A Population Pharmacokinetic Study to Compare a Novel Empagliflozin L-Proline Formulation with Its Conventional Formulation in Healthy Subjects
Abstract
1. Introduction
2. Results
2.1. Demographics and Datasets
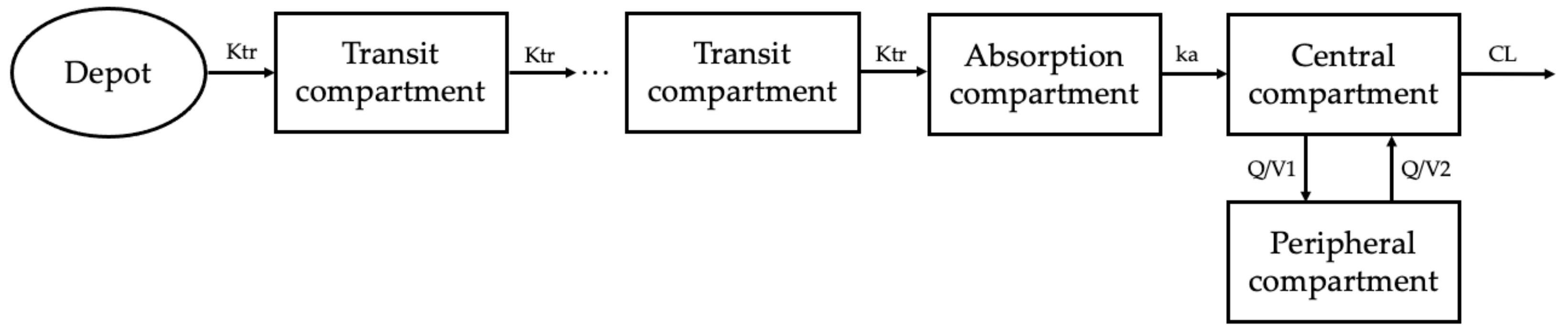
2.2. Base Model Development
2.3. Covariate Analysis
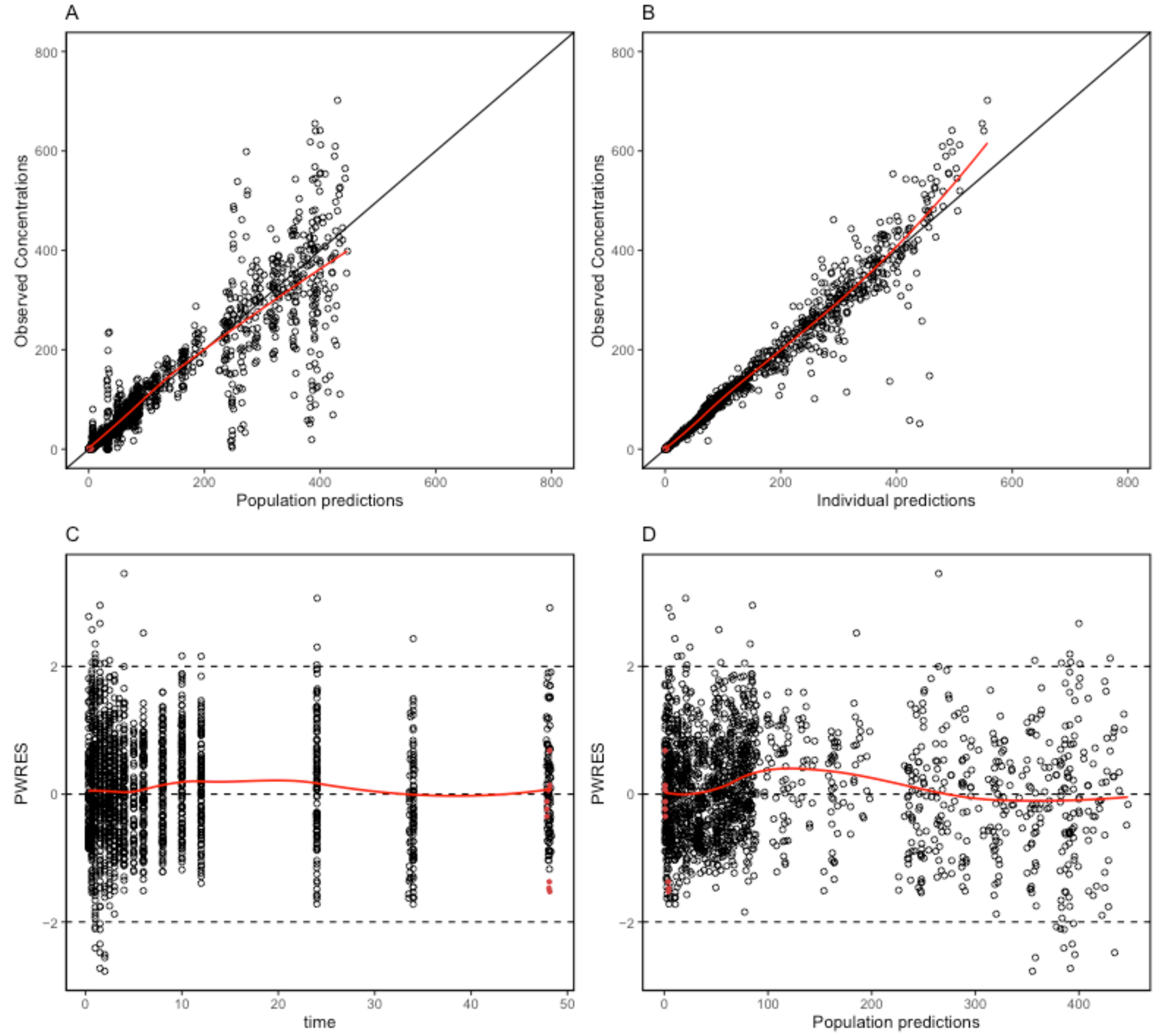
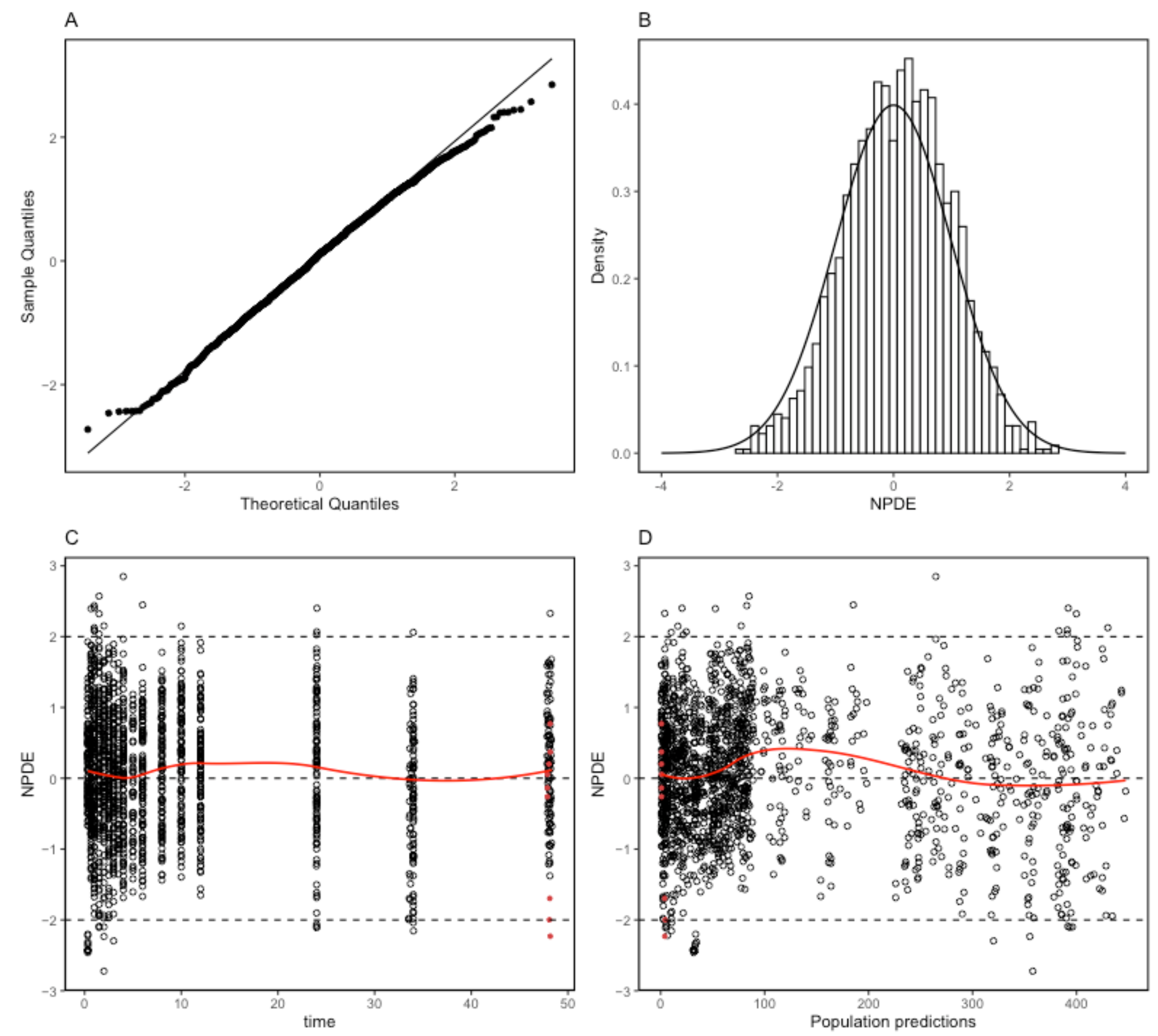
2.4. Model Evaluation
3. Discussion
4. Material and Methods
4.1. Data and Study Population
4.2. Sample Collection and Analytical Methodology
4.3. Base Model Development
4.4. Covariate Analysis
4.5. Final Model Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ha, K.H.; Lee, K.A.; Han, K.D.; Moon, M.K.; Kim, D.J. Diabetes screening in South Korea: A new estimate of the number needed to screen to detect diabetes. Korean J. Intern. Med. 2023, 38, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Marin-Penalver, J.J.; Martin-Timon, I.; Sevillano-Collantes, C.; Del Canizo-Gomez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Lee, S.Y.; Sohn, S.Y.; Ahn, J.; Han, K.; Lee, J.H. Type 2 Diabetes and Its Association With Psychiatric Disorders in Young Adults in South Korea. JAMA Netw. Open 2023, 6, e2319132. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Zielinski, A.; Roach, A.H.; Jende, J.A.; Householder, A.M.; Cole, E.E.; Atway, S.A.; Amornyard, M.; Accursi, M.L.; Shieh, S.W.; et al. Pharmacologic treatment of type 2 diabetes: Oral medications. Ann. Pharmacother. 2015, 49, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Meng, Q.; Zhang, H.; Zhang, J.J.; Shi, S.; Guan, Z.; Jiang, X.; Zhang, F.; Lei, H.; Lin, X. 760-P: Discovery of GSBR-1290, a Highly Potent, Orally Available, Novel Small Molecule GLP-1 Receptor Agonist. Diabetes 2023, 72, 760-P. [Google Scholar] [CrossRef]
- Rieg, T.; Vallon, V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia 2018, 61, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, O.N.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Pharmaceutical cocrystals: From serendipity to design to application. Drug Discov. Today 2019, 24, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Kondo, Y.; Ito, K.; Hasebe, M.; Satoh, S.; Terauchi, Y. Comparison of the effects of empagliflozin and glimepiride on endothelial function in patients with type 2 diabetes: A randomized controlled study. PLoS ONE 2022, 17, e0262831. [Google Scholar] [CrossRef] [PubMed]
- Hierro-Bujalance, C.; Garcia-Alloza, M. Empagliflozin reduces brain pathology in Alzheimer’s disease and type 2 diabetes. Neural Regen. Res. 2024, 19, 1189–1190. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Overberg, K.; Ghouse, Z.; Hollmann, M.W.; Weber, N.C.; Coronel, R.; Zuurbier, C.J. Empagliflozin mitigates cardiac hypertrophy through cardiac RSK/NHE-1 inhibition. Biomed. Pharmacother. 2024, 174, 116477. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Hu, C.; Zhu, C.; Wan, X.; Chen, C.; Ji, X.; Qin, Y.; Lu, L.; Guo, X. Empagliflozin alleviates the development of autoimmune myocarditis via inhibiting NF-kappaB-dependent cardiomyocyte pyroptosis. Biomed. Pharmacother. 2024, 170, 115963. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Yu, J.; Zhang, H.; Zhang, J. Empagliflozin attenuates inflammation levels in autoimmune myocarditis through the STAT3 pathway and macrophage phenotype transformation. Mol. Immunol. 2024, 167, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.; Deng, Y.; Li, Y.; Zhang, P.; Wang, Y.; Xu, B. Pharmacokinetics and bioequivalence of a generic empagliflozin tablet versus a brand-named product and the food effects in healthy Chinese subjects. Drug Dev. Ind. Pharm. 2020, 46, 1487–1494. [Google Scholar] [CrossRef]
- Ayoub, B.M.; Mowaka, S.; Elzanfaly, E.S.; Ashoush, N.; Elmazar, M.M.; Mousa, S.A. Pharmacokinetic Evaluation of Empagliflozin in Healthy Egyptian Volunteers Using LC-MS/MS and Comparison with Other Ethnic Populations. Sci. Rep. 2017, 7, 2583. [Google Scholar] [CrossRef] [PubMed]
- Sarashina, A.; Koiwai, K.; Seman, L.J.; Yamamura, N.; Taniguchi, A.; Negishi, T.; Sesoko, S.; Woerle, H.J.; Dugi, K.A. Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in healthy Japanese subjects. Drug Metab. Pharmacokinet. 2013, 28, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Seman, L.; Macha, S.; Nehmiz, G.; Simons, G.; Ren, B.; Pinnetti, S.; Woerle, H.J.; Dugi, K. Empagliflozin (BI 10773), a Potent and Selective SGLT2 Inhibitor, Induces Dose-Dependent Glucosuria in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2013, 2, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.V.; Shete, A.S.; Dabke, A.P.; Kulkarni, P.V.; Sakhare, S.S. Co-crystals: A novel approach to modify physicochemical properties of active pharmaceutical ingredients. Indian J. Pharm. Sci. 2009, 71, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Gadade, D.D.; Pekamwar, S.S. Pharmaceutical Cocrystals: Regulatory and Strategic Aspects, Design and Development. Adv. Pharm. Bull. 2016, 6, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Shan, N.; Perry, M.L.; Weyna, D.R.; Zaworotko, M.J. Impact of pharmaceutical cocrystals: The effects on drug pharmacokinetics. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1255–1271. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical cocrystals: A review of preparations, physicochemical properties and applications. Acta Pharm. Sin. B 2021, 11, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Siahi-Shadbad, M.; Adibkia, K.; Barzegar-Jalali, M. Recent advances in improving oral drug bioavailability by cocrystals. Bioimpacts 2018, 8, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; He, Z.; Zhang, Y.; Fan, Q.; Feng, N. Naringenin Cocrystals Prepared by Solution Crystallization Method for Improving Bioavailability and Anti-hyperlipidemia Effects. AAPS PharmSciTech 2019, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, Z.; Pan, J.; Zhang, S.; Zhou, W. A concise and practical stereoselective synthesis of ipragliflozin L-proline. Beilstein J. Org. Chem. 2017, 13, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Woo, M.R.; Cho, J.H.; Kim, Y.I.; Choi, H.G. Novel dapagliflozin di-L-proline cocrystal-loaded tablet: Preparation, physicochemical characterization, and pharmacokinetics in beagle dogs and mini-pigs. Pharm. Dev. Technol. 2022, 27, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Chung, J.Y.; Yu, K.S.; Park, S.J.; Lee, S. Pharmacokinetic Comparison Between a Fixed-Dose Combination of Empagliflozin L-Proline/Metformin and Empagliflozin/Metformin in Healthy Korean Subjects. Clin. Pharmacol. Drug Dev. 2023, 12, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Bae, S.; Yoon, D.Y.; Park, S.J.; Oh, J.; Cho, J.Y.; Yu, K.S. Comparison of the Pharmacokinetics, Safety, and Tolerability of Two Empagliflozin Formulations in Healthy Korean Subjects. Drug Des. Devel. Ther. 2023, 17, 2137–2145. [Google Scholar] [CrossRef]
- Zandvliet, A.S.; Schellens, J.H.; Beijnen, J.H.; Huitema, A.D. Population pharmacokinetics and pharmacodynamics for treatment optimization in clinical oncology. Clin. Pharmacokinet. 2008, 47, 487–513. [Google Scholar] [CrossRef] [PubMed]
- Aguillon, A.R.; Mascarello, A.; Segretti, N.D.; de Azevedo, H.F.Z.; Guimaraes, C.R.W.; Miranda, L.S.M.; de Souza, R.O.M.A. Synthetic Strategies toward SGLT2 Inhibitors. Org. Process Res. Dev. 2018, 22, 467–488. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Pharmaceutical cocrystals: Walking the talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef]
- Shinozaki, T.; Ono, M.; Higashi, K.; Moribe, K. A Novel Drug-Drug Cocrystal of Levofloxacin and Metacetamol: Reduced Hygroscopicity and Improved Photostability of Levofloxacin. J. Pharm. Sci. 2019, 108, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Kale, D.P.; Ugale, B.; Nagaraja, C.M.; Dubey, G.; Bharatam, P.V.; Bansal, A.K. Molecular Basis of Water Sorption Behavior of Rivaroxaban-Malonic Acid Cocrystal. Mol. Pharm. 2019, 16, 2980–2991. [Google Scholar] [CrossRef]
- Scheen, A.J. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin. Pharmacokinet. 2014, 53, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Riggs, M.M.; Staab, A.; Seman, L.; MacGregor, T.R.; Bergsma, T.T.; Gastonguay, M.R.; Macha, S. Population pharmacokinetics of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with type 2 diabetes. J. Clin. Pharmacol. 2013, 53, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Baron, K.T.; Macha, S.; Broedl, U.C.; Nock, V.; Retlich, S.; Riggs, M. Population Pharmacokinetics and Exposure-Response (Efficacy and Safety/Tolerability) of Empagliflozin in Patients with Type 2 Diabetes. Diabetes Ther. 2016, 7, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Mondick, J.; Riggs, M.; Kaspers, S.; Soleymanlou, N.; Marquard, J.; Nock, V. Population Pharmacokinetic-Pharmacodynamic Analysis to Characterize the Effect of Empagliflozin on Renal Glucose Threshold in Patients With Type 1 Diabetes Mellitus. J. Clin. Pharmacol. 2018, 58, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Riggs, M.M.; Seman, L.J.; Staab, A.; MacGregor, T.R.; Gillespie, W.; Gastonguay, M.R.; Woerle, H.J.; Macha, S. Exposure-response modelling for empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in patients with type 2 diabetes. Br. J. Clin. Pharmacol. 2014, 78, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Kristoffersson, A.N.; Friberg, L.E.; Nyberg, J. Inter occasion variability in individual optimal design. J. Pharmacokinet. Pharmacodyn. 2015, 42, 735–750. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services Food and Drug Administration. Population Pharmacokinetics Guidance for Industry; U.S. Department of Health and Human Services Food and Drug Administration: White Oak, MD, USA, 2022. [Google Scholar]
- Lindstrom, M.L.; Bates, D.M. Nonlinear mixed effects models for repeated measures data. Biometrics 1990, 46, 673–687. [Google Scholar] [CrossRef]
- Sheiner, L.B.; Ludden, T.M. Population pharmacokinetics/dynamics. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Savic, R.M.; Mentre, F.; Lavielle, M. Implementation and evaluation of the SAEM algorithm for longitudinal ordered categorical data with an illustration in pharmacokinetics-pharmacodynamics. AAPS J. 2011, 13, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, J.A.; Jonsson, S.; Karlsson, M.O.; Nielsen, E.I. Handling interoccasion variability in model-based dose individualization using therapeutic drug monitoring data. Br. J. Clin. Pharmacol. 2019, 85, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Ayral, G.; Si Abdallah, J.F.; Magnard, C.; Chauvin, J. A novel method based on unbiased correlations tests for covariate selection in nonlinear mixed effects models: The COSSAC approach. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 318–329. [Google Scholar] [CrossRef] [PubMed]

| Physicochemical Parameters (Units) | Study A (n = 27) | Study B (n = 27) | All (n = 54) | |||
|---|---|---|---|---|---|---|
| Median [Min–Max] | Mean ± SD | Median [Min–Max] | Mean ± SD | Median [Min–Max] | Mean ± SD | |
| Age (Year) | 29 [20–50] | 30 ± 7.38 | 27 [21–47] | 30.22 ± 7.71 | 27.5 [20–50] | 30.26 ± 7.48 |
| Height (cm) | 171.2 [156.3–186.3] | 170.83 ± 7.01 | 172.4 [155.2–186.4] | 171.60 ± 7.64 | 171.5 [155.2–186.4] | 171.21 ± 7.28 |
| Weight (kg) | 72.3 [55.6–82.1] | 69.62 ± 7.74 | 70.1 [56.1–83.4] | 69.76 ± 8.05 | 72.1 [55.6–83.4] | 69.69 ± 7.82 |
| BMI (kg/m2) | 24.2 [19.5–26.4] | 23.84 ± 2.15 | 23.9 [19.8–26.9] | 23.65 ± 1.95 | 24.1 [19.5–26.9] | 23.75 ± 2.03 |
| Glucose (mg/dL) | 87 [79–96] | 87.33 ± 5.67 | 87 [78–101] | 88.07 ± 6.34 | 87 [78–101] | 87.70 ± 5.97 |
| Protein (g/dL) | 6.7 [6.2–7.2] | 6.67 ± 0.25 | 6.7 [6.3–7.4] | 6.71 ± 0.26 | 6.7 [6.2–7.4] | 6.69 ± 0.25 |
| ALP (IU/L) | 52 [30–83] | 54.44 ± 14.19 | 59 [36–81] | 58.63 ± 11.98 | 55.5 [30–83] | 56.54 ± 13.18 |
| AST (IU/L) | 17 [12–27] | 17.37 ± 4.1 | 17 [13–35] | 17.85 ± 4.88 | 17 [12–35] | 17.61 ± 4.47 |
| ALT (IU/L) | 17 [8–47] | 18.56 ± 8.93 | 16 [7–64] | 19.44 ± 12.20 | 16.5 [7–64] | 19.00 ± 10.60 |
| LDH (IU/L) | 147 [128–219] | 155.11 ± 23.04 | 147 [113–185] | 145 ± 19.80 | 147 [113–219] | 150.06 ± 21.88 |
| eGFR (mL/min/1.73 m2) | 115 [74.4–143.9] | 111.57 ± 15.79 | 111.9 [74.2–138.7] | 110.77 ± 15.74 | 112.35 [74.2–143.9] | 111.17 ± 15.62 |
| Population Parameter (Unit) | Value | RSE (%) | Median of Bootstrap * (95% CI) | Shrinkage a |
|---|---|---|---|---|
| Fixed effects | ||||
| Ktr (h−1) | 9.19 | 12.4 | 8.94 (6.766–11.728) | 3.79% |
| Mtt (h) | 0.63 | 4.26 | 0.64 (0.526–0.725) | −0.239% |
| Ka (h−1) | 0.26 | 3.42 | 0.25 (0.23–0.275) | 1.7% |
| CL (L/h) | 8.21 | 1.78 | 8.21 (7.945–8.465) | 1.28% |
| V1 (L) | 0.6 | 6.67 | 0.64 (0–1.889) | |
| V2 (L) | 44.6 | 2.51 | 43.93 (41.255–47.171) | 1.09% |
| Q (L/h) | 4.92 | 5.09 | 4.66 (3.979–5.456) | |
| βCL_logWT | 0.64 | 24.5 | 0.64 (0.366–0.926) | |
| βV2_logWT | 0.57 | 30.3 | 0.54 (0.221–0.851) | |
| Interindividual variability (IIV) b | ||||
| ωKa (%) | 0.17 (17.12) | 10.8 | 0.16 (0.116–0.208) | |
| ωCL (%) | 0.12 (12.04) | 10.9 | 0.12 (0.101–0.141) | |
| ωV2 (%) | 0.13 (13.06) | 12.0 | 0.13 (0.1–0.172) | |
| Interoccasion Variability (IOV) b | ||||
| γKtr (%) | 1.17 (171.20) | 7.43 | 1.11 (0.858–1.334) | |
| γMtt (%) | 0.42 (43.92) | 7.15 | 0.44 (0.359–0.528) | |
| γKa (%) | 0.029 (2.90) | 34.3 | 0.04 (0.023–0.062) | |
| γCL (%) | 0.043 (4.30) | 16.7 | 0.04 (0.025–0.06) | |
| Residual variability | ||||
| b | 0.16 | 2.01 | 0.16 (0.141–0.175) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Yu, K.-S.; Nam, D.H.; Oh, J. A Population Pharmacokinetic Study to Compare a Novel Empagliflozin L-Proline Formulation with Its Conventional Formulation in Healthy Subjects. Pharmaceuticals 2024, 17, 522. https://doi.org/10.3390/ph17040522
Jiang X, Yu K-S, Nam DH, Oh J. A Population Pharmacokinetic Study to Compare a Novel Empagliflozin L-Proline Formulation with Its Conventional Formulation in Healthy Subjects. Pharmaceuticals. 2024; 17(4):522. https://doi.org/10.3390/ph17040522
Chicago/Turabian StyleJiang, Xu, Kyung-Sang Yu, Dong Hyuk Nam, and Jaeseong Oh. 2024. "A Population Pharmacokinetic Study to Compare a Novel Empagliflozin L-Proline Formulation with Its Conventional Formulation in Healthy Subjects" Pharmaceuticals 17, no. 4: 522. https://doi.org/10.3390/ph17040522
APA StyleJiang, X., Yu, K.-S., Nam, D. H., & Oh, J. (2024). A Population Pharmacokinetic Study to Compare a Novel Empagliflozin L-Proline Formulation with Its Conventional Formulation in Healthy Subjects. Pharmaceuticals, 17(4), 522. https://doi.org/10.3390/ph17040522






