Real-World Safety Data of the Orphan Drug Onasemnogene Abeparvovec (Zolgensma®) for the SMA Rare Disease: A Pharmacovigilance Study Based on the EMA Adverse Event Reporting System
Abstract
1. Introduction
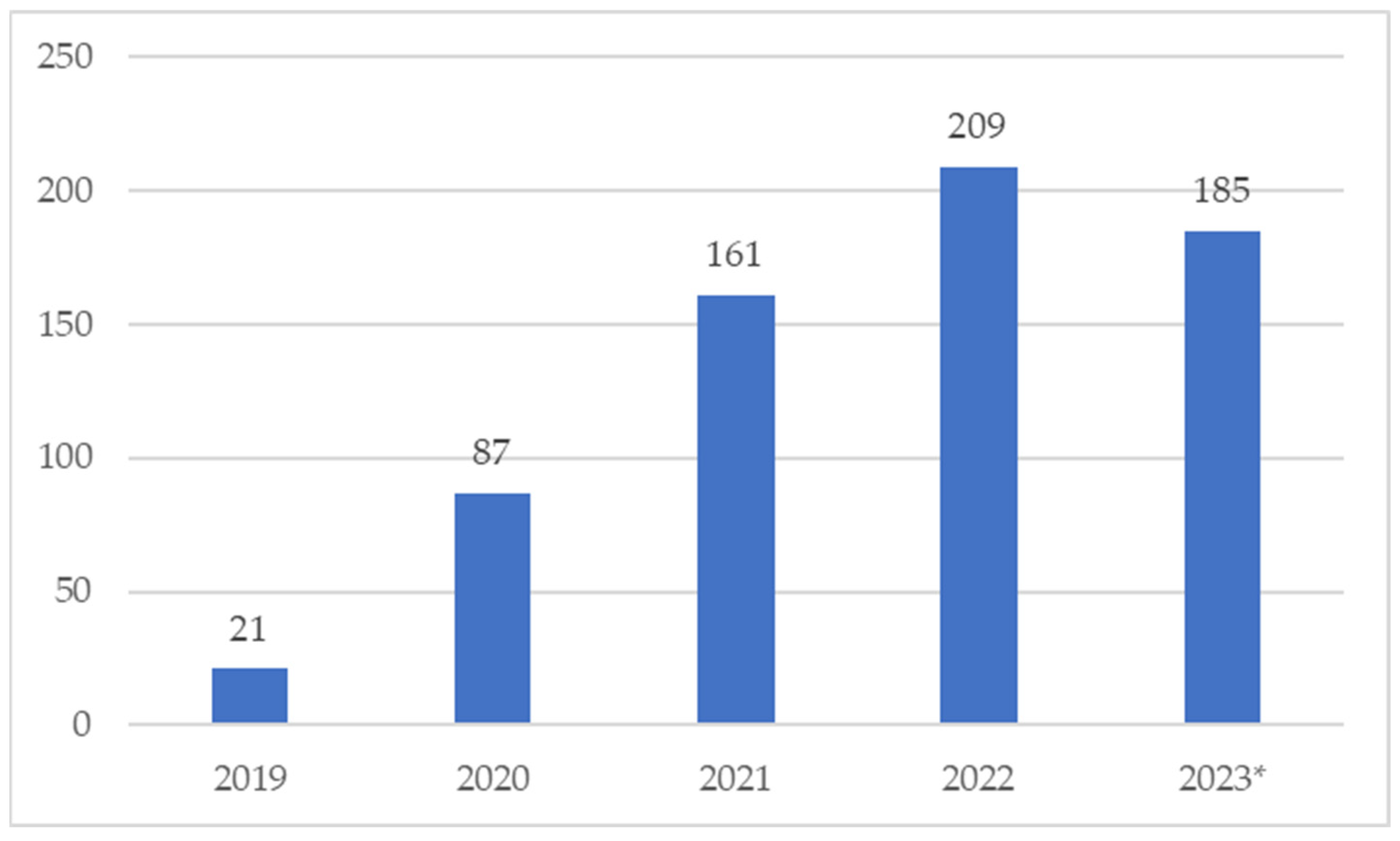
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaytow, H.; Huang, Y.T.; Gillingwater, T.H.; Faller, K.M.E. The Role of Survival Motor Neuron Protein (SMN) in Protein Homeostasis. Cell. Mol. Life Sci. 2018, 75, 3877–3894. [Google Scholar] [CrossRef]
- Verhaart, I.E.C.; Robertson, A.; Wilson, I.J.; Aartsma-Rus, A.; Cameron, S.; Jones, C.C.; Cook, S.F.; Lochmüller, H. Prevalence, Incidence and Carrier Frequency of 5q-Linked Spinal Muscular Atrophy-a Literature Review. Orphanet J. Rare Dis. 2017, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.F.; Kwon, J.M. Spinal Muscular Atrophy: Past, Present, and Future. Neoreviews 2019, 20, e437–e451. [Google Scholar] [CrossRef]
- Oliveira, D.; Sarkar, P.S.; Zeng, C.-W.; Tedesco, B.; Dimitriadi, M.; Rashid, S. Autophagy in Spinal Muscular Atrophy: From Pathogenic Mechanisms to Therapeutic Approaches. Front. Cell. Neurosci 2024, 17, 1307636. [Google Scholar] [CrossRef]
- Younger, D.S.; Mendell, J.R. Childhood Spinal Muscular Atrophy. Handb. Clin. Neurol. 2023, 196, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S.J.; Kissel, J.T. Spinal Muscular Atrophy. Neurol. Clin. 2015, 33, 831–846. [Google Scholar] [CrossRef]
- Arnold, E.S.; Fischbeck, K.H. Spinal Muscular Atrophy. Handb. Clin. Neurol. 2018, 148, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Sarret, C. Pathogenesis and Therapeutic Targets in Spinal Muscular Atrophy (SMA). Arch. Pédiatrie 2020, 27, 7S3–7S8. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, S.; Boccanegra, B.; Vitturi, G.; Trifirò, G.; De Luca, A. Pharmacological Therapies of Spinal Muscular Atrophy: A Narrative Review of Preclinical, Clinical–Experimental, and Real-World Evidence. Brain Sci. 2023, 13, 1446. [Google Scholar] [CrossRef]
- Hagenacker, T.; Schara-Schmidt, U. Gene Replacement Therapy in Spinal Muscular Atrophy: Filling the Data Gaps. Lancet Reg. Health—Eur. 2024, 37, 100822. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaidy, S.; Pickard, A.S.; Kotha, K.; Alfano, L.N.; Lowes, L.; Paul, G.; Church, K.; Lehman, K.; Sproule, D.M.; Dabbous, O.; et al. Health Outcomes in Spinal Muscular Atrophy Type 1 Following AVXS-101 Gene Replacement Therapy. Pediatr. Pulmonol. 2019, 54, 179–185. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Summary of the Risk Management for Zolgensma® (onasemnogene abeparvovec). Last updated:18/10/2023. Available online: https://www.ema.europa.eu/en/documents/rmp-summary/zolgensma-epar-risk-management-plan-summary_en.pdf (accessed on 3 March 2024).
- Zinzi, A.; Gaio, M.; Liguori, V.; Ruggiero, R.; Tesorone, M.; Rossi, F.; Rafaniello, C.; Capuano, A. Safety Monitoring of MRNA COVID-19 Vaccines in Children Aged 5 to 11 Years by Using EudraVigilance Pharmacovigilance Database: The CoVaxChild Study. Vaccines 2023, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, R.; Balzano, N.; Di Napoli, R.; Fraenza, F.; Pentella, C.; Riccardi, C.; Donniacuo, M.; Tesorone, M.; Danesi, R.; Del Re, M.; et al. Do Peripheral Neuropathies Differ among Immune Checkpoint Inhibitors? Reports from the European Post-Marketing Surveillance Database in the Past 10 Years. Front. Immunol. 2023, 14, 1134436. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.R.; Sarpatwari, A.; Desai, R.J.; Bohn, J.M.; Khan, N.F.; Kesselheim, A.S.; Fischer, M.A.; Gagne, J.J.; Connolly, J.G. Effect of Lawyer-Submitted Reports on Signals of Disproportional Reporting in the Food and Drug Administration’s Adverse Event Reporting System. Drug Saf. 2019, 42, 85–93. [Google Scholar] [CrossRef]
- Toki, T.; Ono, S. Spontaneous Reporting on Adverse Events by Consumers in the United States: An Analysis of the Food and Drug Administration Adverse Event Reporting System Database. Drugs—Real World Outcomes 2018, 5, 117–128. [Google Scholar] [CrossRef]
- Sienkiewicz, K.; Burzyńska, M.; Rydlewska-Liszkowska, I.; Sienkiewicz, J.; Gaszyńska, E. The Importance of Direct Patient Reporting of Adverse Drug Reactions in the Safety Monitoring Process. Int. J. Environ. Res. Public Health 2022, 19, 413. [Google Scholar] [CrossRef]
- Herdeiro, M.T.; Figueiras, A.; Polónia, J.; Gestal-Otero, J.J. Physicians’ Attitudes and Adverse Drug Reaction Reporting: A Case-Control Study in Portugal. Drug Saf. 2005, 28, 825–833. [Google Scholar] [CrossRef]
- Hoffman, K.B.; Demakas, A.R.; Dimbil, M.; Tatonetti, N.P.; Erdman, C.B. Stimulated Reporting: The Impact of US Food and Drug Administration-Issued Alerts on the Adverse Event Reporting System (FAERS). Drug Saf. 2014, 37, 971–980. [Google Scholar] [CrossRef]
- Castel, E.S.; Ginsburg, L.R.; Zaheer, S.; Tamim, H. Understanding Nurses’ and Physicians’ Fear of Repercussions for Reporting Errors: Clinician Characteristics, Organization Demographics, or Leadership Factors? BMC Health Serv. Res. 2015, 15, 326. [Google Scholar] [CrossRef]
- Bihan, K.; Lebrun-Vignes, B.; Funck-Brentano, C.; Salem, J.E. Uses of Pharmacovigilance Databases: An Overview. Therapie 2020, 75, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Ailani, J.; Smith, T.R.; Abdrabboh, A.; Xue, F.; Navetta, M.S. Pharmacovigilance: Reporting Requirements throughout a Product’s Lifecycle. Ther. Adv. Drug Saf. 2022, 13, 20420986221125006. [Google Scholar] [CrossRef] [PubMed]
- European Medicine Agency. New Gene Therapy to Treat Spinal Muscolar Atrophy; European Medicine Agency: Amsterdam, The Netherlands, 2020; Volume 31.
- Martini, N.; Trifirò, G.; Capuano, A.; Corrao, G.; Corrao, G.; Racagni, G.; Pani, L. Expert Opinion on Real World Evidence RWE in Drug Development and Usage. Pharmadvances 2020, 2, 41–50. [Google Scholar] [CrossRef]
- Pani, L.; Cicchetti, A.; De Luca, A.; Mennini, F.S.; Mini, E.; Nocentini, G.; Racagni, G.; Jommi, C. Pricing for Multi Indication Medicines: A Discussion with Italian Experts. Pharmadvances 2022, 4, 163–170. [Google Scholar] [CrossRef]
- Nuijten, M. Pricing Zolgensma—The World’s Most Expensive Drug. J. Mark. Access Health Policy 2022, 10, 2022353. [Google Scholar] [CrossRef]
- Dean, R.; Jensen, I.; Cyr, P.; Miller, B.; Maru, B.; Sproule, D.M.; Feltner, D.E.; Wiesner, T.; Malone, D.C.; Bischof, M.; et al. An Updated Cost-Utility Model for Onasemnogene Abeparvovec (Zolgensma®) in Spinal Muscular Atrophy Type 1 Patients and Comparison with Evaluation by the Institute for Clinical and Effectiveness Review (ICER). J. Mark. Access Heal. Policy 2021, 9, 1889841. [Google Scholar] [CrossRef]
- Mitsue Ivama-Brummell, A.; Wagner, A.K.; Lúcia, V.; Pepe, E.; Naci, H. Ultraexpensive Gene Therapies, Industry Interests and the Right to Health: The Case of Onasemnogene Abeparvovec in Brazil Commentary. BMJ Glob. Health 2022, 7, 8637. [Google Scholar] [CrossRef]
- Dias Fernandes, B.; D’Athayde Rodrigues, F.; Cardoso Cirilo, H.N.; Borges, S.S.; Krug, B.C.; Fernandes Probst, L.; Zimmermann, I. Economic Evaluation Cost-Effectiveness of Onasemnogene Abeparvovec Compared with Nusinersen and Risdiplam in Patients with Spinal Muscular Atrophy Type 1 in Brazil. Value Health Reg. Issues 2024, 40, 108–117. [Google Scholar] [CrossRef]
- Dangouloff, T.; Botty, C.; Beaudart, C.; Servais, L.; Hiligsmann, M. Systematic Literature Review of the Economic Burden of Spinal Muscular Atrophy and Economic Evaluations of Treatments. Orphanet J. Rare Dis. 2021, 16, 47. [Google Scholar] [CrossRef]
- Malone, D.C.; Dean, R.; Arjunji, R.; Jensen, I.; Cyr, P.; Miller, B.; Maru, B.; Sproule, D.M.; Feltner, D.E.; Dabbous, O. Cost-Effectiveness Analysis of Using Onasemnogene Abeparvocec (AVXS-101) in Spinal Muscular Atrophy Type 1 Patients. J. Mark. Access Health Policy 2019, 7, 1601484. [Google Scholar] [CrossRef]
- Zhuang, W.; Lu, M.; Wu, Y.; Chen, Z.; Wang, M.; Wang, X.; Guan, S.; Lin, W. Safety Concerns with Nusinersen, Risdiplam, and Onasemnogene Abeparvovec in Spinal Muscular Atrophy: A Real-World Pharmacovigilance Study. Clin. Drug Investig. 2023, 43, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Harrington, M.A.; Porter, B. Sex Difference in Spinal Muscular Atrophy Patients—Are Males More Vulnerable? J. Neuromuscul. Dis. 2023, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Antonacci, Y.; Liguori, M. Sex and Gender Differences in Neurodegenerative Diseases: Challenges for Therapeutic Opportunities. Int. J. Mol. Sci. 2023, 24, 6354. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Ruggiero, R.; Sportiello, L.; Pentella, C.; Gaio, M.; Pinto, A.; Rafaniello, C. Did the COVID-19 Pandemic Affect Contrast Media-Induced Adverse Drug Reaction’s Reporting? A Pharmacovigilance Study in Southern Italy. J. Clin. Med. 2022, 11, 5104. [Google Scholar] [CrossRef]
- Scavone, C.; Carnovale, C.; Ruggiero, R.; Radice, S.; Scatigna, M.; Racagni, G.; Mugelli, A.; Rossi, F.; Clementi, E.; Capuano, A. On the Policy of the Italian Government in the Discovery, Development, and Access to Medicines. Clin. Ther. 2018, 40, 1931–1940. [Google Scholar] [CrossRef]
- Day, J.W.; Mendell, J.R.; Mercuri, E.; Finkel, R.S.; Strauss, K.A.; Kleyn, A.; Tauscher-Wisniewski, S.; Tukov, F.F.; Reyna, S.P.; Chand, D.H. Clinical Trial and Postmarketing Safety of Onasemnogene Abeparvovec Therapy. Drug Saf. 2021, 44, 1109. [Google Scholar] [CrossRef]
- Blair, H.A. Onasemnogene Abeparvovec: A Review in Spinal Muscular Atrophy. CNS Drugs 2022, 36, 995–1005. [Google Scholar] [CrossRef]
- Chand, D.; Mohr, F.; Mcmillan, H.; Tukov, F.F.; Montgomery, K.; Kleyn, A.; Sun, R.; Tauscher-Wisniewski, S.; Kaufmann, P.; Kullak-Ublick, G. Hepatotoxicity Following Administration of Onasemnogene Abeparvovec (AVXS-101) for the Treatment of Spinal Muscular Atrophy. J. Hepatol. 2020, 74, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Day, J.W.; Finkel, R.S.; Chiriboga, C.A.; Connolly, A.M.; Crawford, T.O.; Darras, B.T.; Iannaccone, S.T.; Kuntz, N.L.; Peña, L.D.M.; Shieh, P.B.; et al. Onasemnogene Abeparvovec Gene Therapy for Symptomatic Infantile-Onset Spinal Muscular Atrophy in Patients with Two Copies of SMN2 (STR1VE): An Open-Label, Single-Arm, Multicentre, Phase 3 Trial. Artic. Lancet Neurol 2021, 20, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; Mcmillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene Abeparvovec for Presymptomatic Infants with Two Copies of SMN2 at Risk for Spinal Muscular Atrophy Type 1: The Phase III SPR1NT Trial. Nat. Med. 2022, 28, 1381–1389. [Google Scholar] [CrossRef]
- García-Abeijon, P.; Costa, C.; Taracido, M.; Herdeiro, M.T.; Torre, C.; Figueiras, A. Factors Associated with Underreporting of Adverse Drug Reactions by Health Care Professionals: A Systematic Review Update. Drug Saf. 2023, 46, 625–636. [Google Scholar] [CrossRef]
- Biagi, C.; Montanaro, N.; Buccellato, E.; Roberto, G.; Vaccheri, A.; Motola, D. Underreporting in Pharmacovigilance: An Intervention for Italian GPs (Emilia-Romagna Region). Eur. J. Clin. Pharmacol. 2013, 69, 237–244. [Google Scholar] [CrossRef]
- Pellegrino, P.; Carnovale, C.; Cattaneo, D.; Perrone, V.; Antoniazzi, S.; Pozzi, M.; Napoleone, E.; Filograna, M.R.; Clementi, E.; Radice, S. Pharmacovigilance Knowledge in Family Paediatricians. A Survey Study in Italy. Health Policy 2013, 113, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Shariff, M.; Bhat, V.; DeSimone, C.; Deshmukh, A. Atrial Fibrillation after Vaccination for COVID-19: Analysis of the Vaccine Adverse Event Reporting System. J. Interv. Card. Electrophysiol. 2022, 65, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gonzalez, E.; Herdeiro, M.T.; Figueiras, A. Determinants of Under-Reporting of Adverse Drug Reactions A Systematic Review. Drug Saf. 2009, 32, 19–31. [Google Scholar] [CrossRef]
- Van Alstyne, M.; Tattoli, I.; Delestrée, N.; Recinos, Y.; Workman, E.; Shihabuddin, L.S.; Zhang, C.; Mentis, G.Z.; Pellizzoni, L. Gain of Toxic Function by Long-Term AAV9-Mediated SMN Overexpression in the Sensory-Motor Circuit. Nat. Neurosci. 2021, 24, 930. [Google Scholar] [CrossRef] [PubMed]

| Overall (n = 661) | |
|---|---|
| Age Group | |
| 0–1 month (neonates) | 61 (9.2%) |
| 2 months–2 years (infants) | 396 (59.9%) |
| 3–11 years (children) | 76 (11.5%) |
| 12–17 years (adolescents) | 1 (0.2%) |
| 18–64 years (adults) | 1 (0.2%) |
| Not specified | 126 (19.1%) |
| Patient Sex | |
| Female | 287 (43.4%) |
| Male | 249 (37.7%) |
| Not specified | 125 (18.9%) |
| Reporter Type | |
| Healthcare professional | 585 (88.5%) |
| Non-healthcare professional | 76 (11.5%) |
| Country | |
| European Economic Area | 319 (48.3%) |
| Non-European Economic Area | 342 (51.7%) |
| Concomitant Drugs per ICSR | |
| 0 | 407 (61.6%) |
| 1 | 143 (21.6%) |
| 2 | 49 (7.4%) |
| 3 | 33 (5.0%) |
| 4 | 12 (1.8%) |
| ≥5 | 17 (2.6%) |
| Suspected Drugs per ICSR | |
| 1 | 609 (92.1%) |
| 2 | 42 (6.4%) |
| 3 | 8 (1.2%) |
| 4 | 1 (0.2%) |
| ≥5 | 1 (0.2%) |
| Overall ADRs (n = 2744) | |
|---|---|
| ADR Seriousness Criteria | |
| Caused/prolonged hospitalization | 588 (21.4%) |
| Disabling | 2 (0.1%) |
| Life-threatening | 118 (4.3%) |
| Not serious | 1185 (43.1%) |
| Other medically important condition | 721 (26.3%) |
| Results in death | 130 (4.7%) |
| ADR Outcome | |
| Fatal | 130 (4.7%) |
| Not recovered/not resolved | 188 (6.9%) |
| Recovered/resolved | 676 (24.6%) |
| Recovered/resolved with sequelae | 10 (0.4%) |
| Recovering/resolving | 238 (8.7%) |
| Unknown | 1502 (54.7%) |
| Reported Adverse Events | Overall ADRs (n = 2744) | Overall ICSRs (n = 661) | |
|---|---|---|---|
| n | % per Total ADRs | % per Total ICSRs | |
| Pyrexia | 173 | 6.30% | 26.17% |
| Vomiting | 141 | 5.10% | 21.33% |
| Aspartate aminotransferase increased | 129 | 4.70% | 19.52% |
| Alanine aminotransferase increased | 120 | 4.40% | 18.15% |
| Thrombocytopenia | 118 | 4.30% | 17.85% |
| Transaminases increased | 91 | 3.30% | 13.77% |
| Hepatic enzyme increased | 77 | 2.80% | 11.65% |
| Decreased appetite | 50 | 1.80% | 7.56% |
| Platelet count decreased | 49 | 1.80% | 7.41% |
| Troponin I increased | 40 | 1.50% | 6.05% |
| Pneumonia | 36 | 1.30% | 5.45% |
| Liver function test increased | 35 | 1.30% | 5.30% |
| Hypertransaminasaemia | 30 | 1.10% | 4.54% |
| Asthenia | 29 | 1.10% | 4.39% |
| Dyspnoea | 25 | 0.90% | 3.78% |
| Gamma-glutamyltransferase increased | 23 | 0.80% | 3.48% |
| Blood lactate dehydrogenase increased | 22 | 0.80% | 3.33% |
| Body temperature increased | 22 | 0.80% | 3.33% |
| Nausea | 21 | 0.80% | 3.18% |
| Apathy | 19 | 0.70% | 2.87% |
| Thrombotic microangiopathy | 19 | 0.70% | 2.87% |
| Adverse Events Belonging to the Investigation MedDRA SOC (n = 982) | n | % |
|---|---|---|
| Aspartate aminotransferase increased | 129 | 13.10% |
| Alanine aminotransferase increased | 120 | 12.20% |
| Transaminases increased | 91 | 9.30% |
| Hepatic enzyme increased | 77 | 7.80% |
| Platelet count decreased | 49 | 5.00% |
| Troponin I increased | 40 | 4.10% |
| Liver function test increased | 35 | 3.60% |
| Gamma-glutamyltransferase increased | 23 | 2.30% |
| Blood lactate dehydrogenase increased | 22 | 2.20% |
| Body temperature increased | 22 | 2.20% |
| Troponin increased | 18 | 1.80% |
| Oxygen saturation decreased | 15 | 1.50% |
| Troponin T increased | 15 | 1.50% |
| C-reactive protein increased | 14 | 1.40% |
| Heart rate increased | 13 | 1.30% |
| Monocyte count increased | 13 | 1.30% |
| Blood bilirubin increased | 11 | 1.10% |
| Adverse Events Belonging to the Cardiac Disorders MedDRA SOC (n = 59) | n | % |
|---|---|---|
| Tachycardia | 14 | 23.70% |
| Bradycardia | 11 | 18.60% |
| Cardiac arrest | 9 | 15.30% |
| Cardio-respiratory arrest | 4 | 6.80% |
| Tachyarrhythmia | 3 | 5.10% |
| Arrhythmia | 2 | 3.40% |
| Cardiac failure | 2 | 3.40% |
| Pericardial effusion | 2 | 3.40% |
| Pericarditis | 2 | 3.40% |
| Bradyarrhythmia | 1 | 1.70% |
| Cardiac disorder | 1 | 1.70% |
| Cardiomegaly | 1 | 1.70% |
| Myocardial hypoxia | 1 | 1.70% |
| Myocardial injury | 1 | 1.70% |
| Pulseless electrical activity | 1 | 1.70% |
| Sinus tachycardia | 1 | 1.70% |
| Toxic cardiomyopathy | 1 | 1.70% |
| Ventricular extrasystoles | 1 | 1.70% |
| Ventricular hypertrophy | 1 | 1.70% |
| Adverse Events Belonging to the Hepatobiliary Disorders MedDRA SOC | n | % |
|---|---|---|
| Hypertransaminasaemia | 30 | 25.90% |
| Liver disorder | 10 | 8.60% |
| Acute hepatic faliure | 9 | 7.80% |
| Hepatic cytolysis | 9 | 7.80% |
| Abnormal hepatic function | 8 | 6.90% |
| Hepatitis | 8 | 6.90% |
| Hepatotoxicity | 6 | 5.20% |
| Drug-induced liver injury | 5 | 4.30% |
| Jaundice | 4 | 3.40% |
| Cholestasis | 3 | 2.60% |
| Hepatic failure | 3 | 2.60% |
| Hepatomegaly | 3 | 2.60% |
| Liver injury | 3 | 2.60% |
| Gallbladder enlargement | 2 | 1.70% |
| Hepatic fibrosis | 2 | 1.70% |
| Hyperbilirubinaemia | 2 | 1.70% |
| Ocular icterus | 2 | 1.70% |
| Autoimmune hepatitis | 1 | 0.90% |
| Cholangitis | 1 | 0.90% |
| Hepatic steatosis | 1 | 0.90% |
| Hepatosplenomegaly | 1 | 0.90% |
| Ischaemic hepatitis | 1 | 0.90% |
| Liver tenderness | 1 | 0.90% |
| Subacute hepatic failure | 1 | 0.90% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggiero, R.; Balzano, N.; Nicoletti, M.M.; di Mauro, G.; Fraenza, F.; Campitiello, M.R.; Rossi, F.; Capuano, A. Real-World Safety Data of the Orphan Drug Onasemnogene Abeparvovec (Zolgensma®) for the SMA Rare Disease: A Pharmacovigilance Study Based on the EMA Adverse Event Reporting System. Pharmaceuticals 2024, 17, 394. https://doi.org/10.3390/ph17030394
Ruggiero R, Balzano N, Nicoletti MM, di Mauro G, Fraenza F, Campitiello MR, Rossi F, Capuano A. Real-World Safety Data of the Orphan Drug Onasemnogene Abeparvovec (Zolgensma®) for the SMA Rare Disease: A Pharmacovigilance Study Based on the EMA Adverse Event Reporting System. Pharmaceuticals. 2024; 17(3):394. https://doi.org/10.3390/ph17030394
Chicago/Turabian StyleRuggiero, Rosanna, Nunzia Balzano, Maria Maddalena Nicoletti, Gabriella di Mauro, Federica Fraenza, Maria Rosaria Campitiello, Francesco Rossi, and Annalisa Capuano. 2024. "Real-World Safety Data of the Orphan Drug Onasemnogene Abeparvovec (Zolgensma®) for the SMA Rare Disease: A Pharmacovigilance Study Based on the EMA Adverse Event Reporting System" Pharmaceuticals 17, no. 3: 394. https://doi.org/10.3390/ph17030394
APA StyleRuggiero, R., Balzano, N., Nicoletti, M. M., di Mauro, G., Fraenza, F., Campitiello, M. R., Rossi, F., & Capuano, A. (2024). Real-World Safety Data of the Orphan Drug Onasemnogene Abeparvovec (Zolgensma®) for the SMA Rare Disease: A Pharmacovigilance Study Based on the EMA Adverse Event Reporting System. Pharmaceuticals, 17(3), 394. https://doi.org/10.3390/ph17030394




