánchez. 2024. "The Autoxidized Mixture of (-)-Epicatechin Contains Procyanidins and Shows Antiproliferative and Apoptotic Activity in Breast Cancer Cells" Pharmaceuticals 17, no. 2: 258. https://doi.org/10.3390/ph17020258
APA StyleOsorio-Cruz, Y., Olivares-Corichi, I. M., Correa-Basurto, J., González-Garrido, J. A., Pereyra-Vergara, F., Rivera, G., & García-Sánchez, J. R. (2024). The Autoxidized Mixture of (-)-Epicatechin Contains Procyanidins and Shows Antiproliferative and Apoptotic Activity in Breast Cancer Cells. Pharmaceuticals, 17(2), 258. https://doi.org/10.3390/ph17020258