Therapeutic Drug Monitoring in Psychiatry: Enhancing Treatment Precision and Patient Outcomes
Abstract
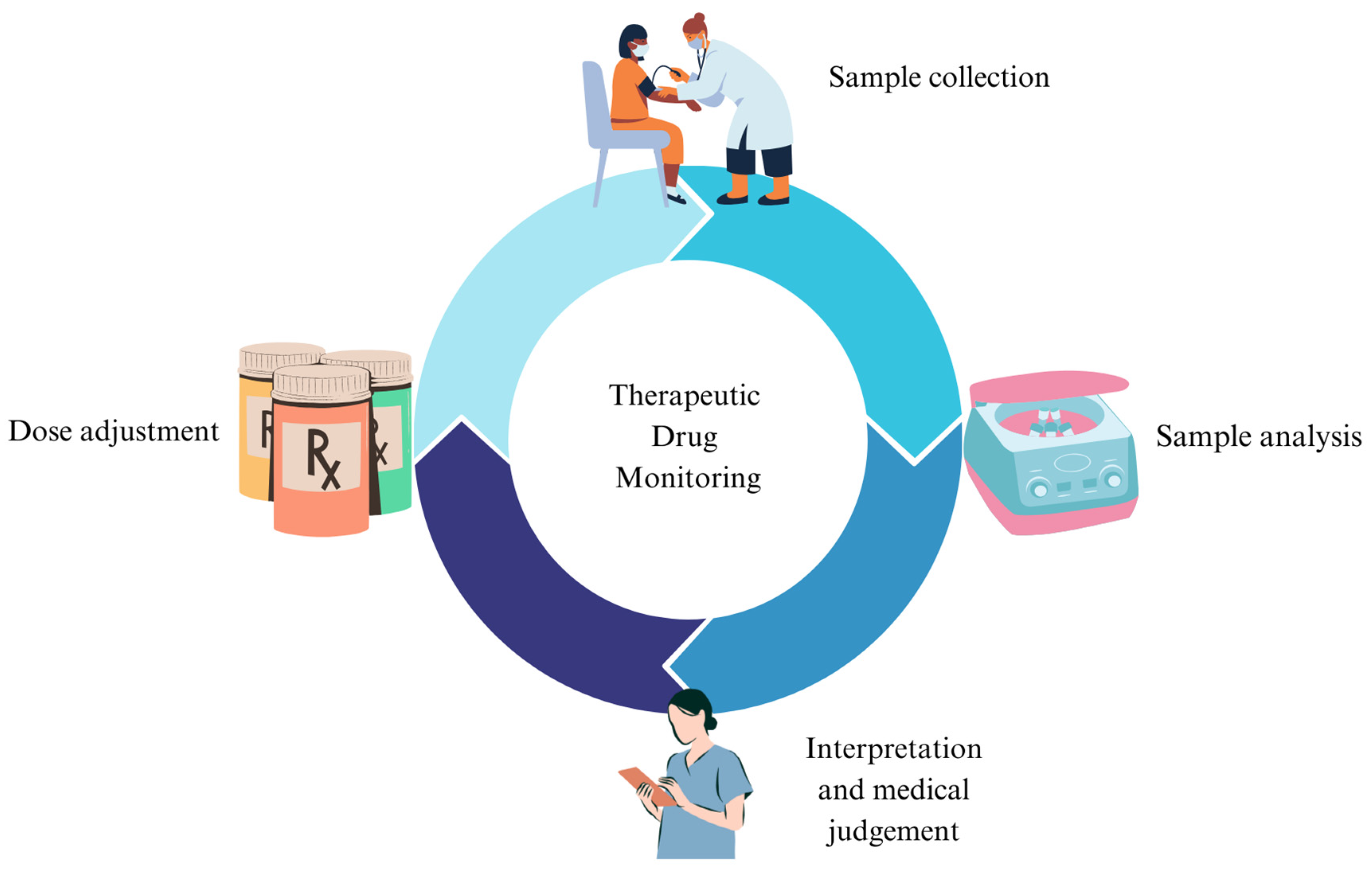
:1. Introduction
2. TDM and Mood Stabilizers
3. TDM and Antipsychotics
4. TDM and Antidepressant Medications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schoretsanitis, G.; Paulzen, M.; Unterecker, S.; Schwarz, M.; Conca, A.; Zernig, G.; Gründer, G.; Haen, E.; Baumann, P.; Bergemann, N.; et al. TDM in Psychiatry and Neurology: A Comprehensive Summary of the Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology, Update 2017; a Tool for Clinicians. World J. Biol. Psychiatry 2018, 19, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.E.; Marks, V. Value of Plasma-Lithium Monitoring. Lancet 1971, 1, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Pippenger, C.E. Therapeutic Drug Monitoring Assay Development to Improve Efficacy and Safety. Epilepsy Res. 2006, 68, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; Hiemke, C.; Ulrich, S.; Eckermann, G.; Gaertner, I.; Gerlach, M.; Kuss, H.-J.; Laux, G.; Müller-Oerlinghausen, B.; Rao, M.L.; et al. The AGNP-TDM Expert Group Consensus Guidelines: Therapeutic Drug Monitoring in Psychiatry. Pharmacopsychiatry 2004, 37, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.-M.; Li, X.; Wang, Z.; Qin, J.; Jiang, D.; Tian, P.; Yang, P.; Zhao, R. Status and Quality of Guidelines for Therapeutic Drug Monitoring Based on AGREE II Instrument. Clin. Pharmacokinet. 2023, 62, 1201–1217. [Google Scholar] [CrossRef]
- Aringhieri, S.; Carli, M.; Kolachalam, S.; Verdesca, V.; Cini, E.; Rossi, M.; McCormick, P.J.; Corsini, G.U.; Maggio, R.; Scarselli, M. Molecular Targets of Atypical Antipsychotics: From Mechanism of Action to Clinical Differences. Pharmacol. Ther. 2018, 192, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Schoretsanitis, G.; Kane, J.M.; Correll, C.U.; Marder, S.R.; Citrome, L.; Newcomer, J.W.; Robinson, D.G.; Goff, D.C.; Kelly, D.L.; Freudenreich, O.; et al. Blood Levels to Optimize Antipsychotic Treatment in Clinical Practice: A Joint Consensus Statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft Für Neuropsychopharmakologie Und Pharmakopsychiatrie. J. Clin. Psychiatry 2020, 81, 3649. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C. Clinical Utility of Drug Measurement and Pharmacokinetics—Therapeutic Drug Monitoring in Psychiatry. Eur. J. Clin. Pharmacol. 2008, 64, 159–166. [Google Scholar] [CrossRef]
- Burke, M.J.; Preskorn, S.H. Therapeutic Drug Monitoring of Antidepressants. Clin. Pharmacokinet. 1999, 37, 147–165. [Google Scholar] [CrossRef]
- Eilers, R. Therapeutic Drug Monitoring for the Treatment of Psychiatric Disorders. Clin. Pharmacokinet. 1995, 29, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Al Mutarid, M.; Alhossan, A.; Khan, T.; Alyami, M.G.; Almutared, K.M.; Alshiban, M.; Alyami, A.H.D.; Alyami, M.M.M.; AlKulayb, J.A.H.; Alyami, D.S.; et al. Knowledge and Attitude of Healthcare Practitioners toward Therapeutic Drug Monitoring Practices in the Najran Region, Kingdom of Saudi Arabia. Cureus 2022, 14, e32214. [Google Scholar] [CrossRef] [PubMed]
- Eryılmaz, G.; Hızlı Sayar, G.; Gül, I.G.; Noyan, C.O.; Özten, E.; Darçın, A.E.; Yorbik, Ö.; Dilbaz, N. Therapeutic Drug Monitoring: Perspectives of Psychiatrists in Turkey. Int. J. Psychiatry Clin. Pract. 2015, 19, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Guo, G.-X.; Sun, C.; Zhang, J.; Rong, Z.; He, J.; Sun, Z.; Yan, F.; Tang, Y.; Wang, C.; et al. Therapeutic Drug Monitoring of Psychotropic Drugs in China. Ther. Drug Monit. 2013, 35, 816–822. [Google Scholar] [CrossRef]
- Stephan, P.L.; Etzensberger, M.; Sirot, J. Arzneimittelspiegel Als Pharmakotherapeutisches Werkzeug Bei Der Behandlung Mit Psychopharmaka. Praxis 2006, 95, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Molden, E. Therapeutic Drug Monitoring of Clozapine in Adults with Schizophrenia: A Review of Challenges and Strategies. Expert. Opin. Drug Metab. Toxicol. 2021, 17, 1211–1221. [Google Scholar] [CrossRef]
- Vázquez, G.H.; Bahji, A.; Undurraga, J.; Tondo, L.; Baldessarini, R.J. Efficacy and Tolerability of Combination Treatments for Major Depression: Antidepressants plus Second-Generation Antipsychotics vs. Esketamine vs. Lithium. J. Psychopharmacol. 2021, 35, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Sunny, S.; Prabhu, S.; Chand, S.; Up, N.; Susan, C.; Joel, J.J. Assessment of Drug-Drug Interactions among Patients with Psychiatric Disorders: A Clinical Pharmacist-Led Study. Clin. Epidemiol. Glob. Health 2022, 13, 100930. [Google Scholar] [CrossRef]
- Aburamadan, H.A.R.; Sridhar, S.B.; Tadross, T.M. Assessment of Potential Drug Interactions among Psychiatric Inpatients Receiving Antipsychotic Therapy of a Secondary Care Hospital, United Arab Emirates. J. Adv. Pharm. Technol. Res. 2021, 12, 45–51. [Google Scholar] [CrossRef]
- Sandson, N. Important Drug-Drug Interactions for the Addiction Psychiatrist. Psychiatr. Clin. N. Am. 2022, 45, 431–450. [Google Scholar] [CrossRef]
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 Guidelines for the Management of Patients with Bipolar Disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef]
- Nierenberg, A.A.; Agustini, B.; Köhler-Forsberg, O.; Cusin, C.; Katz, D.; Sylvia, L.G.; Peters, A.; Berk, M. Diagnosis and Treatment of Bipolar Disorder: A Review. JAMA 2023, 330, 1370–1380. [Google Scholar] [CrossRef]
- Parkin, G.M.; McCarthy, M.J.; Thein, S.H.; Piccerillo, H.L.; Warikoo, N.; Granger, D.A.; Thomas, E.A. Saliva Testing as a Means to Monitor Therapeutic Lithium Levels in Patients with Psychiatric Disorders: Identification of Clinical and Environmental Covariates, and Their Incorporation into a Prediction Model. Bipolar Disord. 2021, 23, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.; Qassem, M.; Triantis, I.F.; Kyriacou, P.A. Advances in Therapeutic Monitoring of Lithium in the Management of Bipolar Disorder. Sensors 2022, 22, 736. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Gupta, Y.K.; Singh, M.; Joshi, R.; Tiwari, P.; Kaleekal, T.; Tripathi, M. Correlation of Saliva and Serum Free Valproic Acid Concentrations in Persons with Epilepsy. Seizure 2015, 25, 187–190. [Google Scholar] [CrossRef]
- Guo, M.; Shao, L.; Chen, X.; Li, H.; Wang, L.; Pan, Y.; Tang, D. Assay of Dried Blood Spot from Finger Prick for Sodium Valproate via Ink Auxiliary Headspace Gas Chromatography Mass Spectrometry. J. Chromatogr. A 2019, 1601, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Namera, A.; Uekusa, K.; Saito, T.; Yoshimoto, K.; Ishiuchi, N.; Murata, K.; Nagao, M. A Method for Determining Valproic Acid in Human Whole Blood and Urine via Gas Chromatography-Mass Spectrometry and Small-Scale Inter-Laboratory Trial. Leg. Med. 2022, 59, 102133. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Spencer, E.P.; Berry, D.J. Therapeutic Drug Monitoring of Antiepileptic Drugs in Epilepsy: A 2018 Update. Ther. Drug Monit. 2018, 40, 526–548. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Singh, M.; Kaleekal, T.; Gupta, Y.K.; Tripathi, M. Concentration of Antiepileptic Drugs in Persons with Epilepsy: A Comparative Study in Serum and Saliva. Int. J. Neurosci. 2016, 126, 972–978. [Google Scholar] [CrossRef]
- Chen, N.; Yuan, Y.; Lu, P.; Wang, L.; Zhang, X.; Chen, H.; Ma, P. Detection of Carbamazepine in Saliva Based on Surface-Enhanced Raman Spectroscopy. Biomed. Opt. Express 2021, 12, 7673. [Google Scholar] [CrossRef]
- Rezaei Kahkha, M.R.; Oveisi, A.R.; Kaykhaii, M.; Rezaei Kahkha, B. Determination of Carbamazepine in Urine and Water Samples Using Amino-Functionalized Metal–Organic Framework as Sorbent. Chem. Cent. J. 2018, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Chen, F. Determination of Carbamazepine in Human Urine and Serum Samples by High-performance Liquid Chromatography with Post-column Ru(Bipy)-Ce(SO4)2 Chemiluminescence Detection. Luminescence 2013, 28, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Erarpat, S.; Bodur, S.; Ayyıldız, M.F.; Günkara, Ö.T.; Erulaş, F.; Chormey, D.S.; Turak, F.; Budak, T.B.; Bakırdere, S. Accurate and Simple Determination of Oxcarbazepine in Human Plasma and Urine Samples Using Switchable-hydrophilicity Solvent in GC–MS. Biomed. Chromatogr. 2020, 34, e4915. [Google Scholar] [CrossRef] [PubMed]
- Incecayir, T.; Agabeyoglu, I.; Gucuyener, K. Comparison of Plasma and Saliva Concentrations of Lamotrigine in Healthy Volunteers. Arzneimittelforschung 2011, 57, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Tsiropoulos, I.; Kristensen, O.; Klitgaard, N.A. Saliva and Serum Concentration of Lamotrigine in Patients with Epilepsy. Ther. Drug Monit. 2000, 22, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Trnavska, Z.; Krejcova, H.; Tkaczykovam; Salcmanova, Z.; Elis, J. Pharmacokinetics of Lamotrigine (Lamictal) in Plasma and Saliva. Eur. J. Drug Metab. Pharmacokinet. 1991, 3, 211–215. [Google Scholar]
- Milosheska, D.; Roškar, R.; Vovk, T.; Lorber, B.; Grabnar, I.; Trontelj, J. An LC-MS/MS Method for Quantification of Lamotrigine and Its Main Metabolite in Dried Blood Spots. Pharmaceuticals 2024, 17, 449. [Google Scholar] [CrossRef]
- Houston, J.P.; Tohen, M.; Degenhardt, E.K.; Jamal, H.H.; Liu, L.L.L.; Ketter, T.A. Olanzapine-Divalproex Combination versus Divalproex Monotherapy in the Treatment of Bipolar Mixed Episodes: A Double-Blind, Placebo-Controlled Study. J. Clin. Psychiatry 2009, 70, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Ercis, M.; Ozerdem, A.; Singh, B. When and How to Use Lithium Augmentation for Treating Major Depressive Disorder. J. Clin. Psychiatry 2023, 84, 23ac14813. [Google Scholar] [CrossRef]
- Licht, R.W. Lithium: Still a Major Option in the Management of Bipolar Disorder. CNS Neurosci. Ther. 2012, 18, 219–226. [Google Scholar] [CrossRef]
- Gitlin, M. Lithium Side Effects and Toxicity: Prevalence and Management Strategies. Int. J. Bipolar Disord. 2016, 4, 1–10. [Google Scholar] [CrossRef]
- Nolen, W.A.; Licht, R.W.; Young, A.H.; Malhi, G.S.; Tohen, M.; Vieta, E.; Kupka, R.W.; Zarate, C.; Nielsen, R.E.; Baldessarini, R.J.; et al. What Is the Optimal Serum Level for Lithium in the Maintenance Treatment of Bipolar Disorder? A Systematic Review and Recommendations from the ISBD/IGSLI Task Force on Treatment with Lithium. Bipolar Disord. 2019, 21, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S.; Reddy, M.S. Brief Communication Serum Lithium Levels: Ideal Time for Sample Collection! Are We Doing It Right? Indian J. Psychol. Med. 2014, 36, 346–347. [Google Scholar] [CrossRef]
- Malhi, G.S.; Gershon, S.; Outhred, T. Lithiumeter: Version 2.0. Bipolar Disord. 2016, 18, 631–641. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Pattanaseri, K.; Hidalgo-Mazzei, D.; Taylor, D.; Young, A.H. Is Lithium Monitoring NICE? Lithium Monitoring in a UK Secondary Care Setting. J. Psychopharmacol. 2018, 32, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.M.; Haddad, P.M.; Ferrier, I.N.; Aronson, J.K.; Barnes, T.; Cipriani, A.; Coghill, D.R.; Fazel, S.; Geddes, J.R.; Grunze, H.; et al. Evidence-Based Guidelines for Treating Bipolar Disorder: Revised Third Edition Recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2016, 30, 495–553. [Google Scholar] [CrossRef]
- Pérez de Mendiola, X.; Hidalgo-Mazzei, D.; Vieta, E.; González-Pinto, A. Overview of Lithium’s Use: A Nationwide Survey. Int. J. Bipolar Disord. 2021, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Parkin, G.M.; Thomas, E.A. Provider Perspectives on the Current Use of Lithium Medications and Lithium Monitoring Practices for Psychiatric Conditions. Neuropsychiatr. Dis. Treat. 2022, 18, 2083–2093. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Tsai, S.-Y.; Wang, L.-J.; Liang, C.-S.; Carvalho, A.F.; Solmi, M.; Vieta, E.; Lin, P.-Y.; Hu, C.-A.; Kao, H.-Y. Predicting Serum Levels of Lithium-Treated Patients: A Supervised Machine Learning Approach. Biomedicines 2021, 9, 1558. [Google Scholar] [CrossRef]
- Ooba, N.; Tsutsumi, D.; Kobayashi, N.; Hidaka, S.; Hayashi, H.; Obara, T.; Satoh, M.; Kubota, K.; Fukuoka, N. Prevalence of Therapeutic Drug Monitoring for Lithium and the Impact of Regulatory Warnings: Analysis Using Japanese Claims Database. Ther. Drug Monit. 2018, 40, 252–256. [Google Scholar] [CrossRef]
- Rej, S.; Herrmann, N.; Gruneir, A.; Jandoc, R.; McArthur, E.; Dixon, S.; Garg, A.X. Blood Lithium Monitoring Practices in a Population-Based Sample of Older Adults. J. Clin. Psychiatry 2018, 79, 10458. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, E.; Bazire, S.; Anderson, T.; Wood, J.; Grassby, P.; Desborough, J.A. Impact of Active Monitoring on Lithium Management in Norfolk. Ther. Adv. Psychopharmacol. 2013, 3, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, V.; Al-Sukhni, M.; Lawson, A.; Chandler, G. Lithium Prescribing and Therapeutic Drug Monitoring in Bipolar Disorder: A Survey of Current Practices and Perspectives. J. Psychiatr. Pract. 2020, 26, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, C.; Duff, C.J.; Scargill, J.; Green, L.; Holland, D.; Heald, A.H.; Fryer, A.A. Serum Lithium Test Requesting across Three UK Regions: An Evaluation of Adherence to Monitoring Guidelines. BMC Psychiatry 2021, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Carli, M.; Risaliti, E.; Francomano, M.; Kolachalam, S.; Longoni, B.; Bocci, G.; Maggio, R.; Scarselli, M. A 5-Year Study of Lithium and Valproic Acid Drug Monitoring in Patients with Bipolar Disorders in an Italian Clinical Center. Pharmaceuticals 2022, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- Tomson, T.; Battino, D.; Perucca, E. The Remarkable Story of Valproic Acid. Lancet Neurol. 2016, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Hakami, T. Neuropharmacology of Antiseizure Drugs. Neuropsychopharmacol. Rep. 2021, 41, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Nanau, R.M.; Neuman, M.G. Adverse Drug Reactions Induced by Valproic Acid. Clin. Biochem. 2013, 46, 1323–1338. [Google Scholar] [CrossRef]
- Shakerdi, L.; Ryan, A. Drug-Induced Hyperammonaemia. J. Clin. Pathol. 2023, 76, 501–509. [Google Scholar] [CrossRef]
- Tomson, T.; Battino, D.; Perucca, E. Teratogenicity of Antiepileptic Drugs. Curr. Opin. Neurol. 2019, 32, 246–252. [Google Scholar] [CrossRef]
- Bromley, R.; Adab, N.; Bluett-Duncan, M.; Clayton-Smith, J.; Christensen, J.; Edwards, K.; Greenhalgh, J.; Hill, R.A.; Jackson, C.F.; Khanom, S.; et al. Monotherapy Treatment of Epilepsy in Pregnancy: Congenital Malformation Outcomes in the Child. Cochrane Database Syst. Rev. 2023, 8, CD010224. [Google Scholar] [CrossRef] [PubMed]
- Collins-Yoder, A.; Lowell, J. Valproic Acid: Special Considerations and Targeted Monitoring. J. Neurosci. Nurs. 2017, 49, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Damegunta, S.R. Time Matters!: When Is the Right Time to Estimate Serum Valproic Acid Levels? Indian J. Psychol. Med. 2014, 36, 349–350. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Lai, E.C.-C.; Chen, Y.-C.B.; Kao, H.-Y. Valproic Acid Monitoring: Serum Prediction Using a Machine Learning Framework from Multicenter Real-World Data. J. Affect. Disord. 2024, 347, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Paholpak, P.; Paholpak, S.; Patanasethanant, D.; Rangseekajee, P.; Patjanasoontorn, N. Rate of Serum Valproate Concentration Monitoring in Patients with Bipolar Disorder Type I at Srinagarind Hospital Outpatient Clinic. J. Med. Assoc. Thai 2016, 99, 1153–1160. [Google Scholar]
- Shaikh, A.S.; Liu, H.; Li, Y.; Cao, L.; Guo, R. Therapeutic Drug Monitoring of Valproic Acid. Pak. J. Pharm. Sci. 2018, 31, 1773–1776. [Google Scholar]
- Machino, A.; Jitsuiki, H.; Okamoto, Y.; Izumitani, S.; Kimura, Y.; Suzuki, K.; Tanaka, T.; Inoue, T.; Koyama, T.; Wada, K.; et al. The Valproate Serum Level in Maintenance Therapy for Bipolar Disorder in Japan. Hiroshima J. Med. Sci. 2013, 62, 7–12. [Google Scholar]
- Biso, L.; Carli, M.; Kolachalam, S.; Monticelli, G.; Calabrò, P.F.; di Paolo, A.; Giorgi, F.S.; Bocci, G.; Scarselli, M. A 5-Year Study of Antiseizure Medications (ASMs) Monitoring in Patients with Neuropsychiatric Disorders in an Italian Clinical Center. Pharmaceuticals 2023, 16, 945. [Google Scholar] [CrossRef] [PubMed]
- Grunze, A.; Amann, B.L.; Grunze, H. Efficacy of Carbamazepine and Its Derivatives in the Treatment of Bipolar Disorder. Medicina 2021, 57, 433. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, R.; Chen, Q.; Li, M.; Lin, W.; Cui, L. Effect of Carbamazepine on the Bone Health of People with Epilepsy: A Systematic Review and Meta-Analysis. J. Int. Med. Res. 2020, 48, 300060520902608. [Google Scholar] [CrossRef]
- Jentink, J.; Dolk, H.; Loane, M.A.; Morris, J.K.; Wellesley, D.; Garne, E.; De Jong-van Den Berg, L. Intrauterine Exposure to Carbamazepine and Specific Congenital Malformations: Systematic Review and Case-Control Study. BMJ (Online) 2010, 341, 1261. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Lee, P.M.Y.; Li, F.; Li, J. Prenatal Carbamazepine Exposure and Academic Performance in Adolescents: A Population-Based Cohort Study. Neurology 2023, 100, e728–e738. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.M.; Donnelly, A. Carbamazepine-10,11-Epoxide in Therapeutic Drug Monitoring. Ther. Drug Monit. 1998, 20, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Burianová, I.; Bořecká, K. Routine Therapeutic Monitoring of the Active Metabolite of Carbamazepine: Is It Really Necessary? Clin. Biochem. 2015, 48, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.N.; Nguyen, L.H.; Fitz-Gerald, M.J.; Crane, E.; Lewis, K.; Pierre, S.S.; Kaye, A.D.; Kaye, A.M.; Kaye, J.S.; Kaye, R.J.; et al. Lamotrigine and Stevens-Johnson Syndrome Prevention. Psychopharmacol. Bull. 2021, 51, 96–114. [Google Scholar] [PubMed]
- Mannapperuma, U.; Galappatthy, P.; Jayakody, R.L.; Mendis, J.; de Silva, V.A.; Hanwella, R. Safety Monitoring of Treatment in Bipolar Disorder in a Tertiary Care Setting in Sri Lanka and Recommendations for Improved Monitoring in Resource Limited Settings. BMC Psychiatry 2019, 19, 194. [Google Scholar] [CrossRef] [PubMed]
- Grześk, G.; Stolarek, W.; Kasprzak, M.; Grześk, E.; Rogowicz, D.; Wiciński, M.; Krzyżanowski, M. Therapeutic Drug Monitoring of Carbamazepine: A 20-Year Observational Study. J. Clin. Med. 2021, 10, 5396. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, S.N.; Ko, J.Y.; Katzow, J.J. Oxcarbazepine Treatment of Refractory Bipolar Disorder: A Retrospective Chart Review. Bipolar Disord. 2002, 4, 70–74. [Google Scholar] [CrossRef]
- Vieta, E.; Sanchez-Moreno, J. Acute and Long-Term Treatment of Mania. Dialogues Clin. Neurosci. 2008, 10, 165–179. [Google Scholar] [CrossRef]
- Kishi, T.; Ikuta, T.; Matsuda, Y.; Sakuma, K.; Okuya, M.; Mishima, K.; Iwata, N. Mood Stabilizers and/or Antipsychotics for Bipolar Disorder in the Maintenance Phase: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Mol. Psychiatry 2021, 26, 4146–4157. [Google Scholar] [CrossRef]
- Montouris, G. Safety of the Newer Antiepileptic Drug Oxcarbazepine during Pregnancy. Curr. Med. Res. Opin. 2005, 21, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Wellington, K.; Goa, K.L. Oxcarbazepine: An Update of Its Efficacy in the Management of Epilepsy. CNS Drugs 2001, 15, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Bring, P.; Ensom, M.H.H. Does Oxcarbazepine Warrant Therapeutic Drug Monitoring? A Critical Review. Clin. Pharmacokinet. 2008, 47, 767–778. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar Disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Kanner, A.M. Lamotrigine-Induced Rash: Can We Stop Worrying? Epilepsy Curr. 2005, 5, 190–191. [Google Scholar] [CrossRef] [PubMed]
- French, J.A.; Perucca, E.; Sander, J.W.; Bergfeldt, L.; Baulac, M.; Auerbach, D.S.; Keezer, M.; Thijs, R.D.; Devinsky, O.; Vossler, D.G.; et al. FDA Safety Warning on the Cardiac Effects of Lamotrigine: An Advisory from the Ad Hoc ILAE/AES Task Force. Epilepsia Open 2021, 6, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Husein, N.; Thijs, R.D.; Bunschoten, J.W.; Keezer, M.R.; Sander, J.W. Concerns about Lamotrigine. Lancet Neurol. 2021, 20, 418–419. [Google Scholar] [CrossRef]
- Pariente, G.; Leibson, T.; Shulman, T.; Adams-Webber, T.; Barzilay, E.; Nulman, I. Pregnancy Outcomes Following In Utero Exposure to Lamotrigine: A Systematic Review and Meta-Analysis. CNS Drugs 2017, 31, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Tomson, T.; Battino, D.; Bonizzoni, E.; Craig, J.; Lindhout, D.; Perucca, E.; Sabers, A.; Thomas, S.V.; Vajda, F. Comparative Risk of Major Congenital Malformations with Eight Different Antiepileptic Drugs: A Prospective Cohort Study of the EURAP Registry. Lancet Neurol. 2018, 17, 530–538. [Google Scholar] [CrossRef]
- Kagawa, S.; Mihara, K.; Nakamura, A.; Nemoto, K.; Suzuki, T.; Nagai, G.; Kondo, T. Relationship between Plasma Concentrations of Lamotrigine and Its Early Therapeutic Effect of Lamotrigine Augmentation Therapy in Treatment-Resistant Depressive Disorder. Ther. Drug Monit. 2014, 36, 730–733. [Google Scholar] [CrossRef]
- Kikkawa, A.; Kitamura, Y.; Aiba, T.; Hiraki, K.; Sendo, T. Correlation between the Efficacy of Lamotrigine and the Serum Lamotrigine Level during the Remission Phase of Acute Bipolar II Depression: A Naturalistic and Unblinded Prospective Pilot Study. Biol. Pharm. Bull. 2017, 40, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Douglas-Hall, P.; Dzahini, O.; Gaughran, F.; Bile, A.; Taylor, D. Variation in Dose and Plasma Level of Lamotrigine in Patients Discharged from a Mental Health Trust. Ther. Adv. Psychopharmacol. 2017, 7, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Unholzer, S.; Haen, E. Retrospective Analysis of Therapeutic Drug Monitoring Data for Treatment of Bipolar Disorder with Lamotrigine. Pharmacopsychiatry 2015, 48, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Chouchana, M.; Delage, C.; Godin, O.; Fontan, J.-E.; Bellivier, F.; Gard, S.; Aubin, V.; Belzeaux, R.; Dubertret, C.; Haffen, E.; et al. Factors Associated with Lamotrigine Concentration/Dose Ratio in Individuals with Bipolar Disorders. Eur. Neuropsychopharmacol. 2023, 73, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y. Update on Typical and Atypical Antipsychotic Drugs. Annu. Rev. Med. 2013, 64, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Carli, M.; Kolachalam, S.; Longoni, B.; Pintaudi, A.; Baldini, M.; Aringhieri, S.; Fasciani, I.; Annibale, P.; Maggio, R.; Scarselli, M. Atypical Antipsychotics and Metabolic Syndrome: From Molecular Mechanisms to Clinical Differences. Pharmaceuticals 2021, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, N.; Lanctôt, K.L. Do Atypical Antipsychotics Cause Stroke? CNS Drugs 2005, 19, 91–103. [Google Scholar] [CrossRef]
- Luft, B.; Taylor, D. A Review of Atypical Antipsychotic Drugs versus Conventional Medication in Schizophrenia. Expert. Opin. Pharmacother. 2006, 7, 1739–1748. [Google Scholar] [CrossRef]
- Alsabhan, J.F.; Almalag, H.M.; Aljafali, L.; Alnughamish, H.; Almutlaq, G. Prescribing Pattern of Antipsychotics for Patients with Schizophrenia Using the Total Daily Dose Online Tool. Saudi Pharm. J. 2023, 31, 101837. [Google Scholar] [CrossRef]
- Bastaki, K.; El Anbari, M.; Ghuloum, S.; Jithesh, P.V. Prescription Pattern and Off-Label Use of Antipsychotics in a Middle Eastern Population. Front. Pharmacol. 2021, 12, 753845. [Google Scholar] [CrossRef]
- Eloff, I.; Esterhuysen, W.; Odayar, K. Antipsychotic Use in a Resource-Limited Setting: Findings in an Eastern Cape Psychiatric Hospital. S. Afr. J. Psychiatry 2017, 23, 1093. [Google Scholar] [CrossRef] [PubMed]
- Ayenew, W.; Asmamaw, G.; Bitew, T. Antipsychotic Polypharmacy Among Patients with Schizophrenia in Africa: A Systematic Review and Meta-Analysis. Int. J. Neuropsychopharmacol. 2021, 24, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Scarselli, M.; Annibale, P.; Gerace, C.; Radenovic, A. Enlightening G-Protein-Coupled Receptors on the Plasma Membrane Using Super-Resolution Photoactivated Localization Microscopy. Biochem. Soc. Trans. 2013, 41, 191–196. [Google Scholar] [CrossRef]
- Chan, Y.-C.; Derflinger, B.A. Use of Therapeutic Drug Monitoring (TDM) for Antipsychotics to Avoid Polypharmacy in the Treatment of Schizophrenia. Psychiatry Res. Case Rep. 2023, 2, 100130. [Google Scholar] [CrossRef]
- Kapur, S.; Zipursky, R.; Jones, C.; Remington, G.; Houle, S. Relationship between Dopamine D(2) Occupancy, Clinical Response, and Side Effects: A Double-Blind PET Study of First-Episode Schizophrenia. Am. J. Psychiatry 2000, 157, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Takeuchi, H.; Graff-Guerrero, A.; Suzuki, T.; Watanabe, K.; Mamo, D.C. Predicting Dopamine D₂ Receptor Occupancy from Plasma Levels of Antipsychotic Drugs: A Systematic Review and Pooled Analysis. J. Clin. Psychopharmacol. 2011, 31, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Takeuchi, H.; Graff-Guerrero, A.; Suzuki, T.; Watanabe, K.; Mamo, D.C. Dopamine D2 Receptor Occupancy and Clinical Effects: A Systematic Review and Pooled Analysis. J. Clin. Psychopharmacol. 2011, 31, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, M.; Kacirova, I.; Urinovska, R. Therapeutic Drug Monitoring of Atypical Antipsychotic Drugs. Acta Pharm. 2014, 64, 387–401. [Google Scholar] [CrossRef]
- Nakajima, S.; Uchida, H.; Bies, R.R.; Caravaggio, F.; Suzuki, T.; Plitman, E.; Mar, W.; Gerretsen, P.; Pollock, B.G.; Mulsant, B.H.; et al. Dopamine D 2/3 Receptor Occupancy Following Dose Reduction Is Predictable with Minimal Plasma Antipsychotic Concentrations: An Open-Label Clinical Trial. Schizophr. Bull. 2015, 42, 212–219. [Google Scholar] [CrossRef]
- Lako, I.M.; Van Den Heuvel, E.R.; Knegtering, H.; Bruggeman, R.; Taxis, K. Estimating Dopamine D2 Receptor Occupancy for Doses of 8 Antipsychotics: A Meta-Analysis. J. Clin. Psychopharmacol. 2013, 33, 675–681. [Google Scholar] [CrossRef]
- Gunes, A.; Spina, E.; Dahl, M.-L.; Scordo, M.G. ABCB1 Polymorphisms Influence Steady-State Plasma Levels of 9-Hydroxyrisperidone and Risperidone Active Moiety. Ther. Drug Monit. 2008, 30, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Gründer, G.; Yokoi, F.; Offord, S.J.; Ravert, H.T.; Dannals, R.F.; Salzmann, J.K.; Szymanski, S.; Wilson, P.D.; Howard, D.R.; Wong, D.F. Time Course of 5-HT2A Receptor Occupancy in the Human Brain after a Single Oral Dose of the Putative Antipsychotic Drug MDL 100,907 Measured by Positron Emission Tomography. Neuropsychopharmacology 1997, 17, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Mamo, D.; Kapur, S.; Shammi, C.M.; Papatheodorou, G.; Mann, S.; Therrien, F.; Remington, G. A PET Study of Dopamine D2 and Serotonin 5-HT2 Receptor Occupancy in Patients with Schizophrenia Treated with Therapeutic Doses of Ziprasidone. Am. J. Psychiatry 2004, 161, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Alberati, D.; Moreau, J.L.; Lengyel, J.; Hauser, N.; Mory, R.; Borroni, E.; Pinard, E.; Knoflach, F.; Schlotterbeck, G.; Hainzl, D.; et al. Glycine Reuptake Inhibitor RG1678: A Pharmacologic Characterization of an Investigational Agent for the Treatment of Schizophrenia. Neuropharmacology 2012, 62, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Matsui-Sakata, A.; Ohtani, H.; Sawada, Y. Receptor Occupancy-Based Analysis of the Contributions of Various Receptors to Antipsychotics-Induced Weight Gain and Diabetes Mellitus. Drug Metab. Pharmacokinet. 2005, 20, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Prior, T.I.; Chue, P.S.; Tibbo, P.; Baker, G.B. Drug Metabolism and Atypical Antipsychotics. Eur. Neuropsychopharmacol. 1999, 9, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Rostami-Hodjegan, A.; Amin, A.M.; Spencer, E.P.; Lennard, M.S.; Tucker, G.T.; Flanagan, R.J. Influence of Dose, Cigarette Smoking, Age, Sex, and Metabolic Activity on Plasma Clozapine Concentrations: A Predictive Model and Nomograms to Aid Clozapine Dose Adjustment and to Assess Compliance in Individual Patients. J. Clin. Psychopharmacol. 2004, 24, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.; McLaren, A.; Galanos, J.; Copolov, D. The Clinical Use of Plasma Clozapine Levels. Aust. N. Z. J. Psychiatry 1998, 32, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.V.; Kane, J.M. Plasma Levels of Second-Generation Antipsychotics and Clinical Response in Acute Psychosis: A Review of the Literature. Schizophr. Res. 2013, 147, 368–374. [Google Scholar] [CrossRef]
- Jerling, M.; Lindström, L.; Bondesson, U.; Bertilsson, L. Fluvoxamine Inhibition and Carbamazepine Induction of the Metabolism of Clozapine: Evidence from a Therapeutic Drug Monitoring Service. Ther. Drug Monit. 1994, 16, 368–374. [Google Scholar] [CrossRef]
- Spina, E.; de Leon, J. Metabolic Drug Interactions with Newer Antipsychotics: A Comparative Review. Basic Clin. Pharmacol. Toxicol. 2007, 100, 4–22. [Google Scholar] [CrossRef]
- Perry, P.J.; Miller, D.D.; Arndt, S.V.; Cadoret, R.J. Clozapine and Norclozapine Plasma Concentrations and Clinical Response of Treatment-Refractory Schizophrenic Patients. Am. J. Psychiatry 1991, 148, 231–235. [Google Scholar] [PubMed]
- Kronig, M.H.; Munne, R.A.; Szymanski, S.; Safferman, A.Z.; Pollack, S.; Cooper, T.; Kane, J.M.; Lieberman, J.A. Plasma Clozapine Levels and Clinical Response for Treatment-Refractory Schizophrenic Patients. Am. J. Psychiatry 1995, 152, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Mauri, M.C.; Volonteri, L.S.; Colasanti, A.; Fiorentini, A.; De Gaspari, I.F.; Bareggi, S.R. Clinical Pharmacokinetics of Atypical Antipsychotics: A Critical Review of the Relationship between Plasma Concentrations and Clinical Response. Clin. Pharmacokinet. 2007, 46, 359–388. [Google Scholar] [CrossRef] [PubMed]
- Wohkittel, C.; Gerlach, M.; Taurines, R.; Wewetzer, C.; Unterecker, S.; Burger, R.; Schreck, D.; Mehler-Wex, C.; Romanos, M.; Egberts, K. Relationship between Clozapine Dose, Serum Concentration, and Clinical Outcome in Children and Adolescents in Clinical Practice. J. Neural Transm. 2016, 123, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Aichhorn, W.; Whitworth, A.B.; Weiss, E.M.; Marksteiner, J. Second-Generation Antipsychotics. Drug Saf. 2006, 29, 587–598. [Google Scholar] [CrossRef]
- Kelly, D.L.; Conley, R.R.; Tamminga, C.A. Differential Olanzapine Plasma Concentrations by Sex in a Fixed-Dose Study. Schizophr. Res. 1999, 40, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Bergemann, N.; Frick, A.; Parzer, P.; Kopitz, J. Olanzapine Plasma Concentration, Average Daily Dose, and Interaction with Co-Medication in Schizophrenic Patients. Pharmacopsychiatry 2004, 37, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Olesen, O.V.; Linnet, K. Olanzapine Serum Concentrations in Psychiatric Patients given Standard Doses: The Influence of Comedication. Ther. Drug Monit. 1999, 21, 87–90. [Google Scholar] [CrossRef]
- Gex-Fabry, M.; Balant-Gorgia, A.E.; Balant, L.P. Therapeutic Drug Monitoring of Olanzapine: The Combined Effect of Age, Gender, Smoking, and Comedication. Ther. Drug Monit. 2003, 25, 46–53. [Google Scholar] [CrossRef]
- Bergemann, N.; Kopitz, J.; Kress, K.R.; Frick, A. Plasma Amisulpride Levels in Schizophrenia or Schizoaffective Disorder. Eur. Neuropsychopharmacol. 2004, 14, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Hart, X.M.; Hiemke, C.; Eichentopf, L.; Lense, X.M.; Clement, H.W.; Conca, A.; Faltraco, F.; Florio, V.; Grüner, J.; Havemann-Reinecke, U.; et al. Therapeutic Reference Range for Aripiprazole in Schizophrenia Revised: A Systematic Review and Metaanalysis. Psychopharmacology 2022, 239, 3377–3391. [Google Scholar] [CrossRef]
- Tien, Y.; Huang, H.-P.; Liao, D.-L.; Huang, S.-C. Dose-Response Analysis of Aripiprazole in Patients with Schizophrenia in Taiwan. Ther. Adv. Psychopharmacol. 2022, 12, 204512532211132. [Google Scholar] [CrossRef] [PubMed]
- Dziurkowska, E.; Wesolowski, M. Simultaneous Quantification of Antipsychotic and Antiepileptic Drugs and Their Metabolites in Human Saliva Using UHPLC-DAD. Molecules 2019, 24, 2953. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, C.; Gonçalves, J.; Soares, S.; Rosado, T.; Araujo, A.R.T.S.; Passarinha, L.A.; Barroso, M.; Gallardo, E. Evaluation of Antipsychotic Drugs’ Stability in Oral Fluid Samples. Molecules 2023, 28, 2030. [Google Scholar] [CrossRef] [PubMed]
- Dziurkowska, E.; Kosinska, S.; Plenis, A.; Wesolowski, M. A New Method for the Determination of Amisulpride in a Small Volume (200 ΜL) of Human Saliva Using LC-DAD Supported by SPE. Separations 2023, 10, 277. [Google Scholar] [CrossRef]
- Miller, J.; Wehring, H.; McMahon, R.P.; DiPaula, B.A.; Love, R.C.; Morris, A.A.; Raley, H.; Feldman, S.; Kelly, D.L. Urine Testing for Antipsychotics: A Pilot Trial for a Method to Determine Detection Levels. Hum. Psychopharmacol. Clin. Exp. 2015, 30, 350–355. [Google Scholar] [CrossRef]
- Jacobs, C.M.; Wagmann, L.; Meyer, M.R. Development, Validation, and Application of a Quantitative Volumetric Absorptive Microsampling–Based Method in Finger Prick Blood by Means of LC-HRMS/MS Applicable for Adherence Monitoring of Antipsychotics. Anal. Bioanal. Chem. 2021, 413, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Shi, Z.; Wu, Y.; Lv, J.; Deng, P.; Liu, G.; An, Z.; Che, Z.; Lu, Y.; Shan, J.; et al. Wireless, Noninvasive Therapeutic Drug Monitoring System for Saliva Measurement toward Medication Management of Schizophrenia. Biosens. Bioelectron. 2023, 234, 115363. [Google Scholar] [CrossRef]
- Piacentino, D. Therapeutic Drug Monitoring of Antidepressants: An Underused but Potentially Valuable Tool in Primary. Front. Psychiatry 2022, 13, 867840. [Google Scholar] [CrossRef]
- Fiaturi, N.; Greenblatt, D.J. Therapeutic Drug Monitoring of Antidepressants. Handb. Exp. Pharmacol. 2019, 250, 115–133. [Google Scholar] [CrossRef]
- Müller, M.J.; Dragicevic, A.; Fric, M.; Gaertner, I.; Grasmäder, K.; Härtter, S.; Hermann, E.; Kuss, H.J.; Laux, G.; Oehl, W.; et al. Therapeutic Drug Monitoring of Tricyclic Antidepressants: How Does It Work under Clinical Conditions? Pharmacopsychiatry 2003, 36, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; Ulrich, S.; Eckermann, G.; Gerlach, M.; Kuss, H.-J.; Laux, G.; Müller-Oerlinghausen, B.; Rao, M.L.; Riederer, P.; Zernig, G.; et al. The AGNP-TDM Expert Group Consensus Guidelines: Focus on Therapeutic Monitoring of Antidepressants. Dialogues Clin. Neurosci. 2005, 7, 231–247. [Google Scholar] [CrossRef]
- Preskorn, S.H. Dose-Effect and Concentration-Effect Relationships with New Antidepressants. Psychopharmacol. Ser. 1993, 10, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Ostad Haji, E.; Tadić, A.; Wagner, S.; Dragicevic, A.; Müller, M.J.; Boland, K.; Rao, M.-L.; Fric, M.; Laux, G.; Hiemke, C. Association between Citalopram Serum Levels and Clinical Improvement of Patients with Major Depression. J. Clin. Psychopharmacol. 2011, 31, 281–286. [Google Scholar] [CrossRef]
- Ostad Haji, E.; Mann, K.; Dragicevic, A.; Müller, M.J.; Boland, K.; Rao, M.-L.; Fric, M.; Laux, G.; Hiemke, C. Potential Cost-Effectiveness of Therapeutic Drug Monitoring for Depressed Patients Treated with Citalopram. Ther. Drug Monit. 2013, 35, 396–401. [Google Scholar] [CrossRef]
- Waldschmitt, C.; Vogel, F.; Pfuhlmann, B.; Hiemke, C. Duloxetine Serum Concentrations and Clinical Effects. Data from a Therapeutic Drug Monitoring (TDM) Survey. Pharmacopsychiatry 2009, 42, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.R.; Kuhlmann, I.B.; Pottegård, A.; Damkier, P. Therapeutic Drug Monitoring of Venlafaxine in an Everyday Clinical Setting: Analysis of Age, Sex and Dose Concentration Relationships. Basic Clin. Pharmacol. Toxicol. 2017, 121, 298–302. [Google Scholar] [CrossRef]
- Grasmäder, K.; Verwohlt, P.L.; Kühn, K.-U.; Frahnert, C.; Hiemke, C.; Dragicevic, A.; von Widdern, O.; Zobel, A.; Maier, W.; Rao, M.L. Relationship between Mirtazapine Dose, Plasma Concentration, Response, and Side Effects in Clinical Practice. Pharmacopsychiatry 2005, 38, 113–117. [Google Scholar] [CrossRef]
- López-Jaramillo, C.; Díaz-Zuluaga, A.M.; de Leon, J.; Schoretsanitis, G.; Paulzen, M.; Unterecker, S.; Schwarz, M.; Conca, A.; Zernig, G.; Gründer, G.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology. Psiquiatr. Biol. 2020, 27, 83–95. [Google Scholar] [CrossRef]
- Dziurkowska, E.; Wesolowski, M. Isolation of Antidepressants and Their Metabolites from Saliva Using Supported Liquid Extraction (SLE). Biomedicines 2023, 11, 708. [Google Scholar] [CrossRef]
- Soares, S.; Rosado, T.; Barroso, M.; Gallardo, E. New Method for the Monitoring of Antidepressants in Oral Fluid Using Dried Spot Sampling. Pharmaceuticals 2021, 14, 1284. [Google Scholar] [CrossRef]
- Marasca, C.; Protti, M.; Mandrioli, R.; Atti, A.R.; Armirotti, A.; Cavalli, A.; De Ronchi, D.; Mercolini, L. Whole Blood and Oral Fluid Microsampling for the Monitoring of Patients under Treatment with Antidepressant Drugs. J. Pharm. Biomed. Anal. 2020, 188, 113384. [Google Scholar] [CrossRef]
- Johannesson, N.; Bergquist, J. Rapid On-Line Extraction and Quantification of Escitalopram from Urine Using Sol–Gel Columns and Mass Spectrometric Detection. J. Pharm. Biomed. Anal. 2007, 43, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Badulla, W.F.S.; Atkoşar, Z.; Arli, G.; Şener, E. Application of LC–ESI-MS/MS Method for Analysis of Escitalopram Oxalate in Human Urine and Pharmaceutical Dosage Forms. J. Chromatogr. Sci. 2020, 58, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ulu, S.T.; Tuncel, M. Determination of Bupropion Using Liquid Chromatography with Fluorescence Detection in Pharmaceutical Preparations, Human Plasma and Human Urine. J. Chromatogr. Sci. 2012, 50, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; He, Y.; Chen, F. Determination of Fluvoxamine Maleate in Human Urine and Human Serum Using Alkaline KMnO4–Rhodamine B Chemiluminescence. Luminescence 2017, 32, 1077–1083. [Google Scholar] [CrossRef]
- Hoskins, J.M.; Shenfield, G.M.; Gross, A.S. A modified hplc method for rapi detection of moclobemide and it N-oxide metabolite in human urin. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 521–529. [Google Scholar] [CrossRef]
- Sarıkaya, M.; Ulusoy, H.I.; Morgul, U.; Ulusoy, S.; Tartaglia, A.; Yılmaz, E.; Soylak, M.; Locatelli, M.; Kabir, A. Sensitive Determination of Fluoxetine and Citalopram Antidepressants in Urine and Wastewater Samples by Liquid Chromatography Coupled with Photodiode Array Detector. J. Chromatogr. A 2021, 1648, 462215. [Google Scholar] [CrossRef]
- Agrawal, N.; Marco-Peiró, S.; Esteve-Romero, J.; Durgbanshi, A.; Bose, D.; Peris-Vicente, J.; Carda-Broch, S. Determination of Paroxetine in Blood and Urine Using Micellar Liquid Chromatography with Electrochemical Detection. J. Chromatogr. Sci. 2014, 52, 1217–1223. [Google Scholar] [CrossRef]
- Bishnoi, S.; Sharma, A.; Singhal, R.; Goyal, R.N. Edge Plane Pyrolytic Graphite as a Sensing Surface for the Determination of Fluvoxamine in Urine Samples of Obsessive-Compulsive Disorder Patients. Biosens. Bioelectron. 2020, 168, 112489. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, A.; Farajzadeh, M.A.; Yaripour, S.; Afshar Mogaddam, M.R. Determination of Tricyclic Antidepressants in Human Urine Samples by the Three-Step Sample Pretreatment Followed by HPLC-UV Analysis: An Efficient Analytical Method for Further Pharmacokinetic and Forensic Studies. EXCLI J. 2018, 17, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Petruczynik, A.; Wróblewski, K.; Wojtanowski, K.; Mroczek, T.; Juchnowicz, D.; Karakuła-Juchnowicz, H.; Tuzimski, T. Comparison of Various Chromatographic Systems for Identification of Vortioxetine in Bulk Drug Substance, Human Serum, Saliva, and Urine Samples by HPLC-DAD and LC-QTOF-MS. Molecules 2020, 25, 2483. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Hao, X.; Yu, J.; Wang, D.; Zhang, J.; Yu, Z.; Gao, F.; Zhou, C. Machine Learning-Based Prediction of Sertraline Concentration in Patients with Depression through Therapeutic Drug Monitoring. Front. Pharmacol. 2024, 15, 1289673. [Google Scholar] [CrossRef] [PubMed]

| Drug | Class | Therapeutic Drug Range (Blood) | TDM AGNP Recommendation Levels | Preferred Sampling Method | Other Sampling Methods |
|---|---|---|---|---|---|
| Lithium | Mood stabilizer | 0.5–1.2 mmol/L | 1 | Plasma | Saliva, urine, sweat, interstitial fluid, dried blood/plasma spots [23,24] |
| Valproate | Antiseizure medication/mood stabilizer/migraine prevention | 50–100 μg/mL | 2 | Plasma | Saliva, urine, dried blood spots [25,26,27] |
| Carbamazepine | Antiseizure medication/mood stabilizer/neuropathic pain | 4–12 μg/mL | 1 | Serum | Saliva, urine [28,29,30,31,32] |
| Oxcarbazepine | Antiseizure medication/mood stabilizer | 10–35 μg/mL | 2 | Plasma | Urine [33] |
| Lamotrigine | Antiseizure medication/mood stabilizer | 3–15 μg/mL | 2 | Plasma | Saliva, dried blood spots [34,35,36,37] |
| Drug | Class | Therapeutic Drug Range (Blood) | TDM AGNP Recommendation Levels | Preferred Sampling Method | Other Sampling Methods |
|---|---|---|---|---|---|
| Amisulpride | SGA | 100–320 ng/mL | 1 | Plasma | Saliva, dried blood spots [136,138] |
| Aripiprazole | TGA | 100–350 ng/mL | 2 | Plasma | Saliva, dried blood spots [134,138] |
| Chlorpromazine | FGA | 30–300 ng/mL | 2 | Plasma | Saliva [135] |
| Clozapine | SGA | 350–600 ng/mL | 1 | Plasma | Saliva, dried blood spots [134,135,136,138] |
| Haloperidol | FGA | 1–10 ng/mL | 1 | Plasma | Saliva, dried blood spots, urine [135,137,138] |
| Olanzapine | SGA | 20–80 ng/mL | 1 | Plasma | Saliva, dried blood spots, urine [134,135,137,138] |
| Quetiapine | SGA | 100–500 ng/mL | 2 | Plasma | Saliva, dried blood spots, urine [134,135,137] |
| Risperidone | SGA | 20–60 ng/mL | 2 | Plasma | Saliva, dried blood spots, urine [134,137,138] |
| Drug | Class | Therapeutic Drug Range (Blood) | TDM AGNP Recommendation Levels | Preferred Sampling Method | Other Sampling Methods |
|---|---|---|---|---|---|
| Amitriptyline | TCA | 80–200 ng/mL | 1 | Plasma | Saliva, urine [162] |
| Bupropion | NDRI | 10–100 ng/mL | 2 | Plasma | Urine [156] |
| Citalopram | SSRI | 50–110 ng/mL | 1 | Plasma, serum | Saliva, dried saliva spots, urine [151,152,153,159] |
| Clomipramine | TCA | 230–450 ng/mL | 1 | Plasma | Urine [162] |
| Desipramine | TCA | 100–300 ng/mL | 2 | Plasma | Urine [162] |
| Duloxetine | SNRI | 30–120 ng/mL | 2 | Plasma | Saliva [151,152] |
| Escitalopram | SSRI | 15–80 ng/mL | 2 | Plasma, serum | Urine [154,155] |
| Fluoxetine | SSRI | 120–500 ng/mL | 3 | Plasma, serum | Urine, dried saliva spots [152,153,159] |
| Fluvoxamine | SSRI | 60–230 ng/mL | 2 | Plasma, serum | Urine [157,161] |
| Imipramine | TCA | 175–300 ng/mL | 1 | Plasma | Urine [162] |
| Mirtazapine | NaSSA | 30–80 ng/mL | 2 | Plasma | Saliva [151] |
| Moclobemide | MAOI | 300–1000 ng/mL | 3 | Plasma | Urine [158] |
| Nortriptyline | TCA | 70–170 ng/mL | 1 | Plasma | Urine [162] |
| Paroxetine | SSRI | 20–65 ng/mL | 3 | Plasma, serum | Urine, dried saliva spots [152,160] |
| Sertraline | SSRI | 10–150 ng/mL | 2 | Plasma, serum | Saliva, dried saliva spots [151,152,153] |
| Venlafaxine | SNRI | 100–400 ng/mL | 2 | Plasma | Saliva, dried saliva spots [151,152] |
| Vortioxetine | SMS/SSRI | 15–60 ng/mL | 2 | Plasma | Saliva, urine [153,163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biso, L.; Aringhieri, S.; Carli, M.; Scarselli, M.; Longoni, B. Therapeutic Drug Monitoring in Psychiatry: Enhancing Treatment Precision and Patient Outcomes. Pharmaceuticals 2024, 17, 642. https://doi.org/10.3390/ph17050642
Biso L, Aringhieri S, Carli M, Scarselli M, Longoni B. Therapeutic Drug Monitoring in Psychiatry: Enhancing Treatment Precision and Patient Outcomes. Pharmaceuticals. 2024; 17(5):642. https://doi.org/10.3390/ph17050642
Chicago/Turabian StyleBiso, Letizia, Stefano Aringhieri, Marco Carli, Marco Scarselli, and Biancamaria Longoni. 2024. "Therapeutic Drug Monitoring in Psychiatry: Enhancing Treatment Precision and Patient Outcomes" Pharmaceuticals 17, no. 5: 642. https://doi.org/10.3390/ph17050642
APA StyleBiso, L., Aringhieri, S., Carli, M., Scarselli, M., & Longoni, B. (2024). Therapeutic Drug Monitoring in Psychiatry: Enhancing Treatment Precision and Patient Outcomes. Pharmaceuticals, 17(5), 642. https://doi.org/10.3390/ph17050642









