Patchouli Alcohol Protects the Heart against Diabetes-Related Cardiomyopathy through the JAK2/STAT3 Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. PatA Attenuates Diabetes-Induced Cardiac Injury and Dysfunction in Mice
2.2. PatA Attenuates Diabetes-Induced Cardiac Injury and Myocardial Fibrosis in Mice
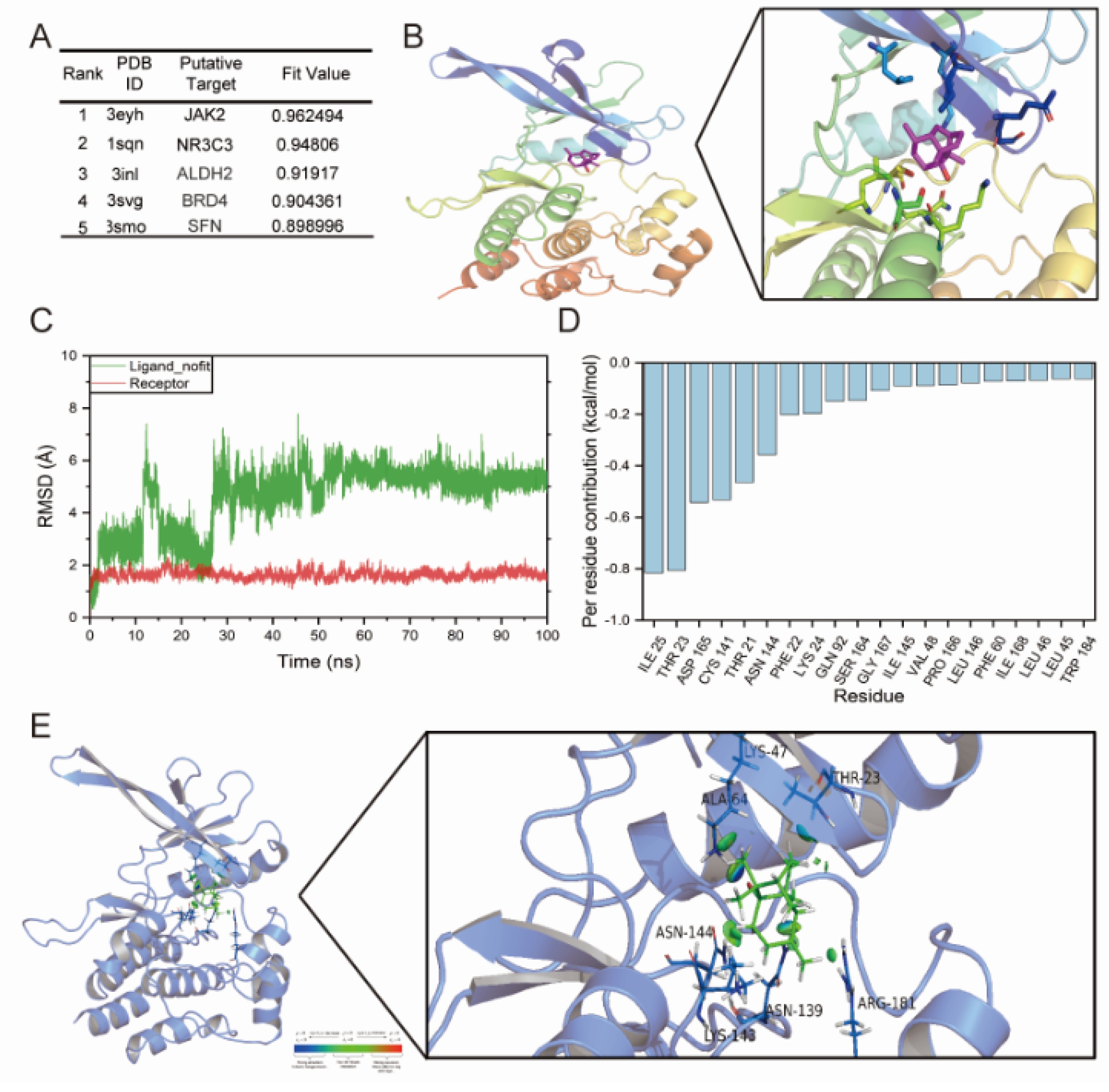
2.3. JAK2 May Be a Potential Target of PatA
2.4. PatA Alleviates DCM by Inhibiting the JAK2/STAT3 Pathway
2.5. PatA Alleviates HG + PA-Induced Fibrotic and Inflammatory Responses in Myocardial H9C2 Cells
2.6. Inhibition of JAK2 Relieves HG + PA Induced-Myocardial Injury
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Cell Culture
4.3. Animals and Animal Models
4.4. Echocardiography
4.5. Real-Time Quantitative PCR (RT-qPCR) Assay
4.6. Measurement of Biomarkers
4.7. Histopathological Analysis
4.8. Western Blot Analysis
4.9. Methyl Thiazole Tetrazolium (MTT) Assay
4.10. RNA Sequencing and Data Analysis
4.11. Molecular Docking and Dynamics Simulation
4.12. Rhodamine–Phalloidin (RP) Staining
4.13. Statistical Analysis
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BNP | brain natriuretic peptide |
| BSA | bovine serum albumin |
| CK-MB | creatine kinase isoenzymes |
| CMC-Na | sodium carboxymethyl cellulose |
| COL1 | collagen Type I |
| DCM | diabetic cardiomyopathy |
| DEGs | the differentially expressed genes |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | dimethyl sulfoxide |
| ECL reagent | enhanced chemiluminescence reagent |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| H&E | hematoxylin and eosin |
| HG | high-concentration glucose |
| IL6 | interleukin 6 |
| JAK2-IN | JAK2 inhibitor |
| LDH | lactate dehydrogenase |
| MTT | methyl thiazolyl tetrazoliumlactate |
| OCT | optimal cutting temperature |
| PatA | patchouli alcohol |
| PVDF | polyvinylidene fluoride |
| qPCR | quantitative polymerase chain reaction |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| STZ | streptozotocin |
| T1DM | type 1 diabetes mellitus |
| TBST | Tris-buffered saline with Tween-20 |
| TGF-β | transforming growth factor beta |
| TL | tibia lengtht |
| TNF-α | tumor necrosis factor-α |
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972, 30, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef]
- Bouthoorn, S.; Valstar, G.B.; Gohar, A.; den Ruijter, H.M.; Reitsma, H.B.; Hoes, A.W.; Rutten, F.H. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis. Diabetes Vasc. Dis. Res. 2018, 15, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Konduracka, E.; Cieslik, G.; Galicka-Latala, D.; Rostoff, P.; Pietrucha, A.; Latacz, P.; Gajos, G.; Malecki, M.T.; Nessler, J. Myocardial dysfunction and chronic heart failure in patients with long-lasting type 1 diabetes: A 7-year prospective cohort study. Acta Diabetol. 2013, 50, 597–606. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Scappaticcio, L.; Cirillo, P.; Maio, A.; Carotenuto, R.; Maiorino, M.I.; Bellastella, G.; Esposito, K. Glycemic Control and the Heart: The Tale of Diabetic Cardiomyopathy Continues. Biomolecules 2022, 12, 272. [Google Scholar] [CrossRef]
- Gilbert, R.E.; Krum, H. Heart failure in diabetes: Effects of anti-hyperglycaemic drug therapy. Lancet 2015, 385, 2107–2117. [Google Scholar] [CrossRef]
- Turnbull, F.M.; Abraira, C.; Anderson, R.J.; Byington, R.P.; Chalmers, J.P.; Duckworth, W.C.; Evans, G.W.; Gerstein, H.C.; Holman, R.R.; Moritz, T.E.; et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009, 52, 2288–2298. [Google Scholar] [CrossRef]
- Zhan, J.; Chen, C.; Wang, D.W.; Li, H. Hyperglycemic memory in diabetic cardiomyopathy. Front. Med. 2022, 16, 25–38. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Singh, G.B.; Khullar, M. Nitric oxide synthases and diabetic cardiomyopathy. Nitric Oxide 2014, 43, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E.; Singh, J. Structural changes in the myocardium during diabetes-induced cardiomyopathy. Heart Fail. Rev. 2014, 19, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Elia, E.; Ministrini, S.; Carbone, F.; Montecucco, F. Diabetic cardiomyopathy and inflammation: Development of hostile microenvironment resulting in cardiac damage. Minerva Cardiol. Angiol. 2022, 70, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Shaver, A.; Nichols, A.; Thompson, E.; Mallick, A.; Payne, K.; Jones, C.; Manne, N.D.; Sundaram, S.; Shapiro, J.I.; Sodhi, K. Role of Serum Biomarkers in Early Detection of Diabetic Cardiomyopathy in the West Virginian Population. Int. J. Med. Sci. 2016, 13, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; DeMarco, V.G.; Sowers, J.R. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat. Rev. Endocrinol. 2016, 12, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Liu, L.; Song, F.H.; Gao, S.J.; Wu, J.Y.; Li, D.Y.; Zhang, L.Q.; Liu, D.Q.; Zhou, Y.Q.; Mei, W. Targeting the JAK2/STAT3 signaling pathway for chronic pain. Aging Dis. 2024, 15, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Montero, P.; Milara, J.; Roger, I.; Cortijo, J. Role of JAK/STAT in Interstitial Lung Diseases; Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2021, 22, 6211. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Farzeen, I.; Ashraf, A.; Aslam, B.; Ijaz, M.U.; Hayat, S.; Sarfraz, M.H.; Zafar, S.; Zafar, N.; Unuofin, J.O.; et al. A Comprehensive Review on Pharmacological Activities of Pachypodol: A Bioactive Compound of an Aromatic Medicinal Plant Pogostemon Cablin Benth. Molecules 2023, 28, 3469. [Google Scholar] [CrossRef]
- Pyun, D.H.; Kim, T.J.; Park, S.Y.; Lee, H.J.; Abd El-Aty, A.M.; Jeong, J.H.; Jung, T.W. Patchouli alcohol ameliorates skeletal muscle insulin resistance and NAFLD via AMPK/SIRT1-mediated suppression of inflammation. Mol. Cell. Endocrinol. 2021, 538, 111464. [Google Scholar] [CrossRef]
- Hu, G.; Peng, C.; Xie, X.; Zhang, S.; Cao, X. Availability, Pharmaceutics, Security, Pharmacokinetics, and Pharmacological Activities of Patchouli Alcohol. Evid. Based Complement. Alterna. Med. 2017, 2017, 4850612. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gan, Y.; Li, M.; Chen, L.; Liang, J.; Zhuo, J.; Luo, H.; Xu, N.; Wu, X.; Wu, Q.; et al. Patchouli alcohol attenuates 5-fluorouracil-induced intestinal mucositis via TLR2/MyD88/NF-kB pathway and regulation of microbiota. Biomed. Pharmacother. 2020, 124, 109883. [Google Scholar] [CrossRef]
- Berretta, A.A.; Silveira, M.A.D.; Cóndor Capcha, J.M.; De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed. Pharmacother. 2020, 131, 110622. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.; Verstovsek, S. JAK Inhibition for the Treatment of Myelofibrosis: Limitations and Future Perspectives. Hemasphere 2020, 4, e424. [Google Scholar] [CrossRef] [PubMed]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.W.; de Boer, R.A. From Inflammation to Fibrosis-Molecular and Cellular Mechanisms of Myocardial Tissue Remodelling and Perspectives on Differential Treatment Opportunities. Curr. Heart Fail. Rep. 2017, 14, 235–250. [Google Scholar] [CrossRef]
- Pickup, J.C.; Mattock, M.B.; Chusney, G.D.; Burt, D. NIDDM as a disease of the innate immune system: Association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997, 40, 1286–1292. [Google Scholar] [CrossRef]
- Filardi, T.; Ghinassi, B.; Di Baldassarre, A.; Tanzilli, G.; Morano, S.; Lenzi, A.; Basili, S.; Crescioli, C. Cardiomyopathy Associated with Diabetes: The Central Role of the Cardiomyocyte. Int. J. Mol. Sci. 2019, 20, 3299. [Google Scholar] [CrossRef]
- Parim, B.; Sathibabu Uddandrao, V.V.; Saravanan, G. Diabetic cardiomyopathy: Molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail. Rev. 2019, 24, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.S.; Jaferi, G.A.; Narang, R.M.; Regan, T.J. Preclinical abnormality of left ventricular function in diabetes mellitus. Am. Heart J. 1975, 89, 153–158. [Google Scholar] [CrossRef]
- Kuwahara, F.; Kai, H.; Tokuda, K.; Kai, M.; Takeshita, A.; Egashira, K.; Imaizumi, T. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation 2002, 106, 130–135. [Google Scholar] [CrossRef]
- Schimmel, K.; Ichimura, K.; Reddy, S.; Haddad, F.; Spiekerkoetter, E. Cardiac Fibrosis in the Pressure Overloaded Left and Right Ventricle as a Therapeutic Target. Front. Cardiovasc. Med. 2022, 9, 886553. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Transforming growth factor-β in myocardial disease. Nat. Rev. Cardiol. 2022, 19, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Meng, K.; Pu, Y.; Zhang, X. Transforming growth factor beta (TGF-β) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Res. Clin. Pract. 2017, 133, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, S.N.; Girardot, M.; Pecquet, C. Mining for JAK-STAT mutations in cancer. Trends Biochem. Sci. 2008, 33, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lang, X.; Li, X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef] [PubMed]
- Mahdiani, S.; Omidkhoda, N.; Rezaee, R.; Heidari, S.; Karimi, G. Induction of JAK2/STAT3 pathway contributes to protective effects of different therapeutics against myocardial ischemia/reperfusion. Biomed. Pharmacother. 2022, 155, 113751. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yin, B.; Ye, Y.; Dekhel, O.; Xiong, X.; Jian, Z.; Gu, L. The bidirectional role of the JAK2/STAT3 signaling pathway and related mechanisms in cerebral ischemia-reperfusion injury. Exp. Neurol. 2021, 341, 113690. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Zhao, S.; Wang, C.; Li, Y.; Duan, H.J. Fluvastatin inhibits activation of JAK and STAT proteins in diabetic rat glomeruli and mesangial cells under high glucose conditions. Acta Pharmacol. Sin. 2007, 28, 1938–1946. [Google Scholar] [CrossRef]
- Mao, T.; Chen, H.; Hong, L.; Li, J. Pigment epithelium-derived factor inhibits high glucose-induced JAK/STAT signalling pathway activation in human glomerular mesangial cells. Saudi Med. J. 2013, 34, 793–800. [Google Scholar]
- Perner, F.; Perner, C.; Ernst, T.; Heidel, F.H. Roles of JAK2 in Aging, Inflammation, Hematopoiesis and Malignant Transformation. Cells 2019, 8, 854. [Google Scholar] [CrossRef]
- Danese, S.; Argollo, M.; Le Berre, C.; Peyrin-Biroulet, L. JAK selectivity for inflammatory bowel disease treatment: Does it clinically matter? Gut 2019, 68, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Luo, W.; Khan, Z.A.; Wu, G.; Xuan, L.; Shan, P.; Lin, K.; Chen, T.; Wang, J.; Hu, X.; et al. Celastrol Attenuates Angiotensin II-Induced Cardiac Remodeling by Targeting STAT3. Circ. Res. 2020, 126, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Huang, L.; Wang, J.; Zhuang, F.; Xu, Z.; Yin, H.; Qian, Y.; Liang, G.; Zheng, C.; Wang, Y. Inhibition of EGFR-STAT3 attenuates cardiomyopathy in streptozotocin-induced type 1 diabetes. J. Endocrinol. 2019, 242, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Khadrawy, S.M.; El Sayed, R.A. Umbelliferone attenuates diabetic cardiomyopathy by suppression of JAK/STAT signaling pathway through amelioration of oxidative stress and inflammation in rats. J. Biochem. Mol. Toxicol. 2023, 37, e23296. [Google Scholar] [CrossRef] [PubMed]
- Qaed, E.; Wang, J.; Almoiliqy, M.; Song, Y.; Liu, W.; Chu, P.; Alademi, S.; Alademi, M.; Li, H.; Alshwmi, M.; et al. Phosphocreatine Improves Cardiac Dysfunction by Normalizing Mitochondrial Respiratory Function through JAK2/STAT3 Signaling Pathway In Vivo and In Vitro. Oxid. Med. Cell. Longev. 2019, 2019, 6521218. [Google Scholar] [CrossRef] [PubMed]
- Kimes, B.W.; Brandt, B.L. Properties of a clonal muscle cell line from rat heart. Exp. Cell Res. 1976, 98, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.J.; Borthwick, G.M.; Arthur, H.M. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ding, C.; Zou, C.; Xiong, Z.; Zhu, W.; Qian, J.; Xu, M.; Zhang, A.; Liang, G. A novel salviadione derivative, compound 15a, attenuates diabetes-induced renal injury by inhibiting NF-κB-mediated inflammatory responses. Toxicol. Appl. Pharmacol. 2020, 409, 115322. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, S.Y.; Lou, H. Patchouli alcohol protects against myocardial ischaemia-reperfusion injury by regulating the Notch1/Hes1 pathway. Pharm. Biol. 2022, 60, 949–957. [Google Scholar] [CrossRef]
- Yeo, H.; Yeo, E.J.; Shin, M.J.; Choi, Y.J.; Lee, C.H.; Kwon, H.Y.; Kim, D.W.; Eum, W.S.; Choi, S.Y. Protective effects of Tat-DJ-1 protein against streptozotocin-induced diabetes in a mice model. BMB Rep. 2018, 51, 362–367. [Google Scholar] [CrossRef]
- Wang, S.; Kamat, A.; Pergola, P.; Swamy, A.; Tio, F.; Cusi, K. Metabolic factors in the development of hepatic steatosis and altered mitochondrial gene expression in vivo. Metabolism 2011, 60, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, K.J.; Regensteiner, J.G.; Bauer, T.A.; Brown, M.S.; Dorosz, J.L.; Hull, A.; Zeitler, P.; Draznin, B.; Reusch, J.E. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J. Clin. Endocrinol. Metab. 2010, 95, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Cree-Green, M.; Newcomer, B.R.; Brown, M.S.; Baumgartner, A.D.; Bergman, B.; Drew, B.; Regensteiner, J.G.; Pyle, L.; Reusch, J.E.; Nadeau, K.J. Delayed skeletal muscle mitochondrial ADP recovery in youth with type 1 diabetes relates to muscle insulin resistance. Diabetes 2015, 64, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Schauer, I.E.; Snell-Bergeon, J.K.; Bergman, B.C.; Maahs, D.M.; Kretowski, A.; Eckel, R.H.; Rewers, M. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diabetes 2011, 60, 306–314. [Google Scholar] [CrossRef] [PubMed]





| Parameters | Ctrl | STZ | STZ- PatA-5 mg/kg | STZ- PatA-15 mg/kg |
|---|---|---|---|---|
| n = 6 | n = 6 | n = 6 | n = 6 | |
| EF (%) | 81.672 ± 0.046 | 76.483 ± 0.023 * | 77.882 ± 0.025 | 80.263 ± 0.021 # |
| FS (%) | 44.450 ± 0.052 | 39.121 ± 0.020 * | 40.523 ± 0.023 | 42.753 ± 0.020 # |
| LVIDs (mm) | 1.517 ± 0.231 | 1.583 ± 0.183 * | 1.8 ± 0.126 | 1.683 ± 0.231 # |
| LVIDd (mm) | 2.717 ± 0.183 | 2.6 ± 0.283 * | 3.033 ± 0.288 | 2.933 ± 0.327 # |
| LVFWs (mm) | 0.867 ± 0.052 | 0.783 ± 0.041 * | 0.817 ± 0.075 | 0.8 ± 0.063 # |
| LVFWd (mm) | 0.667 ± 0.082 | 0.6 ± 0.063 * | 0.6 ± 0.063 | 0.633 ± 0.052 # |
| LVPWs (mm) | 0.85 ± 0.055 | 0.8 ± 0 * | 0.8 ± 0.063 | 0.833 ± 0.051 # |
| LVPWd (mm) | 0.65 ± 0.055 | 0.6 ± 0.063 * | 0.633 ± 0.051 | 0.617 ± 0.041 # |
| Gene Name | Species | Primer Sequence (5′-3′) | |
|---|---|---|---|
| Tgfb1 | Mouse | Forward | CTCCCGTGGCTTCTAGTGC |
| Reversed | GCCTTAGTTTGGACAGGATCTG | ||
| Co11a1 | Mouse | Forward | AATGGTGCTCCTGGTATTGC |
| Reversed | GGTCCTCGTTTTCCTTCTT | ||
| Co14 | Mouse | Forward | ATCGGATACTCCTTCCTCATGC |
| Reversed | CCAGGGGAGACTAGGGACTG | ||
| β-actin | Mouse | Forward | GGCTGTATTCCCCTCCATCG |
| Reversed | CCAGTTGGTAACAATGCCATGT | ||
| Tnfa | Mouse | Forward Reversed | CAGGGGCCACCACGCTCTTC TTTGTGAGTGTGAGGGTCTGG |
| Il6 | Mouse | Forward Reversed | TAGTCCTTCCTACCCCAATTTCC TTGGTCCTTAGCCACTCCTTC |
| Il1b | Mouse | Forward Reversed | ACTCCTTAGTCCTCGGCCA CCATCAGAGGCAAGGAGGAA |
| Col1a1 | Rat | Forward Reversed | GACATCCCTGAAGTCAGCTGC TCCCTTGGGTCCCTCGAC |
| Tgfb1 | Rat | Forward Reversed | GCAACAACGCAATCTATGAC CCTGTATTCCGTCTCCTT |
| Co14 | Rat | Forward Reversed | TAGGTGTCAGCAATTAGGCAGG TCACTTCAAGCATAGTGGTCCG |
| β-actin | Rat | Forward Reversed | TTCGCGTAGAAGAAGGCACAT CTTCCCATTCGTCAGTTTCCTC |
| Tnfa | Rat | Forward Reversed | GCCTCTTCTCATTTCCTGCTCGT CTCCGCTTGGTGGTTTGC |
| Il6 | Rat | Forward Reversed | AGCCACTGCCTTCCCTACTTC AGTGCATCATCGCTGTTCATACAAT |
| Il1b | Rat | Forward Reversed | GCAACTGTTCCTGAACTCAACT ATCTTTTGGGGTCCGTCAACT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, L.; Lou, S.; Fang, Y.; Wang, X.; Zhu, W.; Liang, G.; Lee, K.; Luo, W.; Zhuang, Z. Patchouli Alcohol Protects the Heart against Diabetes-Related Cardiomyopathy through the JAK2/STAT3 Signaling Pathway. Pharmaceuticals 2024, 17, 631. https://doi.org/10.3390/ph17050631
Ji L, Lou S, Fang Y, Wang X, Zhu W, Liang G, Lee K, Luo W, Zhuang Z. Patchouli Alcohol Protects the Heart against Diabetes-Related Cardiomyopathy through the JAK2/STAT3 Signaling Pathway. Pharmaceuticals. 2024; 17(5):631. https://doi.org/10.3390/ph17050631
Chicago/Turabian StyleJi, Lijun, Shuaijie Lou, Yi Fang, Xu Wang, Weiwei Zhu, Guang Liang, Kwangyoul Lee, Wu Luo, and Zaishou Zhuang. 2024. "Patchouli Alcohol Protects the Heart against Diabetes-Related Cardiomyopathy through the JAK2/STAT3 Signaling Pathway" Pharmaceuticals 17, no. 5: 631. https://doi.org/10.3390/ph17050631
APA StyleJi, L., Lou, S., Fang, Y., Wang, X., Zhu, W., Liang, G., Lee, K., Luo, W., & Zhuang, Z. (2024). Patchouli Alcohol Protects the Heart against Diabetes-Related Cardiomyopathy through the JAK2/STAT3 Signaling Pathway. Pharmaceuticals, 17(5), 631. https://doi.org/10.3390/ph17050631







