New Light on Plants and Their Chemical Compounds Used in Polish Folk Medicine to Treat Urinary Diseases
Abstract
1. Introduction
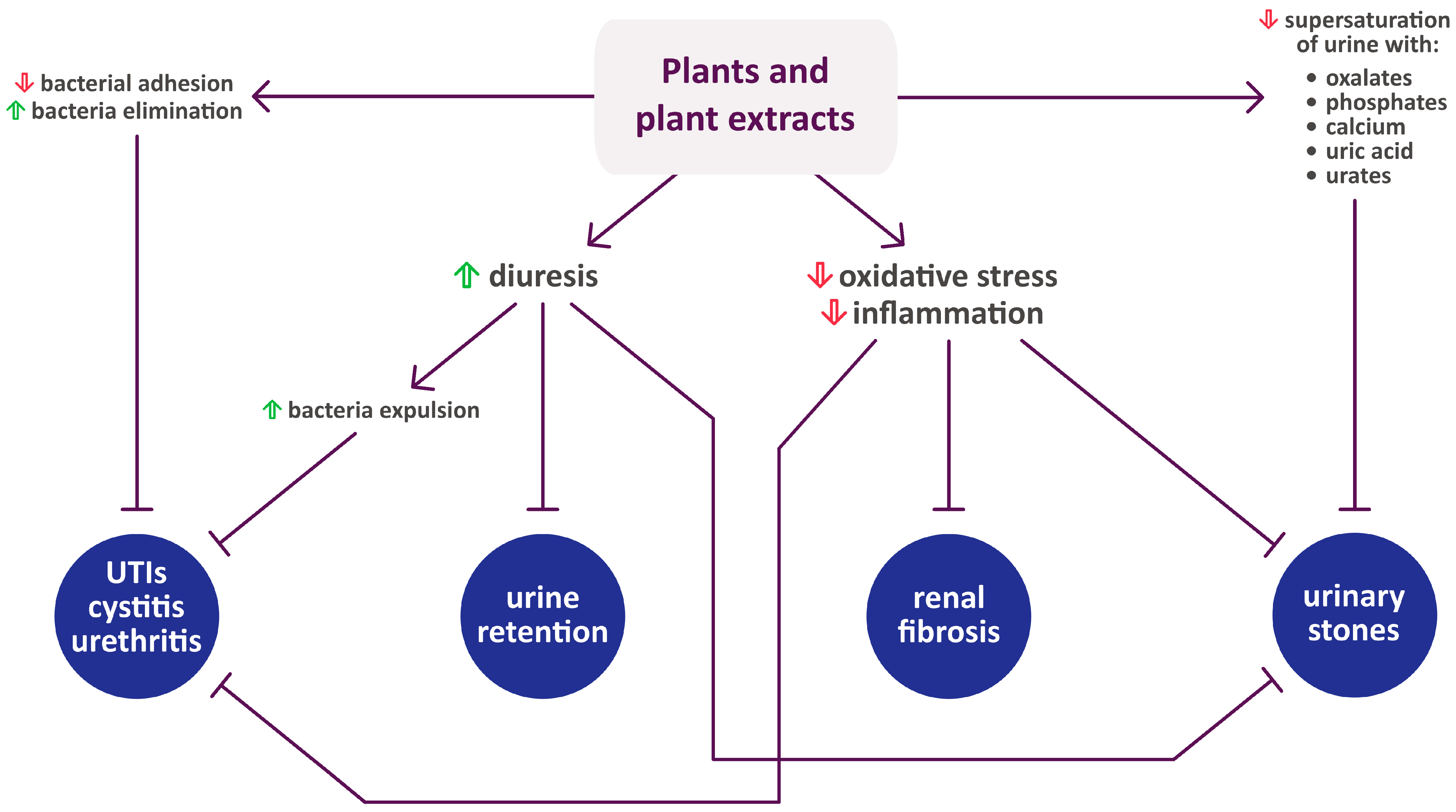
2. Plants Whose Therapeutic Effect on the Urinary Tract Has Been Confirmed by Scientific Findings (in Alphabetical Order)
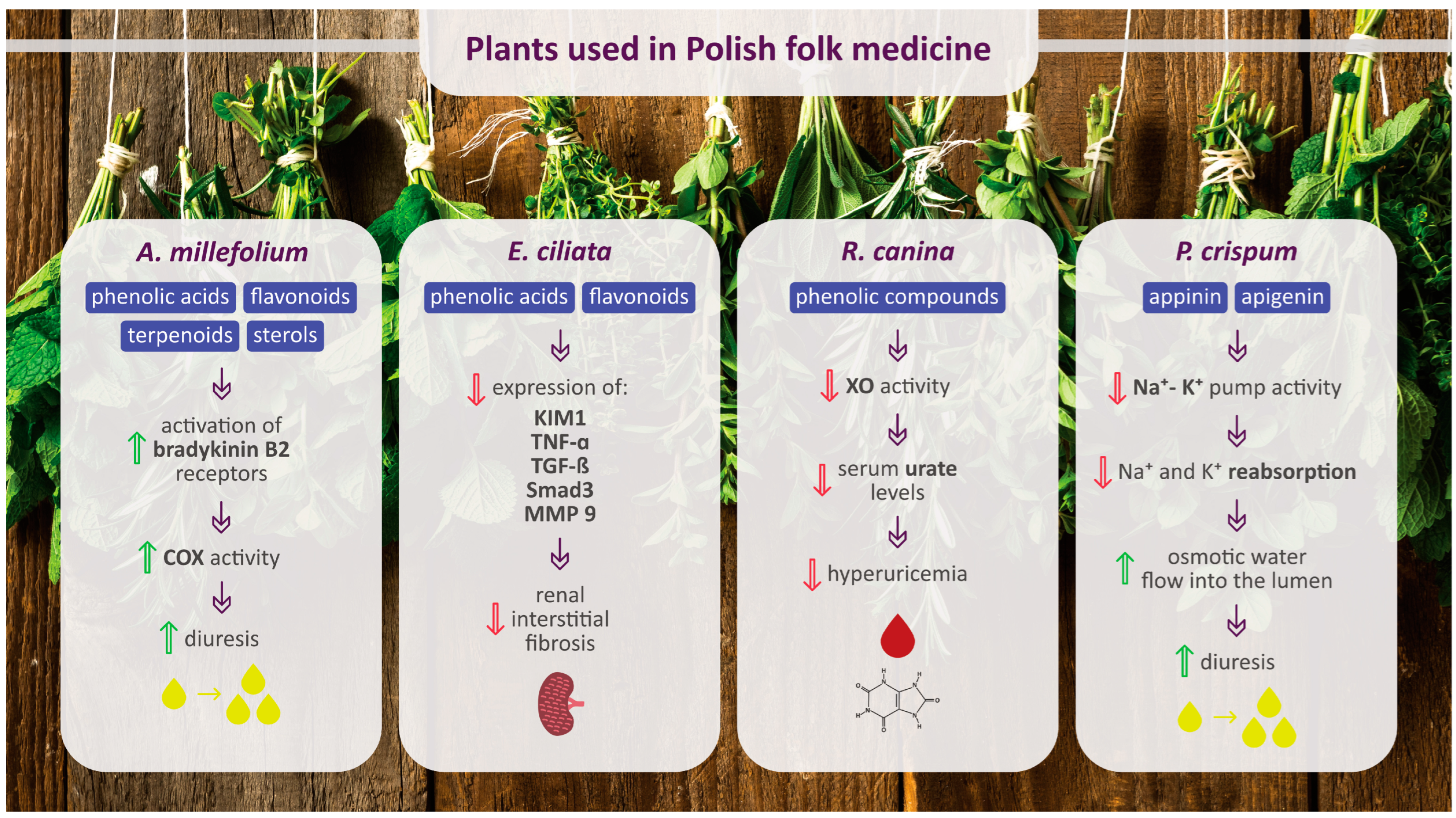
2.1. Achillea millefolium L.
2.2. Acorus calamus L.
2.3. Aegopodium podagraria L.
2.4. Amaranthus retroflexus L.
2.5. Apium graveolens L.
2.6. Arctostaphylos uva-ursi (L.) Spreng.
2.7. Betula pendula Roth and Betula pubescens Ehrh.
2.8. Bidens tripartita L.
2.9. Carum carvi L.
2.10. Daucus carota L.
2.11. Elsholtzia ciliata (Thunb.) Hyl.
2.12. Elymus repens (L.) Gould
2.13. Equisetum arvense L.
2.14. Foeniculum vulgare Mill.
2.15. Fraxinus excelsior L.
2.16. Humulus lupulus L.
2.17. Juniperus communis L.
2.18. Lycopodium clavatum L.
2.19. Nigella sativa L.
2.20. Petroselinum crispum (Mill.) Fuss
2.21. Plantago major L.
2.22. Rosa canina L.
2.23. Sambucus ebulus L. and Sambucus nigra L.
2.24. Taraxacum campylodes G.E.Haglund
2.25. Urtica dioica L. and Urtica urens L.
2.26. Vaccinium myrtillus L.
2.27. Viola tricolor L.
3. Phytochemicals Important in the Treatment of Urinary Diseases
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lee, J.B.L.; Neild, G.H. Urinary Tract Infection. Medicine 2007, 35, 423–428. [Google Scholar] [CrossRef]
- Weissman, S.J.; Warren, J.W.; Mobley, H.I.T.; Donnenberg, M.S. Host–Pathogen Interactions and Host Defense Mechanisms. In Diseases of the Kidney and Urinary Tract; Schrier, R.W., Ed.; Wolters Kluwer Health, Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 816–831. [Google Scholar]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Romero, V.; Akpinar, H.; Assimos, D.G. Kidney Stones: A Global Picture of Prevalence, Incidence, and Associated Risk Factors. Rev. Urol. 2010, 12, e86–e96. [Google Scholar] [PubMed]
- Gambaro, G.; Croppi, E.; Bushinsky, D.; Jaeger, P.; Cupisti, A.; Ticinesi, A.; Mazzaferro, S.; D’Addessi, A.; Ferraro, P.M. The Risk of Chronic Kidney Disease Associated with Urolithiasis and Its Urological Treatments: A Review. J. Urol. 2017, 198, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Wilson, D.M.; O’Fallon, W.M.; Malek, R.S.; Kurland, L.T. Renal Stone Epidemiology: A 25-Year Study in Rochester, Minnesota. Kidney Int. 1979, 16, 624–631. [Google Scholar] [CrossRef]
- Abbagani, S.; Gundimeda, S.D.; Varre, S.; Ponnala, D.; Mundluru, H.P. Kidney Stone Disease: Etiology and Evaluation. Int. J. Appl. Biol. Pharm. 2010, 1, 175–182. [Google Scholar]
- Hesse, A.; Tiselius, H.-G.; Siener, R.; Hoppe, B. Urinary Stones: Diagnosis, Treatment, and Prevention of Recurrence; S. Karger AG: Basel, Switzerland, 2009; ISBN 978-3-8055-9149-2. [Google Scholar]
- Keshavarzi, B.; Yavar Ashayeri, N.; Moore, F.; Irani, D.; Asadi, S.; Zarasvandi, A.; Salari, M. Mineralogical Composition of Urinary Stones and Their Frequency in Patients: Relationship to Gender and Age. Minerals 2016, 6, 131. [Google Scholar] [CrossRef]
- Yarnell, E. Botanical Medicines for the Urinary Tract. World J. Urol. 2002, 20, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Awang, D.V.C. Chapter Four: Kidney, Urinary Tract, and Prostate Problems. In Tyler’s Herbs of Choice: The Therapeutic Use of Phytomedicinals; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2009; pp. 59–76. [Google Scholar]
- Tylkowa, D. Medycyna Ludowa w Kulturze Wsi Karpat Polskich; Ossolineum: Wrocław, Poland, 1989. [Google Scholar]
- Penkala-Gawęcka, D. Medycyna Komplementarna w Polsce i Jej Badanie. Lud 1991, 74, 43–54. [Google Scholar]
- Piątkowski, W.; Majchrowska, A. Health, Illness and Dying in Polish Folk Medicine. Health Sci. 2015, 5, 214–224. [Google Scholar]
- Ghelani, H.; Chapala, M.; Jadav, P. Diuretic and Antiurolithiatic Activities of an Ethanolic Extract of Acorus calamus L. Rhizome in Experimental Animal Models. J. Tradit. Complement. Med. 2016, 6, 431–436. [Google Scholar] [CrossRef]
- Dimkov, P. Bulgarian Folk Medicine. Naturopathy and Natural Life; Publishing House of Bulgarian Academy of Science: Sofia, Bulgaria, 1977. [Google Scholar]
- Dévora Gutiérrez, S.; Hernández-Luis, F.; Martín-Herrera, D.; Morales Marrero, C.C.; Abdala, S. Diuretic Activity of Sambucus nigra L. ssp. Palmensis (Link) R. Bolli, an Endemic Canary Islands Species. Bol. Latinoam. Caribe Plantas Med. Aromat. 2023, 22, 500–507. [Google Scholar] [CrossRef]
- Lamer-Zarawska, E.; Kowal-Gierczak, B.; Niedworok, J. Fitoterapia i Leki Roślinne; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2007. [Google Scholar]
- Johri, R.K. Cuminum cyminum and Carum carvi: An Update. Pharmacogn. Rev. 2011, 5, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ismail, J.; Shebaby, W.N.; Daher, J.; Boulos, J.C.; Taleb, R.; Daher, C.F.; Mroueh, M. The Wild Carrot (Daucus carota): A Phytochemical and Pharmacological Review. Plants 2023, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- El Bardai, S.; Lyoussi, B.; Wibo, M.; Morel, N. Pharmacological Evidence of Hypotensive Activity of Marrubium vulgare and Foeniculum vulgare in Spontaneously Hypertensive Rat. Clin. Exp. Hypertens. 2001, 23, 329–343. [Google Scholar] [CrossRef]
- Shokry, A.A.; El-Shiekh, R.A.; Kamel, G.; Bakr, A.F.; Ramadan, A. Bioactive Phenolics Fraction of Hedera helix L. (Common Ivy Leaf) Standardized Extract Ameliorates LPS-Induced Acute Lung Injury in the Mouse Model through the Inhibition of Proinflammatory Cytokines and Oxidative Stress. Heliyon 2022, 8, e09477. [Google Scholar] [CrossRef] [PubMed]
- Maseehullah, M.; Zakir, M.; Anas, M.; Kazmi, M.H. Ethno-Pharmacology of Asaroon (Asarum europaeum L.) with Special Reference to Unani System of Medicine. J. Complement. Integr. Med. 2022, 19, 181–192. [Google Scholar] [CrossRef]
- Mohammed, G.J.; Hameed, I.H. Pharmacological Activities: Hepatoprotective, Cardio Protective, Anti-Cancer and Anti-Microbial Activity of (Raphanus raphanistrum subsp. sativus): A Review. Indian J. Public Health Res. Dev. 2018, 9, 212. [Google Scholar] [CrossRef]
- Mayer, J.G. The History of Valerian and Hops. Z. Phytother. 2003, 24, 70–81. [Google Scholar]
- Mohammadhosseini, M.; Sarker, S.D.; Akbarzadeh, A. Chemical Composition of the Essential Oils and Extracts of Achillea Species and Their Biological Activities: A Review. J. Ethnopharmacol. 2017, 199, 257–315. [Google Scholar] [CrossRef]
- Sezik, E.; Yesilada, E.; Shadidoyatov, H.; Kulivey, Z.; Nigmatullaev, A.M.; Aripov, H.N.; Takaishi, Y.; Takeda, Y.; Honda, G. Folk Medicine in Uzbekistan. J. Ethnopharmacol. 2004, 92, 197–207. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Makarova, M.N.; Kosman, V.M.; Faustova, N.M.; Tesakova, S.V.; Makarov, V.G.; Galambosi, B. Anti-Inflammatory Activity of a HPLC-Fingerprinted Aqueous Infusion of Aerial Part of Bidens tripartita L. Phytomedicine 2010, 17, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Sowa, I.; Mołdoch, J.; Paduch, R.; Strzemski, M.; Szkutnik, J.; Tyszczuk-Rotko, K.; Dresler, S.; Szczepanek, D.; Wójciak, M. Polyphenolic Composition of Carlina acaulis L. Extract and Cytotoxic Potential against Colorectal Adenocarcinoma and Cervical Cancer Cells. Molecules 2023, 28, 6148. [Google Scholar] [CrossRef]
- Satmbekova, D.; Srivedavyasasri, R.; Orazbekov, Y.; Omarova, R.; Datkhayev, U.; Ross, S.A. Chemical and biological studies on Cichorium intybus L. Nat. Prod. Res. 2018, 32, 1343–1347. [Google Scholar] [CrossRef]
- Saeedi, M.; Khanavi, M.; Shahsavari, K.; Manayi, A. Matricaria chamomilla: An Updated Review on Biological Activities of the Plant and Constituents. Res. J. Pharmacogn. 2024, 11, 109–136. [Google Scholar] [CrossRef]
- Robertovna, G.E.; Alexeevich, K.D.; Alexeevich, S.A.; Petrovna, G.M.; Kenzhebaevna, O.K. A Traditional Medicine Plant, Onopordum acanthium L. (Asteraceae): Chemical Composition and Pharmacological Research. Plants 2019, 8, 40. [Google Scholar] [CrossRef]
- Sharef, A.Y.; Hamdi, B.A.; Alrawi, R.A.; Ahmad, H.O. Onopordum acanthium L. Extract Attenuates Pancreatic β-Cells and Cardiac Inflammation in Streptozocin-Induced Diabetic Rats. PLoS ONE 2023, 18, e0280464. [Google Scholar] [CrossRef]
- Ilhan, M.; Dereli, F.T.G.; Tümen, I.; Akkol, E.K. Anti-Inflammatory and Antinociceptive Features of Bryonia alba L.: As a Possible Alternative in Treating Rheumatism. Open Chem. 2019, 17, 23–30. [Google Scholar] [CrossRef]
- Carneiro, D.M.; Freire, R.C.; Honório, T.C.; Zoghaib, I.; Cardoso, F.F.; Tresvenzol, L.M.; de Paula, J.R.; Sousa, A.L.; Jardim, P.C.; Cunha, L.C. Randomized, Double-Blind Clinical Trial to Assess the Acute Diuretic Effect of Equisetum arvense (Field Horsetail) in Healthy Volunteers. Evid.-Based Complement. Altern. Med. 2014, 2014, 760683. [Google Scholar] [CrossRef]
- Kozlowski, J. Arctostaphyllos Uva-Ursi Spreng. [Common Bearberry]—An Indispensable Drug Plant [Ecology; Conservation]. Wiadomości Zielar. 1984, 9, 15–16. (In Polish) [Google Scholar]
- Şöhretoğlu, D.; Renda, G. The Polyphenolic Profile of Oak (Quercus) Species: A Phytochemical and Pharmacological Overview. Phytochem. Rev. 2020, 19, 1379–1426. [Google Scholar] [CrossRef]
- Ejaz, A.; Waliat, S.; Afzaal, M.; Saeed, F.; Ahmad, A.; Din, A.; Ateeq, H.; Asghar, A.; Shah, Y.A.; Rafi, A.; et al. Biological Activities, Therapeutic Potential, and Pharmacological Aspects of Blackcurrants (Ribes nigrum L.): A Comprehensive Review. Food Sci. Nutr. 2023, 11, 5799–5817. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Khaksarian, M.; Rafieian-Kopaei, M.; Abbasi, N. Overview of the Therapeutic Effects of Origanum Vulgare and Hypericum Perforatum Based on Iran’s Ethnopharmacological Documents. J. Clin. Diagn. Res. 2018, 12, FE1–FE4. [Google Scholar] [CrossRef]
- Pourmirzaee Sheikhali Kelayeh, T.; Abedinzade, M.; Ghorbani, A. A Review on Biological Effects of Lamium album (White Dead Nettle) and Its Components. J. Herbmed Pharmacol. 2019, 8, 185–193. [Google Scholar] [CrossRef]
- Bharatan, V. Homeopathy and Systematics: A Systematic Analysis of the Therapeutic Effects of the Plant Species Used in Homeopathy. Homeopathy 2008, 97, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Iossifova, T. Chemical Components of Fraxinus Species. Fitoterapia 2007, 78, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Casadebaig, J.; Jacob, M.; Cassanas, G.; Gaudy, D.; Baylac, G.; Puech, A. Physicochemical and Pharmacological Properties of Spray-Dried Powders from Fraxinus excelsior Leaf Extracts. J. Ethnopharmacol. 1989, 26, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Eddouks, M.; Maghrani, M.; Zeggwagh, N.-A.; Haloui, M.; Michel, J.-B. Fraxinus excelsior L. Evokes a Hypotensive Action in Normal and Spontaneously Hypertensive Rats. J. Ethnopharmacol. 2005, 99, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Păltinean, R.; Mocan, A.; Vlase, L.; Gheldiu, A.-M.; Crișan, G.; Ielciu, I.; Voștinaru, O.; Crișan, O. Evaluation of Polyphenolic Content, Antioxidant and Diuretic Activities of Six Fumaria Species. Molecules 2017, 22, 639. [Google Scholar] [CrossRef]
- Abate, L.; Bachheti, R.K.; Tadesse, M.G.; Bachheti, A. Ethnobotanical Uses, Chemical Constituents, and Application of Plantago lanceolata L. J. Chem. 2022, 2022, 1532031. [Google Scholar] [CrossRef]
- Samuelsen, A.B. The Traditional Uses, Chemical Constituents and Biological Activities of Plantago major L. A Review. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Duwiejua, M.; Zeitlin, I.; Gray, A.; Waterman, P. The Anti-Inflammatory Compounds of Polygonum bistorta: Isolation and Characterisation. Planta Med. 1999, 65, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Gleńsk, M.; Dudek, M.K.; Ciach, M.; Włodarczyk, M. Isolation and Structural Determination of Flavan-3-Ol Derivatives from the Polypodium vulgare L. Rhizomes Water Extract. Nat. Prod. Res. 2021, 35, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Zaoui, A.; Cherrah, Y.; Lacaille-Dubois, M.A.; Settaf, A.; Amarouch, H.; Hassar, M. Diuretic and Hypotensive Effects of Nigella sativa in the Spontaneously Hypertensive Rat. Therapie 2000, 55, 379–382. [Google Scholar] [PubMed]
- Ali, B.H.; Blunden, G. Pharmacological and Toxicological Properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, A.; Peeters, L.; Foubert, K.; Piazza, S.; Vanden Berghe, W.; Hermans, N.; Pieters, L. In Vitro Biotransformation and Anti-Inflammatory Activity of Constituents and Metabolites of Filipendula ulmaria. Pharmaceutics 2023, 15, 1291. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Pizza, C.; Piacente, S.; Montoro, P. Combination of LC–MS Based Metabolomics and Antioxidant Activity for Evaluation of Bioactive Compounds in Fragaria vesca Leaves from Italy. J. Pharm. Biomed. Anal. 2018, 150, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Li, X.; Huang, X.; Liu, F.; Zhang, Z.; Cao, L. The Identification of SQS/SQE/OSC Gene Families in Regulating the Biosynthesis of Triterpenes in Potentilla Anserina. Molecules 2023, 28, 2782. [Google Scholar] [CrossRef] [PubMed]
- Gençdağ, E.; Görgüç, A.; Yılmaz, F.M. Valorization of Sweet Cherry (Prunus Avium) Wastes as a Source of Advanced Bioactive Compounds. In Mediterranean Fruits Bio-Wastes; Springer International Publishing: Cham, Switzerland, 2022; pp. 559–579. [Google Scholar]
- Sulimanec, A.; Kragić;, K.; Sekovanić;, A.; Jurasović;, J.; Panjkota Krbavčić;, I.; Vahčić;, N.; Vidaković, A.; Poljak, I.; Rumora Samarin, I. Chemical Characterization and Antioxidant Potential of the Rowan (Sorbus aucuparia L.) Fruits from Alpine-Dinaric Region of Croatia. Food Technol. Biotechnol. 2023, 61, 465–474. [Google Scholar] [CrossRef]
- Mocan, A.; Crişan, G.; Vlase, L.; Ivanescu, B.; Bădărău, A.S.; Arsene, A.L. Phytochemical Investigations on Four Galium Species (Rubiaceae) from Romania. Farmacia 2016, 64, 95–99. [Google Scholar]
- Jayakody, J.R.A.C.; Ratnasoori, W.D.; Fernando, W.A.N.A.; Weeraseker, K.R. Diuretic Activity of Leaves Extract of Hot Water Infusion of Ruta graveolens L. in Rats. J. Pharmacol. Toxicol. 2011, 6, 525–532. [Google Scholar] [CrossRef]
- Tahri, A.; Yamani, S.; Legssyer, A.; Aziz, M.; Mekhfi, H.; Bnouham, M.; Ziyyat, A. Acute Diuretic, Natriuretic and Hypotensive Effects of a Continuous Perfusion of Aqueous Extract of Urtica dioica in the Rat. J. Ethnopharmacol. 2000, 73, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Rimkiene, S.; Ragazinskiene, O.; Savickiene, N. The Cumulation of Wild Pansy (Viola tricolor L.) Accessions: The Possibility of Species Preservation and Usage in Medicine. Medicina 2003, 39, 411–416. [Google Scholar] [PubMed]
- Toiu, A.; Muntean, E.; Oniga, I.; Voştinaru, O.; Tămaş, M. Pharmacognostic Research on Viola tricolor L. (Violaceae). Rev. Med. Chir. Soc. Med. Nat. Iasi 2009, 113, 264–267. [Google Scholar] [PubMed]
- De Souza, P.; Crestani, S.; da Silva, R.D.; Gasparotto, F.; Kassuya, C.A.; da Silva-Santos, J.E.; Gasparotto, A., Jr. Involvement of Bradykinin and Prostaglandins in the Diuretic Effects of Achillea millefolium L. (Asteraceae). J. Ethnopharmacol. 2013, 149, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Koyro, O.O.; Tovchiga, O.V.; Stepanova, S.I.; Shtrygol, Y. Study of the Composition of the Goutweed Flowers Essential Oil, Its Renal Effects and Influence on Uric Acid Exchange. Pharmacogn. Commun. 2012, 2, 46–49. [Google Scholar] [CrossRef]
- Tovchiga, O.V. Renal Effects of Goutweed (Aegopodium podagraria L.) Preparations in Rats with the Metabolic Disorders Induced by Fructose and Hydrochlorothiazide. Ukr. Biopharm. J. 2014, 4, 60–66. [Google Scholar]
- Al Jawad, F.H.; Al Razzuqi, R.A.M.; Al Jeboori, A.A. Apium Graveolens Accentuates Urinary Ca+2 Excretions in Experimental Model of Nephrocalcinosis. Int. J. Green Pharm. 2011, 5, 100–102. [Google Scholar] [CrossRef]
- Rafsanjany, N.; Lechtenberg, M.; Petereit, F.; Hensel, A. Antiadhesion as a Functional Concept for Protection against Uropathogenic Escherichia coli: In Vitro Studies with Traditionally Used Plants with Antiadhesive Activity against Uropathognic Escherichia coli. J. Ethnopharmacol. 2013, 145, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Gaybullaev, A.; Kariev, S. Phytotherapy of Calcium urolithiasis with Extracts of Medicinal Plants: Changes of Diuresis, Urine PH and Crystalluria. ATI-Appl. Technol. Innov. 2012, 7, 59–66. [Google Scholar] [CrossRef]
- Lahlou, S.; Tahraoui, A.; Israili, Z.; Lyoussi, B. Diuretic Activity of the Aqueous Extracts of Carum carvi and Tanacetum vulgare in Normal Rats. J. Ethnopharmacol. 2007, 110, 458–463. [Google Scholar] [CrossRef]
- Sodimbaku, V.; Pujari, L.; Mullangi, R.; Marri, S. Carrot (Daucus carota L.): Nephroprotective against Gentamicin-Induced Nephrotoxicity in Rats. Indian J. Pharmacol. 2016, 48, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Kim, Y.-J.; Seo, C.-S.; Kim, H.-T.; Park, S.-R.; Lee, M.-Y.; Jung, J.-Y. Elsholtzia ciliata (Thunb.) Hylander Attenuates Renal Inflammation and Interstitial Fibrosis via Regulation of TGF-ß and Smad3 Expression on Unilateral Ureteral Obstruction Rat Model. Phytomedicine 2016, 23, 331–339. [Google Scholar] [CrossRef]
- Brardi, S.; Imperiali, P.; Cevenini, G.; Verdacchi, T.; Ponchietti, R. Effects of the Association of Potassium citrate and Agropyrum repens in Renal Stone Treatment: Results of a Prospective Randomized Comparison with Potassium citrate. Arch. Ital. Urol. Androl. 2012, 84, 61–67. [Google Scholar]
- Al-Snafi, A.E. Chemical Constituents and Pharmacological Importance of Agropyron repens—A Review. Res. J. Pharmacol. Toxicol. 2015, 1, 37–41. [Google Scholar]
- Stanić, G.; Samaržija, I.; Blažević, N. Time-Dependent Diuretic Response in Rats Treated with Juniper Berry Preparations. Phytother. Res. 1998, 12, 494–497. [Google Scholar] [CrossRef]
- Ribeiro, R.D.; de Barros, F.; de Melo, M.M.; Muniz, C.; Chieia, S.; das Graças Wanderley, M.; Gomes, C.; Trolin, G. Acute Diuretic Effects in Conscious Rats Produced by Some Medicinal Plants Used in the State of São Paulo, Brasil. J. Ethnopharmacol. 1988, 24, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Kreydiyyeh, S.I.; Usta, J. Diuretic Effect and Mechanism of Action of Parsley. J. Ethnopharmacol. 2002, 79, 353–357. [Google Scholar] [CrossRef]
- Alyami, F.A.; Rabah, D.M. Effect of Drinking Parsley Leaf Tea on Urinary Composition and Urinary Stones’ Risk Factors. Saudi J. Kidney Dis. Transpl. 2011, 22, 511–514. [Google Scholar]
- Saeidi, J.; Bozorgi, H.; Zendehdel, A.; Mehrzad, J. Therapeutic Effects of Aqueous Extracts of Petroselinum sativum on Ethylene Glycol-Induced Kidney Calculi in Rats. Urol. J. 2012, 9, 361–366. [Google Scholar]
- Abdul Aziz, S.; Lee See, T.; Yew Khuay, L.; Osman, K.; Azman Abu Bakar, M.; Kebangsaan Malaysia, U.; Raja Muda Abdul Aziz, J.; Lumpur Malaysia, K. In Vitro Effects of Plantago Major Extract on Urolithiasis. Malays. J. Med. Sci. 2005, 12, 22–26. [Google Scholar]
- Tayefi-Nasrabadi, H.; Sadigh-Eteghad, S.; Aghdam, Z. The Effects of the Hydroalcohol Extract of Rosa canina L. Fruit on Experimentally Nephrolithiasic Wistar Rats. Phytother. Res. 2012, 26, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Beaux, D.; Fleurentin, J.; Mortier, F. Effect of Extracts of Orthosiphon stamineus Benth, Hieracium pilosella L., Sambucus nigra L. and Arctostaphylos uva-ursi (L.) spreng. in Rats. Phytother. Res. 1999, 13, 222–225. [Google Scholar] [CrossRef]
- Walz, B.; Chrubasik, S. Impact of a Proprietary Concentrate of Sambucus nigra L. on Urinary PH. Phytother. Res. 2008, 22, 977–978. [Google Scholar] [CrossRef] [PubMed]
- Rácz–Kotilla, E.; Racz, G.; Solomon, A. The Action of Taraxacum Officinale Extracts on the Body Weight and Diuresis of Laboratory Animals. Planta Med. 1974, 26, 212–217. [Google Scholar] [CrossRef]
- Benedek, B.; Kopp, B. Achillea millefolium L. s.l. Revisited: Recent Findings Confirm the Traditional Use. Wien. Med. Wochenschr. 2007, 157, 312–314. [Google Scholar] [CrossRef]
- Saeidnia, S.; Gohari, A.; Mokhber-Dezfuli, N.; Kiuchi, F. A Review on Phytochemistry and Medicinal Properties of the Genus Achillea. Daru 2011, 19, 173–186. [Google Scholar]
- Kuźniewski, E.; Augustyn-Puziewicz, J. Przewodnik Ziołolecznictwa Ludowego; PWN Wydawnictwo Naukowe: Warszawa, Poland, 1984. [Google Scholar]
- Rutkowski, L. Klucz Do Oznaczania Roślin Naczyniowych Polski Niżowej; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2006. [Google Scholar]
- Osweiler, G.D.; Buck, W.B.; Bicknell, E.J. Production of Perirenal Edema in Swine with Amaranthus retroflexus. Am. J. Vet. Res. 1969, 30, 557–566. [Google Scholar]
- Kessell, A.; Boulton, J.; Krebs, G.; Quinn, J. Acute Renal Failure Associated with Amaranthus Species Ingestion by Lambs. Aust. Vet. J. 2015, 93, 208–213. [Google Scholar] [CrossRef]
- Fazal, S.S.; K Singla, R. Review on the Pharmacognostical & Pharmacological Characterization of Apium graveolens Linn. Indo Glob. J. Pharm. Sci. 2012, 2, 36–42. [Google Scholar] [CrossRef]
- Kooti, W.; Mansouri, E.; Ghasemiboroon, M.; Harizi, M.; Ashtary-Larky, D.; Afrisham, R. The Effects of Hydroalcoholic Extract of Apium graveolens Leaf on the Number of Sexual Cells and Testicular Structure in Rat. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e17532. [Google Scholar] [CrossRef] [PubMed]
- Das, S. Natural Therapeutics for Urinary Tract Infections—A Review. Futur. J. Pharm. Sci. 2020, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Haslam, E. Natural Polyphenols (Vegetable Tannins) as Drugs: Possible Modes of Action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Medicinal Plants of the Genus Betula—Traditional Uses and a Phytochemical–Pharmacological Review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef] [PubMed]
- Gründemann, C.; Gruber, C.W.; Hertrampf, A.; Zehl, M.; Kopp, B.; Huber, R. An Aqueous Birch Leaf Extract of Betula Pendula Inhibits the Growth and Cell Division of Inflammatory Lymphocytes. J. Ethnopharmacol. 2011, 136, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Raudonė, L.; Raudonis, R.; Janulis, V.; Viškelis, P. Quality Evaluation of Different Preparations of Dry Extracts of Birch (Betula pendula Roth) Leaves. Nat. Prod. Res. 2014, 28, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Sosnowska, K.; Tomczykowa, M.; Winnicka, K.; Kasacka, I.; Tomczyk, M. In Vivo Anti-Inflammatory and Anti-Allergic Activities of Cynaroside Evaluated by Using Hydrogel Formulations. Biomed. Pharmacother. 2020, 121, 109681. [Google Scholar] [CrossRef] [PubMed]
- Hojden, B. Marzymięta Grzebieniasta—Zapomniany Użyteczny Chwast. Wiadomości Zielar. 1995, 37, 7–8. [Google Scholar]
- Wang, F.; Liu, X.; Chen, Y.; An, Y.; Zhao, W.; Wang, L.; Tian, J.; Kong, D.; Xu, Y.; Ba, Y.; et al. Elsholtzia ciliata (Thunb.) Hyland: A Review of Phytochemistry and Pharmacology. Molecules 2022, 27, 6411. [Google Scholar] [CrossRef]
- Kasote, D.M.; Jagtap, S.D.; Thapa, D.; Khyade, M.S.; Russell, W.R. Herbal Remedies for Urinary Stones Used in India and China: A Review. J. Ethnopharmacol. 2017, 203, 55–68. [Google Scholar] [CrossRef]
- Mamedova, K.T.; Gysejnova, I.D. Effect of Equisetum arvense L. on Diuresis. Dokl. Akad. Nauk. Azerbaidzhana 1996, 51, 175–179. [Google Scholar]
- Asgarpanah, J.; Roohi, E. Jinous Asgarpanah Phytochemistry and Pharmacological Properties of Equisetum arvense L. J. Med. Plants Res. 2012, 6, 3689–3693. [Google Scholar] [CrossRef]
- Sandhu, N.S.; Kaur, S.; Chopra, D. Equisetum arvense: Pharmacology and Phytochemistry—A Review. AJPCR 2010, 3, 146–150. [Google Scholar]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculum Vulgare: A Comprehensive Review of Its Traditional Use, Phytochemistry, Pharmacology, and Safety. Arab. J. Chem. 2016, 9, S1574–S1583. [Google Scholar] [CrossRef]
- Zanoli, P.; Zavatti, M. Pharmacognostic and Pharmacological Profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Biswas, S.; Ranjan Madhu, N.; Roy Karmakar, S.; Jyoti Biswas, S.; Jyoti Biswas, S. A Better Understanding of Pharmacological Activities and Uses of Phytochemicals of Lycopodium clavatum: A Review. J. Pharmacogn. Phytochem. 2014, 3, 207–210. [Google Scholar]
- Kong, L.D.; Cai, Y.; Huang, W.W.; Cheng, C.H.K.; Tan, R.X. Inhibition of Xanthine Oxidase by Some Chinese Medicinal Plants Used to Treat Gout. J. Ethnopharmacol. 2000, 73, 199–207. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Abbasabadi, Z.; Ardekani, M.R.S.; Rahimi, R.; Farzaei, F. Parsley: A Review of Ethnopharmacology, Phytochemistry and Biological Activities. J. Tradit. Chin. Med. 2013, 33, 815–826. [Google Scholar] [CrossRef]
- Sõukand, R.; Quave, C.L.; Pieroni, A.; Pardo-de-Santayana, M.; Tardío, J.; Kalle, R.; Łuczaj, Ł.; Svanberg, I.; Kolosova, V.; Aceituno-Mata, L.; et al. Plants Used for Making Recreational Tea in Europe: A Review Based on Specific Research Sites. J. Ethnobiol. Ethnomed. 2013, 9, 58. [Google Scholar] [CrossRef]
- Edwards, N.L. The Role of Hyperuricemia in Vascular Disorders. Curr. Opin. Rheumatol. 2009, 21, 132–137. [Google Scholar] [CrossRef]
- Jiménez, P.; Tejero, J.; Cordoba-Diaz, D.; Quinto, E.J.; Garrosa, M.; Gayoso, M.J.; Girbés, T. Ebulin from Dwarf Elder (Sambucus ebulus L.): A Mini-Review. Toxins 2015, 7, 648–658. [Google Scholar] [CrossRef]
- Lis, B.; Grabek-Lejko, D. Dandelion (Taraxacum officinale)—Potential Health Benefits. Nauka Przyr. Technol. 2016, 10, 37. [Google Scholar] [CrossRef]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A Review on Its Phytochemical and Pharmacological Profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef]
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse Biological Activities of Dandelion. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef] [PubMed]
- González-Castejón, M.; García-Carrasco, B.; Fernández-Dacosta, R.; Dávalos, A.; Rodriguez-Casado, A. Reduction of Adipogenesis and Lipid Accumulation by Taraxacum officinale (Dandelion) Extracts in 3T3L1 Adipocytes: An In Vitro Study. Phytother. Res. 2014, 28, 745–752. [Google Scholar] [CrossRef]
- Joshi, B.C.; Mukhija, M.; Kalia, A.N. Pharmacognostical Review of Urtica dioica L. Int. J. Green Pharm. 2014, 8, 201–209. [Google Scholar]
- Upton, R. Stinging Nettles Leaf (Urtica dioica L.): Extraordinary Vegetable Medicine. J. Herb. Med. 2013, 3, 9–38. [Google Scholar] [CrossRef]
- Ieri, F.; Martini, S.; Innocenti, M.; Mulinacci, N. Phenolic Distribution in Liquid Preparations of Vaccinium myrtillus L. and Vaccinium vitis idaea L. Phytochem. Anal. 2013, 24, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.; Zając, M. Atlas Rozmieszczenia Roślin Naczyniowych w Polsce; Pracownia Choronologii Komputerowej Instytutu Botaniki Uniwersytetu Jagiellońskiego: Kraków, Poland, 2001. [Google Scholar]
- Shen, P.; Deng, X.; Li, T.; Chen, X.; Wu, X. Demethylzeylasteral Protects against Renal Interstitial Fibrosis by Attenuating Mitochondrial Complex I-Mediated Oxidative Stress. J. Ethnopharmacol. 2024, 327, 117986. [Google Scholar] [CrossRef]
- Mohammad, A.; Laboulaye, M.A.; Shenhar, C.; Dobberfuhl, A.D. Mechanisms of Oxidative Stress in Interstitial Cystitis/Bladder Pain Syndrome. Nat. Rev. Urol. 2024. [Google Scholar] [CrossRef]
- Hong, S.Y.; Qin, B.L. The Protective Role of Dietary Polyphenols in Urolithiasis: Insights into Antioxidant Effects and Mechanisms of Action. Nutrients 2023, 15, 3753. [Google Scholar] [CrossRef]
- Lien, E.J.; Lien, L.L.; Wang, R.; Wang, J. Phytochemical Analysis of Medicinal Plants with Kidney Protective Activities. Chin. J. Integr. Med. 2012, 18, 790–800. [Google Scholar] [CrossRef]
- Mehta, A. Pharmacology of Medicinal Plants with Antioxidant Activity. In Plants as a Source of Natural Antioxidants; Dubey, N.K., Ed.; CAB International: Oxfordshire, UK, 2015; pp. 225–244. [Google Scholar]
- Cela-López, J.M.; Camacho Roldán, C.J.; Gómez-Lizarraga, G.; Martínez, V. Effects of Itxasol© Components on Gene Expression in Bacteria Related to Infections of the Urinary Tract and to the Inflammation Process. Int. J. Mol. Sci. 2021, 22, 12655. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines; Pharmaceutical Press: London, UK, 2007. [Google Scholar]
- Talapatra, S.K.; Talapatra, B. Chemistry of Plant Natural Products; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-642-45409-7. [Google Scholar]
- Zeng, X.; Xi, Y.; Jiang, W. Protective Roles of Flavonoids and Flavonoid-Rich Plant Extracts against Urolithiasis: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2125–2135. [Google Scholar] [CrossRef]
- Pawar, A.; Deshmukh, C.; Bhanudas, B.; Ghodasara, J. Inhibitory Effect of Rutin and Curcumin on Experimentally-Induced Calcium Oxalate Urolithiasis in Rats. Pharmacogn. Res. 2010, 2, 388. [Google Scholar] [CrossRef] [PubMed]
- Kappel, V.D.; Zanatta, L.; Postal, B.G.; Silva, F.R.M.B. Rutin Potentiates Calcium Uptake via Voltage-Dependent Calcium Channel Associated with Stimulation of Glucose Uptake in Skeletal Muscle. Arch. Biochem. Biophys. 2013, 532, 55–60. [Google Scholar] [CrossRef]
- Titko, T.; Perekhoda, L.; Drapak, I.; Tsapko, Y. Modern Trends in Diuretics Development. Eur. J. Med. Chem. 2020, 208, 112855. [Google Scholar] [CrossRef]
- Boeing, T.; da Silva, L.M.; Mariott, M.; de Andrade, S.F.; de Souza, P. Diuretic and Natriuretic Effect of Luteolin in Normotensive and Hypertensive Rats: Role of Muscarinic Acetylcholine Receptors. Pharmacol. Rep. 2017, 69, 1121–1124. [Google Scholar] [CrossRef]
- Schlickmann, F.; Boeing, T.; Mariano, L.N.; da Silva, R.D.; da Silva, L.M.; de Andrade, S.F.; de Souza, P.; Cechinel-Filho, V. Gallic Acid, a Phenolic Compound Isolated from Mimosa bimucronata (DC.) Kuntze Leaves, Induces Diuresis and Saluresis in Rats. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 649–655. [Google Scholar] [CrossRef]
- Hostettmann, K.; Marston, A. Saponins; Cambridge University Press: Cambridge, UK, 1995; ISBN 9780521329705. [Google Scholar]
- Ożarowski, A. Ziołolecznictwo. Poradnik Dla Lekarzy; Państwowy Zakład Wydawnictw Lekarskich: Warszawa, Poland, 1982. [Google Scholar]
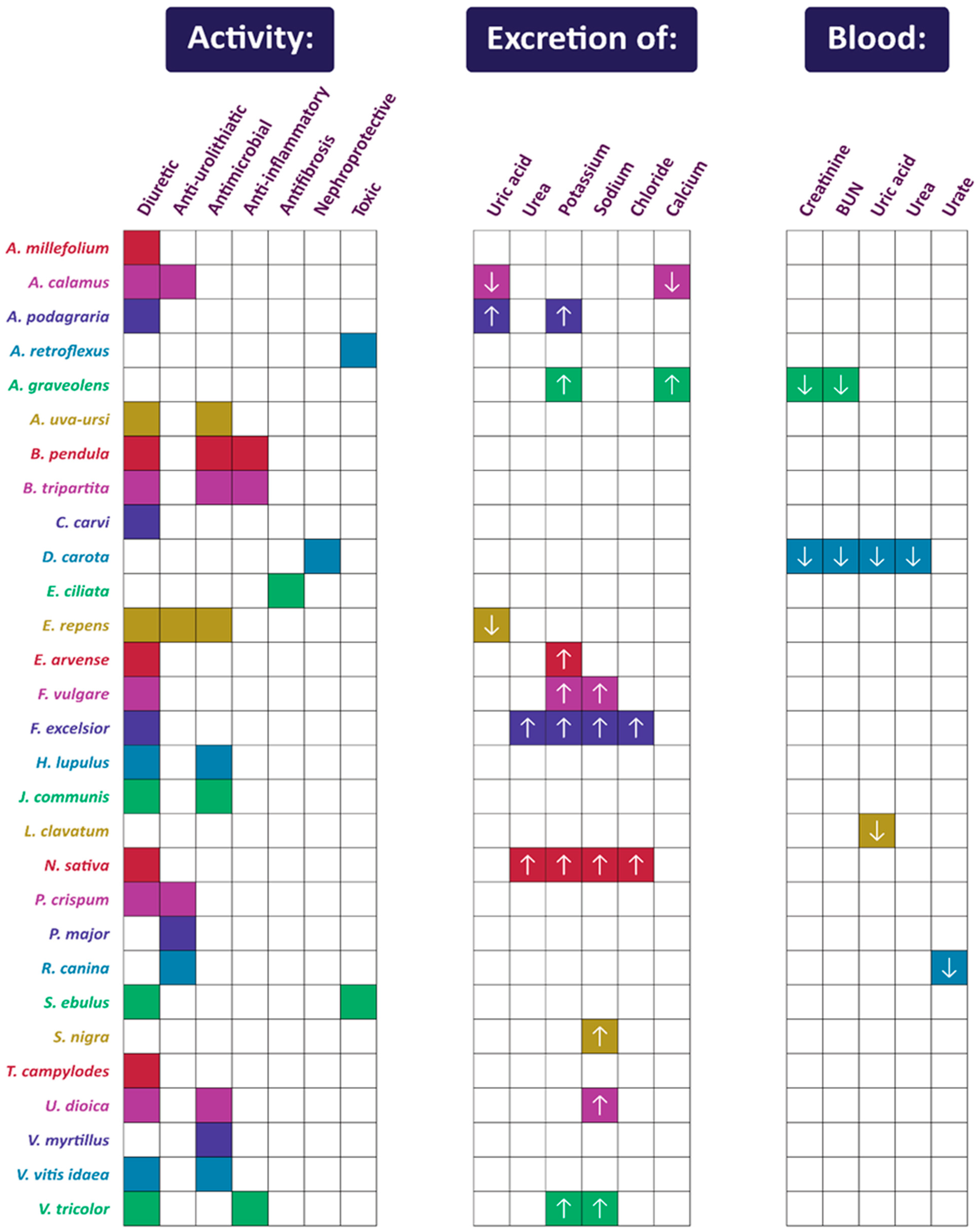
| Name of the Species (Family) | Polish Name | English Common Name | Plant Part | Form of Administration in Folk Medicine | Utilization | References |
|---|---|---|---|---|---|---|
| Acorus calamus L. (Acoraceae) | tatarak pospolity | sweet flag | Rz | infusion, decoction | diuretic (urinary stones) | [15] |
| Sambucus ebulus L. (Adoxaceae) | bez hebd | dwarf elder | Ro | infusion, decoction | diuretic (kidney diseases and edema) | [16] |
| Sambucus nigra L. (Adoxaceae) | dziki bez czarny | elder | Fl, Ba, Lf | infusion, as tea | diuretic (urethritis) | [17] |
| Apium graveolens L. (Apiaceae) | seler zwyczajny | celery root | Lf, Ro | infusion, as tea | diuretic (bladder diseases) | [18] |
| Carum carvi L. (Apiaceae) | kminek zwyczajny | meridian fennel | Fr | decoction, as tea | diuretic | [19] |
| Daucus carota L. (Apiaceae) | marchew zwyczajna | Queen Anne’s lace | Ro | fresh | diuretic (edema) | [20] |
| Foeniculum vulgare Mill. (Apiaceae) | koper włoski | fennel | Fr | fresh, decoction, as tea | diuretic | [21] |
| Levisticum officinale W.D.J. Koch (Apiaceae) | lubczyk ogrodowy | lovage | Ba, Lf | decoction | diuretic (cystitis) | [18] |
| Petroselinum crispum (Mill.) Fuss (Apiaceae) | pietruszka zwyczajna | parsley | WP | fresh, infusion, decoction | diuretic (ischuria) | [18] |
| Hedera helix L. (Araliaceae) | bluszcz pospolity | ivy | Lf | infusion | anti-inflammatory (kidney stones) | [22] |
| Asarum europaeum L. (Aristolochiaceae) | kopytnik pospolity | asarabacca | Rz | decoction | analgesic (cystitis, edema) | [23] |
| Betula pendula Roth (Betulaceae) | brzoza brodawkowata | silver birch | Lf | infusion, as tea | diuretic (cystitis) | [18] |
| Betula pubescens Ehrh. (Betulaceae) | brzoza omszona | downy birch | Lf | infusion | diuretic (cystitis) | [18] |
| Raphanus raphanistrum subsp. sativus (L.) Domin (Brassicaceae) | rzodkiew świrzepa | wild radish | Ro | fresh | diuretic (bladder diseases) | [24] |
| Humulus lupulus L. (Cannabaceae) | chmiel zwyczajny | hop | Fl | decoction | diuretic (cystitis) | [25] |
| Achillea millefolium L. (Compositae) | krwawnik pospolity | yarrow | AP | infusion | analgesic (bladder and kidney diseases) | [26] |
| Bidens tripartita L. (Compositae) | uczep trójlistkowy | three-lobe beggarticks | AP | infusion | diuretic (ischuria, urinary stones) | [27,28] |
| Carlina acaulis L. (Compositae) | dziewięćsił bezłodygowy | stemless carline thistle | Ro | decoction | diuretic, anti-inflammatory (ischuria, urethritis, cystitis) | [29] |
| Cichorium intybus L. (Compositae) | cykoria podróżnik | chicory | Ro | decoction | diuretic (ischuria) | [30] |
| Matricaria chamomilla L. (Compositae) | rumianek pospolity | chamomile | In | infusion, as tea | anti-inflammatory (nephritis, urethritis, cystitis) | [31] |
| Onopordum acanthium L. (Compositae) | popłoch pospolity | cotton thistle | Lf | infusion | anti-inflammatory (inflammation of the urinary tract) | [32,33] |
| Solidago virgaurea L. (Compositae) | nawłoć pospolita | European goldenrod | FS | decoction | diuretic (kidney stones, cystitis) | [18] |
| Taraxacum campylodes G.E.Haglund (Compositae) | mniszek lekarski | dandelion | Ro | decoction | diuretic (edema) | [18] |
| Bryonia alba L. (Cucurbitaceae) | przestęp biały | white bryony | Ro | decoction | diuretic (edema) | [34] |
| Juniperus communis L. (Cupressaceae) | jałowiec pospolity | juniper | JB | infusion, decoction | diuretic (urinary diseases, hematuria, edema) | [18] |
| Equisetum arvense L. (Equisetaceae) | skrzyp polny | horsetail | AP | decoction | diuretic (kidney stones, urine retention) | [18,35] |
| Arctostaphylos uva-ursi (L.) Spreng. (Ericaceae) | mącznica lekarska | bearberry | Lf | decoction | antiseptic (kidney stones, inflammation of the urinary tract) | [36] |
| Vaccinium myrtillus L. (Ericaceae) | borówka czarna | bilberry | Lf, Fr | decoction, fresh (fruit) | diuretic (urine retention) | [18] |
| Vaccinium vitis-idaea L. (Ericaceae) | borówka brusznica | lingonberry | Lf, Fr | decoction, fruit juice | diuretic, antiseptic (kidney stones, inflammation of the urinary tract) | [18] |
| Quercus robur L. (Fagaceae) | dąb szypułkowy | pedunculate oak | Ba | decoction | anti-inflammatory (urethritis, cystitis) | [37] |
| Ribes nigrum L. (Grossulariaceae) | porzeczka czarna | blackcurrant | AP, Fr | infusion, juice, as tea | anti-inflammatory (kidney stones, cystitis, kidney diseases) | [38] |
| Hypericum perforatum L. (Hypericaceae) | dziurawiec zwyczajny | perforate St John’s-wort | AP | infusion, decoction, as tea | diuretic (kidney diseases) | [39] |
| Lamium album L. (Lamiaceae) | jasnota biała | white nettle | Fl | infusion | anti-inflammatory (urethritis, cystitis) | [40] |
| Cytisus scoparius (L.) Link (Leguminosae) | żarnowiec miotlasty | broom | AP | decoction | diuretic (edema) | [41] |
| Ononis spinosa L. (Leguminosae) | wilżyna ciernista | spiny restharrow | Ro | decoction | diuretic (kidney/bladder stones) | [18] |
| Fraxinus excelsior L. (Oleaceae) | jesion wyniosły | ash | Ba, Lf | decoction, infusion | diuretic, anti-inflammatory (urethritis, cystitis) | [42,43,44] |
| Fumaria officinalis L. (Papaveraceae) | dymnica pospolita | fumitory | AP | infusion | diuretic (bladder diseases) | [45] |
| Plantago lanceolata L. (Plantaginaceae) | babka lancetowata | English plantain | AP | infusion | anti-inflammatory (cystitis) | [46] |
| Plantago major L. (Plantaginaceae) | babka zwyczajna | broadleaf plantain | AP, Lf | infusion | diuretic (urological diseases) | [47] |
| Elymus repens (L.) Gould (Poaceae) | perz właściwy | couch grass | Rz | decoction, infusion | diuretic (edema, urinary stones) | [18] |
| Polygonum bistorta L. (Polygonaceae) | rdest wężownik | bistort | AP, Rz | infusion | anti-inflammatory (urethritis, cystitis, hematuria) | [48] |
| Polypodium vulgare L. (Polypodiaceae) | paprotka zwyczajna | polypody | Rz | infusion | diuretic (kidney diseases) | [49] |
| Nigella sativa L. (Ranunculaceae) | czarnuszka siewna | black caraway | Se | infusion | diuretic, anti-inflammatory (urethritis, cystitis) | [50,51] |
| Filipendula ulmaria (L.) Maxim. (Rosaceae) | wiązówka błotna | meadowsweet | Fl | infusion | diuretic, anti-inflammatory (urethritis, cystitis) | [52] |
| Fragaria vesca L. (Rosaceae) | poziomka pospolita | wild strawberry | AP, Fr | decoction, as tea | diuretic (kidney/bladder stones) | [53] |
| Potentilla anserina L. (Rosaceae) | pięciornik gęsi | silverweed | AP | infusion | anti-inflammatory, diuretic (edema) | [54] |
| Prunus avium (L.) L. (Rosaceae) | wiśnia ptasia | wild cherry | LS | infusion | diuretic (urinary stones) | [55] |
| Sorbus aucuparia L. (Rosaceae) | jarząb pospolity | rowan | Fr, Fl | infusion, jam | diuretic (urinary stones) | [56] |
| Galium aparine L. (Rubiaceae) | przytulia czepna | cleavers | AP | infusion | diuretic (kidney stones) | [57] |
| Ruta graveolens L. (Rutaceae) | ruta zwyczajna | herb-of-grace | Lf | infusion | diuretic (edema) | [58] |
| Urtica dioica L. (Urticaceae) | pokrzywa zwyczajna | stinging nettle | WP | infusion, as tea | diuretic (urinary stones) | [59] |
| Viola tricolor L. (Violaceae) | fiołek trójbarwny | heartsease | AP | infusion, as tea | diuretic (cystitis) | [60,61] |
| Scientific Name | Model | Extract Used | Dose | Control/ Reference | Duration/ Total Number of Animals or Participants | UV | UNa | Other Effects Found | Citations |
|---|---|---|---|---|---|---|---|---|---|
| Achillea millefolium L. | conscious rats—Wistar | aqueous | 125, 250, 500 mg/kg | 5% Tween 80 aqueous solution (1 mL/kg) /HCTZ (10 mg/kg) | 8 h/50 (5 per group) | 0 | 0 | - | [62] |
| hydro-ethanolic | 30–300 mg/kg | +ve | +ve | ||||||
| dichloromethane subfractions | 10, 30 mg/kg | +ve | +ve | ||||||
| Acorus calamus L. | conscious rats—Wistar | ethanolic | 250, 500, 750 mg/kg | sodium carboxymethyl cellulose (0.5%; 10 mL/kg)/furosemide (15 mg/kg) | 7 h/30 (6 per group) | +ve | +ve | - | [15] |
| urolithiatic rats | ethanolic | 750 mg/kg | sodium carboxymethyl cellulose (1%)/Cystone (750 mg/kg, p.o.) | 28 d/24 (6 per group) | +ve | −ve | antiurolithiatic | ||
| Aegopodium podagraria L. | conscious mice | essential oil | 1 mg/kg | the initial state/ olimetin (1 mg/kg) | 3 d | +ve | - | uricosuric | [63] |
| conscious rats | aqueous | 1g/kg | water/HCTZ (20 mg/kg) | 8 w/42 (6 per group) | −ve | +ve | - | [64] | |
| tincture | 1 and 5 mL/kg | 0 | +ve | ||||||
| Apium graveolens L. | conscious rabbits | fresh celery | 8 g/kg | water/- | 10 d/16 (8 per group) | +ve | +ve | - | [65] |
| Betula spp. | in vitro bladder cell line T24 | hydro-ethanolic | 9.4% | - | no data | - | - | antiadhesive against UPEC strain 2980 | [66] |
| Bidens tripartita L. | Human | aqueous | 1 mL/kg | - | 2 m/24 | +ve | - | - | [67] |
| Carum carvi L. | conscious rats—Wistar | aqueous | 100 mg/kg | water/ furosemide (10 mg/kg) | 1–24 h/20 (5 per group) | +ve | +ve | - | [68] |
| 8d | +ve | +ve | |||||||
| Daucus carota L. | conscious rats—Wistar | crude extract | 200, 400 mg/kg/day | normal saline (i.p.) and 0.5% carboxymethyl cellulose/- | 8 d/24 (6 per group) | - | - | nephro- protective | [69] |
| Elsholtzia ciliata (Thunb.) Hyl. | conscious rats— Sprague Dawley | ethanolic | 300 and 500 mg/kg | water/ captopril 200 mg/kg | 14 d/50 (10 per group) | - | - | anti-inflammatory, protective against renal fibrotic disease | [70] |
| Elymus repens (L.) Gould | human | dry extract/twice a day | 100 mg | combination of different drugs | 5 m/50 (25 per group) | - | 0 | protective against renal stone formation | [71] |
| in vitro bladder cell line T24 | hydro-ethanolic | 9.5% | - | - | - | - | antiadhesive against UPEC strain 2980 | [66] | |
| human | ethanolic | 20% (60 drops 3 times daily) | - | 99 | - | - | mictirition problems reduction | [72] | |
| Equisetum arvense L. | human | dry extract | 900 mg/day | corn starch, 900 mg/day or HCTZ (25 mg/day) | 4 d each stage/10 d washout interval/ 36 (6 per group) | +ve | -ve | - | [35] |
| Foeniculum vulgare Mill. | conscious rats—SHR and Wistar-Kyoto | dry | 190 mg/kg | water | 5 d/61 | +ve | +ve (in SHR) | - | [21] |
| Fraxinus excelsior L. | conscious rats—Wistar | spray-dried powders from aqueous and alcoholic extracts | no data | water + 3% gum arabic | 6 h/25 (5 per group) | - | +ve | - | [43] |
| conscious rats—SHR and Wistar-Kyoto | aqueous | 20 mg/kg | water | 21 d/25 (5 per group) | +ve | +ve | - | [44] | |
| Juniperus communis L. | conscious rats—Wistar | 0.1% water solution of essential oil and 0.01% water solution of terinen-4-ol | 5 mL/100 g b.w. | water or water + 0.2% Tween 20/Moduretic (5 mg HCTZ and 50 mg amyloride)/ ADH | 3 d/28 (min. 5 per group) | +ve | - | - | [73] |
| Nigella sativa L. | conscious rats—SHR | dichloromethane extract | 0.6 mL/kg/day | furosemide (5 mg/kg/day) | 15 d | +ve | +ve | - | [50] |
| Petroseli-num crispum (Mill.) Fuss | conscious rats—Wistar | Hydro-ethanolic (50:50, v/v) | 40 mL/kg | 0.9% NaCl, 40 mL/kg | 4 h | +ve | +ve | - | [74] |
| conscious rats— Sprague–Dawley | aqueous 20% | - | water/ furosemide (0.6 mM) and amiloride (1mM) | 24 h/6 | +ve | - | inhibition of Na+-K+ pump | [75] | |
| human | aqueous (tea) | 1200 mL/day | bottled water | 2 w/20 (10 per group) | 0 | 0 | - | [76] | |
| conscious rats—Wistar | aqueous | 200 and 600 mg/kg | untreated/1% ethylene glycol | 30 d/36 (6 per group) | - | - | antiurolithiatic | [77] | |
| Plantago major L. | in vitro calcium oxalate crystals | ethanolic | 100 ppm to 350 ppm | water + DMSO/ allopurinol and potassium citrate | - | - | - | - | [78] |
| Rosa canina L. | conscious rats—Wistar | aqueous | 65 mg/ mL | water/ potassium citrate | 30 d/50 (10 per group) | - | - | antinephrolithiatic | [79] |
| Sambucus nigra L. | conscious rats— Sprague–Dawley | aqueous | 50 mg/kg | 0.45% saline/HCTZ (10 mg/kg) | 24 h/5 groups | +ve | +ve | - | [80] |
| human | Concentrate (120 g berries + flower juice and extract from 3.9 g of dried flowers) | 200 mL/ day | - | 7 d/11 | - | - | lack of effect on urine pH | [81] | |
| Taraxacum campylodes G.E.Haglund | conscious rats and mice | aqueous | 0.5–6% (50 mL/kg) | No data/furosemide (80 mg/kg) | 30 d/40 (20 per group) | +ve | +ve | - | [82] |
| Urtica dioica L. | anaesthetized rats—Wistar | aqueous hydro-ethanolic | 4 mg/kg/h or 24 mg/kg/h | 0.9% NaCl/ furosemide (2 mg/kg/h) | 30 min/22 | +ve | +ve | - | [59] |
| in vitro bladder cell line T24 | 11.2% | - | - | - | - | antiadhesive against UPEC strain 2980 | [66] |
| Specific Name | The Most Important Phytochemicals | References |
|---|---|---|
| Apium graveolens L. | flavonoids (apiin, apigenin, isoquercitrin), coumarins (apiumetin, apigravin, apiumoside, bergapten, celereoside, celerin), volatile oils (limonene, selenine), choline ascorbate, fatty acids | [10,125] |
| Arctostaphylos uva-ursi (L.) Spreng. | flavonols (myricetin, quercetin), iridoids (asperuloside, monotropein), phenolic glycosides (arbutin, methyl-arbutin), tannins (corilagin pyranoside, ellagic and gallic acids), terpenoids (amyrin, lupeol, uvaol, ursolic acid) | [10,125] |
| Betula pendula Roth | flavonoids (luteolin, myricetin, quercetin), saponins, tannins, triterpenes, volatile oil (α-betulenol), resin, chlorogenic acid | [10,18] |
| Elymus repens (L.) Gould | carbohydrates (glucose, fructose, mannitol, inositol, mucilaginous substances, triticin, pectin), flavonoids (tricin), volatile oils (agropyrene) | [125] |
| Equisetum arvense L. | flavonoids (luteolin, quercetin, apigenin, kaempferol), acids (coffee-tartaric acid, chlorogenic acid), triterpenoids (taraxerol isobauerenol, germanicol, oleanolic acid, ursolic acid, and betulinic acid), saponins, alkaloids (nicotine, palustrine, palustrinine), silicon, potassium | [18,102,122] |
| Fraxinus excelsior L. | flavonoids (quercetin, rhamnetin, rutin, isoquercetrin, kaempferol, astragalin), coumarin glucosides (esculin, fraxin), iridoids, coumarins, lignans, phenolic acids (p-hydroxybenzoic acid, protocatehuic acid, vanillic acid), phenylpropanoid glucosides (coniferin, syringin), triterpenes, mannitol | [42] |
| Juniperus communis L. | acids (ascorbic acid, diterpene acids, glucuronic acid), flavonoids (quercetin, isoquercitrin, amentoflavone, apigenin), tannins (gallocatechin, epigallocatechin, proanthocyanidins), volatile oils (pinene, myrcene, sabinene), junionone, resins | [125] |
| Lycopodium clavatum L. | flavonoids (apigenin), acids (vanillic, coumaric, ferulic acids and syringic acid), alkaloids (lycopodine) | [105,122] |
| Matricaria chamomilla L. | coumarins (umbelliferone, herniarin), flavonoids (apiin, apigenin, apigetrin, quercetin, quercimeritrin, luteolin, rutin), vilatile oils (α-bisabolol, chamazulene), amino acids, anthemic acid, choline, polysaccharide, tannin, triterpene hydrocarbons | [125] |
| Ononis spinosa L. | triterpenoids, flavonoids, tannins, volatile oil (carvone, trans-anethole) | [10,18] |
| Petroselinum crispum (Mill.) Fuss | flavonoids (glycosides of apigenin, luteolin), furanocoumarins (bergapten, oxypeucedanin), volatile oil (myristicin, apiole, tetramethoxyallylbenzene, terpenes, alcohols, aldehydes, ketones), proteins, fixed oil, oleo-resin, carbohydrates, vitamins (especially C and A) | [10,125] |
| Plantago major L. | acids (caffeic acid, benzoic acid, chlorogenic acid, p-coumaric acid, cinnamic acid, ferulic acid, fumaric acid, neochlorogenic acid, gentisic acid, p-hydroxybenzoic acid, syringic acid, salicylic acid, ursolic acid, oleanolic acid, vanillic acid, ascorbic acid), amino acids (asparaginę, DL-α-alanine, L-histidine, DL-leucine, DL-lysine, tryptophan, serine), alkaloids (boschniakine) carbohydrates, flavonoids (baicalein, apigenin, scutellarein, homoplantaginin, baicalin, nepitrin, hispidulin, luteolin, plantagoside), iridoids (aucubin), tannins, choline, allantoin, invertin, and emulsin | [125] |
| Sambucus nigra L. | flavonols (kaempferol, quercetin), triterpenes (α- and β-amyrin, ursolic and oleanolic acids), volatile oils (alkanes, fatty acids), tannin, chlorogenic acid, mucilage, pectin, sugar, and plastocynin | [125] |
| Solidago virgaurea L. | flavonoids (kaempferol, quercetin, isorhamnetin), saponins, acids (chlorogenic acid, caffeic acid, ferulic acid), tannins, volatile oils, fructans | [10,18] |
| Taraxacum campylodes G.E.Haglund | acids and phenols (p-hydroxyphenylacetic acid, caffeic acid, chlorogenic acid, monocaffeoyl tartaric acids, cichoric acid, taraxacoside, linolenic acid, linoleic acid, oleic acid, and palmitic acid), coumarins (aesculin, cichoriin), flavonoids (luteolin-7-diglucosides, luteolin-7-glucoside), resin, potassium, terpenoids (eudesmanolides, sesquiterpene lactones taraxinic acid esterified with glucose), carotenoids, vitamin A, choline, pectin, inulin, phytosterols | [10,125] |
| Urtica dioica L. | acids (caffeic, carbonic, caffeoylmalic, chlorogenic, silicic, formic, citric, glyceric, fumaric, malic, phosphoric, oxalic, quinic, succinic), amines (acetylcholine, betaine, choline, histamine, lecithin, serotonin), flavonol glycosides (isorhamnetin, kaempferol, quercetin), lignans, choline acetyltransferase, scopoletin, β-sitosterol, tannin | [10,125] |
| Vaccinium myrtillus L. | Fruit: anthocyanins (glycosides of delphinidin, petunidin, cyanidin, peonidin, malvidin), polyphenols (catechin, epicatechin, tannins), pectins, vitamin C | [125] |
| Leaf: flavonoids (quercetin and its glycosides), phenolic acids (p-coumaric, caffeic, p-hydroxybenzoic), tannins, iridoids | ||
| Vaccinium vitis-idaea L. | phenolic glycosides (arbutin), tannins, flavonoids (quercetin, myricetin), triterpenoids (ursolic acid) | [18,60] |
| Viola tricolor L. | flavonoids (violaquercitrin, rutin, violanthin, saponaretin, scoparin, orientin, vicenin, anthocyanidin glycosides), coumarins (umbelliferone), saponins, phenol carboxylic acids (protocatechuic acid, trans-caffeic acid, p-coumaric acid), salicylic acid and its derivatives such as the methyl ester and violutoside, mucilages, tannins, carotenoids (violaxanthin), ascorbic acid | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olas, B.; Różański, W.; Urbańska, K.; Sławińska, N.; Bryś, M. New Light on Plants and Their Chemical Compounds Used in Polish Folk Medicine to Treat Urinary Diseases. Pharmaceuticals 2024, 17, 435. https://doi.org/10.3390/ph17040435
Olas B, Różański W, Urbańska K, Sławińska N, Bryś M. New Light on Plants and Their Chemical Compounds Used in Polish Folk Medicine to Treat Urinary Diseases. Pharmaceuticals. 2024; 17(4):435. https://doi.org/10.3390/ph17040435
Chicago/Turabian StyleOlas, Beata, Waldemar Różański, Karina Urbańska, Natalia Sławińska, and Magdalena Bryś. 2024. "New Light on Plants and Their Chemical Compounds Used in Polish Folk Medicine to Treat Urinary Diseases" Pharmaceuticals 17, no. 4: 435. https://doi.org/10.3390/ph17040435
APA StyleOlas, B., Różański, W., Urbańska, K., Sławińska, N., & Bryś, M. (2024). New Light on Plants and Their Chemical Compounds Used in Polish Folk Medicine to Treat Urinary Diseases. Pharmaceuticals, 17(4), 435. https://doi.org/10.3390/ph17040435






