The Role of Small Molecules Containing Fluorine Atoms in Medicine and Imaging Applications
Abstract
:1. Introduction
2. Common Reactions Incorporating Fluorine in Small Molecules
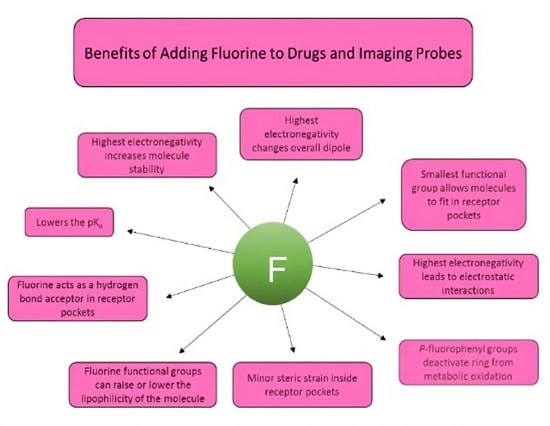
3. Characteristics of Pharmaceuticals Featuring Fluorine and Examples
3.1. Metabolic Oxidation
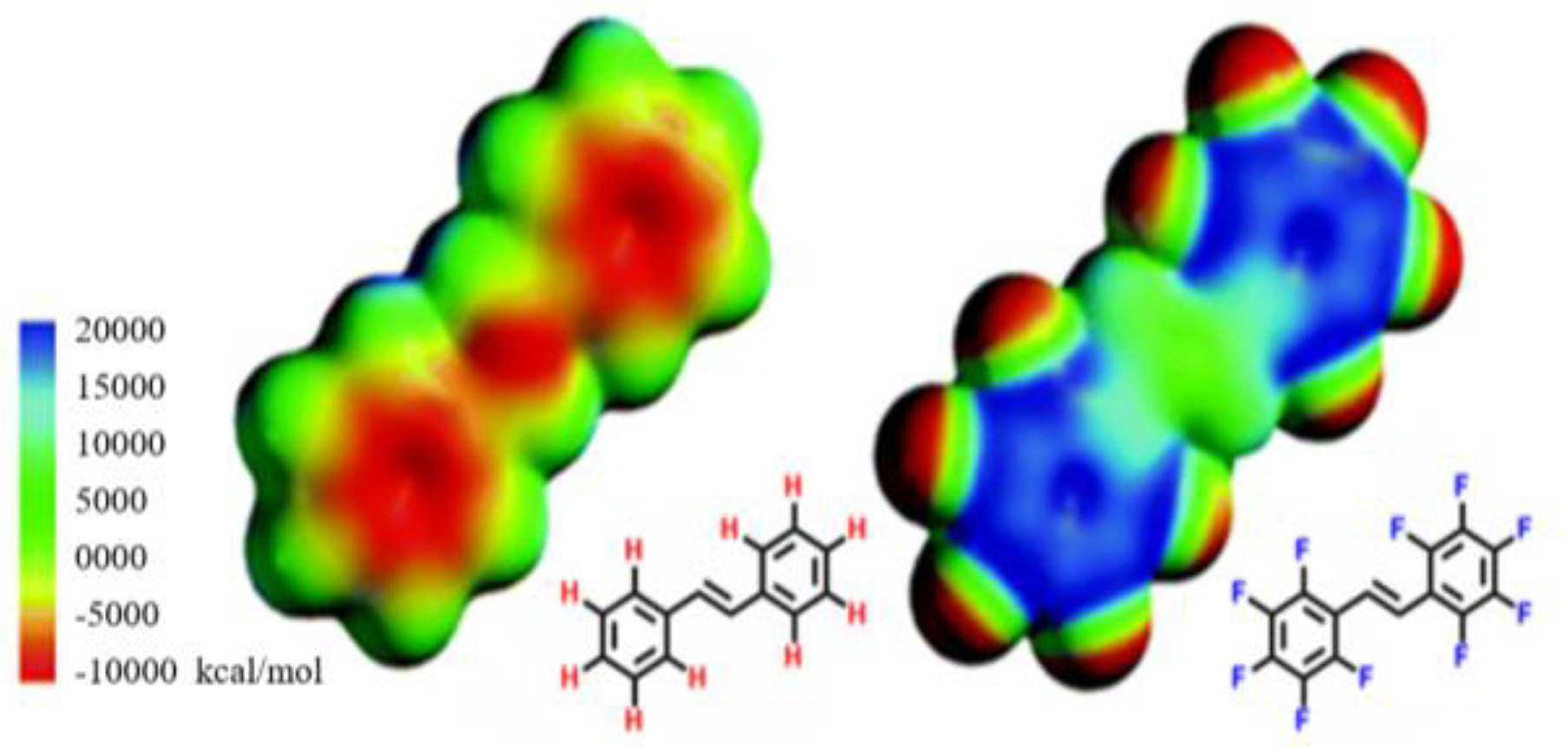
3.2. Electronic Considerations
3.3. Size Considerations
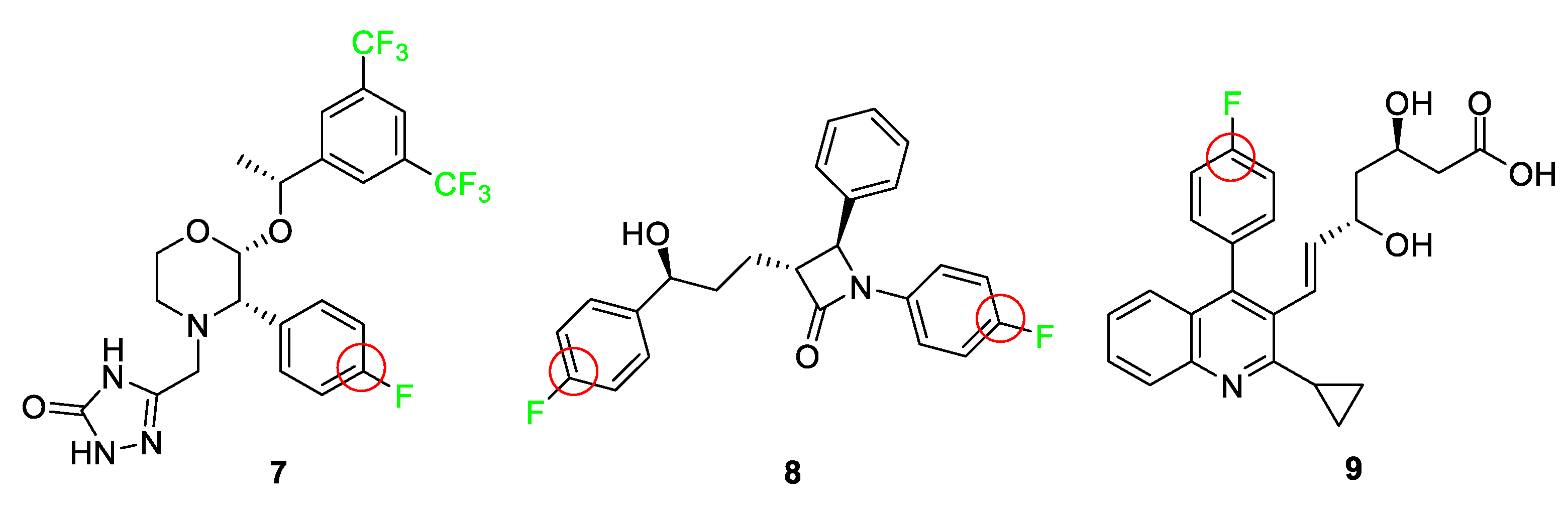
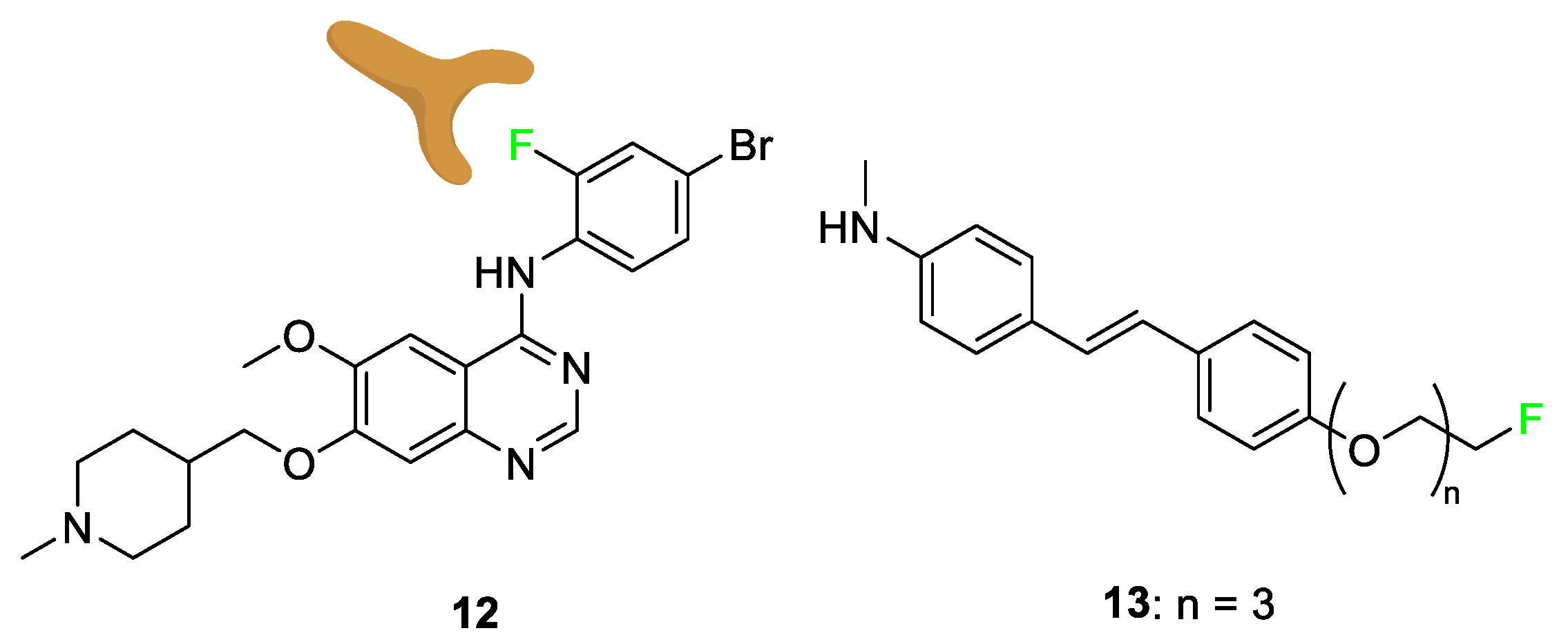
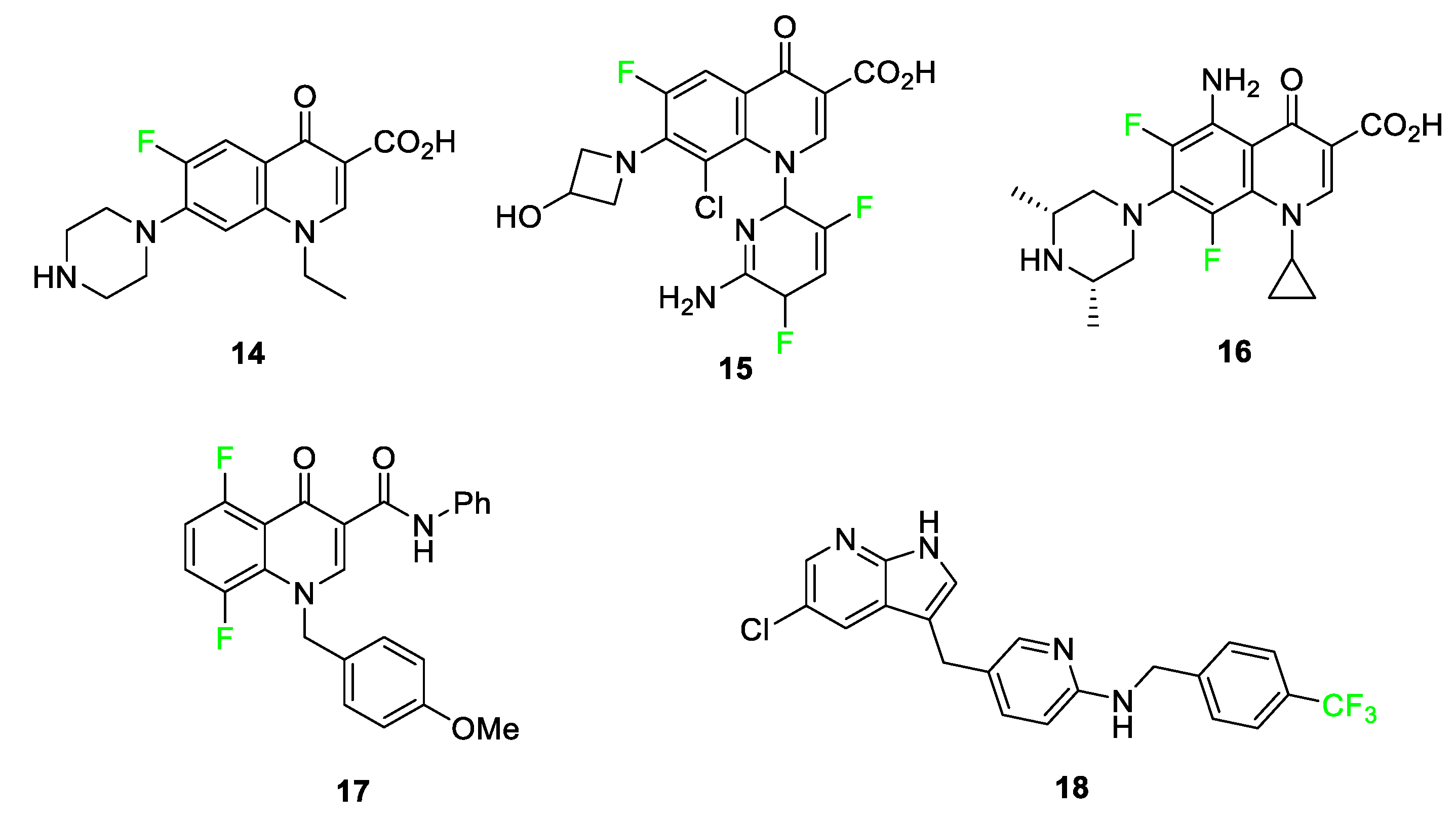
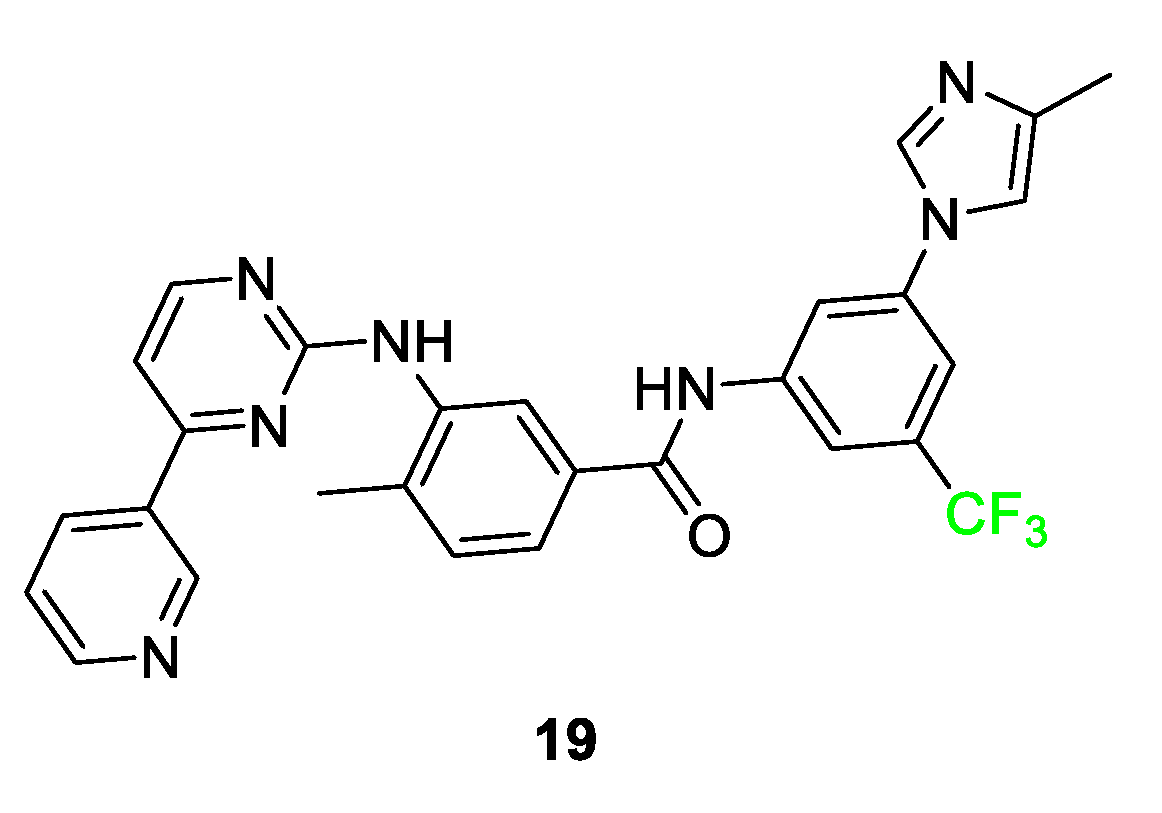
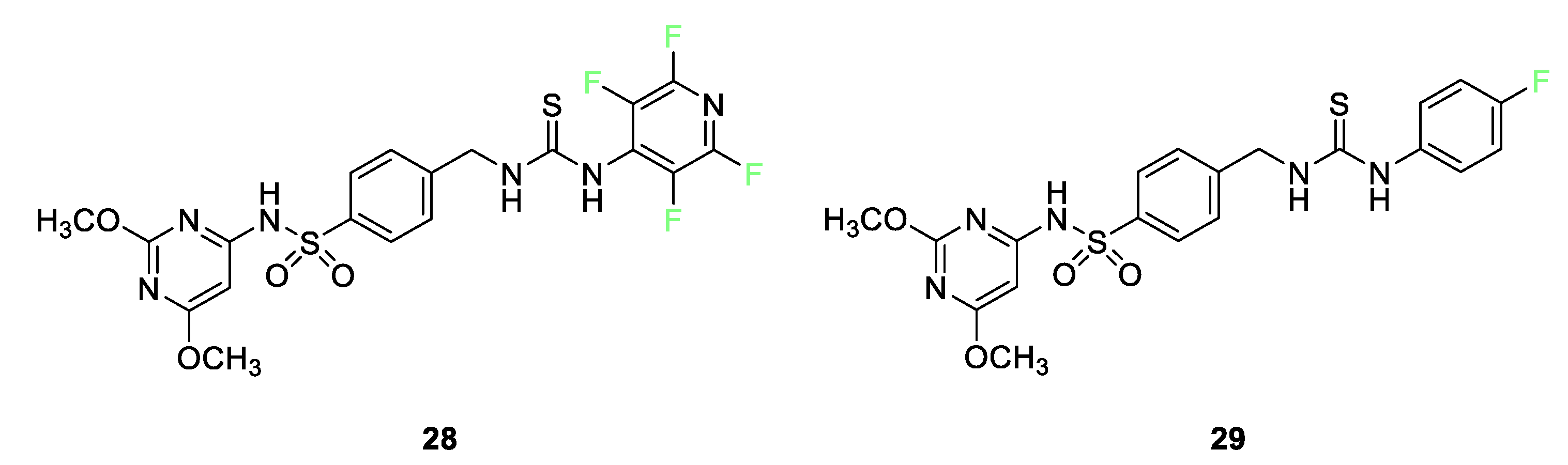
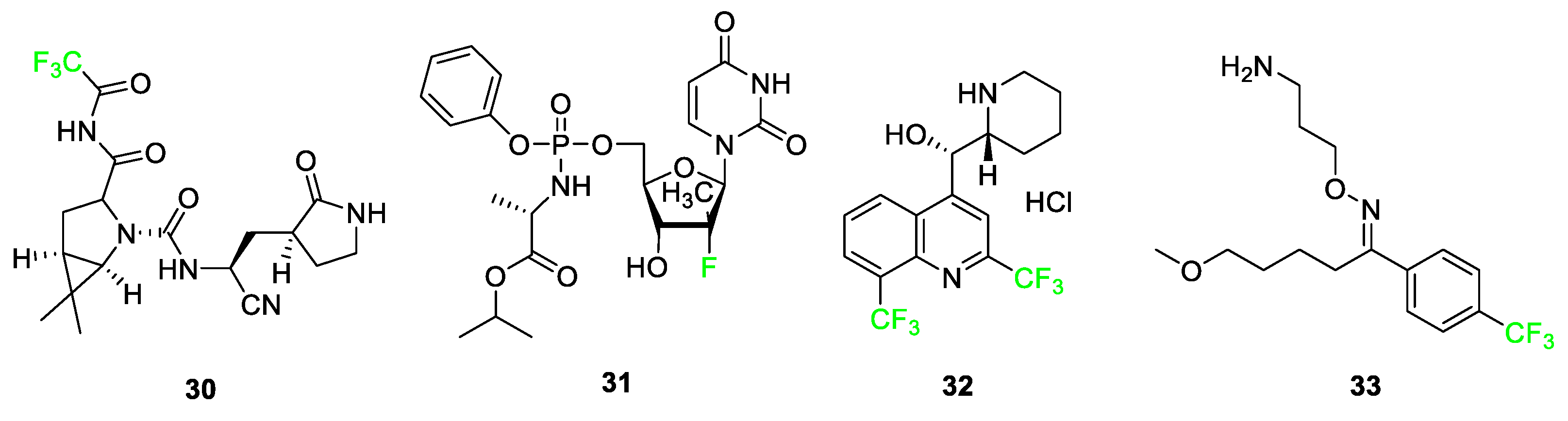
3.4. Examples of Pharmaceuticals Containing Fluorine

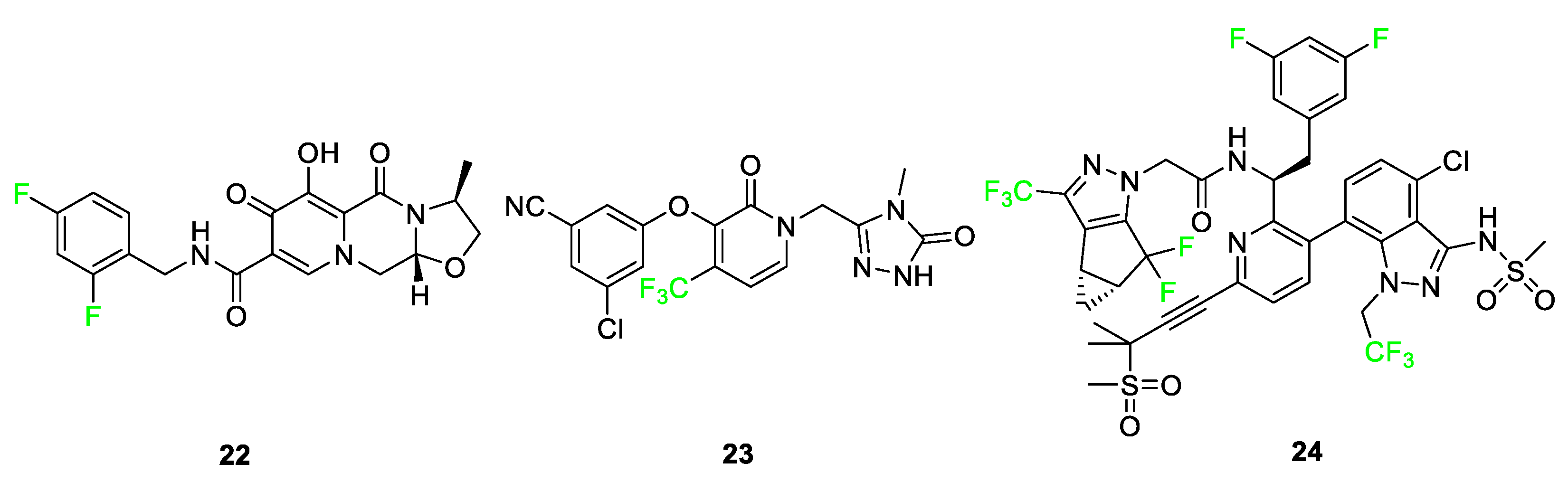
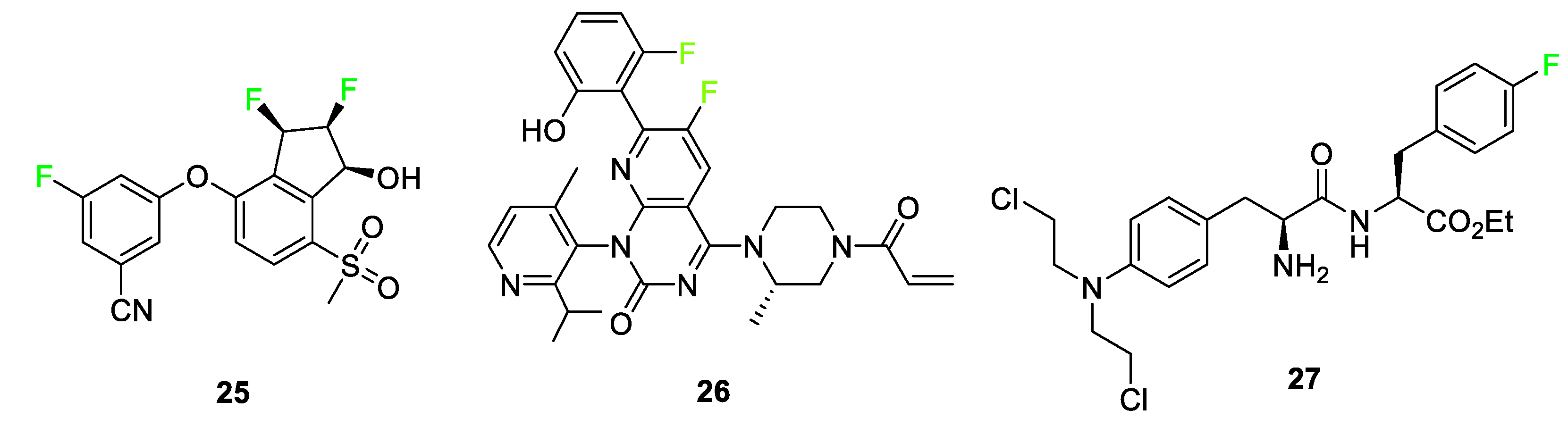
4. Fluorine Incorporated into Dye Imaging Agents
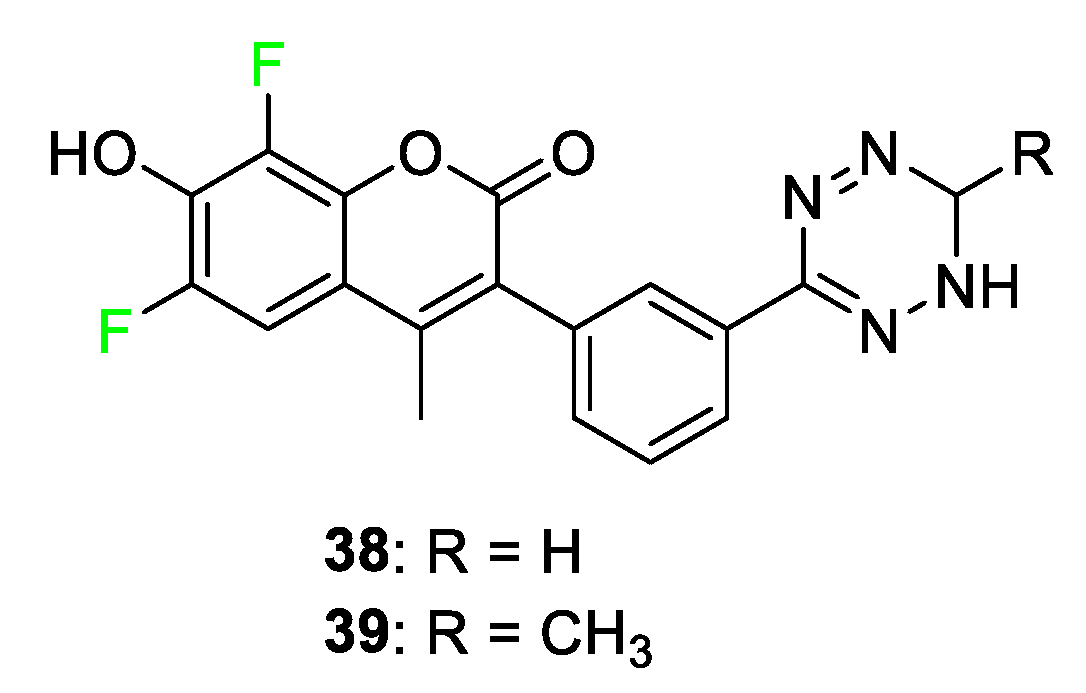
4.1. Coumarin Dyes
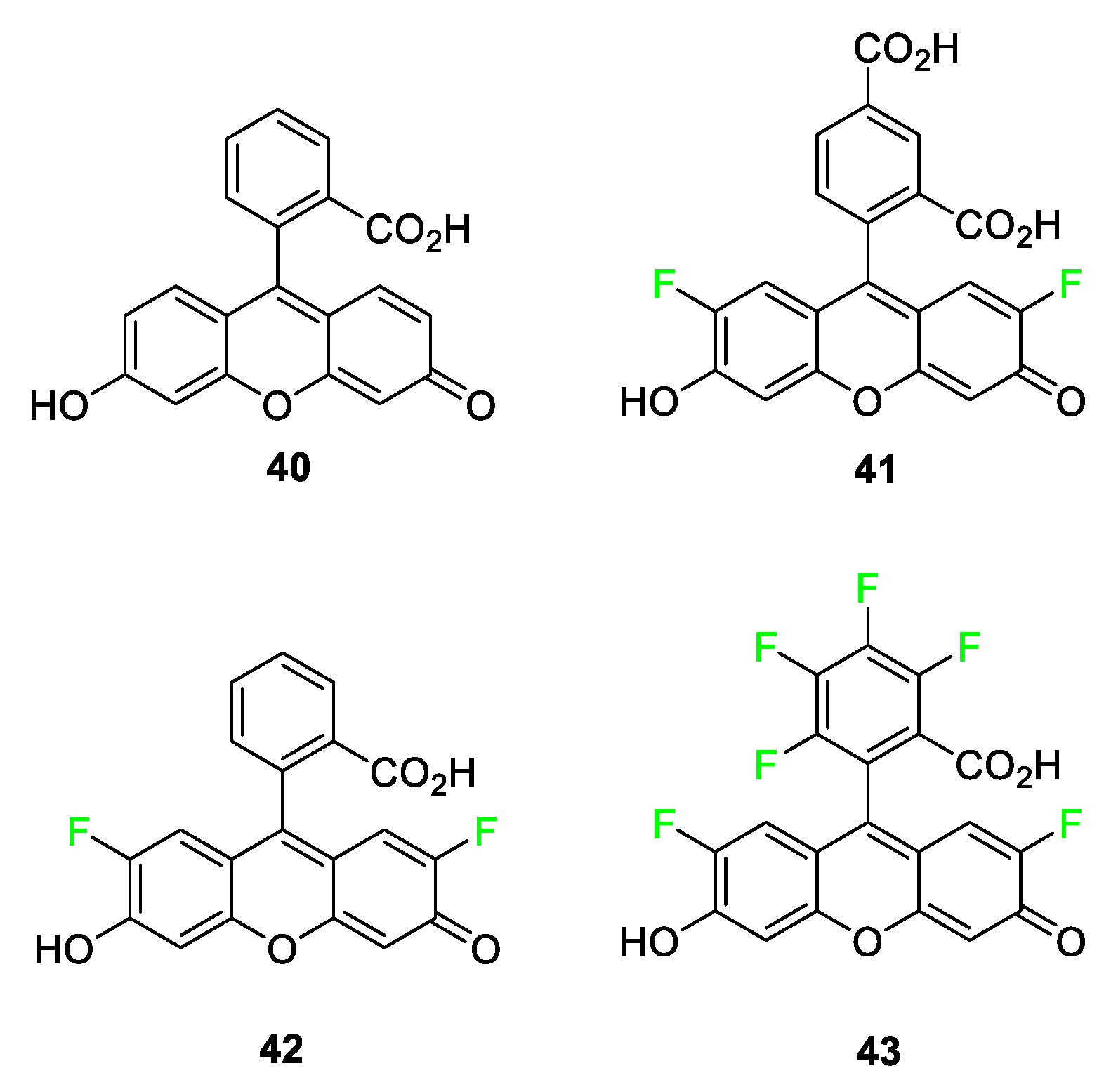
4.2. Fluorescein/Rhodamine Dyes
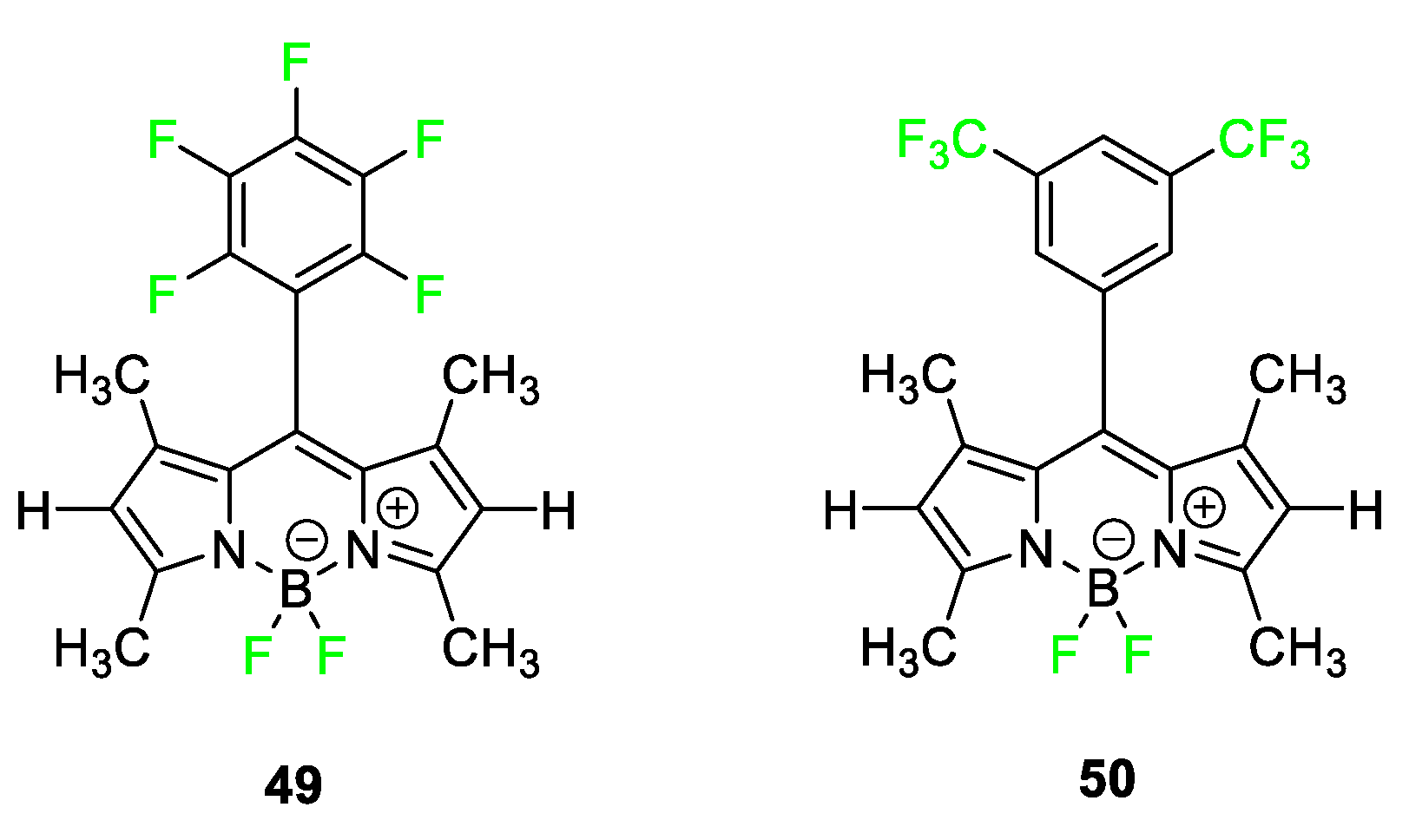
4.3. Boron–Dipyrromethene Dyes
4.4. Carbocyanine Dyes
4.5. Squaraine Dyes
5. Fluorine Incorporated into Molecules for Early Disease Detection Imaging Agents
Fluorine in PET
6. Summary and Outlook
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzym. Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Han, J.; Sorochinsky, A.; Landa, A.; Butler, G.; Soloshonok, V.A. The Latest FDA-Approved Pharmaceuticals Containing Fragments of Tailor-Made Amino Acids and Fluorine. Pharmaceuticals 2022, 15, 999. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, Z.; Dhawan, G.; Zhang, W.; Sorochinsky, A.E.; Butler, G.; Soloshonok, V.A.; Han, J. Fluorine-containing drugs approved by the FDA in 2021. Chin. Chem. Lett. 2022, 34, 107578. [Google Scholar] [CrossRef]
- Arntson, K.E.; Pomerantz, W.C.K. Protein-Observed Fluorine NMR: A Bioorthogonal Approach for Small Molecule Discovery. J. Med. Chem. 2016, 59, 5158–5171. [Google Scholar] [CrossRef] [PubMed]
- Smart, B.E. Fluorine substituent effects (on bioactivity). J. Fluor. Chem. 2001, 109, 3–11. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef]
- Reichenbächer, K.; Süss, H.I.; Hulliger, J. Fluorine in crystal engineering—“The little atom that could”. Chem. Soc. Rev. 2005, 34, 22–30. [Google Scholar] [CrossRef]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Aradi, K.; Kiss, L. Carbotrifluoromethylations of C−C Multiple Bonds (Excluding Aryl-and Alkynyltrifluoromethylations). Chem. A Eur. J. 2023, 29, e202203499. [Google Scholar] [CrossRef] [PubMed]
- Alle, T.; Joyasawal, S.; Oukoloff, K.; Long, K.; Owyang, Z.; Francisco, K.R.; Cahard, D.; Huryn, D.M.; Ballatore, C. Structure-property relationships of fluorinated carboxylic acid bioisosteres. Bioorganic Med. Chem. Lett. 2023, 91, 129363. [Google Scholar] [CrossRef]
- Han, S.; Lu, Y. Fluorine in anti-HIV drugs approved by FDA from 1981 to 2023. Eur. J. Med. Chem. 2023, 258, 115586. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.M.; Shu, Y.-Z.; Zhuo, X.; Meanwell, N.A. Metabolic and Pharmaceutical Aspects of Fluorinated Compounds. J. Med. Chem. 2020, 63, 6315–6386. [Google Scholar] [CrossRef]
- Casa, S.; Henary, M. Synthesis and Applications of Selected Fluorine-Containing Fluorophores. Molecules 2021, 26, 1160. [Google Scholar] [CrossRef]
- Hyun, H.; Park, M.H.; Owens, E.A.; Wada, H.; Henary, M.; Handgraaf, H.J.M.; Vahrmeijer, A.L.; Frangioni, J.V.; Choi, H.S. Structure-inherent targeting of near-infrared fluorophores for parathyroid and thyroid gland imaging. Nat. Med. 2015, 21, 192–197. [Google Scholar] [CrossRef]
- Zhou, P.; Zou, J.; Tian, F.; Shang, Z. Fluorine Bonding—How Does It Work In Protein−Ligand Interactions? J. Chem. Inf. Model. 2009, 49, 2344–2355. [Google Scholar] [CrossRef]
- Britton, R.; Gouverneur, V.; Lin, J.-H.; Meanwell, M.; Ni, C.; Pupo, G.; Xiao, J.-C.; Hu, J. Contemporary synthetic strategies in organofluorine chemistry. Nat. Rev. Methods Primers 2021, 1, 47. [Google Scholar] [CrossRef]
- Liang, T.; Neumann, C.N.; Ritter, T. Introduction of Fluorine and Fluorine-Containing Functional Groups. Angew. Chem. Int. Ed. 2013, 52, 8214–8264. [Google Scholar] [CrossRef]
- Preparation of Highly Fluorinated Compounds. In Fluorine in Organic Chemistry; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2004; pp. 23–46.
- Zhou, G.; Tian, Y.; Zhao, X.; Dan, W. Selective Fluorination of 4-Substituted 2-Aminopyridines and Pyridin-2(1H)-ones in Aqueous Solution. Org. Lett. 2018, 20, 4858–4861. [Google Scholar] [CrossRef] [PubMed]
- Lerman, O.; Tor, Y.; Hebel, D.; Rozen, S. A novel electrophilic fluorination of activated aromatic rings using acetyl hypofluorite, suitable also for introducing fluorine-18 into benzene. J. Org. Chem. 1984, 49, 806–813. [Google Scholar] [CrossRef]
- Furuya, T.; Kamlet, A.S.; Ritter, T. Catalysis for fluorination and trifluoromethylation. Nature 2011, 473, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Sloop, J.C.; Holder, C.; Henary, M. Selective Incorporation of Fluorine in Pyrazoles. Eur. J. Org. Chem. 2015, 2015, 3405–3422. [Google Scholar] [CrossRef]
- Yang, X.; Sun, R.; Li, S.; Zheng, X.; Yuan, M.; Xu, B.; Jiang, W.; Chen, H.; Fu, H.; Li, R. Regioselective Direct C–H Trifluoromethylation of Pyridine. Org. Lett. 2020, 22, 7108–7112. [Google Scholar] [CrossRef]
- Liang, D.; Li, Y.; Gao, S.; Li, R.; Li, X.; Wang, B.; Yang, H. Amide-assisted radical strategy: Metal-free direct fluorination of arenes in aqueous media. Green Chem. 2017, 19, 3344–3349. [Google Scholar] [CrossRef]
- Park, B.K.; Kitteringham, N.R.; O’Neill, P.M. Metabolism of Fluorine-Containing Drugs. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 443–470. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.-E.F.; Kamel, A.M.; Clarimont, C. Improving the decision-making process in the structural modification of drug candidates: Enhancing metabolic stability. Drug Discov. Today 2004, 9, 1020–1028. [Google Scholar] [CrossRef]
- Hagmann, W.K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef]
- Schoepfer, J.; Jahnke, W.; Berellini, G.; Buonamici, S.; Cotesta, S.; Cowan-Jacob, S.W.; Dodd, S.; Drueckes, P.; Fabbro, D.; Gabriel, T.; et al. Discovery of Asciminib (ABL001), an Allosteric Inhibitor of the Tyrosine Kinase Activity of BCR-ABL1. J. Med. Chem. 2018, 61, 8120–8135. [Google Scholar] [CrossRef]
- Szarek, M.; Amarenco, P.; Callahan, A.; DeMicco, D.; Fayyad, R.; Goldstein, L.B.; Laskey, R.; Sillesen, H.; Welch, K.M. Atorvastatin Reduces First and Subsequent Vascular Events Across Vascular Territories: The SPARCL Trial. J. Am. Coll. Cardiol. 2020, 75, 2110–2118. [Google Scholar] [CrossRef]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, X.; Cheng, W.; Han, Y.; Mu, Q.; Li, J.; He, S. Effects of escitalopram oxalate plus low-dose trazodone on psychological state and quality of life in patients with treatment-refractory depression. Am. J. Transl. Res. 2023, 15, 1905–1912. [Google Scholar] [PubMed]
- Larsen, M.A.B.; Plenge, P.; Andersen, J.; Eildal, J.N.N.; Kristensen, A.S.; Bøgesø, K.P.; Gether, U.; Strømgaard, K.; Bang-Andersen, B.; Loland, C.J. Structure–activity relationship studies of citalopram derivatives: Examining substituents conferring selectivity for the allosteric site in the 5-HT transporter. Br. J. Pharmacol. 2016, 173, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Chandra, G.; Singh, D.V.; Mahato, G.K.; Patel, S. Fluorine-a small magic bullet atom in the drug development: Perspective to FDA approved and COVID-19 recommended drugs. Chem. Pap. 2023, 77, 4085–4106. [Google Scholar] [CrossRef] [PubMed]
- Ailani, J.; Lipton, R.B.; Goadsby, P.J.; Guo, H.; Miceli, R.; Severt, L.; Finnegan, M.; Trugman, J.M. Atogepant for the Preventive Treatment of Migraine. N. Engl. J. Med. 2021, 385, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, H.H.; Scadding, G.K. Seasonal allergic rhinitis: Fluticasone propionate and fluticasone furoate therapy evaluated. J. Asthma Allergy 2010, 3, 19–28. [Google Scholar] [PubMed]
- Kumar, R.S.; Jain, M.K.; Kushwaha, J.S.; Patil, S.; Patil, V.; Ghatak, S.; Sanmukhani, J.; Mittal, R. Efficacy and Safety of Fluticasone Furoate and Oxymetazoline Nasal Spray: A Novel First Fixed Dose Combination for the Management of Allergic Rhinitis with Nasal Congestion. J. Asthma Allergy 2022, 15, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, P.; Chen, B.; Li, X.; Luo, X.; Chen, X.; Xiang, M.; Li, L.; Zhao, S.; Xiao, X.; et al. Intranasal fluticasone furoate in pediatric allergic rhinitis: Randomized controlled study. Pediatr. Res. 2021, 89, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, M.; Mao, Y. Efficacy of Fluticasone and Salmeterol Dry Powder in Treating Patients with Bronchial Asthma and Its Effect on Inflammatory Factors and Pulmonary Function. Evid. Based Complement. Altern. Med. Ecam 2022, 2022, 8555417. [Google Scholar] [CrossRef]
- Prabhudesai, P.; Singh, B.P.; Agrawal, G.; Singh, A.K.; Jadhav, A.Y.; Patil, S.R.; Bhagat, S.; Patil, S.; Barkate, H. Fluticasone Furoate/Vilanterol Use Trends and Characteristics in Patients with Obstructive Airway Disease: A Real-World Study of 10,374 Patients from India. Cureus 2023, 15, e34825. [Google Scholar] [CrossRef]
- Crim, C.; Pierre, L.N.; Daley-Yates, P.T. A review of the pharmacology and pharmacokinetics of inhaled fluticasone propionate and mometasone furoate. Clin. Ther. 2001, 23, 1339–1354. [Google Scholar] [CrossRef]
- Ali, S.; Zhou, J. Highlights on U.S. FDA-approved fluorinated drugs over the past five years (2018–2022). Eur. J. Med. Chem. 2023, 256, 115476. [Google Scholar] [CrossRef]
- Lin, H.-C.; Saputra, F.; Audira, G.; Lai, Y.-H.; Roldan, M.J.M.; Alos, H.C.; Aventurado, C.A.; Vasquez, R.D.; Tsai, G.-J.; Lim, K.-H.; et al. Investigating Potential Cardiovascular Toxicity of Two Anti-Leukemia Drugs of Asciminib and Ponatinib in Zebrafish Embryos. Int. J. Mol. Sci. 2022, 23, 11711. [Google Scholar] [CrossRef]
- Deeks, E.D. Asciminib: First Approval. Drugs 2022, 82, 219–226. [Google Scholar] [CrossRef]
- Rizzo, C.; Amata, S.; Pibiri, I.; Pace, A.; Buscemi, S.; Palumbo Piccionello, A. FDA-Approved Fluorinated Heterocyclic Drugs from 2016 to 2022. Int. J. Mol. Sci. 2023, 24, 7728. [Google Scholar] [CrossRef] [PubMed]
- Dubowchik, G.M.; Conway, C.M.; Xin, A.W. Blocking the CGRP Pathway for Acute and Preventive Treatment of Migraine: The Evolution of Success. J. Med. Chem. 2020, 63, 6600–6623. [Google Scholar] [CrossRef] [PubMed]
- Martelletti, P.; Cipolla, F.; Capi, M.; Curto, M.; Lionetto, L. Atogepant. Calcitonin gene-related peptide (CGRP) receptor antagonist, Preventive treatment of migraine. Drugs Futur. 2020, 45, 285. [Google Scholar] [CrossRef]
- Benedetto Tiz, D.; Bagnoli, L.; Rosati, O.; Marini, F.; Sancineto, L.; Santi, C. New Halogen-Containing Drugs Approved by FDA in 2021: An Overview on Their Syntheses and Pharmaceutical Use. Molecules 2022, 27, 1643. [Google Scholar] [CrossRef] [PubMed]
- Di Fabio, R.; Alvaro, G.; Griffante, C.; Pizzi, D.A.; Donati, D.; Mattioli, M.; Cimarosti, Z.; Guercio, G.; Marchioro, C.; Provera, S.; et al. Discovery and Biological Characterization of (2R,4S)-1′-Acetyl-N-{(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethyl}-2-(4-fluoro-2-methylphenyl)-N-methyl-4,4′-bipiperidine-1-carboxamide as a New Potent and Selective Neurokinin 1 (NK1) Receptor Antagonist Clinical Candidate. J. Med. Chem. 2011, 54, 1071–1079. [Google Scholar]
- Wang, D.-S.; Hu, M.-T.; Wang, Z.-Q.; Ren, C.; Qiu, M.-Z.; Luo, H.-Y.; Jin, Y.; Fong, W.P.; Wang, S.-B.; Peng, J.-W.; et al. Effect of Aprepitant for the Prevention of Chemotherapy-Induced Nausea and Vomiting in Women: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e215250. [Google Scholar] [CrossRef]
- Van Heek, M.; Farley, C.; Compton, D.S.; Hoos, L.; Alton, K.B.; Sybertz, E.J.; Davis, H.R., Jr. Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663. Br. J. Pharmacol. 2000, 129, 1748–1754. [Google Scholar] [CrossRef]
- Igel, M.; Sudhop, T.; von Bergmann, K. Pharmacology of 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Inhibitors (Statins), Including Rosuvastatin and Pitavastatin. J. Clin. Pharmacol. 2002, 42, 835–845. [Google Scholar] [CrossRef]
- Hak Lee, S.; Chung, N.; Kwan, J.; Kim, D.-I.; Ho Kim, W.; Jeong Kim, C.; Seung Kim, H.; Hoon Park, S.; Seog Seo, H.; Gu Shin, D.; et al. Comparison of the efficacy and tolerability of pitavastatin and atorvastatin: An 8-week, multicenter, randomized, open-label, dose-titration study in korean patients with hypercholesterolemia. Clin. Ther. 2007, 29, 2365–2373. [Google Scholar] [CrossRef]
- Xu, Q.; Deng, Y.; Xiao, J.; Liu, X.; Zhou, M.; Ren, Z.; Peng, J.; Tang, Y.; Jiang, Z.; Tang, Z.; et al. Three Musketeers for Lowering Cholesterol: Statins, Ezetimibe and Evolocumab. Curr. Med. Chem. 2021, 28, 1025–1041. [Google Scholar] [CrossRef]
- Saito, Y. Pitavastatin: An overview. Atheroscler. Suppl. 2011, 12, 271–276. [Google Scholar] [CrossRef]
- Saito, Y.; Yamada, N.; Teramoto, T.; Itakura, H.; Hata, Y.; Nakaya, N.; Mabuchi, H.; Tushima, M.; Sasaki, J.; Goto, Y.; et al. Clinical Efficacy of Pitavastatin, a New 3-Hydroxy-3-methylglutaryl Coenzyme A Reductase Inhibitor, in Patients with Hyperlipidemia. Arzneimittelforschung 2002, 52, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Yagi, Y.; Kimura, H.; Okuda, H.; Ono, M.; Nakamoto, Y.; Togashi, K.; Saji, H. Evaluation of [18F]pitavastatin as a positron emission tomography tracer for in vivo organic transporter polypeptide function. Nucl. Med. Biol. 2019, 74–75, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wakeling, A.E.; Dukes, M.; Bowler, J. A Potent Specific Pure Antiestrogen with Clinical Potential. Cancer Res. 1991, 51, 3867–3873. [Google Scholar] [PubMed]
- Troup, R.I.; Jeffries, B.; Saudain, R.E.-B.; Georgiou, E.; Fish, J.; Scott, J.S.; Chiarparin, E.; Fallan, C.; Linclau, B. Skipped Fluorination Motifs: Synthesis of Building Blocks and Comparison of Lipophilicity Trends with Vicinal and Isolated Fluorination Motifs. J. Org. Chem. 2021, 86, 1882–1900. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, B.; Wang, Z.; Felstead, H.R.; Le Questel, J.-Y.; Scott, J.S.; Chiarparin, E.; Graton, J.; Linclau, B. Systematic Investigation of Lipophilicity Modulation by Aliphatic Fluorination Motifs. J. Med. Chem. 2020, 63, 1002–1031. [Google Scholar] [CrossRef] [PubMed]
- Kürschner, M.; Nielsen, K.; von Langen, J.R.G.; Schenk, W.A.; Zimmermann, U.; Sukhorukov, V.L. Effect of Fluorine Substitution on the Interaction of Lipophilic Ions with the Plasma Membrane of Mammalian Cells. Biophys. J. 2000, 79, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Yerien, D.E.; Bonesi, S.; Postigo, A. Fluorination methods in drug discovery. Org. Biomol. Chem. 2016, 14, 8398–8427. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, B.; Wang, Z.; Graton, J.; Holland, S.D.; Brind, T.; Greenwood, R.D.R.; Le Questel, J.-Y.; Scott, J.S.; Chiarparin, E.; Linclau, B. Reducing the Lipophilicity of Perfluoroalkyl Groups by CF2–F/CF2–Me or CF3/CH3 Exchange. J. Med. Chem. 2018, 61, 10602–10618. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.J.; Wedge, S.R. ZD6474–a novel inhibitor of VEGFR and EGFR tyrosine kinase activity. Br. J. Cancer 2005, 92, S6–S13. [Google Scholar] [CrossRef] [PubMed]
- Kung, H.F.; Choi, S.R.; Qu, W.; Zhang, W.; Skovronsky, D. 18F Stilbenes and Styrylpyridines for PET Imaging of Aβ Plaques in Alzheimer’s Disease: A Miniperspective. J. Med. Chem. 2010, 53, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Ragab, F.A.; Heiba, H.I.; Arafa, R.K.; El-Hossary, E.M. Docking study, in vitro anticancer screening and radiosensitizing evaluation of some new fluorine-containing quinoline and pyrimidoquinoline derivatives bearing a sulfonamide moiety. Med. Chem. Res. 2011, 20, 388–400. [Google Scholar] [CrossRef]
- Kathrotiya, H.G.; Patel, M.P. Synthesis and identification of β-aryloxyquinoline based diversely fluorine substituted N-aryl quinolone derivatives as a new class of antimicrobial, antituberculosis and antioxidant agents. Eur. J. Med. Chem. 2013, 63, 675–684. [Google Scholar] [CrossRef]
- Mistry, S.N.; Valant, C.; Sexton, P.M.; Capuano, B.; Christopoulos, A.; Scammells, P.J. Synthesis and Pharmacological Profiling of Analogues of Benzyl Quinolone Carboxylic Acid (BQCA) as Allosteric Modulators of the M1 Muscarinic Receptor. J. Med. Chem. 2013, 56, 5151–5172. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Li, J.; Liu, Y. Exploratory Process Development of Pexidartinib through the Tandem Tsuji–Trost Reaction and Heck Coupling. Synthesis 2019, 51, 2564–2571. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Giles, F.; Gattermann, N.; Bhalla, K.; Alimena, G.; Palandri, F.; Ossenkoppele, G.J.; Nicolini, F.-E.; O’Brien, S.G.; Litzow, M.; et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome–positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood 2007, 110, 3540–3546. [Google Scholar] [CrossRef]
- Kuang, Y.-H.; Shen, T.; Chen, X.; Sodani, K.; Hopper-Borge, E.; Tiwari, A.K.; Lee, J.W.K.K.; Fu, L.-W.; Chen, Z.-S. Lapatinib and erlotinib are potent reversal agents for MRP7 (ABCC10)-mediated multidrug resistance. Biochem. Pharmacol. 2010, 79, 154–161. [Google Scholar] [CrossRef]
- Popovici-Muller, J.; Lemieux, R.M.; Artin, E.; Saunders, J.O.; Salituro, F.G.; Travins, J.; Cianchetta, G.; Cai, Z.; Zhou, D.; Cui, D.; et al. Discovery of AG-120 (Ivosidenib): A First-in-Class Mutant IDH1 Inhibitor for the Treatment of IDH1 Mutant Cancers. ACS Med. Chem. Lett. 2018, 9, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.S. Roles of Fluorine in Drug Design and Drug Action. Lett. Drug Des. Discov. 2019, 16, 1089–1109. [Google Scholar] [CrossRef]
- Dabur, M.; Loureiro, J.A.; Pereira, M.C. Fluorinated Molecules and Nanotechnology: Future ‘Avengers’ against the Alzheimer’s Disease? Int. J. Mol. Sci. 2020, 21, 2989. [Google Scholar] [CrossRef]
- Dhillon, S. Melphalan Flufenamide (Melflufen): First Approval. Drugs 2021, 81, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, F.; Wennerberg, J. Melflufen: A Journey from Discovery to Multi-Kilogram Production. In Complete Accounts of Integrated Drug Discovery and Development: Recent Examples from the Pharmaceutical Industry Volume 3; American Chemical Society: Washington, DC, USA, 2020; Volume 1369, pp. 157–177. [Google Scholar]
- Mei, H.; Han, J.; Fustero, S.; Medio-Simon, M.; Sedgwick, D.M.; Santi, C.; Ruzziconi, R.; Soloshonok, V.A. Fluorine-Containing Drugs Approved by the FDA in 2018. Chem. A Eur. J. 2019, 25, 11797–11819. [Google Scholar] [CrossRef]
- El-Gaby, M.; Ammar, Y.A.; IH El-Qaliei, M.; Hussein, M.F.; Faraghally, F.A. Sulfonamides: Synthesis and the recent applications in Medicinal Chemistry. Egypt. J. Chem. 2020, 63, 5289–5327. [Google Scholar]
- Berrino, E.; Michelet, B.; Martin-Mingot, A.; Carta, F.; Supuran, C.T.; Thibaudeau, S. Modulating the Efficacy of Carbonic Anhydrase Inhibitors through Fluorine Substitution. Angew. Chem. Int. Ed. 2021, 60, 23068–23082. [Google Scholar] [CrossRef]
- Vullo, D.; Scozzafava, A.; Pastorekova, S.; Pastorek, J.; Supuran, C.T. Carbonic anhydrase inhibitors: Inhibition of the tumor-associated isozyme IX with fluorine-containing sulfonamides. The first subnanomolar CA IX inhibitor discovered. Bioorg. Med. Chem. Lett. 2004, 14, 2351–2356. [Google Scholar] [CrossRef]
- Pastorekova, S.; Vullo, D.; Casini, A.; Scozzafava, A.; Pastorek, J.; Nishimori, I.; Supuran, C.T. Carbonic anhydrase inhibitors: Inhibition of the tumor-associated isozymes IX and XII with polyfluorinated aromatic/heterocyclic sulfonamides. J. Enzym. Inhib. Med. Chem. 2005, 20, 211–217. [Google Scholar] [CrossRef]
- Pacchiano, F.; Aggarwal, M.; Avvaru, B.S.; Robbins, A.H.; Scozzafava, A.; McKenna, R.; Supuran, C.T. Selective hydrophobic pocket binding observed within the carbonic anhydrase II active site accommodate different 4-substituted-ureido-benzenesulfonamides and correlate to inhibitor potency. Chem. Commun. 2010, 46, 8371–8373. [Google Scholar] [CrossRef] [PubMed]
- Compain, G.; Martin-Mingot, A.; Maresca, A.; Thibaudeau, S.; Supuran, C.T. Superacid synthesis of halogen containing N-substituted-4-aminobenzene sulfonamides: New selective tumor-associated carbonic anhydrase inhibitors. Bioorg. Med. Chem. 2013, 21, 1555–1563. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef]
- Welsher, K.; Sherlock, S.P.; Dai, H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc. Natl. Acad. Sci. USA 2011, 108, 8943–8948. [Google Scholar] [CrossRef]
- Drexhage, K.H. Fluorescence Efficiency of Laser Dyes. J. Res. Natl. Bur. Stand. 1976, 80A, 421–428. [Google Scholar] [CrossRef]
- Meimetis, L.G.; Carlson, J.C.T.; Giedt, R.J.; Kohler, R.H.; Weissleder, R. Ultrafluorogenic Coumarin–Tetrazine Probes for Real-Time Biological Imaging. Angew. Chem. Int. Ed. 2014, 53, 7531–7534. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, L.; Lundeen, G.W. Radiationless Transitions in Xanthene Dyes. J. Chem. Phys. 1966, 44, 1711–1712. [Google Scholar] [CrossRef]
- Fink, D.W.; Willis, C.R. Structure and Internal Mixing in Xanthene Dyes. J. Chem. Phys. 1970, 53, 4720–4722. [Google Scholar] [CrossRef]
- Urano, Y.; Kamiya, M.; Kanda, K.; Ueno, T.; Hirose, K.; Nagano, T. Evolution of Fluorescein as a Platform for Finely Tunable Fluorescence Probes. J. Am. Chem. Soc. 2005, 127, 4888–4894. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, Y.-S.; Xu, W.-N.; Zhao, Y.-F.; Xie, H.-Y.; Li, H.-J.; Chen, X.-F.; Zhao, R.-S.; Guo, D.-S. Far-red to near-infrared fluorescent probes based on silicon-substituted xanthene dyes for sensing and imaging. TrAC Trends Anal. Chem. 2020, 122, 115704. [Google Scholar] [CrossRef]
- Sun, W.-C.; Gee, K.R.; Klaubert, D.H.; Haugland, R.P. Synthesis of Fluorinated Fluoresceins. J. Org. Chem. 1997, 62, 6469–6475. [Google Scholar] [CrossRef]
- Mitronova, G.Y.; Belov, V.N.; Bossi, M.L.; Wurm, C.A.; Meyer, L.; Medda, R.; Moneron, G.; Bretschneider, S.; Eggeling, C.; Jakobs, S.; et al. New Fluorinated Rhodamines for Optical Microscopy and Nanoscopy. Chem. A Eur. J. 2010, 16, 4477–4488. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. Molecular Imaging in Cancer. Science 2006, 312, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Peng, X.; Jiang, M.; Wu, T.; Chen, K.; Yang, Z.; Chen, S.; Zhou, X.; Zheng, X.; Jiang, Z.-X. Synthesis of trifluoromethylated aza-BODIPYs as fluorescence-19F MRI dual imaging and photodynamic agents. Org. Biomol. Chem. 2022, 20, 3335–3341. [Google Scholar] [CrossRef] [PubMed]
- Hecht, M.; Fischer, T.; Dietrich, P.; Kraus, W.; Descalzo, A.B.; Unger, W.E.; Rurack, K. Fluorinated Boron-Dipyrromethene (BODIPY) Dyes: Bright and Versatile Probes for Surface Analysis. ChemistryOpen 2013, 2, 25–38. [Google Scholar] [CrossRef]
- Vives, G.; Giansante, C.; Bofinger, R.; Raffy, G.; Guerzo, A.D.; Kauffmann, B.; Batat, P.; Jonusauskas, G.; McClenaghan, N.D. Facile functionalization of a fully fluorescent perfluorophenyl BODIPY: Photostable thiol and amine conjugates. Chem. Commun. 2011, 47, 10425–10427. [Google Scholar] [CrossRef]
- Kani, R.; Kubota, Y.; Inuzuka, T.; Funabiki, K. Aromatic fluorine atom-induced highly amine-sensitive trimethine cyanine dye showing colorimetric and ratiometric fluorescence change. RSC Adv. 2022, 12, 25587–25592. [Google Scholar] [CrossRef]
- Nanjunda, R.; Owens, E.A.; Mickelson, L.; Dost, T.L.; Stroeva, E.M.; Huynh, H.T.; Germann, M.W.; Henary, M.M.; Wilson, W.D. Selective G-Quadruplex DNA Recognition by a New Class of Designed Cyanines. Molecules 2013, 18, 13588–13607. [Google Scholar] [CrossRef]
- Bisht, R.; Sudhakar, V.; Mele Kavungathodi, M.F.; Karjule, N.; Nithyanandhan, J. Fused Fluorenylindolenine-Donor-Based Unsymmetrical Squaraine Dyes for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 26335–26347. [Google Scholar] [CrossRef] [PubMed]
- Al-horaibi, S.A.; Asiri, A.M.; El-Shishtawy, R.M.; Gaikwad, S.T.; Rajbhoj, A.S. Indoline and benzothiazole-based squaraine dye-sensitized solar cells containing bis-pendent sulfonate groups: Synthesis, characterization and solar cell performance. J. Mol. Struct. 2019, 1195, 591–597. [Google Scholar] [CrossRef]
- Galliano, S.; Novelli, V.; Barbero, N.; Smarra, A.; Viscardi, G.; Borrelli, R.; Sauvage, F.; Barolo, C. Dicyanovinyl and Cyano-Ester Benzoindolenine Squaraine Dyes: The Effect of the Central Functionalization on Dye-Sensitized Solar Cell Performance. Energies 2016, 9, 486. [Google Scholar] [CrossRef]
- Tatarets, A.L.; Fedyunyayeva, I.A.; Dyubko, T.S.; Povrozin, Y.A.; Doroshenko, A.O.; Terpetschnig, E.A.; Patsenker, L.D. Synthesis of water-soluble, ring-substituted squaraine dyes and their evaluation as fluorescent probes and labels. Anal. Chim. Acta 2006, 570, 214–223. [Google Scholar] [CrossRef]
- Markova, L.I.; Terpetschnig, E.A.; Patsenker, L.D. Comparison of a series of hydrophilic squaraine and cyanine dyes for use as biological labels. Dye. Pigment. 2013, 99, 561–570. [Google Scholar] [CrossRef]
- Yadav, Y.; Owens, E.; Nomura, S.; Fukuda, T.; Baek, Y.; Kashiwagi, S.; Choi, H.S.; Henary, M. Ultrabright and Serum-Stable Squaraine Dyes. J. Med. Chem. 2020, 63, 9436–9445. [Google Scholar] [CrossRef] [PubMed]
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine Dyes: Molecular Design for Different Applications and Remaining Challenges. Bioconjug. Chem. 2020, 31, 194–213. [Google Scholar] [CrossRef]
- Mayerhöffer, U.; Gsänger, M.; Stolte, M.; Fimmel, B.; Würthner, F. Synthesis and Molecular Properties of Acceptor-Substituted Squaraine Dyes. Chem. A Eur. J. 2013, 19, 218–232. [Google Scholar] [CrossRef]
- Fukuda, T.; Yokomizo, S.; Casa, S.; Monaco, H.; Manganiello, S.; Wang, H.; Lv, X.; Ulumben, A.D.; Yang, C.; Kang, M.-W.; et al. Fast and Durable Intraoperative Near-infrared Imaging of Ovarian Cancer Using Ultrabright Squaraine Fluorophores. Angew. Chem. Int. Ed. 2022, 61, e202117330. [Google Scholar] [CrossRef]
- Renard, B.-L.; Aubert, Y.; Asseline, U. Fluorinated squaraine as near-IR label with improved properties for the labeling of oligonucleotides. Tetrahedron Lett. 2009, 50, 1897–1901. [Google Scholar] [CrossRef]
- Etzioni, R.; Urban, N.; Ramsey, S.; McIntosh, M.; Schwartz, S.; Reid, B.; Radich, J.; Anderson, G.; Hartwell, L. The case for early detection. Nat. Rev. Cancer 2003, 3, 243–252. [Google Scholar] [CrossRef]
- Ametamey, S.M.; Honer, M.; Schubiger, P.A. Molecular Imaging with PET. Chem. Rev. 2008, 108, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, O.; Kiesewetter, D.; Chen, X. Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjugate Chem. 2015, 26, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Coenen, H.H. Fluorine-18 labeling methods: Features and possibilities of basic reactions. In PET Chemistry: The driving Force in Molecular Imaging; Springer: Berlin/Heidelberg, Germany, 2007; pp. 15–50. [Google Scholar]
- Ido, T.; Wan, C.N.; Casella, V.; Fowler, J.S.; Wolf, A.P.; Reivich, M.; Kuhl, D.E. Labeled 2-deoxy-D-glucose analogs. 18F-labeled 2-deoxy-2-fluoro-D-glucose, 2-deoxy-2-fluoro-D-mannose and 14C-2-deoxy-2-fluoro-D-glucose. J. Label. Compd. Radiopharm. 1978, 14, 175–183. [Google Scholar] [CrossRef]
- Hamacher, K.; Coenen, H.H.; Stöcklin, G. Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. J. Nucl. Med. 1986, 27, 235–238. [Google Scholar] [PubMed]
- Grierson, J.; Link, J.; Mathis, C.; Rasey, J.; Krohn, K. A Radiosynthesis of fluorine- 18 Fluoromisonidazole. J. Nucl. Med. 1989, 30, 343–350. [Google Scholar]
- Shen, B.; Ehrlichmann, W.; Uebele, M.; Machulla, H.; Reischl, G. Automated synthesis of n.c.a. [18F]FDOPA via nucleophilic aromatic substitution with [18F]fluoride. Appl. Radiat. Isot. 2009, 67, 1650–1653. [Google Scholar] [CrossRef]
- Choi, S.R.; Golding, G.; Zhuang, Z.; Zhang, W.; Lim, N.; Hefti, F.; Benedum, T.E.; Kilbourn, M.R.; Skovronsky, D.; Kung, H.F. Preclinical Properties of 18F-AV-45: A PET Agent for Aβ Plaques in the Brain. J. Nucl. Med. 2009, 50, 1887–1894. [Google Scholar] [CrossRef]
- Zhang, W.; Oya, S.; Kung, M.-P.; Hou, C.; Maier, D.L.; Kung, H.F. F-18 Polyethyleneglycol stilbenes as PET imaging agents targeting Aβ aggregates in the brain. Nucl. Med. Biol. 2005, 32, 799–809. [Google Scholar] [CrossRef]
- Qiao, L.; Fisher, E.; McMurray, L.; Milicevic Sephton, S.; Hird, M.; Kuzhuppilly-Ramakrishnan, N.; Williamson, D.J.; Zhou, X.; Werry, E.; Kassiou, M.; et al. Radiosynthesis of (R,S)-[18F]GE387: A Potential PET Radiotracer for Imaging Translocator Protein 18 kDa (TSPO) with Low Binding Sensitivity to the Human Gene Polymorphism rs6971. ChemMedChem 2019, 14, 982–993. [Google Scholar] [CrossRef]
- Alauddin, M.M.; Conti, P.S.; Fissekis, J.D. Synthesis of [18F]-labeled 2′-deoxy-2′-fluoro-5-methyl-1-β-D-arabinofuranosyluracil ([18F]-FMAU). J. Label. Compd. Radiopharm. 2002, 45, 583–590. [Google Scholar] [CrossRef]
- Hendricks, J.A.; Keliher, E.J.; Wan, D.; Hilderbrand, S.A.; Weissleder, R.; Mazitschek, R. Synthesis of [18F]BODIPY: Bifunctional Reporter for Hybrid Optical/Positron Emission Tomography Imaging. Angew. Chem. Int. Ed. 2012, 51, 4603–4606. [Google Scholar] [CrossRef]
- Huang, X.; Gillies, R.J.; Tian, H. Synthesis of [18F] 4-amino-N-(3-chloro-4-fluorophenyl)-N′-hydroxy-1,2,5-oxadiazole-3-carboximidamide (IDO5L): A novel potential PET probe for imaging of IDO1 expression. J. Label. Compd. Radiopharm. 2015, 58, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Sromek, A.W.; Zhang, S.; Akurathi, V.; Packard, A.B.; Li, W.; Alagille, D.; Morley, T.J.; Baldwin, R.; Tamagnan, G.; Neumeyer, J.L. Convenient synthesis of 18F-radiolabeled R-(−)-N-n-propyl-2-(3-fluoropropanoxy-11-hydroxynoraporphine. J. Label. Compd. Radiopharm. 2014, 57, 725–729. [Google Scholar] [CrossRef]
- Graham, T.J.A.; Lambert, R.F.; Ploessl, K.; Kung, H.F.; Doyle, A.G. Enantioselective Radiosynthesis of Positron Emission Tomography (PET) Tracers Containing [18F]Fluorohydrins. J. Am. Chem. Soc. 2014, 136, 5291–5294. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.K.; Ugaz, C.R.; Li, W.; Doyle, A.G. PyFluor: A Low-Cost, Stable, and Selective Deoxyfluorination Reagent. J. Am. Chem. Soc. 2015, 137, 9571–9574. [Google Scholar] [CrossRef]
- An, F.-F.; Kommidi, H.; Chen, N.; Ting, R. A Conjugate of Pentamethine Cyanine and 18F as a Positron Emission Tomography/Near-Infrared Fluorescence Probe for Multimodality Tumor Imaging. Int. J. Mol. Sci. 2017, 18, 1214. [Google Scholar] [CrossRef]
- Heo, Y.-A. Flotufolastat F 18: Diagnostic First Approval. Mol. Diagn. Ther. 2023, 27, 631–636. [Google Scholar] [CrossRef]
- Michler, E.; Kaiser, D.; Eleftheriadou, K.; Falkenburger, B.; Kotzerke, J.; Hoberück, S. Comparison of 6-[18F]FDOPA PET with Nigrosome 1 detection in patients with parkinsonism. EJNMMI Res. 2021, 11, 16. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Liu, T. Recent Advances in Synthetic Methodologies to Form C-18F Bonds. Front. Chem. 2022, 10, 883866. [Google Scholar] [CrossRef]
- Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.-G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23, 5023. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.; Young, R.J. Future challenges and opportunities with fluorine in drugs? Med. Chem. Res. 2023, 32, 1231–1234. [Google Scholar] [CrossRef]





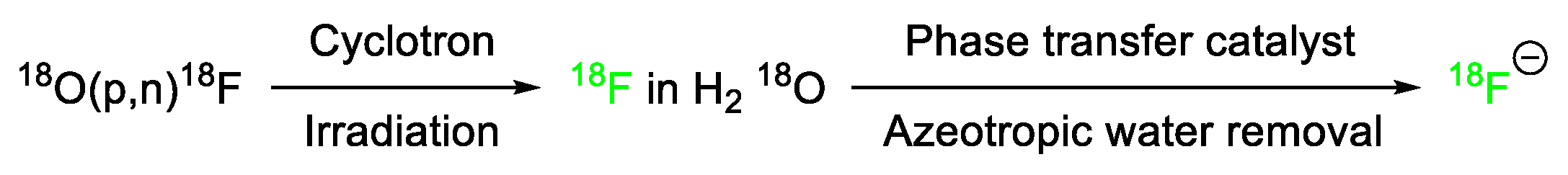
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henary, E.; Casa, S.; Dost, T.L.; Sloop, J.C.; Henary, M. The Role of Small Molecules Containing Fluorine Atoms in Medicine and Imaging Applications. Pharmaceuticals 2024, 17, 281. https://doi.org/10.3390/ph17030281
Henary E, Casa S, Dost TL, Sloop JC, Henary M. The Role of Small Molecules Containing Fluorine Atoms in Medicine and Imaging Applications. Pharmaceuticals. 2024; 17(3):281. https://doi.org/10.3390/ph17030281
Chicago/Turabian StyleHenary, Emily, Stefanie Casa, Tyler L. Dost, Joseph C. Sloop, and Maged Henary. 2024. "The Role of Small Molecules Containing Fluorine Atoms in Medicine and Imaging Applications" Pharmaceuticals 17, no. 3: 281. https://doi.org/10.3390/ph17030281