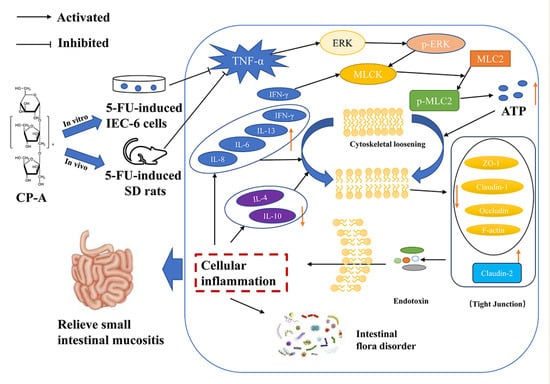
An Inulin-Type Fructan CP-A from Codonopsis pilosula Alleviated 5-Fluorouracil-Induced Intestinal Mucositis via the ERK/MLCK/MLC2 Pathway and Regulation of Gut Microbiota
Abstract
:1. Introduction
2. Results
2.1. Cytotoxic Effects of CP-A on IEC-6 Cells
2.2. Protective Effects of CP-A on 5-FU-Induced Intestinal Mucositis in Rats
2.3. Effect of CP-A on Inflammatory Cytokines
2.4. Effect of CP-A on ERK/MLCK/MLC2 Signaling Pathway
2.5. Effect of CP-A on Intestinal Mucosal Barrier Proteins
2.6. Effect of CP-A on Microbial Diversity
2.7. Taxonomic Composition Analysis of Microbial Community
2.8. LEfSe Analysis of Microbial Community
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cells Culture and MTT Assay
4.3. Total RNA Extraction and Quantitative Real-Time PCR
4.4. Experimental Animals
4.5. Establishment of 5-FU-Induced Intestinal Mucositis
4.6. Histopathological Evaluation
4.7. Immunohistochemical Staining
4.8. ELISA Analysis
4.9. Western Blotting Experiments
4.10. Gut Microbiota Analysis
4.11. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IM | Intestinal mucositis |
| 5-FU | 5-fluorouracil |
| IHC | Immunohistochemical |
| RT-PCR | Real-time PCR |
| ERK | Extracellular-regulated protein kinases |
| MLCK | Myosin light chain kinase |
| MLC2 | Myosin light chain 2 |
| ELISA | Enzyme-linked immunosorbent assay |
| TNF-α | Tumor necrosis factor-alpha |
| IFN-γ | Interferon gamma |
| ZO-1 | Zonula occludens-1 |
| IL | Interleukin |
| BTC | Bifid. Triple Viable Capsules Dissolving at Intestines |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DMSO | Dimethyl sulfoxide |
| PBS | Phosphate-buffered saline |
| HE | Hematoxylin and eosin |
| PCoA | Principal coordinate analysis |
| LEfSe | LDA effect Size |
| FBS | Fetal bovine serum |
| BSA | Bovine serum albumin |
| SPF | Specific pathogen-free |
| SD | Sprague–Dawley |
| TCM | Traditional Chinese Medicine |
| IEC-6 | Rat intestinal epithelial cell line 6 |
| PVDF | Polyvinylidene difluoride |
| ECL | Electro-chemiluminescence |
References
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.A.; Wanderley, C.W.S.; Wong, D.V.T.; Mota, J.M.S.C.; Leite, C.A.V.G.; Souza, M.H.L.P.; Cunha, F.Q.; Lima-Júnior, R.C.P. Irinotecan- and 5-fluorouracil-induced intestinal mucositis: Insights into pathogenesis and therapeutic perspectives. Cancer Chemother. Pharmacol. 2016, 78, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.; Cunningham, D.; Roth, A.D.; Navarro, M.; James, R.D.; Karasek, P.; Jandik, P.; Iveson, T.; Carmichael, J.; Alakl, M.; et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet 2000, 355, 1041–1047. [Google Scholar] [CrossRef]
- Wang, J.; Feng, W.; Zhang, S.; Chen, L.; Sheng, Y.; Tang, F.; He, J.; Xu, X.; Ao, H.; Peng, C. Ameliorative effect of Atractylodes macrocephala essential oil combined with Panax ginseng total saponins on 5-fluorouracil induced diarrhea is associated with gut microbial modulation. J. Ethnopharmacol. 2019, 238, 111887. [Google Scholar] [CrossRef]
- Lalla, R.V.; Schubert, M.M.; Bensadoun, R.-J.; Keefe, D. Anti-inflammatory agents in the management of alimentary mucositis. Support. Care Cancer 2006, 14, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Keefe, D.M. Intestinal mucositis: Mechanisms and management. Curr. Opin. Oncol. 2007, 19, 323–327. [Google Scholar] [CrossRef]
- Sharma, R.; Tobin, P.; Clarke, S.J. Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. Lancet Oncol. 2005, 6, 93–102. [Google Scholar] [CrossRef]
- Cai, B.; Pan, J.; Chen, H.; Chen, X.; Ye, Z.; Yuan, H.; Sun, H.; Wan, P. Oyster polysaccharides ameliorate intestinal mucositis and improve metabolism in 5-fluorouracil-treated S180 tumour-bearing mice. Carbohydr. Polym. 2020, 256, 117545. [Google Scholar] [CrossRef]
- Wu, J.; Gan, Y.; Li, M.; Chen, L.; Liang, J.; Zhuo, J.; Luo, H.; Xu, N.; Wu, X.; Wu, Q.; et al. Patchouli alcohol attenuates 5-fluorouracil-induced intestinal mucositis via TLR2/MyD88/NF-kB pathway and regulation of microbiota. Biomed. Pharmacother. 2020, 124, 109883. [Google Scholar] [CrossRef]
- Zeisel, M.B.; Dhawan, P.; Baumert, T.F. Tight junction proteins in gastrointestinal and liver disease. Gut 2018, 68, 547–561. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, F.; Li, R.; Liu, Y.; Wang, X.; Zhang, X.; Xu, C.; Li, Y.; Guo, Y.; Yao, Q. Berberine regulates fecal metabolites to ameliorate 5-fluorouracil induced intestinal mucositis through modulating gut microbiota. Biomed. Pharmacother. 2020, 124, 109829. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-L.; Lu, L.; Wang, X.-S.; Qin, L.-Y.; Wang, P.; Qiu, S.-P.; Wu, H.; Huang, F.; Zhang, B.-B.; Shi, H.-L.; et al. Alteration of Gut Microbiota and Inflammatory Cytokine/Chemokine Profiles in 5-Fluorouracil Induced Intestinal Mucositis. Front. Cell. Infect. Microbiol. 2017, 7, 455. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Wu, D.; Li, L.; Li, J.; Xu, Y. TBHQ attenuates ferroptosis against 5-fluorouracil-induced intestinal epithelial cell injury and intestinal mucositis via activation of Nrf2. Cell. Mol. Biol. Lett. 2021, 26, 48. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Hayashi, S.; Kitahara, Y.; Nagasawa, K.; Aono, H.; Shibata, J.; Utsumi, D.; Amagase, K.; Kadowaki, M. Saireito (TJ-114), a Japanese traditional herbal medicine, reduces 5-fluorouracil-induced intestinal mucositis in mice by inhibiting cytokine-mediated apoptosis in intestinal crypt cells. PLoS ONE 2015, 10, e0116213. [Google Scholar] [CrossRef] [PubMed]
- Atiq, A.; Shal, B.; Naveed, M.; Khan, A.; Ali, J.; Zeeshan, S.; Al-Sharari, S.D.; Kim, Y.S.; Khan, S. Diadzein ameliorates 5-fluorouracil-induced intestinal mucositis by suppressing oxidative stress and inflammatory mediators in rodents. Eur. J. Pharmacol. 2019, 843, 292–306. [Google Scholar] [CrossRef]
- Su, J.; Li, C.; Yu, X.; Yang, G.; Deng, J.; Su, Z.; Zeng, H.; Chen, J.; Zhang, X.; Lai, X. Protective Effect of Pogostone on 2,4,6-Trinitrobenzenesulfonic Acid-Induced Experimental Colitis via Inhibition of T Helper Cell. Front. Pharmacol. 2017, 8, 829. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Zhang, C.; Fang, X.; Wang, J.; Li, Y.; Wang, J.; Wang, L.; Dong, J.; Zhou, Z.; Li, C. Dual role of Ca(2+)-activated Cl(-) channel transmembrane member 16A in lipopolysaccharide-induced intestinal epithelial barrier dysfunction in vitro. Cell Death Dis. 2020, 11, 404. [Google Scholar] [CrossRef]
- Du, L.; Kim, J.J.; Shen, J.; Dai, N. Crosstalk between Inflammation and ROCK/MLCK Signaling Pathways in Gastrointestinal Disorders with Intestinal Hyperpermeability. Gastroenterol. Res. Pract. 2016, 2016, 7374197. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Guo, S.; Ye, D.; Ma, T.Y. TNF-alpha modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am. J. Pathol. 2013, 183, 1871–1884. [Google Scholar] [CrossRef]
- Weber, C.R.; Raleigh, D.R.; Su, L.; Shen, L.; Sullivan, E.A.; Wang, Y.; Turner, J.R. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J. Biol. Chem. 2010, 285, 12037–12046. [Google Scholar] [CrossRef]
- Chen, S.; Qian, K.; Zhang, G.; Zhang, M. Akkermansia muciniphila and its outer membrane protein Amuc_1100 prophylactically attenuate 5-fluorouracil-induced intestinal mucositis. Biochem. Biophys. Res. Commun. 2022, 614, 34–40. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, S.H.; Bi, Q.; Liang, L.; Wang, Y.F.; Yang, X.X.; Gu, W.; Yu, J. Volatile Oil from Amomi Fructus Attenuates 5-Fluorouracil-Induced Intestinal Mucositis. Front. Pharmacol. 2017, 8, 786. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Liu, X.-J.; Qiu, J.-M.; Zeng, X.-L.; Bao, H.-R.; Shu, J. Effects of Astragalus and Codonopsis pilosula polysaccharides on alveolar macrophage phagocytosis and inflammation in chronic obstructive pulmonary disease mice exposed to PM2.5. Environ. Toxicol. Pharmacol. 2016, 48, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Xu, Y.; Chang, C.; Qiu, Z.; Hu, J.; Wu, Y.; Zhang, B.; Zheng, G. Extraction, characterization and anti-inflammatory activities of an inulin-type fructan from Codonopsis pilosula. Int. J. Biol. Macromol. 2020, 163, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.M.; Liu, J.S.; Wang, M.; Cao, T.T.; Qi, Y.D.; Zhang, B.G.; Sun, X.B.; Liu, H.T.; Xiao, P.G. Traditional uses, phytochemistry, pharmacology and toxicology of Codonopsis: A review. J. Ethnopharmacol. 2018, 219, 50–70. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yan, H.; Li, C.; Wen, F.; Jize, X.; Zhang, C.; Liu, S.; Zhao, Y.; Fu, Y.; Li, L.; et al. A Pectic Polysaccharide from Codonopsis pilosula Alleviates Inflammatory Response and Oxidative Stress of Aging Mice via Modulating Intestinal Microbiota-Related Gut–Liver Axis. Antioxidants 2023, 12, 1781. [Google Scholar] [CrossRef]
- Liu, J.-H.; Hsieh, C.-H.; Liu, C.-Y.; Chang, C.-W.; Chen, Y.-J.; Tsai, T.-H. Anti-inflammatory effects of Radix Aucklandiae herbal preparation ameliorate intestinal mucositis induced by 5-fluorouracil in mice. J. Ethnopharmacol. 2021, 271, 113912. [Google Scholar] [CrossRef] [PubMed]
- Korenaga, D.; Honda, M.; Yasuda, M.; Inutsuka, S.; Nozoe, T.; Tashiro, H. Increased intestinal permeability correlates with gastrointestinal toxicity among formulations of the fluorouracil analogue tegafur in rats. Eur. Surg. Res. 2002, 34, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Tefas, C.; Ciobanu, L.; Berce, C.; Meșter, A.; Onica, S.; Toma, C.; Tanțău, M.; Taulescu, M. Beneficial effect of oral administration of zinc sulfate on 5-fluorouracil-induced gastrointestinal mucositis in rats. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11365–11373. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, B.; Lu, Y.; Xu, L.; Wang, Y.-J.; Cai, B.-Y.; Yao, Q.-H. RNA-seq and In Vitro Experiments Reveal the Protective Effect of Curcumin against 5-Fluorouracil-Induced Intestinal Mucositis via IL-6/STAT3 Signaling Pathway. J. Immunol. Res. 2021, 2021, 8286189. [Google Scholar] [CrossRef]
- Xiang, D.-C.; Yang, J.-Y.; Xu, Y.-J.; Zhang, S.; Li, M.; Zhu, C.; Zhang, C.-L.; Liu, D. Protective effect of Andrographolide on 5-Fu induced intestinal mucositis by regulating p38 MAPK signaling pathway. Life Sci. 2020, 252, 117612. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Xiong, B.; Chen, H.; Wang, X.; Zhang, D. Discoidin domain receptor 1(DDR1) promote intestinal barrier disruption in Ulcerative Colitis through tight junction proteins degradation and epithelium apoptosis. Pharmacol. Res. 2022, 183, 106368. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Huang, S.; Fu, Y.; Xu, B.; Liu, C.; Wang, Q.; Luo, S.; Nong, F.; Wang, X.; Huang, S.; Chen, J.; et al. Wogonoside alleviates colitis by improving intestinal epithelial barrier function via the MLCK/pMLC2 pathway. Phytomedicine 2020, 68, 153179. [Google Scholar] [CrossRef]
- Wu, J.; Gan, Y.; Luo, H.; Xu, N.; Chen, L.; Li, M.; Guan, F.; Su, Z.; Lin, Z.; Xie, J.; et al. β-Patchoulene Ameliorates Water Transport and the Mucus Barrier in 5-Fluorouracil-Induced Intestinal Mucositis Rats via the cAMP/PKA/CREB Signaling Pathway. Front. Pharmacol. 2021, 12, 689491. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.-M. Exotoxins and endotoxins: Inducers of inflammatory cytokines. Toxicon 2018, 149, 45–53. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, N.; Kishimoto, T. Inhibition of IL-6 for the treatment of inflammatory diseases. Curr. Opin. Pharmacol. 2004, 4, 386–391. [Google Scholar] [CrossRef]
- Kucharzik, T.; Lügering, N.; Pauels, H.G.; Domschke, W.; Stoll, R. IL-4, IL-10 and IL-13 down-regulate monocyte-chemoattracting protein-1 (MCP-1) production in activated intestinal epithelial cells. Clin. Exp. Immunol. 1998, 111, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, J.; Mei, Q.; Han, L.; Huang, J. Myosin Light Chain Kinase Inhibitor Inhibits Dextran Sulfate Sodium-Induced Colitis in Mice. Dig. Dis. Sci. 2012, 58, 107–114. [Google Scholar] [CrossRef]
- Shao, Y.-Y.; Zhao, Y.-N.; Sun, Y.-F.; Guo, Y.; Zhang, X.; Chang, Z.-P.; Hou, R.-G.; Gao, J. Investigation of the internalization and transport mechanism of Codonopsis Radix polysaccharide both in mice and Caco-2 cells. Int. J. Biol. Macromol. 2022, 215, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Zhao, C.L.; Li, Y.S.; Yang, Y.B.; Luo, J.G.; Zhang, C.; Wang, L. Moutai Distiller’s grains Polyphenol extracts and rutin alleviate DSS-induced colitis in mice: Modulation of gut microbiota and intestinal barrier function (R2). Heliyon 2023, 9, e22186. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2018, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; He, J.; Li, H.; You, J.; Qin, H. Alterations in intestinal microbiota of colorectal cancer patients receiving radical surgery combined with adjuvant CapeOx therapy. Sci. China Life Sci. 2019, 62, 1178–1193. [Google Scholar] [CrossRef]
- Kim, E.; Kim, D.-B.; Park, J.-Y. Changes of Mouse Gut Microbiota Diversity and Composition by Modulating Dietary Protein and Carbohydrate Contents: A Pilot Study. Prev. Nutr. Food Sci. 2016, 21, 57–61. [Google Scholar] [CrossRef]
- Gong, H.; Gan, X.; Qin, B.; Chen, J.; Zhao, Y.; Qiu, B.; Chen, W.; Yu, Y.; Shi, S.; Li, T.; et al. Structural characteristics of steamed Polygonatum cyrtonema polysaccharide and its bioactivity on colitis via improving the intestinal barrier and modifying the gut microbiota. Carbohydr. Polym. 2024, 327, 121669. [Google Scholar] [CrossRef]
- Li, X.; Lv, H.; Shi, F.; Song, J.; Zhang, Z. The potential therapeutic effects of hydroxypropyl cellulose on acute murine colitis induced by DSS. Carbohydr. Polym. 2022, 289, 119430. [Google Scholar] [CrossRef]
- Xu, D.; Wu, Q.; Liu, W.; Hu, G.; Meng, H.; Wang, J. Therapeutic efficacy and underlying mechanisms of Gastrodia elata polysaccharides on dextran sulfate sodium-induced inflammatory bowel disease in mice: Modulation of the gut microbiota and improvement of metabolic disorders. Int. J. Biol. Macromol. 2023, 248, 125919. [Google Scholar] [CrossRef]
- Lunken, G.R.; Tsai, K.; Schick, A.; Lisko, D.J.; Cook, L.; Vallance, B.A.; Jacobson, K. Prebiotic Enriched Exclusive Enteral Nutrition Suppresses Colitis via Gut Microbiome Modulation and Expansion of Anti-inflammatory T Cells in a Mouse Model of Colitis. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1251–1266. [Google Scholar] [CrossRef]
- Qasim, M.; Wrage, M.; Nüse, B.; Mattner, J. Shigella Outer Membrane Vesicles as Promising Targets for Vaccination. Int. J. Mol. Sci. 2022, 23, 994. [Google Scholar] [CrossRef]
- Li, X.; Tang, X.; Chen, M.; Wang, S.; Tong, C.; Xu, J.; Xie, G.; Ma, B.; Zou, Y.; Wang, Y.; et al. Intramuscular therapeutic doses of enrofloxacin affect microbial community structure but not the relative abundance of fluoroquinolones resistance genes in swine manure. Sci. Total. Environ. 2024, 913, 169794. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Zhu, Z.; Yang, F.; Cao, L.; Gao, J. Structure Features and Anti-Gastric Ulcer Effects of Inulin-Type Fructan CP-A from the Roots of Codonopsis pilosula (Franch.) Nannf. Molecules 2017, 22, 2258. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Rapa, S.F.; Salviati, E.; Bertamino, A.; Auriemma, G.; Cascioferro, S.; Autore, G.; Quaroni, A.; Campiglia, P.; Marzocco, S. Bioactive Polyphenols from Pomegranate Juice Reduce 5-Fluorouracil-Induced Intestinal Mucositis in Intestinal Epithelial Cells. Antioxidants 2020, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Author Correction: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 1091. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Li, D.; Wang, J.; Cheng, Z.; Wang, C.; Zhang, X.; Xu, X.; Gao, J. An Inulin-Type Fructan CP-A from Codonopsis pilosula Alleviated 5-Fluorouracil-Induced Intestinal Mucositis via the ERK/MLCK/MLC2 Pathway and Regulation of Gut Microbiota. Pharmaceuticals 2024, 17, 297. https://doi.org/10.3390/ph17030297
Zhou J, Li D, Wang J, Cheng Z, Wang C, Zhang X, Xu X, Gao J. An Inulin-Type Fructan CP-A from Codonopsis pilosula Alleviated 5-Fluorouracil-Induced Intestinal Mucositis via the ERK/MLCK/MLC2 Pathway and Regulation of Gut Microbiota. Pharmaceuticals. 2024; 17(3):297. https://doi.org/10.3390/ph17030297
Chicago/Turabian StyleZhou, Jiangtao, Deyun Li, Jiajing Wang, Zhuoyang Cheng, Changjian Wang, Xuepeng Zhang, Xiexin Xu, and Jianping Gao. 2024. "An Inulin-Type Fructan CP-A from Codonopsis pilosula Alleviated 5-Fluorouracil-Induced Intestinal Mucositis via the ERK/MLCK/MLC2 Pathway and Regulation of Gut Microbiota" Pharmaceuticals 17, no. 3: 297. https://doi.org/10.3390/ph17030297
APA StyleZhou, J., Li, D., Wang, J., Cheng, Z., Wang, C., Zhang, X., Xu, X., & Gao, J. (2024). An Inulin-Type Fructan CP-A from Codonopsis pilosula Alleviated 5-Fluorouracil-Induced Intestinal Mucositis via the ERK/MLCK/MLC2 Pathway and Regulation of Gut Microbiota. Pharmaceuticals, 17(3), 297. https://doi.org/10.3390/ph17030297