Potential Mechanism of Tibetan Medicine Liuwei Muxiang Pills against Colorectal Cancer: Network Pharmacology and Bioinformatics Analyses
Abstract
:1. Introduction
2. Results
2.1. Acquisition of the Drug Targets and Disease Targets
2.2. Potential Target Prediction of LWMX Pills in CRC and Construction of Protein–Protein Interaction (PPI) and Drug–Disease Networks
2.3. Functional Enrichment Analysis and Construction of Ingredient–Target–Pathway Network
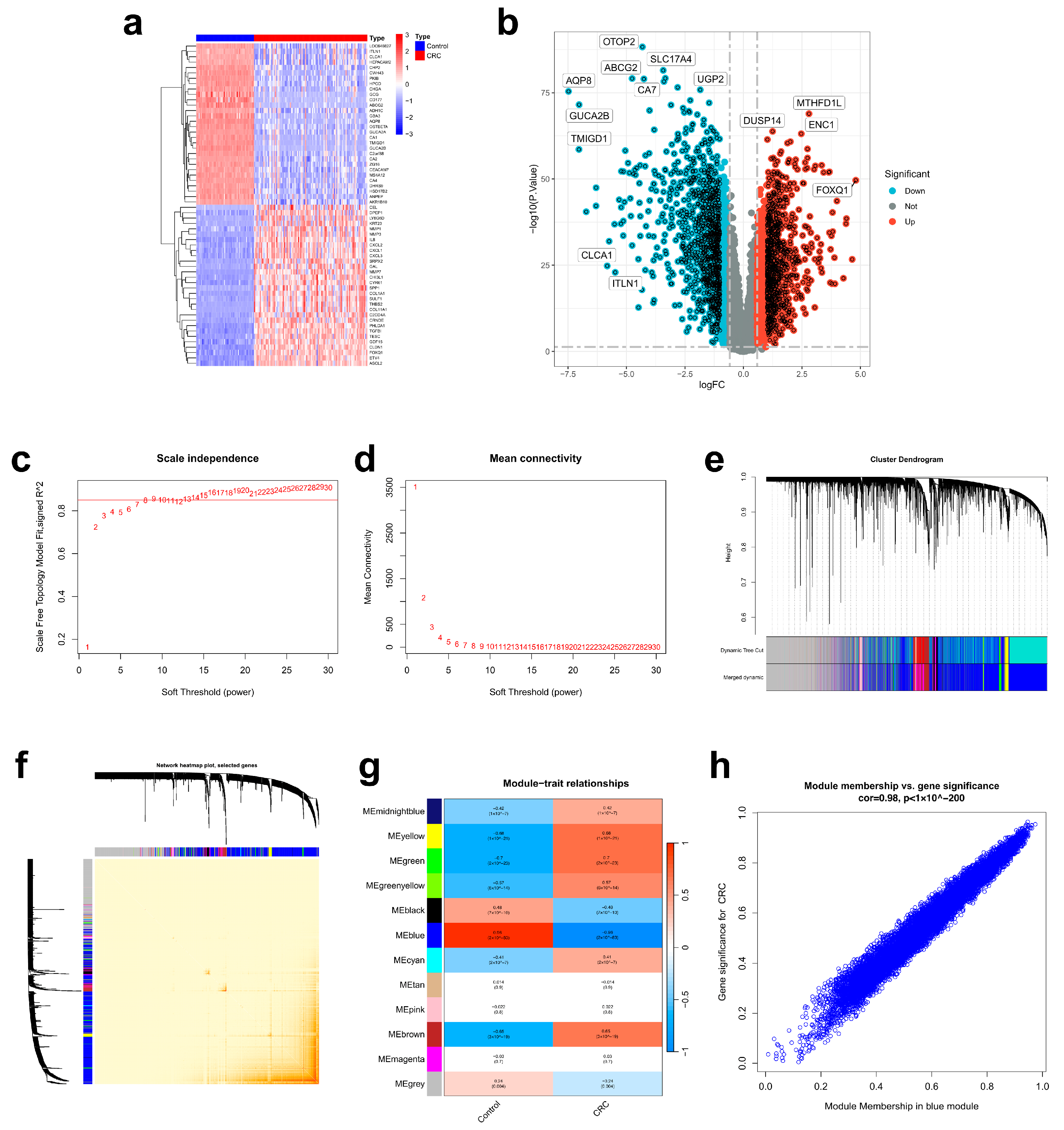
2.4. Determination of Target Hub Genes with Machine Learning
2.5. Immune Infiltration Analysis
2.6. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Acquisition of Relevant Targets of LWMX Pills
4.2. Acquisition of CRC-Related Targets
4.3. Potential Target Prediction of LWMX Pills in CRC Treatment
4.4. Construction of PPI Network and Drug–Disease Network
4.5. Functional Enrichment Analysis and Construction of Ingredient–Target–Pathway Network
4.6. Determination of Hub Genes with Machine Learning
4.7. Immune Infiltration Analysis
4.8. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LWMX pills | Liuwei Muxiang pills |
| CRC | colorectal cancer |
| TTM | traditional Tibetan medicine |
| TCMSP | Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform |
| DEGs | differentially expressed genes |
| WGCNA | weighted gene correlation network analysis |
| PPI | Protein–protein interaction |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| GO | Gene Ontology |
| BP | biological process |
| CC | cellular component |
| MF | molecular function |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LASSO | least absolute shrinkage and selection operator |
| SVM-RFE | support vector machine-recursive feature elimination |
| RF | random forest |
| VIP | variable importance |
| GEO | Gene Expression Omnibus |
| TOM | topological overlap matrix |
| MM | module membership |
| GS | gene significance |
| CIBERSORT | cell-type identification by estimating relative subsets of RNA transcripts |
| PDB | Protein Data Bank |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, X.; Liu, J.; Ma, Y.; Tan, J.; Wang, W.; Hu, J.; Fu, X.; Xu, L.; Yu, F.; et al. Shenlingcao oral liquid for patients with non-small cell lung cancer receiving adjuvant chemotherapy after radical resection: A multicenter randomized controlled trial. Phytomedicine 2023, 113, 154723. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Chen, Y.; Bi, J.; Shang, Q.; Liu, H.; Wang, J.B.; Tan, L.; Wang, J.; Chen, Y.; Li, Q.; et al. Entecavir plus Biejia-Ruangan compound reduces the risk of hepatocellular carcinoma in Chinese patients with chronic hepatitis B. J. Hepatol. 2022, 77, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, Y.; Liu, Y.; Ye, X.; Ji, X.; Sun, L.; Gao, F.; Zhang, Q.; Li, Y.; Zhu, B.; et al. Fuzheng Jiedu Xiaoji formulation inhibits hepatocellular carcinoma progression in patients by targeting the AKT/CyclinD1/p21/p27 pathway. Phytomedicine 2021, 87, 153575. [Google Scholar] [CrossRef]
- Fu, K.; Xu, M.; Zhou, Y.; Li, X.; Wang, Z.; Liu, X.; Meng, X.; Zeng, Y.; Zhang, H. The Status quo and way forwards on the development of Tibetan medicine and the pharmacological research of tibetan materia Medica. Pharmacol. Res. 2020, 155, 104688. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhao, C.C.; Yi, H.; Geng, Z.J.; Wu, X.Y.; Zhang, Y.; Liu, Y.; Fan, G. Traditional Tibetan Medicine in Cancer Therapy by Targeting Apoptosis Pathways. Front. Pharmacol. 2020, 11, 976. [Google Scholar] [CrossRef]
- Chen, X.; Shen, K.; Deng, Y.; Mo, J.; Ni, J.; Hendi, M.; Chen, S.; Wang, L.; Si, J. A Randomized Double-blind Clinical Trial of Weierkang Pills for the Treatment of Chronic Atrophic Gastritis. J. Clin. Gastroenterol. 2023, 57, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Baser, R.E.; Li, S.Q.; Hou, Y.N.; Chong, K.; Zhang, Y.L.; Hoque, I.; Bao, T.; Mao, J.J. Tibetan Herbal Pain-Relieving Plaster for Chronic Musculoskeletal Pain among Cancer Survivors: Study Protocol of a Randomized, Double-Blind, Placebo-Controlled Trial. Front. Pharmacol. 2022, 13, 878371. [Google Scholar] [CrossRef]
- Bauer-Wu, S.; Lhundup, T.; Tidwell, T.; Lhadon, T.; Ozawa-de Silva, C.; Dolma, J.; Dorjee, P.; Neshar, D.R.; Sangmo, R.; Yeshi, T. Tibetan medicine for cancer: An overview and review of case studies. Integr. Cancer Ther. 2014, 13, 502–512. [Google Scholar] [CrossRef]
- Ji, P.; Zhao, N.S.; Wu, F.L.; Wei, Y.M.; Laba, C.D.; Wujin, C.M.; Hua, Y.L.; Yuan, Z.W.; Yao, W.L. Mechanisms predictive of Tibetan Medicine Sophora moorcroftiana alkaloids for treatment of lung cancer based on the network pharmacology and molecular docking. BMC Complement. Med. Ther. 2024, 24, 47. [Google Scholar] [CrossRef]
- Yang, X.; Man, D.; Zhao, P.; Li, X. Identification of the therapeutic mechanism of the saffron crocus on glioma through network pharmacology and bioinformatics analysis. Med. Oncol. 2023, 40, 296. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.Y.; Zheng, J.H.; Li, S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Dhondrup, R.; Tidwell, T.; Zhang, X.; Feng, X.; Lobsang, D.; Hua, Q.; Geri, D.; Suonan, D.C.; Fan, G.; Samdrup, G. Tibetan medicine Liuwei Muxiang pills (LWMX pills) effectively protects mice from chronic non-atrophic gastritis. Phytomedicine 2023, 115, 154826. [Google Scholar] [CrossRef]
- Renqing, D.; Feng, X.; Luosang, D.; Hua, Q.; Ying, C.; Liu, J.; Sangjie, Z.; Geri, D.; Caidanduojie, S.; Sanzhi, J. Network Pharmacology Combined with Molecular Docking to Study the Molecular Mechanism of Tibetan Medicine Liuwei Muxiang Pill in the Treatment of Gastric Cancer. Mod. Tradit. Chin. Med. Mater. Medica-World Sci. Technol. 2022, 24, 309–319. [Google Scholar]
- Liu, D.; Lü, L.; Zeng, R.; Lu, J.; Zhang, J.; Liu, Y.; Zhang, Z.; Ren, Y. Study on the mechanism of Liuwei Muxiang pills on the treatment of experimental gastric ulcer in rats. West China J. Pharm. Sci. 2016, 31, 257–259. [Google Scholar] [CrossRef]
- Hsin, K.Y.; Ghosh, S.; Kitano, H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS ONE 2013, 8, e83922. [Google Scholar] [CrossRef]
- Johdi, N.A.; Sukor, N.F. Colorectal Cancer Immunotherapy: Options and Strategies. Front. Immunol. 2020, 11, 1624. [Google Scholar] [CrossRef]
- Pu, W.L.; Zhang, M.Y.; Bai, R.Y.; Sun, L.K.; Li, W.H.; Yu, Y.L.; Zhang, Y.; Song, L.; Wang, Z.X.; Peng, Y.F.; et al. Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomed. Pharmacother. 2020, 121, 109552. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Hochnadel, I.; Petriv, N.; Franke, R.; Schmidt, J.; Limanska, N.; Tugai, A.; Jedicke, N.; Broenstrup, M.; Manns, M.P.; et al. Elucidating the mechanism behind and investigating the efficacy of Traditional Chinese Medicine and Traditional Tibetan Medicine in combination with standard therapeutics in hepatocellular carcinoma and cholangiocarcinoma in vitro. Front. Pharmacol. 2022, 13, 906468. [Google Scholar] [CrossRef]
- Tortora, K.; Femia, A.P.; Romagnoli, A.; Sineo, I.; Khatib, M.; Mulinacci, N.; Giovannelli, L.; Caderni, G. Pomegranate By-Products in Colorectal Cancer Chemoprevention: Effects in Apc-Mutated Pirc Rats and Mechanistic Studies In Vitro and Ex Vivo. Mol. Nutr. Food Res. 2018, 62, 1700401. [Google Scholar] [CrossRef]
- Chen, X.X.; Khyeam, S.; Zhang, Z.J.; Zhang, K.Y. Granatin B and punicalagin from Chinese herbal medicine pomegranate peels elicit reactive oxygen species-mediated apoptosis and cell cycle arrest in colorectal cancer cells. Phytomedicine 2022, 97, 153923. [Google Scholar] [CrossRef]
- Berdowska, I.; Matusiewicz, M.; Fecka, I. Punicalagin in Cancer Prevention-Via Signaling Pathways Targeting. Nutrients 2021, 13, 2733. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X. Phyllanthus emblica Fruit Extract Activates Spindle Assembly Checkpoint, Prevents Mitotic Aberrations and Genomic Instability in Human Colon Epithelial NCM460 Cells. Int. J. Mol. Sci. 2016, 17, 1437. [Google Scholar] [CrossRef]
- Liu, J.H.; Hsieh, C.H.; Liu, C.Y.; Chang, C.W.; Chen, Y.J.; Tsai, T.H. Anti-inflammatory effects of Radix Aucklandiae herbal preparation ameliorate intestinal mucositis induced by 5-fluorouracil in mice. J. Ethnopharmacol. 2021, 271, 113912. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Xu, B. Network pharmacology and bioinformatics to identify the molecular mechanisms of Gleditsiae Spina against colorectal cancer. Curr. Res. Toxicol. 2023, 5, 100139. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Su, Y.; Hu, J.; Sun, J.; Zheng, M.; Huang, Z. Exploring the potential mechanisms of Yi-Yi-Fu-Zi-Bai-Jiang-San therapy on the immune-inflamed phenotype of colorectal cancer via combined network pharmacology and bioinformatics analyses. Comput. Biol. Med. 2023, 166, 107432. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Liu, J.; Zhao, Y.; Liu, W. Network pharmacology prediction and molecular docking-based strategy to discover the potential pharmacological mechanism of action of Wang Bu Liu Xing (Semen vaccariae) for colorectal cancer. J. Gastrointest. Oncol. 2023, 14, 504–515. [Google Scholar] [CrossRef]
- Liang, Z.X.; Liu, H.S.; Wang, F.W.; Xiong, L.; Zhou, C.; Hu, T.; He, X.W.; Wu, X.J.; Xie, D.; Wu, X.R.; et al. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization. Cell Death Dis. 2019, 10, 829. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Lin, H.Y.; Zhang, Y.; Chen, W.F. miR-200b-3p mitigates oxaliplatin resistance via targeting TUBB3 in colorectal cancer. J. Gene Med. 2020, 22, e3178. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, S.; He, L.; Fu, Q.; Liao, L.; Chen, L.; Ding, X. Knockdown of BUB1B Inhibits the Proliferation, Migration, and Invasion of Colorectal Cancer by Regulating the JNK/c-Jun Signaling Pathway. Cancer Biother. Radiopharm. 2023. ahead of print. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Ding, Y.; Feng, G. CAMSAP2 promotes colorectal cancer cell migration and invasion through activation of JNK/c-Jun/MMP-1 signaling pathway. Sci. Rep. 2022, 12, 16899. [Google Scholar] [CrossRef]
- Yan, C.; Huang, W.Y.; Boudreau, J.; Mayavannan, A.; Cheng, Z.; Wang, J. IL-17R deletion predicts high-grade colorectal cancer and poor clinical outcomes. Int. J. Cancer 2019, 145, 548–558. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Yang, X.; Zhang, Y.; Lu, Y.; Li, Y. The expression and diagnostic value of serum levels of EphA2 and VEGF-A in patients with colorectal cancer. Cancer Biomark. 2021, 31, 399–408. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Zhang, F.; Hu, X.; Bi, F.; Li, K.; Yu, H.; Zhao, Y.; Teng, X.; Li, J.; et al. m6A methylated EphA2 and VEGFA through IGF2BP2/3 regulation promotes vasculogenic mimicry in colorectal cancer via PI3K/AKT and ERK1/2 signaling. Cell Death Dis. 2022, 13, 483. [Google Scholar] [CrossRef]
- Gao, F.; Zhou, L.; Li, M.; Liu, W.; Yang, S.; Li, W. Inhibition of ERKs/Akt-Mediated c-Fos Expression Is Required for Piperlongumine-Induced Cyclin D1 Downregulation and Tumor Suppression in Colorectal Cancer Cells. Onco Targets Ther. 2020, 13, 5591–5603. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, Z.; Gu, X.; Feng, L.; Xu, M.; Zhang, X. miR-92b-3p Regulates Cell Cycle and Apoptosis by Targeting CDKN1C, Thereby Affecting the Sensitivity of Colorectal Cancer Cells to Chemotherapeutic Drugs. Cancers 2021, 13, 3323. [Google Scholar] [CrossRef]
- Yang, R.; Tan, J.; Liu, Z.; Shen, X.; Hu, Y. Lappaol F regulates the cell cycle by activating CDKN1C/p57 in human colorectal cancer cells. Pharm. Biol. 2023, 61, 337–344. [Google Scholar] [CrossRef]
- Reichman, H.; Itan, M.; Rozenberg, P.; Yarmolovski, T.; Brazowski, E.; Varol, C.; Gluck, N.; Shapira, S.; Arber, N.; Qimron, U.; et al. Activated Eosinophils Exert Antitumorigenic Activities in Colorectal Cancer. Cancer Immunol. Res. 2019, 7, 388–400. [Google Scholar] [CrossRef]
- Gatault, S.; Delbeke, M.; Driss, V.; Sarazin, A.; Dendooven, A.; Kahn, J.E.; Lefèvre, G.; Capron, M. IL-18 Is Involved in Eosinophil-Mediated Tumoricidal Activity against a Colon Carcinoma Cell Line by Upregulating LFA-1 and ICAM-1. J. Immunol. 2015, 195, 2483–2492. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Wei, H.; Liu, Y.; Li, N. Mast cells in colorectal cancer tumour progression, angiogenesis, and lymphangiogenesis. Front. Immunol. 2023, 14, 1209056. [Google Scholar] [CrossRef]
- Liu, C.; Li, P.; Qu, Z.; Xiong, W.; Liu, A.; Zhang, S. Advances in the Antagonism of Epigallocatechin-3-gallate in the Treatment of Digestive Tract Tumors. Molecules 2019, 24, 1726. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform 2014, 6, 13. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, W.; Huang, C.; Li, Y.; Yu, H.; Wang, Y.; Duan, J.; Ling, Y. A novel chemometric method for the prediction of human oral bioavailability. Int. J. Mol. Sci. 2012, 13, 6964–6982. [Google Scholar] [CrossRef]
- Maphetu, N.; Unuofin, J.O.; Masuku, N.P.; Olisah, C.; Lebelo, S.L. Medicinal uses, pharmacological activities, phytochemistry, and the molecular mechanisms of Punica granatum L. (pomegranate) plant extracts: A review. Biomed. Pharmacother. 2022, 153, 113256. [Google Scholar] [CrossRef]
- Chen, J.; Ge, S. Study on chemical constituents and prescription of Tibetan medicine Veronica eriogyne H. Winkl. Guid. J. Tradit. Chin. Med. Pharmacol. 2017, 23, 46–47. [Google Scholar] [CrossRef]
- UniProt, C. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef]
- Diez-Villanueva, A.; Sanz-Pamplona, R.; Sole, X.; Cordero, D.; Crous-Bou, M.; Guino, E.; Lopez-Doriga, A.; Berenguer, A.; Ausso, S.; Pare-Brunet, L.; et al. COLONOMICS—Integrative omics data of one hundred paired normal-tumoral samples from colon cancer patients. Sci. Data 2022, 9, 595. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Choi, Y.J.; Kim, I.K.; Lee, H.S.; Kim, H.; Baik, S.H.; Kim, N.K.; Lee, K.Y. LASSO-Based Machine Learning Algorithm for Prediction of Lymph Node Metastasis in T1 Colorectal Cancer. Cancer Res. Treat. 2021, 53, 773–783. [Google Scholar] [CrossRef]
- Sanz, H.; Valim, C.; Vegas, E.; Oller, J.M.; Reverter, F. SVM-RFE: Selection and visualization of the most relevant features through non-linear kernels. BMC Bioinform. 2018, 19, 432. [Google Scholar] [CrossRef]
- Hu, J.; Szymczak, S. A review on longitudinal data analysis with random forest. Brief. Bioinform. 2023, 24, bbad002. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]





| Full Target Name | Target Acronym | Degree | Betweenness Centrality | Closeness Centrality |
|---|---|---|---|---|
| Interleukin-1 beta | IL1B | 90 | 0.035500531 | 0.868852459 |
| Prostaglandin G/H synthase 2 | PTGS2 | 89 | 0.035478698 | 0.861788618 |
| Transcription factor Jun | JUN | 89 | 0.035418858 | 0.861788618 |
| MAP kinase-activated protein kinase 3 | MAPK3 | 85 | 0.030411643 | 0.834645669 |
| Peroxisome proliferator-activated receptor gamma | PPARG | 85 | 0.026629396 | 0.834645669 |
| Myc proto-oncogene protein | MYC | 83 | 0.026441028 | 0.821705426 |
| Heat shock protein HSP 90-beta | HSP90AB1 | 82 | 0.026165911 | 0.815384615 |
| Protein c-Fos | FOS | 80 | 0.034046611 | 0.796992481 |
| ATP-dependent translocase ABCB1 | ABCB1 | 79 | 0.025695306 | 0.796992481 |
| Interleukin-1 alpha | IL1A | 72 | 0.016995181 | 0.757142857 |
| G1/S-specific cyclin-D1 | CCND1 | 71 | 0.015427766 | 0.75177305 |
| Heme oxygenase 1 | HMOX1 | 67 | 0.011811346 | 0.726027397 |
| Glucocorticoid receptor | NR3C1 | 67 | 0.02356451 | 0.731034483 |
| Angiotensinogen | AGT | 66 | 0.014954789 | 0.721088435 |
| Fatty acid synthase | FASN | 65 | 0.020256702 | 0.716216216 |
| Cyclin-dependent kinase inhibitor 1 | CDKN1A | 64 | 0.017355073 | 0.716216216 |
| Broad substrate specificity ATP-binding cassette transporter ABCG2 | ABCG2 | 64 | 0.010097723 | 0.711409396 |
| Poly [ADP-ribose] polymerase 1 | PARP1 | 60 | 0.008521068 | 0.692810458 |
| Cyclin-dependent kinase 4 | CDK4 | 59 | 0.014661166 | 0.692810458 |
| Pro-glucagon | GCG | 59 | 0.009433137 | 0.688311688 |
| Ingredient Code | Ingredient Name | Degree | Betweenness Centrality | Closeness Centrality |
|---|---|---|---|---|
| MOL000098 | Quercetin | 46 | 0.028875395 | 0.393665158 |
| MOL006821 | (-)-Epigallocatechin-3-gallate | 30 | 0.034899926 | 0.404651163 |
| MOL000006 | Luteolin | 24 | 0.009673829 | 0.373390558 |
| SLZ2 | Palmitoleic acid | 22 | 0.011690763 | 0.397260274 |
| SLZ5 | Oleic acid | 21 | 0.010631126 | 0.395454545 |
| SLZ11 | 9,12-hexadecadienoic acid | 19 | 0.009826783 | 0.397260274 |
| SLZ6 | Linoleic acid | 19 | 0.008236272 | 0.391891892 |
| SLZ1 | Palmitic acid-13C | 19 | 0.009049947 | 0.376623377 |
| SLZ9 | Gondoic acid | 18 | 0.008683739 | 0.390134529 |
| SLZ10 | Linolenic acid | 18 | 0.008140464 | 0.390134529 |
| SLZ3 | Margaric acid | 18 | 0.007711383 | 0.375 |
| SLZ7 | Γ-linolenic acid | 18 | 0.009737845 | 0.391891892 |
| BXG3 | Protocatechuic acid | 18 | 0.020991044 | 0.379912664 |
| SLZ4 | Stearic acid-1-13C | 17 | 0.00703053 | 0.373390558 |
| SLZ12 | L-aspartic acid | 15 | 0.005323021 | 0.345238095 |
| SLZ13 | L-glutamic acid | 15 | 0.005323021 | 0.345238095 |
| SLZ14 | Sericic acid | 14 | 0.00715489 | 0.381578947 |
| SLZ8 | Arachidic acid | 13 | 0.003773497 | 0.348 |
| MOL000358 | Beta-sitosterol | 10 | 0.004651937 | 0.335907336 |
| MOL000422 | Kaempferol | 10 | 0.003127066 | 0.341176471 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, S.; Liang, X.; Wang, Z.; Jin, H.; Zou, L.; Yang, J. Potential Mechanism of Tibetan Medicine Liuwei Muxiang Pills against Colorectal Cancer: Network Pharmacology and Bioinformatics Analyses. Pharmaceuticals 2024, 17, 429. https://doi.org/10.3390/ph17040429
Qi S, Liang X, Wang Z, Jin H, Zou L, Yang J. Potential Mechanism of Tibetan Medicine Liuwei Muxiang Pills against Colorectal Cancer: Network Pharmacology and Bioinformatics Analyses. Pharmaceuticals. 2024; 17(4):429. https://doi.org/10.3390/ph17040429
Chicago/Turabian StyleQi, Shaochong, Xinyu Liang, Zijing Wang, Haoran Jin, Liqun Zou, and Jinlin Yang. 2024. "Potential Mechanism of Tibetan Medicine Liuwei Muxiang Pills against Colorectal Cancer: Network Pharmacology and Bioinformatics Analyses" Pharmaceuticals 17, no. 4: 429. https://doi.org/10.3390/ph17040429




