Anthocyanins from Lycium ruthenicum Murray Mitigate Cadmium-Induced Oxidative Stress and Testicular Toxicity by Activating the Keap1/Nrf2 Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. Total Anthocyanin Content
2.2. Phytochemical Analysis of Anthocyanins
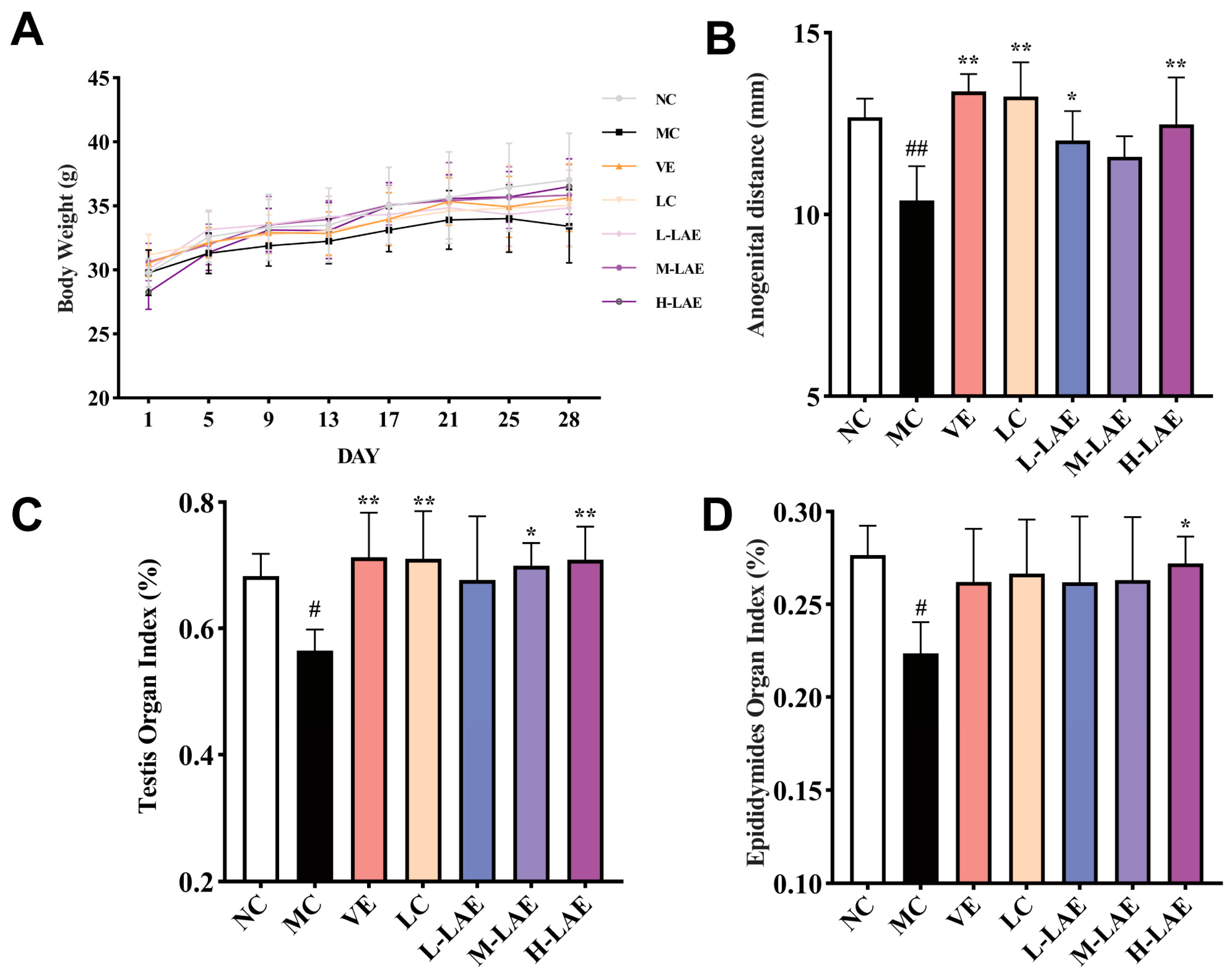
2.3. Therapeutic LAE Mitigates Cd-Induced Effects on Weight, Anogenital Distance, Testis, and Epididymal Organ Indices
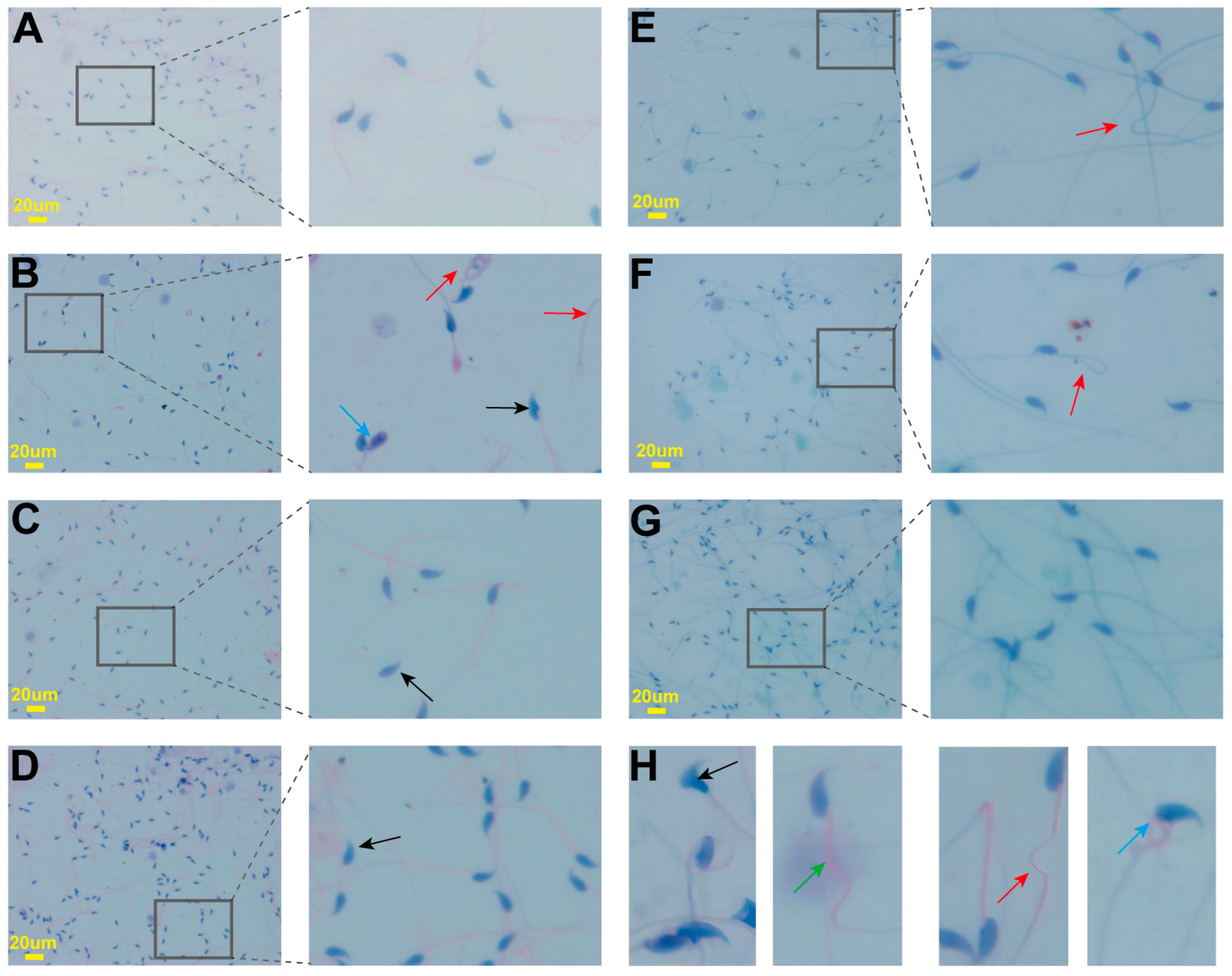
2.4. Effects on Sperm Parameters
2.5. Effects on Sex Hormones
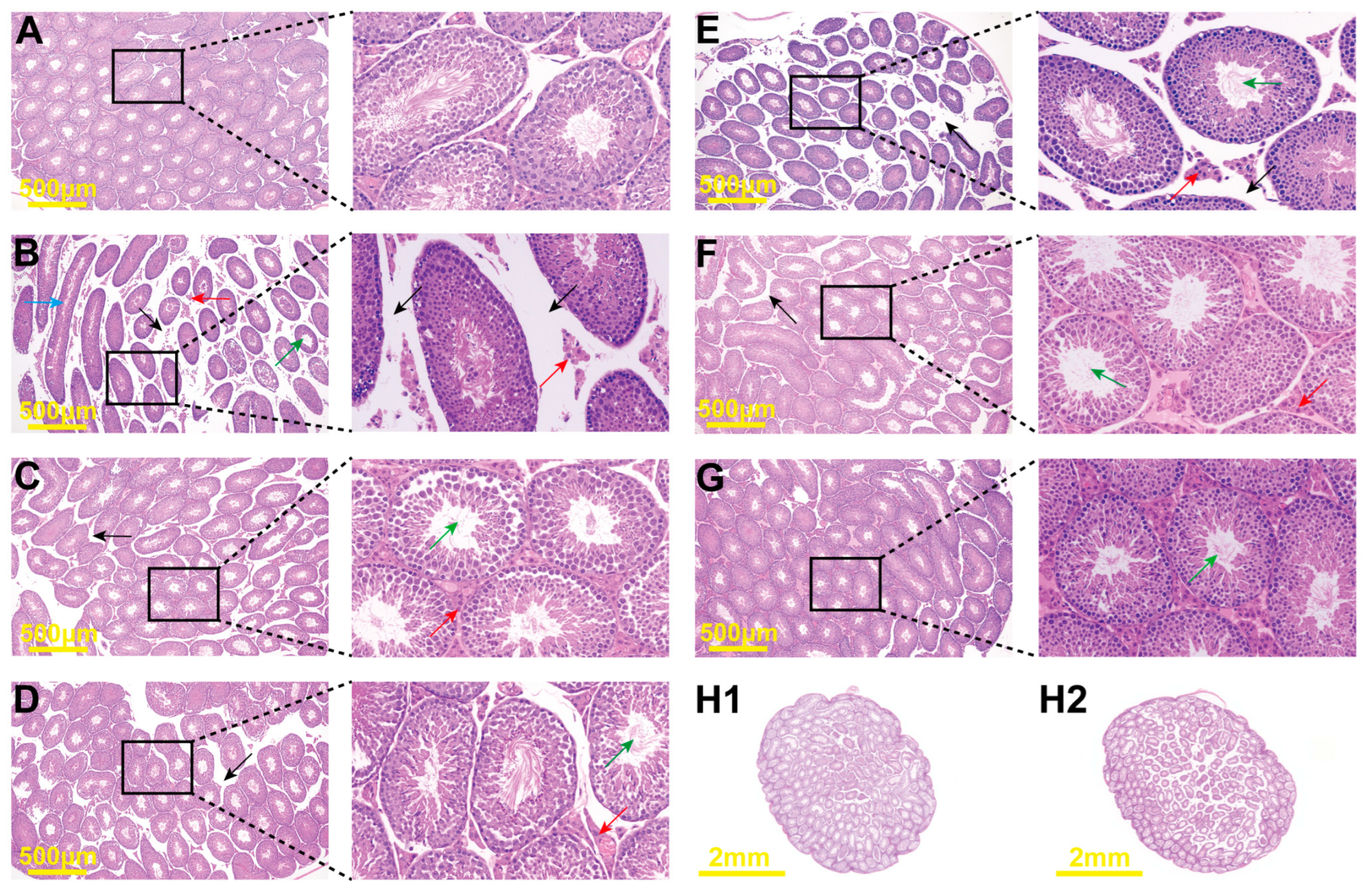
2.6. Histopathological Changes
2.7. Effects on Testicular Marker Enzymes
2.8. Therapeutic LAE Alleviated Cd-Induced Oxidative Stress
2.9. Effects of the Keap1/Nrf2 Signaling Pathway on Gene Expression
2.10. Effects of the Keap1/Nrf2 Signaling Pathway on Protein Expression
3. Discussion
4. Materials and Methods
4.1. Preparation of LAE
4.2. Materials and Chemicals
4.3. Determination of Total Anthocyanin Content
4.4. Characterization of Anthocyanins
4.5. Animals and Experimental Design
4.6. Sample Preparation
4.7. Analysis of Sperm Parameters
4.8. Determination of the Concentration of Hormones
4.9. Determination of Oxidative/Antioxidant Parameters
4.10. Determination of Testicular Marker Enzymes
4.11. Analysis of ROS
4.12. Histopathological Observation
4.13. Gene Expression
4.14. Immunohistochemistry
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhao, X.; Jiao, J.; Li, Y.; Wei, M. Surface-Modified Biochar with Polydentate Binding Sites for the Removal of Cadmium. Int. J. Mol. Sci. 2019, 20, 1775. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Foti, C.; Milea, D.; Sammartano, S. Speciation of Cadmium in the Environment. Met. Ions Life Sci. 2013, 11, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.; Hoyo, C.; Mattingly, C.J.; Luo, Y.; Tzeng, J.-Y.; Murphy, S.K.; Buchwalter, D.B.; Planchart, A. Cadmium Exposure Increases the Risk of Juvenile Obesity: A Human and Zebrafish Comparative Study. Int. J. Obes. 2018, 42, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Li, Q.; Luo, X.; Wu, J. Identification for Heavy Metals Exposure on Osteoarthritis among Aging People and Machine Learning for Prediction: A Study Based on NHANES 2011–2020. Front. Public Health 2022, 10, 906774. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.; Gadi, S.; Lin, M.; Qi, Q.; Niture, S.S.; Moore, J.T.; Bodnar, W.; Fernando, R.A.; Levine, K.E.; Kumar, D. Cadmium Modulates Steatosis, Fibrosis, and Oncogenic Signaling in Liver Cancer Cells by Activating Notch and AKT/mTOR Pathways. Environ. Toxicol. 2023, 38, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Kendall, M.D.; Osborn, D. Cadmium and Mercury Nephrotoxicity. Nature 1983, 304, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-B.; Zhu, H.-L.; Xiong, Y.-W.; Lv, J.; Huang, Y.-C.; Wang, H. Environmental Cadmium Exposure during Gestation Impairs Fetal Brain and Cognitive Function of Adult Offspring via Reducing Placenta-Derived E2 Level. Chemosphere 2022, 307, 135668. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Li, D.; Wang, Y.; Wei, S. The Burden of Coronary Heart Disease and Stroke Attributable to Dietary Cadmium Exposure in Chinese Adults, 2017. Sci. Total Environ. 2022, 825, 153997. [Google Scholar] [CrossRef]
- Lin, X.; Peng, L.; Xu, X.; Chen, Y.; Zhang, Y.; Huo, X. Connecting Gastrointestinal Cancer Risk to Cadmium and Lead Exposure in the Chaoshan Population of Southeast China. Environ. Sci. Pollut. Res. 2018, 25, 17611–17619. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.D. The Blood-Testis Barrier and Its Implications for Male Contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef]
- Ali, W.; Ma, Y.; Zhu, J.; Zou, H.; Liu, Z. Mechanisms of Cadmium-Induced Testicular Injury: A Risk to Male Fertility. Cells 2022, 11, 3601. [Google Scholar] [CrossRef]
- Yi, L.; Shang, X.-J.; Lv, L.; Wang, Y.; Zhang, J.; Quan, C.; Shi, Y.; Liu, Y.; Zhang, L. Cadmium-Induced Apoptosis of Leydig Cells Is Mediated by Excessive Mitochondrial Fission and Inhibition of Mitophagy. Cell Death Dis. 2022, 13, 928. [Google Scholar] [CrossRef]
- Nna, V.U.; Ujah, G.A.; Mohamed, M.; Etim, K.B.; Igba, B.O.; Augustine, E.R.; Osim, E.E. Cadmium Chloride–Induced Testicular Toxicity in Male Wistar Rats; Prophylactic Effect of Quercetin, and Assessment of Testicular Recovery Following Cadmium Chloride Withdrawal. Biomed. Pharmacother. 2017, 94, 109–123. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Zheng, G.; Chen, Y.; Huang, M.; Zhang, L.; Lin, X. Protective Effect of Lycium Barbarum Polysaccharides against Cadmium-Induced Testicular Toxicity in Male Mice. Food Funct. 2017, 8, 2322–2330. [Google Scholar] [CrossRef] [PubMed]
- Lacorte, L.M.; Rinaldi, J.C.; Justulin, L.A.; Delella, F.K.; Moroz, A.; Felisbino, S.L. Cadmium Exposure Inhibits MMP2 and MMP9 Activities in the Prostate and Testis. Biochem. Biophys. Res. Commun. 2015, 457, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Kheradmand, A.; Alirezaei, M.; Dezfoulian, O. Biochemical and Histopathological Evaluations of Ghrelin Effects following Cadmium Toxicity in the Rat Testis. Andrologia 2015, 47, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Mori, C.; Lee, J.-Y.; Tokumoto, M.; Satoh, M. Cadmium Toxicity Is Regulated by Peroxisome Proliferator-Activated Receptor δ in Human Proximal Tubular Cells. Int. J. Mol. Sci. 2022, 23, 8652. [Google Scholar] [CrossRef]
- Siu, E.R.; Mruk, D.D.; Porto, C.S.; Cheng, C.Y. Cadmium-Induced Testicular Injury. Toxicol. Appl. Pharmacol. 2009, 238, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-H.; Chen, S.-T.; Liang, C.; Shi, Y.-H.; Chen, Q.-S. Effects of Cadmium Exposure on Leydig Cells and Blood Vessels in Mouse Testis. Int. J. Environ. Res. Public Health 2022, 19, 2416. [Google Scholar] [CrossRef]
- Squadrito, F.; Micali, A.; Rinaldi, M.; Irrera, N.; Marini, H.; Puzzolo, D.; Pisani, A.; Lorenzini, C.; Valenti, A.; Laurà, R.; et al. Polydeoxyribonucleotide, an Adenosine-A2A Receptor Agonist, Preserves Blood Testis Barrier from Cadmium-Induced Injury. Front. Pharmacol. 2016, 7, 537. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, T.; Nie, G.; Hu, R.; Pi, S.; Wei, Z.; Wang, C.; Xing, C.; Hu, G. Cadmium and Molybdenum Co-Induce Pyroptosis via ROS/PTEN/PI3K/AKT Axis in Duck Renal Tubular Epithelial Cells. Environ. Pollut. 2021, 272, 116403. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Zhang, D.D. Mechanistic Studies of the Nrf2-Keap1 Signaling Pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 Signaling in Oxidative and Reductive Stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Henderson, P.; Hale, T.W.; Shum, S.; Habersang, R.W. N-Acetylcysteine Therapy of Acute Heavy Metal Poisoning in Mice. Vet. Hum. Toxicol. 1985, 27, 522–525. [Google Scholar] [PubMed]
- Baba, H.; Tsuneyama, K.; Yazaki, M.; Nagata, K.; Minamisaka, T.; Tsuda, T.; Nomoto, K.; Hayashi, S.; Miwa, S.; Nakajima, T.; et al. The Liver in Itai-Itai Disease (Chronic Cadmium Poisoning): Pathological Features and Metallothionein Expression. Mod. Pathol. 2013, 26, 1228–1234. [Google Scholar] [CrossRef]
- Jones, M.M.; Basinger, M.A.; Topping, R.J.; Gale, G.R.; Jones, S.G.; Holscher, M.A. Meso-2,3-Dimercaptosuccinic Acid and Sodium N-Benzyl-N-Dithiocarboxy-D-Glucamine as Antagonists for Cadmium Intoxication. Arch. Toxicol. 1988, 62, 29–36. [Google Scholar] [CrossRef]
- Jones, S.G.; Basinger, M.A.; Jones, M.M.; Gibbs, S.J. A Comparison of Diethyldithiocarbamate and EDTA as Antidotes for Acute Cadmium Intoxication. Res. Commun. Chem. Pathol. Pharmacol. 1982, 38, 271–278. [Google Scholar]
- Kamenova, K.; Gluhcheva, Y.; Dorkov, P.; Ivanova, J. Comparative Assessment of the Effects of Meso-2,3-Dimercaptosuccinic Acid and Salinomycin on Spleen Function of Cadmium-Exposed Mice. Environ. Sci. Pollut. Res. Int. 2019, 26, 33304–33310. [Google Scholar] [CrossRef]
- Lamas, G.A.; Navas-Acien, A.; Mark, D.B.; Lee, K.L. Heavy Metals, Cardiovascular Disease, and the Unexpected Benefits of Chelation Therapy. J. Am. Coll. Cardiol. 2016, 67, 2411–2418. [Google Scholar] [CrossRef]
- Venditti, M.; Ben Rhouma, M.; Romano, M.Z.; Messaoudi, I.; Reiter, R.J.; Minucci, S. Evidence of Melatonin Ameliorative Effects on the Blood-Testis Barrier and Sperm Quality Alterations Induced by Cadmium in the Rat Testis. Ecotoxicol. Environ. Saf. 2021, 226, 112878. [Google Scholar] [CrossRef]
- Yu, H.-T.; Zhen, J.; Leng, J.-Y.; Cai, L.; Ji, H.-L.; Keller, B.B. Zinc as a Countermeasure for Cadmium Toxicity. Acta Pharmacol. Sin. 2021, 42, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. The Role of Selenium in Arsenic and Cadmium Toxicity: An Updated Review of Scientific Literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, B.M.; Kim, H.S. Potential Protective Roles of Curcumin against Cadmium-Induced Toxicity and Oxidative Stress. J. Toxicol. Environ. Health B Crit. Rev. 2021, 24, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, Z.; Yang, D.; Jiang, X.; Sun, J.; Tian, L.; Hu, J.; Wu, B.; Bai, W. Cyanidin-3-O-Glucoside Restores Spermatogenic Dysfunction in Cadmium-Exposed Pubertal Mice via Histone Ubiquitination and Mitigating Oxidative Damage. J. Hazard. Mater. 2020, 387, 121706. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of Plant Pigments: Anthocyanins, Betalains and Carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit-A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef]
- Barreto, N.M.B.; Pimenta, N.G.; Braz, B.F.; Freire, A.S.; Santelli, R.E.; Oliveira, A.C.; Bastos, L.H.P.; Cardoso, M.H.W.M.; Monteiro, M.; Diogenes, M.E.L.; et al. Organic Black Beans (Phaseolus vulgaris L.) from Rio de Janeiro State, Brazil, Present More Phenolic Compounds and Better Nutritional Profile Than Nonorganic. Foods 2021, 10, 900. [Google Scholar] [CrossRef]
- Yun, D.; Yan, Y.; Liu, J. Isolation, Structure and Biological Activity of Polysaccharides from the Fruits of Lycium Ruthenicum Murr: A Review. Carbohydr. Polym. 2022, 291, 119618. [Google Scholar] [CrossRef]
- Yang, D.; Ran, Y.; Li, X.; Jiang, X.; Chen, J.; Sun, J.; Tian, L.; Teerds, K.; Bai, W. Cyanidin-3-O-Glucoside Ameliorates Cadmium Induced Uterine Epithelium Proliferation in Mice. J. Hazard. Mater. 2022, 425, 127571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, Y.; Nisar, T.; Zou, L.; Yang, X.; Niu, P.; Sun, L.; Guo, Y. Comparison and Multivariate Statistical Analysis of Anthocyanin Composition in Lycium Ruthenicum Murray from Different Regions to Trace Geographical Origins: The Case of China. Food Chem. 2018, 246, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Bravo, J.; Beltrán, J.F.; Huard, N.; Saavedra, K.; Saavedra, N.; Alvear, M.; Lanas, F.; Salazar, L.A. Anthocyanins Found in Pinot Noir Waste Induce Target Genes Related to the Nrf2 Signalling in Endothelial Cells. Antioxidants 2022, 11, 1239. [Google Scholar] [CrossRef] [PubMed]
- Česlová, L.; Kalendová, P.; Dubnová, L.; Pernica, M.; Fischer, J. The Effect of Sample Pretreatment on the Anthocyanin Content in Czech Wild Elderberry (Sambucus nigra L.). Molecules 2023, 28, 6690. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, T.; Patel, H.; Akande, O.E.; Galam, D.C.A. Total Anthocyanin Content of Strawberry and the Profile Changes by Extraction Methods and Sample Processing. Foods 2022, 11, 1072. [Google Scholar] [CrossRef]
- Martins, D.R.D.S.; Lescano, C.H.; Justo, A.F.O.; Vicente, J.M.; Santos, S.H.S.; Aguilar, C.M.; Borges, A.; Pires de Oliveira, I.; Sanjinez-Argandoña, E.J. Effect of Different Extraction Methods on Anthocyanin Content in Hibiscus sabdariffa L. and Their Antiplatelet and Vasorelaxant Properties. Plant Foods Hum. Nutr. 2023, 78, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Shao, H.; Zhang, Z.; Ng, J.C.; Peng, C. Malathion-Induced Testicular Toxicity Is Associated with Spermatogenic Apoptosis and Alterations in Testicular Enzymes and Hormone Levels in Male Wistar Rats. Environ. Toxicol. Pharmacol. 2015, 39, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Choong, G.; Liu, Y.; Templeton, D.M. Interplay of Calcium and Cadmium in Mediating Cadmium Toxicity. Chem.-Biol. Interact. 2014, 211, 54–65. [Google Scholar] [CrossRef]
- Park, Y.-J.; Pang, M.-G. Mitochondrial Functionality in Male Fertility: From Spermatogenesis to Fertilization. Antioxidants 2021, 10, 98. [Google Scholar] [CrossRef]
- Kvist, K.; Clasen-Linde, E.; Cortes, D.; Petersen, B.L.; Thorup, J. Adult Immunohistochemical Markers Fail to Detect Intratubular Germ Cell Neoplasia in Prepubertal Boys with Cryptorchidism. J. Urol. 2014, 191, 1084–1089. [Google Scholar] [CrossRef]
- Peereboom-Stegeman, J.H.; Melet, J.; Peereboom, J.W.; Hooghwinkel, G.J. Influence of Chronic Cd Intoxication on the Alkaline Phosphatase Activity of Liver and Kidney; Biochemical, Histochemical and Histological Investigations. Toxicology 1979, 14, 67–80. [Google Scholar] [CrossRef]
- Sharma, P.; Goyal, P.K. Ameliorative Effect of Green Tea Catechin Against Cadmium Chloride-Induced Testicular Toxicity in Mice. J. Environ. Pathol. Toxicol. Oncol. 2015, 34, 335–352. [Google Scholar] [CrossRef]
- Hamden, K.; Carreau, S.; Marki, F.A.; Masmoudi, H.; El Feki, A. Positive Effects of Green Tea on Hepatic Dysfunction, Lipid Peroxidation and Antioxidant Defence Depletion Induced by Cadmium. Biol. Res. 2008, 41, 331–339. [Google Scholar] [CrossRef]
- Li, J.L.; Wang, Q.Y.; Luan, H.Y.; Kang, Z.C.; Wang, C.B. Effects of L-Carnitine against Oxidative Stress in Human Hepatocytes: Involvement of Peroxisome Proliferator-Activated Receptor Alpha. J. Biomed. Sci. 2012, 19, 32. [Google Scholar] [CrossRef]
- Ma, L.; Mo, J.; Chen, Y.; Li, L.; Xie, L.; Chen, X.; Li, X.; Wang, Y.; Lin, Z.; Ge, R.S. In Utero Cadmium and Dibutyl Phthalate Combination Exposure Worsens the Defects of Fetal Testis in Rats. Environ. Pollut. 2020, 265, 114842. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, Y.; Yang, L.; Ma, C.; Fei, Y.; Ding, J.; Song, W.; Tong, W.-M.; Niu, Y.; Li, H. ALKBH5 in Mouse Testicular Sertoli Cells Regulates Cdh2 mRNA Translation to Maintain Blood-Testis Barrier Integrity. Cell. Mol. Biol. Lett. 2022, 27, 101. [Google Scholar] [CrossRef]
- Tafakori, V.; Zadmard, R.; Tabandeh, F.; Amoozegar, M.A.; Ahmadian, G. Equilibrium Isotherm, Kinetic Modeling, Optimization, and Characterization Studies of Cadmium Adsorption by Surface-Engineered Escherichia coli. Iran. Biomed. J. 2017, 21, 380. [Google Scholar]
- Jm, M. Cellular Mechanisms of Cadmium Toxicity Related to the Homeostasis of Essential Metals. Biomet. Int. J. Role Met. Ions Biol. Biochem. Med. 2010, 23, 877–896. [Google Scholar] [CrossRef]
- Wang, Y.; Luan, G.; Zhou, W.; Meng, J.; Wang, H.; Hu, N.; Suo, Y. Subcritical Water Extraction, UPLC-Triple-TOF/MS Analysis and Antioxidant Activity of Anthocyanins from Lycium ruthenicum Murr. Food Chem. 2018, 249, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Klusóczki, Á.; Oláh, B.; Hosszú, D.; Fenyvesi, F.; Remenyik, J.; Homoki, J.; Gyöngyösi, A.; Bácskay, I.; Váradi, J. Effectiveness of Anthocyanin-Rich Sour Cherry Extract on Gliadin-Induced Caco-2 Barrier Damage. Nutrients 2023, 15, 4022. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-Y.; Chen, H.; Zirkin, B. Sirt1 and Nrf2: Regulation of Leydig Cell Oxidant/Antioxidant Intracellular Environment and Steroid Formation. Biol. Reprod. 2021, 105, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, P.; Yang, X.; Wang, W.; Zhang, J.; He, Y.; Zhang, W.; Jing, T.; Wang, B.; Lin, R. SIRT1 Inhibits Inflammatory Response Partly through Regulation of NLRP3 Inflammasome in Vascular Endothelial Cells. Mol. Immunol. 2016, 77, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Li, L.; Yanqun, X.; Cravotto, G.; Luo, Z. Ultrasonic-Assisted Modifications of Macroporous Resin to Improve Anthocyanin Purification from a Pyrus communis Var. Starkrimson Extract. Ultrason. Sonochem. 2020, 62, 104853. [Google Scholar] [CrossRef] [PubMed]
- Paul, I.D.; Das, M. Effect of Freeze, Microwave-Convective Hot Air, Vacuum and Dehumidified Air Drying on Total Phenolics Content, Anthocyanin Content and Antioxidant Activity of Jamun (Syzygium cumini L.) Pulp. J. Food Sci. Technol. 2018, 55, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, Q.; Belwal, T.; Huang, H.; Zou, L.; Lv, W.; Luo, Z. Effect of Advanced/Hybrid Oxidation Process Involving Ultrasonication and Ultraviolet Radiation (Sonophotolysis) on Anthocyanin Stability: Degradation Kinetics and Mechanism. Food Chem. 2022, 370, 131083. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yan, Y.; Ran, L.; Mi, J.; Sun, Y.; Lu, L.; Gao, Y.; Zeng, X.; Cao, Y. Isolation, Antioxidant Property and Protective Effect on PC12 Cell of the Main Anthocyanin in Fruit of Lycium ruthenicum Murray. J. Funct. Foods 2017, 30, 97–107. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Kafache, D.; Deli, M.; Galani, B.R.T.; Agume, A.N.; Bouba, A.A.; Njintang, N.Y. Physicochemical and in Vitro Antioxidant Properties of Juice and Cake Filters from Carissa edulis Vahl Fruits. J. Explor. Res. Pharmacol. 2022, 7, 133–144. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, S.; Zhou, W.; Meng, J.; Deng, K.; Zhou, H.; Hu, N.; Suo, Y. Rapid Qualitative and Quantitative Analyses of Eighteen Phenolic Compounds from Lycium ruthenicum Murray by UPLC-Q-Orbitrap MS and Their Antioxidant Activity. Food Chem. 2018, 269, 150–156. [Google Scholar] [CrossRef]
- Lu, L.; Mi, J.; Jin, B.; Zhang, L.; Luo, Q.; Li, X.; Yan, Y.; Cao, Y. Inhibitory Effects of the Anthocyanins from Lycium ruthenicum Murray on Angiotensin-I-Converting Enzyme: In Vitro and Molecular Docking Studies. J. Sci. Food Agric. 2023, 103, 7164–7175. [Google Scholar] [CrossRef]
- Caballero-García, A.; Noriega-González, D.C.; Roche, E.; Drobnic, F.; Córdova, A. Effects of L-Carnitine Intake on Exercise-Induced Muscle Damage and Oxidative Stress: A Narrative Scoping Review. Nutrients 2023, 15, 2587. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Pan, Y.; Gao, X.; Chen, H. Hypoglycemic and Hypolipidemic Effects of Anthocyanins Extract from Black Soybean Seed Coat in High Fat Diet and Streptozotocin-Induced Diabetic Mice. Food Funct. 2018, 9, 426–439. [Google Scholar] [CrossRef]
- Wang, B.; Cui, S.; Mao, B.; Zhang, Q.; Tian, F.; Zhao, J.; Tang, X.; Chen, W. Cyanidin Alleviated CCl4-Induced Acute Liver Injury by Regulating the Nrf2 and NF-κB Signaling Pathways. Antioxidants 2022, 11, 2383. [Google Scholar] [CrossRef] [PubMed]
- Olaniyan, O.T.; Ojewale, A.O.; Eweoya, O.O.; Adedoyin, A.A.; Adesanya, O.A.; Adeoye, A.O.; Okeniran, O.S. Modulatory Role of Vitamin E on Proton Pump (ATPase) Activity of Cadmium Chloride-Induced Testicular Damage in Wistar Rats. BioMed Res. Int. 2021, 2021, 4615384. [Google Scholar] [CrossRef]
- Solis-Cruz, B.; Hernandez-Patlan, D.; Petrone, V.M.; Pontin, K.P.; Latorre, J.D.; Beyssac, E.; Hernandez-Velasco, X.; Merino-Guzman, R.; Owens, C.; Hargis, B.M.; et al. Evaluation of Cellulosic Polymers and Curcumin to Reduce Aflatoxin B1 Toxic Effects on Performance, Biochemical, and Immunological Parameters of Broiler Chickens. Toxins 2019, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, X.; Zhang, P.; Chen, Z.; Guo, Z.; Shang, X. Reproductive Toxicity Investigation of Silica Nanoparticles in Male Pubertal Mice. Environ. Sci. Pollut. Res. 2022, 29, 36640–36654. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Yang, T.; Ye, X.; Calafat, A.M.; Hauser, R. Urinary Concentrations of Parabens and Serum Hormone Levels, Semen Quality Parameters, and Sperm DNA Damage. Environ. Health Perspect. 2011, 119, 252–257. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Lu, J.-C.; Tang, S.-S. Effects of Six Kinds of Sperm Staining Methods on Human Sperm Size and Evaluation of Their Staining Effects. J. Clin. Lab. Anal. 2022, 36, e24794. [Google Scholar] [CrossRef]
- Gill, K.; Machalowski, T.; Harasny, P.; Kups, M.; Grabowska, M.; Duchnik, E.; Sipak, O.; Fraczek, M.; Kurpisz, M.; Kurzawa, R.; et al. Male Infertility Coexists with Decreased Sperm Genomic Integrity and Oxidative Stress in Semen Irrespective of Leukocytospermia. Antioxidants 2022, 11, 1987. [Google Scholar] [CrossRef]
- Johnsen, S.G. Testicular Biopsy Score Count—A Method for Registration of Spermatogenesis in Human Testes: Normal Values and Results in 335 Hypogonadal Males. Hormones 1970, 1, 2–25. [Google Scholar] [CrossRef]
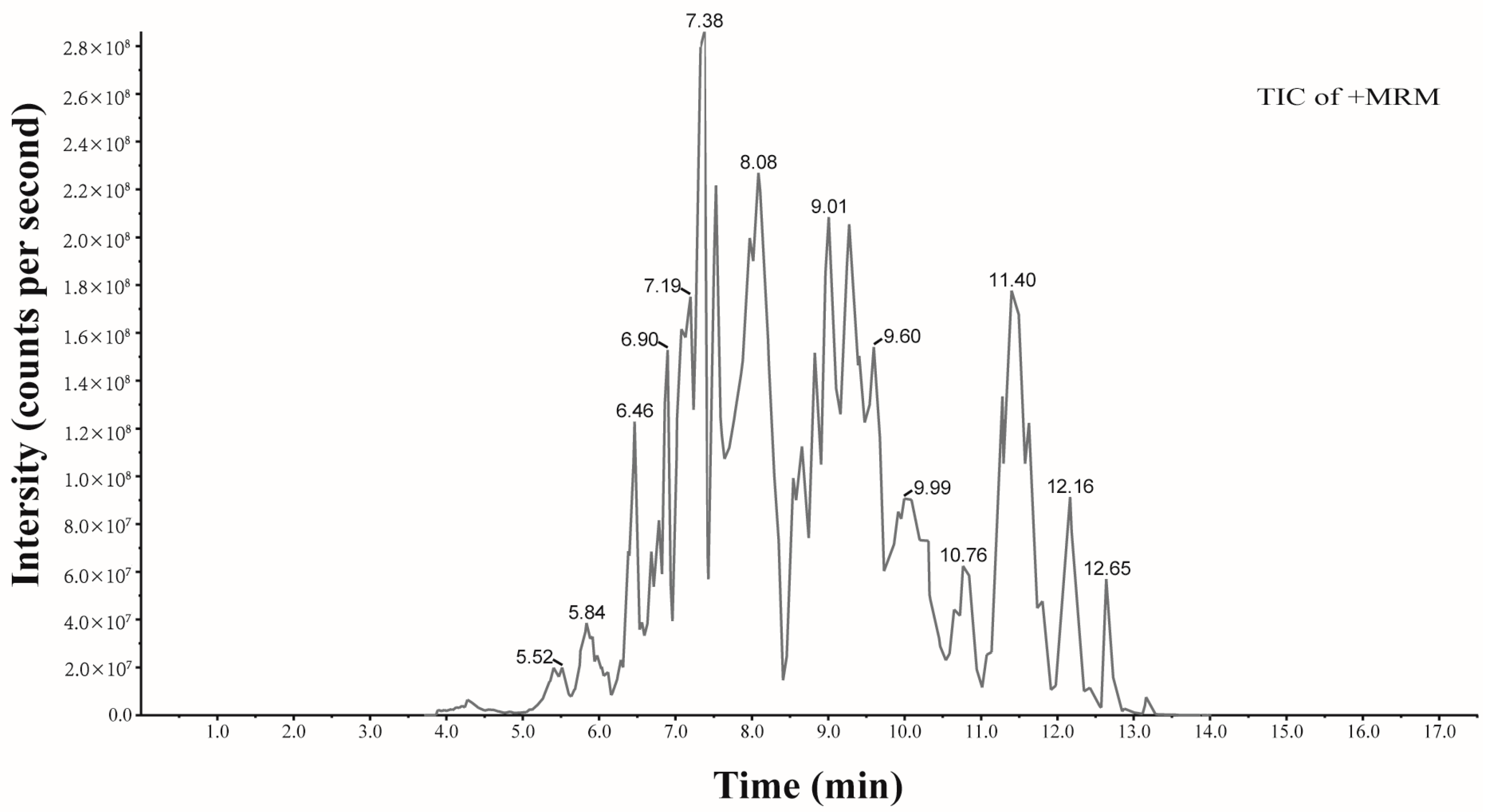








| Identification | tR | Formula | Ionization Model | Molecular | Content (μg/g) |
|---|---|---|---|---|---|
| Procyanidin B1 | 4.21 | C30H26O12 | [M+H]+ | 578.14 | 2.21 |
| Cyanidin-3,5-O-diglucoside | 5.89 | C27H31O16+ | [M]+ | 611.16 | 45.13 |
| Procyanidin B2 | 5.96 | C30H26O12 | [M+H]+ | 578.14 | 5.05 |
| Delphinidin-3-O-sophoroside | 6.46 | C27H31O17+ | [M]+ | 627.16 | 16.40 |
| Delphinidin-3-O-galactoside | 6.50 | C21H21O12+ | [M]+ | 465.10 | 80.22 |
| Cyanidin-3-gentiobioside | 6.90 | C27H31O16+ | [M+H]+ | 611.16 | 6.76 |
| Delphinidin-3-O-glucoside | 6.95 | C21H21O12+ | [M]+ | 465.10 | 51.98 |
| Cyanidin-3-O-arabinosidase-glucoside | 7.15 | C26H29O15+ | [M+H]+ | 581.15 | 1.90 |
| Cyanidin-3-O-galactoside | 7.43 | C21H21O11+ | [M]+ | 449.11 | 169.64 |
| Delphinidin-3-O-rutinoside-5-O-glucoside | 7.48 | C33H41O21+ | [M]+ | 773.21 | 28.77 |
| Delphinidin-3-O-rutinoside | 7.57 | C27H31O16+ | [M]+ | 611.16 | 23.11 |
| Cyanidin-3-O-sambubioside | 8.11 | C26H29O15+ | [M]+ | 581.15 | 19.49 |
| Pelargonidin-3-O-galactoside | 8.32 | C21H21O10+ | [M]+ | 433.11 | 3.55 |
| Petunidin-3-O-sophoroside | 8.60 | C28H33O17+ | [M]+ | 641.17 | 5.77 |
| Cyanidin-3-O-rutinoside | 8.62 | C27H31O15+ | [M]+ | 595.17 | 181.40 |
| Petunidin-3-O-galactoside | 8.63 | C22H23O12+ | [M]+ | 479.12 | 23.00 |
| Petunidin-3-O-glucoside | 8.64 | C22H23O12+ | [M]+ | 479.12 | 12.14 |
| Peonidin-3-O-galactoside | 9.02 | C22H23O11+ | [M]+ | 463.12 | 12.17 |
| Petunidin-3-O-sophoroside-glucoside-5-O-sambubioside | 9.06 | C45H61O31+ | [M+H]+ | 1097.32 | 3.38 |
| Cyanidin-3-O-(6″-O-acetyl-2″-O-xylosyl) glucoside | 9.53 | C28H31O16+ | [M+H]+ | 623.16 | 13.59 |
| Malvidin-3-O-galactoside | 9.54 | C23H25O12+ | [M]+ | 493.13 | 58.48 |
| Cyanidin-3-O-xyloside | 10.24 | C20H19O10+ | [M]+ | 419.10 | 57.73 |
| Malvidin-3-O-rutinoside | 10.32 | C29H35O16+ | [M]+ | 639.19 | 130.37 |
| Delphinidin-3-O-(6″-O-coumaroyl) rhamnoside-5-O-glucoside | 10.42 | C36H37O18+ | [M+H]+ | 757.20 | 9.86 |
| Cyanidin-3-O-(6-O-malonyl-beta-D-glucoside) | 10.77 | C24H23O14+ | [M]+ | 535.11 | 11.43 |
| Petunidin-3-O-rutinoside-5-O-rhamnoside | 10.97 | C34H43O20+ | [M+H]+ | 771.23 | 4.11 |
| Quercetin-3-O-glucoside | 11.71 | C21H20O12 | [M+H]+ | 464.10 | 155.23 |
| Cyanidin-3-O-(6″-O-caffeoyl) rhamnoside | 12.65 | C30H27O13+ | [M+H]+ | 595.15 | 8.65 |
| Petunidin-3-O-(6-O-p-coumaroyl)-glucoside | 12.74 | C31H29O14+ | [M]+ | 625.16 | 5.00 |
| Naringenin | 13.17 | C15H12O5 | [M+H]+ | 272.07 | 11.55 |
| Score | Standard |
|---|---|
| 10 | Complete spermatogenesis and perfect tubule |
| 9 | Many spermatozoa present, but germinal epithelium disorganized with marked sloughing or obliteration of lumen |
| 8 | Only a few (<5–10) late spermatids |
| 7 | No late spermatids, but many early spermatids |
| 6 | No spermatozoa and only few spermatids (<5–10) present |
| 5 | No spermatozoa, no spermatids, but several or many spermatocytes present |
| 4 | Only few spermatocytes (<5) and no spermatids or spermatozoa present |
| 3 | Spermatogonia are the only germ cells present |
| 2 | No germ cells but Sertoli cells are present |
| 1 | No cells in tubular section |
| Gene | Species | Forward (5′-3′) | Reverse (3′-5′) |
|---|---|---|---|
| β-actin | Mouse | TATAAAACCCGGCGGCGCA | CATCCATGGCGAACTGGTGG |
| Nrf2 | Mouse | ACCTCTGCTGCAAGTAGCCT | TGGGCAACCATCACTCTGCT |
| Keap1 | Mouse | ACAGCAGCGTGGAGAGATATG | GTTAAGCCGGTTAGTCCCGT |
| HO-1 | Mouse | GCTAGCCTGGTGCAAGATACT | AAGCTGAGAGTGAGGACCCA |
| NQO-1 | Mouse | AGGATGGGAGGTACTCGAATC | AGGCGTCCTTCCTTATATGCTA |
| SOD2 | Mouse | TCGCTCTTCAGCCTGCACTG | AGCCTCCAGCAACTCTCCTTTG |
| SOD3 | Mouse | TGAGAAGATAGGCGACACGC | TGGCTGATGGTTGTACCCTG |
| CAT | Mouse | CGCAATTCACACCTACACGC | GGGAGAATCCATCCAGCGTT |
| GCLC | Mouse | GGGGTGACGAGGTGGAGTA | GTTGGGGTTTGTCCTCTCCC |
| GCLM | Mouse | CAATGACCCGAAAGAACTGCTC | TTCCCCTGCTCTTCACGATG |
| BACH1 | Mouse | AGAGGGAGTGAGTCACCTG | TCACTGTCATCGACCGGAG |
| SIRT1 | Mouse | TCGGCTACCGAGGTCCATA | TAACAATCTGCCACAGCGTC |
| NOX4 | Mouse | ACCAAATGTTGGGCGATTGTG | GATGAGGCTGCAGTTGAGGT |
| GPX1 | Mouse | AGTCCACCGTGTATGCCTTCT | GAGACGCGACATTCTCAATGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, M.; Lu, J.; Xue, H.; Lou, Y.; Li, S.; Liu, T.; Ding, Z.; Chen, X. Anthocyanins from Lycium ruthenicum Murray Mitigate Cadmium-Induced Oxidative Stress and Testicular Toxicity by Activating the Keap1/Nrf2 Signaling Pathway. Pharmaceuticals 2024, 17, 322. https://doi.org/10.3390/ph17030322
Dong M, Lu J, Xue H, Lou Y, Li S, Liu T, Ding Z, Chen X. Anthocyanins from Lycium ruthenicum Murray Mitigate Cadmium-Induced Oxidative Stress and Testicular Toxicity by Activating the Keap1/Nrf2 Signaling Pathway. Pharmaceuticals. 2024; 17(3):322. https://doi.org/10.3390/ph17030322
Chicago/Turabian StyleDong, Mingran, Juan Lu, Hongwei Xue, Yang Lou, Shuyang Li, Tao Liu, Zimian Ding, and Xi Chen. 2024. "Anthocyanins from Lycium ruthenicum Murray Mitigate Cadmium-Induced Oxidative Stress and Testicular Toxicity by Activating the Keap1/Nrf2 Signaling Pathway" Pharmaceuticals 17, no. 3: 322. https://doi.org/10.3390/ph17030322
APA StyleDong, M., Lu, J., Xue, H., Lou, Y., Li, S., Liu, T., Ding, Z., & Chen, X. (2024). Anthocyanins from Lycium ruthenicum Murray Mitigate Cadmium-Induced Oxidative Stress and Testicular Toxicity by Activating the Keap1/Nrf2 Signaling Pathway. Pharmaceuticals, 17(3), 322. https://doi.org/10.3390/ph17030322








