Apigenin Alleviates Autistic-like Stereotyped Repetitive Behaviors and Mitigates Brain Oxidative Stress in Mice
Abstract
:1. Introduction
2. Results
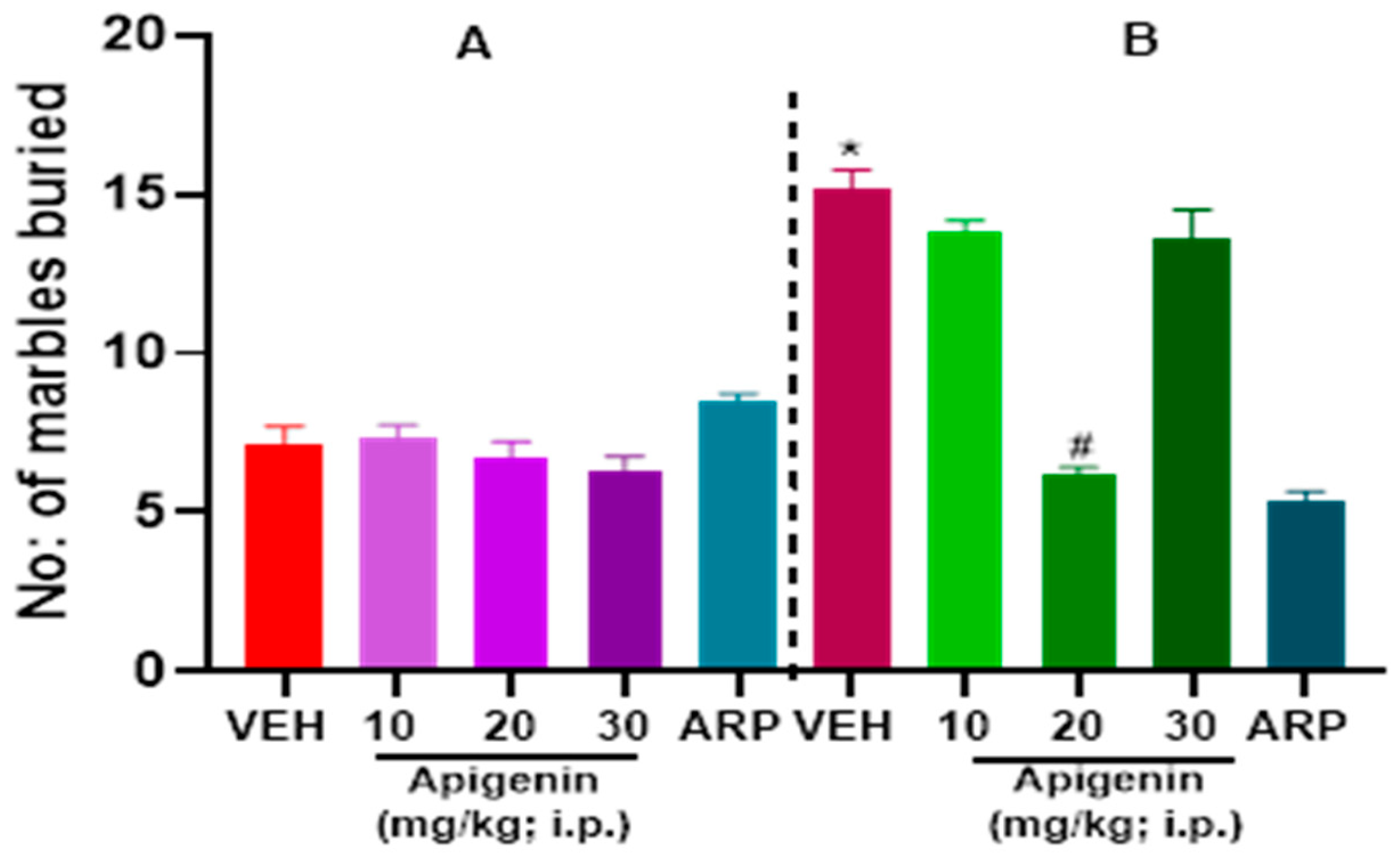
2.1. APG and ARP Significantly Reduced Burying Behaviors of BTBR Mice in MBT
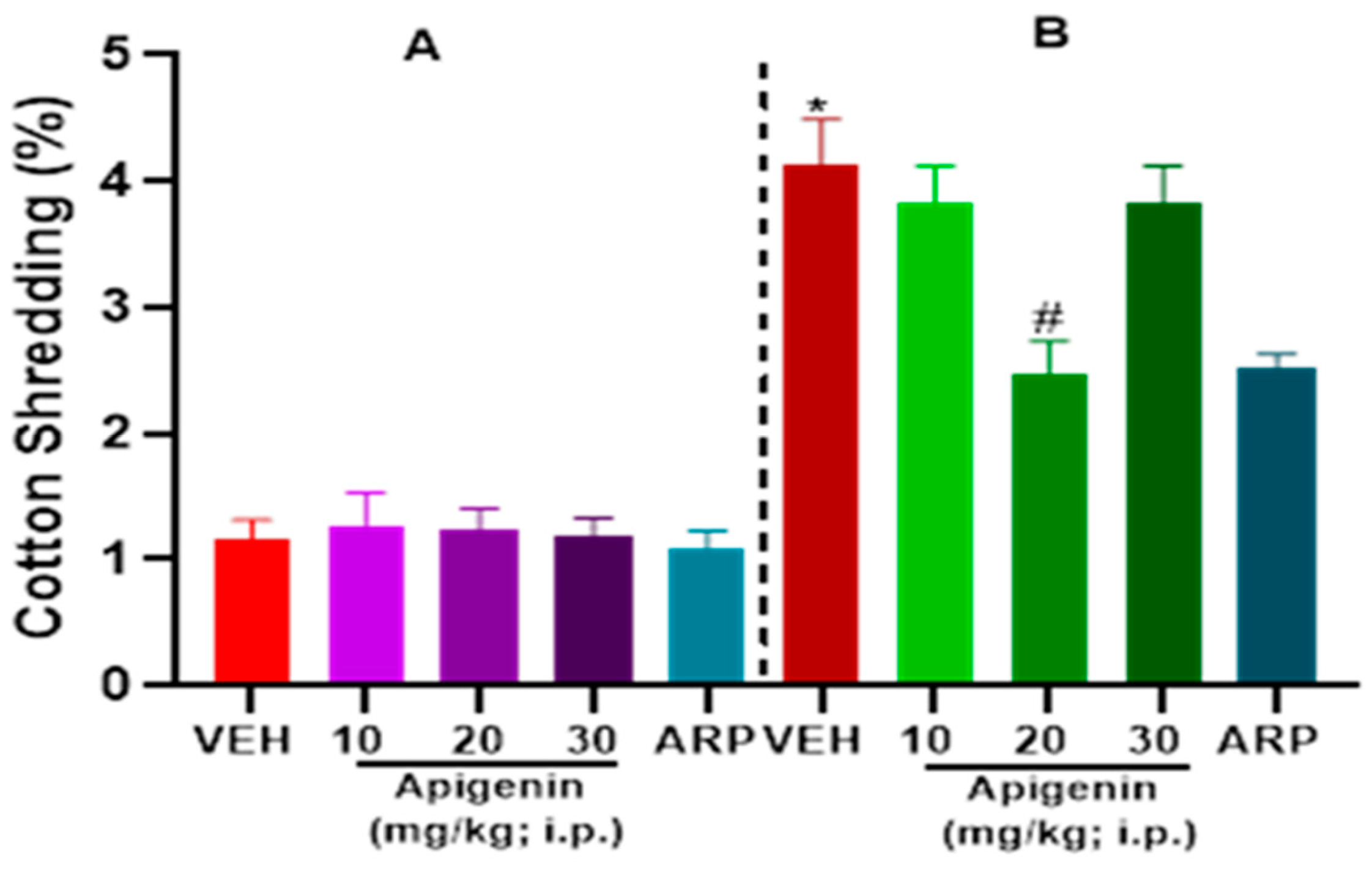
2.2. APG and ARP Significantly Decreased Cotton Shredding Behaviors of BTBR Mice in CST
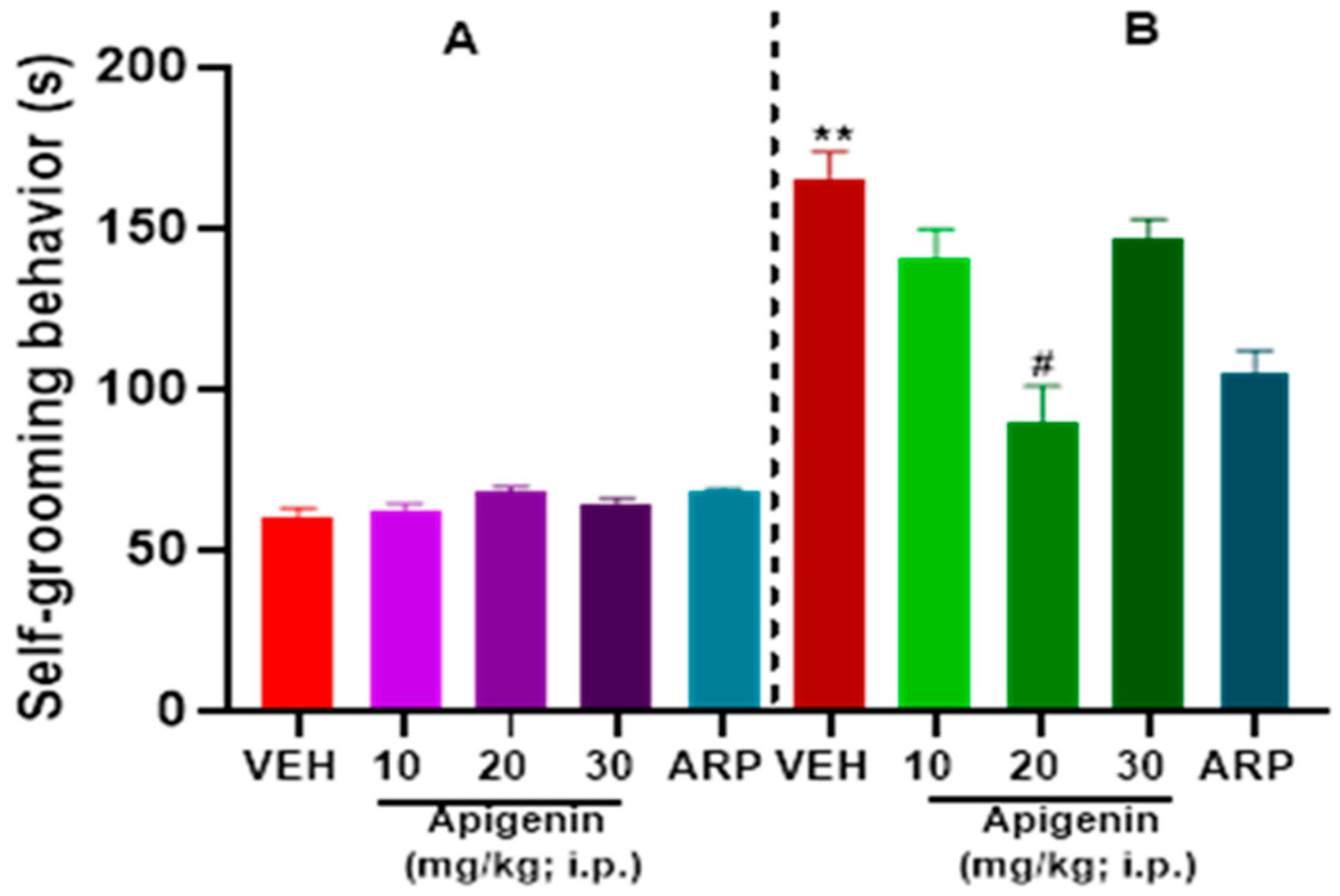
2.3. APG and ARP Reduced Self-Grooming Behaviors of BTBR Mice
2.4. Effects of APG and ARP on Anxiety Levels and Locomotion of Tested Mice in OFT
2.5. APG and ARP Mitigated Disturbed Oxidative Levels in Hippocampus and Cerebellum of Treated BTBR Mice
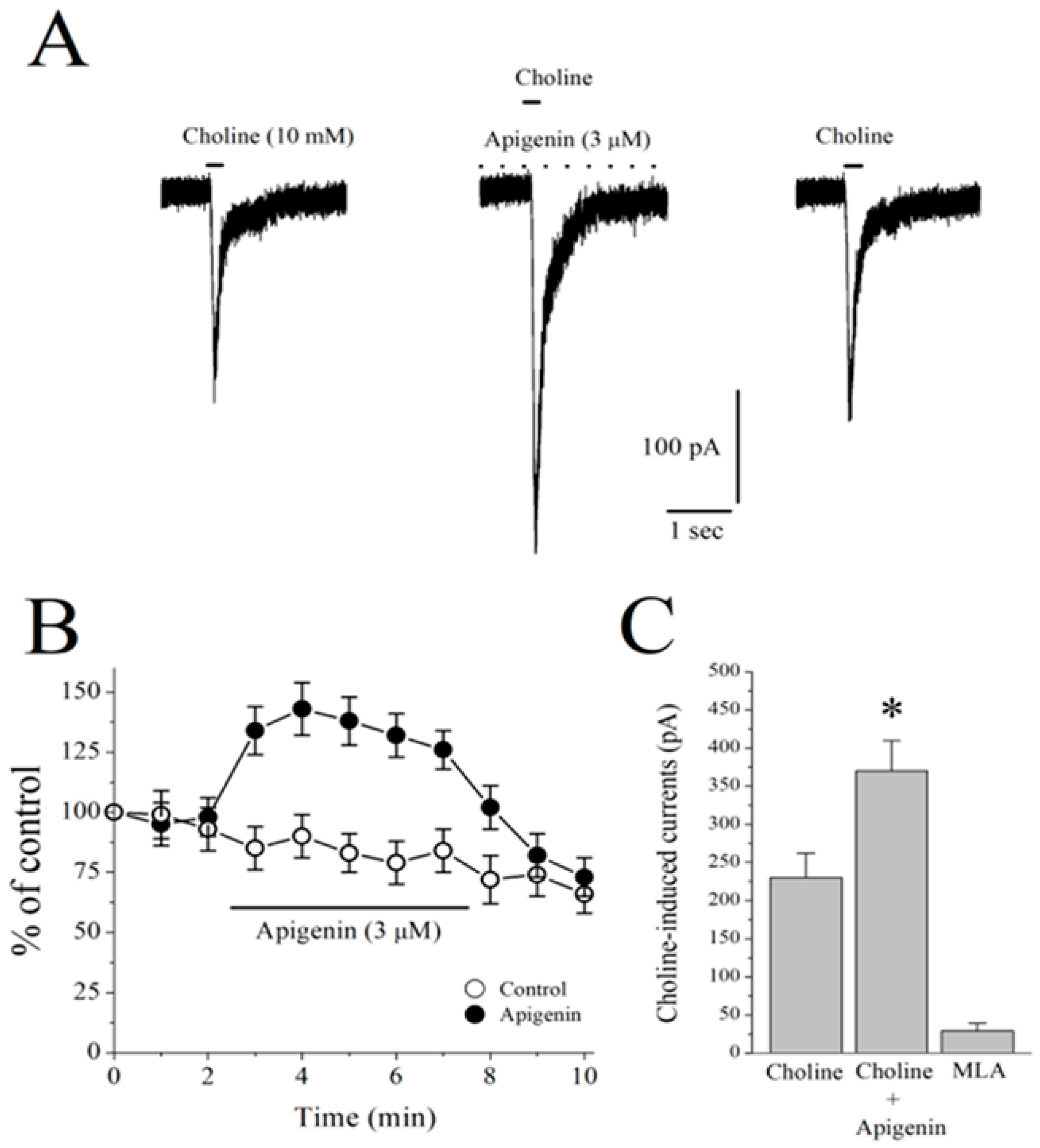
2.6. Effects of APG on α7-nACh Receptors in the CA1 Region of Stratum Radiatum Interneurons in Mice Hippocampal Brain Slices
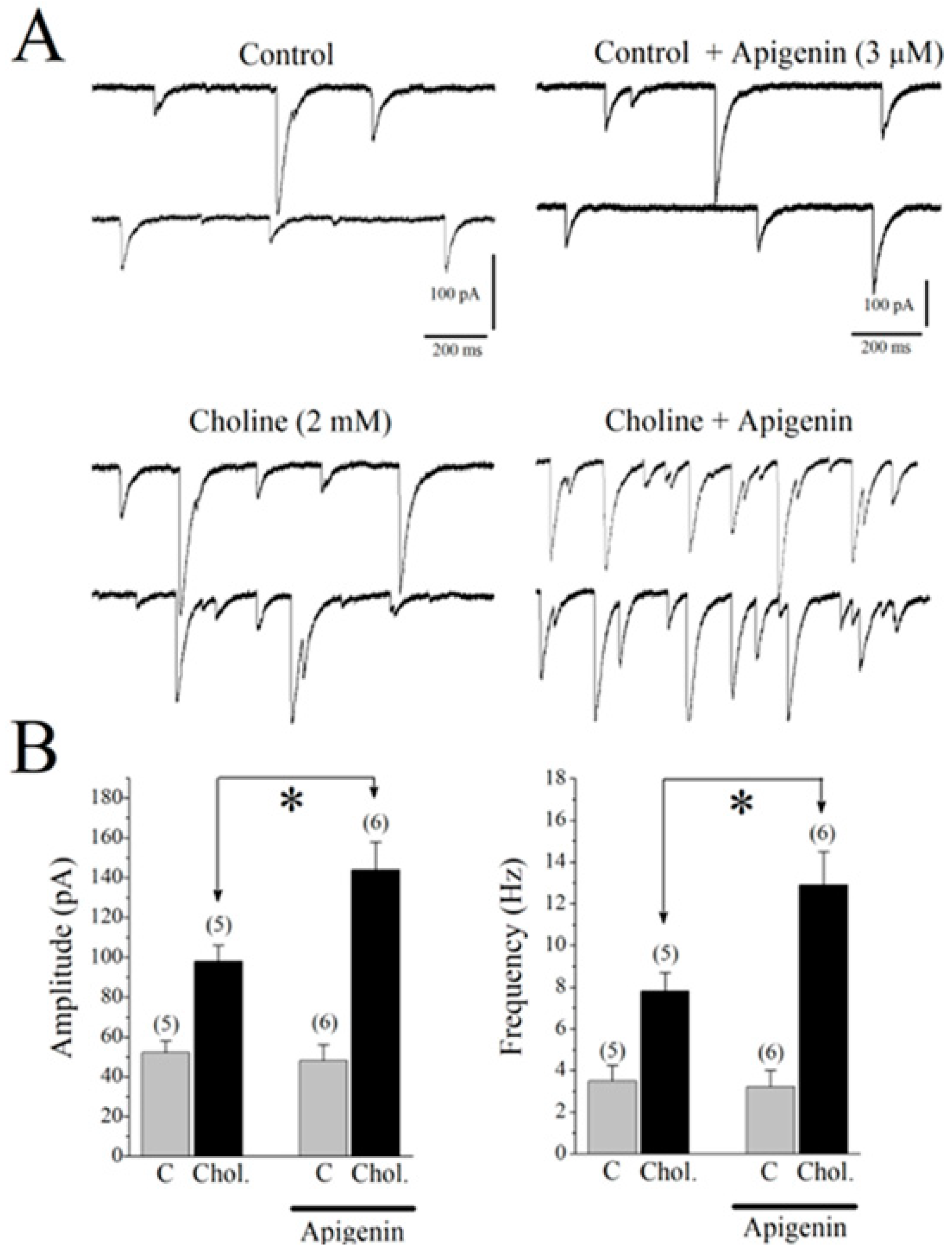
2.7. Effects of APG on Choline-Induced GABA Responses in CA1 Pyramidal Neurons of Mice Hippocampal Slices
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Experimental Plan
4.4. Behavior Tests
4.4.1. Marble Burying Test (MBT)
4.4.2. Cotton Shredding Test (CST)
4.4.3. Spontaneous Self-Grooming Behavior Test (SGT)
4.4.4. Open Field Test (OFT)
4.5. Biochemical Assessments
4.5.1. Catalase (CAT) Assay
4.5.2. Superoxide Dismutase (SOD) Assay
4.6. Recordings from Hippocampal Slices
4.7. Statistical Analyses
5. Conclusions
6. Study Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouzat, C.; Lasala, M.; Nielsen, B.E.; Corradi, J.; Esandi, M.D.C. Molecular function of alpha7 nicotinic receptors as drug targets. J. Physiol. 2018, 596, 1847–1861. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, V.; Yarkov, A.; Aliev, G. Positive modulators of the alpha7 nicotinic receptor against neuroinflammation and cognitive impairment in Alzheimer’s disease. Prog. Neurobiol. 2016, 144, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Almeida, L.E.; Spornick, N.A.; Kenyon, N.; Kamimura, S.; Khaibullina, A.; Nouraie, M.; Quezado, Z.M. Modulation of social deficits and repetitive behaviors in a mouse model of autism: The role of the nicotinic cholinergic system. Psychopharmacology 2015, 232, 4303–4316. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V., Jr.; Callahan, P.M. alpha7 nicotinic acetylcholine receptors as therapeutic targets in schizophrenia: Update on animal and clinical studies and strategies for the future. Neuropharmacology 2020, 170, 108053. [Google Scholar] [CrossRef]
- Deutsch, S.I.; Burket, J.A. An Evolving Therapeutic Rationale for Targeting the alpha7 Nicotinic Acetylcholine Receptor in Autism Spectrum Disorder. Curr. Top. Behav. Neurosci. 2020, 45, 167–208. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Jayaprakash, P.; Stark, H.; Lazewska, D.; Kiec-Kononowicz, K.; Sadek, B. Simultaneous Blockade of Histamine H3 Receptors and Inhibition of Acetylcholine Esterase Alleviate Autistic-Like Behaviors in BTBR T+ tf/J Mouse Model of Autism. Biomolecules 2020, 10, 1251. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Martin-Ruiz, C.; Graham, A.; Court, J.; Jaros, E.; Perry, R.; Iversen, P.; Bauman, M.; Perry, E. Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain 2002, 125, 1483–1495. [Google Scholar] [CrossRef]
- Martin-Ruiz, C.M.; Lee, M.; Perry, R.H.; Baumann, M.; Court, J.A.; Perry, E.K. Molecular analysis of nicotinic receptor expression in autism. Brain Res. Mol. Brain Res. 2004, 123, 81–90. [Google Scholar] [CrossRef]
- Eissa, N.; Sadeq, A.; Sasse, A.; Sadek, B. Role of Neuroinflammation in Autism Spectrum Disorder and the Emergence of Brain Histaminergic System. Lessons Also for BPSD? Front. Pharmacol. 2020, 11, 886. [Google Scholar] [CrossRef]
- Pardo, M.; Beurel, E.; Jope, R.S. Cotinine administration improves impaired cognition in the mouse model of Fragile X syndrome. Eur. J. Neurosci. 2017, 45, 490–498. [Google Scholar] [CrossRef]
- Oginsky, M.F.; Cui, N.; Zhong, W.; Johnson, C.M.; Jiang, C. Alterations in the cholinergic system of brain stem neurons in a mouse model of Rett syndrome. Am. J. Physiol. Cell Physiol. 2014, 307, C508–C520. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.L.; Adams, C.E.; Stevens, K.E.; Chow, K.H.; Freedman, R.; Patterson, P.H. The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain Behav. Immun. 2015, 46, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.S.; van Schalkwyk, G.I.; Lopez, M.O.; Volkmar, F.R.; Picciotto, M.R.; Sukhodolsky, D.G. An Exploratory Trial of Transdermal Nicotine for Aggression and Irritability in Adults with Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 2748–2757. [Google Scholar] [CrossRef] [PubMed]
- Yasui, D.H.; Scoles, H.A.; Horike, S.; Meguro-Horike, M.; Dunaway, K.W.; Schroeder, D.I.; Lasalle, J.M. 15q11.2–13.3 chromatin analysis reveals epigenetic regulation of CHRNA7 with deficiencies in Rett and autism brain. Hum. Mol. Genet. 2011, 20, 4311–4323. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, H.G.; Kusek, G.K.; Yang, M.; Phoenix, J.L.; Bolivar, V.J.; Crawley, J.N. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008, 7, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Abrams, D.N.; Zhang, J.Y.; Weber, M.D.; Katz, A.M.; Clarke, A.M.; Silverman, J.L.; Crawley, J.N. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiol. Behav. 2012, 107, 649–662. [Google Scholar] [CrossRef]
- McTighe, S.M.; Neal, S.J.; Lin, Q.; Hughes, Z.A.; Smith, D.G. The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex. PLoS ONE 2013, 8, e62189. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, R.F.; Tran, M.B.; Hogenkamp, D.J.; Ayala, N.L.; Johnstone, T.; Dunnigan, A.J.; Gee, T.K.; Gee, K.W. Allosteric modulation of nicotinic and GABAA receptor subtypes differentially modify autism-like behaviors in the BTBR mouse model. Neuropharmacology 2017, 126, 38–47. [Google Scholar] [CrossRef]
- Mahmood, H.M.; Aldhalaan, H.M.; Alshammari, T.K.; Alqasem, M.A.; Alshammari, M.A.; Albekairi, N.A.; AlSharari, S.D. The Role of Nicotinic Receptors in the Attenuation of Autism-Related Behaviors in a Murine BTBR T + tf/J Autistic Model. Autism Res. 2020, 13, 1311–1334. [Google Scholar] [CrossRef]
- Gzielo, K.; Nikiforuk, A. The Effects of Positive Allosteric Modulators of α7-nAChR on Social Play Behavior in Adolescent Rats Prenatally Exposed to Valproic Acid. Pharmaceuticals 2022, 15, 1417. [Google Scholar] [CrossRef]
- Melrose, J. The Potential of Flavonoids and Flavonoid Metabolites in the Treatment of Neurodegenerative Pathology in Disorders of Cognitive Decline. Antioxidants 2023, 12, 663. [Google Scholar] [CrossRef]
- Savino, R.; Medoro, A.; Ali, S.; Scapagnini, G.; Maes, M.; Davinelli, S. The Emerging Role of Flavonoids in Autism Spectrum Disorder: A Systematic Review. J. Clin. Med. 2023, 12, 3520. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Chagas, M.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef] [PubMed]
- Ximenis, M.; Mulet, J.; Sala, S.; Sala, F.; Criado, M.; Gonzalez-Muniz, R.; Perez de Vega, M.J. Natural Polyhydroxy Flavonoids, Curcuminoids, and Synthetic Curcumin Analogs as alpha7 nAChRs Positive Allosteric Modulators. Int. J. Mol. Sci. 2021, 22, 973. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, W.; Yang, K.S.; Sadek, B.; Oz, M. Apigenin and Structurally Related Flavonoids Allosterically Potentiate the Function of Human α7-Nicotinic Acetylcholine Receptors Expressed in SH-EP1 Cells. Cells 2021, 10, 1110. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Attia, S.M.; Alshammari, M.A.; Alzahrani, K.S.; Bakheet, S.A. Increased oxidative stress in the cerebellum and peripheral immune cells leads to exaggerated autism-like repetitive behavior due to deficiency of antioxidant response in BTBR T + tf/J mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Yui, K.; Kawasaki, Y.; Yamada, H.; Ogawa, S. Oxidative Stress and Nitric Oxide in Autism Spectrum Disorder and Other Neuropsychiatric Disorders. CNS Neurol. Disord. Drug Targets 2016, 15, 587–596. [Google Scholar] [CrossRef]
- Cheng, Q.; Yakel, J.L. The effect of alpha7 nicotinic receptor activation on glutamatergic transmission in the hippocampus. Biochem. Pharmacol. 2015, 97, 439–444. [Google Scholar] [CrossRef]
- Riedel, G.; Kang, S.H.; Choi, D.Y.; Platt, B. Scopolamine-induced deficits in social memory in mice: Reversal by donepezil. Behav. Brain Res. 2009, 204, 217–225. [Google Scholar] [CrossRef]
- Friedman, S.D.; Shaw, D.W.; Artru, A.A.; Dawson, G.; Petropoulos, H.; Dager, S.R. Gray and white matter brain chemistry in young children with autism. Arch. Gen. Psychiatry 2006, 63, 786–794. [Google Scholar] [CrossRef]
- Mukaetova-Ladinska, E.B. Silent lives: Why do we fail community-dwelling people with dementia? Age Ageing 2017, 46, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Horvathova, K.; Novotny, L.; Tothova, D.; Vachalkova, A. Determination of free radical scavenging activity of quercetin, rutin, luteolin and apigenin in H2O2-treated human ML cells K562. Neoplasma 2004, 51, 395–399. [Google Scholar] [PubMed]
- Liu, R.; Zhang, T.; Yang, H.; Lan, X.; Ying, J.; Du, G. The flavonoid apigenin protects brain neurovascular coupling against amyloid-beta25–35-induced toxicity in mice. J. Alzheimers Dis. 2011, 24, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Zou, S.; Shi, Y.; Mao, Q.; Chen, Y. Apigenin attenuates PM2.5-induced airway hyperresponsiveness and inflammation by down-regulating NF-kappaB in murine model of asthma. Int. J. Clin. Exp. Pathol. 2019, 12, 3700–3709. [Google Scholar] [PubMed]
- Thomas, S.D.; Jha, N.K.; Jha, S.K.; Sadek, B.; Ojha, S. Pharmacological and Molecular Insight on the Cardioprotective Role of Apigenin. Nutrients 2023, 15, 385. [Google Scholar] [CrossRef]
- Vargo, M.A.; Voss, O.H.; Poustka, F.; Cardounel, A.J.; Grotewold, E.; Doseff, A.I. Apigenin-induced-apoptosis is mediated by the activation of PKCdelta and caspases in leukemia cells. Biochem. Pharmacol. 2006, 72, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, J.L.; Liu, R.; Li, X.X.; Li, J.F.; Zhang, L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kregiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Alam, W.; Rocca, C.; Khan, H.; Hussain, Y.; Aschner, M.; De Bartolo, A.; Amodio, N.; Angelone, T.; Cheang, W.S. Current Status and Future Perspectives on Therapeutic Potential of Apigenin: Focus on Metabolic-Syndrome-Dependent Organ Dysfunction. Antioxidants 2021, 10, 1643. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Lv, Z.; Yu, M. LC-MS/MS Determination of Apigenin in Rat Plasma and Application to Pharmacokinetic Study. Curr. Pharm. Biotechnol. 2021, 22, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Yuan, Z.; Guo, W.; Meng, Y.; Cui, Y.; Kong, D.; Zhang, L.; Wang, N. LC-MS/MS determination and pharmacokinetic study of five flavone components after solvent extraction/acid hydrolysis in rat plasma after oral administration of Verbena officinalis L. extract. J. Ethnopharmacol. 2011, 135, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert. Opin. Drug Metab. Toxicol. 2017, 13, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J.; He, M.; Lee, A.; Cho, E. Apigenin Ameliorates Scopolamine-Induced Cognitive Dysfunction and Neuronal Damage in Mice. Molecules 2021, 26, 5192. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.F.; Buxhoeveden, D.; Gomez, J. Disruption in the inhibitory architecture of the cell minicolumn: Implications for autism. Neuroscientist 2003, 9, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Eissa, N.; Awad, M.A.; Jayaprakash, P.; Zhong, S.; Stolting, F.; Stark, H.; Sadek, B. The histamine H3R and dopamine D2R/D3R antagonist ST-713 ameliorates autism-like behavioral features in BTBR T+tf/J mice by multiple actions. Biomed. Pharmacother. 2021, 138, 111517. [Google Scholar] [CrossRef]
- Gaikwad, U.; Parle, M. Combination of aripiprazole and ethanol attenuates marble-burying behavior in mice. Acta Pol. Pharm. 2011, 68, 435–440. [Google Scholar] [PubMed]
- Burda, K.; Czubak, A.; Kus, K.; Nowakowska, E.; Ratajczak, P.; Zin, J. Influence of aripiprazole on the antidepressant, anxiolytic and cognitive functions of rats. Pharmacol. Rep. 2011, 63, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Citraro, R.; Davoli, A.; Gallelli, L.; Di Paola, E.D.; De Sarro, G. Ameliorating effects of aripiprazole on cognitive functions and depressive-like behavior in a genetic rat model of absence epilepsy and mild-depression comorbidity. Neuropharmacology 2013, 64, 371–379. [Google Scholar] [CrossRef]
- Abhishek, M.; Rubal, S.; Rohit, K.; Rupa, J.; Phulen, S.; Gurjeet, K.; Raj, S.A.; Manisha, P.; Alka, B.; Ramprasad, P.; et al. Neuroprotective effect of the standardised extract of Bacopa monnieri (BacoMind) in valproic acid model of autism spectrum disorder in rats. J. Ethnopharmacol. 2022, 293, 115199. [Google Scholar] [CrossRef]
- Bertolino, B.; Crupi, R.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Beneficial Effects of Co-Ultramicronized Palmitoylethanolamide/Luteolin in a Mouse Model of Autism and in a Case Report of Autism. CNS Neurosci. Ther. 2017, 23, 87–98. [Google Scholar] [CrossRef] [PubMed]
- de Mattos, B.D.S.; Soares, M.S.P.; Spohr, L.; Pedra, N.S.; Teixeira, F.C.; de Souza, A.A.; Stefanello, F.M.; Baldissarelli, J.; Gamaro, G.D.; Spanevello, R.M. Quercetin prevents alterations of behavioral parameters, delta-aminolevulinic dehydratase activity, and oxidative damage in brain of rats in a prenatal model of autism. Int. J. Dev. Neurosci. 2020, 80, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Elesawy, R.O.; El-Deeb, O.S.; Eltokhy, A.K.; Arakeep, H.M.; Ali, D.A.; Elkholy, S.S.; Kabel, A.M. Postnatal baicalin ameliorates behavioral and neurochemical alterations in valproic acid-induced rodent model of autism: The possible implication of sirtuin-1/mitofusin-2/ Bcl-2 pathway. Biomed. Pharmacother. 2022, 150, 112960. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Choi, T.Y.; Ryu, H.G.; Lee, D.; Lee, S.H.; Choi, S.Y.; Kim, K.T. Autism-like behavior caused by deletion of vaccinia-related kinase 3 is improved by TrkB stimulation. J. Exp. Med. 2017, 214, 2947–2966. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Sarma, S.J.; Marshall, B.L.; Liu, Y.; Kinkade, J.A.; Bellamy, M.M.; Mao, J.; Helferich, W.G.; Schenk, A.K.; Bivens, N.J.; et al. Developmental exposure of California mice to endocrine disrupting chemicals and potential effects on the microbiome-gut-brain axis at adulthood. Sci. Rep. 2020, 10, 10902. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, R.; Hajizadeh Moghaddam, A.; Zare, M. Hesperetin and it nanocrystals ameliorate social behavior deficits and oxido-inflammatory stress in rat model of autism. Int. J. Dev. Neurosci. 2018, 69, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Bhandari, R.; Kuhad, A. Effects of catechin on a rodent model of autism spectrum disorder: Implications for the role of nitric oxide in neuroinflammatory pathway. Psychopharmacology 2021, 238, 3249–3271. [Google Scholar] [CrossRef]
- Serra, D.; Henriques, J.F.; Sousa, F.J.; Laranjo, M.; Resende, R.; Ferreira-Marques, M.; de Freitas, V.; Silva, G.; Peca, J.; Dinis, T.C.P.; et al. Attenuation of Autism-like Behaviors by an Anthocyanin-Rich Extract from Portuguese Blueberries via Microbiota-Gut-Brain Axis Modulation in a Valproic Acid Mouse Model. Int. J. Mol. Sci. 2022, 23, 9259. [Google Scholar] [CrossRef]
- Tassinari, M.; Mottolese, N.; Galvani, G.; Ferrara, D.; Gennaccaro, L.; Loi, M.; Medici, G.; Candini, G.; Rimondini, R.; Ciani, E.; et al. Luteolin Treatment Ameliorates Brain Development and Behavioral Performance in a Mouse Model of CDKL5 Deficiency Disorder. Int. J. Mol. Sci. 2022, 23, 8719. [Google Scholar] [CrossRef]
- Trovo, L.; Fuchs, C.; De Rosa, R.; Barbiero, I.; Tramarin, M.; Ciani, E.; Rusconi, L.; Kilstrup-Nielsen, C. The green tea polyphenol epigallocatechin-3-gallate (EGCG) restores CDKL5-dependent synaptic defects in vitro and in vivo. Neurobiol. Dis. 2020, 138, 104791. [Google Scholar] [CrossRef]
- Angoa-Perez, M.; Kane, M.J.; Briggs, D.I.; Francescutti, D.M.; Kuhn, D.M. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J. Vis. Exp. 2013, 82, 50978. [Google Scholar] [CrossRef]
- Eissa, N.; Azimullah, S.; Jayaprakash, P.; Jayaraj, R.L.; Reiner, D.; Ojha, S.K.; Beiram, R.; Stark, H.; Lazewska, D.; Kiec-Kononowicz, K.; et al. The dual-active histamine H3 receptor antagonist and acetylcholine esterase inhibitor E100 ameliorates stereotyped repetitive behavior and neuroinflammmation in sodium valproate induced autism in mice. Chem. Biol. Interact. 2019, 312, 108775. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Azimullah, S.; Jayaprakash, P.; Jayaraj, R.L.; Reiner, D.; Ojha, S.K.; Beiram, R.; Stark, H.; Lazewska, D.; Kiec-Kononowicz, K.; et al. The Dual-Active Histamine H3 Receptor Antagonist and Acetylcholine Esterase Inhibitor E100 Alleviates Autistic-Like Behaviors and Oxidative Stress in Valproic Acid Induced Autism in Mice. Int. J. Mol. Sci. 2020, 21, 3996. [Google Scholar] [CrossRef] [PubMed]
- Dorninger, F.; Zeitler, G.; Berger, J. Nestlet Shredding and Nest Building Tests to Assess Features of Psychiatric Disorders in Mice. Bio-Protoc. 2020, 10, e3863. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Tolu, S.S.; Barkan, C.L.; Crawley, J.N. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology 2010, 35, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M. Assessing nest building in mice. Nat. Protoc. 2006, 1, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Stewart, A.M.; Song, C.; Berridge, K.C.; Graybiel, A.M.; Fentress, J.C. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 2016, 17, 45–59. [Google Scholar] [CrossRef]
- Eissa, N.; Venkatachalam, K.; Jayaprakash, P.; Falkenstein, M.; Dubiel, M.; Frank, A.; Reiner-Link, D.; Stark, H.; Sadek, B. The Multi-Targeting Ligand ST-2223 with Histamine H3 Receptor and Dopamine D2/D3 Receptor Antagonist Properties Mitigates Autism-Like Repetitive Behaviors and Brain Oxidative Stress in Mice. Int. J. Mol. Sci. 2021, 22, 1947. [Google Scholar] [CrossRef] [PubMed]
- Abrahim, N.N.; Kanthimathi, M.S.; Abdul-Aziz, A. Piper betle shows antioxidant activities, inhibits MCF-7 cell proliferation and increases activities of catalase and superoxide dismutase. BMC Complement. Altern. Med. 2012, 12, 220. [Google Scholar] [CrossRef]
- Somanadhan, B.; Varughese, G.; Palpu, P.; Sreedharan, R.; Gudiksen, L.; Smitt, U.W.; Nyman, U. An ethnopharmacological survey for potential angiotensin converting enzyme inhibitors from Indian medicinal plants. J. Ethnopharmacol. 1999, 65, 103–112. [Google Scholar] [CrossRef]
- Alzaabi, A.H.; Howarth, L.; El Nebrisi, E.; Syed, N.; Susan Yang, K.H.; Howarth, F.C.; Oz, M. Capsaicin inhibits the function of alpha7-nicotinic acetylcholine receptors expressed in Xenopus oocytes and rat hippocampal neurons. Eur. J. Pharmacol. 2019, 857, 172411. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, P.; Isaev, D.; Shabbir, W.; Lorke, D.E.; Sadek, B.; Oz, M. Curcumin Potentiates alpha7 Nicotinic Acetylcholine Receptors and Alleviates Autistic-Like Social Deficits and Brain Oxidative Stress Status in Mice. Int. J. Mol. Sci. 2021, 22, 7251. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.; Yang, K.S.; Isaev, D.; Nebrisi, E.E.; Syed, N.; Khan, N.; Howarth, C.F.; Sadek, B.; Oz, M. Thujone inhibits the function of alpha7-nicotinic acetylcholine receptors and impairs nicotine-induced memory enhancement in one-trial passive avoidance paradigm. Toxicology 2017, 384, 23–32. [Google Scholar] [CrossRef] [PubMed]
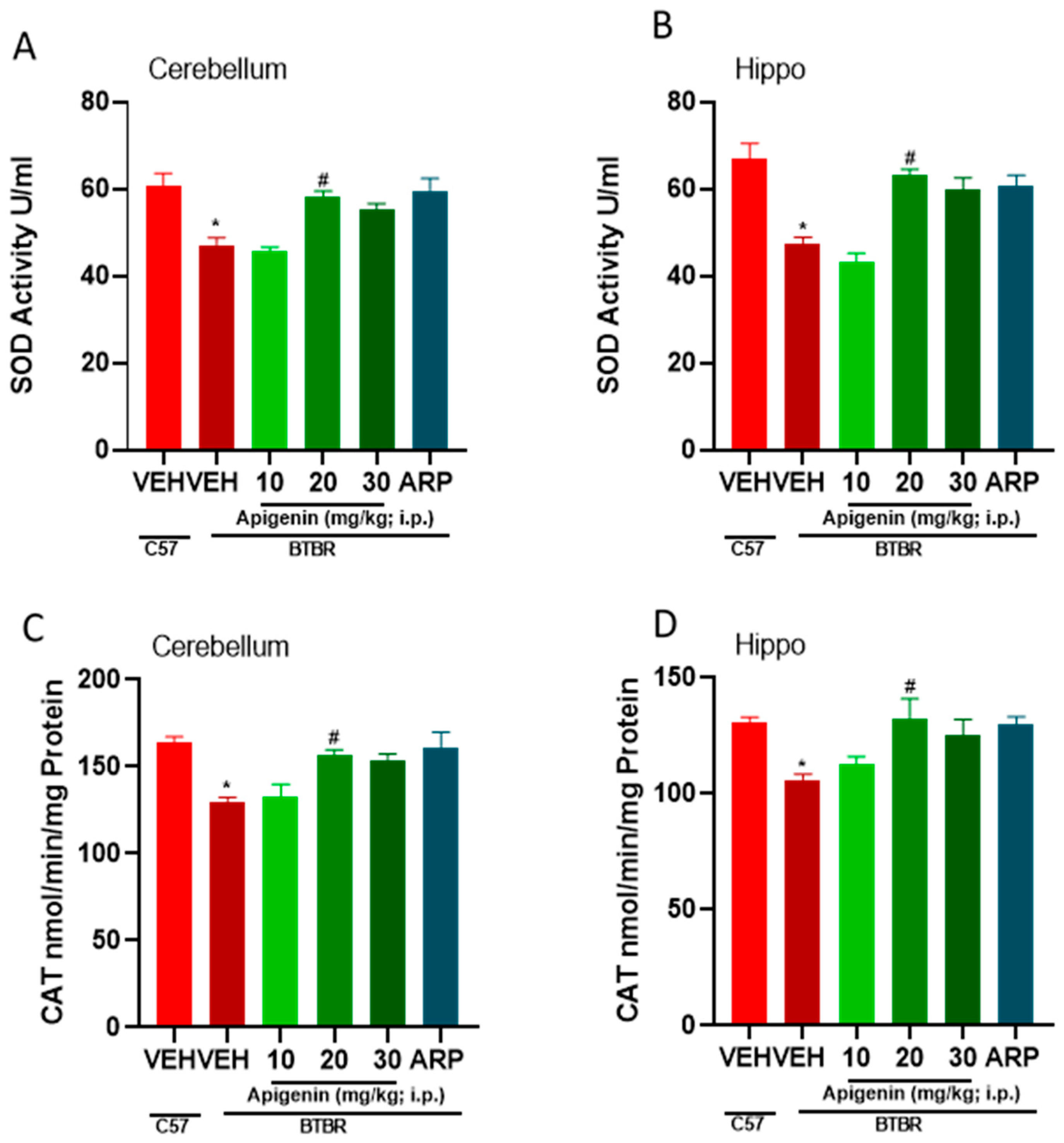
| Group | Center Time (s) | Periphery Time (s) | Travelled Distance (cm) |
|---|---|---|---|
| VEH (C57) | 31.64 ± 3.27 | 561.63 ± 4.91 | 2621 ± 197.57 |
| VEH (BTBR) | 66.15 ± 4.29 ** | 536.31 ± 7.27 ** | 4201 ± 171.23 ** |
| APG (10 mg/kg)/BTBR | 61.02 ± 5.63 | 539.69 ± 11.73 | 3979 ± 118.98 |
| APG (20 mg/kg)/BTBR | 58.971 ± 6.72 | 542.22 ± 10.28 | 4036 ± 191.26 |
| APG (30 mg/kg)/BTBR | 55.98 ± 6.97 | 535.92 ± 12.71 | 3878 ± 352.18 |
| ARP (1 mg/kg)/BTBR | 39.84 ± 7.21 # | 566.65 ± 9.89 | 4063 ± 181.09 |
| C57 Mice (VEH) | BTBR Mice | |||||
|---|---|---|---|---|---|---|
| (VEH) | APG (10 mg/kg) | APG (20 mg/kg) | APG (30 mg/kg) | ARP (1 mg/kg) | ||
| SOD | ||||||
| Hippocampus | 66.4 ± 5.4 | 49.5 ± 0.5 * | 43.36 ± 1.90 | 54.42 ± 1.54 | 49.74 ± 1.03 | 51.8 ± 2.0 |
| Cerebellum | 60.6 ± 3.1 | 49.7 ± 1.3 * | 45.83 ± 0.84 | 58.15 ± 1.40 # | 51.38 ± 2.13 | 56.4 ± 1.1 |
| CAT | ||||||
| Hippocampus | 131.7 ± 3.4 | 111.4 ± 4.8 * | 112.4 ± 3.60 | 117.7 ± 8.03 | 110.9 ± 5.41 | 118.3 ± 6.5 |
| Cerebellum | 156.7 ± 7.29 | 130. 7 ± 3.3 * | 139.5 ± 8.46 | 155.2 ± 1.602 # | 145.8 ± 3.22 | 159.2 ± 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayaprakash, P.; Isaev, D.; Yang, K.-H.S.; Beiram, R.; Oz, M.; Sadek, B. Apigenin Alleviates Autistic-like Stereotyped Repetitive Behaviors and Mitigates Brain Oxidative Stress in Mice. Pharmaceuticals 2024, 17, 482. https://doi.org/10.3390/ph17040482
Jayaprakash P, Isaev D, Yang K-HS, Beiram R, Oz M, Sadek B. Apigenin Alleviates Autistic-like Stereotyped Repetitive Behaviors and Mitigates Brain Oxidative Stress in Mice. Pharmaceuticals. 2024; 17(4):482. https://doi.org/10.3390/ph17040482
Chicago/Turabian StyleJayaprakash, Petrilla, Dmytro Isaev, Keun-Hang Susan Yang, Rami Beiram, Murat Oz, and Bassem Sadek. 2024. "Apigenin Alleviates Autistic-like Stereotyped Repetitive Behaviors and Mitigates Brain Oxidative Stress in Mice" Pharmaceuticals 17, no. 4: 482. https://doi.org/10.3390/ph17040482







