Anti-Inflammatory Effects of GPR55 Agonists and Antagonists in LPS-Treated BV2 Microglial Cells
Abstract
1. Introduction
2. Results
2.1. Effects of KIT C, ML-193, and O-1602 on Cell Viability
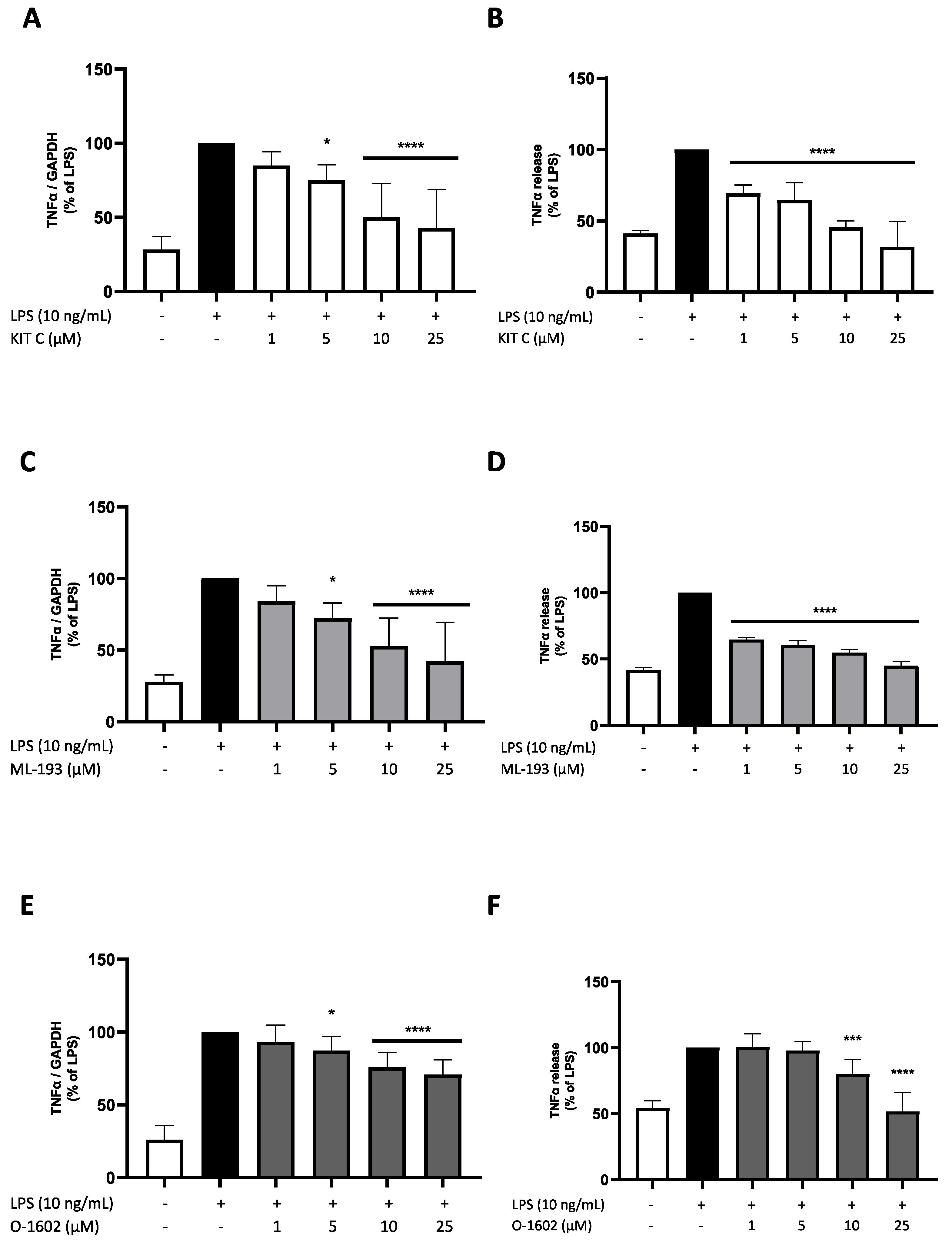
2.2. Effects of KIT C, ML-193, and O-1602 on TNF-α Expression and Release
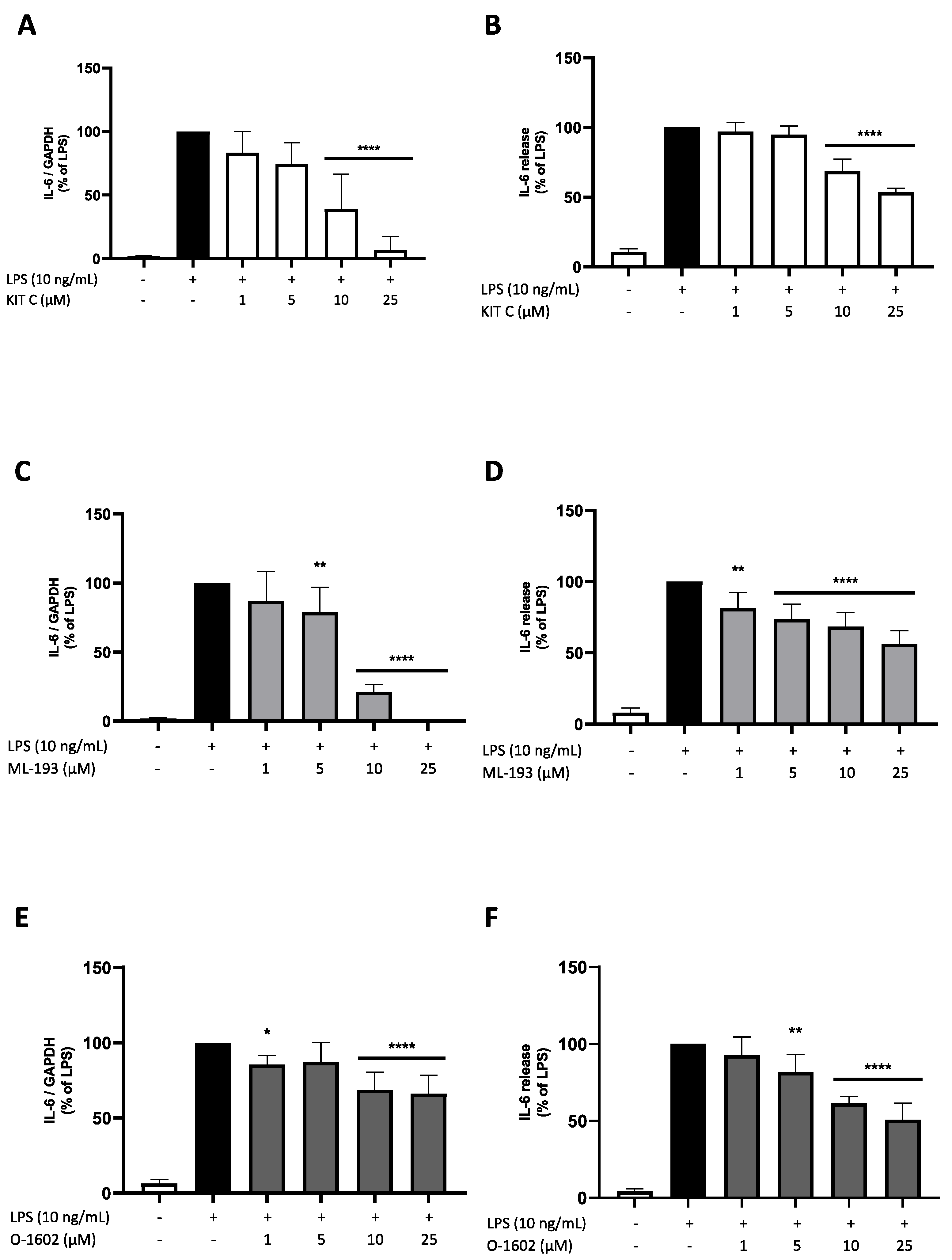
2.3. Effects of KIT C, ML-193, and O-1602 on IL-6 Expression and Release
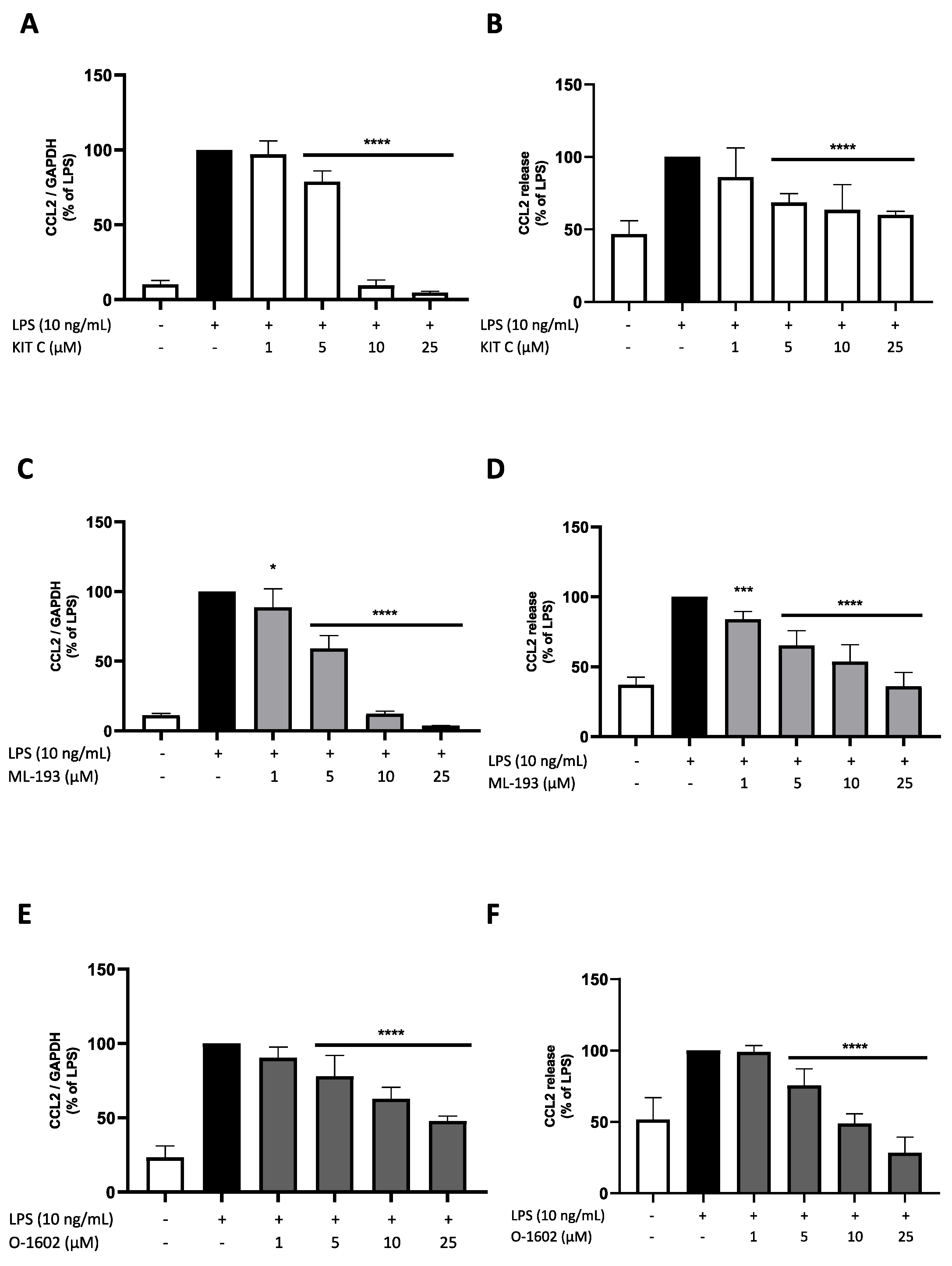
2.4. Effects of KIT C, ML-193, and O-1602 on CCL2 Expression and Release
2.5. Effects of KIT C, ML-193, and O-1602 on CCL3 Expression and Release
2.6. Effects of KIT C, ML-193, and O-1602 on CXCL2 Expression and Release
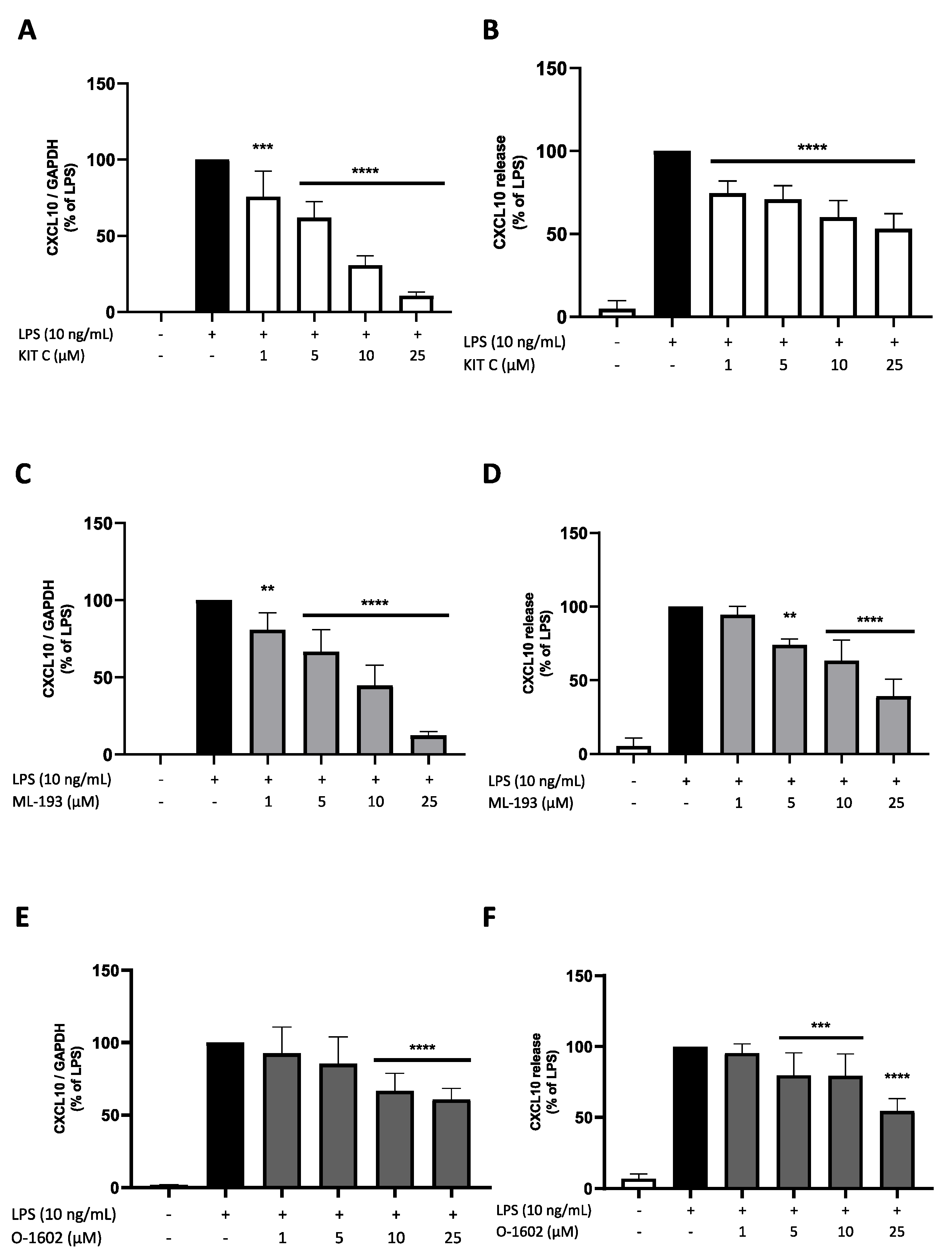
2.7. Effects of KIT C, ML-193, and O-1602 on CXCL10 Expression and Release
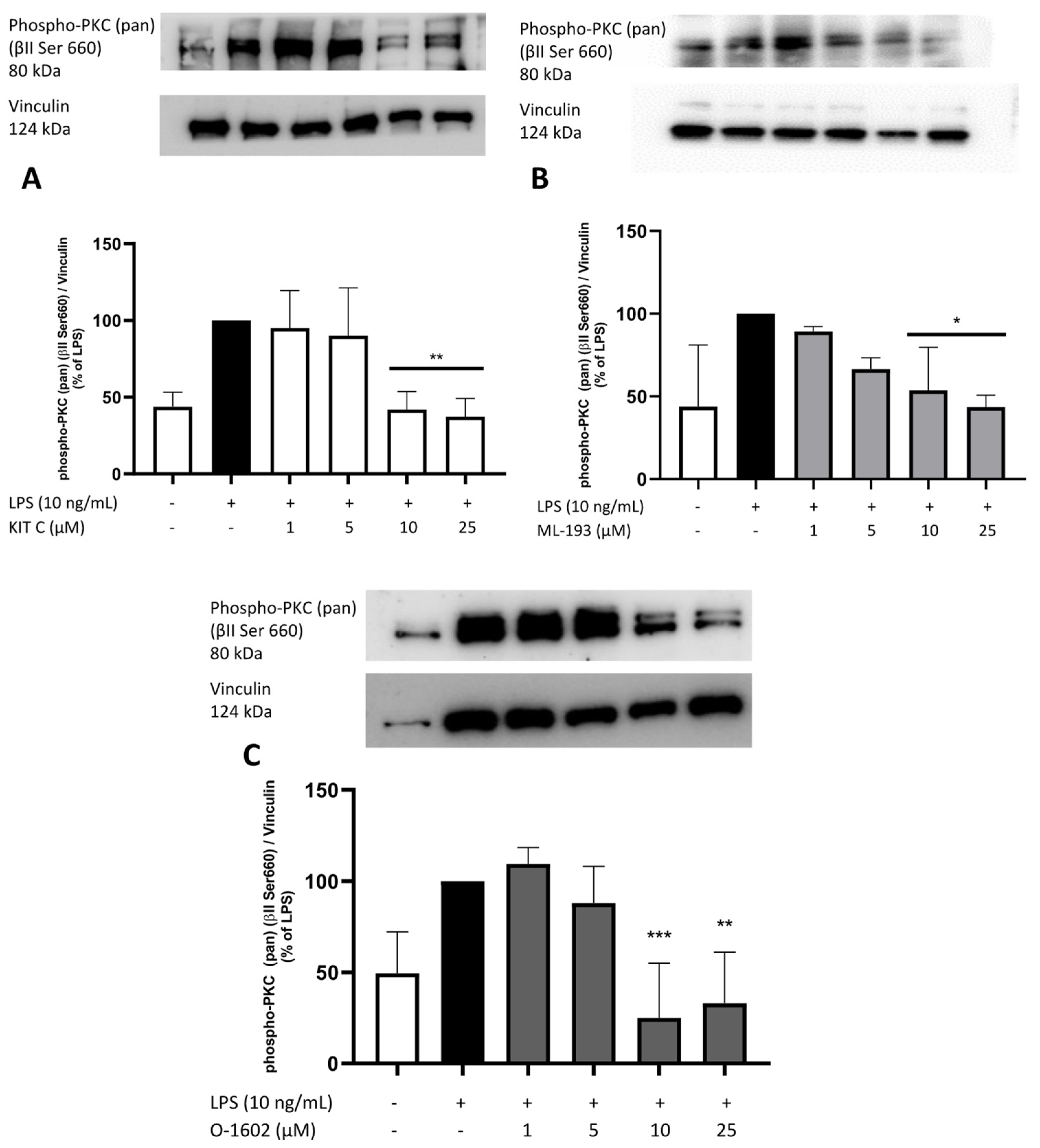
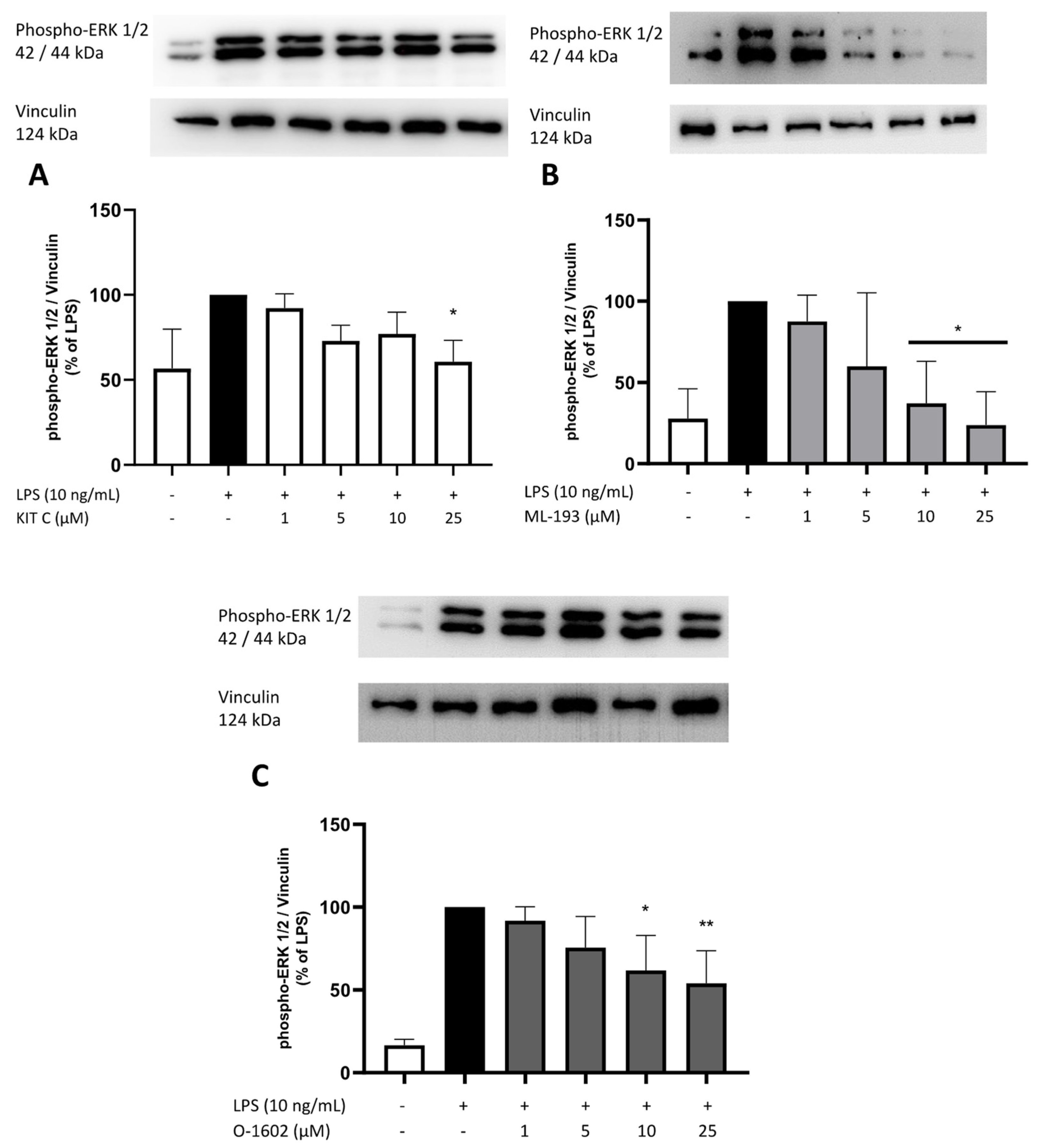
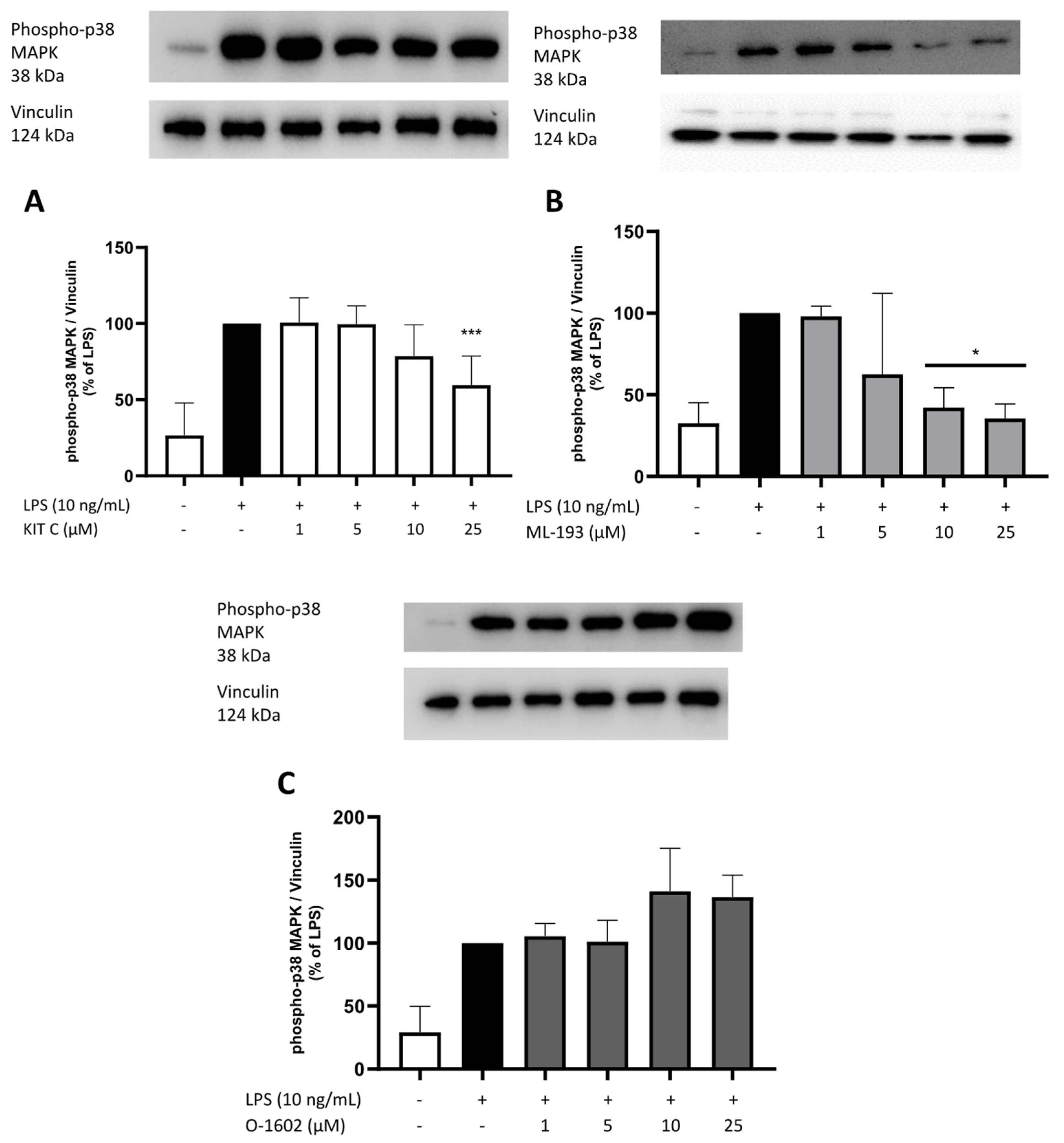
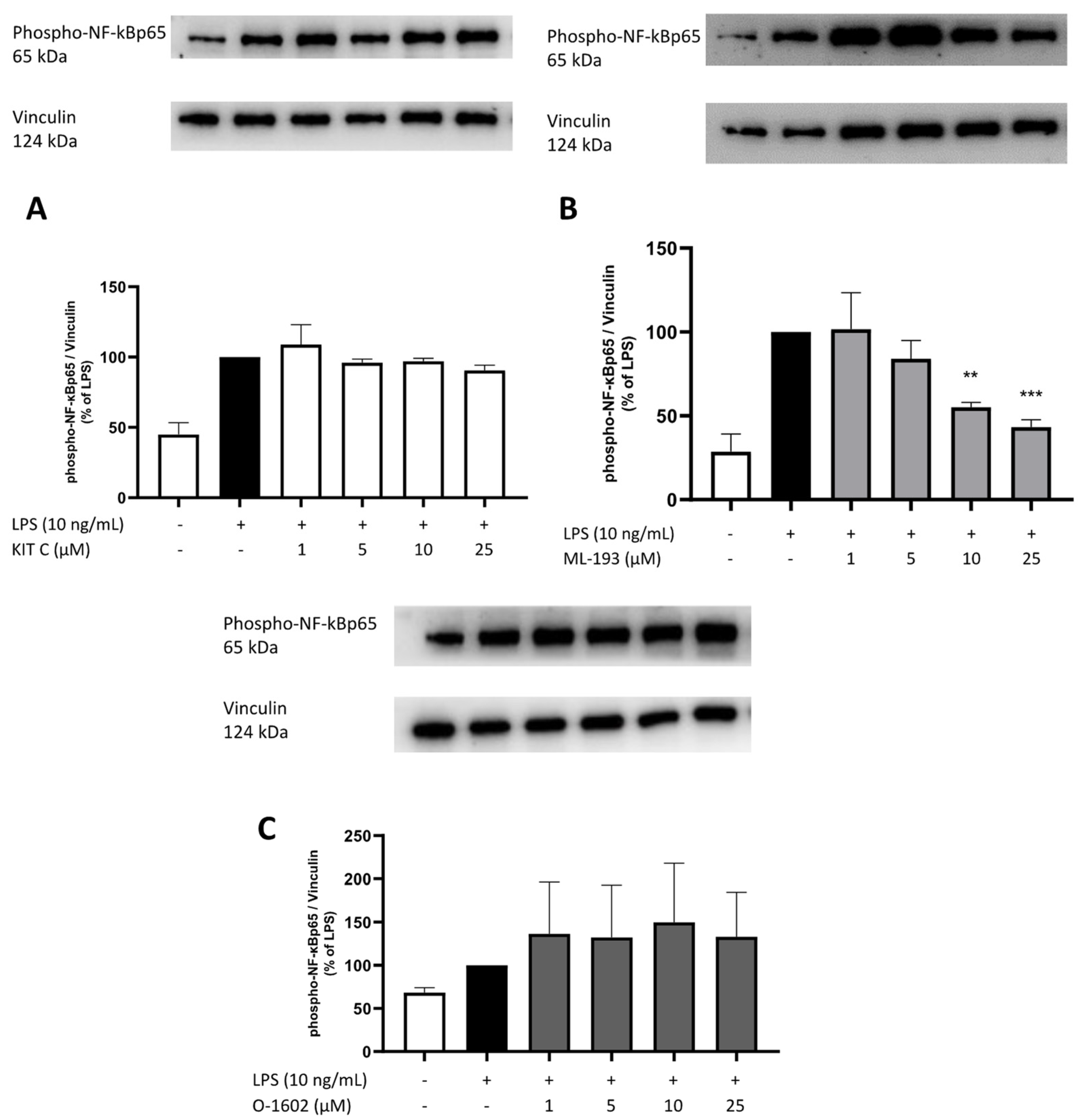
2.8. Effects of KIT C, ML-193, and O-1602 on Phosphorylation of PKC (pan) (βII Ser660), p38 MAPK, ERK 1/2, and NF-κB
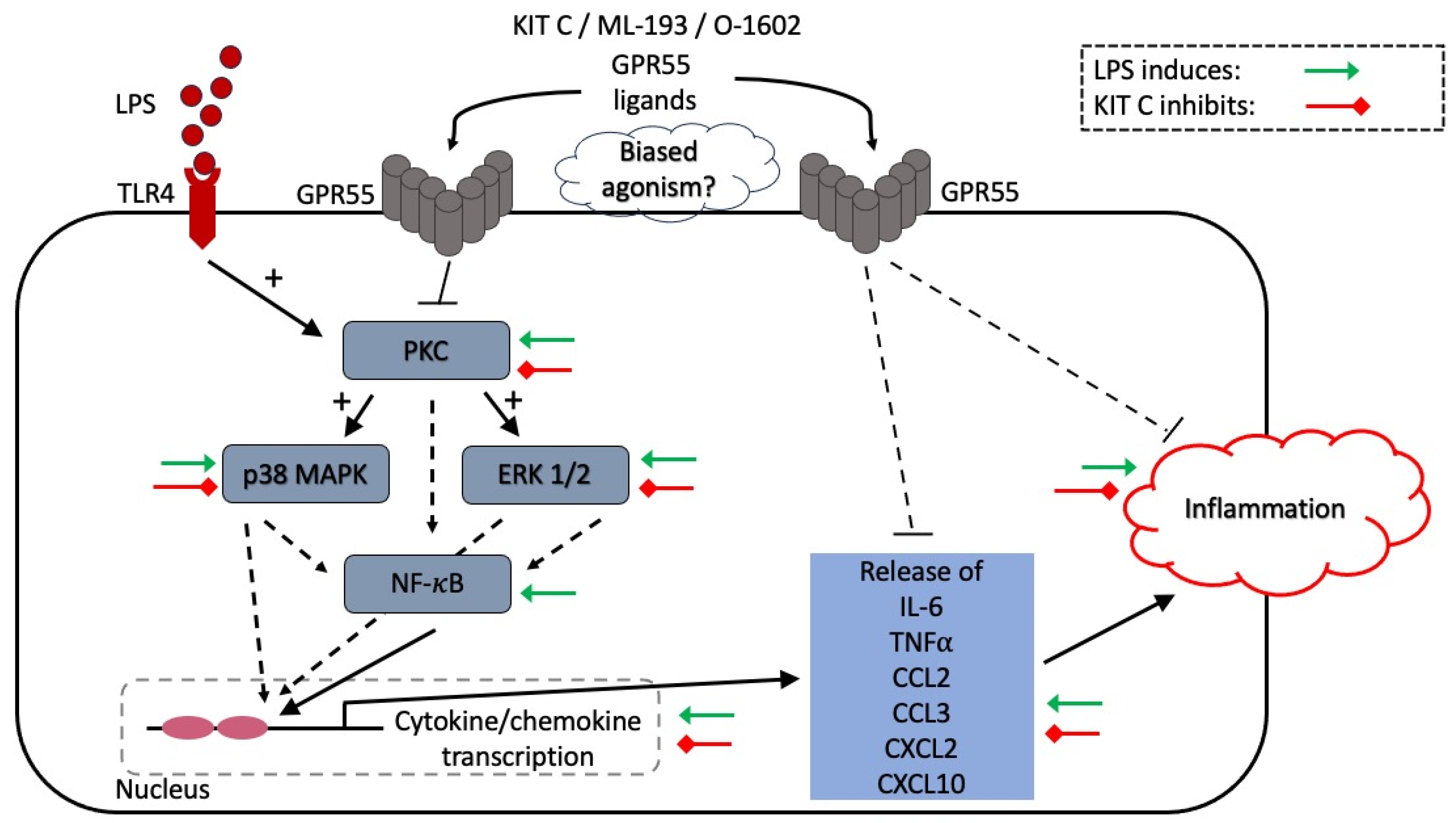
3. Discussion
4. Materials and Methods
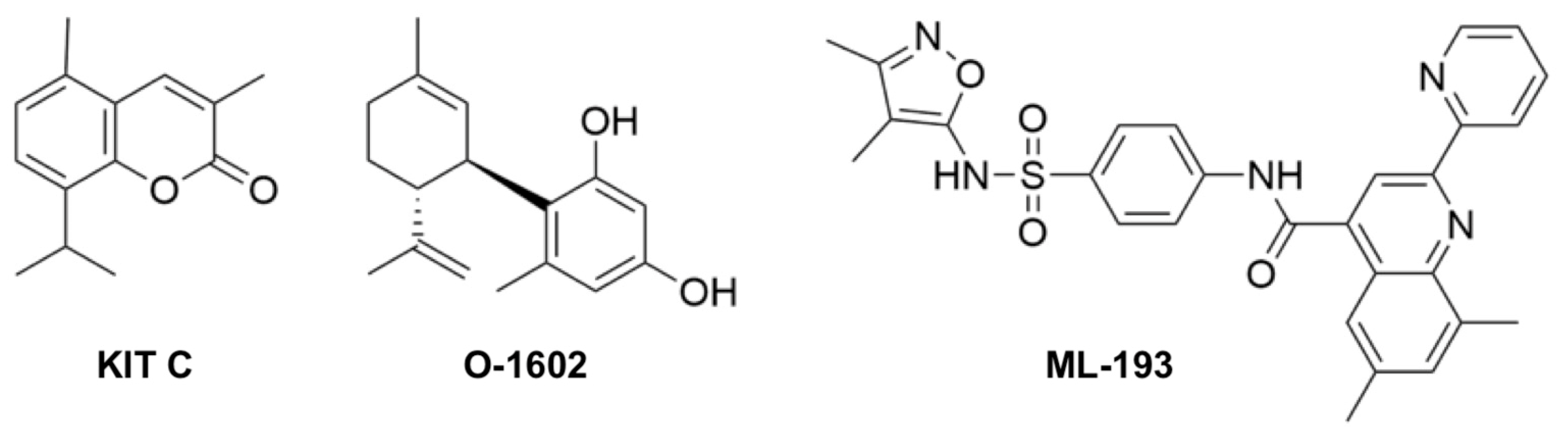
4.1. Chemicals
4.2. BV2 Microglial Cell Culture
4.3. Cell Viability Assay
4.4. Determination of Cytokine and Chemokine Release
4.5. RNA Isolation and Quantitative PCR
4.6. Immunoblotting
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voet, S.; Prinz, M.; van Loo, G. Microglia in Central Nervous System Inflammation and Multiple Sclerosis Pathology. Trends Mol. Med. 2019, 25, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.Y.; Nam, J.H.; Yoon, G.; Lee, J.Y.; Nam, Y.; Kang, H.J.; Cho, H.J.; Kim, J.; Hoe, H.S. Ibrutinib suppresses LPS-induced neuroinflammatory responses in BV2 microglial cells and wild-type mice. J. Neuroinflammation 2018, 15, 271. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Kim, I.; Akther, M.; Choi, D. Importance of GPCR-Mediated Microglial Activation in Alzheimer’ s Disease. Front. Cell. Neurosci. 2018, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Kirkley, K.S.; Popichak, K.A.; Afzali, M.F.; Legare, M.E.; Tjalkens, R.B. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J. Neuroinflammation 2017, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Craft, J.M.; Watterson, D.M.; Van Eldik, L.J. Neuroinflammation: A potential therapeutic target. Expert Opin. Ther. Targets 2005, 9, 8222. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Davis, K.L. Inflammatory mechanisms in Alzheimer’s disease: Implications for therapy. Am. J. Psychiatry 1994, 151, 1105–1113. [Google Scholar] [PubMed]
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Wang, D.; Zhang, J.; Zhang, F. NSAID exposure and risk of Alzheimer’s disease: An updated meta-analysis from cohort studies. Front. Aging Neurosci. 2018, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zong, Y.; Zhu, L.; Wang, W.; Han, Y. Chemokines in patients with Alzheimer’ s disease: A meta-analysis. Front. Aging Neurosci. 2021, 15, 1047810. [Google Scholar] [CrossRef]
- Esposito, E.; Di Matteo, V.; Benigno, A.; Pierucci, M.; Crescimanno, G.; Di Giovanni, G. Non-steroidal anti-inflammatory drugs in Parkinson’s disease. Exp. Neurol. 2007, 205, 295–312. [Google Scholar] [CrossRef]
- Yuan, N.; Chen, Y.; Xia, Y.; Dai, J.; Liu, C. Inflammation-related biomarkers in major psychiatric disorders: A cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl. Psychiatry 2019, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with c-reactive protein, IL-1, and IL-6, A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Lehto, S.M.; Niskanen, L.; Herzig, K.H.; Tolmunen, T.; Huotari, A.; Viinamäki, H.; Koivumaa-Honkanen, H.; Honkalampi, K.; Ruotsalainen, H.; Hintikka, J. Serum chemokine levels in major depressive disorder. Psychoneuroendocrinology 2010, 35, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.N.; Rizavi, H.S.; Bhaumik, R.; Zhang, H. Chemokines gene expression in the prefrontal cortex of depressed suicide victims and normal control subjects. Brain Behav. Immun. 2021, 94, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, V.M.; Stanton, E.H.; Nothdurfter, C.; Rupprecht, R.; Wetzel, C.H. The role of chemokines in the pathophysiology of major depressive disorder. Int. J. Mol. Sci. 2019, 20, 2283. [Google Scholar] [CrossRef]
- Marichal-Cancino, B.A.; Fajardo-Valdez, A.; Ruiz-Contreras, A.E.; Mendez-Díaz, M.; Prospero-García, O. Advances in the Physiology of GPR55 in the Central Nervous System. Curr. Neuropharmacol. 2016, 15, 771–778. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, J.; Lehmann, C. GPR55—A putative “type 3” cannabinoid receptor in inflammation. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Saliba, S.W.; Jauch, H.; Gargouri, B.; Keil, A.; Hurrle, T.; Volz, N.; Mohr, F.; van der Stelt, M.; Bräse, S.; Fiebich, B.L. Anti-neuroinflammatory effects of GPR55 antagonists in LPS-activated primary microglial cells. J. Neuroinflammation 2018, 15, 322. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Lanuti, M.; De Bardi, M.; Battistini, L.; Maccarrone, M. The differential characterization of GPR55 receptor in human peripheral blood reveals a distinctive expression in monocytes and NK cells and a proinflammatory role in these innate cells. Int. Immunol. 2015, 27, 153–160. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Lauckner, J.E.; Jensen, J.B.; Chen, H.Y.; Lu, H.C.; Hille, B.; Mackie, K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. USA 2008, 105, 2699–2704. [Google Scholar] [CrossRef] [PubMed]
- Reggio, P.H.; Shore, D.M. The therapeutic potential of orphan GPCRs, GPR35 and GPR55. Front. Pharmacol. 2015, 6, 139382. [Google Scholar]
- Henstridge, C.M.; Balenga, N.A.; Schröder, R.; Kargl, J.K.; Platzer, W.; Martini, L.; Arthur, S.; Penman, J.; Whistler, J.L.; Kostenis, E.; et al. GPR55 ligands promote receptor coupling to multiple signalling pathways. Br. J. Pharmacol. 2010, 160, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Andradas, C.; Caffarel, M.M.; Pérez-Gómez, E.; Salazar, M.; Lorente, M.; Velasco, G.; Guzmán, M.; Sánchez, C. The orphan G protein-coupled receptor GPR55 promotes cancer cell proliferation via ERK. Oncogene 2011, 30, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Feng, J.Y.; Li, Y.Y.; Yuece, B.; Lin, X.H.; Yu, L.Y.; Li, Y.N.; Feng, Y.J.; Storr, M. Anti-inflammatory role of cannabidiol and O-1602 in cerulein-induced acute pancreatitis in mice. Pancreas 2013, 42, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Saliba, S.W.; Gläser, F.; Deckers, A.; Keil, A.; Hurrle, T.; Apweiler, M.; Ferver, F.; Volz, N.; Endres, D.; Bräse, S.; et al. Effects of a novel gpr55 antagonist on the arachidonic acid cascade in lps-activated primary microglial cells. Int. J. Mol. Sci. 2021, 22, 2503. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Hajizadeh Moghaddam, A.; Roohbakhsh, A. Central Administration of GPR55 Receptor Agonist and Antagonist Modulates Anxiety-Related Behaviors in Rats. Fundam. Clin. Pharmacol. 2015, 29, 185–190. [Google Scholar] [CrossRef]
- Hill, J.D.; Zuluaga-Ramirez, V.; Gajghate, S.; Winfield, M.; Sriram, U.; Rom, S.; Persidsky, Y. Activation of GPR55 induces neuroprotection of hippocampal neurogenesis and immune responses of neural stem cells following chronic, systemic inflammation. Brain Behav. Immun. 2019, 76, 165–181. [Google Scholar] [CrossRef]
- Wróbel, A.; Serefko, A.; Szopa, A.; Ulrich, D.; Poleszak, E.; Rechberger, T. O-1602, an Agonist of Atypical Cannabinoid Receptors GPR55, Reverses the Symptoms of Depression and Detrusor Overactivity in Rats Subjected to Corticosterone Treatment. Front. Pharmacol. 2020, 11, 1002. [Google Scholar] [CrossRef]
- Schicho, R.; Bashashati, M.; Bawa, M.; McHugh, D.; Saur, D.; Hu, H.M.; Zimmer, A.; Lutz, B.; Mackie, K.; Bradshaw, H.B.; et al. The atypical cannabinoid O-1602 protects against experimental colitis and inhibits neutrophil recruitment. Inflamm. Bowel Dis. 2011, 17, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Apweiler, M.; Streyczek, J.; Saliba, S.W.; Collado, J.A.; Hurrle, T.; Gräßle, S.; Muñoz, E.; Normann, C.; Hellwig, S.; Bräse, S.; et al. Functional Selectivity of Coumarin Derivates Acting via GPR55 in Neuroinflammation. Int. J. Mol. Sci. 2022, 23, 959. [Google Scholar] [CrossRef]
- Fylaktakidou, K.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolaides, D. Natural and Synthetic Coumarin Derivatives with Anti-Inflammatory / Antioxidant Activities. Curr. Pharm. Des. 2005, 10, 3813–3833. [Google Scholar] [CrossRef] [PubMed]
- Rempel, V.; Volz, N.; Gläser, F.; Nieger, M.; Bräse, S.; Müller, C.E. Antagonists for the orphan G-protein-coupled receptor GPR55 based on a coumarin scaffold. J. Med. Chem. 2013, 56, 4798–4810. [Google Scholar] [CrossRef] [PubMed]
- Apweiler, M.; Saliba, S.W.; Streyczek, J.; Hurrle, T.; Gräßle, S.; Bräse, S.; Fiebich, B.L. Targeting oxidative stress: Novel coumarin-based inverse agonists of GPR55. Int. J. Mol. Sci. 2021, 22, 11665. [Google Scholar] [CrossRef] [PubMed]
- Apweiler, M.; Streyczek, J.; Saliba, S.W.; Ditrich, J.; Muñoz, E.; Fiebich, B.L. Anti-Inflammatory and Anti-Oxidative Effects of AM404 in IL-1β-Stimulated SK-N-SH Neuroblastoma Cells. Front. Pharmacol. 2021, 12, 789074. [Google Scholar] [CrossRef] [PubMed]
- Henn, A.; Lund, S.; Hedtjärn, M.; Schrattenholz, A.; Pörzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. Altern. Anim. Exp. 2009, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Horvath, R.J.; Nutile-McMenemy, N.; Alkaitis, M.S.; DeLeo, J.A. Differential migration, LPS-induced cytokine, chemokine, and NO expression in immortalized BV-2 and HAPI cell lines and primary microglial cultures. J. Neurochem. 2008, 107, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Curzytek, K.; Leśkiewicz, M. Targeting the CCL2—CCR2 axis in depressive disorders. Pharmacol. Rep. 2021, 73, 1052–1062. [Google Scholar] [CrossRef]
- Menten, P.; Wuyts, A.; Van Damme, J. Macrophage inflammatory protein-1. Int. J. Biochem. Cell Biol. 2002, 13, 455–481. [Google Scholar] [CrossRef]
- Amanzada, A.; Moriconi, F.; Mansuroglu, T.; Cameron, S.; Ramadori, G.; Malik, I.A. Induction of chemokines and cytokines before neutrophils and macrophage recruitment in different regions of rat liver after TAA administration. Lab. Investig. 2014, 94, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Koper, O.; Kamińska, J.; Sawicki, K.; Kemona, H. CXCL9, CXCL10, CXCL11, and Their Receptor (CXCR3) in Neuroinflammation and Neurodegeneration. Adv. Clin. Exp. Med. 2018, 27, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C. Regulation of the ABC kinases by phosphorylation: Protein kinase C as a paradigm. Biochem. J. 2003, 370, 361–371. [Google Scholar] [CrossRef]
- Neuman, I.; Cooke, M.; Lemiña, N.A.; Kazanietz, M.G.; Cornejo Maciel, F. 5-oxo-ETE activates migration of H295R adrenocortical cells via MAPK and PKC pathways. Prostaglandins Other Lipid Mediat. 2019, 144, 106346. [Google Scholar] [CrossRef]
- Jubaidi, F.F.; Zainalabidin, S.; Taib, I.S.; Abdul Hamid, Z.; Mohamad Anuar, N.N.; Jalil, J.; Nor, N.A.M.; Budin, S.B. The Role of PKC-MAPK Signalling Pathways in the Development of Hyperglycemia-Induced Cardiovascular Complications. Int. J. Mol. Sci. 2022, 23, 8582. [Google Scholar] [CrossRef]
- Lu, W.; Tang, S.; Li, A.; Huang, Q.; Dou, M.; Zhang, Y.; Hu, X.; Chang, R.C.C.; Wong, G.T.C.; Huang, C. The role of PKC/PKR in aging, Alzheimer’s disease, and perioperative neurocognitive disorders. Front. Aging Neurosci. 2022, 14, 973068. [Google Scholar] [CrossRef] [PubMed]
- Machado-Vieira, R.; Salvadore, G.; DiazGranados, N.; Ibrahim, L.; Latov, D.; Wheeler-Castillo, C.; Baumann, J.; Henter, I.D.; Zarate, C.A. New therapeutic targets for mood disorders. Sci. World J. 2010, 10, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Olajide, O.A.; Bhatia, H.S.; Oliveira ACPDe Wright, C.W.; Fiebich, B.L. Inhibition of Neuroinflammation in LPS-Activated Microglia by Cryptolepine. Evid.-Based Complement. Altern. Med. 2013, 2013, 459723. [Google Scholar] [CrossRef] [PubMed]
- Morroni, F.; Sita, G.; Tarozzi, A.; Rimondini, R.; Hrelia, P. Early effects of Aβ1-42 oligomers injection in mice: Involvement of PI3K/Akt/GSK3 and MAPK/ERK1/2 pathways. Behav. Brain Res. 2016, 314, 106–115. [Google Scholar] [CrossRef]
- Sun, E.; Motolani, A.; Campos, L.; Lu, T. The Pivotal Role of NF-kB in the Pathogenesis and Therapeutics of Alzheimer’ s Disease. Int. J. Mol. Sci. 2022, 23, 8972. [Google Scholar] [CrossRef]
- Buckwalter, M.S.; Wyss-coray, T. Modelling neuroinflammatory phenotypes in vivo. J. Neuroinflammation 2004, 12, 10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lehnardt, S. Innate immunity and neuroinflammation in the CNS: The role of microglia in toll-like receptor-mediated neuronal injury. Glia 2010, 58, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Hirsch, E.C. Neuroinflammatory processes in Parkinson’ s disease. Park. Relat. Disord. 2005, 11, 9–15. [Google Scholar]
- Monfared, R.V.; Alhassen, W.; Truong, T.M.; Gonzales, M.A.M.; Vachirakorntong, V.; Chen, S.; Baldi, P.; Civelli, O.; Alachkar, A. Transcriptome Profiling of Dysregulated GPCRs Reveals and Age-Disease Interactions. Cells 2021, 10, 2967. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef] [PubMed]
- Pålsson-McDermott, E.M.; O’Neill, L.A.J. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; Doykan, C.E.; et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 2014, 17, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Hornik, T.C.; Neniskyte, U.; Brown, G.C. Inflammation induces multinucleation of Microglia via PKC inhibition of cytokinesis, generating highly phagocytic multinucleated giant cells. J. Neurochem. 2014, 128, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Moscat, J.; Diaz-meco, M.T.; Rennert, P. NF-κ B activation by protein kinase C isoforms and B-cell function. EMBO Rep. 2003, 4, 31–36. [Google Scholar] [CrossRef]
- Trushin, S.A.; Pennington, K.N.; Carmona, E.M.; Asin, S.; Savoy, D.N.; Billadeau, D.D.; Paya, C.V. Protein Kinase Cα (PKCα) Acts Upstream of PKCθ To Activate IκB Kinase and NF-κB in T Lymphocytes. Mol. Cell. Biol. 2003, 23, 7068–7081. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Ee, S.L.; Im, K.L. Glycoprotein Isolated from Rhus verniciflua S TOKES Inhibits Inflammation-Related Protein and Nitric Oxide Production in LPS-Stimulated RAW 264.7 Cells. Biol. Pharm. Bull. 2007, 30, 111–116. [Google Scholar]
- Muresan, Z.; Muresan, V. The Amyloid-β Precursor Protein Is Phosphorylated via Distinct Pathways during Differentiation, Mitosis, Stress, and Degeneration. Mol. Biol. Cell 2007, 18, 3835–3844. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Fourquet, S.; Guerois, R.; Biard, D.; Toledano, M.B. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J. Biol. Chem. 2010, 285, 8463–8471. [Google Scholar] [CrossRef]
- Baldwin, A.S. The NF-κB and IκB proteins: New discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–681. [Google Scholar] [CrossRef]
- Santulli, G.; Nakashima, R.; Yuan, Q.; Marks, A.R. Intracellular calcium release channels: An update. J. Physiol. 2017, 595, 3041–3051. [Google Scholar] [CrossRef]
- de Oliveira, A.C.P.; Yousif, N.M.; Bhatia, H.S.; Hermanek, J.; Huell, M.; Fiebich, B.L. Poly(I:C) Increases the expression of mPGES-1 and COX-2 in rat primary microglia. J. Neuroinflammation 2016, 13, 11. [Google Scholar] [CrossRef]
- Wooten, M.W. Mini-Review Function for NF-kB in Neuronal Survival: Regulation by Atypical Protein Kinase C. J. Neurosci. Res. 1999, 58, 607–611. [Google Scholar] [CrossRef]
- Qu, Y.; Li, J.; Qin, Q.; Wang, D.; Zhao, J.; An, K.; Mao, Z.; Min, Z.; Xiong, Y.; Li, J.; et al. A systematic review and meta-analysis of inflammatory biomarkers in Parkinson’s disease. NPJ Park. Dis. 2023, 9, 18. [Google Scholar] [CrossRef]
- Domingues, C.; da Cruz e Silva, O.A.B.; Henriques, A.G. Impact of Cytokines and Chemokines on Alzheimer’s Disease Neuropathological Hallmarks. Curr. Alzheimer Res. 2017, 14, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Ting, E.Y.C.; Yang, A.C.; Tsai, S.J. Role of interleukin-6 in depressive disorder. Int. J. Mol. Sci. 2020, 21, 2194. [Google Scholar] [CrossRef] [PubMed]
- Roohi, E.; Jaafari, N.; Hashemian, F. On inflammatory hypothesis of depression: What is the role of IL-6 in the middle of the chaos? J. Neuroinflammation 2021, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Medina-Vera, D.; Rosell-Valle, C.; López-Gambero, A.J.; Navarro, J.A.; Zambrana-Infantes, E.N.; Rivera, P.; Santín, L.J.; Suarez, J.; Rodríguez de Fonseca, F. Imbalance of endocannabinoid/lysophosphatidylinositol receptors marks the severity of alzheimer’s disease in a preclinical model: A therapeutic opportunity. Biology 2020, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Wang, X.; Wu, Y.; Hu, J.; Li, Y.; Jin, S.; Wu, X. Activation of GPR55 attenuates cognitive impairment, oxidative stress, neuroinflammation, and synaptic dysfunction in a streptozotocin-induced Alzheimer’s mouse model. Pharmacol. Biochem. Behav. 2022, 214, 173340. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiang, X.T.; Hu, J.; Wu, Y.M.; Li, Y.Y.; Jin, S.Y.; Wu, X. Pharmacological Activation of GPR55 Improved Cognitive Impairment Induced by Lipopolysaccharide in Mice. J. Mol. Neurosci. 2022, 72, 1656–1669. [Google Scholar] [CrossRef]
- Joly-Amado, A.; Hunter, J.; Quadri, Z.; Zamudio, F.; Rocha-Rangel, P.V.; Chan, D.; Kesarwani, A.; Nash, K.; Lee, D.C.; Morgan, D.; et al. CCL2 Overexpression in the Brain Promotes Glial Activation and Accelerates Tau Pathology in a Mouse Model of Tauopathy. Front. Immunol. 2020, 11, 997. [Google Scholar] [CrossRef]
- Cudaback, E.; Yang, Y.; Montine, T.J.; Keene, C.D. APOE genotype-dependent modulation of astrocyte chemokine CCL3 production. Glia 2015, 63, 51–65. [Google Scholar] [CrossRef]
- Mahad, D.J.; Howell, S.J.L.; Woodroofe, M.N. Expression of chemokines in the CSF and correlation with clinical disease activity in patients with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2002, 72, 498–502. [Google Scholar]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Festari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Beers, D.R.; Hooten, K.G.; Sieglaff, D.H.; Zhang, A.; Kalyana-Sundaram, S.; Traini, C.M.; Halsey, W.S.; Hughes, A.M.; Sathe, G.M.; et al. Characterization of gene expression phenotype in amyotrophic lateral sclerosis monocytes. JAMA Neurol. 2017, 74, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, I.; Abdollahi, A.; Shamsizadeh, A.; Allahtavakoli, M.; Roohbakhsh, A. The effect of intra-striatal administration of GPR55 agonist (LPI) and antagonist (ML193) on sensorimotor and motor functions in a Parkinson’s disease rat model. Acta Neuropsychiatr. 2021, 33, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, H.; Sun, H.; Zhu, J.; Jew, C.P.; Wager-Miller, J.; Straiker, A.; Spencer, C.; Bradshaw, H.; Mackie, K.; et al. GPR55, a G-Protein Coupled Receptor for Lysophosphatidylinositol, Plays a Role in Motor Coordination. PLoS ONE 2013, 8, e60314. [Google Scholar] [CrossRef]
- Celorrio, M.; Rojo-Bustamante, E.; Fernández-Suárez, D.; Sáez, E.; Estella-Hermoso de Mendoza, A.; Müller, C.E.; Ramírez, M.J.; Oyarzábal, J.; Franco, R.; Aymerich, M.S. GPR55, A therapeutic target for Parkinson’s disease? Neuropharmacology 2017, 125, 319–332. [Google Scholar] [CrossRef]
- Shen, S.; Li, B. The Protective Effects of GPR55 against Hippocampal Neuroinflammation and Neurogenic Damage in CSDS Mice. Res Sq [Internet]: 1–30. Available online: https://www.researchsquare.com/article/rs-829457/latest?utm_source=researcher_app&utm_medium=referral&utm_campaign=RESR_MRKT_Researcher_inbound (accessed on 18 April 2024).
- Hurrle, T.; Jung, N.; Bräse, S. Chemotion Repository Homepage. Available online: https://www.chemotion-repository.net/home/ (accessed on 18 April 2024).
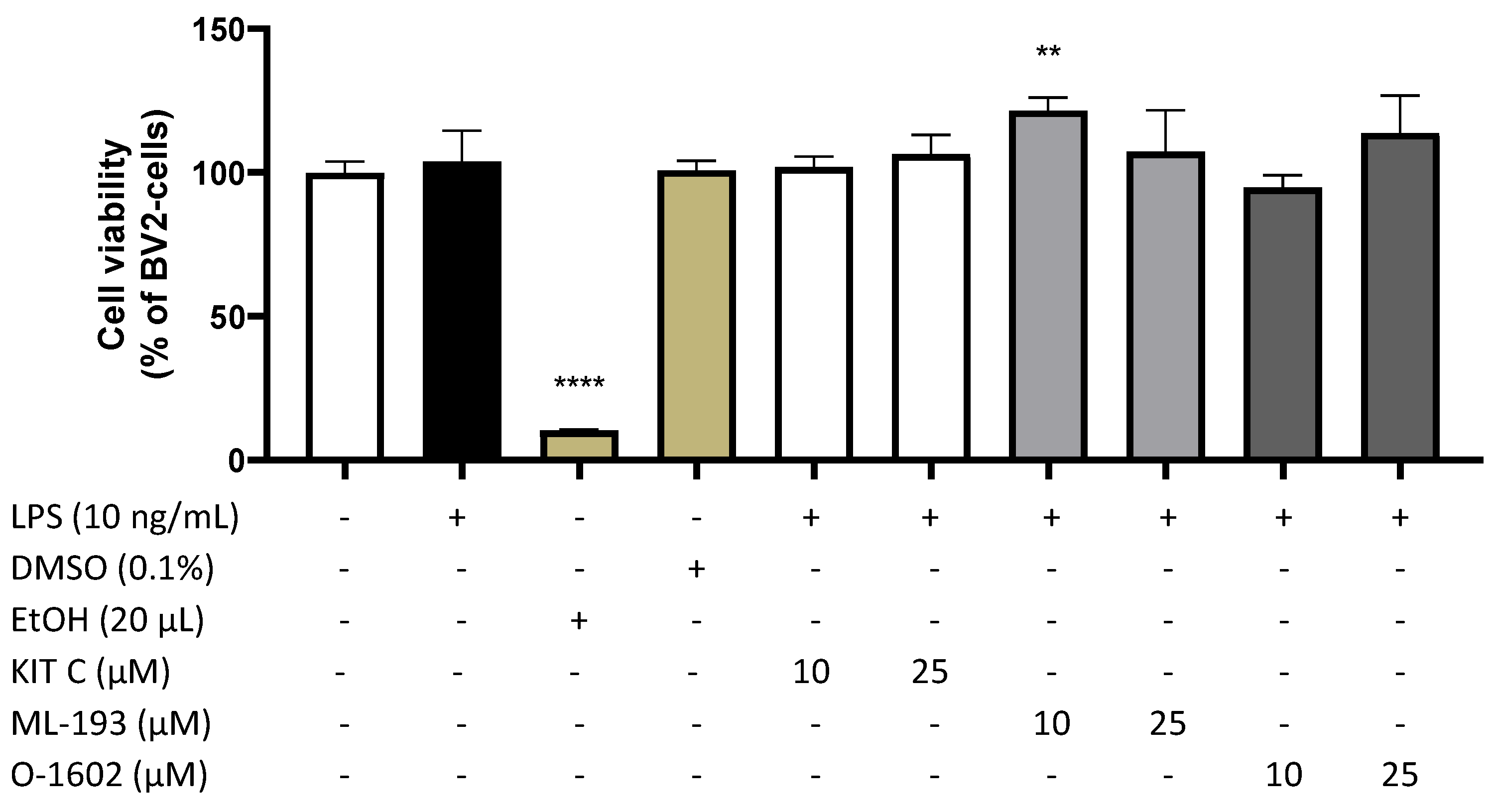


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Apweiler, M.; Normann, C.; Grathwol, C.W.; Hurrle, T.; Gräßle, S.; Jung, N.; Bräse, S.; Fiebich, B.L. Anti-Inflammatory Effects of GPR55 Agonists and Antagonists in LPS-Treated BV2 Microglial Cells. Pharmaceuticals 2024, 17, 674. https://doi.org/10.3390/ph17060674
Sun L, Apweiler M, Normann C, Grathwol CW, Hurrle T, Gräßle S, Jung N, Bräse S, Fiebich BL. Anti-Inflammatory Effects of GPR55 Agonists and Antagonists in LPS-Treated BV2 Microglial Cells. Pharmaceuticals. 2024; 17(6):674. https://doi.org/10.3390/ph17060674
Chicago/Turabian StyleSun, Lu, Matthias Apweiler, Claus Normann, Christoph W. Grathwol, Thomas Hurrle, Simone Gräßle, Nicole Jung, Stefan Bräse, and Bernd L. Fiebich. 2024. "Anti-Inflammatory Effects of GPR55 Agonists and Antagonists in LPS-Treated BV2 Microglial Cells" Pharmaceuticals 17, no. 6: 674. https://doi.org/10.3390/ph17060674
APA StyleSun, L., Apweiler, M., Normann, C., Grathwol, C. W., Hurrle, T., Gräßle, S., Jung, N., Bräse, S., & Fiebich, B. L. (2024). Anti-Inflammatory Effects of GPR55 Agonists and Antagonists in LPS-Treated BV2 Microglial Cells. Pharmaceuticals, 17(6), 674. https://doi.org/10.3390/ph17060674





