Enhanced Anti-Inflammatory Activity of Tilianin Based on the Novel Amorphous Nanocrystals
Abstract
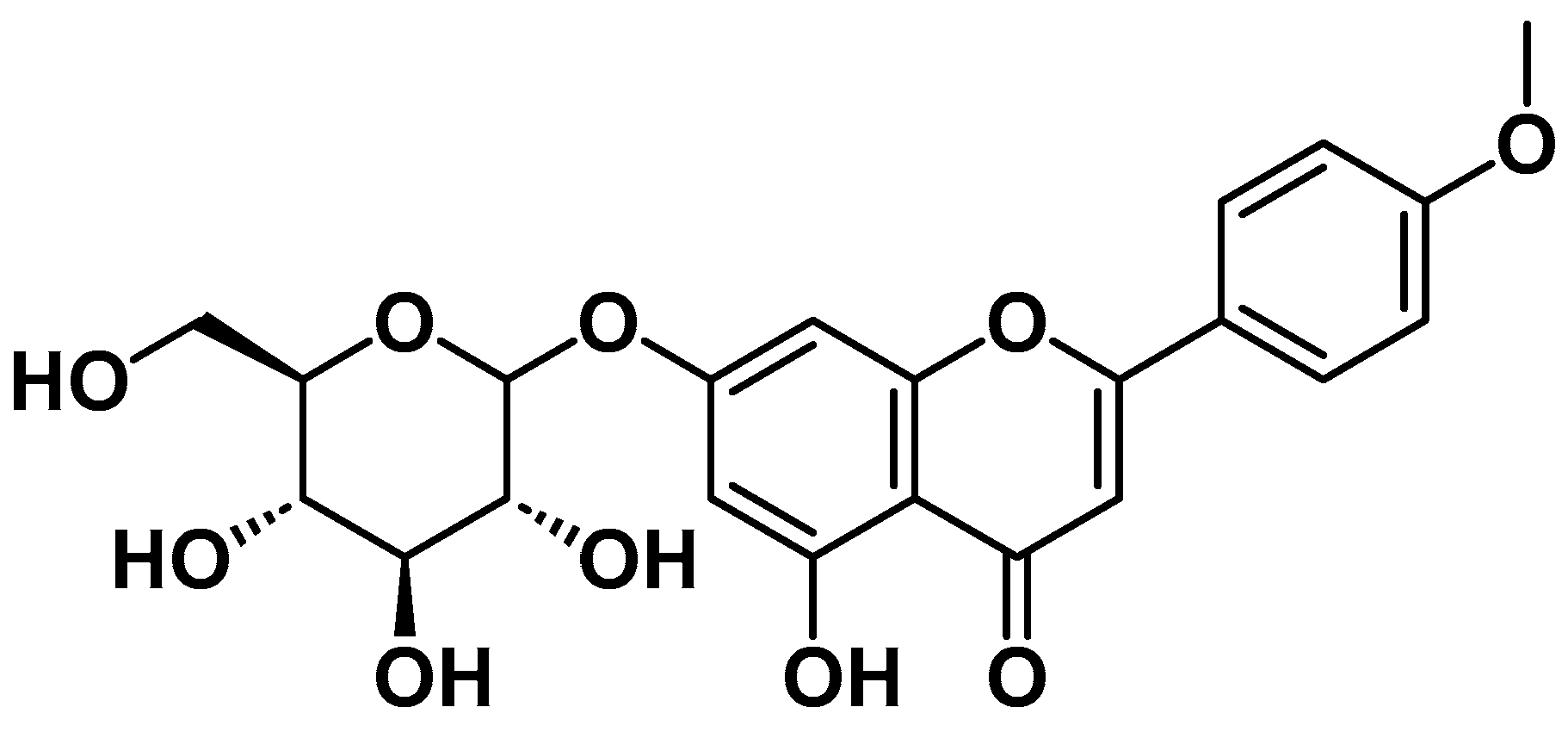
1. Introduction
2. Results and Discussion
2.1. Formulation Optimization
2.1.1. Selection of Organic Solvents, Stabilizers, and Process Parameters
2.1.2. Optimization Using the CCD Method
2.2. Characterization of Til NCs
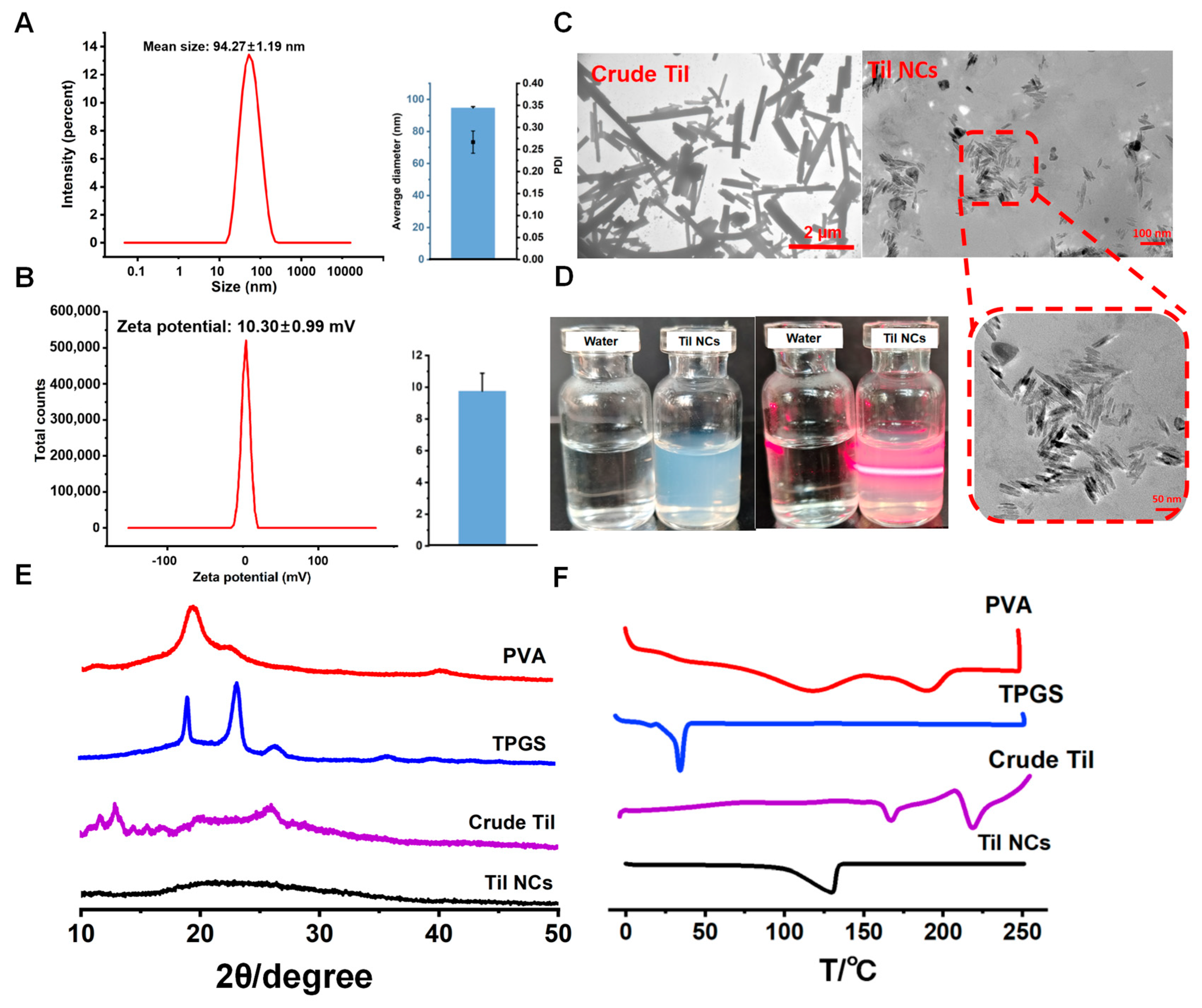
2.2.1. Particle Size and Morphology
2.2.2. Crystalline Characterization
2.3. Stability and Saturation Solubility
2.4. In Vitro Release of Til NCs
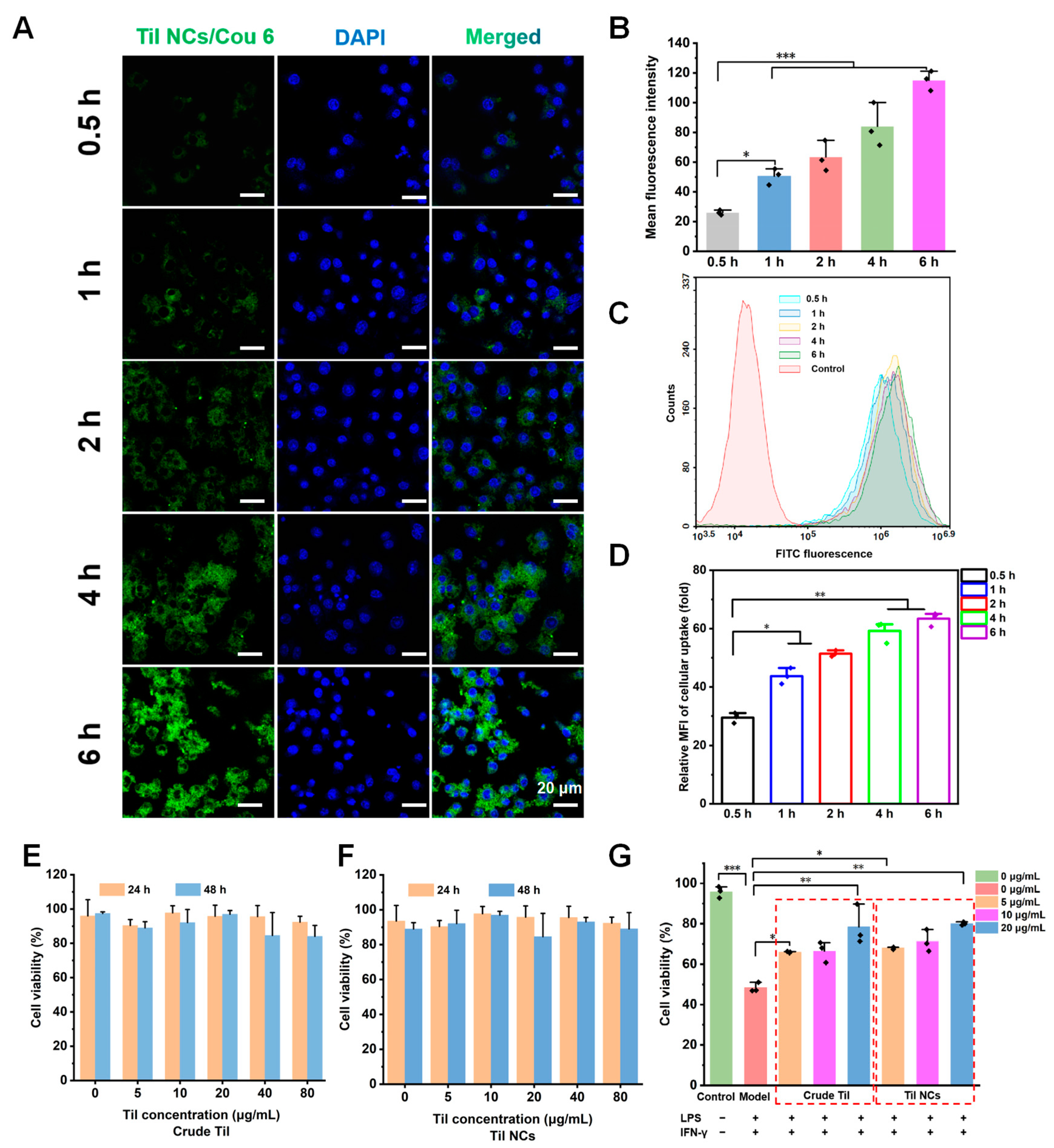
2.5. The Cellular Uptake and Cytotoxicity of Til NCs
2.6. Study of Macrophage Polarization
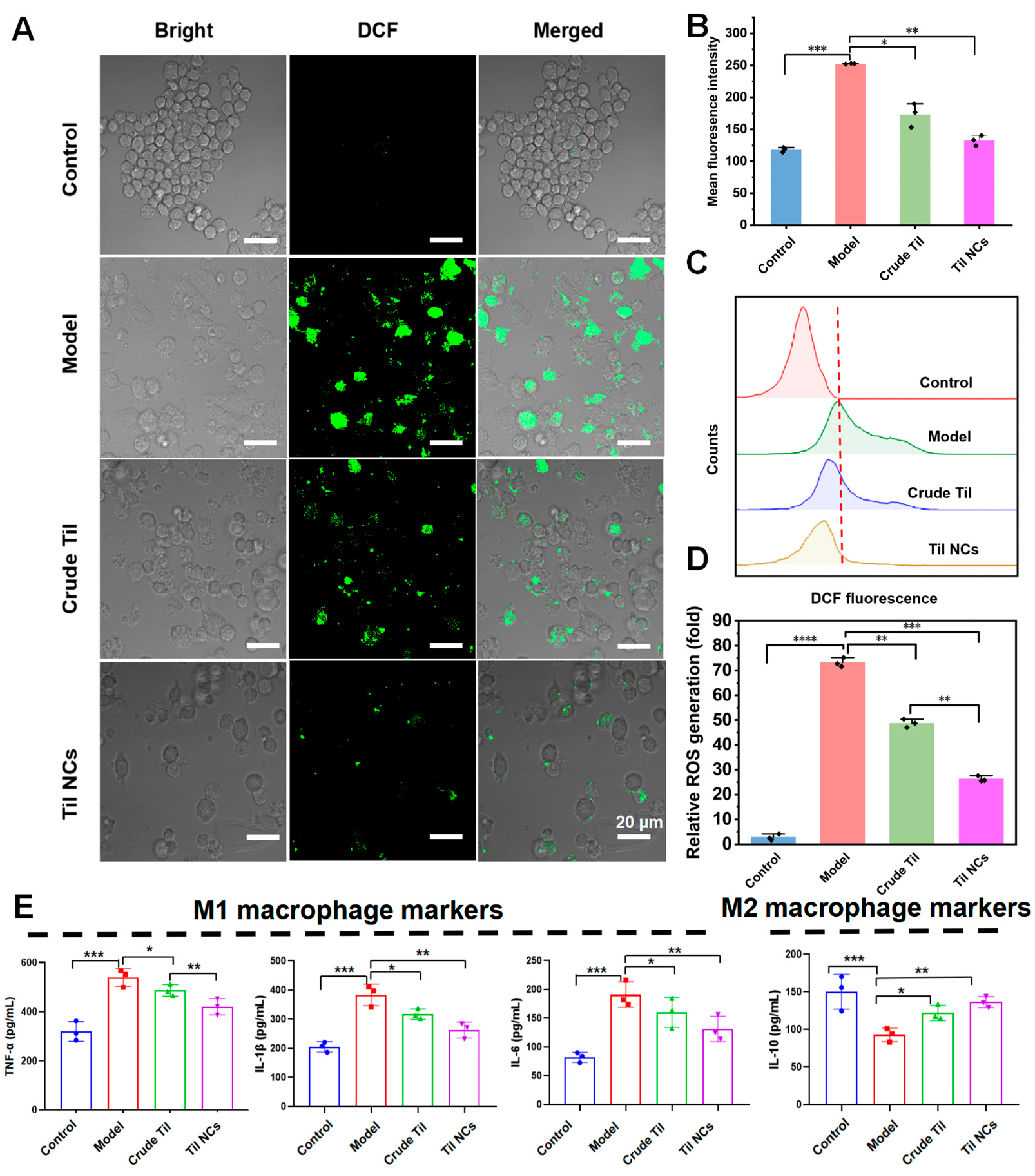
2.7. Anti-ROS and Anti-Inflammatory Factor Study
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Til NCs
3.2.2. Formulation Optimization of Til NCs
3.3. Characterization of Til NCs
3.4. Stability and Saturation Solubility of Til NCs
3.5. In Vitro Release
3.6. Cell Culture
3.7. Cellular Uptake
3.8. Cytotoxicity Analysis
3.9. Macrophage Polarization Analysis
3.10. Assessment of Intracellular ROS Generation
3.11. Cytokine Assays
3.12. Analytical Methods
3.12.1. HPLC Analysis
3.12.2. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
References
- Selenge, E.; Murata, T.; Tanaka, S.; Sasaki, K.; Batkhuu, J.; Yoshizaki, F. Monoterpene glycosides, phenylpropanoids, and acacetin glycosides from Dracocephalum foetidum. Phytochemistry 2014, 101, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Abreu, O.; Torres-Piedra, M.; García-Jiménez, S.; Ibarra-Barajas, M.; Villalobos-Molina, R.; Montes, S.; Rembao, D.; Estrada-Soto, S. Dose-dependent antihypertensive determination and toxicological studies of tilianin isolated from Agastache mexicana. J. Ethnopharmacol. 2013, 146, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Cao, P.; Wang, J.; Kang, W. Analysis of tilianin and acacetin in Agastache rugosa by high-performance liquid chromatography with ionic liquids-ultrasound based extraction. Chem. Cent. J. 2016, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- García-Díaz, J.A.; Navarrete-Vázquez, G.; García-Jiménez, S.; Hidalgo-Figueroa, S.; Almanza-Pérez, J.C.; Alarcón-Aguilar, F.J.; Gómez-Zamudio, J.; Cruz, M.; Ibarra-Barajas, M.; Estrada-Soto, S. Antidiabetic, antihyperlipidemic and anti-inflammatory effects of tilianin in streptozotocin-nicotinamide diabetic rats. Biomed. Pharmacother. 2016, 83, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Choi, J.; Seo, Y.; Lee, Y.; Lee, M.; Park, J.; Oh, G. We-P14:489 Inhibitory effects of tilianin on the expression of inducible nitric oxide synthase in low density lipoprotein receptor deficiency mice. Exp. Mol. Med. 2006, 7, 455. [Google Scholar] [CrossRef]
- Oh, H.M.; Kang, Y.J.; Lee, Y.S.; Park, M.K.; Kim, S.H.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Chang, K.C. Protein kinase G-dependent heme oxygenase-1 induction by Agastache rugosa leaf extract protects RAW264.7 cells from hydrogen peroxide-induced injury. J. Ethnopharmacol. 2006, 103, 229–235. [Google Scholar] [CrossRef] [PubMed]
- González-Trujano, M.E.; Ponce-Muñoz, H.; Hidalgo-Figueroa, S.; Navarrete-Vázquez, G.; Estrada-Soto, S. Depressant effects of Agastache mexicana methanol extract and one of major metabolites tilianin. Restor. Neurol. Neurosci. 2015, 33, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Cao, P.; Wang, J.; Kang, W. Tilianin mediates neuroprotection against ischemic injury by attenuating CaMKII-dependent mitochondrion-mediated apoptosis and MAPK/NF-kappa B signaling. Life Sci. 2019, 216, 233–245. [Google Scholar]
- Zeng, C.; Jiang, W.; Zheng, R.; He, C.; Li, J.; Xing, J. Cardioprotection of tilianin ameliorates myocardial ischemia-reperfusion injury: Role of the apoptotic signaling pathway. PLoS ONE 2018, 13, e0193845. [Google Scholar] [CrossRef]
- Gálvez, J.; Estrada-Reyes, R.; Benítez-King, G.; Araujo, G.; Orozco, S.; Fernández-Mas, R.; Almazán, S.; Calixto, E. Involvement of the GABAergic system in the neuroprotective and sedative effects of acacetin 7-O-glucoside in rodents. Restor. Neurol. Neurosci. 2015, 33, 683–700. [Google Scholar] [CrossRef]
- Dong, T.; Chen, X.; Xu, H.; Song, Y.; Wang, H.; Gao, Y.; Wang, J.; Du, R.; Lou, H.; Dong, T. Mitochondrial metabolism mediated macrophage polarization in chronic lung diseases. Pharmacol. Ther. 2022, 239, 108208. [Google Scholar] [CrossRef]
- Feng, N.; Liang, L.; Fan, M.; Du, Y.; Chen, C.; Jiang, R.; Yu, D.; Yang, Y.; Zhang, M.; Deng, L.; et al. Treating Autoimmune Inflammatory Diseases with an siERN1-Nanoprodrug That Mediates Macrophage Polarization and Blocks Toll-like Receptor Signaling. ACS Nano 2021, 15, 15874–15891. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bai, Y.; Shen, S.; Tao, X.; Ma, C.; Fu, B.; Dai, Q.; Wu, J.; Meng, Z.; Sun, Q.; et al. An oral nano-antioxidant for targeted treatment of inflammatory bowel disease by regulating macrophage polarization and inhibiting ferroptosis of intestinal cells. Chem. Eng. J. 2023, 465, 142940. [Google Scholar] [CrossRef]
- Wang, S.; Yang, S.; Chen, Y.; Chen, Y.; Li, R.; Han, S.; Kamili, A.; Wu, Y.; Zhang, W. Ginsenoside Rb2 Alleviated Atherosclerosis by Inhibiting M1 Macrophages Polarization Induced by MicroRNA-216a. Front. Pharmacol. 2021, 12, 764130. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Müller, E.; Christopoulos, P.F.; Halder, S.; Lunde, A.; Beraki, K.; Speth, M.; Øynebråten, I.; Corthay, A. Toll-Like Receptor Ligands and Interferon-γ Synergize for Induction of Antitumor M1 Macrophages. Front. Immunol. 2017, 8, 1383. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2016, 79, 541. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Hilhorst, M.; Harrison, D.G.; Goronzy, J.J.; Weyand, C.M. Macrophages in vascular inflammation—From atherosclerosis to vasculitis. Autoimmunity 2015, 48, 139–151. [Google Scholar] [CrossRef]
- Lafuse, W.P.; Wozniak, D.J.; Rajaram, M.V.S. Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair. Cells 2020, 10, 51. [Google Scholar] [CrossRef]
- Kim, S.Y.; Nair, M.G. Macrophages in wound healing: Activation and plasticity. Immunol. Cell Biol. 2019, 97, 258–267. [Google Scholar] [CrossRef]
- Sun, K.; Li, Y.-Y.; Jin, J. A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair. Signal Transduct. Target Ther. 2021, 6, 16. [Google Scholar] [CrossRef]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.; Wei, Y.; Wei, X. Epigenetic regulation of macrophages: From homeostasis maintenance to host defense. Cell Mol. Immunol. 2020, 17, 36–49. [Google Scholar] [CrossRef]
- Nam, K.-W.; Kim, J.; Hong, J.-J.; Choi, J.-H.; Mar, W.; Cho, M.-H.; Kim, Y.-M.; Oh, S.-R.; Lee, H.-K.; Nam, K.-H.; et al. Inhibition of cytokine-induced IκB kinase activation as a mechanism contributing to the anti-atherogenic activity of tilianin in hyperlipidemic mice. Atherosclerosis 2005, 180, 27–35. [Google Scholar] [CrossRef]
- Shen, W.; Anwaier, G.; Cao, Y.; Lian, G.; Chen, C.; Liu, S.; Tuerdi, N.; Qi, R. Atheroprotective Mechanisms of Tilianin by Inhibiting Inflammation Through Down-Regulating NF-κB Pathway and Foam Cells Formation. Front. Physiol. 2019, 10, 825. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Z. Acute Lung Injury (ALI): Role of Tilianin in an in-vitro LPS Induced Alveolar Macrophages Cells and in-vivo C57BL/6 Mice Model. J. Environ. Pathol. Toxicol. Oncol. 2020, 39, 335–344. [Google Scholar] [CrossRef]
- Zeng, C. Optimization and in vitro evaluation of TAT and PEG co-modified tilianin-loaded composite phospholipid liposomes. Chin. Herb. Med. 2018, 49, 5061–5069. [Google Scholar]
- Silva, A.D.A.; Sarcinelli, M.A.; Patricio, B.F.d.C.; Chaves, M.H.d.C.; Lima, L.M.; Parreiras, P.M.; Pinto, P.d.F.; Prado, L.D.; Rocha, H.V.A. Pharmaceutical development of micro and nanocrystals of a poorly water-soluble drug: Dissolution rate enhancement of praziquantel. J. Drug Deliv. Sci. Technol. 2023, 81, 104–260. [Google Scholar] [CrossRef]
- Bianchi, M.B.; Zhang, C.; Catlin, E.; Sandri, G.; Calderon, M.; Larraneta, E.; Donnelly, R.F.; Picchio, M.L.; Paredes, A.J. Bioadhesive eutectogels supporting drug nanocrystals for long-acting delivery to mucosal tissues. Mater. Today Bio 2022, 17, 100471. [Google Scholar] [CrossRef]
- Zingale, E.; Bonaccorso, A.; Carbone, C.; Musumeci, T.; Pignatello, R. Drug Nanocrystals: Focus on Brain Delivery from Therapeutic to Diagnostic Applications. Pharmaceutics 2022, 14, 691. [Google Scholar] [CrossRef]
- Guo, M.; Qin, S.; Wang, S.; Sun, M.; Yang, H.; Wang, X.; Fan, P.; Jin, Z. Herbal Medicine Nanocrystals: A Potential Novel Therapeutic Strategy. Molecules 2023, 28, 6370. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hood, Z.D.; Qiu, J.; Guan, B.; Xia, Y. Continuous Production of Water-Soluble Nanocrystals through Anti-Solvent Precipitation in a Fluidic Device. ChemNanoMat 2019, 5, 1131–1136. [Google Scholar] [CrossRef]
- Shaikh, F.; Patel, M.; Patel, V.; Patel, A.; Shinde, G.; Shelke, S.; Pathan, I. Formulation and optimization of cilnidipine loaded nanosuspension for the enhancement of solubility, dissolution and bioavailability. J. Drug Deliv. Sci. Technol. 2022, 69, 103066. [Google Scholar] [CrossRef]
- Lv, Y.; Wu, W.; Corpstein, C.D.; Li, T.; Lu, Y. Biological and intracellular fates of drug nanocrystals through different delivery routes: Recent development enabled by bioimaging and PK modeling. Adv. Drug Deliv. Rev. 2022, 188, 114466. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, H.; Jung, S.; Seo, H.; Nida, S.K.; Yoo, S.-Y.; Lee, J. Pharmaceutical Strategies for Stabilizing Drug Nanocrystals. Curr. Pharm. Des. 2018, 24, 2362–2374. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Desai, A.; Kansara, V.; Vyas, B. Core Shell Lipid-Polymer Hybrid Nanoparticles for Oral Bioavailability Enhancement of Ibrutinib via Lymphatic Uptake. AAPS PharmSciTech 2023, 24, 142. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante de Freitas, P.G.; Rodrigues Arruda, B.; Araújo Mendes, M.G.; Barroso de Freitas, J.V.; da Silva, M.E.; Sampaio, T.L.; Petrilli, R.; Eloy, J.O. Resveratrol-Loaded Polymeric Nanoparticles: The Effects of D-α-Tocopheryl Polyethylene Glycol 1000 Succinate (TPGS) on Physicochemical and Biological Properties against Breast Cancer In Vitro and In Vivo. Cancers 2023, 15, 2802. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Fu, J.; Ao, H.; Wang, W.; Wang, X. Enhancement of oral bioavailability of quercetin by metabolic inhibitory nanosuspensions compared to conventional nanosuspensions. Drug Deliv. 2021, 28, 1226–1236. [Google Scholar] [CrossRef]
- Soisuwan, S.; Teeranachaideekul, V.; Wongrakpanich, A.; Langguth, P.; Junyaprasert, V.B. Impact of uncharged and charged stabilizers on in vitro drug performances of clarithromycin nanocrystals. Eur. J. Pharm. Biopharm. 2019, 137, 68–76. [Google Scholar] [CrossRef]
- Weiss, A.M.; Macke, N.; Zhang, Y.; Calvino, C.; Esser-Kahn, A.P.; Rowan, S.J. In Vitro and in Vivo Analyses of the Effects of Source, Length, and Charge on the Cytotoxicity and Immunocompatibility of Cellulose Nanocrystals. ACS Biomater. Sci. Eng. 2021, 7, 1450–1461. [Google Scholar] [CrossRef]
- Gil-González, E.; Perejón, A.; Sánchez-Jiménez, P.E.; Medina-Carrasco, S.; Kupčík, J.; Šubrt, J.; Criado, J.M.; Pérez-Maqueda, L.A. Crystallization Kinetics of Nanocrystalline Materials by Combined X-ray Diffraction and Differential Scanning Calorimetry Experiments. Cryst. Growth Des. 2018, 18, 3107–3116. [Google Scholar] [CrossRef]
- Yousefi, M.; Mohammadi, V.G.; Shadnoush, M.; Khorshidian, N.; Mortazavian, A.M. Zingiber officinale essential oil-loaded chitosan-tripolyphosphate nanoparticles: Fabrication, characterization and in-vitro antioxidant and antibacterial activities. Food Sci. Technol. Int. 2022, 28, 592–602. [Google Scholar] [CrossRef]
- Ji, X.; Song, Z.; He, J.; Guo, S.; Chen, Y.; Wang, H.; Zhang, J.; Xu, X.; Liu, J. NIMA-related kinase 7 amplifies NLRP3 inflammasome pro-inflammatory signaling in microglia/macrophages and mice models of spinal cord injury. Exp. Cell Res. 2021, 398, 112418. [Google Scholar] [CrossRef]
- Howait, M.; Albassam, A.; Yamada, C.; Sasaki, H.; Bahammam, L.; Azuma, M.M.; Cintra, L.T.A.; Satoskar, A.R.; Yamada, S.; White, R.; et al. Elevated Expression of Macrophage Migration Inhibitory Factor Promotes Inflammatory Bone Resorption Induced in a Mouse Model of Periradicular Periodontitis. J. Immunol. 2019, 202, 2035–2043. [Google Scholar] [CrossRef]
- He, R.; Liu, M.; Zou, Z.; Wang, M.; Wang, Z.; Ju, X.; Hao, G. Anti-inflammatory activity of peptides derived from millet bran in vitro and in vivo. Food Funct. 2022, 13, 1881–1889. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, W.; Zhao, X.; Xian, Y.; Wu, W.; Zhang, X.; Zhao, N.; Xu, F.; Wang, C. Natural Melanin/Alginate Hydrogels Achieve Cardiac Repair through ROS Scavenging and Macrophage Polarization. Adv. Sci. 2021, 8, e2100505. [Google Scholar] [CrossRef]
- Kou, L.; Huang, H.; Tang, Y.; Sun, M.; Li, Y.; Wu, J.; Zheng, S.; Zhao, X.; Chen, D.; Luo, Z.; et al. Opsonized nanoparticles target and regulate macrophage polarization for osteoarthritis therapy: A trapping strategy. J. Control. Release Off. J. Control. Release Soc. 2022, 347, 237–255. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, J.; Gao, J.; Zhang, D.; Lin, D.; Lin, J. Polysaccharides of Plantago asiatica enhance antitumor activity via regulating macrophages to M1-like phenotype. Biomed. Pharmacother. 2023, 159, 114246. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zou, Q.; Li, W.; Chen, H. Intracellular Interaction of Hydroxyapatite-Based Nanocrystals with Uniform Shape and Traceable Fluorescence. Inorg. Chem. 2018, 57, 13739–13748. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhu, L. Anisotropic morphology, formation mechanisms, and fluorescence properties of zirconia nanocrystals. Sci. Rep. 2020, 10, 115–298. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Ho, Y.C.; Lee, M.W.; Tseng, C.C.; Lee, S.S.; Hsieh, M.K.; Chen, H.H.; Lee, C.Y.; Wu, S.W.; Kuan, Y.H. 1-Nitropyrene Induced Reactive Oxygen Species-Mediated Apoptosis in Macrophages through AIF Nuclear Translocation and AMPK/Nrf-2/HO-1 Pathway Activation. Oxid. Med. Cell. Longev. 2021, 9, 314–342. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ma, M.; Zhu, X.; Li, M.; Guo, M.; Liu, P.; He, Z.; Fu, Q. Effectiveness of idebenone nanorod formulations in the treatment of Alzheimer’s disease. Journal of controlled release. Off. J. Control. Release Soc. 2021, 336, 169–180. [Google Scholar] [CrossRef] [PubMed]

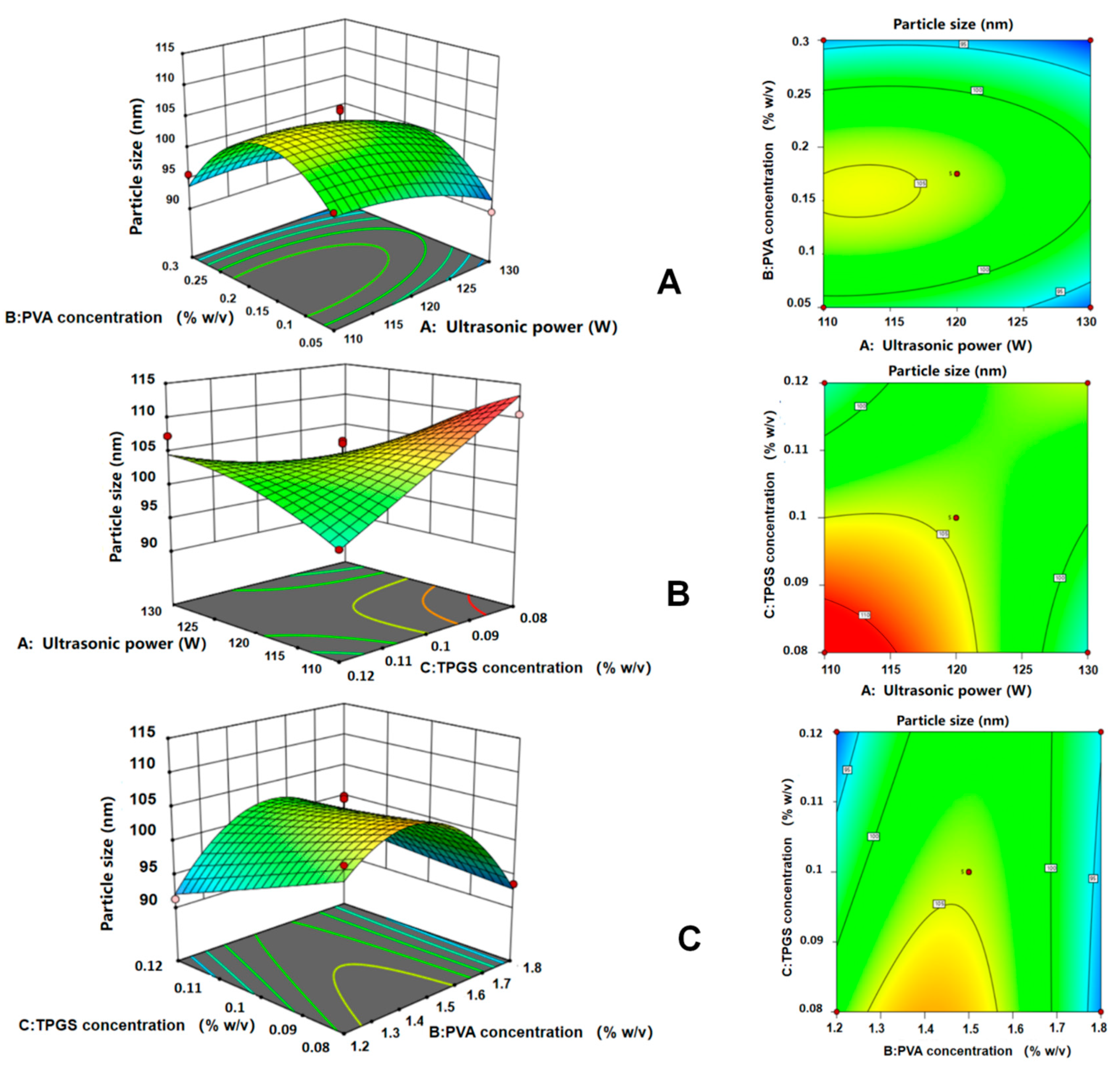


| No. | Argument | Response Value | ||
|---|---|---|---|---|
| X1 (W) | X2 (%, w/v) | X3 (%, w/v) | Y (nm) | |
| 1 | 120 | 0.3 | 0.12 | 93.2 |
| 2 | 120 | 0.175 | 0.1 | 103.4 |
| 3 | 130 | 0.05 | 0.1 | 90.1 |
| 4 | 120 | 0.3 | 0.08 | 93.6 |
| 5 | 130 | 0.175 | 0.12 | 107.3 |
| 6 | 120 | 0.175 | 0.1 | 101.7 |
| 7 | 120 | 0.175 | 0.1 | 106.7 |
| 8 | 120 | 0.175 | 0.1 | 103.8 |
| 9 | 120 | 0.05 | 0.12 | 91.3 |
| 10 | 110 | 0.175 | 0.08 | 110.6 |
| 11 | 120 | 0.175 | 0.1 | 106.4 |
| 12 | 110 | 0.3 | 0.1 | 95.7 |
| 13 | 110 | 0.05 | 0.1 | 99.4 |
| 14 | 130 | 0.3 | 0.1 | 90.1 |
| 15 | 120 | 0.05 | 0.08 | 104.6 |
| 16 | 110 | 0.175 | 0.12 | 96.9 |
| 17 | 130 | 0.175 | 0.08 | 95.9 |
| Source | Particle Size (nm) | |||
|---|---|---|---|---|
| Sum of Squares | df | F-Value | p-Value (prob > F) | |
| Model | 647.78 | 9 | 9.79 | 0.0033 |
| X1 | 46.08 | 1 | 6.27 | 0.0408 |
| X2 | 20.48 | 1 | 2.78 | 0.1391 |
| X3 | 32.00 | 1 | 4.35 | 0.0754 |
| X1X2 | 3.42 | 1 | 0.4653 | 0.5171 |
| X1X3 | 157.50 | 1 | 21.42 | 0.0024 |
| X2X3 | 41.60 | 1 | 5.66 | 0.0490 |
| X12 | 13.30 | 1 | 1.81 | 0.2206 |
| X22 | 324.40 | 1 | 44.11 | 0.0003 |
| X32 | 0.0221 | 1 | 0.0030 | 0.9578 |
| Residual | 51.48 | 7 | ||
| Lack of Fit | 33.93 | 3 | 2.58 | 0.1912 |
| Pure Error | 17.55 | 4 | ||
| Cor Total | 699.26 | 16 | ||
| Factor | Value |
|---|---|
| Organic solvent | DMF, AC:DMF (1:1, 2:1), EtOH:DMF (1:1, 2:1), 70% EtOH |
| Oil–water phase ratio | 1:2, 1:5, 1:10, 1:20, 1:30 |
| Ultrasonic power (W) | 100, 110, 120, 130, 140, 150, 160 |
| Ultrasonic time (min) | 5, 10, 15, 20, 25, 30, 35 |
| Spatial stabilizer | PVP K30, PVA, F68, F127 |
| Content of spatial stabilizer (%, w/v) | 0.1, 0.2, 0.3, 0.4, 0.5 |
| Ion stabilizer | sodium deoxycholate, SDS, TPGS, CTAB |
| Content of ion stabilizer (%, w/v) | 0.050, 0.075, 0.100, 0.150, 0.200 |
| Element | Level | ||
|---|---|---|---|
| Low (−1) | Middle (0) | High (+1) | |
| X1, Ultrasonic power (W) | 110 | 120 | 130 |
| X2, PVA concentration (%, w/v) | 0.05 | 0.175 | 0.3 |
| X3, TPGS concentration (%, w/v) | 0.08 | 0.1 | 0.12 |
| Dependent Variables | Goal | ||
| Y, Particle size (nm) | Minimize | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Guo, M.; He, Z.; Luo, Y.; He, X.; Huang, C.; Yuan, Y.; Zhao, Y.; Song, X.; Wang, X. Enhanced Anti-Inflammatory Activity of Tilianin Based on the Novel Amorphous Nanocrystals. Pharmaceuticals 2024, 17, 654. https://doi.org/10.3390/ph17050654
Sun M, Guo M, He Z, Luo Y, He X, Huang C, Yuan Y, Zhao Y, Song X, Wang X. Enhanced Anti-Inflammatory Activity of Tilianin Based on the Novel Amorphous Nanocrystals. Pharmaceuticals. 2024; 17(5):654. https://doi.org/10.3390/ph17050654
Chicago/Turabian StyleSun, Min, Mengran Guo, Zhongshan He, Yaoyao Luo, Xi He, Chuansheng Huang, Yong Yuan, Yunli Zhao, Xiangrong Song, and Xinchun Wang. 2024. "Enhanced Anti-Inflammatory Activity of Tilianin Based on the Novel Amorphous Nanocrystals" Pharmaceuticals 17, no. 5: 654. https://doi.org/10.3390/ph17050654
APA StyleSun, M., Guo, M., He, Z., Luo, Y., He, X., Huang, C., Yuan, Y., Zhao, Y., Song, X., & Wang, X. (2024). Enhanced Anti-Inflammatory Activity of Tilianin Based on the Novel Amorphous Nanocrystals. Pharmaceuticals, 17(5), 654. https://doi.org/10.3390/ph17050654








