Preparation of a Zirconium-89 Labeled Clickable DOTA Complex and Its Antibody Conjugate
Abstract
:1. Introduction
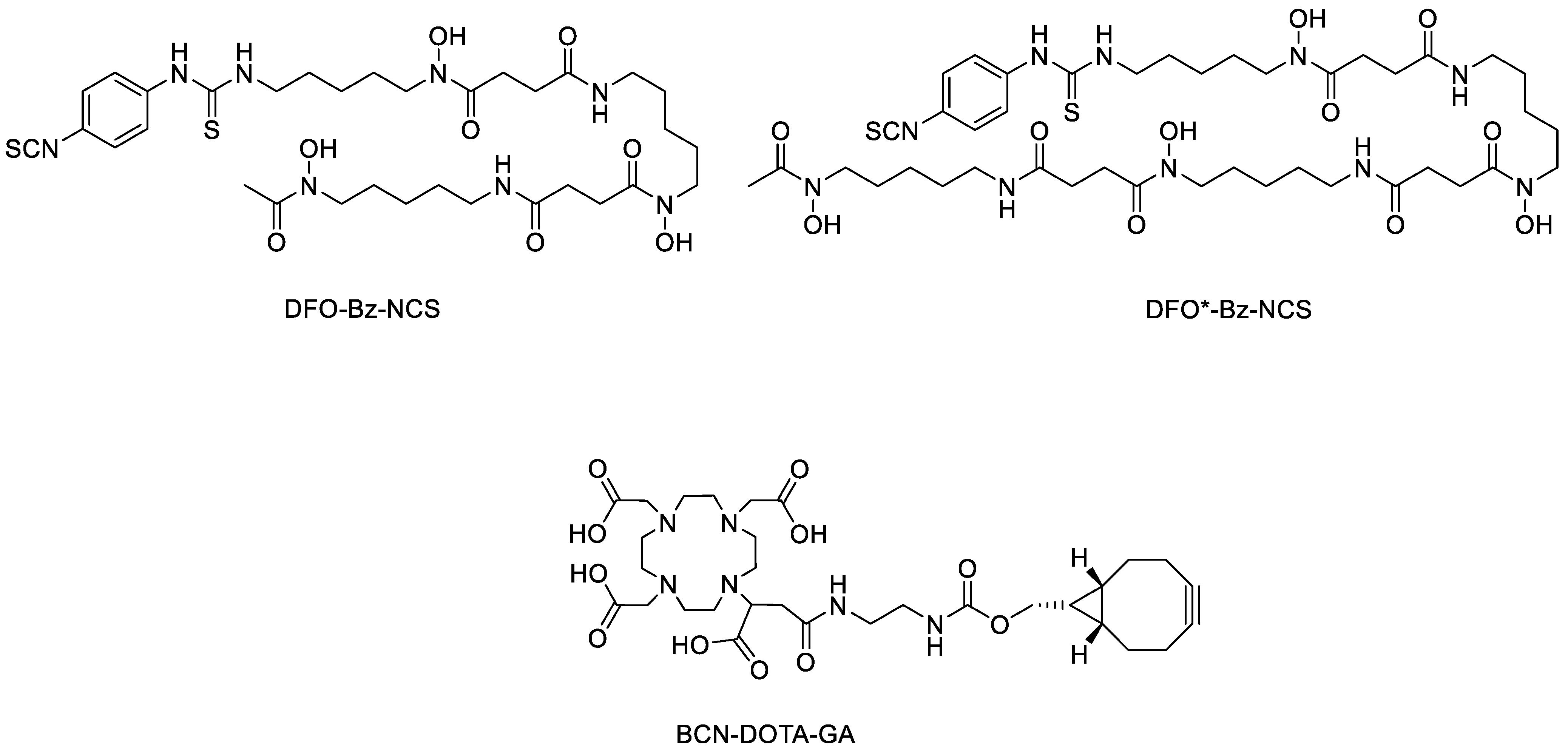
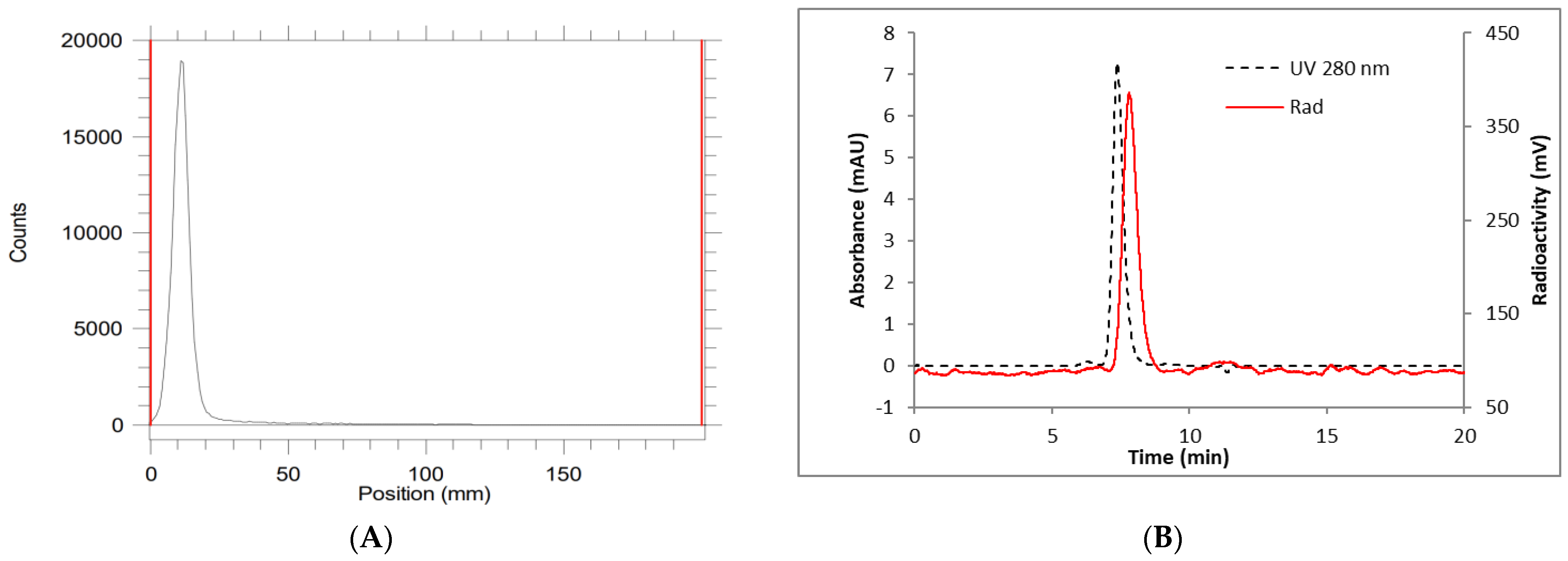
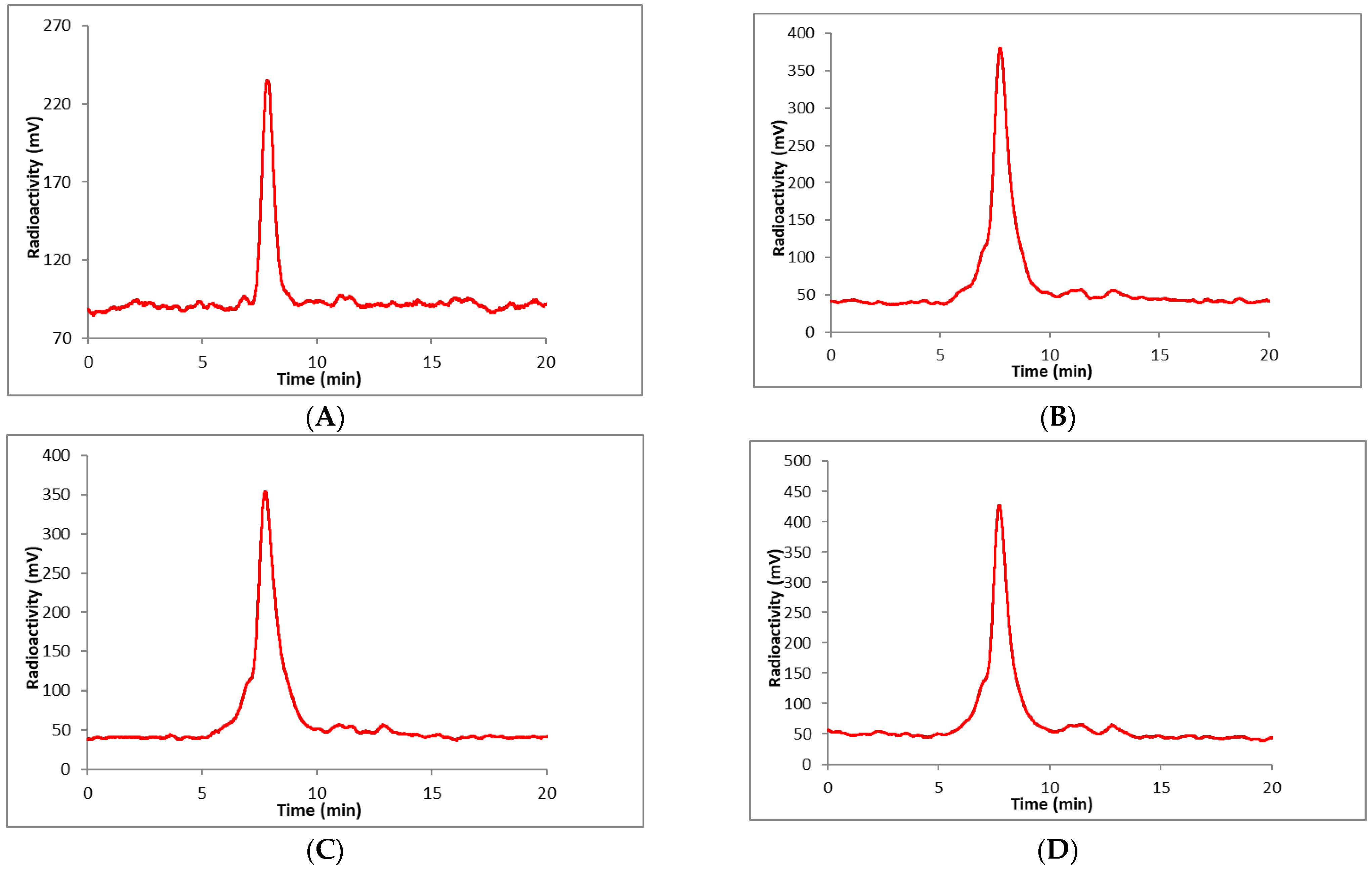
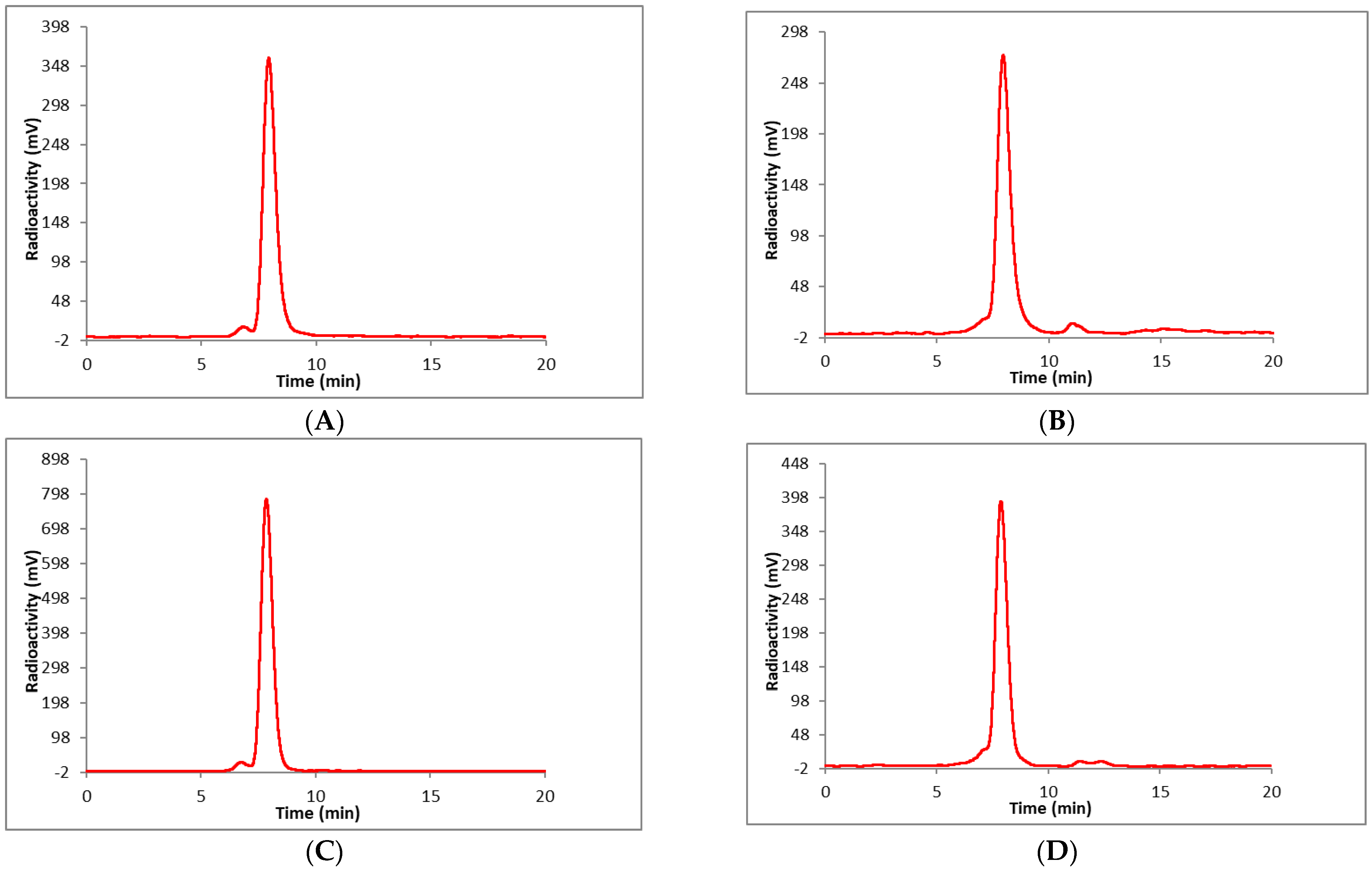
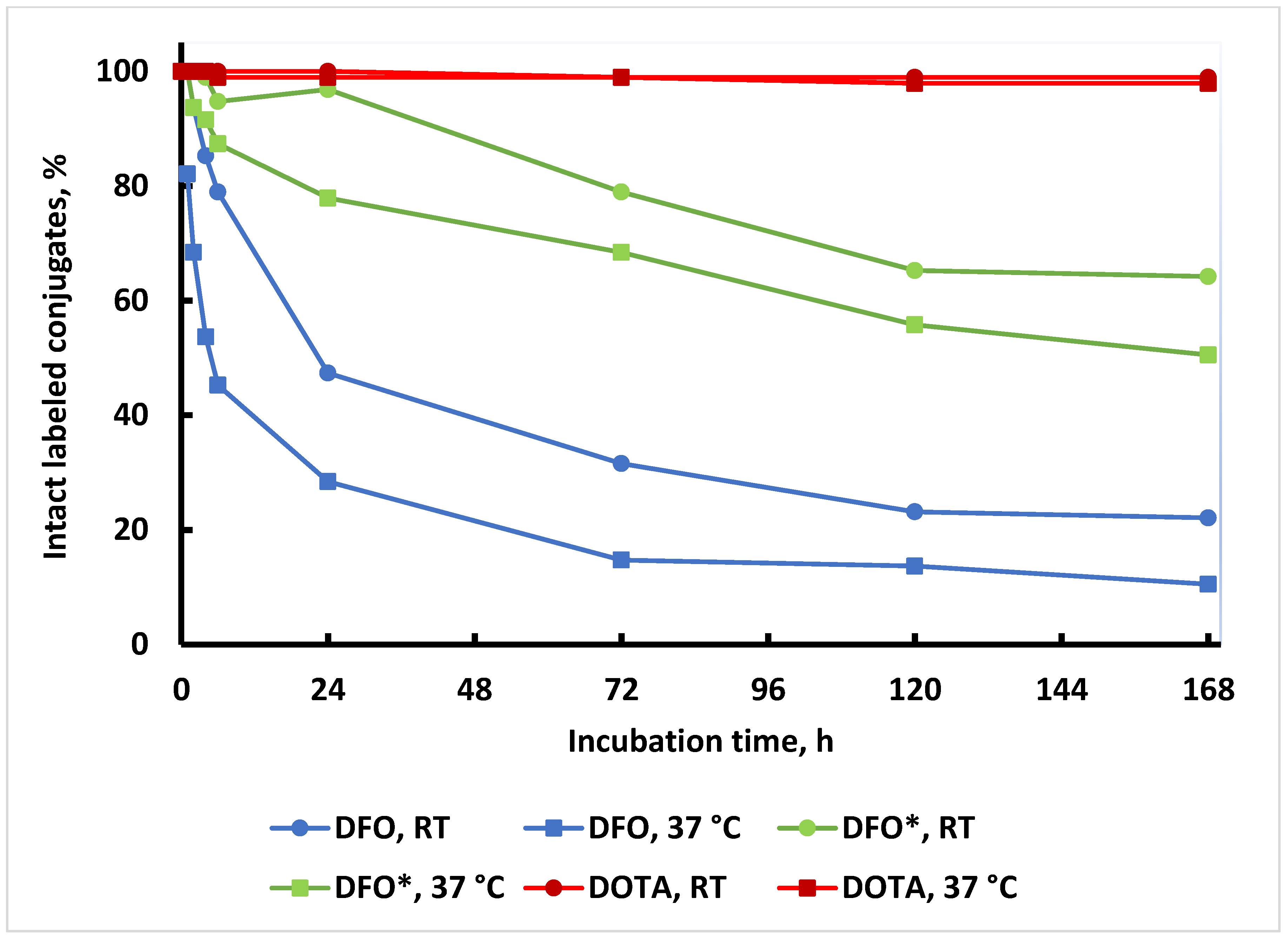
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Synthesis of DFO-PAN and DFO*-PAN
4.2. Synthesis of [89Zr]Zr-DFO-PAN and [89Zr]Zr-DFO*-PAN
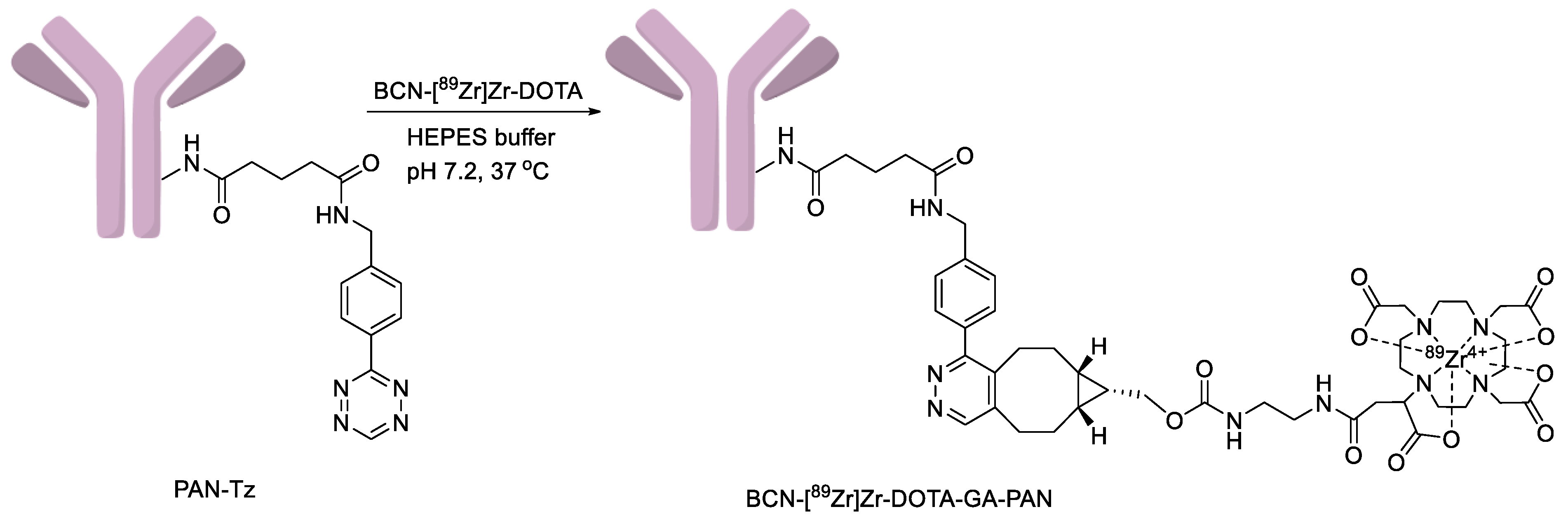
4.3. Synthesis of PAN-Tz
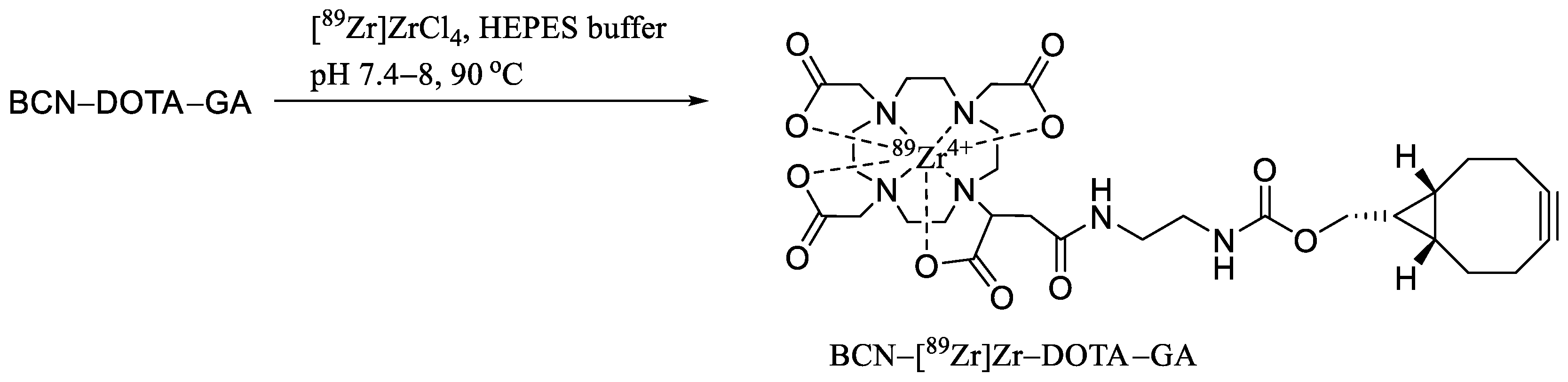
4.4. Synthesis of BCN-[89Zr]Zr-DOTA-GA-PAN
4.5. Storage and In Vitro Serum Stability
4.6. EDTA Challenge Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duclos, V.; Iep, A.; Gomez, L.; Goldfarb, L.; Besson, F.L. PET Molecular Imaging: A Holistic Review of Current Practice and Emerging Perspectives for Diagnosis, Therapeutic Evaluation and Prognosis in Clinical Oncology. Int. J. Mol. Sci. 2021, 22, 4159. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.P.; Pomper, M.G. Molecular imaging in oncology: Current impact and future directions. CA Cancer J. Clin. 2022, 72, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Haider, A.; Jeppesen, T.E.; Josephson, L.; Liang, S.H. Radiochemistry for positron emission tomography. Nat. Commun. 2023, 14, 3257. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.D.; Drake, C.; Ippisch, R.C.; Moore, M.; Sutcliffe, J.L. Fully automated peptide radiolabeling from [18F]fluoride t. RSC Adv. 2019, 9, 8638–8649. [Google Scholar] [CrossRef] [PubMed]
- Koatale, P.C.; Welling, M.M.; Ndlovu, H.; Kgatle, M.; Sipho Mdanda, S.; Mdlophane, A.; Okem, A.; Takyi-Williams, J.; Sathekge, M.M.; Ebenhan, T. Insights into Peptidoglycan-Targeting Radiotracers for Imaging Bacterial Infections: Updates, Challenges, and Future Perspectives. ACS Infect Dis. 2024, 10, 270–286. [Google Scholar] [CrossRef] [PubMed]
- Jagoda, E.M.; Vasalatiy, O.; Basuli, F.; Opina, A.C.L.; Williams, M.R.; Wong, K.; Lane, K.C.; Adler, S.; Ton, A.T.; Szajek, L.P.; et al. Immuno-PET Imaging of the Programmed Cell Death-1 Ligand (PD-L1) Using a Zirconium-89 Labeled Therapeutic Antibody, Avelumab. Mol. Imaging 2019, 18, 1536012119829986. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-G.; Haaparanta, M.; Solin, O. Oxime formation for fluorine-18 labeling of peptides and proteins for positron emission tomography (PET) imaging: A review. J. Fluor. Chem. 2012, 143, 49–56. [Google Scholar] [CrossRef]
- Olberg, D.E.; Hjelstuen, O.K. Labeling strategies of peptides with 18F for positron emission tomography. Curr. Top. Med. Chem. 2010, 10, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Menke-van der Houven van Oordt, C.W.; Gootjes, E.C.; Huisman, M.C.; Vugts, D.J.; Roth, C.; Luik, A.M.; Mulder, E.R.; Schuit, R.C.; Boellaard, R.; Hoekstra, O.S.; et al. 89Zr-cetuximab PET imaging in patients with advanced colorectal cancer. Oncotarget 2015, 6, 30384–30393. [Google Scholar] [CrossRef]
- Van de Watering, F.C.; Rijpkema, M.; Perk, L.; Brinkmann, U.; Oyen, W.J.; Boerman, O.C. Zirconium-89 labeled antibodies: A new tool for molecular imaging in cancer patients. Biomed. Res. Int. 2014, 2014, 203601. [Google Scholar] [CrossRef]
- Fischer, G.; Seibold, U.; Schirrmacher, R.; Wängler, B.; Wängler, C. 89Zr, a radiometal nuclide with high potential for molecular imaging with PET: Chemistry, applications and remaining challenges. Molecules 2013, 18, 6469–6490. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.K.; Park, B.N.; Ryu, E.K.; An, Y.S.; Lee, S.J. Current Perspectives on 89Zr-PET Imaging. Int. J. Mol. Sci. 2020, 21, 4309. [Google Scholar] [CrossRef] [PubMed]
- Poot, A.J.; Adamzek, K.W.A.; Windhorst, A.D.; Vosjan, M.J.W.D.; Kropf, S.; Wester, H.-J.; van Dongen, G.A.M.S.; Vugts, D.J. Fully Automated 89Zr Labeling and Purification of Antibodies. J. Nucl. Med. 2019, 60, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Abou, D.S.; Longtine, M.; Fears, A.; Benabdallah, N.; Unnerstall, R.; Johnston, H.; Shim, K.; Hasson, A.; Zhang, H.; Ulmert, D.; et al. Evaluation of Candidate Theranostics for 227Th/89Zr Paired Radioimmunotherapy of Lymphoma. J. Nucl. Med. 2023, 64, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Sharifian, M.; Sadeghi, M.; Alirezapour, B.; Yarmohammadi, M.; Ardaneh, K. Modeling and experimental data of zirconium-89 production yield. Appl. Radiat. Isot. 2017, 130, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, G.; Beaino, W.; Windhorst, A.D.; Zwezerijnen, G.J.C.; Oprea-Lager, D.E.; Hendrikse, N.H.; van Kuijk, C.; Boellaard, R.; Huisman, M.C.; Vugts, D.J. The Role of 89Zr-Immuno-PET in Navigating and Derisking the Development of Biopharmaceuticals. J. Nucl. Med. 2021, 62, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, S.M.; Endepols, H.; Fischer, T.; Tawadros, S.-G.; Hohberg, M.; Zimmermanns, B.; Dietlein, F.; Neumaier, B.; Drzezga, A.; Dietlein, M.; et al. Translational Development of a Zr-89-Labeled Inhibitor of Prostate-specific Membrane Antigen for PET Imaging in Prostate Cancer. Mol. Imaging Biol. 2022, 24, 115–125. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Hyman, D.M.; Ross, D.S.; Corben, A.; Chandarlapaty, S.; Goldfarb, S.; McArthur, H.; Erinjeri, J.P.; Solomon, S.B.; Kolb, H.; et al. Detection of HER2-Positive Metastases in Patients with HER2-Negative Primary Breast Cancer Using 89Zr-Trastuzumab PET/CT. J. Nucl. Med. 2016, 57, 1523–1528. [Google Scholar] [CrossRef]
- Price, E.W.; Carnazza, K.E.; Carlin, S.D.; Cho, A.; Edwards, K.J.; Sevak, K.K.; Glaser, J.M.; de Stanchina, E.; Janjigian, Y.Y.; Lewis, J.S. 89Zr-DFO-AMG102 Immuno-PET to Determine Local Hepatocyte Growth Factor Protein Levels in Tumors for Enhanced Patient Selection. J. Nucl. Med. 2017, 58, 1386–1394. [Google Scholar] [CrossRef]
- Jauw, Y.W.S.; O’Donoghue, J.A.; Zijlstra, J.M.; Hoekstra, O.S.; Menke-van der Houven van Oordt, C.W.; Morschhauser, F.; Carrasquillo, J.A.; Zweegman, S.; Pandit-Taskar, N.; Lammertsma, A.A.; et al. 89Zr-Immuno-PET: Toward a Noninvasive Clinical Tool to Measure Target Engagement of Therapeutic Antibodies In Vivo. J. Nucl. Med. 2019, 60, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, N.B.; Pandya, D.N.; Wadas, T.J. Recent Advances in Zirconium-89 Chelator Development. Molecules 2018, 23, 638. [Google Scholar] [CrossRef] [PubMed]
- Jagoda, E.M.; Lang, L.; Bhadrasetty, V.; Histed, S.; Williams, M.; Kramer-Marek, G.; Mena, E.; Rosenblum, L.; Marik, J.; Tinianow, J.N.; et al. Immuno-PET of the hepatocyte growth factor receptor Met using the 1-armed antibody onartuzumab. J. Nucl. Med. 2012, 53, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Bauman, A.; Mari, C.; Fischer, C.A.; Blacque, O.; Häussinger, D.; Gasser, G.; Mindt, T.L. An octadentate bifunctional chelating agent for the development of stable zirconium-89 based molecular imaging probes. ChemComm 2014, 50, 11523–11525. [Google Scholar] [CrossRef] [PubMed]
- Sarbisheh, E.K.; Salih, A.K.; Raheem, S.J.; Lewis, J.S.; Price, E.W. A High-Denticity Chelator Based on Desferrioxamine for Enhanced Coordination of Zirconium-89. Inorg. Chem. 2020, 59, 11715–11727. [Google Scholar] [CrossRef] [PubMed]
- Deri, M.A.; Ponnala, S.; Zeglis, B.M.; Pohl, G.; Dannenberg, J.J.; Lewis, J.S.; Francesconi, L.C. Alternative chelator for 89Zr radiopharmaceuticals: Radiolabeling and evaluation of 3,4,3-(LI-1,2-HOPO). J. Med. Chem. 2014, 57, 4849–4860. [Google Scholar] [CrossRef] [PubMed]
- Abou, D.S.; Ku, T.; Smith-Jones, P.M. In Vivo biodistribution and accumulation of 89Zr in mice. Nucl. Med. Biol. 2011, 38, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Summers, K.L.; Sarbisheh, E.K.; Zimmerling, A.; Cotelesage, J.J.H.; Pickering, I.J.; George, G.N.; Price, E.W. Structural Characterization of the Solution Chemistry of Zirconium(IV) Desferrioxamine: A Coordination Sphere Completed by Hydroxides. Inorg. Chem. 2020, 59, 17443–17452. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Hyman, D.M.; Lyashchenko, S.K.; Lewis, J.S.; Carrasquillo, J.A. 89Zr-Trastuzumab PET/CT for Detection of Human Epidermal Growth Factor Receptor 2-Positive Metastases in Patients With Human Epidermal Growth Factor Receptor 2-Negative Primary Breast Cancer. Clin. Nucl. Med. 2017, 42, 912–917. [Google Scholar] [CrossRef]
- Racow, E.E.; Kreinbihl, J.J.; Cosby, A.G.; Yang, Y.; Pandey, A.; Boros, E.; Johnson, C.J. General Approach to Direct Measurement of the Hydration State of Coordination Complexes in the Gas Phase: Variable Temperature Mass Spectrometry. J. Am. Chem. Soc. 2019, 141, 14650–14660. [Google Scholar] [CrossRef]
- Holland, J.P. Predicting the Thermodynamic Stability of Zirconium Radiotracers. Inorg. Chem. 2020, 59, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Meijs, W.E.; Herscheid, J.D.; Haisma, H.J.; Pinedo, H.M. Evaluation of desferal as a bifunctional chelating agent for labeling antibodies with Zr-89. Int. J. Rad. Appl. Instrum. A 1992, 43, 1443–1447. [Google Scholar] [CrossRef]
- Chomet, M.; Schreurs, M.; Bolijn, M.J.; Verlaan, M.; Beaino, W.; Brown, K.; Poot, A.J.; Windhorst, A.D.; Gill, H.; Marik, J.; et al. Head-to-head comparison of DFO* and DFO chelators: Selection of the best candidate for clinical 89Zr-immuno-PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Vugts, D.J.; Klaver, C.; Sewing, C.; Poot, A.J.; Adamzek, K.; Huegli, S.; Mari, C.; Visser, G.W.M.; Valverde, I.E.; Gasser, G.; et al. Comparison of the octadentate bifunctional chelator DFO*-pPhe-NCS and the clinically used hexadentate bifunctional chelator DFO-pPhe-NCS for 89Zr-immuno-PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Seibold, U.; Wängler, B.; Wängler, C. Rational Design, Development, and Stability Assessment of a Macrocyclic Four-Hydroxamate-Bearing Bifunctional Chelating Agent for 89Zr. ChemMedChem 2017, 12, 1555–1571. [Google Scholar] [CrossRef]
- Deri, M.A.; Ponnala, S.; Kozlowski, P.; Burton-Pye, B.P.; Cicek, H.T.; Hu, C.; Lewis, J.S.; Francesconi, L.C. p-SCN-Bn-HOPO: A Superior Bifunctional Chelator for 89Zr ImmunoPET. Bioconjug Chem. 2015, 26, 2579–2591. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.K.; Dominguez Garcia, M.; Raheem, S.J.; Ahiahonu, W.K.; Price, E.W. DFO-Km: A Modular Chelator as a New Chemical Tool for the Construction of Zirconium-89-Based Radiopharmaceuticals. Inorg. Chem. 2023, 62, 20806–20819. [Google Scholar] [CrossRef]
- Salih, A.K.; Raheem, S.J.; Garcia, M.D.; Ahiahonu, W.K.; Price, E.W. Design, Synthesis, and Evaluation of DFO-Em: A Modular Chelator with Octadentate Chelation for Optimal Zirconium-89 Radiochemistry. Inorg. Chem. 2022, 61, 20964–20976. [Google Scholar] [CrossRef]
- Khozeimeh Sarbisheh, E.; Summers, K.L.; Salih, A.K.; Cotelesage, J.J.H.; Zimmerling, A.; Pickering, I.J.; George, G.N.; Price, E.W. Radiochemical, Computational, and Spectroscopic Evaluation of High-Denticity Desferrioxamine Derivatives DFO2 and DFO2p toward an Ideal Zirconium-89 Chelate Platform. Inorg. Chem. 2023, 62, 2637–2651. [Google Scholar] [CrossRef]
- Richardson-Sanchez, T.; Tieu, W.; Gotsbacher, M.P.; Telfer, T.J.; Codd, R. Exploiting the biosynthetic machinery of Streptomyces pilosus to engineer a water-soluble zirconium(iv) chelator. Org. Biomol. Chem. 2017, 15, 5719–5730. [Google Scholar] [CrossRef]
- Roy, J.; Jagoda, E.M.; Basuli, F.; Vasalatiy, O.; Phelps, T.E.; Wong, K.; Ton, A.T.; Hagemann, U.B.; Cuthbertson, A.S.; Cole, P.E.; et al. In Vitro and In Vivo Comparison of 3,2-HOPO Versus Deferoxamine-Based Chelation of Zirconium-89 to the Antimesothelin Antibody Anetumab. Cancer Biother. Radiopharm. 2021, 36, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Damerow, H.; Hübner, R.; Judmann, B.; Schirrmacher, R.; Wängler, B.; Fricker, G.; Wängler, C. Side-by-Side Comparison of Five Chelators for 89Zr-Labeling of Biomolecules: Investigation of Chemical/Radiochemical Properties and Complex Stability. Cancers 2021, 13, 6349. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Tann, M. Highly variable biodistribution of 68Ga labeled somatostatin analogues 68Ga-DOTA-NOC and 68Ga-DOTA-TATE in neuroendocrine tumors: Clinical implications for somatostatin receptor directed PET/CT. Hepatobiliary Surg. Nutr. 2022, 11, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Bluemel, C.; Allen-Auerbach, M.S.; Higuchi, T.; Herrmann, K. 68Gallium- and 90Yttrium-/177Lutetium: “theranostic twins” for diagnosis and treatment of NETs. Ann. Nucl. Med. 2015, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Li, L.; Chea, J.; Hu, W.; Poku, E.; Ebner, T.; Bowles, N.; Wong, J.Y.C.; Yazaki, P.J.; Sligar, S.; et al. Antibody Targeted PET Imaging of 64Cu-DOTA-Anti-CEA PEGylated Lipid Nanodiscs in CEA Positive Tumors. Bioconjug Chem. 2020, 31, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, L.; Boschi, A.; Cittanti, C.; Martini, P.; Panareo, S.; Tonini, E.; Nieri, A.; Urso, L.; Caracciolo, M.; Lodi, L.; et al. 90Y/177Lu-DOTATOC: From Preclinical Studies to Application in Humans. Pharmaceutics 2021, 13, 1463. [Google Scholar] [CrossRef]
- Werner, R.A.; Weich, A.; Kircher, M.; Solnes, L.B.; Javadi, M.S.; Higuchi, T.; Buck, A.K.; Pomper, M.G.; Rowe, S.P.; Lapa, C. The theranostic promise for Neuroendocrine Tumors in the late 2010s—Where do we stand, where do we go? Theranostics 2018, 8, 6088–6100. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Peitl, P.K.; Velikyan, I. Current Status of Radiopharmaceuticals for the Theranostics of Neuroendocrine Neoplasms. Pharmaceuticals 2017, 10, 30. [Google Scholar] [CrossRef]
- Pandya, D.N.; Bhatt, N.; Yuan, H.; Day, C.S.; Ehrmann, B.M.; Wright, M.; Bierbach, U.; Wadas, T.J. Zirconium tetraazamacrocycle complexes display extraordinary stability and provide a new strategy for zirconium-89-based radiopharmaceutical development. Chem. Sci. 2017, 8, 2309–2314. [Google Scholar] [CrossRef]
- Privé, B.M.; Derks, Y.H.W.; Rosar, F.; Franssen, G.M.; Peters, S.M.B.; Khreish, F.; Bartholomä, M.; Maus, S.; Gotthardt, M.; Laverman, P.; et al. 89Zr-labeled PSMA ligands for pharmacokinetic PET imaging and dosimetry of PSMA-617 and PSMA-I&T: A preclinical evaluation and first in man. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2064–2076. [Google Scholar] [CrossRef]
- Lee, Y.S.; O’Connor, R.D.; Vasalatiy, O. Investigation of Two Zr-p-NO2Bn-DOTA Isomers via NMR and Quantum Chemical Studies. Eur. J. Inorg. Chem. 2023, 26, e202300439. [Google Scholar] [CrossRef] [PubMed]
- Vosjan, M.J.; Perk, L.R.; Visser, G.W.; Budde, M.; Jurek, P.; Kiefer, G.E.; van Dongen, G.A. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protoc. 2010, 5, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Meares, C.F.; McCall, M.J.; Reardan, D.T.; Goodwin, D.A.; Diamanti, C.I.; McTigue, M. Conjugation of antibodies with bifunctional chelating agents: Isothiocyanate and bromoacetamide reagents, methods of analysis, and subsequent addition of metal ions. Anal. Biochem. 1984, 142, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Jagoda, E.M.; Basuli, F.; Olkowski, C.; Weiss, I.; Phelps, T.E.; Wong, K.; Ton, A.T.; Lane, K.C.; Adler, S.; Butcher, D.; et al. Immuno-PET Imaging of Siglec-15 Using the Zirconium-89-Labeled Therapeutic Antibody, NC318. Mol. Imaging 2023, 2023, 3499655. [Google Scholar] [CrossRef]
- Feiner, I.V.J.; Brandt, M.; Cowell, J.; Demuth, T.; Vugts, D.; Gasser, G.; Mindt, T.L. The Race for Hydroxamate-Based Zirconium-89 Chelators. Cancers 2021, 13, 4466. [Google Scholar] [CrossRef]
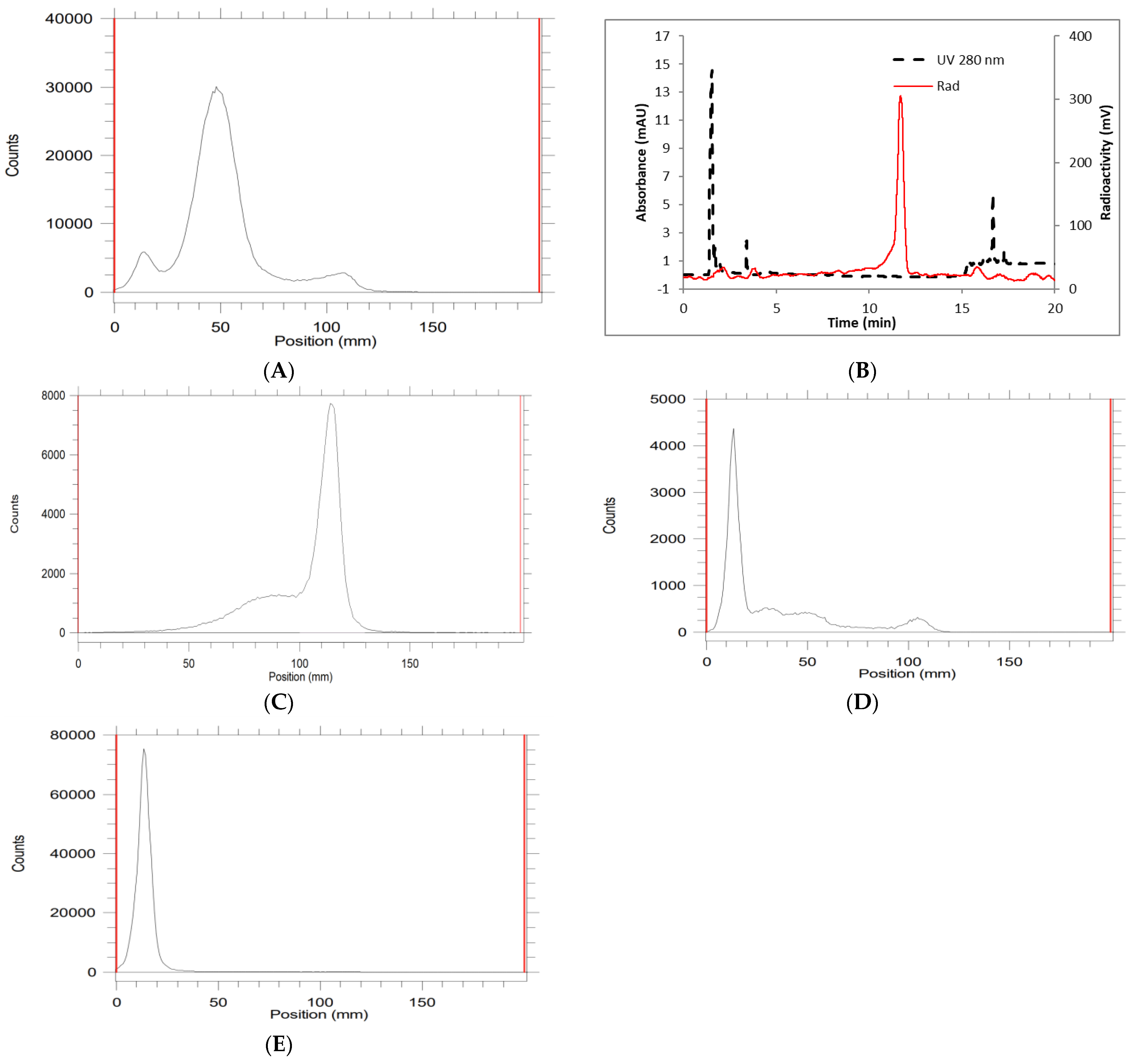
| Entry a | Amount of Ligand (µg) | Amount of Zirconium-89 (mCi) | Temperature (°C) | Yield (%) by Radio TLC |
|---|---|---|---|---|
| 1 | 10 | 1 | 80 | <20 |
| 2 | 10 | 1 | 90 | <20 |
| 3 | 30 | 1 | 80 | 95 |
| 4 | 30 | 1 | 90 | 98 |
| 5 | 30 | 2 | 90 | 92 |
| 6 | 30 | 3 | 90 | 83 |
| 7 | 30 | 4 | 90 | 74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basuli, F.; Vasalatiy, O.; Shi, J.; Lane, K.C.; Escorcia, F.E.; Swenson, R.E. Preparation of a Zirconium-89 Labeled Clickable DOTA Complex and Its Antibody Conjugate. Pharmaceuticals 2024, 17, 480. https://doi.org/10.3390/ph17040480
Basuli F, Vasalatiy O, Shi J, Lane KC, Escorcia FE, Swenson RE. Preparation of a Zirconium-89 Labeled Clickable DOTA Complex and Its Antibody Conjugate. Pharmaceuticals. 2024; 17(4):480. https://doi.org/10.3390/ph17040480
Chicago/Turabian StyleBasuli, Falguni, Olga Vasalatiy, Jianfeng Shi, Kelly C. Lane, Freddy E. Escorcia, and Rolf E. Swenson. 2024. "Preparation of a Zirconium-89 Labeled Clickable DOTA Complex and Its Antibody Conjugate" Pharmaceuticals 17, no. 4: 480. https://doi.org/10.3390/ph17040480
APA StyleBasuli, F., Vasalatiy, O., Shi, J., Lane, K. C., Escorcia, F. E., & Swenson, R. E. (2024). Preparation of a Zirconium-89 Labeled Clickable DOTA Complex and Its Antibody Conjugate. Pharmaceuticals, 17(4), 480. https://doi.org/10.3390/ph17040480






