Insulin Treatment Does Not Prevent EARLY Autonomic Cardiovascular and Diastolic Dysfunctions in Streptozotocin-Induced Diabetic Rats †
Abstract
:1. Introduction
2. Results
2.1. Glycemia, Insulin Levels, and Body Mass Evaluations
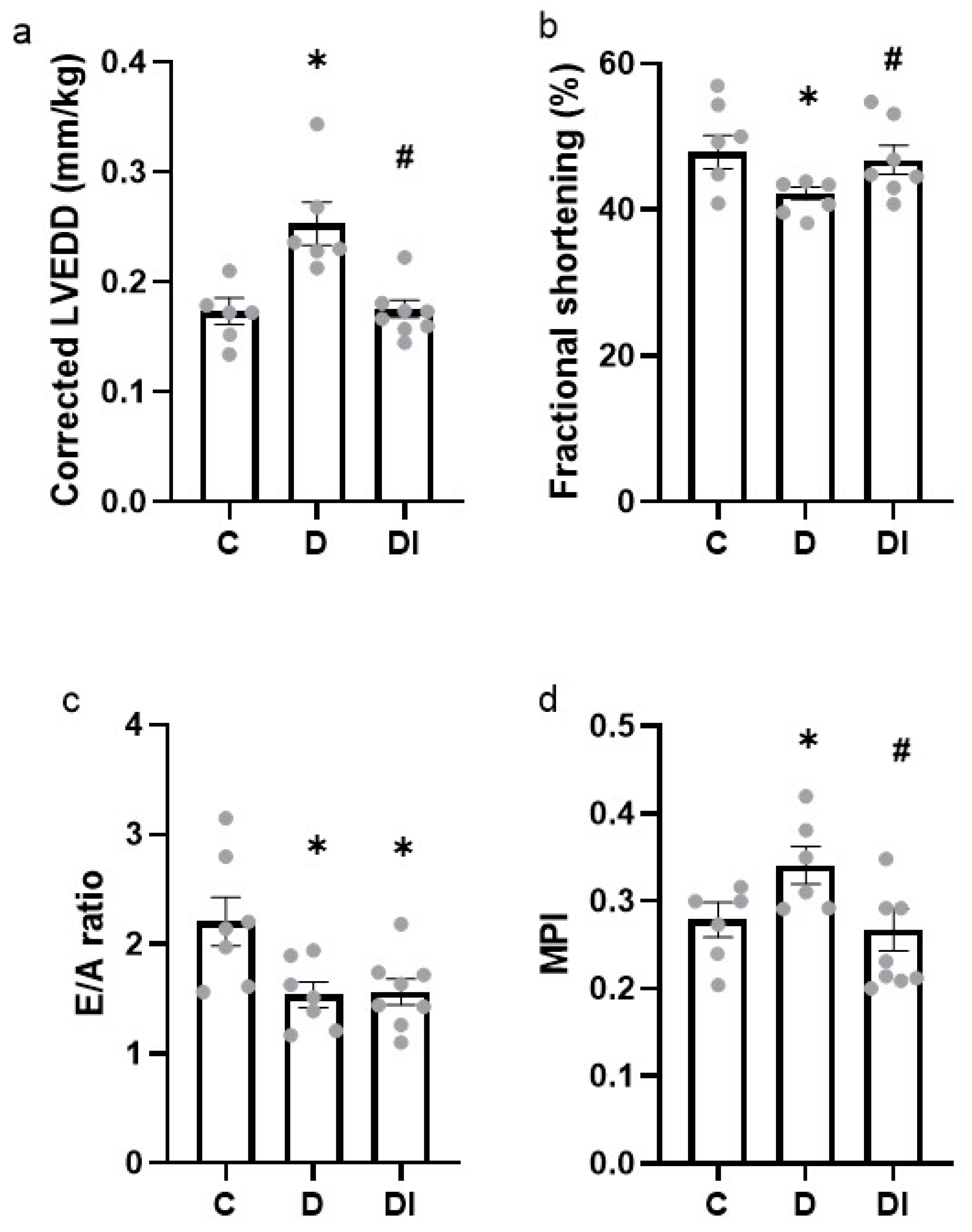
2.2. Echocardiography
2.2.1. Cardiac Morphometry
2.2.2. Systolic Function
2.2.3. Diastolic Function
2.2.4. Global Function
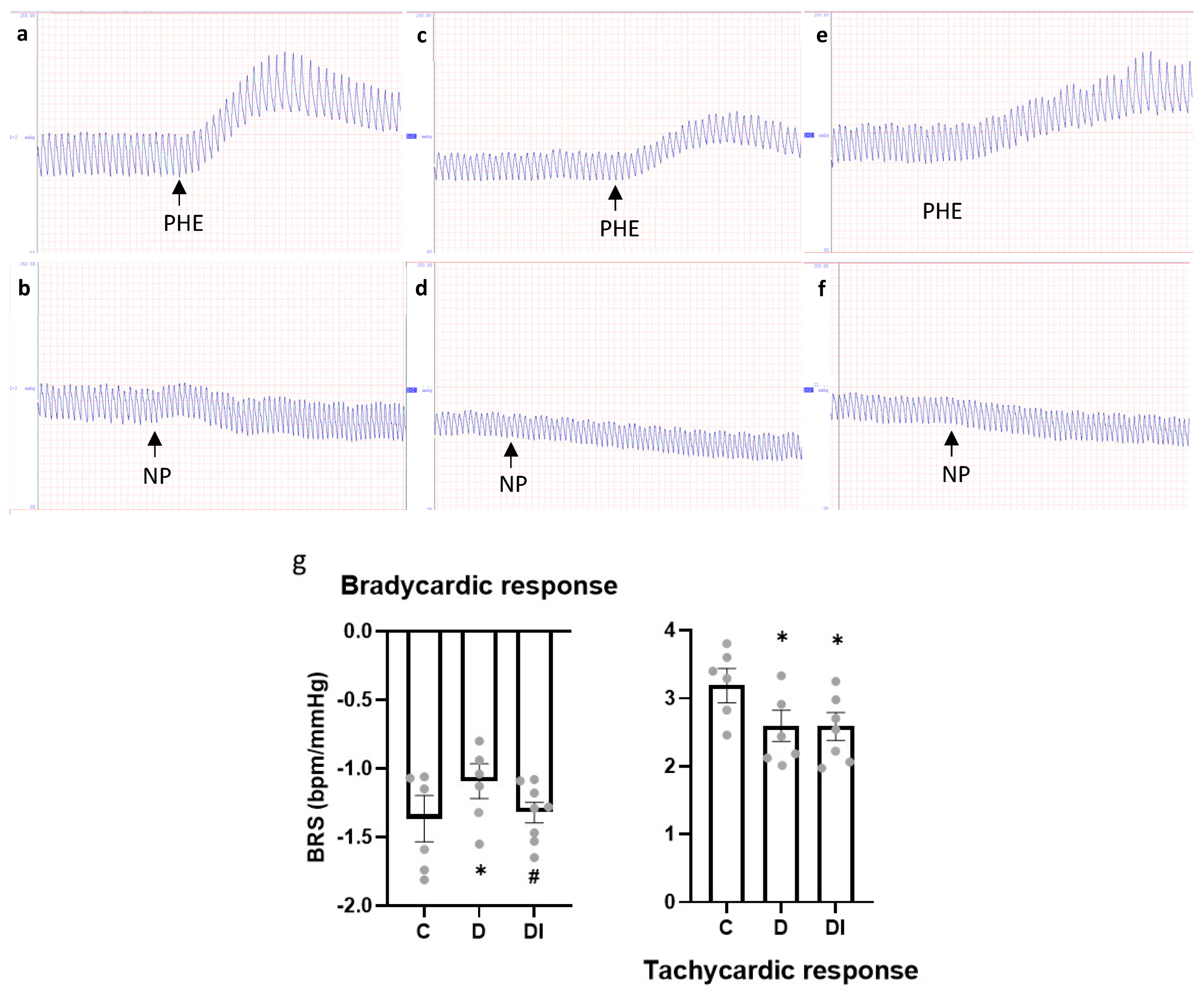
2.3. Hemodynamic and Baroreflex Sensitivity Assessment
2.4. Autonomic Control of Heart Rate
2.5. Cardiovascular Autonomic Modulation
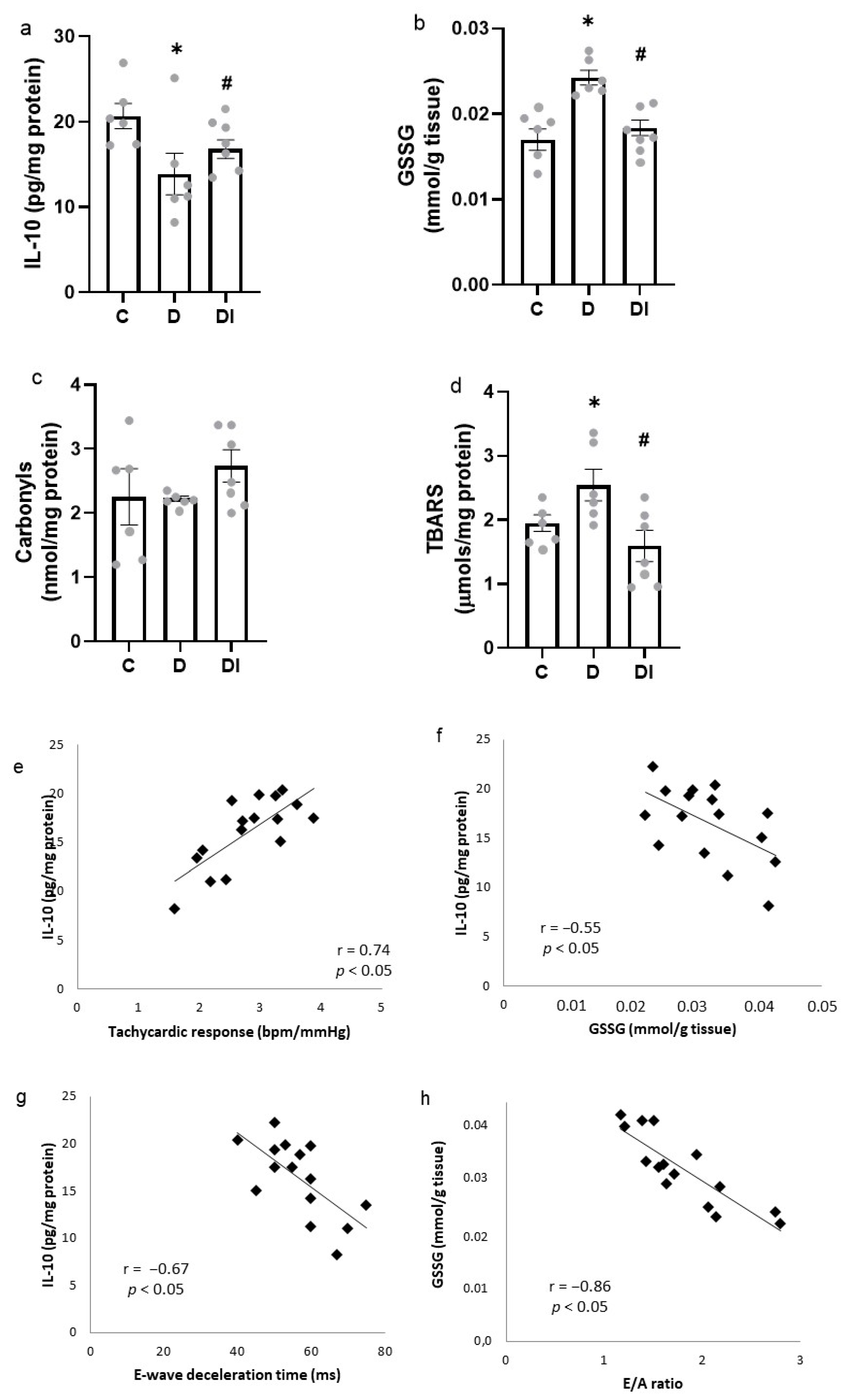
2.6. Inflammatory Mediators
2.7. Oxidative Stress Analysis
2.8. Pearson Correlation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Diabetes Induction and Evaluation of Glycemia, Insulin Levels, and Body Mass
4.3. Insulin Replacement
4.4. Echocardiography
4.5. Hemodynamic and Baroreflex Sensitivity Assessment
4.6. Autonomic Control of Heart Rate
4.7. Cardiovascular Autonomic Modulation
4.8. Tissue Preparation
4.9. Inflammatory Mediators
4.10. Oxidative Stress Analysis
4.10.1. Thiobarbituric Acid Reactive Substances (TBARS) Assay
4.10.2. Protein Oxidation by Carbonyl Assay
4.11. Glutathione Redox Balance
4.12. Antioxidant Enzyme Activities
4.13. Total Antioxidant Capacity (TRAP) Assay
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malmberg, K.; Yusuf, S.; Gerstein, H.C.; Brown, J.; Zhao, F.; Hunt, D.; Piegas, L.; Calvin, J.; Keltai, M.; Budaj, A. Impact of Diabetes on Long-Term Prognosis in Patients with Unstable Angina and Non-Q-Wave Myocardial Infarction: Results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation 2000, 102, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Laing, S.P.; Swerdlow, A.J.; Slater, S.D.; Burden, A.C.; Morris, A.; Waugh, N.R.; Gatling, W.; Bingley, P.J.; Patterson, C.C. Mortality from Heart Disease in a Cohort of 23,000 Patients with Insulin-Treated Diabetes. Diabetologia 2003, 46, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.; Svensson, A.-M.; Kosiborod, M.; Gudbjörnsdottir, S.; Pivodic, A.; Wedel, H.; Dahlqvist, S.; Clements, M.; Rosengren, A. Glycemic Control and Excess Mortality in Type 1 Diabetes. N. Engl. J. Med. 2014, 371, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Snell-Bergeon, J.K.; Maahs, D.M. Diabetes: Elevated Risk of Mortality in Type 1 Diabetes Mellitus. Nat. Rev. Endocrinol. 2015, 11, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Norhammar, A.; Malmberg, K. Heart Failure and Glucose Abnormalities: An Increasing Combination with Poor Functional Capacity and Outcome. Eur. Heart J. 2000, 21, 1293–1294. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, S.; Cai, L. Diabetic Cardiomyopathy and Its Mechanisms: Role of Oxidative Stress and Damage. J. Diabetes Investig. 2014, 5, 623–634. [Google Scholar] [CrossRef]
- Yagihashi, S.; Sima, A.A. Diabetic Autonomic Neuropathy in BB Rat. Ultrastructural and Morphometric Changes in Parasympathetic Nerves. Diabetes 1986, 35, 733–743. [Google Scholar] [CrossRef]
- Monckton, G.; Pehowich, E. Autonomic Neuropathy in the Streptozotocin Diabetic Rat. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 1980, 7, 135–142. [Google Scholar] [CrossRef]
- Wichi, R.; Malfitano, C.; Rosa, K.; De Souza, S.B.; Salemi, V.; Mostarda, C.; De Angelis, K.; Irigoyen, M.C. Noninvasive and Invasive Evaluation of Cardiac Dysfunction in Experimental Diabetes in Rodents. Cardiovasc. Diabetol. 2007, 6, 14. [Google Scholar] [CrossRef]
- De Angelis, K.L.; Oliveira, A.R.; Dall’Ago, P.; Peixoto, L.R.; Gadonski, G.; Lacchini, S.; Fernandes, T.G.; Irigoyen, M.C. Effects of Exercise Training on Autonomic and Myocardial Dysfunction in Streptozotocin-Diabetic Rats. Braz. J. Med. Biol. Res. 2000, 33, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Harthmann, A.D.; De Angelis, K.; Costa, L.P.; Senador, D.; Schaan, B.D.; Krieger, E.M.; Irigoyen, M.-C. Exercise Training Improves Arterial Baro- and Chemoreflex in Control and Diabetic Rats. Auton. Neurosci. Basic Clin. 2007, 133, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R.; Evans, G.W.; Gerstein, H.C.; Fonseca, V.; Fleg, J.L.; Hoogwerf, B.J.; Genuth, S.; Grimm, R.H.; Corson, M.A.; Prineas, R.; et al. Effects of Cardiac Autonomic Dysfunction on Mortality Risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. Diabetes Care 2010, 33, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. Physiology and Immunology of the Cholinergic Antiinflammatory Pathway. J. Clin. Investig. 2007, 117, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, S.J.; Levin, D.; Looker, H.C.; Lindsay, R.S.; Wild, S.H.; Joss, N.; Leese, G.; Leslie, P.; McCrimmon, R.J.; Metcalfe, W.; et al. Estimated Life Expectancy in a Scottish Cohort with Type 1 Diabetes, 2008–2010. JAMA 2015, 313, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Mameli, C.; Mazzantini, S.; Ben Nasr, M.; Fiorina, P.; Scaramuzza, A.E.; Zuccotti, G.V. Explaining the Increased Mortality in Type 1 Diabetes. World J. Diabetes 2015, 6, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Le Douairon Lahaye, S.; Rebillard, A.; Zguira, M.S.; Malardé, L.; Saïag, B.; Gratas-Delamarche, A.; Carré, F.; Bekono, F.R. Effects of Exercise Training Combined with Insulin Treatment on Cardiac NOS1 Signaling Pathways in Type 1 Diabetic Rats. Mol. Cell Biochem. 2011, 347, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Schaible, T.F.; Malhotra, A.; Bauman, W.A.; Scheuer, J. Left Ventricular Function after Chronic Insulin Treatment in Diabetic and Normal Rats. J. Mol. Cell Cardiol. 1983, 15, 445–458. [Google Scholar] [CrossRef]
- Tahiliani, A.G.; Lopaschuk, G.D.; McNeill, J.H. Effect of Insulin Treatment on Long-Term Diabetes-Induced Alteration of Myocardial Function. Gen. Pharmacol. 1984, 15, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Tate, M.; Deo, M.; Cao, A.H.; Hood, S.G.; Huynh, K.; Kiriazis, H.; Du, X.-J.; Julius, T.L.; Figtree, G.A.; Dusting, G.J.; et al. Insulin Replacement Limits Progression of Diabetic Cardiomyopathy in the Low-Dose Streptozotocin-Induced Diabetic Rat. Diabetes Vasc. Dis. Res. 2017, 14, 423–433. [Google Scholar] [CrossRef]
- Diaz-Valencia, P.A.; Bougnères, P.; Valleron, A.-J. Global Epidemiology of Type 1 Diabetes in Young Adults and Adults: A Systematic Review. BMC Public Health 2015, 15, 255. [Google Scholar] [CrossRef]
- Huynh, K.; Bernardo, B.C.; McMullen, J.R.; Ritchie, R.H. Diabetic Cardiomyopathy: Mechanisms and New Treatment Strategies Targeting Antioxidant Signaling Pathways. Pharmacol. Ther. 2014, 142, 375–415. [Google Scholar] [CrossRef]
- Schilling, J.D.; Mann, D.L. Diabetic Cardiomyopathy: Bench to Bedside. Heart Fail. Clin. 2012, 8, 619–631. [Google Scholar] [CrossRef]
- Maeda, C.Y.; Fernandes, T.G.; Timm, H.B.; Irigoyen, M.C. Autonomic Dysfunction in Short-Term Experimental Diabetes. Hypertension 1995, 26, 1100–1104. [Google Scholar] [CrossRef]
- De Angelis, K.; Schaan, B.D.; Maeda, C.Y.; Dall’Ago, P.; Wichi, R.B.; Irigoyen, M.C. Cardiovascular Control in Experimental Diabetes. Braz. J. Med. Biol. Res. 2002, 35, 1091–1100. [Google Scholar] [CrossRef]
- Mostarda, C.; Rogow, A.; Silva, I.C.M.; De La Fuente, R.N.; Jorge, L.; Rodrigues, B.; Heeren, M.V.; Caldini, E.G.; De Angelis, K.; Irigoyen, M.C. Benefits of Exercise Training in Diabetic Rats Persist after Three Weeks of Detraining. Auton. Neurosci. Basic Clin. 2009, 145, 11–16. [Google Scholar] [CrossRef]
- Irigoyen, M.-C.; Paulini, J.; Flores, L.J.F.; Flues, K.; Bertagnolli, M.; Moreira, E.D.; Consolim-Colombo, F.; Belló-Klein, A.; De Angelis, K. Exercise Training Improves Baroreflex Sensitivity Associated with Oxidative Stress Reduction in Ovariectomized Rats. Hypertension 2005, 46, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Freitas, S.C.; Dourado, P.M.; Sanches, I.C.; Machi, J.F.; Irigoyen, M.C.; De angelis, K. Abstract 16595: Insulin Replacement Attenuates Autonomic Impairment but Did Not Prevent Early Diastolic Dysfunction in a Model of Type 1 Diabetes. Circulation 2015, 132, A16595. [Google Scholar] [CrossRef]
- Irigoyen, M.-C.; De Angelis, K.; Dos Santos, F.; Dartora, D.R.; Rodrigues, B.; Consolim-Colombo, F.M. Hypertension, Blood Pressure Variability, and Target Organ Lesion. Curr. Hypertens. Rep. 2016, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, V.E.; Dragoumanis, C.; Theodorou, V.; Gargaretas, C.; Pneumatikos, I. Relation of Heart Rate Variability to Serum Levels of C-Reactive Protein, Interleukin 6, and 10 in Patients with Sepsis and Septic Shock. J. Crit. Care 2009, 24, 625.e1–625.e7. [Google Scholar] [CrossRef]
- Malfitano, C.; Barboza, C.A.; Mostarda, C.; da Palma, R.K.; Santos, C.P.d.; Rodrigues, B.; Freitas, S.C.F.; Bello-Klein, A.; Llesuy, S.; Irigoyen, M.-C.; et al. Diabetic Hyperglycemia Attenuates Sympathetic Dysfunction and Oxidative Stress after Myocardial Infarction in Rats. Cardiovasc. Diabetol. 2014, 13, 131. [Google Scholar] [CrossRef]
- Xu, X.; Kobayashi, S.; Chen, K.; Timm, D.; Volden, P.; Huang, Y.; Gulick, J.; Yue, Z.; Robbins, J.; Epstein, P.N.; et al. Diminished Autophagy Limits Cardiac Injury in Mouse Models of Type 1 Diabetes. J. Biol. Chem. 2013, 288, 18077–18092. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.; da Costa Martins, P.A.; Bitsch, N.; Pintelon, I.; De Meyer, G.R.; Martinet, W.; Schrijvers, D.M. Defective Autophagy in Vascular Smooth Muscle Cells Accelerates Senescence and Promotes Neointima Formation and Atherogenesis. Autophagy 2015, 11, 2014–2032. [Google Scholar] [CrossRef] [PubMed]
- El-Osta, A.; Brasacchio, D.; Yao, D.; Pocai, A.; Jones, P.L.; Roeder, R.G.; Cooper, M.E.; Brownlee, M. Transient High Glucose Causes Persistent Epigenetic Changes and Altered Gene Expression during Subsequent Normoglycemia. J. Exp. Med. 2008, 205, 2409–2417. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Rerup, C.C. Drugs Producing Diabetes through Damage of the Insulin Secreting Cells. Pharmacol. Rev. 1970, 22, 485–518. [Google Scholar] [PubMed]
- David-Silva, A.; Freitas, H.S.; Okamoto, M.M.; Sabino-Silva, R.; Schaan, B.D.; Machado, U.F. Hepatocyte Nuclear Factors 1α/4α and Forkhead Box A2 Regulate the Solute Carrier 2A2 (Slc2a2) Gene Expression in the Liver and Kidney of Diabetic Rats. Life Sci. 2013, 93, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Mostarda, C.T.; Rodrigues, B.; de Moraes, O.A.; Moraes-Silva, I.C.; Arruda, P.B.O.; Cardoso, R.; Scapini, K.B.; Dos Santos, F.; De Angelis, K.; Irigoyen, M.C. Low Intensity Resistance Training Improves Systolic Function and Cardiovascular Autonomic Control in Diabetic Rats. J. Diabetes Complicat. 2014, 28, 273–278. [Google Scholar] [CrossRef]
- Soares, P.P.d.S.; da Nóbrega, A.C.L.; Ushizima, M.R.; Irigoyen, M.C.C. Cholinergic Stimulation with Pyridostigmine Increases Heart Rate Variability and Baroreflex Sensitivity in Rats. Auton. Neurosci. Basic Clin. 2004, 113, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ishise, H.; Asanoi, H.; Ishizaka, S.; Joho, S.; Kameyama, T.; Umeno, K.; Inoue, H. Time Course of Sympathovagal Imbalance and Left Ventricular Dysfunction in Conscious Dogs with Heart Failure. J. Appl. Physiol. 1998, 84, 1234–1241. [Google Scholar] [CrossRef]
- Conti, F.F.; Brito, J.d.O.; Bernardes, N.; Dias, D.d.S.; Malfitano, C.; Morris, M.; Llesuy, S.F.; Irigoyen, M.-C.; Angelis, K.D. Positive Effect of Combined Exercise Training in a Model of Metabolic Syndrome and Menopause: Autonomic, Inflammatory, and Oxidative Stress Evaluations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1532–R1539. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Reznick, A.Z.; Packer, L. [38] Oxidative Damage to Proteins: Spectrophotometric Method for Carbonyl Assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar] [CrossRef]
- Anderson, M.E. Determination of Glutathione and Glutathione Disulfide in Biological Samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Flohé, L.; Günzler, W.A. Assays of Glutathione Peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Lissi, E.; Salim-Hanna, M.; Pascual, C.; del Castillo, M.D. Evaluation of Total Antioxidant Potential (TRAP) and Total Antioxidant Reactivity from Luminol-Enhanced Chemiluminescence Measurements. Free Radic. Biol. Med. 1995, 18, 153–158. [Google Scholar] [CrossRef] [PubMed]

| C | D | DI | |
|---|---|---|---|
| Glycemia (mg/dL) | |||
| Initial | 121 ± 6.9 | 370 ± 21.4 * | 392 ± 22.8 * |
| First week | 125 ± 8.3 | 411 ± 45.5 * | 153 ± 14.9 #‡ |
| Second week | 156 ± 17.8 | 488 ± 33.1 * | 135 ± 19.9 #‡ |
| Third week | 127 ± 5.3 | 471 ± 25.8 * | 113 ± 18.3 #‡ |
| Fourth week | 112 ± 8.1 | 456 ± 44.6 * | 103 ± 15.9 #‡ |
| Insulin (ng/mL) | |||
| Fourth week | 0.69 ± 0.08 | 0.13 ± 0.02 * | 1.64 ± 0.71 |
| Body mass (g) | |||
| Initial | 265 ± 6.0 | 264 ± 6.3 | 268 ± 4.8 |
| First week | 297 ± 6.4 | 259 ± 12.3 * | 304 ± 5.8 #‡ |
| Second week | 312 ± 7.1 ‡ | 274 ± 14.5 * | 336 ± 6.2 #‡¥ |
| Third week | 335 ± 10.1 ‡¥$ | 278 ± 16.6 * | 371 ± 4.6 #‡¥$ |
| Fourth week | 346 ± 10.1 ‡¥$ | 279 ± 18.9 * | 383 ± 5.3 #‡¥$† |
| Mass gain | 80.7 ± 10.0 | 15.4 ± 17.9 * | 115.0 ± 6.0 # |
| C | D | DI | |
|---|---|---|---|
| Morphometric | |||
| LVEDD (cm) | 0.61 ± 0.04 | 0.64 ± 0.04 | 0.68 ± 0.03 |
| LVPWd (cm) | 0.119 ± 0.003 | 0.096 ± 0.007 * | 0.124 ± 0.004 # |
| RWT | 0.40 ± 0.01 | 0.30 ± 0.02 * | 0.38 ± 0.01 # |
| LV mass (g) | 1.04 ± 0.04 | 0.82 ± 0.03 * | 1.04 ± 0.03 # |
| Systolic function | |||
| Ejection fraction (%) | 81.62 ± 2.10 | 84.78 ± 2.31 | 79.03 ± 2.81 |
| Cardiac output (mL/min) | 149 ± 18 | 126 ± 13 | 176 ± 10 # |
| Stroke volume (mL) | 0.48 ± 0.04 | 0.42 ± 0.03 | 0.57 ± 0.02 # |
| Diastolic function | |||
| IVRT (ms) | 20.00 ± 2.24 | 24.17 ± 1.68 | 22.75 ± 1.48 |
| IVRT normalized by RR | 1.29 ± 0.11 | 1.68 ± 0.11 * | 1.60 ± 0.08 * |
| E-wave (m/s) | 0.79 ± 0.04 | 0.71 ± 0.04 | 0.76 ± 0.03 |
| A-wave (m/s) | 0.36 ± 0.05 | 0.48 ± 0.05 | 0.47 ± 0.04 |
| E-wave deceleration time (ms) | 51.75 ± 3.14 | 62.16 ± 2.44 * | 60.33 ± 2.87 * |
| E-wave deceleration time normalized by RR | 3.86 ± 0.25 | 4.29 ± 0.28 | 4.27 ± 0.22 |
| C | D | DI | |
|---|---|---|---|
| MAP (mmHg) | 109 ± 2 | 100 ± 3 * | 114 ± 1 # |
| SAP (mmHg) | 122 ± 3 | 109 ± 3 * | 125 ± 2 # |
| DAP (mmHg) | 94 ± 2 | 87 ± 3 * | 99 ± 1 # |
| HR (bpm) | 345 ± 4 | 318 ± 6 * | 348 ± 5 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, S.C.F.; Dutra, M.R.H.; Dourado, P.M.M.; Miranda, V.H.d.M.; dos Santos, C.P.; Sanches, I.C.; Irigoyen, M.-C.; De Angelis, K. Insulin Treatment Does Not Prevent EARLY Autonomic Cardiovascular and Diastolic Dysfunctions in Streptozotocin-Induced Diabetic Rats. Pharmaceuticals 2024, 17, 577. https://doi.org/10.3390/ph17050577
Freitas SCF, Dutra MRH, Dourado PMM, Miranda VHdM, dos Santos CP, Sanches IC, Irigoyen M-C, De Angelis K. Insulin Treatment Does Not Prevent EARLY Autonomic Cardiovascular and Diastolic Dysfunctions in Streptozotocin-Induced Diabetic Rats. Pharmaceuticals. 2024; 17(5):577. https://doi.org/10.3390/ph17050577
Chicago/Turabian StyleFreitas, Sarah C. F., Marina R. H. Dutra, Paulo M. M. Dourado, Victor Hugo de Martins Miranda, Camila P. dos Santos, Iris C. Sanches, Maria-Cláudia Irigoyen, and Kátia De Angelis. 2024. "Insulin Treatment Does Not Prevent EARLY Autonomic Cardiovascular and Diastolic Dysfunctions in Streptozotocin-Induced Diabetic Rats" Pharmaceuticals 17, no. 5: 577. https://doi.org/10.3390/ph17050577
APA StyleFreitas, S. C. F., Dutra, M. R. H., Dourado, P. M. M., Miranda, V. H. d. M., dos Santos, C. P., Sanches, I. C., Irigoyen, M.-C., & De Angelis, K. (2024). Insulin Treatment Does Not Prevent EARLY Autonomic Cardiovascular and Diastolic Dysfunctions in Streptozotocin-Induced Diabetic Rats. Pharmaceuticals, 17(5), 577. https://doi.org/10.3390/ph17050577







