Targeting Common Inflammatory Mediators in Experimental Severe Asthma and Acute Lung Injury
Abstract
:1. Introduction
2. Results
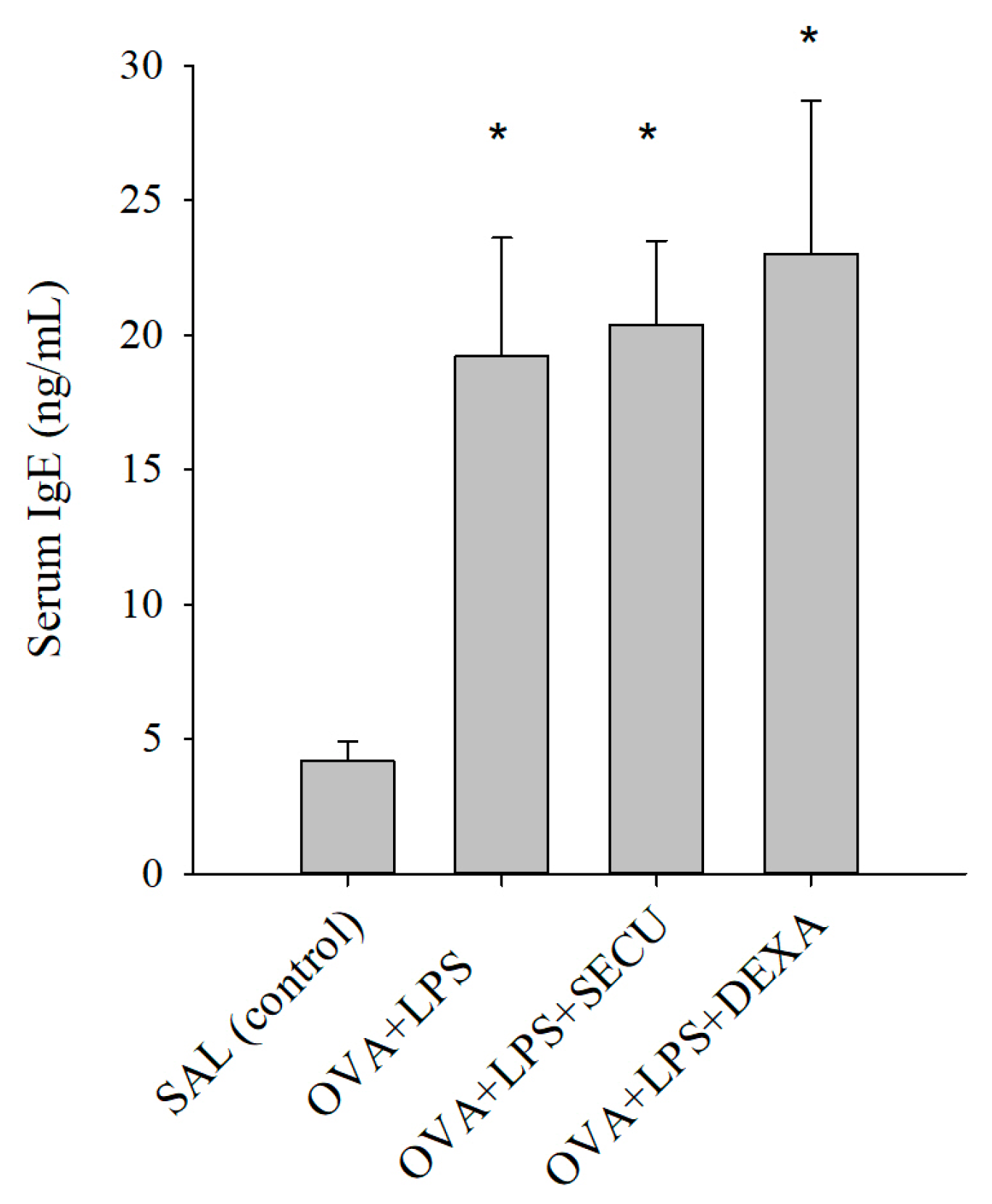
2.1. Effects of SECU on the Level of Ovalbumin (OVA)-Specific IgE in Serum
2.2. Effects of Secukinumab on Bronchoalveolar Lavage Fluid (BALF)
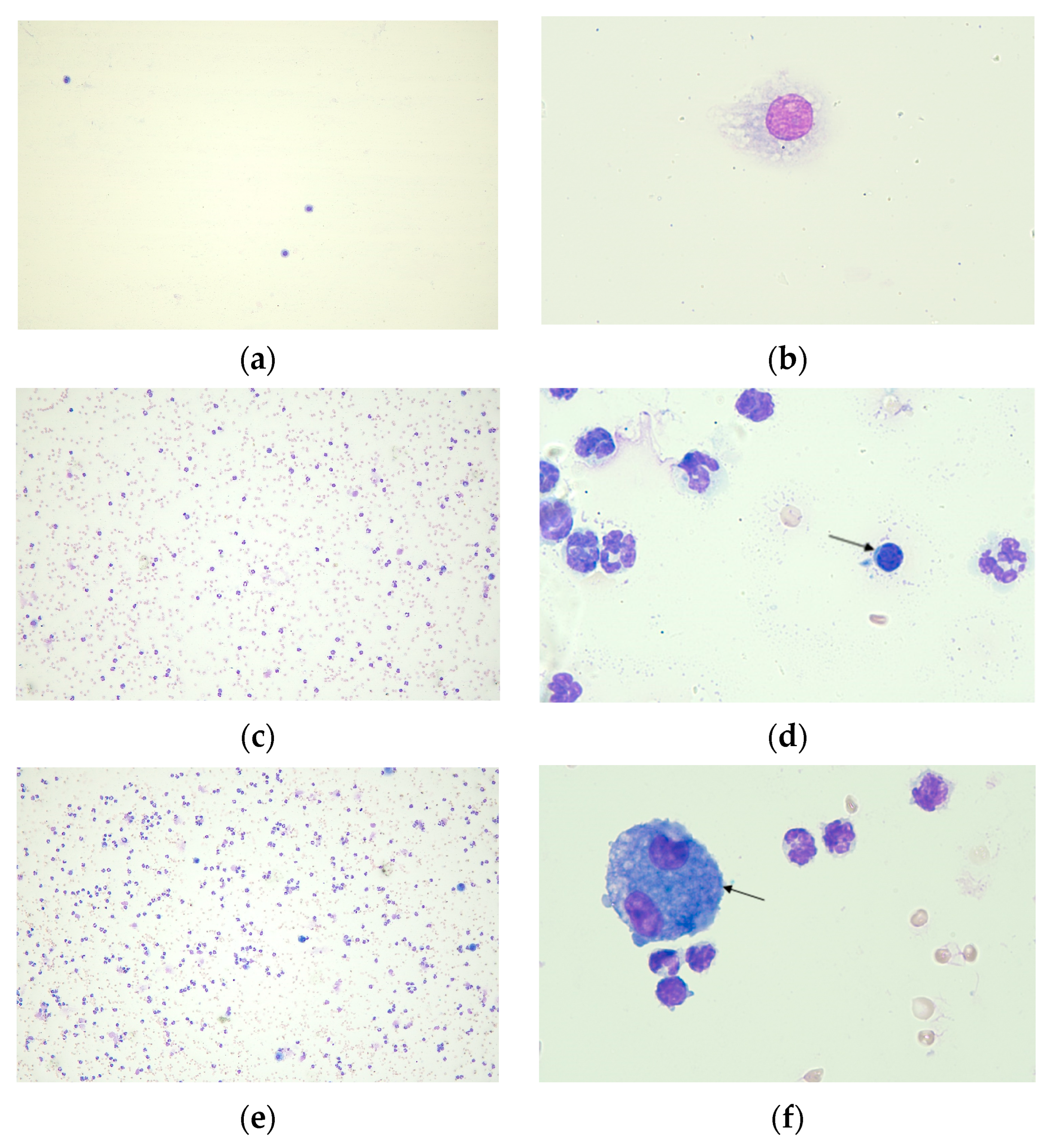
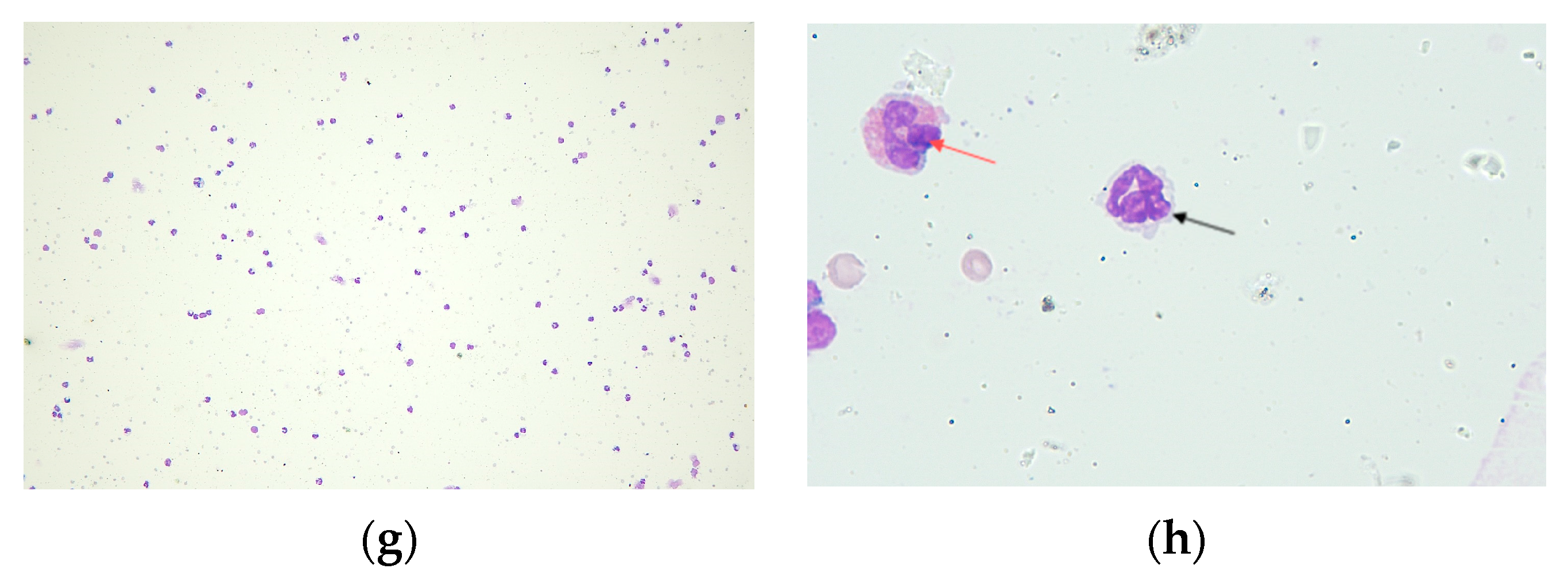
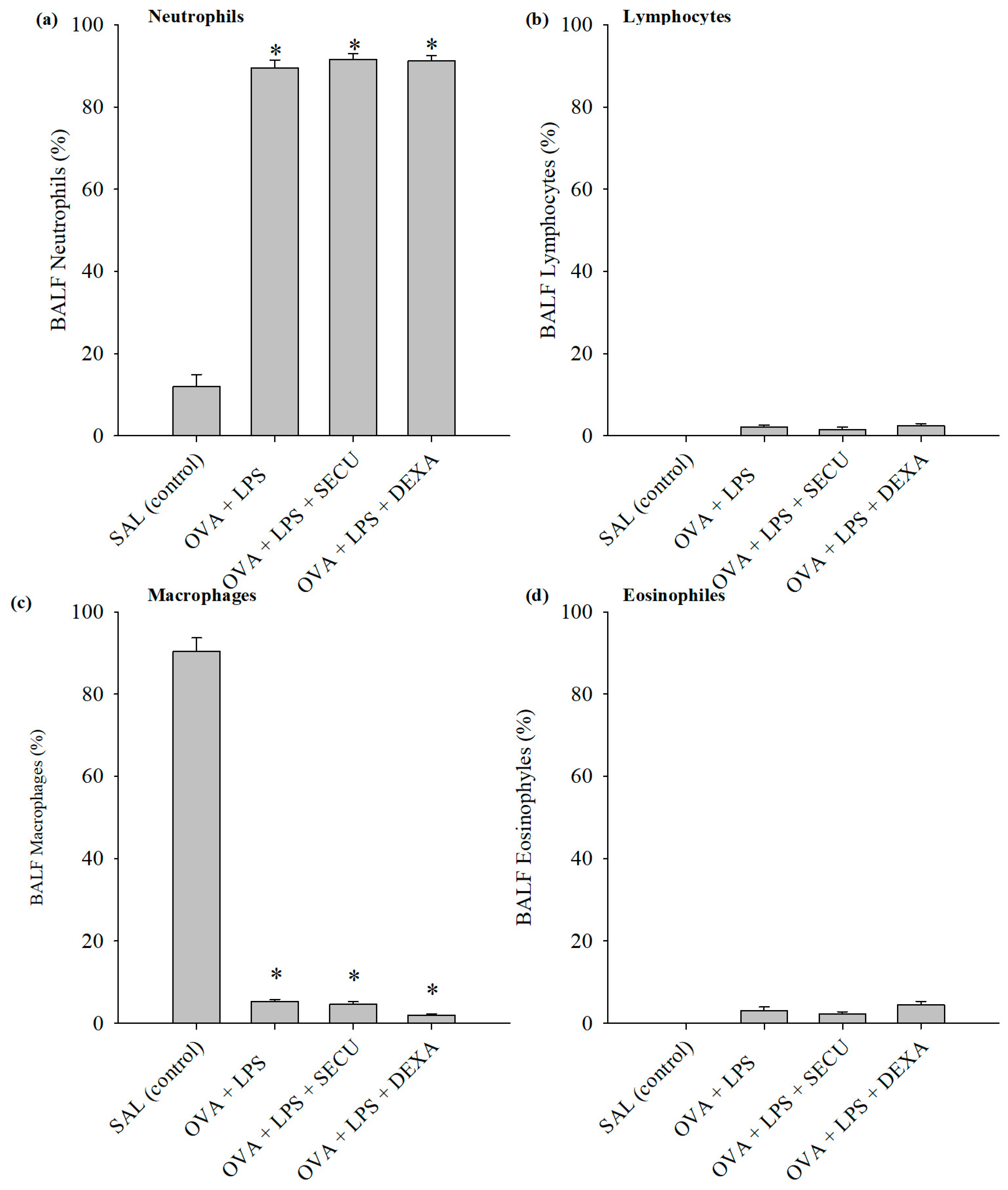
2.2.1. Cell Count in BALF
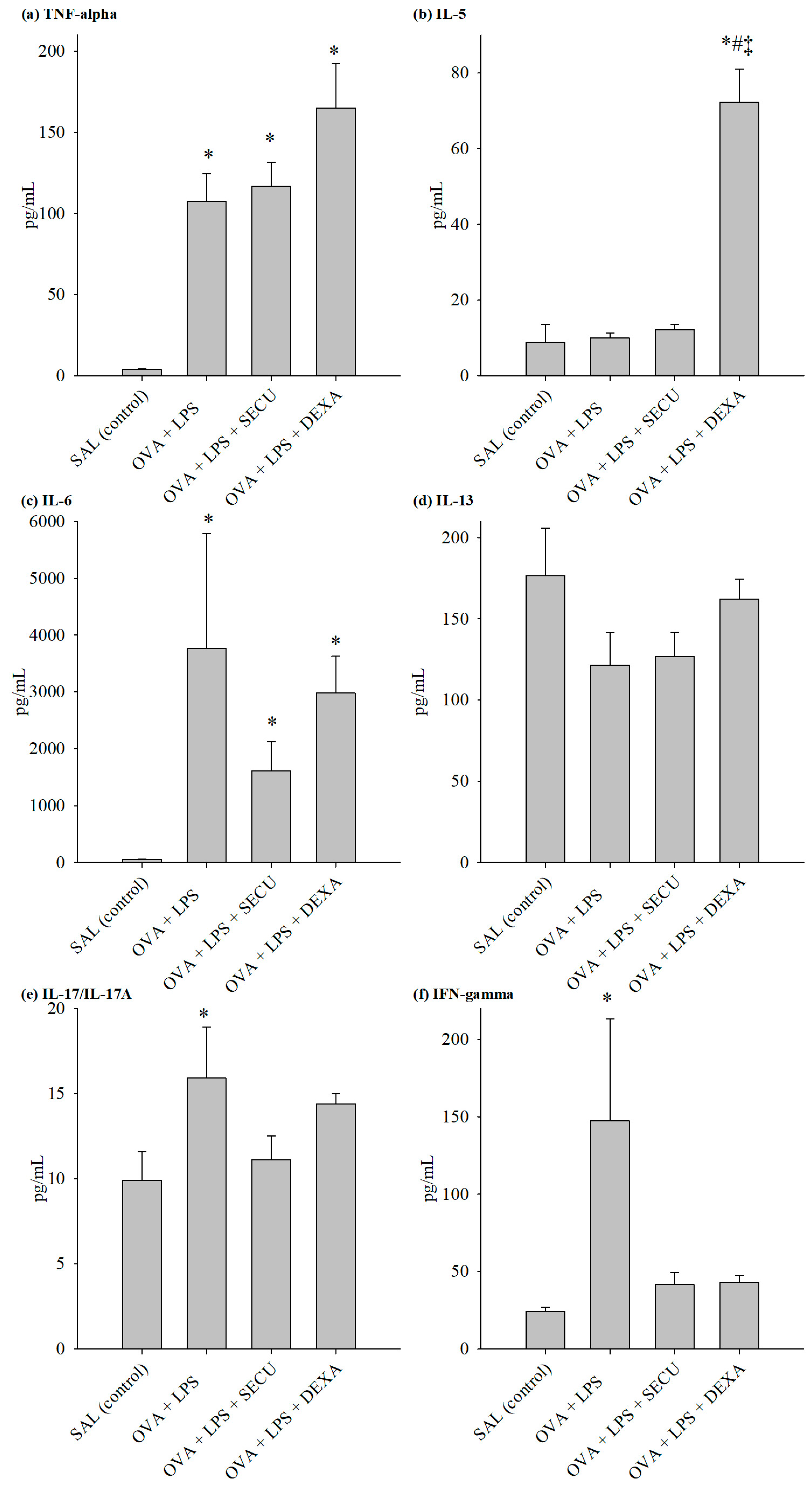
2.2.2. Cytokines in BALF
2.2.3. Cytokines in Lung Tissue Homogenate (LTH)
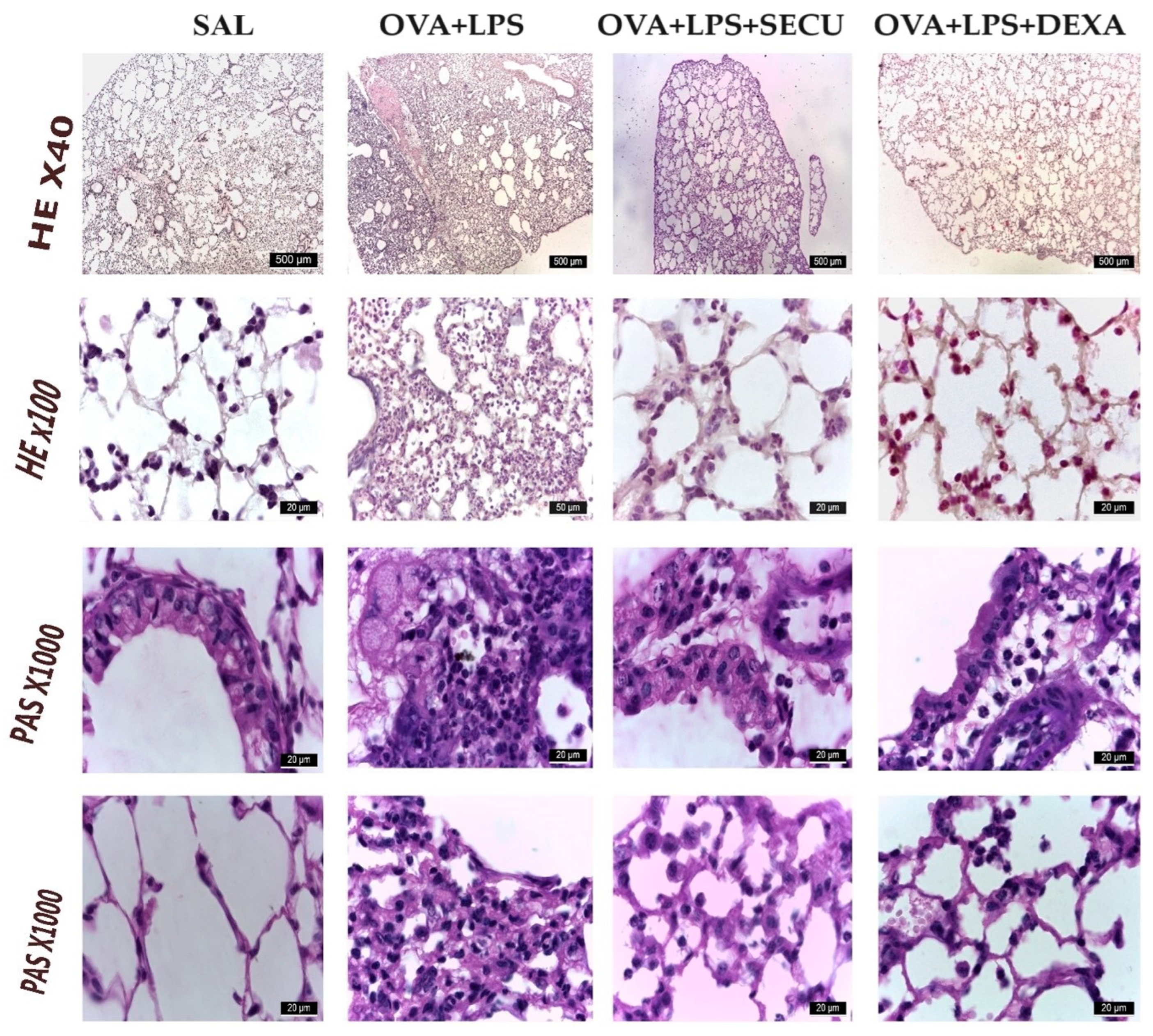
2.2.4. Histological Analysis
3. Discussion
- there is a limited number of exposure groups in our study, and at least two other exposure groups, with LPS and OVA only, could have been helpful to understand better how the combined treatment deviates from the individual pathological condition; however, the study design was decided based on the already existing comparative results [1,5,45] for OVA and LPS and OVA + LPS; moreover, the goal of the 3Rs principles (Replacement, Reduction and Refinement) was followed;
- secondary to binding the LPS binding protein (LBP), LPS:LBP complexes ligate the TLR4 receptor located on the surface of many cell types, inducing a strong proinflammatory reaction with the upregulation of cytokine expression [46]; the results interpretation have to be completed with a certain degree of caution because mice share with humans only approximately 50% homology of the TLR4 receptor [34];
- overreliance on murine models can often be difficult to extrapolate to the complex and uniquely human disease of asthma; moreover, the use of female only animals may raise the question of the potential effect on male mice;
- this study lacks a deeper mechanistic exploration of how SECU modulates the immune response in the context of asthma and ALI, but the link between intra-/inter-cellular signaling and the level of cytokines/inflammatory markers is already approached in asthma [47,48,49,50] or in ALI [51] by other authors;
- Secukinumab is able to neutralize cynomolgus, rhesus and marmoset monkey IL-17A but not the rodent IL-17A.
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Animal Experimental Design
- SAL group (normal control), which received inhalations with a sterile saline solution;
- OVA + LPS group (positive control for disease), which received inhalations of an ovalbumin solution and LPS instillation;
- OVA + LPS + SECU group, which received inhalations of an ovalbumin solution, LPS instillation and treatment with Secukinumab (10 mg/kg, subcutaneous);
- OVA + LPS + DEXA group (positive control for treatment), which received inhalations of an ovalbumin solution, LPS instillation and treatment with Dexamethasone (1 mg/kg, intraperitoneal).
4.2.1. Sensitization Protocol
4.2.2. ALI Induction and Treatment
4.2.3. Blood Sampling for OVA-Specific IgE and BALF
4.2.4. Lung Homogenate Analysis
4.2.5. Histological Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camargo, L.D.N.; Righetti, R.F.; Aristóteles, L.R.D.C.R.B.; Dos Santos, T.M.; de Souza, F.C.R.; Fukuzaki, S.; Cruz, M.M.; Alonso-Vale, M.I.C.; Saraiva-Romanholo, B.M.; Prado, C.M.; et al. Effects of Anti-IL-17 on Inflammation, Remodeling, and Oxidative Stress in an Experimental Model of Asthma Exacerbated by LPS. Front. Immunol. 2018, 8, 1835. [Google Scholar] [CrossRef]
- Camargo, L.D.N.; Santos, T.M.D.; Andrade, F.C.P.D.; Fukuzaki, S.; Dos Santos Lopes, F.D.T.Q.; De Arruda Martins, M.; Prado, C.M.; Leick, E.A.; Righetti, R.F.; Tiberio, I.D.F.L.C. Bronchial Vascular Remodeling Is Attenuated by Anti-IL-17 in Asthmatic Responses Exacerbated by LPS. Front. Pharmacol. 2020, 11, 1269. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, T.M.; Righetti, R.F.; Rezende, B.G.; Campos, E.C.; Camargo, L.D.N.; Saraiva-Romanholo, B.M.; Fukuzaki, S.; Prado, C.M.; Leick, E.A.; Martins, M.A.; et al. Effect of anti-IL17 and/or Rho-kinase inhibitor treatments on vascular remodeling induced by chronic allergic pulmonary inflammation. Ther. Adv. Respir. Dis. 2020, 14, 1753466620962665. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.M.D.; Righetti, R.F.; Camargo, L.D.N.; Saraiva-Romanholo, B.M.; Aristoteles, L.R.C.R.B.; De Souza, F.C.R.; Fukuzaki, S.; Alonso-Vale, M.I.C.; Cruz, M.; Prado, C.M.; et al. Effect of Anti-IL17 Antibody Treatment Alone and in Combination With Rho-Kinase Inhibitor in a Murine Model of Asthma. Front. Physiol. 2018, 9, 1183. [Google Scholar] [CrossRef] [PubMed]
- Righetti, R.F.; Dos Santos, T.M.; Camargo, L.D.N.; Aristóteles, L.R.C.R.B.; Fukuzaki, S.; De Souza, F.C.R.; Santana, F.P.R.; De Agrela, M.V.R.; Cruz, M.M.; Alonso-Vale, M.I.C.; et al. Protective Effects of Anti-IL17 on Acute Lung Injury Induced by LPS in Mice. Front. Pharmacol. 2018, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.R.; Blakey, J.D. Why are people with asthma susceptible to pneumonia? A review of factors related to upper airway bacteria. Respirology 2019, 24, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.; Al-Haddad, M. Acute lung injury and acute respiratory distress syndrome. Contin. Educ. Anaesth. Crit. Care Pain. 2009, 9, 152–156. [Google Scholar] [CrossRef]
- Comhair, S.A.; Erzurum, S.C. Redox Control of Asthma: Molecular Mechanisms and Therapeutic Opportunities. Antioxidants Redox Signal. 2010, 12, 93–124. [Google Scholar] [CrossRef]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, O. Oxidative stress in asthma. World Allergy Organ J. 2011, 4, 151–158. [Google Scholar] [CrossRef]
- Metnitz, P.G.H.; Bartens, C.; Fischer, M.; Fridrich, P.; Steltzer, H.; Druml, W. Antioxidant status in patients with acute respiratory distress syndrome. Intensiv. Care Med. 1999, 25, 180–185. [Google Scholar] [CrossRef]
- Bezerra, F.S.; Lanzetti, M.; Nesi, R.T.; Nagato, A.C.; e Silva, C.P.; Kennedy-Feitosa, E.; Melo, A.C.; Cattani-Cavalieri, I.; Porto, L.C.; Valenca, S.S. Oxidative Stress and Inflammation in Acute and Chronic Lung Injuries. Antioxidants 2023, 12, 548. [Google Scholar] [CrossRef]
- McGovern, T.K.; Chen, M.; Allard, B.; Larsson, K.; Martin, J.G.; Adner, M. Neutrophilic oxidative stress mediates organic dust-induced pulmonary inflammation and airway hyperresponsiveness. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L155–L165. [Google Scholar] [CrossRef]
- Pham, D.L.; Ban, G.; Kim, S.; Shin, Y.S.; Ye, Y.; Chwae, Y.; Park, H. Neutrophil autophagy and extracellular DNA traps contribute to airway inflammation in severe asthma. Clin. Exp. Allergy 2017, 47, 57–70. [Google Scholar] [CrossRef]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000 2015, 69, 142–159. [Google Scholar] [CrossRef]
- Zijlstra, G.J.; Hacken, N.H.T.T.; Hoffmann, R.F.; van Oosterhout, A.J.M.; Heijink, I.H. Interleukin-17A induces glucocorticoid insensitivity in human bronchial epithelial cells. Eur. Respir. J. 2012, 39, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Chang, M.M.J.; Velichko, S.; Thai, P.; Hung, L.Y.; Huang, F.; Phuong, N.; Chen, Y.; Wu, R. NF-κB mediates IL-1β- and IL-17A-induced MUC5B expression in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2011, 45, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Melton, A.C.; Chen, C.; Engler, M.B.; Huang, K.E.; Ren, X.; Wang, Y.; Bernstein, X.; Li, J.T.; Atabai, K.; et al. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat. Med. 2012, 18, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.B.; Bhat, S.G.; Bhandary, Y.P. Curcumin attenuates IL-17A mediated pulmonary SMAD dependent and non-dependent mechanism during acute lung injury in vivo. Mol. Biol. Rep. 2020, 47, 5643–5649. [Google Scholar] [CrossRef] [PubMed]
- Flierl, M.A.; Rittirsch, D.; Gao, H.; Hoesel, L.M.; Nadeau, B.A.; Day, D.E.; Zetoune, F.S.; Sarma, J.V.; Huber-Lang, M.S.; Ferrara, J.L.M.; et al. Adverse functions of IL-17A in experimental sepsis. FASEB J. 2008, 22, 2198–2205. [Google Scholar] [CrossRef]
- Wiche Salinas, T.R.; Zheng, B.; Routy, J.P.; Ancuta, P. Targeting the interleukin-17 pathway to prevent acute respiratory distress syndrome associated with SARS-CoV-2 infection. Respirology 2020, 25, 797–799. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Tong, L.; Wang, J.; Dou, M.; Ji, S.; Bi, J.; Chen, C.; Yang, D.; He, H.; et al. Recovery from acute lung injury can be regulated via modulation of regulatory T cells and Th17 cells. Scand. J. Immunol. 2018, 88, e12715. [Google Scholar] [CrossRef]
- Liu, S.P.; Huang, L.; Flores, J.; Ding, Y.; Li, P.; Peng, J.; Zuo, G.; Zhang, J.H.; Lu, J.; Tang, J.P. Secukinumab attenuates reactive astrogliosis via IL-17RA/(C/EBPβ)/SIRT1 pathway in a rat model of germinal matrix hemorrhage. CNS Neurosci. Ther. 2019, 25, 1151–1161. [Google Scholar] [CrossRef]
- Mohamad, H.E.; Asker, M.E.; Shaheen, M.A.; Baraka, N.M.; Fantoukh, O.I.; Alqahtani, A.; Salama, A.E.; Mahmoud, Y.K. Secukinumab and Black Garlic Downregulate OPG/RANK/RANKL Axis and Devitalize Myocardial Interstitial Fibrosis Induced by Sunitinib in Experimental Rats. Life 2023, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Deng, S.; Ding, Y.; Flores, J.J.; Zhang, X.; Jia, X.; Hu, X.; Peng, J.; Zuo, G.; Zhang, J.H.; et al. Secukinumab attenuates neuroinflammation and neurobehavior defect via PKCβ/ERK/NF-κB pathway in a rat model of GMH. Exp. Neurol. 2023, 360, 114276. [Google Scholar] [CrossRef] [PubMed]
- Oztanir, M.N.; Dogan, M.F.; Turkmen, N.B.; Taslidere, A.; Sahin, Y.; Ciftci, O. Secukinumab ameliorates oxidative damage induced by cerebral ischemia-reperfusion in rats. Turk. Neurosurg. 2022, 32, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Karatas, A.; Celik, C.; Oz, B.; Akar, Z.A.; Etem, E.O.; Dagli, A.F.; Koca, S.S. Secukinumab and metformin ameliorate dermal fibrosis by decreasing tissue interleukin-17 levels in bleomycin-induced dermal fibrosis. Int. J. Rheum. Dis. 2021, 24, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Sun, L.; Gao, G.; Li, Y. Protective effect of Secukinumab on severe sepsis model rats by neutralizing IL-17A to inhibit IKBα/NFκB inflammatory signal pathway. Eur. J. Med. Res. 2022, 27, 206. [Google Scholar] [CrossRef] [PubMed]
- Eshwar, V.; Kamath, A. Assessment of safety profile of secukinumab in real-world scenario using United States food and drug administration adverse event reporting system database. Sci. Rep. 2024, 14, 1222. [Google Scholar] [CrossRef]
- Ci, X.; Chu, X.; Wei, M.; Yang, X.; Cai, Q.; Deng, X. Different Effects of Farrerol on an OVA-Induced Allergic Asthma and LPS-induced Acute Lung Injury. PLoS ONE 2012, 7, e34634. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.O.; Imam, F.; Al-Harbi, M.M.; Ansari, M.A.; Zoheir, K.M.; Korashy, H.M.; Sayed-Ahmed, M.M.; Attia, S.M.; Shabanah, O.A.; Ahmad, S.F. Dexamethasone Attenuates LPS-induced Acute Lung Injury through Inhibition of NF-κB, COX-2, and Pro-inflammatory Mediators. Immunol. Investig. 2016, 45, 349–369. [Google Scholar] [CrossRef]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef]
- Huang, Q.; Han, L.; Lv, R.; Ling, L. Magnolol exerts anti-asthmatic effects by regulating Janus kinase-signal transduction and activation of transcription and Notch signaling pathways and modulating Th1/Th2/Th17 cytokines in ovalbumin-sensitized asthmatic mice. Korean J. Physiol. Pharmacol. 2019, 23, 251. [Google Scholar] [CrossRef]
- Hellings, P.W.; Kasran, A.; Liu, Z.; Vandekerckhove, P.; Wuyts, A.; Overbergh, L.; Mathieu, C.; Ceuppens, J.L. Interleukin-17 Orchestrates the Granulocyte Influx into Airways after Allergen Inhalation in a Mouse Model of Allergic Asthma. Am. J. Respir. Cell Mol. Biol. 2003, 28, 42–50. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L379–L399. [Google Scholar] [CrossRef]
- Ehrentraut, H.; Weisheit, C.K.; Frede, S.; Hilbert, T. Inducing Acute Lung Injury in Mice by Direct Intratracheal Lipopolysaccharide Instillation. JoVE 2019, 6, 59999. [Google Scholar]
- Nguyen, L.T.; Lim, S.; Oates, T.; Chung, K.F. Increase in airway neutrophils after oral but not inhaled corticosteroid therapy in mild asthma. Respir. Med. 2005, 99, 200–207. [Google Scholar] [CrossRef]
- Meagher, L.C.; Cousin, J.M.; Seckl, J.R.; Haslett, C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J. Immunol. 1996, 156, 4422–4428. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.A.; Chupin, C.; Pascariu, M.; Privé, A.; Dagenais, A.; Berthiaume, Y.; Brochiero, E. Dexamethasone fails to improve bleomycin-induced acute lung injury in mice. Physiol. Rep. 2019, 7, e14253. [Google Scholar] [CrossRef]
- Allard, B.; Panariti, A.; Martin, J.G. Alveolar Macrophages in the Resolution of Inflammation, Tissue Repair, and Tolerance to Infection. Front. Immunol. 2018, 9, 1777. [Google Scholar] [CrossRef]
- Su, K.; Bo, L.; Jiang, C.; Deng, X.; Zhao, Y.-Y.; Minshall, R.D.; Hu, G. TLR4 is required for macrophage efferocytosis during resolution of ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L787–L801. [Google Scholar] [CrossRef] [PubMed]
- Gideon, H.P.; Phuah, J.; Junecko, B.A.; Mattila, J.T. Neutrophils express pro- and anti-inflammatory cytokines in granulomas from Mycobacterium tuberculosis-infected cynomolgus macaques. Mucosal Immunol. 2019, 12, 1370–1381. [Google Scholar] [CrossRef]
- Nelms, K.; Keegan, A.D.; Zamorano, J.; Ryan, J.J.; Paul, W.E. THE IL-4 RECEPTOR: Signaling Mechanisms and Biologic Functions. Annu. Rev. Immunol. 1999, 17, 701–738. [Google Scholar] [CrossRef] [PubMed]
- Clutterbuck, E.; Shields, J.G.; Gordon, J.; Smith, S.H.; Boyd, A.; Callard, R.E.; Campbell, H.D.; Young, I.G.; Sanderson, C.J. Recombinant human interleukin 5 is an eosinophil differentiation factor but has no activity in standard human B cell growth factor assays. Eur. J. Immunol. 1987, 17, 1743–1750. [Google Scholar] [CrossRef]
- Mattoli, S.; Stacey, M.A.; Sun, G.; Bellini, A.; Marini, M. Eotaxin expression and eosinophilic inflammation in asthma. Biochem. Biophys. Res. Commun. 1997, 236, 299–301. [Google Scholar] [CrossRef]
- Mac Sharry, J.; Shalaby, K.H.; Marchica, C.; Farahnak, S.; Chieh-Li, T.; Lapthorne, S.; Qureshi, S.T.; Shanahan, F.; Martin, J.G. Concomitant Exposure to Ovalbumin and Endotoxin Augments Airway Inflammation but Not Airway Hyperresponsiveness in a Murine Model of Asthma. PLoS ONE 2014, 9, e98648. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Lajoie, S.; Lewkowich, I.P.; Suzuki, Y.; Clark, J.R.; Sproles, A.A.; Dienger, K.; Budelsky, A.L.; Wills-Karp, M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat. Immunol. 2010, 11, 928–935. [Google Scholar] [CrossRef]
- Peters, K.; Ernst, S.; Peters, M. Interaction of Interleukin-17A with a Th2 Response in a Mouse Model of Allergic Airway Inflammation. Cells 2023, 12, 1774. [Google Scholar] [CrossRef] [PubMed]
- Ritzmann, F.; Lunding, L.P.; Bals, R.; Wegmann, M.; Beisswenger, C. IL-17 Cytokines and Chronic Lung Diseases. Cells 2022, 11, 2132. [Google Scholar] [CrossRef]
- Tan, H.L.; Rosenthal, M. IL-17 in lung disease: Friend or foe? Thorax 2013, 68, 788–790. [Google Scholar] [CrossRef]
- Ding, Q.; Liu, G.Q.; Zeng, Y.Y.; Zhu, J.J.; Liu, Z.Y.; Zhang, X.; Huang, J.A. Role of IL-17 in LPS-induced acute lung injury: An in vivo study. Oncotarget 2017, 8, 93704–93711. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, Y.; Yang, X.; Guo, D.; Wang, Y.; Wang, J.; Wang, R.; Wang, C. Lipopolysaccharides promote a shift from Th2-derived airway eosinophilic inflammation to Th17-derived neutrophilic inflammation in an ovalbumin-sensitized murine asthma model. J. Asthma 2017, 54, 447–455. [Google Scholar] [CrossRef]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.V.; et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients with Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA 2020, 324, 1307–1316. [Google Scholar] [CrossRef]
- Zhao, J.; Lloyd, C.M.; Noble, A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol. 2013, 6, 335–346. [Google Scholar] [CrossRef]
- Nanzer, A.M.; Chambers, E.S.; Ryanna, K.; Richards, D.F.; Black, C.; Timms, P.M.; Martineau, A.R.; Griffiths, C.J.; Corrigan, C.J.; Hawrylowicz, C.M. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1α,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J. Allergy Clin. Immunol. 2013, 132, 297–304.e3. [Google Scholar] [CrossRef]
- Flores-Torres, A.S.; Samarasinghe, A.E. Impact of Therapeutics on Unified Immunity During Allergic Asthma and Respiratory Infections. Front. Allergy 2022, 3, 852067. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma—GINA [Internet]. 2023 GINA Main Report. Available online: https://ginasthma.org/2023-gina-main-report/ (accessed on 25 February 2024).
- Camargo, L.D.N.; Righetti, R.F.; de Almeida, F.M.; dos Santos, T.M.; Fukuzaki, S.; Martins, N.A.B.; Barbeiro, M.C.; Saraiva-Romanholo, B.M.; Lopes, F.D.T.Q.D.S.; Leick, E.A.; et al. Modulating asthma–COPD overlap responses with IL-17 inhibition. Front. Immunol. 2023, 14, 1271342. [Google Scholar] [CrossRef] [PubMed]
- Long, M.E.; Mallampalli, R.K.; Horowitz, J.C. Pathogenesis of pneumonia and acute lung injury. Clin. Sci. 2022, 136, 747–769. [Google Scholar] [CrossRef] [PubMed]
- Melgert, B.N.; Postma, D.S.; Kuipers, I.; Geerlings, M.; Luinge, M.A.; van der Strate, B.W.A.; Kerstjens, H.A.M.; Timens, W.; Hylkema, M.N. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin. Exp. Allergy 2005, 35, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Debeuf, N.; Haspeslagh, E.; Van Helden, M.; Hammad, H.; Lambrecht, B.N. Mouse Models of Asthma. Curr. Protoc. Mouse Biol. 2016, 6, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, Z. Establishment of different experimental asthma models in mice. Exp. Ther. Med. 2018, 15, 2492–2498. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Netto, K.G.; Zhou, L.; Liu, X.; Wang, M.; Zhang, G.; Foster, P.S.; Li, F.; Yang, M. Single-cell transcriptomic analysis reveals the immune landscape of lung in steroid-resistant asthma exacerbation. Proc. Natl. Acad. Sci. USA 2021, 118, e2005590118. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, A.B.; Deodhar, A.; Mcinnes, I.B.; Baraliakos, X.; Reich, K.; Schreiber, S.; Bao, W.; Marfo, K.; Richards, H.B.; Pricop, L.; et al. Long-term Safety of Secukinumab Over Five Years in Patients with Moderate-to-severe Plaque Psoriasis, Psoriatic Arthritis and Ankylosing Spondylitis: Update on Integrated Pooled Clinical Trial and Post-marketing Surveillance Data. Acta Derm. Venereol. 2022, 102, adv00698. [Google Scholar] [CrossRef] [PubMed]


| SAL | OVA + LPS | OVA + LPS + SECU | OVA + LPS + DEXA | |
|---|---|---|---|---|
| VEGF (pg/mL) | 129.7 ± 12.8 | 354.7 ± 82.0 | 384.2 ± 47.0 * | 390.3 ± 40.4 * |
| IL-4 (pg/mL) | 6.1 ± 1.9 | 8.2 ± 1.8 | 9.6 ± 3.4 | 56.1 ± 5.2 *#‡ |
| SAL | OVA + LPS | OVA + LPS + SECU | OVA + LPS + DEXA | |
|---|---|---|---|---|
| VEGF (pg/mL) | 4155.9 ± 232.0 | 3134.1 ± 256.0 * | 3811.2 ± 210.8 | 3696.4 ± 234.4 |
| IL-4 (pg/mL) | 102.3 ± 10.5 | 57.5 ± 8.3 * | 76.9 ± 13.0 | 130.7 ± 11.0 #‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicovan, A.G.; Petrescu, D.C.; Cretu, A.; Ghiciuc, C.M.; Constantinescu, D.; Iftimi, E.; Strugariu, G.; Ancuta, C.M.; Caratașu, C.-C.; Solcan, C.; et al. Targeting Common Inflammatory Mediators in Experimental Severe Asthma and Acute Lung Injury. Pharmaceuticals 2024, 17, 338. https://doi.org/10.3390/ph17030338
Vicovan AG, Petrescu DC, Cretu A, Ghiciuc CM, Constantinescu D, Iftimi E, Strugariu G, Ancuta CM, Caratașu C-C, Solcan C, et al. Targeting Common Inflammatory Mediators in Experimental Severe Asthma and Acute Lung Injury. Pharmaceuticals. 2024; 17(3):338. https://doi.org/10.3390/ph17030338
Chicago/Turabian StyleVicovan, Andrei Gheorghe, Diana Cezarina Petrescu, Aurelia Cretu, Cristina Mihaela Ghiciuc, Daniela Constantinescu, Elena Iftimi, Georgiana Strugariu, Codrina Mihaela Ancuta, Cezar-Cătălin Caratașu, Carmen Solcan, and et al. 2024. "Targeting Common Inflammatory Mediators in Experimental Severe Asthma and Acute Lung Injury" Pharmaceuticals 17, no. 3: 338. https://doi.org/10.3390/ph17030338
APA StyleVicovan, A. G., Petrescu, D. C., Cretu, A., Ghiciuc, C. M., Constantinescu, D., Iftimi, E., Strugariu, G., Ancuta, C. M., Caratașu, C.-C., Solcan, C., & Stafie, C. S. (2024). Targeting Common Inflammatory Mediators in Experimental Severe Asthma and Acute Lung Injury. Pharmaceuticals, 17(3), 338. https://doi.org/10.3390/ph17030338







