Exploring the Potential Biological Activities of Pyrazole-Based Schiff Bases as Anti-Diabetic, Anti-Alzheimer’s, Anti-Inflammatory, and Cytotoxic Agents: In Vitro Studies with Computational Predictions
Abstract
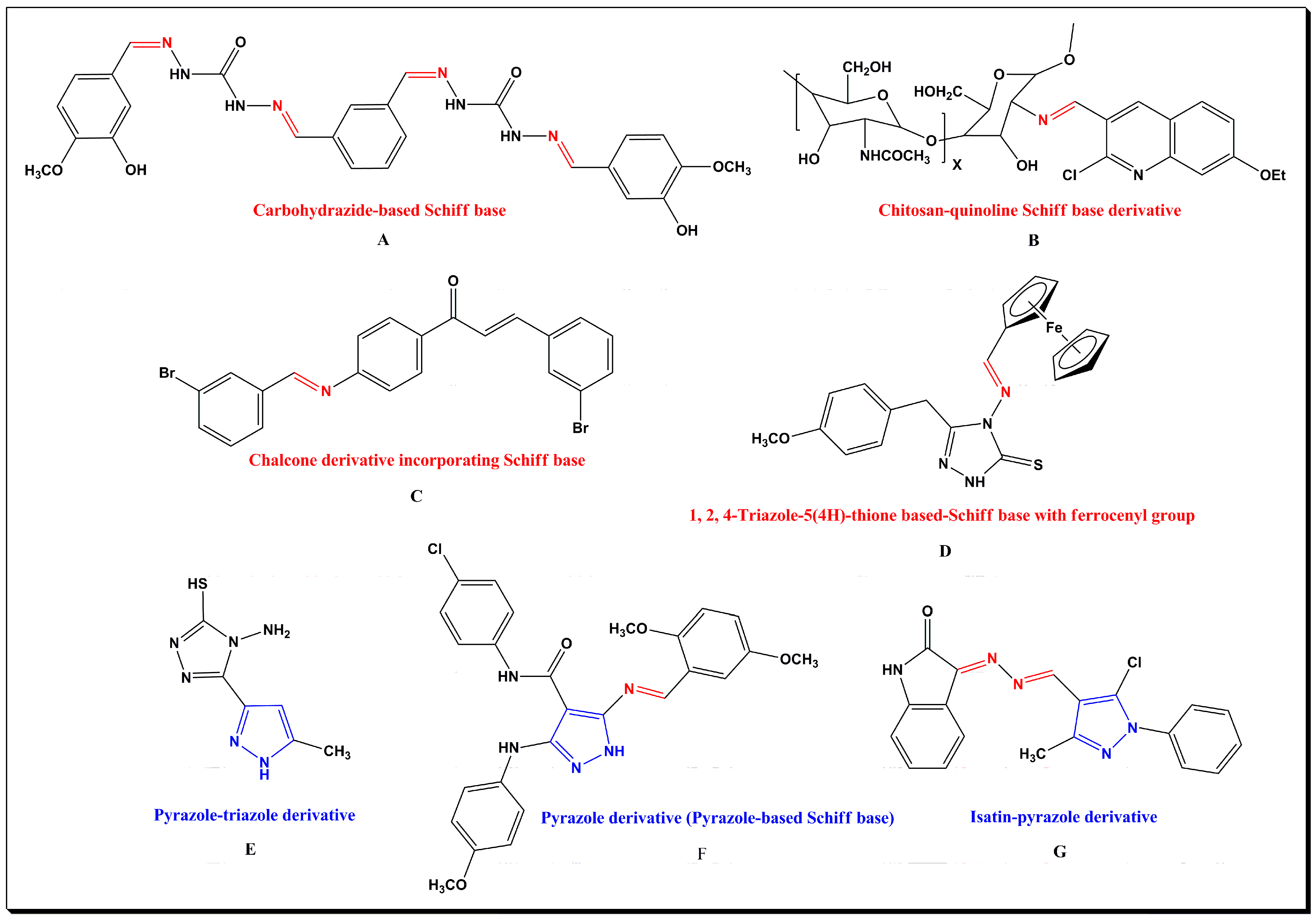
:1. Introduction
2. Results
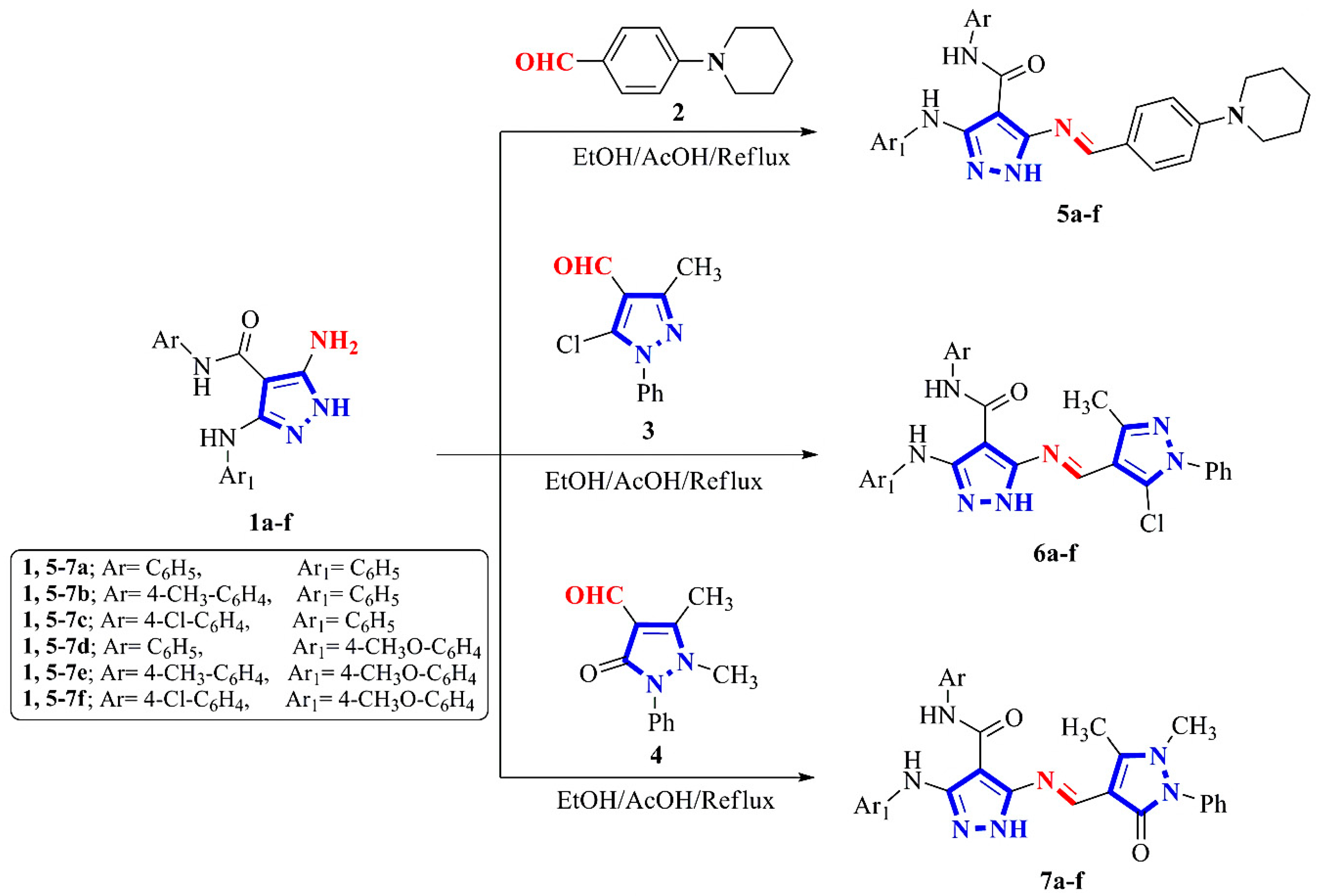
2.1. Chemistry
2.2. Biological Evaluations
2.2.1. The Antioxidant Activity
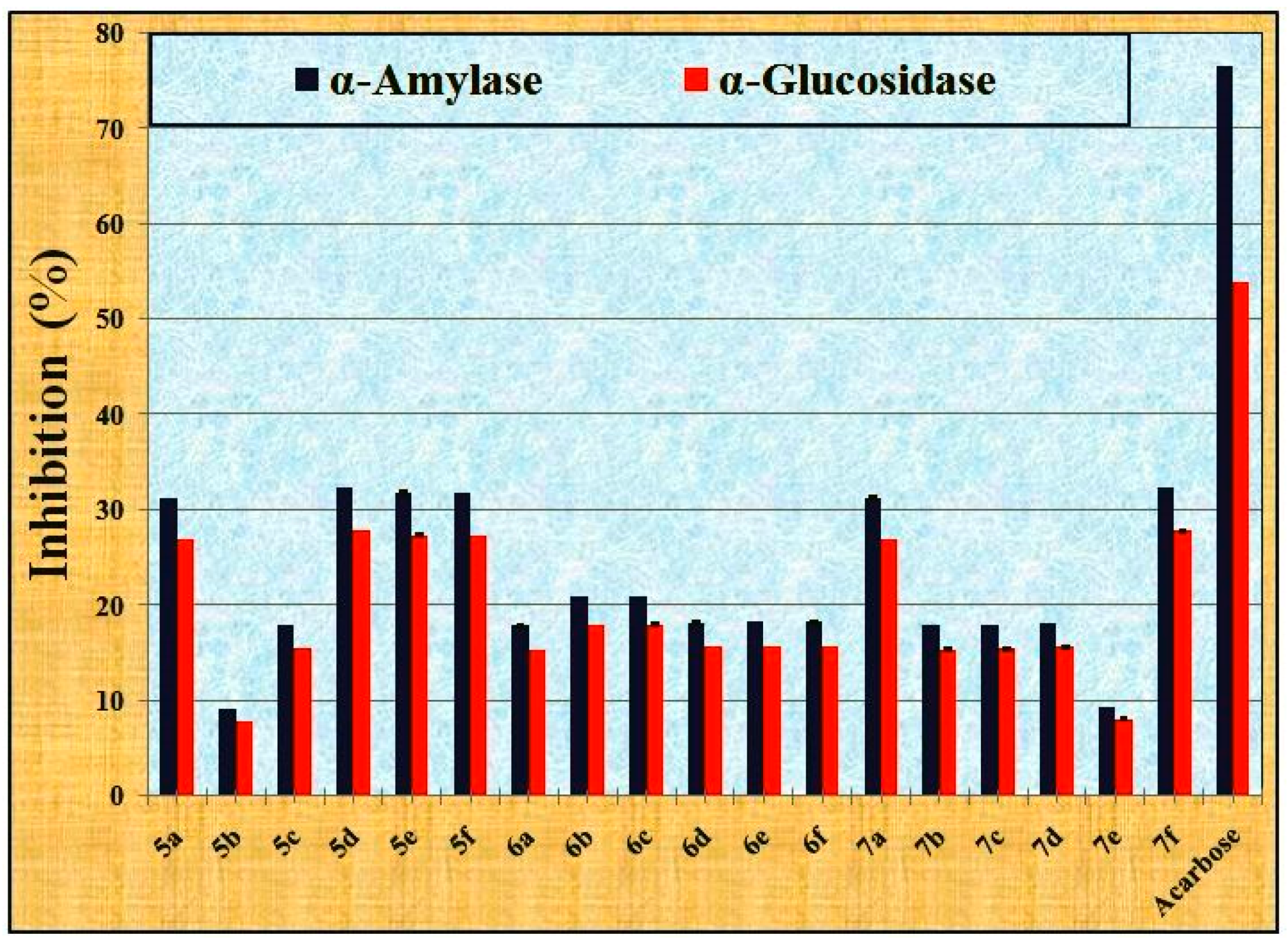
2.2.2. The Anti-Diabetic Activity
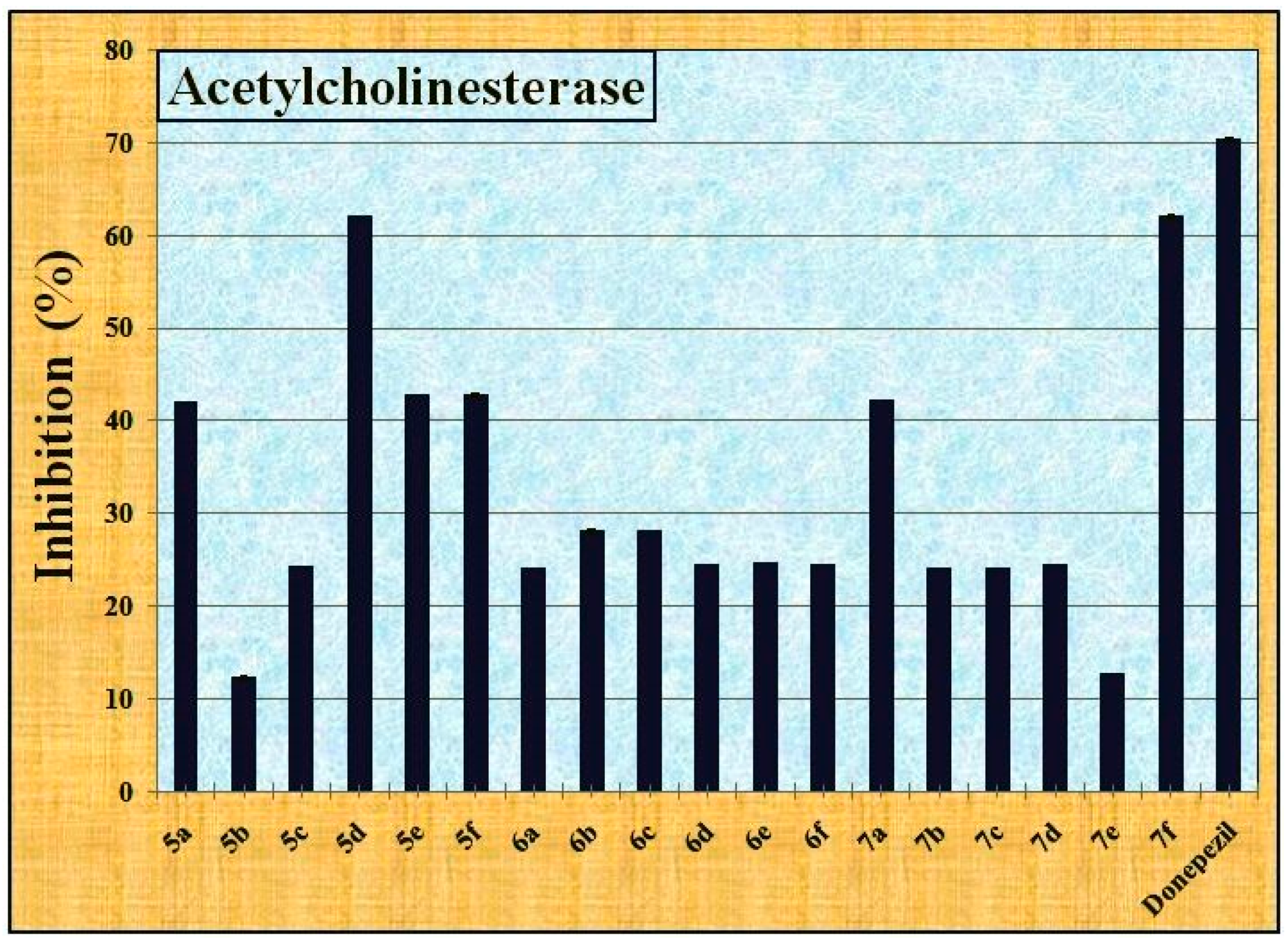
2.2.3. The Anti-Alzheimer’s Activity
2.2.4. The Anti-Inflammatory Activity
2.2.5. Cytotoxic Activity
2.2.6. The Enzymatic Activity
2.3. Computational Prediction (ADMT Properties)
3. Discussion
- -
- The absorption results of the pyrazole-based Schiff bases (5a, 5d, 5e, 5f, 7a, and 7f) indicate high values (well-absorbed molecules) ranging from 91.357% to 96.711% for intestinal absorption. Skin permeability (Kp) refers to skin absorption and the rate of drug candidates penetrating the skin. The skin permeability of the Schiff bases indicates low values less than −2.5, therefore, showing good skin permeability (log Kp = −2.735) and the ability to penetrate through the outermost layer of the epidermal skin.
- -
- The distribution results conclude that (i) the blood–brain permeation barrier (BBB permeability) predicted results indicate that these Schiff bases exhibit a poor distribution, with values lower than −1. The blood–brain barrier (BBB) regulates the permeability of drugs to the brain. A poor distribution refers to impaired drug delivery into the brain. Therefore, the medicinal efficacy of the drugs decreases. (ii) The central nervous system permeability (CNS permeability) indicates that the four Schiff bases (5a, 5d, 5e, and 5f) show high penetration, but two Schiff bases (7a and 7f) show moderate penetration.
- -
- Drug metabolism is one of the essential factors in drug disposition. The five enzymes play a crucial role in the metabolic processes of drugs in the liver. The results indicated that the six Schiff bases (5a, 5d, 5e, 5f, 7a, and 7f) are non-inhibitors of the CYP2D6 enzyme. Also, three Schiff bases (5e, 5f, and 7f) are non-inhibitors of the CYP1A2 enzyme. Therefore, the Schiff bases are well-metabolized molecules in the liver, can be eliminated from the body, and have no potential adverse effects.
- -
- The prediction of the toxicity of pyrazole-based Schiff bases (5a, 5d, 5e, 5f, 7a, and 7f) suggests that all Schiff bases are non-mutagenic except 7f. Additionally, none of the Schiff bases (5a, 5d, 5e, 5f, 7a, and 7f) induce skin sensitization. Therefore, these compounds are considered safe.
4. Materials and Methods
4.1. Chemistry
4.2. Biological Evaluations
4.2.1. The Antioxidant Activity
4.2.2. The Anti-Diabetic Activity
4.2.3. The Anti-Alzheimer’s Activity
4.2.4. The Anti-Inflammatory Activity
4.2.5. Cytotoxic Activity
4.2.6. The Enzymatic Activity
4.2.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Leszek, J.; Mikhaylenko, E.V.; Belousov, D.M.; Koutsouraki, E.; Szczechowiak, K.; Kobusiak-Prokopowicz, M.; Mysiak, A.; Diniz, B.S.; Somasundaram, S.G.; Kirkland, C.E.; et al. The links between cardiovascular diseases and Alzheimer’s disease. Curr. Neuropharmacol. 2021, 19, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Teles, F.; Collman, R.G.; Mominkhan, D.; Wang, Y. Viruses, periodontitis, and comorbidities. Periodontology 2000, 89, 190–206. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, L.H.; Mohammed, A.F.; Abdelrahman, M.H.; Trembleau, L.; Youssif, B.G. Design, Synthesis, and Biological Evaluation of Indole-2-carboxamides as Potential Multi-Target Antiproliferative Agents. Pharmaceuticals 2023, 16, 1039. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, V.; Ahamed, A.; Radhakrishnan, S.; Gaafar, A.R.Z.; Gurusamy, R.; Akbar, I. Synthesis of anthraquinone-connected coumarin derivatives via grindstone method and their evaluation of antibacterial, antioxidant, tyrosinase inhibitory activities with molecular docking, and DFT calculation studies. Heliyon 2024, 10, e25168. [Google Scholar] [CrossRef]
- Almehizia, A.A.; Aboulthana, W.M.; Naglah, A.M.; Hassan, A.S. In vitro biological studies and computational prediction-based analyses of pyrazolo[1,5-a]pyrimidine derivatives. RSC Adv. 2024, 14, 8397–8408. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Bhalla, A. Sustainable synthetic endeavors of pharmaceutically active Schiff bases and their metal complexes: A review on recent reports. Tetrahedron 2024, 153, 133836. [Google Scholar] [CrossRef]
- Khan, E.; Hanif, M.; Akhtar, M.S. Schiff bases and their metal complexes with biologically compatible metal ions; biological importance, recent trends and future hopes. Rev. Inorg. Chem. 2022, 42, 307–325. [Google Scholar] [CrossRef]
- Tsacheva, I.; Todorova, Z.; Momekova, D.; Momekov, G.; Koseva, N. Pharmacological activities of Schiff bases and their derivatives with low and high molecular phosphonates. Pharmaceuticals 2023, 16, 938. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, I.; Ahmad, M.; Saleem, M.; Ahmed, A. Pharmaceutical significance of Schiff bases: An overview. Future J. Pharm. Sci. 2024, 10, 16. [Google Scholar] [CrossRef]
- Mukhtar, S.S.; Hassan, A.S.; Morsy, N.M.; Hafez, T.S.; Hassaneen, H.M.; Saleh, F.M. Overview on synthesis, reactions, applications, and biological activities of Schiff bases. Egypt. J. Chem. 2021, 64, 6541–6554. [Google Scholar] [CrossRef]
- Çavuş, M.S.; Yakan, H.; Başkan, C.; Muğlu, H.; Babacan, A.A. Schiff bases based on thio/carbohydrazide: Synthesis, spectroscopic characterization, DFT, antimicrobial, DNA interactions and cytotoxicity studies. J. Mol. Struct. 2024, 1304, 137655. [Google Scholar] [CrossRef]
- Abdel-Baky, Y.M.; Omer, A.M.; El-Fakharany, E.M.; Ammar, Y.A.; Abusaif, M.S.; Ragab, A. Developing a new multi-featured chitosan-quinoline Schiff base with potent antibacterial, antioxidant, and antidiabetic activities: Design and molecular modeling simulation. Sci. Rep. 2023, 13, 22792. [Google Scholar] [CrossRef] [PubMed]
- Koçyiğit, Ü.M.; Gezegen, H.; Taslimi, P. Synthesis, characterization, and biological studies of chalcone derivatives containing Schiff bases: Synthetic derivatives for the treatment of epilepsy and Alzheimer’s disease. Arch. Pharm. 2020, 353, 2000202. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Hassan, M.; Rafiq, M.; Abbas, Q.; Ishaq, A.; Shahzadi, S.; Seo, S.Y.; Saleem, M. Microwave-assisted synthesis, in vivo anti-inflammatory and in vitro anti-oxidant activities, and molecular docking study of new substituted Schiff base derivatives. Pharm. Chem. J. 2018, 52, 424–437. [Google Scholar] [CrossRef]
- Aziz, H.; Zahoor, A.F.; Ahmad, S. Pyrazole bearing molecules as bioactive scaffolds: A review. J. Chil. Chem. Soc. 2020, 65, 4746–4753. [Google Scholar] [CrossRef]
- Brullo, C.; Rapetti, F.; Bruno, O. Pyrazolyl-ureas as interesting scaffold in medicinal chemistry. Molecules 2020, 25, 3457. [Google Scholar] [CrossRef]
- Alam, M.A. Pyrazole: An emerging privileged scaffold in drug discovery. Future Med. Chem. 2023, 15, 2011–2023. [Google Scholar] [CrossRef]
- Bastos, I.M.; Rebelo, S.; Silva, V.L. A review of poly (ADP-ribose) polymerase-1 (PARP1) role and its inhibitors bearing pyrazole or indazole core for cancer therapy. Biochem. Pharmacol. 2024, 221, 116045. [Google Scholar] [CrossRef] [PubMed]
- Mortada, S.; Karrouchi, K.; Hamza, E.H.; Oulmidi, A.; Bhat, M.A.; Mamad, H.; Aalilou, Y.; Radi, S.; Ansar, M.H.; Masrar, A.; et al. Synthesis, structural characterizations, in vitro biological evaluation and computational investigations of pyrazole derivatives as potential antidiabetic and antioxidant agents. Sci. Rep. 2024, 14, 1312. [Google Scholar] [CrossRef] [PubMed]
- Alkahtani, H.M.; Almehizia, A.A.; Al-Omar, M.A.; Obaidullah, A.J.; Zen, A.A.; Hassan, A.S.; Aboulthana, W.M. In Vitro Evaluation and Bioinformatics Analysis of Schiff Bases Bearing Pyrazole Scaffold as Bioactive Agents: Antioxidant, Anti-Diabetic, Anti-Alzheimer, and Anti-Arthritic. Molecules 2023, 28, 7125. [Google Scholar] [CrossRef]
- Hassan, A.S.; Morsy, N.M.; Aboulthana, W.M.; Ragab, A. Exploring novel derivatives of isatin-based Schiff bases as multi-target agents: Design, synthesis, in vitro biological evaluation, and in silico ADMET analysis with molecular modeling simulations. RSC Adv. 2023, 13, 9281–9303. [Google Scholar] [CrossRef] [PubMed]
- Abdelazeem, N.M.; Aboulthana, W.M.; Hassan, A.S.; Almehizia, A.A.; Naglah, A.M.; Alkahtani, H.M. Synthesis, in silico ADMET prediction analysis, and pharmacological evaluation of sulfonamide derivatives tethered with pyrazole or pyridine as anti-diabetic and anti-Alzheimer’s agents. Saudi Pharm. J. 2024, 23, 102025. [Google Scholar] [CrossRef]
- Elgiushy, H.R.; Mohamed, S.H.; Taha, H.; Sawaf, H.; Hassan, Z.; Abou-Taleb, N.A.; El-Labbad, E.M.; Hassan, A.S.; Abouzid, K.A.; Hammad, S.F. Identification of a promising hit from a new series of pyrazolo[1,5-a]pyrimidine based compounds as a potential anticancer agent with potent CDK1 inhibitory and pro-apoptotic properties through a multistep in vitro assessment. Bioorg. Chem. 2022, 120, 105646. [Google Scholar] [CrossRef] [PubMed]
- Morsy, N.M.; Hassan, A.S.; Hafez, T.S.; Mahran, M.R.; Sadawe, I.A.; Gbaj, A.M. Synthesis, antitumor activity, enzyme assay, DNA binding and molecular docking of Bis-Schiff bases of pyrazoles. J. Iran. Chem. Soc. 2021, 18, 47–59. [Google Scholar] [CrossRef]
- Hassan, A.S.; Osman, S.A.; Hafez, T.S. 5-Phenyl-2-furaldehyde: Synthesis, reactions and biological activities. Egypt. J. Chem. 2015, 58, 113–139. [Google Scholar]
- Hassan, A.S. Mixed isatin with 3-(2-(aryl)hydrazono)acetylacetone Mn(II), Co(II) and Ni(II) complexes: Antibacterial evaluation and molecular properties prediction. Bull. Chem. Soc. Ethiop. 2020, 34, 533–541. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; Khatab, T.K.; Hassan, A.S. Copper-based glass-ceramic as an efficient catalyst in the synthesis of pyrazolo[1,5-a]pyrimidine under solvent-free condition with docking validation as COVID-19 main protease (Mpro) inhibitor. Bull. Chem. Soc. Ethiop. 2021, 35, 185–196. [Google Scholar] [CrossRef]
- Khatab, T.K.; Hassan, A.S.; Hafez, T.S. V2O5/SiO2 as an efficient catalyst in the synthesis of 5-aminopyrazole derivatives under solvent free condition. Bull. Chem. Soc. Ethiop. 2019, 33, 135–142. [Google Scholar] [CrossRef]
- Hassan, A.S.; Hafez, T.S.; Osman, S.A. Synthesis, characterization, and cytotoxicity of some new 5-aminopyrazole and pyrazolo[1,5-a]pyrimidine derivatives. Sci. Pharm. 2015, 83, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Soliman, D.H.; Eldehna, W.M.; Ghabbour, H.A.; Kabil, M.M.; Abdel-Aziz, M.M.; Abdel-Aziz, H.A. Novel 6-phenylnicotinohydrazide derivatives: Design, synthesis and biological evaluation as a novel class of antitubercular and antimicrobial agents. Biol. Pharm. Bull. 2017, 40, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-J.; Shi, Y.-Q. Synthesis and crystal structure of 5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-carbaldehyde. J. Chem. Crystallogr. 2011, 41, 1816–1819. [Google Scholar] [CrossRef]
- Mukhtar, S.S.; Hassan, A.S.; Morsy, N.M.; Hafez, T.S.; Saleh, F.M.; Hassaneen, H.M. Design, synthesis, molecular prediction and biological evaluation of pyrazole-azomethine conjugates as antimicrobial agents. Synth. Commun. 2021, 51, 1564–1580. [Google Scholar] [CrossRef]
- Hassan, A.S.; Askar, A.A.; Naglah, A.M.; Almehizia, A.A.; Ragab, A. Discovery of new Schiff bases tethered pyrazole moiety: Design, synthesis, biological evaluation, and molecular docking study as dual targeting DHFR/DNA gyrase inhibitors with immunomodulatory activity. Molecules 2020, 25, 2593. [Google Scholar] [CrossRef]
- Ali, S.A.; Awad, S.M.; Said, A.M.; Mahgoub, S.; Taha, H.; Ahmed, N.M. Design, synthesis, molecular modelling and biological evaluation of novel 3-(2-naphthyl)-1-phenyl-1H-pyrazole derivatives as potent antioxidants and 15-Lipoxygenase inhibitors. J. Enzyme Inhib. Med. Chem. 2020, 35, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, G.; Prakash, T.B.; Sravya, G.; Padmavathi, V.; Padmaja, A. Synthesis and antioxidant activity of bis unsaturated sulfones, bispyrroles, and bispyrazoles. Res. Chem. Interm. 2015, 41, 8815–8828. [Google Scholar] [CrossRef]
- Achutha, D.K.; Kameshwar, V.H.; Ningappa, M.B.; Kariyappa, A.K. Synthesis and in vitro biological evaluation for antioxidant, anti-inflammatory activities and molecular docking studies of novel pyrazole derivatives. Biointer. Res. Appl. Chem. 2017, 7, 2040–2047. [Google Scholar]
- Hassan, A.S.; Aboulthana, W.M. Synthesis, In Vitro Biological Investigation, and In Silico Analysis of Pyrazole-Based Derivatives as Multi-target Agents. Egypt. J. Chem. 2023, 66, 441–455. [Google Scholar] [CrossRef]
- Zhong, G.; Shen, J.; Chen, Z.; Lin, Z.; Long, L.; Wu, J.; Long, C.; Huang, S.; Lian, P.; Luo, G. Antioxidant and Antitumor Activities of Newly Synthesized Hesperetin Derivatives. Molecules 2022, 27, 879. [Google Scholar] [CrossRef] [PubMed]
- Matta, R.; Pochampally, J.; Dhoddi, B.N.; Bhookya, S.; Bitla, S.; Akkiraju, A.G. Synthesis, antimicrobial and antioxidant activity of triazole, pyrazole containing thiazole derivatives and molecular docking studies on COVID-19. BMC Chem. 2023, 17, 61. [Google Scholar] [CrossRef]
- Shankar, B.; Jalapathi, P.; Anil, V.; Kumar, K.; Saikrishna, B.; Karunakarrao, K. Synthesis and biological evaluation of new 2-(6-alkyl-pyrazin-2-yl)-1H-benz[d]imidazoles as potent anti-inflammatory and anti-oxidant agents. Med. Chem. Res. 2017, 26, 1835–1846. [Google Scholar] [CrossRef]
- Nikookar, H.; Khanaposhtani, M.M.; Imanparast, S.; Faramarzi, M.A.; Ranjbar, P.R.; Mahdavi, M.; Larijani, B. Design, synthesis and in vitro α-glucosidase inhibition of novel dihydropyrano[3,2-c]quinoline derivatives as potential anti-diabetic agents. Bioorg. Chem. 2018, 77, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Ozil, M.; Emirik, M.; Belduz, A.; Ulker, S. Molecular docking studies and synthesis of novel bis benzimidazole derivatives as inhibitors of α-glucosidase. Bioorg. Med. Chem. 2016, 24, 5103–5114. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, F.; Naureen, S.; Ashraf, M.; Al-Rashida, M.; Jahan, B.; Munawar, M.A.; Khan, M.A. Imidazole-pyrazole hybrids: Synthesis, characterization and in-vitro bio evaluation against α-glucosidase enzyme with molecular docking studies. Bioorg. Chem. 2019, 82, 267–273. [Google Scholar] [CrossRef]
- Pogaku, V.; Gangarapu, K.; Basavoju, S.; Tatapudic, K.K.; Katragadda, S.B. Design, synthesis, molecular modelling, ADME prediction and antihyperglycemic evaluation of new pyrazole-triazolo pyrimidine hybrids as potent α-glucosidase inhibitors. Bioorg. Chem. 2019, 93, 103307. [Google Scholar] [CrossRef]
- Duhan, M.; Singh, R.; Devi, M.; Sindhu, J.; Bhatia, R.; Kumar, A.; Kumar, P. Synthesis, molecular docking and QSAR study of thiazole clubbed pyrazole hybrid as α-amylase inhibitor. J. Biomol. Struct. Dyn. 2019, 39, 91–107. [Google Scholar] [CrossRef]
- Pillai, R.R.; Karrouchi, K.; Fettach, S.; Armaković, S.; Armaković, S.J.; Brik, Y.; Taoufik, J.; Radi, S.; Faouzi, M.E.; Ansar, M. Synthesis, spectroscopic characterization, reactive properties by DFT calculations, molecular dynamics simulations and biological evaluation of Schiff bases tethered 1,2,4-triazole and pyrazole rings. J. Mol. Struct. 2019, 1177, 47–54. [Google Scholar] [CrossRef]
- Tok, F.; Koçyiğit-Kaymakçıoğlu, B.; Sağlık, B.N.; Levent, S.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis and biological evaluation of new pyrazolone Schiff bases as monoamine oxidase and cholinesterase inhibitors. Bioorg. Chem. 2019, 84, 41–50. [Google Scholar] [CrossRef]
- Alam, M.S.; Ahmed, J.U. Synthesis, crystal structure, biological evaluation, in silico ADME properties, enzymatic target prediction and molecular docking studies of pyrazolone-azomethine analogs. J. Mol. Struct. 2023, 1294, 136504. [Google Scholar] [CrossRef]
- Abou-Zied, H.A.; Beshr, E.A.M.; Hayallah, A.M.; Abdel-Aziz, M. Emerging insights into pyrazoline motifs: A comprehensive exploration of biological mechanisms and prospects for future advancements. J. Mol. Struct. 2024, 1296, 136807. [Google Scholar] [CrossRef]
- Gedawy, E.M.; Kassab, A.E.; El Kerdawy, A.M. Design, Synthesis and Biological Evaluation of Novel Pyrazole Sulfonamide Derivatives as Dual COX-2/5-LOX Inhibitors. Eur. J. Med. Chem. 2020, 189, 112066. [Google Scholar] [CrossRef]
- Pohanka, M. Inhibitors of acetylcholinesterase and butyrylcholinesterase meet immunity. Int. J. Mol. Sci. 2014, 15, 9809–9825. [Google Scholar] [CrossRef]
- Kumar, A.; Jain, S.; Parle, M.; Jain, N.; Kumar, P. 3-Aryl-1-phenyl-1H-pyrazole derivatives as new multi target directed ligands for the treatment of Alzheimer’s disease, with acetylcholinesterase and monoamine oxidase inhibitory properties. EXCLI J. 2013, 12, 1030–1042. [Google Scholar] [PubMed]
- Jeon, H.; Yoon, W.J.; Ham, Y.M.; Yoon, S.A.; Kang, S.C. Anti-arthritis effect through the anti-inflammatory effect of Sargassummuticum extract in collagen-induced arthritic (CIA) mice. Molecules 2019, 24, 276. [Google Scholar] [CrossRef]
- Hossain, M.M.; Kabir, M.S.H.; Hasanat, A.; Kabir, M.I.; Chowdhury, T.A.; Kibria, A.S.M.G. Investigation of in vitro anti-arthritic and membrane stabilizing activity of ethanol extracts of three Bangladeshi plants. Pharm. Innov. J. 2015, 4, 76–80. [Google Scholar]
- Hassan, A.S.; Morsy, N.M.; Aboulthana, W.M.; Ragab, A. In vitro enzymatic evaluation of some pyrazolo[1,5-a]pyrimidine derivatives: Design, synthesis, antioxidant, anti-diabetic, anti-Alzheimer, and anti-arthritic activities with molecular modeling simulation. Drug Dev. Res. 2023, 84, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Ayman, R.; Radwan, A.M.; Elmetwally, A.M.; Ammar, Y.A.; Ragab, A. Discovery of novel pyrazole and pyrazolo[1,5-a]pyrimidine derivatives as cyclooxygenase inhibitors (COX-1 and COX-2) using molecular modeling simulation. Arch. Pharm. 2023, 356, e2200395. [Google Scholar] [CrossRef]
- Sivaramakarthikeyan, R.; Iniyaval, S.; Saravanan, V.; Lim, W.M.; Mai, C.W.; Ramalingan, C. Molecular Hybrids Integrated with Benzimidazole and Pyrazole Structural Motifs: Design, Synthesis, Biological Evaluation, and Molecular Docking Studies. ACS Omega 2020, 5, 10089–10098. [Google Scholar] [CrossRef]
- Arya, C.G.; Gondru, R.; Li, Y.; Banothu, J. Coumarin–benzimidazole hybrids: A review of developments in medicinal chemistry. Eur. J. Med. Chem. 2022, 227, 113921. [Google Scholar]
- Joy, M.N.; Guda, M.R.; Zyryanov, G.V. Evaluation of Anti-Inflammatory and Anti-Tubercular Activity of 4-Methyl-7-Substituted Coumarin Hybrids and Their Structure Activity Relationships. Pharmaceuticals 2023, 16, 1326. [Google Scholar] [CrossRef] [PubMed]
- Angre, T.; Kumar, A.; Singh, A.K.; Thareja, S.; Kumar, P. Role of collagen regulators in cancer treatment: A comprehensive review. Anti-Cancer Agents Med. Chem. 2022, 22, 2956–2984. [Google Scholar] [CrossRef] [PubMed]
- Czylkowska, A.; Szczesio, M.; Raducka, A.; Rogalewicz, B.; Kręcisz, P.; Czarnecka, K.; Szymański, P.; Pitucha, M.; Pawlak, T. Cytotoxic Activity against A549 Human Lung Cancer Cells and ADMET Analysis of New Pyrazole Derivatives. Int. J. Mol. Sci. 2021, 22, 6692. [Google Scholar] [CrossRef] [PubMed]
- Nagender, P.; Kumar, R.N.; Reddy, G.M.; Swaroop, D.K.; Poornachandra, Y.; Kumar, C.G.; Narsaiah, B. Synthesis of novel hydrazone and azole functionalized pyrazolo [3, 4-b]pyridine derivatives as promising anticancer agents. Bioorg. Med. Chem. Lett. 2016, 26, 4427–4432. [Google Scholar] [CrossRef] [PubMed]
- Halim, P.A.; Sharkawi, S.M.Z.; Labib, M.B. Novel pyrazole-based COX-2 inhibitors as potential anticancer agents: Design, synthesis, cytotoxic effect against resistant cancer cells, cell cycle arrest, apoptosis induction and dual EGFR/Topo-1 inhibition. Bioorg. Chem. 2023, 131, 106273. [Google Scholar] [CrossRef] [PubMed]
- Fayed, E.A.; Sabour, R.; Harras, M.F.; Mehany, A. Design, synthesis, biological evaluation and molecular modeling of new coumarin derivatives as potent anticancer agents. Med. Chem. Res. 2019, 28, 1284–1297. [Google Scholar] [CrossRef]
- El-Gohary, N.; Gabr, M.; Shaaban, M. Synthesis, molecular modeling and biological evaluation of new pyrazolo[3,4-b]pyridine analogs as potential antimicrobial, antiquorum-sensing and anticancer agents. Bioorg. Chem. 2019, 89, 102976. [Google Scholar] [CrossRef]
- Cao, A.L.; Tang, Q.F.; Zhou, W.C.; Qiu, Y.Y.; Hu, S.J.; Yin, P.H. Ras/ERK signaling pathway is involved in curcumin-induced cell cycle arrest and apoptosis in human gastric carcinoma AGS cells. J. Asian Nat. Prod. Res. 2015, 17, 56–63. [Google Scholar] [CrossRef]
- Harras, M.F.; Sabour, R. Design, synthesis and biological evaluation of novel 1,3,4-trisubstituted pyrazole derivatives as potential chemotherapeutic agents for hepatocellular carcinoma. Bioorg. Chem. 2018, 78, 149–157. [Google Scholar] [CrossRef]
- Lepiarczyk, M.; Kałuża, Z.; Bielawska, A.; Czarnomysy, R.; Gornowicz, A.; Bielawski, K. Cytotoxic activity of octahydro pyrazin[2,1-a:5,4-a’]diisoquinoline derivatives in human breast cancer cells. Arch. Pharm. Res. 2015, 38, 628–641. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Hassan, A.S.; Moustafa, G.O.; Awad, H.M.; Nossier, E.S.; Mady, M.F. Design, Synthesis, Anticancer Evaluation, Enzymatic Assays, and a Molecular Modeling Study of Novel Pyrazole−Indole Hybrids. ACS Omega 2021, 6, 12361–12374. [Google Scholar] [CrossRef]
- Badraoui, R.; Rebai, T.; Elkahoui, S.; Alreshidi, M.; Veettil, N.V.; Noumi, E.; Al-Motair, A.K.; Aouadi, K.; Kadri, A.; De Feo, V.; et al. Allium subhirsutum L. as a Potential Source of Antioxidant and Anticancer Bioactive Molecules: HR-LCMS Phytochemical Profiling, In Vitro and In Vivo Pharmacological Study. Antioxidants 2020, 9, 1003. [Google Scholar] [CrossRef]
- Hassan, A.S. Antimicrobial evaluation, in silico ADMET prediction, molecular docking, and molecular electrostatic potential of pyrazole-isatin and pyrazole-indole hybrid molecules. J. Iran. Chem. Soc. 2022, 19, 3577–3589. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A.H.M. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Wickramaratne, M.N.; Punchihewa, J.; Wickramaratne, D. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement Altern. Med. 2016, 16, 466. [Google Scholar] [CrossRef]
- Pistia-Brueggeman, G.; Hollingsworth, R.I. A preparation and screening strategy for glycosidase inhibitors. Tetrahedron 2001, 57, 8773–8778. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Das, S.; Sureshkumar, P. Effect of methanolic root extract of Blepharispermum subsesssile DC in controlling arthritic activity. Res. J. Biotechnol. 2016, 11, 65–74. [Google Scholar]
- Oyedapo, O.O.; Famurewa, A.J. Antiprotease and Membrane Stabilizing Activities of Extracts of Fagara Zanthoxyloides, Olax Subscorpioides and Tetrapleura Tetraptera. Int. J. Pharmacogn. 1995, 33, 65–69. [Google Scholar] [CrossRef]
- Meera, S.; Ramaiah, N.; Kalidindi, N. Illustration of anti-rheumatic mechanism of rheumavedic capsule. Saudi Pharm. J. 2011, 19, 279–284. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Hassan, A.S.; Awad, H.M.; Magd-El-Din, A.A.; Hafez, T.S. Synthesis and in vitro antitumor evaluation of novel Schiff bases. Med. Chem. Res. 2018, 27, 915–927. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Alzahrani, F.A.; Qari, H.A.; Oves, M. A Novel Approach to Unraveling the Apoptotic Potential of Rutin (Bioflavonoid) via Targeting Jab1 in Cervical Cancer Cells. Molecules 2021, 26, 5529. [Google Scholar] [CrossRef]
| Pyrazole-Based Schiff Bases | Antioxidant Activity | Scavenging Activity | ||
|---|---|---|---|---|
| Total Antioxidant Capacity (TAC, mg Gallic Acid/g) | Iron-Reducing Power (IRP, µg/mL) | DPPH (IC50 µM) | ABTS (%) | |
| 5a * | 52.25 ± 0.19 | 34.27 ± 0.07 | 26.43 ± 0.01 | 75.46 ± 0.05 |
| 5b | 15.26 ± 0.06 | 10.01 ± 0.02 | 59.80 ± 0.03 | 22.03 ± 0.01 |
| 5c | 30.01 ± 0.11 | 19.68 ± 0.04 | 42.86 ± 0.02 | 43.34 ± 0.03 |
| 5d * | 54.13 ± 0.20 | 35.51 ± 0.07 | 23.98 ± 0.01 | 78.18 ± 0.05 |
| 5e * | 53.23 ± 0.20 | 34.91 ± 0.07 | 23.71 ± 0.01 | 76.87 ± 0.05 |
| 5f * | 53.14 ± 0.20 | 34.85 ± 0.07 | 22.83 ± 0.01 | 76.74 ± 0.05 |
| 6a | 29.86 ± 0.11 | 19.58 ± 0.04 | 43.35 ± 0.02 | 43.12 ± 0.03 |
| 6b | 34.93 ± 0.13 | 22.91 ± 0.04 | 36.02 ± 0.02 | 50.45 ± 0.03 |
| 6c | 34.99 ± 0.13 | 22.95 ± 0.05 | 34.58 ± 0.02 | 50.54 ± 0.03 |
| 6d | 30.42 ± 0.11 | 19.95 ± 0.04 | 40.11 ± 0.02 | 43.93 ± 0.03 |
| 6e | 30.52 ± 0.11 | 20.02 ± 0.04 | 38.94 ± 0.02 | 44.08 ± 0.03 |
| 6f | 30.47 ± 0.11 | 19.98 ± 0.04 | 37.60 ± 0.02 | 44.00 ± 0.03 |
| 7a * | 52.34 ± 0.19 | 34.33 ± 0.07 | 24.94 ± 0.01 | 75.59 ± 0.05 |
| 7b | 29.91 ± 0.11 | 19.62 ± 0.04 | 42.45 ± 0.02 | 43.19 ± 0.03 |
| 7c | 29.96 ± 0.11 | 19.65 ± 0.04 | 40.72 ± 0.02 | 43.27 ± 0.03 |
| 7d | 30.36 ± 0.11 | 19.92 ± 0.04 | 40.53 ± 0.02 | 43.85 ± 0.03 |
| 7e | 15.68 ± 0.06 | 10.29 ± 0.02 | 51.98 ± 0.03 | 22.65 ± 0.01 |
| 7f * | 54.04 ± 0.20 | 35.45 ± 0.07 | 21.37 ± 0.01 | 78.05 ± 0.05 |
| STD | Ascorbic Acid | Ascorbic Acid | ||
| 79.10 ± 0.11 | 71.85 ± 0.05 | 29.30 ± 0.01 | 41.25 ± 0.01 | |
| Pyrazole-Based Schiff Bases | Anti-Inflammatory | |
|---|---|---|
| Inhibition (%) | ||
| Protein Denaturation | Proteinase | |
| 5a * | 26.83 ± 0.03 | 23.33 ± 0.03 |
| 5b | 7.83 ± 0.01 | 6.81 ± 0.01 |
| 5c | 15.41 ± 0.02 | 13.40 ± 0.02 |
| 5d * | 27.79 ± 0.03 | 24.17 ± 0.03 |
| 5e * | 27.33 ± 0.03 | 23.76 ± 0.03 |
| 5f * | 27.28 ± 0.03 | 23.72 ± 0.03 |
| 6a | 15.33 ± 0.02 | 13.33 ± 0.02 |
| 6b | 17.94 ± 0.02 | 15.60 ± 0.02 |
| 6c | 17.97 ± 0.02 | 15.62 ± 0.02 |
| 6d | 15.62 ± 0.02 | 13.58 ± 0.02 |
| 6e | 15.67 ± 0.02 | 13.63 ± 0.02 |
| 6f | 15.64 ± 0.02 | 13.60 ± 0.02 |
| 7a * | 26.87 ± 0.03 | 23.37 ± 0.03 |
| 7b | 15.36 ± 0.02 | 13.35 ± 0.02 |
| 7c | 15.38 ± 0.02 | 13.38 ± 0.02 |
| 7d | 15.59 ± 0.02 | 13.56 ± 0.02 |
| 7e | 8.05 ± 0.01 | 4.81 ± 0.01 |
| 7f * | 27.75 ± 0.03 | 24.13 ± 0.03 |
| STD | Diclofenac Sodium | |
| 49.08 ± 0.01 | 46.11 ± 0.02 | |
| Pyrazole-Based Schiff Bases | Lung (A549) | Colon (Caco-2) | Normal Lung (WI-38) | Lung (A549) | Colon (Caco-2) |
|---|---|---|---|---|---|
| (IC50 μM) | Therapeutic Index (TI) | ||||
| 5a | 68.84 ± 0.14 | 60.29 ± 0.14 | 441.69 ± 8.80 | 6.41 | 7.32 |
| 5d | 48.61 ± 0.14 | 62.33 ± 0.14 | 731.72 ± 10.46 | 14.83 | 11.73 |
| 5e | 47.74 ± 0.20 | 40.99 ± 0.20 | 648.12 ± 7.57 | 13.57 | 15.81 |
| 5f | 60.45 ± 0.12 | 61.98 ± 0.12 | 493.07 ± 9.88 | 8.15 | 7.95 |
| 7a | 49.40 ± 0.18 | 42.42 ± 0.18 | 736.26 ± 7.95 | 14.90 | 17.35 |
| 7f | 55.74 ± 0.24 | 49.01 ± 0.24 | 542.51 ± 8.52 | 9.73 | 11.06 |
| Doxorubicin | 36.45 ± 0.16 | 54.94 ± 0.16 | 304.94 ± 4.72 | 8.36 | 5.55 |
| Pyrazole-Based Schiff Bases | Lung (A549) | Colon (Caco-2) | ||
|---|---|---|---|---|
| Caspase-3 (pg/mL) | Bcl-2 (ng/mL) | Caspase-3 (pg/mL) | Bcl-2 (ng/mL) | |
| DMSO | 85.92 ± 0.12 | 9.83 ± 0.05 | 97.06 ± 0.14 | 7.99 ± 0.08 |
| 5a | 184.74 ± 0.26 | 6.55 ± 0.03 | 208.68 ± 0.31 | 5.33 ± 0.05 |
| 5d | 300.73 ± 0.42 | 2.81 ± 0.01 | 242.65 ± 0.36 | 3.20 ± 0.03 |
| 5e | 214.81 ± 0.30 | 3.93 ± 0.02 | 315.45 ± 0.46 | 2.46 ± 0.02 |
| 5f | 236.29 ± 0.33 | 3.57 ± 0.02 | 266.92 ± 0.39 | 2.91 ± 0.03 |
| 7a | 322.21 ± 0.45 | 2.62 ± 0.01 | 363.98 ± 0.54 | 2.13 ± 0.02 |
| 7f | 244.88 ± 0.34 | 3.45 ± 0.02 | 276.62 ± 0.41 | 2.80 ± 0.03 |
| Doxorubicin | 330.80 ± 0.46 | 2.55 ± 0.01 | 373.68 ± 0.55 | 2.08 ± 0.02 |
| Properties | 5a | 5d | 5e | 5f | 7a | 7f | The Ideal Values |
|---|---|---|---|---|---|---|---|
| Absorption | |||||||
| Intestinal absorption (human) | 91.357 | 95.510 | 96.003 | 96.711 | 93.723 | 93.474 | Less than 30% is poorly absorbed |
| Skin permeability (log Kp) | −2.735 | −2.735 | −2.735 | −2.735 | −2.735 | −2.735 | >−2.5 is low |
| Distribution | |||||||
| BBB permeability | −1.318 | −1.530 | −1.550 | −1.704 | −1.433 | −1.818 | Poorly is <−1, high is >0.3 |
| CNS permeability | −1.705 | −1.886 | −1.817 | −1.776 | −2.061 | −2.133 | Penetrate is >−2, unable is <−3 |
| Metabolism | |||||||
| CYP1A2 inhibitor | Yes | Yes | No | No | Yes | No | No |
| CYP2C19 inhibitor | Yes | Yes | Yes | Yes | Yes | Yes | No |
| CYP2C9 inhibitor | Yes | Yes | Yes | Yes | Yes | Yes | No |
| CYP2D6 inhibitor | No | No | No | No | No | No | No |
| CYP3A4 inhibitor | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Toxicity | |||||||
| AMES toxicity | No | No | No | No | No | Yes | No |
| Skin sensitization | No | No | No | No | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naglah, A.M.; Almehizia, A.A.; Al-Wasidi, A.S.; Alharbi, A.S.; Alqarni, M.H.; Hassan, A.S.; Aboulthana, W.M. Exploring the Potential Biological Activities of Pyrazole-Based Schiff Bases as Anti-Diabetic, Anti-Alzheimer’s, Anti-Inflammatory, and Cytotoxic Agents: In Vitro Studies with Computational Predictions. Pharmaceuticals 2024, 17, 655. https://doi.org/10.3390/ph17050655
Naglah AM, Almehizia AA, Al-Wasidi AS, Alharbi AS, Alqarni MH, Hassan AS, Aboulthana WM. Exploring the Potential Biological Activities of Pyrazole-Based Schiff Bases as Anti-Diabetic, Anti-Alzheimer’s, Anti-Inflammatory, and Cytotoxic Agents: In Vitro Studies with Computational Predictions. Pharmaceuticals. 2024; 17(5):655. https://doi.org/10.3390/ph17050655
Chicago/Turabian StyleNaglah, Ahmed M., Abdulrahman A. Almehizia, Asma S. Al-Wasidi, Amirah Senaitan Alharbi, Mohammed H. Alqarni, Ashraf S. Hassan, and Wael M. Aboulthana. 2024. "Exploring the Potential Biological Activities of Pyrazole-Based Schiff Bases as Anti-Diabetic, Anti-Alzheimer’s, Anti-Inflammatory, and Cytotoxic Agents: In Vitro Studies with Computational Predictions" Pharmaceuticals 17, no. 5: 655. https://doi.org/10.3390/ph17050655
APA StyleNaglah, A. M., Almehizia, A. A., Al-Wasidi, A. S., Alharbi, A. S., Alqarni, M. H., Hassan, A. S., & Aboulthana, W. M. (2024). Exploring the Potential Biological Activities of Pyrazole-Based Schiff Bases as Anti-Diabetic, Anti-Alzheimer’s, Anti-Inflammatory, and Cytotoxic Agents: In Vitro Studies with Computational Predictions. Pharmaceuticals, 17(5), 655. https://doi.org/10.3390/ph17050655









