Therapeutic Drug Monitoring in Psychiatry: Enhancing Treatment Precision and Patient Outcomes
Abstract
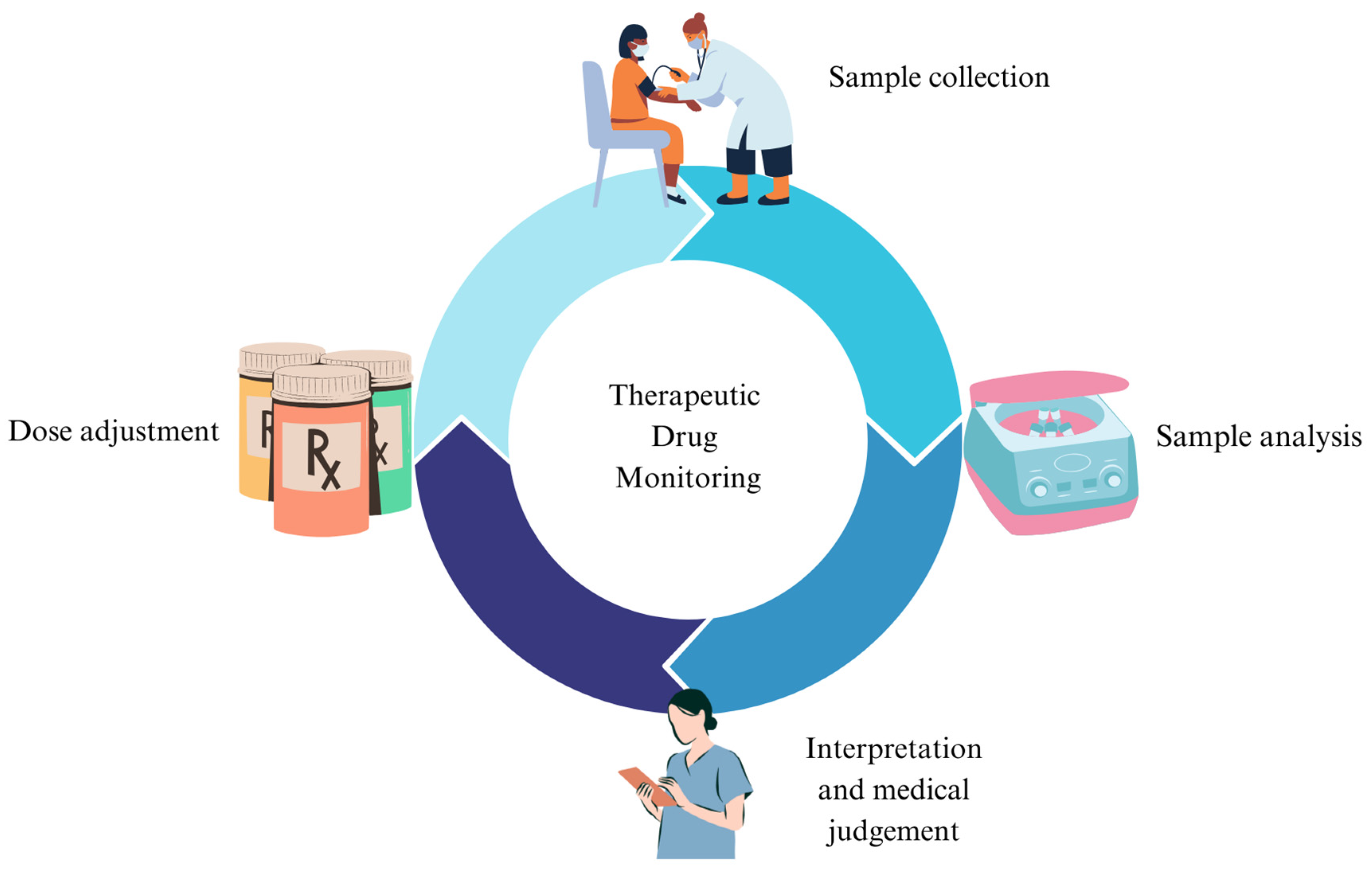
1. Introduction
2. TDM and Mood Stabilizers
3. TDM and Antipsychotics
4. TDM and Antidepressant Medications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schoretsanitis, G.; Paulzen, M.; Unterecker, S.; Schwarz, M.; Conca, A.; Zernig, G.; Gründer, G.; Haen, E.; Baumann, P.; Bergemann, N.; et al. TDM in Psychiatry and Neurology: A Comprehensive Summary of the Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology, Update 2017; a Tool for Clinicians. World J. Biol. Psychiatry 2018, 19, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.E.; Marks, V. Value of Plasma-Lithium Monitoring. Lancet 1971, 1, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Pippenger, C.E. Therapeutic Drug Monitoring Assay Development to Improve Efficacy and Safety. Epilepsy Res. 2006, 68, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; Hiemke, C.; Ulrich, S.; Eckermann, G.; Gaertner, I.; Gerlach, M.; Kuss, H.-J.; Laux, G.; Müller-Oerlinghausen, B.; Rao, M.L.; et al. The AGNP-TDM Expert Group Consensus Guidelines: Therapeutic Drug Monitoring in Psychiatry. Pharmacopsychiatry 2004, 37, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.-M.; Li, X.; Wang, Z.; Qin, J.; Jiang, D.; Tian, P.; Yang, P.; Zhao, R. Status and Quality of Guidelines for Therapeutic Drug Monitoring Based on AGREE II Instrument. Clin. Pharmacokinet. 2023, 62, 1201–1217. [Google Scholar] [CrossRef]
- Aringhieri, S.; Carli, M.; Kolachalam, S.; Verdesca, V.; Cini, E.; Rossi, M.; McCormick, P.J.; Corsini, G.U.; Maggio, R.; Scarselli, M. Molecular Targets of Atypical Antipsychotics: From Mechanism of Action to Clinical Differences. Pharmacol. Ther. 2018, 192, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Schoretsanitis, G.; Kane, J.M.; Correll, C.U.; Marder, S.R.; Citrome, L.; Newcomer, J.W.; Robinson, D.G.; Goff, D.C.; Kelly, D.L.; Freudenreich, O.; et al. Blood Levels to Optimize Antipsychotic Treatment in Clinical Practice: A Joint Consensus Statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft Für Neuropsychopharmakologie Und Pharmakopsychiatrie. J. Clin. Psychiatry 2020, 81, 3649. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C. Clinical Utility of Drug Measurement and Pharmacokinetics—Therapeutic Drug Monitoring in Psychiatry. Eur. J. Clin. Pharmacol. 2008, 64, 159–166. [Google Scholar] [CrossRef]
- Burke, M.J.; Preskorn, S.H. Therapeutic Drug Monitoring of Antidepressants. Clin. Pharmacokinet. 1999, 37, 147–165. [Google Scholar] [CrossRef]
- Eilers, R. Therapeutic Drug Monitoring for the Treatment of Psychiatric Disorders. Clin. Pharmacokinet. 1995, 29, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Al Mutarid, M.; Alhossan, A.; Khan, T.; Alyami, M.G.; Almutared, K.M.; Alshiban, M.; Alyami, A.H.D.; Alyami, M.M.M.; AlKulayb, J.A.H.; Alyami, D.S.; et al. Knowledge and Attitude of Healthcare Practitioners toward Therapeutic Drug Monitoring Practices in the Najran Region, Kingdom of Saudi Arabia. Cureus 2022, 14, e32214. [Google Scholar] [CrossRef] [PubMed]
- Eryılmaz, G.; Hızlı Sayar, G.; Gül, I.G.; Noyan, C.O.; Özten, E.; Darçın, A.E.; Yorbik, Ö.; Dilbaz, N. Therapeutic Drug Monitoring: Perspectives of Psychiatrists in Turkey. Int. J. Psychiatry Clin. Pract. 2015, 19, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Guo, G.-X.; Sun, C.; Zhang, J.; Rong, Z.; He, J.; Sun, Z.; Yan, F.; Tang, Y.; Wang, C.; et al. Therapeutic Drug Monitoring of Psychotropic Drugs in China. Ther. Drug Monit. 2013, 35, 816–822. [Google Scholar] [CrossRef]
- Stephan, P.L.; Etzensberger, M.; Sirot, J. Arzneimittelspiegel Als Pharmakotherapeutisches Werkzeug Bei Der Behandlung Mit Psychopharmaka. Praxis 2006, 95, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Molden, E. Therapeutic Drug Monitoring of Clozapine in Adults with Schizophrenia: A Review of Challenges and Strategies. Expert. Opin. Drug Metab. Toxicol. 2021, 17, 1211–1221. [Google Scholar] [CrossRef]
- Vázquez, G.H.; Bahji, A.; Undurraga, J.; Tondo, L.; Baldessarini, R.J. Efficacy and Tolerability of Combination Treatments for Major Depression: Antidepressants plus Second-Generation Antipsychotics vs. Esketamine vs. Lithium. J. Psychopharmacol. 2021, 35, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Sunny, S.; Prabhu, S.; Chand, S.; Up, N.; Susan, C.; Joel, J.J. Assessment of Drug-Drug Interactions among Patients with Psychiatric Disorders: A Clinical Pharmacist-Led Study. Clin. Epidemiol. Glob. Health 2022, 13, 100930. [Google Scholar] [CrossRef]
- Aburamadan, H.A.R.; Sridhar, S.B.; Tadross, T.M. Assessment of Potential Drug Interactions among Psychiatric Inpatients Receiving Antipsychotic Therapy of a Secondary Care Hospital, United Arab Emirates. J. Adv. Pharm. Technol. Res. 2021, 12, 45–51. [Google Scholar] [CrossRef]
- Sandson, N. Important Drug-Drug Interactions for the Addiction Psychiatrist. Psychiatr. Clin. N. Am. 2022, 45, 431–450. [Google Scholar] [CrossRef]
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 Guidelines for the Management of Patients with Bipolar Disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef]
- Nierenberg, A.A.; Agustini, B.; Köhler-Forsberg, O.; Cusin, C.; Katz, D.; Sylvia, L.G.; Peters, A.; Berk, M. Diagnosis and Treatment of Bipolar Disorder: A Review. JAMA 2023, 330, 1370–1380. [Google Scholar] [CrossRef]
- Parkin, G.M.; McCarthy, M.J.; Thein, S.H.; Piccerillo, H.L.; Warikoo, N.; Granger, D.A.; Thomas, E.A. Saliva Testing as a Means to Monitor Therapeutic Lithium Levels in Patients with Psychiatric Disorders: Identification of Clinical and Environmental Covariates, and Their Incorporation into a Prediction Model. Bipolar Disord. 2021, 23, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.; Qassem, M.; Triantis, I.F.; Kyriacou, P.A. Advances in Therapeutic Monitoring of Lithium in the Management of Bipolar Disorder. Sensors 2022, 22, 736. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Gupta, Y.K.; Singh, M.; Joshi, R.; Tiwari, P.; Kaleekal, T.; Tripathi, M. Correlation of Saliva and Serum Free Valproic Acid Concentrations in Persons with Epilepsy. Seizure 2015, 25, 187–190. [Google Scholar] [CrossRef][Green Version]
- Guo, M.; Shao, L.; Chen, X.; Li, H.; Wang, L.; Pan, Y.; Tang, D. Assay of Dried Blood Spot from Finger Prick for Sodium Valproate via Ink Auxiliary Headspace Gas Chromatography Mass Spectrometry. J. Chromatogr. A 2019, 1601, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Namera, A.; Uekusa, K.; Saito, T.; Yoshimoto, K.; Ishiuchi, N.; Murata, K.; Nagao, M. A Method for Determining Valproic Acid in Human Whole Blood and Urine via Gas Chromatography-Mass Spectrometry and Small-Scale Inter-Laboratory Trial. Leg. Med. 2022, 59, 102133. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Spencer, E.P.; Berry, D.J. Therapeutic Drug Monitoring of Antiepileptic Drugs in Epilepsy: A 2018 Update. Ther. Drug Monit. 2018, 40, 526–548. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Singh, M.; Kaleekal, T.; Gupta, Y.K.; Tripathi, M. Concentration of Antiepileptic Drugs in Persons with Epilepsy: A Comparative Study in Serum and Saliva. Int. J. Neurosci. 2016, 126, 972–978. [Google Scholar] [CrossRef]
- Chen, N.; Yuan, Y.; Lu, P.; Wang, L.; Zhang, X.; Chen, H.; Ma, P. Detection of Carbamazepine in Saliva Based on Surface-Enhanced Raman Spectroscopy. Biomed. Opt. Express 2021, 12, 7673. [Google Scholar] [CrossRef]
- Rezaei Kahkha, M.R.; Oveisi, A.R.; Kaykhaii, M.; Rezaei Kahkha, B. Determination of Carbamazepine in Urine and Water Samples Using Amino-Functionalized Metal–Organic Framework as Sorbent. Chem. Cent. J. 2018, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Chen, F. Determination of Carbamazepine in Human Urine and Serum Samples by High-performance Liquid Chromatography with Post-column Ru(Bipy)-Ce(SO4)2 Chemiluminescence Detection. Luminescence 2013, 28, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Erarpat, S.; Bodur, S.; Ayyıldız, M.F.; Günkara, Ö.T.; Erulaş, F.; Chormey, D.S.; Turak, F.; Budak, T.B.; Bakırdere, S. Accurate and Simple Determination of Oxcarbazepine in Human Plasma and Urine Samples Using Switchable-hydrophilicity Solvent in GC–MS. Biomed. Chromatogr. 2020, 34, e4915. [Google Scholar] [CrossRef] [PubMed]
- Incecayir, T.; Agabeyoglu, I.; Gucuyener, K. Comparison of Plasma and Saliva Concentrations of Lamotrigine in Healthy Volunteers. Arzneimittelforschung 2011, 57, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Tsiropoulos, I.; Kristensen, O.; Klitgaard, N.A. Saliva and Serum Concentration of Lamotrigine in Patients with Epilepsy. Ther. Drug Monit. 2000, 22, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Trnavska, Z.; Krejcova, H.; Tkaczykovam; Salcmanova, Z.; Elis, J. Pharmacokinetics of Lamotrigine (Lamictal) in Plasma and Saliva. Eur. J. Drug Metab. Pharmacokinet. 1991, 3, 211–215. [Google Scholar]
- Milosheska, D.; Roškar, R.; Vovk, T.; Lorber, B.; Grabnar, I.; Trontelj, J. An LC-MS/MS Method for Quantification of Lamotrigine and Its Main Metabolite in Dried Blood Spots. Pharmaceuticals 2024, 17, 449. [Google Scholar] [CrossRef]
- Houston, J.P.; Tohen, M.; Degenhardt, E.K.; Jamal, H.H.; Liu, L.L.L.; Ketter, T.A. Olanzapine-Divalproex Combination versus Divalproex Monotherapy in the Treatment of Bipolar Mixed Episodes: A Double-Blind, Placebo-Controlled Study. J. Clin. Psychiatry 2009, 70, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Ercis, M.; Ozerdem, A.; Singh, B. When and How to Use Lithium Augmentation for Treating Major Depressive Disorder. J. Clin. Psychiatry 2023, 84, 23ac14813. [Google Scholar] [CrossRef]
- Licht, R.W. Lithium: Still a Major Option in the Management of Bipolar Disorder. CNS Neurosci. Ther. 2012, 18, 219–226. [Google Scholar] [CrossRef]
- Gitlin, M. Lithium Side Effects and Toxicity: Prevalence and Management Strategies. Int. J. Bipolar Disord. 2016, 4, 1–10. [Google Scholar] [CrossRef]
- Nolen, W.A.; Licht, R.W.; Young, A.H.; Malhi, G.S.; Tohen, M.; Vieta, E.; Kupka, R.W.; Zarate, C.; Nielsen, R.E.; Baldessarini, R.J.; et al. What Is the Optimal Serum Level for Lithium in the Maintenance Treatment of Bipolar Disorder? A Systematic Review and Recommendations from the ISBD/IGSLI Task Force on Treatment with Lithium. Bipolar Disord. 2019, 21, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S.; Reddy, M.S. Brief Communication Serum Lithium Levels: Ideal Time for Sample Collection! Are We Doing It Right? Indian J. Psychol. Med. 2014, 36, 346–347. [Google Scholar] [CrossRef]
- Malhi, G.S.; Gershon, S.; Outhred, T. Lithiumeter: Version 2.0. Bipolar Disord. 2016, 18, 631–641. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Pattanaseri, K.; Hidalgo-Mazzei, D.; Taylor, D.; Young, A.H. Is Lithium Monitoring NICE? Lithium Monitoring in a UK Secondary Care Setting. J. Psychopharmacol. 2018, 32, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.M.; Haddad, P.M.; Ferrier, I.N.; Aronson, J.K.; Barnes, T.; Cipriani, A.; Coghill, D.R.; Fazel, S.; Geddes, J.R.; Grunze, H.; et al. Evidence-Based Guidelines for Treating Bipolar Disorder: Revised Third Edition Recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2016, 30, 495–553. [Google Scholar] [CrossRef]
- Pérez de Mendiola, X.; Hidalgo-Mazzei, D.; Vieta, E.; González-Pinto, A. Overview of Lithium’s Use: A Nationwide Survey. Int. J. Bipolar Disord. 2021, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Parkin, G.M.; Thomas, E.A. Provider Perspectives on the Current Use of Lithium Medications and Lithium Monitoring Practices for Psychiatric Conditions. Neuropsychiatr. Dis. Treat. 2022, 18, 2083–2093. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Tsai, S.-Y.; Wang, L.-J.; Liang, C.-S.; Carvalho, A.F.; Solmi, M.; Vieta, E.; Lin, P.-Y.; Hu, C.-A.; Kao, H.-Y. Predicting Serum Levels of Lithium-Treated Patients: A Supervised Machine Learning Approach. Biomedicines 2021, 9, 1558. [Google Scholar] [CrossRef]
- Ooba, N.; Tsutsumi, D.; Kobayashi, N.; Hidaka, S.; Hayashi, H.; Obara, T.; Satoh, M.; Kubota, K.; Fukuoka, N. Prevalence of Therapeutic Drug Monitoring for Lithium and the Impact of Regulatory Warnings: Analysis Using Japanese Claims Database. Ther. Drug Monit. 2018, 40, 252–256. [Google Scholar] [CrossRef]
- Rej, S.; Herrmann, N.; Gruneir, A.; Jandoc, R.; McArthur, E.; Dixon, S.; Garg, A.X. Blood Lithium Monitoring Practices in a Population-Based Sample of Older Adults. J. Clin. Psychiatry 2018, 79, 10458. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, E.; Bazire, S.; Anderson, T.; Wood, J.; Grassby, P.; Desborough, J.A. Impact of Active Monitoring on Lithium Management in Norfolk. Ther. Adv. Psychopharmacol. 2013, 3, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, V.; Al-Sukhni, M.; Lawson, A.; Chandler, G. Lithium Prescribing and Therapeutic Drug Monitoring in Bipolar Disorder: A Survey of Current Practices and Perspectives. J. Psychiatr. Pract. 2020, 26, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, C.; Duff, C.J.; Scargill, J.; Green, L.; Holland, D.; Heald, A.H.; Fryer, A.A. Serum Lithium Test Requesting across Three UK Regions: An Evaluation of Adherence to Monitoring Guidelines. BMC Psychiatry 2021, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Carli, M.; Risaliti, E.; Francomano, M.; Kolachalam, S.; Longoni, B.; Bocci, G.; Maggio, R.; Scarselli, M. A 5-Year Study of Lithium and Valproic Acid Drug Monitoring in Patients with Bipolar Disorders in an Italian Clinical Center. Pharmaceuticals 2022, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- Tomson, T.; Battino, D.; Perucca, E. The Remarkable Story of Valproic Acid. Lancet Neurol. 2016, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Hakami, T. Neuropharmacology of Antiseizure Drugs. Neuropsychopharmacol. Rep. 2021, 41, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Nanau, R.M.; Neuman, M.G. Adverse Drug Reactions Induced by Valproic Acid. Clin. Biochem. 2013, 46, 1323–1338. [Google Scholar] [CrossRef]
- Shakerdi, L.; Ryan, A. Drug-Induced Hyperammonaemia. J. Clin. Pathol. 2023, 76, 501–509. [Google Scholar] [CrossRef]
- Tomson, T.; Battino, D.; Perucca, E. Teratogenicity of Antiepileptic Drugs. Curr. Opin. Neurol. 2019, 32, 246–252. [Google Scholar] [CrossRef]
- Bromley, R.; Adab, N.; Bluett-Duncan, M.; Clayton-Smith, J.; Christensen, J.; Edwards, K.; Greenhalgh, J.; Hill, R.A.; Jackson, C.F.; Khanom, S.; et al. Monotherapy Treatment of Epilepsy in Pregnancy: Congenital Malformation Outcomes in the Child. Cochrane Database Syst. Rev. 2023, 8, CD010224. [Google Scholar] [CrossRef] [PubMed]
- Collins-Yoder, A.; Lowell, J. Valproic Acid: Special Considerations and Targeted Monitoring. J. Neurosci. Nurs. 2017, 49, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Damegunta, S.R. Time Matters!: When Is the Right Time to Estimate Serum Valproic Acid Levels? Indian J. Psychol. Med. 2014, 36, 349–350. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Lai, E.C.-C.; Chen, Y.-C.B.; Kao, H.-Y. Valproic Acid Monitoring: Serum Prediction Using a Machine Learning Framework from Multicenter Real-World Data. J. Affect. Disord. 2024, 347, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Paholpak, P.; Paholpak, S.; Patanasethanant, D.; Rangseekajee, P.; Patjanasoontorn, N. Rate of Serum Valproate Concentration Monitoring in Patients with Bipolar Disorder Type I at Srinagarind Hospital Outpatient Clinic. J. Med. Assoc. Thai 2016, 99, 1153–1160. [Google Scholar]
- Shaikh, A.S.; Liu, H.; Li, Y.; Cao, L.; Guo, R. Therapeutic Drug Monitoring of Valproic Acid. Pak. J. Pharm. Sci. 2018, 31, 1773–1776. [Google Scholar]
- Machino, A.; Jitsuiki, H.; Okamoto, Y.; Izumitani, S.; Kimura, Y.; Suzuki, K.; Tanaka, T.; Inoue, T.; Koyama, T.; Wada, K.; et al. The Valproate Serum Level in Maintenance Therapy for Bipolar Disorder in Japan. Hiroshima J. Med. Sci. 2013, 62, 7–12. [Google Scholar]
- Biso, L.; Carli, M.; Kolachalam, S.; Monticelli, G.; Calabrò, P.F.; di Paolo, A.; Giorgi, F.S.; Bocci, G.; Scarselli, M. A 5-Year Study of Antiseizure Medications (ASMs) Monitoring in Patients with Neuropsychiatric Disorders in an Italian Clinical Center. Pharmaceuticals 2023, 16, 945. [Google Scholar] [CrossRef] [PubMed]
- Grunze, A.; Amann, B.L.; Grunze, H. Efficacy of Carbamazepine and Its Derivatives in the Treatment of Bipolar Disorder. Medicina 2021, 57, 433. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, R.; Chen, Q.; Li, M.; Lin, W.; Cui, L. Effect of Carbamazepine on the Bone Health of People with Epilepsy: A Systematic Review and Meta-Analysis. J. Int. Med. Res. 2020, 48, 300060520902608. [Google Scholar] [CrossRef]
- Jentink, J.; Dolk, H.; Loane, M.A.; Morris, J.K.; Wellesley, D.; Garne, E.; De Jong-van Den Berg, L. Intrauterine Exposure to Carbamazepine and Specific Congenital Malformations: Systematic Review and Case-Control Study. BMJ (Online) 2010, 341, 1261. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Lee, P.M.Y.; Li, F.; Li, J. Prenatal Carbamazepine Exposure and Academic Performance in Adolescents: A Population-Based Cohort Study. Neurology 2023, 100, e728–e738. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.M.; Donnelly, A. Carbamazepine-10,11-Epoxide in Therapeutic Drug Monitoring. Ther. Drug Monit. 1998, 20, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Burianová, I.; Bořecká, K. Routine Therapeutic Monitoring of the Active Metabolite of Carbamazepine: Is It Really Necessary? Clin. Biochem. 2015, 48, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.N.; Nguyen, L.H.; Fitz-Gerald, M.J.; Crane, E.; Lewis, K.; Pierre, S.S.; Kaye, A.D.; Kaye, A.M.; Kaye, J.S.; Kaye, R.J.; et al. Lamotrigine and Stevens-Johnson Syndrome Prevention. Psychopharmacol. Bull. 2021, 51, 96–114. [Google Scholar] [PubMed]
- Mannapperuma, U.; Galappatthy, P.; Jayakody, R.L.; Mendis, J.; de Silva, V.A.; Hanwella, R. Safety Monitoring of Treatment in Bipolar Disorder in a Tertiary Care Setting in Sri Lanka and Recommendations for Improved Monitoring in Resource Limited Settings. BMC Psychiatry 2019, 19, 194. [Google Scholar] [CrossRef] [PubMed]
- Grześk, G.; Stolarek, W.; Kasprzak, M.; Grześk, E.; Rogowicz, D.; Wiciński, M.; Krzyżanowski, M. Therapeutic Drug Monitoring of Carbamazepine: A 20-Year Observational Study. J. Clin. Med. 2021, 10, 5396. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, S.N.; Ko, J.Y.; Katzow, J.J. Oxcarbazepine Treatment of Refractory Bipolar Disorder: A Retrospective Chart Review. Bipolar Disord. 2002, 4, 70–74. [Google Scholar] [CrossRef]
- Vieta, E.; Sanchez-Moreno, J. Acute and Long-Term Treatment of Mania. Dialogues Clin. Neurosci. 2008, 10, 165–179. [Google Scholar] [CrossRef]
- Kishi, T.; Ikuta, T.; Matsuda, Y.; Sakuma, K.; Okuya, M.; Mishima, K.; Iwata, N. Mood Stabilizers and/or Antipsychotics for Bipolar Disorder in the Maintenance Phase: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Mol. Psychiatry 2021, 26, 4146–4157. [Google Scholar] [CrossRef]
- Montouris, G. Safety of the Newer Antiepileptic Drug Oxcarbazepine during Pregnancy. Curr. Med. Res. Opin. 2005, 21, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Wellington, K.; Goa, K.L. Oxcarbazepine: An Update of Its Efficacy in the Management of Epilepsy. CNS Drugs 2001, 15, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Bring, P.; Ensom, M.H.H. Does Oxcarbazepine Warrant Therapeutic Drug Monitoring? A Critical Review. Clin. Pharmacokinet. 2008, 47, 767–778. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar Disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Kanner, A.M. Lamotrigine-Induced Rash: Can We Stop Worrying? Epilepsy Curr. 2005, 5, 190–191. [Google Scholar] [CrossRef] [PubMed]
- French, J.A.; Perucca, E.; Sander, J.W.; Bergfeldt, L.; Baulac, M.; Auerbach, D.S.; Keezer, M.; Thijs, R.D.; Devinsky, O.; Vossler, D.G.; et al. FDA Safety Warning on the Cardiac Effects of Lamotrigine: An Advisory from the Ad Hoc ILAE/AES Task Force. Epilepsia Open 2021, 6, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Husein, N.; Thijs, R.D.; Bunschoten, J.W.; Keezer, M.R.; Sander, J.W. Concerns about Lamotrigine. Lancet Neurol. 2021, 20, 418–419. [Google Scholar] [CrossRef]
- Pariente, G.; Leibson, T.; Shulman, T.; Adams-Webber, T.; Barzilay, E.; Nulman, I. Pregnancy Outcomes Following In Utero Exposure to Lamotrigine: A Systematic Review and Meta-Analysis. CNS Drugs 2017, 31, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Tomson, T.; Battino, D.; Bonizzoni, E.; Craig, J.; Lindhout, D.; Perucca, E.; Sabers, A.; Thomas, S.V.; Vajda, F. Comparative Risk of Major Congenital Malformations with Eight Different Antiepileptic Drugs: A Prospective Cohort Study of the EURAP Registry. Lancet Neurol. 2018, 17, 530–538. [Google Scholar] [CrossRef]
- Kagawa, S.; Mihara, K.; Nakamura, A.; Nemoto, K.; Suzuki, T.; Nagai, G.; Kondo, T. Relationship between Plasma Concentrations of Lamotrigine and Its Early Therapeutic Effect of Lamotrigine Augmentation Therapy in Treatment-Resistant Depressive Disorder. Ther. Drug Monit. 2014, 36, 730–733. [Google Scholar] [CrossRef]
- Kikkawa, A.; Kitamura, Y.; Aiba, T.; Hiraki, K.; Sendo, T. Correlation between the Efficacy of Lamotrigine and the Serum Lamotrigine Level during the Remission Phase of Acute Bipolar II Depression: A Naturalistic and Unblinded Prospective Pilot Study. Biol. Pharm. Bull. 2017, 40, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Douglas-Hall, P.; Dzahini, O.; Gaughran, F.; Bile, A.; Taylor, D. Variation in Dose and Plasma Level of Lamotrigine in Patients Discharged from a Mental Health Trust. Ther. Adv. Psychopharmacol. 2017, 7, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Unholzer, S.; Haen, E. Retrospective Analysis of Therapeutic Drug Monitoring Data for Treatment of Bipolar Disorder with Lamotrigine. Pharmacopsychiatry 2015, 48, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Chouchana, M.; Delage, C.; Godin, O.; Fontan, J.-E.; Bellivier, F.; Gard, S.; Aubin, V.; Belzeaux, R.; Dubertret, C.; Haffen, E.; et al. Factors Associated with Lamotrigine Concentration/Dose Ratio in Individuals with Bipolar Disorders. Eur. Neuropsychopharmacol. 2023, 73, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y. Update on Typical and Atypical Antipsychotic Drugs. Annu. Rev. Med. 2013, 64, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Carli, M.; Kolachalam, S.; Longoni, B.; Pintaudi, A.; Baldini, M.; Aringhieri, S.; Fasciani, I.; Annibale, P.; Maggio, R.; Scarselli, M. Atypical Antipsychotics and Metabolic Syndrome: From Molecular Mechanisms to Clinical Differences. Pharmaceuticals 2021, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, N.; Lanctôt, K.L. Do Atypical Antipsychotics Cause Stroke? CNS Drugs 2005, 19, 91–103. [Google Scholar] [CrossRef]
- Luft, B.; Taylor, D. A Review of Atypical Antipsychotic Drugs versus Conventional Medication in Schizophrenia. Expert. Opin. Pharmacother. 2006, 7, 1739–1748. [Google Scholar] [CrossRef]
- Alsabhan, J.F.; Almalag, H.M.; Aljafali, L.; Alnughamish, H.; Almutlaq, G. Prescribing Pattern of Antipsychotics for Patients with Schizophrenia Using the Total Daily Dose Online Tool. Saudi Pharm. J. 2023, 31, 101837. [Google Scholar] [CrossRef]
- Bastaki, K.; El Anbari, M.; Ghuloum, S.; Jithesh, P.V. Prescription Pattern and Off-Label Use of Antipsychotics in a Middle Eastern Population. Front. Pharmacol. 2021, 12, 753845. [Google Scholar] [CrossRef]
- Eloff, I.; Esterhuysen, W.; Odayar, K. Antipsychotic Use in a Resource-Limited Setting: Findings in an Eastern Cape Psychiatric Hospital. S. Afr. J. Psychiatry 2017, 23, 1093. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ayenew, W.; Asmamaw, G.; Bitew, T. Antipsychotic Polypharmacy Among Patients with Schizophrenia in Africa: A Systematic Review and Meta-Analysis. Int. J. Neuropsychopharmacol. 2021, 24, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Scarselli, M.; Annibale, P.; Gerace, C.; Radenovic, A. Enlightening G-Protein-Coupled Receptors on the Plasma Membrane Using Super-Resolution Photoactivated Localization Microscopy. Biochem. Soc. Trans. 2013, 41, 191–196. [Google Scholar] [CrossRef]
- Chan, Y.-C.; Derflinger, B.A. Use of Therapeutic Drug Monitoring (TDM) for Antipsychotics to Avoid Polypharmacy in the Treatment of Schizophrenia. Psychiatry Res. Case Rep. 2023, 2, 100130. [Google Scholar] [CrossRef]
- Kapur, S.; Zipursky, R.; Jones, C.; Remington, G.; Houle, S. Relationship between Dopamine D(2) Occupancy, Clinical Response, and Side Effects: A Double-Blind PET Study of First-Episode Schizophrenia. Am. J. Psychiatry 2000, 157, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Takeuchi, H.; Graff-Guerrero, A.; Suzuki, T.; Watanabe, K.; Mamo, D.C. Predicting Dopamine D₂ Receptor Occupancy from Plasma Levels of Antipsychotic Drugs: A Systematic Review and Pooled Analysis. J. Clin. Psychopharmacol. 2011, 31, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Takeuchi, H.; Graff-Guerrero, A.; Suzuki, T.; Watanabe, K.; Mamo, D.C. Dopamine D2 Receptor Occupancy and Clinical Effects: A Systematic Review and Pooled Analysis. J. Clin. Psychopharmacol. 2011, 31, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, M.; Kacirova, I.; Urinovska, R. Therapeutic Drug Monitoring of Atypical Antipsychotic Drugs. Acta Pharm. 2014, 64, 387–401. [Google Scholar] [CrossRef]
- Nakajima, S.; Uchida, H.; Bies, R.R.; Caravaggio, F.; Suzuki, T.; Plitman, E.; Mar, W.; Gerretsen, P.; Pollock, B.G.; Mulsant, B.H.; et al. Dopamine D 2/3 Receptor Occupancy Following Dose Reduction Is Predictable with Minimal Plasma Antipsychotic Concentrations: An Open-Label Clinical Trial. Schizophr. Bull. 2015, 42, 212–219. [Google Scholar] [CrossRef]
- Lako, I.M.; Van Den Heuvel, E.R.; Knegtering, H.; Bruggeman, R.; Taxis, K. Estimating Dopamine D2 Receptor Occupancy for Doses of 8 Antipsychotics: A Meta-Analysis. J. Clin. Psychopharmacol. 2013, 33, 675–681. [Google Scholar] [CrossRef]
- Gunes, A.; Spina, E.; Dahl, M.-L.; Scordo, M.G. ABCB1 Polymorphisms Influence Steady-State Plasma Levels of 9-Hydroxyrisperidone and Risperidone Active Moiety. Ther. Drug Monit. 2008, 30, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Gründer, G.; Yokoi, F.; Offord, S.J.; Ravert, H.T.; Dannals, R.F.; Salzmann, J.K.; Szymanski, S.; Wilson, P.D.; Howard, D.R.; Wong, D.F. Time Course of 5-HT2A Receptor Occupancy in the Human Brain after a Single Oral Dose of the Putative Antipsychotic Drug MDL 100,907 Measured by Positron Emission Tomography. Neuropsychopharmacology 1997, 17, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Mamo, D.; Kapur, S.; Shammi, C.M.; Papatheodorou, G.; Mann, S.; Therrien, F.; Remington, G. A PET Study of Dopamine D2 and Serotonin 5-HT2 Receptor Occupancy in Patients with Schizophrenia Treated with Therapeutic Doses of Ziprasidone. Am. J. Psychiatry 2004, 161, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Alberati, D.; Moreau, J.L.; Lengyel, J.; Hauser, N.; Mory, R.; Borroni, E.; Pinard, E.; Knoflach, F.; Schlotterbeck, G.; Hainzl, D.; et al. Glycine Reuptake Inhibitor RG1678: A Pharmacologic Characterization of an Investigational Agent for the Treatment of Schizophrenia. Neuropharmacology 2012, 62, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Matsui-Sakata, A.; Ohtani, H.; Sawada, Y. Receptor Occupancy-Based Analysis of the Contributions of Various Receptors to Antipsychotics-Induced Weight Gain and Diabetes Mellitus. Drug Metab. Pharmacokinet. 2005, 20, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Prior, T.I.; Chue, P.S.; Tibbo, P.; Baker, G.B. Drug Metabolism and Atypical Antipsychotics. Eur. Neuropsychopharmacol. 1999, 9, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Rostami-Hodjegan, A.; Amin, A.M.; Spencer, E.P.; Lennard, M.S.; Tucker, G.T.; Flanagan, R.J. Influence of Dose, Cigarette Smoking, Age, Sex, and Metabolic Activity on Plasma Clozapine Concentrations: A Predictive Model and Nomograms to Aid Clozapine Dose Adjustment and to Assess Compliance in Individual Patients. J. Clin. Psychopharmacol. 2004, 24, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.; McLaren, A.; Galanos, J.; Copolov, D. The Clinical Use of Plasma Clozapine Levels. Aust. N. Z. J. Psychiatry 1998, 32, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.V.; Kane, J.M. Plasma Levels of Second-Generation Antipsychotics and Clinical Response in Acute Psychosis: A Review of the Literature. Schizophr. Res. 2013, 147, 368–374. [Google Scholar] [CrossRef]
- Jerling, M.; Lindström, L.; Bondesson, U.; Bertilsson, L. Fluvoxamine Inhibition and Carbamazepine Induction of the Metabolism of Clozapine: Evidence from a Therapeutic Drug Monitoring Service. Ther. Drug Monit. 1994, 16, 368–374. [Google Scholar] [CrossRef]
- Spina, E.; de Leon, J. Metabolic Drug Interactions with Newer Antipsychotics: A Comparative Review. Basic Clin. Pharmacol. Toxicol. 2007, 100, 4–22. [Google Scholar] [CrossRef]
- Perry, P.J.; Miller, D.D.; Arndt, S.V.; Cadoret, R.J. Clozapine and Norclozapine Plasma Concentrations and Clinical Response of Treatment-Refractory Schizophrenic Patients. Am. J. Psychiatry 1991, 148, 231–235. [Google Scholar] [PubMed]
- Kronig, M.H.; Munne, R.A.; Szymanski, S.; Safferman, A.Z.; Pollack, S.; Cooper, T.; Kane, J.M.; Lieberman, J.A. Plasma Clozapine Levels and Clinical Response for Treatment-Refractory Schizophrenic Patients. Am. J. Psychiatry 1995, 152, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Mauri, M.C.; Volonteri, L.S.; Colasanti, A.; Fiorentini, A.; De Gaspari, I.F.; Bareggi, S.R. Clinical Pharmacokinetics of Atypical Antipsychotics: A Critical Review of the Relationship between Plasma Concentrations and Clinical Response. Clin. Pharmacokinet. 2007, 46, 359–388. [Google Scholar] [CrossRef] [PubMed]
- Wohkittel, C.; Gerlach, M.; Taurines, R.; Wewetzer, C.; Unterecker, S.; Burger, R.; Schreck, D.; Mehler-Wex, C.; Romanos, M.; Egberts, K. Relationship between Clozapine Dose, Serum Concentration, and Clinical Outcome in Children and Adolescents in Clinical Practice. J. Neural Transm. 2016, 123, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Aichhorn, W.; Whitworth, A.B.; Weiss, E.M.; Marksteiner, J. Second-Generation Antipsychotics. Drug Saf. 2006, 29, 587–598. [Google Scholar] [CrossRef]
- Kelly, D.L.; Conley, R.R.; Tamminga, C.A. Differential Olanzapine Plasma Concentrations by Sex in a Fixed-Dose Study. Schizophr. Res. 1999, 40, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Bergemann, N.; Frick, A.; Parzer, P.; Kopitz, J. Olanzapine Plasma Concentration, Average Daily Dose, and Interaction with Co-Medication in Schizophrenic Patients. Pharmacopsychiatry 2004, 37, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Olesen, O.V.; Linnet, K. Olanzapine Serum Concentrations in Psychiatric Patients given Standard Doses: The Influence of Comedication. Ther. Drug Monit. 1999, 21, 87–90. [Google Scholar] [CrossRef]
- Gex-Fabry, M.; Balant-Gorgia, A.E.; Balant, L.P. Therapeutic Drug Monitoring of Olanzapine: The Combined Effect of Age, Gender, Smoking, and Comedication. Ther. Drug Monit. 2003, 25, 46–53. [Google Scholar] [CrossRef]
- Bergemann, N.; Kopitz, J.; Kress, K.R.; Frick, A. Plasma Amisulpride Levels in Schizophrenia or Schizoaffective Disorder. Eur. Neuropsychopharmacol. 2004, 14, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Hart, X.M.; Hiemke, C.; Eichentopf, L.; Lense, X.M.; Clement, H.W.; Conca, A.; Faltraco, F.; Florio, V.; Grüner, J.; Havemann-Reinecke, U.; et al. Therapeutic Reference Range for Aripiprazole in Schizophrenia Revised: A Systematic Review and Metaanalysis. Psychopharmacology 2022, 239, 3377–3391. [Google Scholar] [CrossRef]
- Tien, Y.; Huang, H.-P.; Liao, D.-L.; Huang, S.-C. Dose-Response Analysis of Aripiprazole in Patients with Schizophrenia in Taiwan. Ther. Adv. Psychopharmacol. 2022, 12, 204512532211132. [Google Scholar] [CrossRef] [PubMed]
- Dziurkowska, E.; Wesolowski, M. Simultaneous Quantification of Antipsychotic and Antiepileptic Drugs and Their Metabolites in Human Saliva Using UHPLC-DAD. Molecules 2019, 24, 2953. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, C.; Gonçalves, J.; Soares, S.; Rosado, T.; Araujo, A.R.T.S.; Passarinha, L.A.; Barroso, M.; Gallardo, E. Evaluation of Antipsychotic Drugs’ Stability in Oral Fluid Samples. Molecules 2023, 28, 2030. [Google Scholar] [CrossRef] [PubMed]
- Dziurkowska, E.; Kosinska, S.; Plenis, A.; Wesolowski, M. A New Method for the Determination of Amisulpride in a Small Volume (200 ΜL) of Human Saliva Using LC-DAD Supported by SPE. Separations 2023, 10, 277. [Google Scholar] [CrossRef]
- Miller, J.; Wehring, H.; McMahon, R.P.; DiPaula, B.A.; Love, R.C.; Morris, A.A.; Raley, H.; Feldman, S.; Kelly, D.L. Urine Testing for Antipsychotics: A Pilot Trial for a Method to Determine Detection Levels. Hum. Psychopharmacol. Clin. Exp. 2015, 30, 350–355. [Google Scholar] [CrossRef]
- Jacobs, C.M.; Wagmann, L.; Meyer, M.R. Development, Validation, and Application of a Quantitative Volumetric Absorptive Microsampling–Based Method in Finger Prick Blood by Means of LC-HRMS/MS Applicable for Adherence Monitoring of Antipsychotics. Anal. Bioanal. Chem. 2021, 413, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Shi, Z.; Wu, Y.; Lv, J.; Deng, P.; Liu, G.; An, Z.; Che, Z.; Lu, Y.; Shan, J.; et al. Wireless, Noninvasive Therapeutic Drug Monitoring System for Saliva Measurement toward Medication Management of Schizophrenia. Biosens. Bioelectron. 2023, 234, 115363. [Google Scholar] [CrossRef]
- Piacentino, D. Therapeutic Drug Monitoring of Antidepressants: An Underused but Potentially Valuable Tool in Primary. Front. Psychiatry 2022, 13, 867840. [Google Scholar] [CrossRef]
- Fiaturi, N.; Greenblatt, D.J. Therapeutic Drug Monitoring of Antidepressants. Handb. Exp. Pharmacol. 2019, 250, 115–133. [Google Scholar] [CrossRef]
- Müller, M.J.; Dragicevic, A.; Fric, M.; Gaertner, I.; Grasmäder, K.; Härtter, S.; Hermann, E.; Kuss, H.J.; Laux, G.; Oehl, W.; et al. Therapeutic Drug Monitoring of Tricyclic Antidepressants: How Does It Work under Clinical Conditions? Pharmacopsychiatry 2003, 36, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; Ulrich, S.; Eckermann, G.; Gerlach, M.; Kuss, H.-J.; Laux, G.; Müller-Oerlinghausen, B.; Rao, M.L.; Riederer, P.; Zernig, G.; et al. The AGNP-TDM Expert Group Consensus Guidelines: Focus on Therapeutic Monitoring of Antidepressants. Dialogues Clin. Neurosci. 2005, 7, 231–247. [Google Scholar] [CrossRef]
- Preskorn, S.H. Dose-Effect and Concentration-Effect Relationships with New Antidepressants. Psychopharmacol. Ser. 1993, 10, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Ostad Haji, E.; Tadić, A.; Wagner, S.; Dragicevic, A.; Müller, M.J.; Boland, K.; Rao, M.-L.; Fric, M.; Laux, G.; Hiemke, C. Association between Citalopram Serum Levels and Clinical Improvement of Patients with Major Depression. J. Clin. Psychopharmacol. 2011, 31, 281–286. [Google Scholar] [CrossRef]
- Ostad Haji, E.; Mann, K.; Dragicevic, A.; Müller, M.J.; Boland, K.; Rao, M.-L.; Fric, M.; Laux, G.; Hiemke, C. Potential Cost-Effectiveness of Therapeutic Drug Monitoring for Depressed Patients Treated with Citalopram. Ther. Drug Monit. 2013, 35, 396–401. [Google Scholar] [CrossRef]
- Waldschmitt, C.; Vogel, F.; Pfuhlmann, B.; Hiemke, C. Duloxetine Serum Concentrations and Clinical Effects. Data from a Therapeutic Drug Monitoring (TDM) Survey. Pharmacopsychiatry 2009, 42, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.R.; Kuhlmann, I.B.; Pottegård, A.; Damkier, P. Therapeutic Drug Monitoring of Venlafaxine in an Everyday Clinical Setting: Analysis of Age, Sex and Dose Concentration Relationships. Basic Clin. Pharmacol. Toxicol. 2017, 121, 298–302. [Google Scholar] [CrossRef]
- Grasmäder, K.; Verwohlt, P.L.; Kühn, K.-U.; Frahnert, C.; Hiemke, C.; Dragicevic, A.; von Widdern, O.; Zobel, A.; Maier, W.; Rao, M.L. Relationship between Mirtazapine Dose, Plasma Concentration, Response, and Side Effects in Clinical Practice. Pharmacopsychiatry 2005, 38, 113–117. [Google Scholar] [CrossRef]
- López-Jaramillo, C.; Díaz-Zuluaga, A.M.; de Leon, J.; Schoretsanitis, G.; Paulzen, M.; Unterecker, S.; Schwarz, M.; Conca, A.; Zernig, G.; Gründer, G.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology. Psiquiatr. Biol. 2020, 27, 83–95. [Google Scholar] [CrossRef]
- Dziurkowska, E.; Wesolowski, M. Isolation of Antidepressants and Their Metabolites from Saliva Using Supported Liquid Extraction (SLE). Biomedicines 2023, 11, 708. [Google Scholar] [CrossRef]
- Soares, S.; Rosado, T.; Barroso, M.; Gallardo, E. New Method for the Monitoring of Antidepressants in Oral Fluid Using Dried Spot Sampling. Pharmaceuticals 2021, 14, 1284. [Google Scholar] [CrossRef]
- Marasca, C.; Protti, M.; Mandrioli, R.; Atti, A.R.; Armirotti, A.; Cavalli, A.; De Ronchi, D.; Mercolini, L. Whole Blood and Oral Fluid Microsampling for the Monitoring of Patients under Treatment with Antidepressant Drugs. J. Pharm. Biomed. Anal. 2020, 188, 113384. [Google Scholar] [CrossRef]
- Johannesson, N.; Bergquist, J. Rapid On-Line Extraction and Quantification of Escitalopram from Urine Using Sol–Gel Columns and Mass Spectrometric Detection. J. Pharm. Biomed. Anal. 2007, 43, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Badulla, W.F.S.; Atkoşar, Z.; Arli, G.; Şener, E. Application of LC–ESI-MS/MS Method for Analysis of Escitalopram Oxalate in Human Urine and Pharmaceutical Dosage Forms. J. Chromatogr. Sci. 2020, 58, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ulu, S.T.; Tuncel, M. Determination of Bupropion Using Liquid Chromatography with Fluorescence Detection in Pharmaceutical Preparations, Human Plasma and Human Urine. J. Chromatogr. Sci. 2012, 50, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; He, Y.; Chen, F. Determination of Fluvoxamine Maleate in Human Urine and Human Serum Using Alkaline KMnO4–Rhodamine B Chemiluminescence. Luminescence 2017, 32, 1077–1083. [Google Scholar] [CrossRef]
- Hoskins, J.M.; Shenfield, G.M.; Gross, A.S. A modified hplc method for rapi detection of moclobemide and it N-oxide metabolite in human urin. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 521–529. [Google Scholar] [CrossRef]
- Sarıkaya, M.; Ulusoy, H.I.; Morgul, U.; Ulusoy, S.; Tartaglia, A.; Yılmaz, E.; Soylak, M.; Locatelli, M.; Kabir, A. Sensitive Determination of Fluoxetine and Citalopram Antidepressants in Urine and Wastewater Samples by Liquid Chromatography Coupled with Photodiode Array Detector. J. Chromatogr. A 2021, 1648, 462215. [Google Scholar] [CrossRef]
- Agrawal, N.; Marco-Peiró, S.; Esteve-Romero, J.; Durgbanshi, A.; Bose, D.; Peris-Vicente, J.; Carda-Broch, S. Determination of Paroxetine in Blood and Urine Using Micellar Liquid Chromatography with Electrochemical Detection. J. Chromatogr. Sci. 2014, 52, 1217–1223. [Google Scholar] [CrossRef]
- Bishnoi, S.; Sharma, A.; Singhal, R.; Goyal, R.N. Edge Plane Pyrolytic Graphite as a Sensing Surface for the Determination of Fluvoxamine in Urine Samples of Obsessive-Compulsive Disorder Patients. Biosens. Bioelectron. 2020, 168, 112489. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, A.; Farajzadeh, M.A.; Yaripour, S.; Afshar Mogaddam, M.R. Determination of Tricyclic Antidepressants in Human Urine Samples by the Three-Step Sample Pretreatment Followed by HPLC-UV Analysis: An Efficient Analytical Method for Further Pharmacokinetic and Forensic Studies. EXCLI J. 2018, 17, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Petruczynik, A.; Wróblewski, K.; Wojtanowski, K.; Mroczek, T.; Juchnowicz, D.; Karakuła-Juchnowicz, H.; Tuzimski, T. Comparison of Various Chromatographic Systems for Identification of Vortioxetine in Bulk Drug Substance, Human Serum, Saliva, and Urine Samples by HPLC-DAD and LC-QTOF-MS. Molecules 2020, 25, 2483. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Hao, X.; Yu, J.; Wang, D.; Zhang, J.; Yu, Z.; Gao, F.; Zhou, C. Machine Learning-Based Prediction of Sertraline Concentration in Patients with Depression through Therapeutic Drug Monitoring. Front. Pharmacol. 2024, 15, 1289673. [Google Scholar] [CrossRef] [PubMed]

| Drug | Class | Therapeutic Drug Range (Blood) | TDM AGNP Recommendation Levels | Preferred Sampling Method | Other Sampling Methods |
|---|---|---|---|---|---|
| Lithium | Mood stabilizer | 0.5–1.2 mmol/L | 1 | Plasma | Saliva, urine, sweat, interstitial fluid, dried blood/plasma spots [23,24] |
| Valproate | Antiseizure medication/mood stabilizer/migraine prevention | 50–100 μg/mL | 2 | Plasma | Saliva, urine, dried blood spots [25,26,27] |
| Carbamazepine | Antiseizure medication/mood stabilizer/neuropathic pain | 4–12 μg/mL | 1 | Serum | Saliva, urine [28,29,30,31,32] |
| Oxcarbazepine | Antiseizure medication/mood stabilizer | 10–35 μg/mL | 2 | Plasma | Urine [33] |
| Lamotrigine | Antiseizure medication/mood stabilizer | 3–15 μg/mL | 2 | Plasma | Saliva, dried blood spots [34,35,36,37] |
| Drug | Class | Therapeutic Drug Range (Blood) | TDM AGNP Recommendation Levels | Preferred Sampling Method | Other Sampling Methods |
|---|---|---|---|---|---|
| Amisulpride | SGA | 100–320 ng/mL | 1 | Plasma | Saliva, dried blood spots [136,138] |
| Aripiprazole | TGA | 100–350 ng/mL | 2 | Plasma | Saliva, dried blood spots [134,138] |
| Chlorpromazine | FGA | 30–300 ng/mL | 2 | Plasma | Saliva [135] |
| Clozapine | SGA | 350–600 ng/mL | 1 | Plasma | Saliva, dried blood spots [134,135,136,138] |
| Haloperidol | FGA | 1–10 ng/mL | 1 | Plasma | Saliva, dried blood spots, urine [135,137,138] |
| Olanzapine | SGA | 20–80 ng/mL | 1 | Plasma | Saliva, dried blood spots, urine [134,135,137,138] |
| Quetiapine | SGA | 100–500 ng/mL | 2 | Plasma | Saliva, dried blood spots, urine [134,135,137] |
| Risperidone | SGA | 20–60 ng/mL | 2 | Plasma | Saliva, dried blood spots, urine [134,137,138] |
| Drug | Class | Therapeutic Drug Range (Blood) | TDM AGNP Recommendation Levels | Preferred Sampling Method | Other Sampling Methods |
|---|---|---|---|---|---|
| Amitriptyline | TCA | 80–200 ng/mL | 1 | Plasma | Saliva, urine [162] |
| Bupropion | NDRI | 10–100 ng/mL | 2 | Plasma | Urine [156] |
| Citalopram | SSRI | 50–110 ng/mL | 1 | Plasma, serum | Saliva, dried saliva spots, urine [151,152,153,159] |
| Clomipramine | TCA | 230–450 ng/mL | 1 | Plasma | Urine [162] |
| Desipramine | TCA | 100–300 ng/mL | 2 | Plasma | Urine [162] |
| Duloxetine | SNRI | 30–120 ng/mL | 2 | Plasma | Saliva [151,152] |
| Escitalopram | SSRI | 15–80 ng/mL | 2 | Plasma, serum | Urine [154,155] |
| Fluoxetine | SSRI | 120–500 ng/mL | 3 | Plasma, serum | Urine, dried saliva spots [152,153,159] |
| Fluvoxamine | SSRI | 60–230 ng/mL | 2 | Plasma, serum | Urine [157,161] |
| Imipramine | TCA | 175–300 ng/mL | 1 | Plasma | Urine [162] |
| Mirtazapine | NaSSA | 30–80 ng/mL | 2 | Plasma | Saliva [151] |
| Moclobemide | MAOI | 300–1000 ng/mL | 3 | Plasma | Urine [158] |
| Nortriptyline | TCA | 70–170 ng/mL | 1 | Plasma | Urine [162] |
| Paroxetine | SSRI | 20–65 ng/mL | 3 | Plasma, serum | Urine, dried saliva spots [152,160] |
| Sertraline | SSRI | 10–150 ng/mL | 2 | Plasma, serum | Saliva, dried saliva spots [151,152,153] |
| Venlafaxine | SNRI | 100–400 ng/mL | 2 | Plasma | Saliva, dried saliva spots [151,152] |
| Vortioxetine | SMS/SSRI | 15–60 ng/mL | 2 | Plasma | Saliva, urine [153,163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biso, L.; Aringhieri, S.; Carli, M.; Scarselli, M.; Longoni, B. Therapeutic Drug Monitoring in Psychiatry: Enhancing Treatment Precision and Patient Outcomes. Pharmaceuticals 2024, 17, 642. https://doi.org/10.3390/ph17050642
Biso L, Aringhieri S, Carli M, Scarselli M, Longoni B. Therapeutic Drug Monitoring in Psychiatry: Enhancing Treatment Precision and Patient Outcomes. Pharmaceuticals. 2024; 17(5):642. https://doi.org/10.3390/ph17050642
Chicago/Turabian StyleBiso, Letizia, Stefano Aringhieri, Marco Carli, Marco Scarselli, and Biancamaria Longoni. 2024. "Therapeutic Drug Monitoring in Psychiatry: Enhancing Treatment Precision and Patient Outcomes" Pharmaceuticals 17, no. 5: 642. https://doi.org/10.3390/ph17050642
APA StyleBiso, L., Aringhieri, S., Carli, M., Scarselli, M., & Longoni, B. (2024). Therapeutic Drug Monitoring in Psychiatry: Enhancing Treatment Precision and Patient Outcomes. Pharmaceuticals, 17(5), 642. https://doi.org/10.3390/ph17050642









