Exploring the Comprehensive Neuroprotective and Anticancer Potential of Afzelin
Abstract
1. Introduction
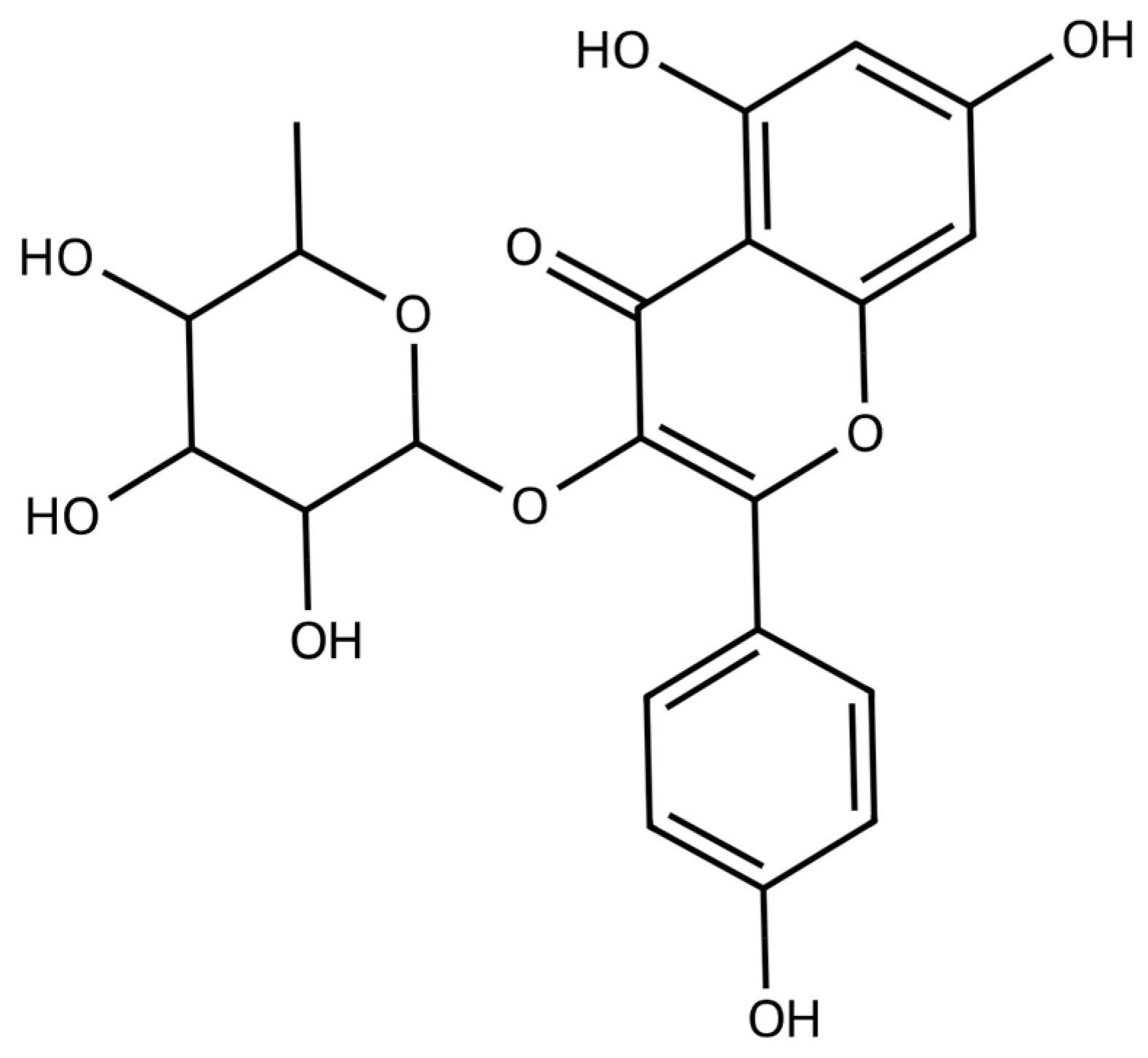
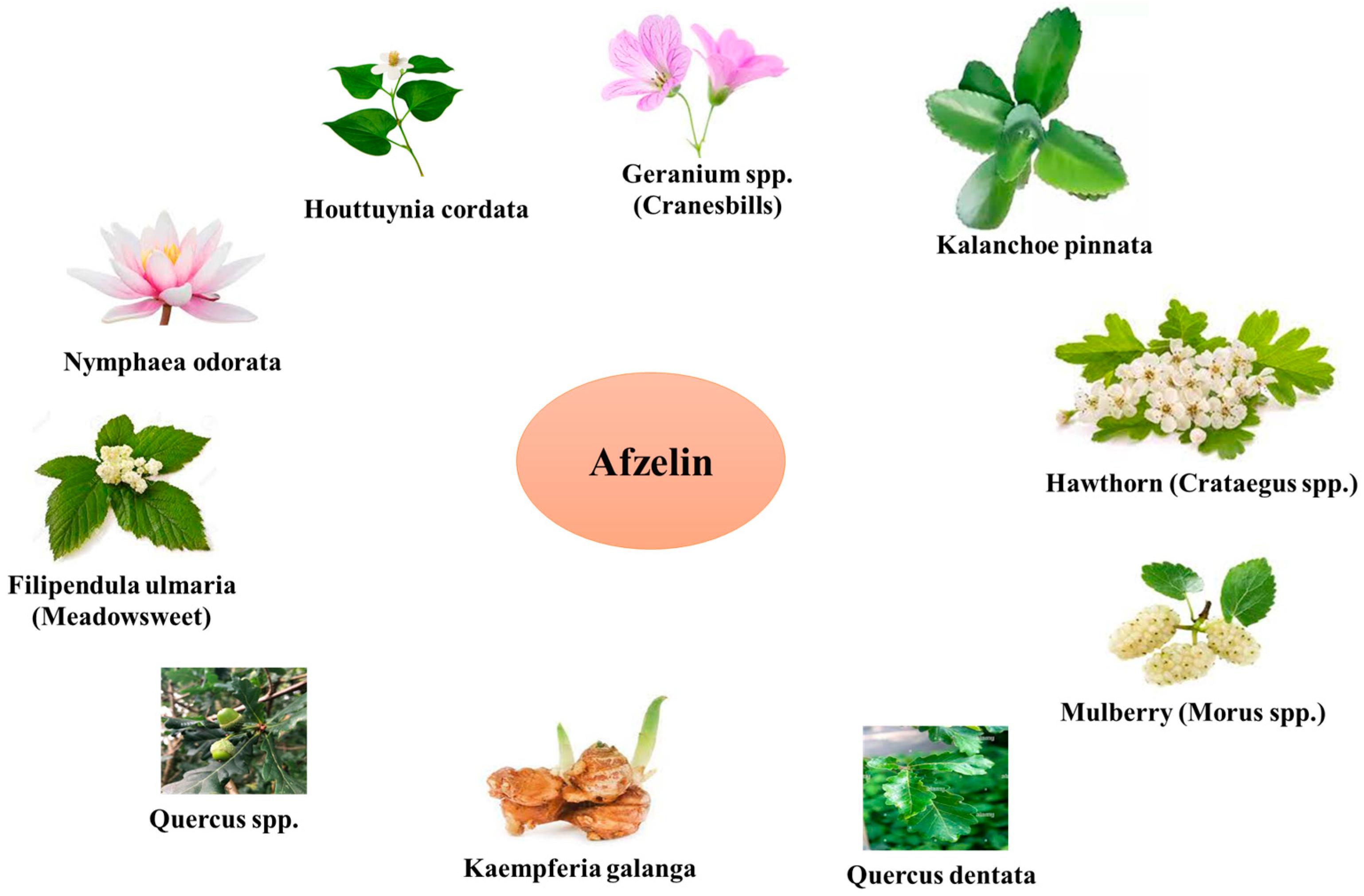
2. Sources and Pharmacological Profile of Afzelin
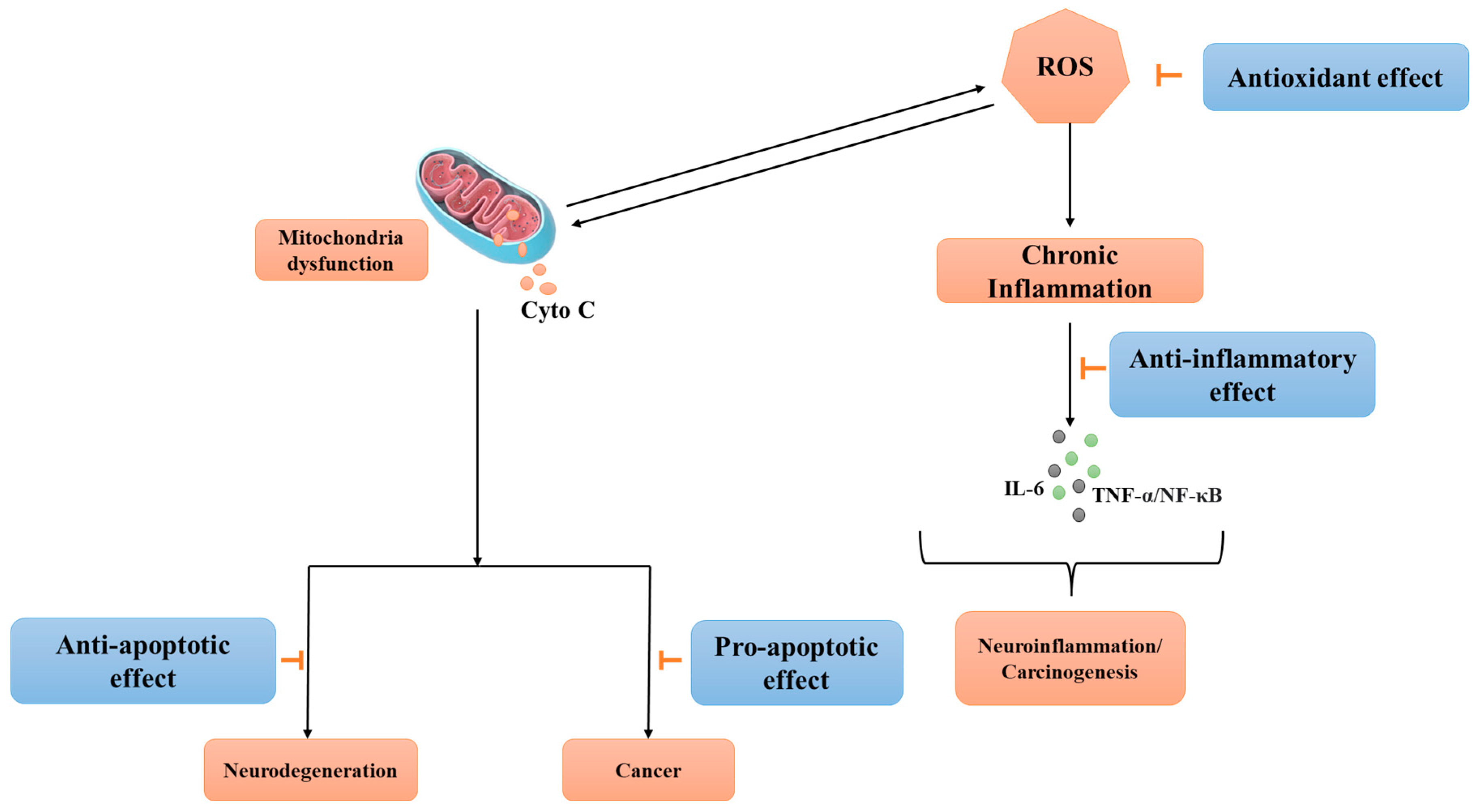
3. Biological Activity of Afzelin
3.1. Antioxidative Effects of Afzelin
- Scavenging of free radicals: Highly reactive chemicals, such as free radicals and ROS, can oxidatively damage biological components like DNA, proteins, and lipids [52]. Afzelin has been found to possess ROS-scavenging characteristics, suggesting it can neutralize the effects of dangerous compounds through interaction with them [53,54].
- Metal chelation: Afzelin also possesses metal chelation capabilities, making it possible for it to bind to metal ions such as those of iron and copper. These metal ions are well-known to have a role in the generation of free radicals via the Fenton and Haber-Weiss reactions. Afzelin is effective in blocking the generation of ROS because of its binding affinity for these metals [55,56,57].
- Activation of nuclear factor erythroid 2-related factor 2 (NRF2) pathway: The NRF2 pathway plays a significant role in the regulation of the antioxidant response in cells via transcriptional control of many target genes involved in the maintenance of oxidation-reduction homeostasis inside the cell. The antioxidative actions of kaempferol and its derivatives, including afzelin, extend to the protection of cellular components like cell membranes and mitochondria from the destructive effects of oxidation. Thereby, afzelin may contribute to the maintenance of proper cellular function by preserving the structural integrity of these components [58].
Anti-Inflammatory Effects of Afzelin
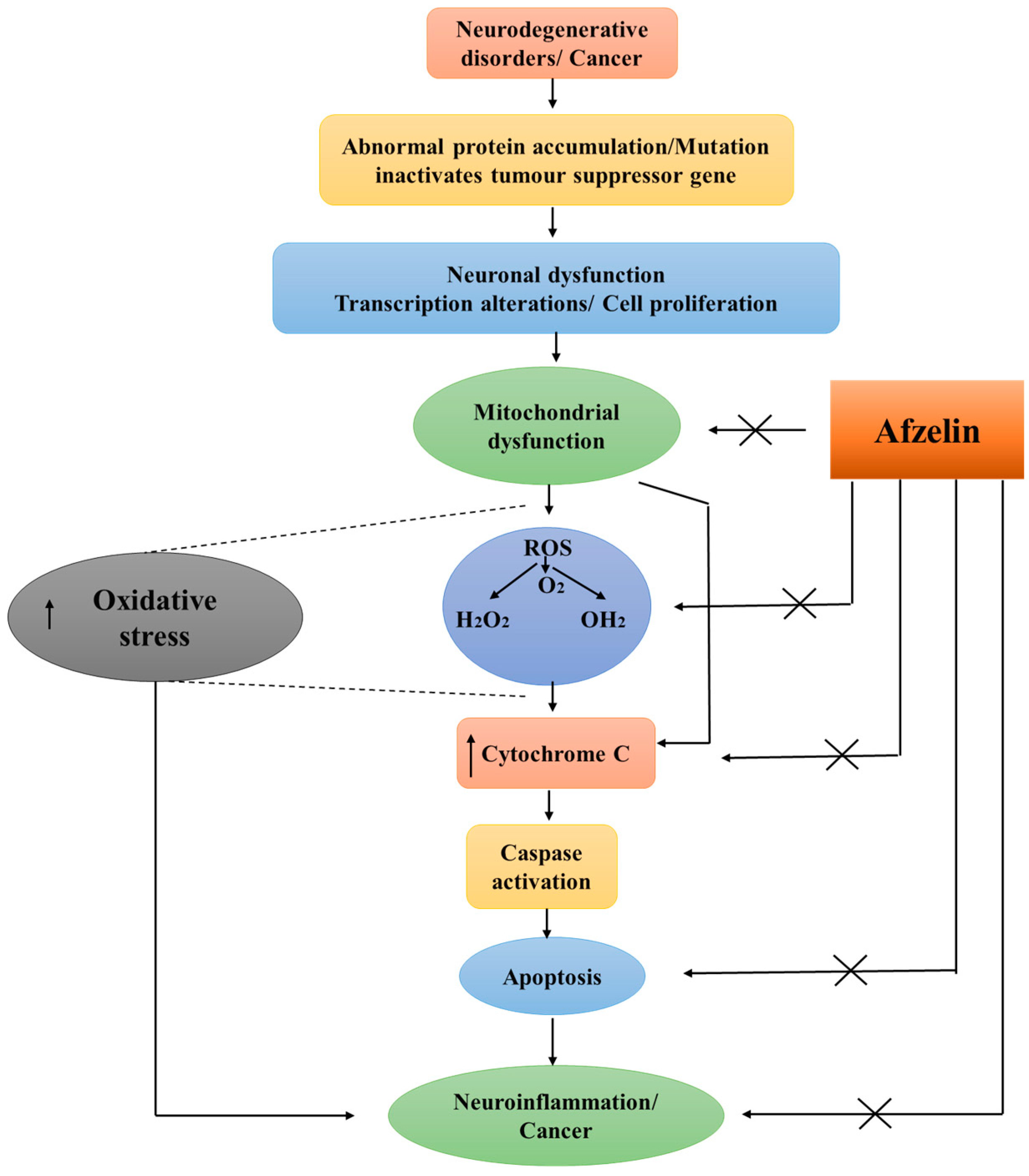
3.2. Neuroprotective and Neurogenic Effects
3.3. Anti-Cancer Effects of Afzelin
4. ADMET Properties of Afzelin
5. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Aβ | amyloid-beta |
| AADC | aromatic L-amino acid decarboxylase |
| ACH | acetylcholine |
| AChE | acetylcholinesterase |
| ADMET | absorption, distribution, metabolism, excretion, and toxicity |
| ALS | amyotrophic lateral sclerosis |
| AR | aldose reductase |
| ARE | antioxidant response element |
| ATP | adenosine triphosphate |
| BBB | blood–brain barrier |
| BCL2 | B-cell lymphoma 2 |
| BDNF | brain-derived neurotrophic factor |
| CLE | hydroalcoholic extract of Copaifera lansdorffii Desf. |
| CNS | central nervous system |
| CO | carbon monoxide |
| CREB | cyclic AMP response element-binding protein |
| DAQ | dopamine quinone |
| DNM1L | dynamin-related protein 1 |
| DQ | DOPAquinone |
| ERK | extracellular signal-regulated kinase |
| FAK | focal adhesion kinase |
| FHF | fulminant hepatic failure |
| FIR | far-infrared |
| FTD | frontotemporal dementia |
| GalN | D-galactosamine |
| GSK-3β | glycogen synthase kinase-3β |
| GSTs | glutathione S-transferases |
| HMGB1 | high-mobility group box 1 |
| HO-1/HO-2 | heme oxygenase-1/2 |
| HPLC | high-performance liquid chromatography |
| IL-1/IL-6 | interleukin-1/6 |
| iNOS | inducible nitric oxide synthase |
| JNK | c-Jun N-terminal kinase |
| LC | locus coeruleus |
| LC-MS/MS | liquid chromatography–tandem mass spectrometry |
| LIMK1 | LIM domain kinase 1 |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MFN2 | mitofusin 2 |
| MRCKα | myotonic dystrophy kinase-related Cdc42-binding kinase α |
| MS | multiple sclerosis |
| MtDNA | mitochondrial DNA |
| NF-κB | nuclear factor kappa B |
| NM | neuromelanin |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| NRF1 | nuclear respiratory factor 1 |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| OMM | outer mitochondrial membrane |
| P75NTR | P75 neurotrophin receptor |
| PARKIN | parkin protein |
| pFAK | phosphorylated focal adhesion kinase |
| PGC-1α | PPAR-γ coactivator 1α |
| PI3K | phosphatidylinositol 3-kinase |
| PINK1 | PTEN-induced putative kinase 1 |
| PMEE | Polygonum minus ethanolic extract |
| RAC1 | Ras-related C3 botulinum toxin substrate 1 |
| ROCK1 | Rho-associated coiled-coil-containing protein kinase |
| ROS | reactive oxygen species |
| SOD1/SOD2 | superoxide dismutase 1/2 |
| SN | substantia nigra |
| TFAM | mitochondrial transcription factor A |
| TH | tyrosine hydroxylase |
| TNBC | triple-negative breast cancer |
| TNF-α | tumor necrosis factor-alpha |
| TRKB | tropomyosin kinase B |
References
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Basic Mechanisms of Neurodegeneration: A Critical Update. J. Cell. Mol. Med. 2010, 14, 457. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Gulisano, W.; Maugeri, D.; Baltrons, M.A.; Fà, M.; Amato, A.; Palmeri, A.; D’Adamio, L.; Grassi, C.; Devanand, D.P.; Honig, L.S.; et al. Role of Amyloid-β and Tau Proteins in Alzheimer’s Disease: Confuting the Amyloid Cascade. J. Alzheimer’s Dis. 2018, 64, S611–S631. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H. Alzheimer’s Disease and the β-Amyloid Peptide. J. Alzheimer’s Dis. 2010, 19, 311. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Manna, J.; Dunbar, G.L. Current Understanding of the Molecular Mechanisms in Parkinson’s Disease: Targets for Potential Treatments. Transl. Neurodegener. 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic Lateral Sclerosis. Nat. Rev. Dis. Prim. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [PubMed]
- Young, J.J.; Lavakumar, M.; Tampi, D.; Balachandran, S.; Tampi, R.R. Frontotemporal Dementia: Latest Evidence and Clinical Implications. Ther. Adv. Psychopharmacol. 2018, 8, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, M.R. Protein Misfolding and Aggregation in Proteinopathies: Causes, Mechanism and Cellular Response. Diseases 2023, 11, 30. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wightman, E.L. Herbal Extracts and Phytochemicals: Plant Secondary Metabolites and the Enhancement of Human Brain Function. Adv. Nutr. 2011, 2, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S. Natural Products and Their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases. Evid.-Based Complement. Altern. Med. 2020, 2020, 6565396. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Tumer, T.B.; Moreira, A.C.; et al. Impact of Natural Compounds on Neurodegenerative Disorders: From Preclinical to Pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Yadav, R.S. Efficacy of Natural Compounds in Neurodegenerative Disorders. Adv. Neurobiol. 2016, 12, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Gielecińska, A.; Kciuk, M.; Mujwar, S.; Celik, I.; Kołat, D.; Kałuzińska-Kołat, Ż.; Kontek, R. Substances of Natural Origin in Medicine: Plants vs. Cancer. Cells 2023, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, C.; Kong, J. Oxidative Stress in Neurodegenerative Diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef] [PubMed]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- da Cunha Germano, B.C.; de Morais, L.C.C.; Idalina Neta, F.; Fernandes, A.C.L.; Pinheiro, F.I.; do Rego, A.C.M.; Araújo Filho, I.; de Azevedo, E.P.; de Paiva Cavalcanti, J.R.L.; Guzen, F.P.; et al. Vitamin E and Its Molecular Effects in Experimental Models of Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 11191. [Google Scholar] [CrossRef] [PubMed]
- Trela-Makowej, A.; Leśkiewicz, M.; Kruk, J.; Żądło, A.; Basta-Kaim, A.; Szymańska, R. Antioxidant and Neuroprotective Activity of Vitamin E Homologues: In Vitro Study. Metabolites 2022, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, U. The Sour Side of Vitamin C Might Mediate Neuroprotective, Anticonvulsive and Antidepressant-like Effects. Med. Hypotheses 2019, 131, 109320. [Google Scholar] [CrossRef] [PubMed]
- Kangisser, L.; Tan, E.; Bellomo, R.; Deane, A.M.; Plummer, M.P. Neuroprotective Properties of Vitamin C: A Scoping Review of Pre-Clinical and Clinical Studies. J. Neurotrauma 2021, 38, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Khatri, N.; Rahman, Z.N.; Menezes, A.A.; Martini, J.; Shehjar, F.; Mujeeb, N.; Shah, Z.A. Neuroprotective Potential of Flavonoids in Brain Disorders. Brain Sci. 2023, 13, 1258. [Google Scholar] [CrossRef] [PubMed]
- Putteeraj, M.; Lim, W.L.; Teoh, S.L.; Yahaya, M.F. Flavonoids and Its Neuroprotective Effects on Brain Ischemia and Neurodegenerative Diseases. Curr. Drug Targets 2018, 19, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Parvin, M.S.; Hasan, M.M.; Rahman, M.A.A.; Islam, M.E. Anti-Tumor and Antioxidant Activity of Kaempferol-3-O-Alpha-L-Rhamnoside (Afzelin) Isolated from Pithecellobium dulce Leaves. BMC Complement. Med. Ther. 2022, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, M.; Kim, J.M.; Lee, M.-K.; Seo, S.J.; Park, K.Y. Afzelin Suppresses Proinflammatory Responses in Particulate Matter-Exposed Human Keratinocytes. Int. J. Mol. Med. 2019, 43, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B.; Kang, J.-W.; Kim, S.-J.; Ahn, J.; Kim, J.; Lee, S.-M. Afzelin Ameliorates D-Galactosamine and Lipopolysaccharide-Induced Fulminant Hepatic Failure by Modulating Mitochondrial Quality Control and Dynamics. Br. J. Pharmacol. 2017, 174, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Lotha, R.; Sundaramoorthy, N.S.; Shamprasad, B.R.; Nagarajan, S.; Sivasubramanian, A. Plant Nutraceuticals (Quercetrin and Afzelin) Capped Silver Nanoparticles Exert Potent Antibiofilm Effect against Food Borne Pathogen Salmonella enterica Serovar Typhi and Curtail Planktonic Growth in Zebrafish Infection Model. Microb. Pathog. 2018, 120, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Cherbuin, N.; Walsh, E.; Baune, B.T.; Anstey, K.J. Oxidative Stress, Inflammation and Risk of Neurodegeneration in a Population Sample. Eur. J. Neurol. 2019, 26, 1347–1354. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ahn, J.; Kim, J.W.; Lee, S.G.; Kim, H.P. Flavonoids from the Aerial Parts of Houttuynia Cordata Attenuate Lung Inflammation in Mice. Arch. Pharm. Res. 2015, 38, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, B.; Liu, X.; Li, Y.; Hou, J.; Chen, S.; Chen, J.; Li, S. Screening of α-Glucosidase Inhibitors from Houttuynia Cordata and Evaluation of the Binding Mechanisms. ChemistrySelect 2020, 5, 8440–8446. [Google Scholar] [CrossRef]
- Zhang, Z.; ElSohly, H.N.; Li, X.-C.; Khan, S.I.; Broedel, S.E.; Raulli, R.E.; Cihlar, R.L.; Burandt, C.; Walker, L.A. Phenolic Compounds from Nymphaea Odorata. J. Nat. Prod. 2003, 66, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.-C.; Sun, J.-M.; Shen, J.-G.; Jin, J.-Z.; Liu, F.; Xu, X.-L.; Chen, L.; Liu, L.-T.; Lv, J.-J. Afzelin Exhibits Anti-Cancer Activity against Androgen-Sensitive LNCaP and Androgen-Independent PC-3 Prostate Cancer Cells through the Inhibition of LIM Domain Kinase 1. Oncol. Lett. 2015, 10, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Caballero, M.E.; Sierra-Ramírez, J.A.; Villalobos-Valencia, R.; Seseña-Méndez, E. Potential of Kalanchoe Pinnata as a Cancer Treatment Adjuvant and an Epigenetic Regulator. Molecules 2022, 27, 6425. [Google Scholar] [CrossRef]
- Mejía-Méndez, J.L.; Bach, H.; Lorenzo-Leal, A.C.; Navarro-López, D.E.; López-Mena, E.R.; Hernández, L.R.; Sánchez-Arreola, E. Biological Activities and Chemical Profiles of Kalanchoe Fedtschenkoi Extracts. Plants 2023, 12, 1943. [Google Scholar] [CrossRef]
- Nascimento, L.B.d.S.; Casanova, L.M.; Costa, S.S. Bioactive Compounds from Kalanchoe Genus Potentially Useful for the Development of New Drugs. Life 2023, 13, 646. [Google Scholar] [CrossRef]
- Yadav, N.P.; Dixit, V.K. Hepatoprotective Activity of Leaves of Kalanchoe Pinnata Pers. J. Ethnopharmacol. 2003, 86, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Mady, M.S.; Ibrahim, R.R.; El-Sayed, E.K.; El-Shazly, M.; Chen, L.-Y.; Lai, K.-H.; El Shaarawy, F.S.; Moharram, F.A. UHPLC-MS Profiles and Antidiarrheal Activity of Quercus coccinea Münchh. and Quercus robur L. Employing In Vivo Technique. Front. Pharmacol. 2023, 14, 1120146. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.A.; Medeiros-Fonseca, B.; Vasconcelos-Nóbrega, C.; Alvarado, A.; Pires, M.J.; Vala, H.; Barros, A.I.R.N.A.; Faustino-Rocha, A.I. Quercus spp. Extract as a Promising Preventive or Therapeutic Strategy for Cancer: A Systematic Review. Mol. Med. Rep. 2023, 28, 175. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, H.; Lee, S.; Hong, M.; Hwang, D. Antioxidant and Anti-Inflammatory Activity of Filipendula Glaberrima Nakai Ethanolic Extract and Its Chemical Composition. Molecules 2022, 27, 4628. [Google Scholar] [CrossRef] [PubMed]
- Marčetić, M.; Samardžić, S.; Ilić, T.; Božić, D.D.; Vidović, B. Phenolic Composition, Antioxidant, Anti-Enzymatic, Antimicrobial and Prebiotic Properties of Prunus spinosa L. Fruits. Foods 2022, 11, 3289. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.S.; Bekbolatova, E.; Cotrim, M.D.; Sakipova, Z.; Ibragimova, L.; Kukula-Koch, W.; Giorno, T.B.S.; Fernandes, P.D.; Fonseca, D.A.; Boylan, F. Chemistry and Pharmacology of the Kazakh Crataegus almaatensis Pojark: An Asian Herbal Medicine. Antioxidants 2019, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Cechinel-Zanchett, C.C.; Bolda Mariano, L.N.; Boeing, T.; da Costa, J.d.C.; Da Silva, L.M.; Bastos, J.K.; Cechinel-Filho, V.; de Souza, P. Diuretic and Renal Protective Effect of Kaempferol 3-O-Alpha-l-Rhamnoside (Afzelin) in Normotensive and Hypertensive Rats. J. Nat. Prod. 2020, 83, 1980–1989. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Kim, J.H.; Kim, M.O.; Lee, S.Y.; Lee, J. Melanocyte-Protective Effect of Afzelin Is Mediated by the Nrf2-ARE Signalling Pathway via GSK-3β Inactivation. Exp. Dermatol. 2017, 26, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Shin, S.; Ryu, D.; Cho, E.; Yoo, J.; Park, D.; Jung, E. Evaluating the Sun Protection Factor of Cosmetic Formulations Containing Afzelin. Chem. Pharm. Bull. 2021, 69, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, P.; Zhou, M.; Wang, T.; Fang, S.; Shang, X.; Fu, X. Geographic Variation in the Chemical Composition and Antioxidant Properties of Phenolic Compounds from Cyclocarya paliurus (Batal) Iljinskaja Leaves. Molecules 2018, 23, 2440. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, K.; Wei, D.; Xiao, H.; Niu, H.; Huang, W. High Anti-Oxidative and Lipid-Lowering Activities of Flavonoid Glycosides-Rich Extract from the Leaves of Zanthoxylum bungeanum in Multi-System. J. Food Nutr. Res. 2015, 3, 62–68. [Google Scholar] [CrossRef]
- Aldana, J.A.; De Grandis, R.A.; Nicolella, H.; Guissoni, A.P.P.; Squarisi, I.; Arruda, C.; Ribeiro, V.P.; Tavares, D.C.; Barcelos, G.R.M.; Antunes, L.M.G.; et al. Evaluation of Cytoprotective Effects of Compounds Isolated from Copaifera langsdorffii Desf. against Induced Cytotoxicity by Exposure to Methylmercury and Lead. Nat. Prod. Res. 2020, 34, 2528–2532. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.T.; Mira, M.L.; Florêncio, M.H.; Jennings, K.R. Iron and Copper Chelation by Flavonoids: An Electrospray Mass Spectrometry Study. J. Inorg. Biochem. 2002, 92, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.J.; Parmigiani-Izquierdo, J.M.; Toledano Gasca, A.; Cabaña-Muñoz, M.E. The Long-Term Algae Extract (Chlorella and Fucus sp.) and Aminosulphurate Supplementation Modulate SOD-1 Activity and Decrease Heavy Metals (Hg++, Sn) Levels in Patients with Long-Term Dental Titanium Implants and Amalgam Fillings Restorations. Antioxidants 2019, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Punia, S.; Mukherjee, T.K. Kaempferol—A Dietary Anticancer Molecule with Multiple Mechanisms of Action: Recent Trends and Advancements. J. Funct. Foods 2017, 30, 203. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, T.; Deore, S.L.; Kide, A.A.; Shende, B.A.; Sharma, R.; Dadarao Chakole, R.; Nemade, L.S.; Kishor Kale, N.; Borah, S.; Shrikant Deokar, S.; et al. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease, and Parkinson’s Disease, Huntington’s Disease and Amyotrophic Lateral Sclerosis—An Updated Review. Mitochondrion 2023, 71, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Induced Pluripotent Stem Cell Models of Parkinson’s Disease. Eur. J. Neurosci. 2019, 49, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Hauser, D.N.; Hastings, T.G. Mitochondrial Dysfunction and Oxidative Stress in Parkinson’s Disease and Monogenic Parkinsonism. Neurobiol. Dis. 2013, 51, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Księżakowska-Łakoma, K.; Żyła, M.; Wilczyński, J.R. Mitochondrial Dysfunction in Cancer. Prz. Menopauzalny 2014, 13, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, J.; Lu, W. The Significance of Mitochondrial Dysfunction in Cancer. Int. J. Mol. Sci. 2020, 21, 5598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, C.; Jiang, Y.; Wang, S.; Wu, X.; Wang, K. PPARγ Coactivator-1α (PGC-1α) Protects Neuroblastoma Cells against Amyloid-Beta (Aβ) Induced Cell Death and Neuroinflammation via NF-κB Pathway. BMC Neurosci. 2017, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Mota, B.C.; Sastre, M. The Role of PGC1α in Alzheimer’s Disease and Therapeutic Interventions. Int. J. Mol. Sci. 2021, 22, 5769. [Google Scholar] [CrossRef] [PubMed]
- Panes, J.D.; Wendt, A.; Ramirez-Molina, O.; Castro, P.A.; Fuentealba, J. Deciphering the Role of PGC-1α in Neurological Disorders: From Mitochondrial Dysfunction to Synaptic Failure. Neural Regen. Res. 2021, 17, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Kaminski, L. The Metabolic Modulator PGC-1α in Cancer. Am. J. Cancer Res. 2019, 9, 198–211. [Google Scholar] [PubMed]
- Kang, I.; Chu, C.T.; Kaufman, B.A. The Mitochondrial Transcription Factor TFAM in Neurodegeneration: Emerging Evidence and Mechanisms. FEBS Lett. 2018, 592, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.M.J.; Moreira, P.I.; Ambrósio, A.F.; Alves, C.H. PINK1/PARKIN Signalling in Neurodegeneration and Neuroinflammation. Acta Neuropathol. Commun. 2020, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Luan, S.; Fan, X.; Wang, J.; Huang, J.; Gao, X.; Han, D. The Emerging Multifaceted Role of PINK1 in Cancer Biology. Cancer Sci. 2022, 113, 4037–4047. [Google Scholar] [CrossRef] [PubMed]
- Denisenko, T.V.; Gogvadze, V.; Zhivotovsky, B. Mitophagy in Carcinogenesis and Cancer Treatment. Discov. Oncol. 2021, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, J.-Y.; Qi, Y.; Park, S.; Lee, H.L.; Yamabe, N.; Kim, H.; Jang, D.S.; Kang, K.S. Phytochemicals from the Flowers of Prunus persica (L.) Batsch: Anti-Adipogenic Effect of Mandelamide on 3T3-L1 Preadipocytes. Bioorg. Med. Chem. Lett. 2021, 49, 128326. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Prajapati, R.; Seong, S.H.; Jung, H.A.; Choi, J.S. Antioxidant and Antineuroinflammatory Mechanisms of Kaempferol-3-O-β-d-Glucuronate on Lipopolysaccharide-Stimulated BV2 Microglial Cells through the Nrf2/HO-1 Signaling Cascade and MAPK/NF-κB Pathway. ACS Omega 2023, 8, 6538–6549. [Google Scholar] [CrossRef] [PubMed]
- Calkins, M.J.; Johnson, D.A.; Townsend, J.A.; Vargas, M.R.; Dowell, J.A.; Williamson, T.P.; Kraft, A.D.; Lee, J.-M.; Li, J.; Johnson, J.A. The Nrf2/ARE Pathway as a Potential Therapeutic Target in Neurodegenerative Disease. Antioxid. Redox Signal. 2009, 11, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.-C. The Nrf2-ARE Pathway: An Indicator and Modulator of Oxidative Stress in Neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, L.; Chen, J.; Li, Q.; Huo, L.; Wang, Y.; Wang, H.; Du, J. Pharmacological Modulation of Nrf2/HO-1 Signaling Pathway as a Therapeutic Target of Parkinson’s Disease. Front. Pharmacol. 2021, 12, 757161. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Narang, R.K.; Singh, S. Role of Nrf2 in Oxidative Stress, Neuroinflammation and Autophagy in Alzheimer’s Disease: Regulation of Nrf2 by Different Signaling Pathways. Curr. Mol. Med. 2023, in press. [Google Scholar] [CrossRef]
- Minj, E.; Yadav, R.K.; Mehan, S. Targeting Abnormal Nrf2/HO-1 Signaling in Amyotrophic Lateral Sclerosis: Current Insights on Drug Targets and Influences on Neurological Disorders. Curr. Mol. Med. 2021, 21, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Soni, D.; Kumar, P. GSK-3β-Mediated Regulation of Nrf2/HO-1 Signaling as a New Therapeutic Approach in the Treatment of Movement Disorders. Pharmacol. Rep. 2022, 74, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lu, H.; Bai, Y. Nrf2 in Cancers: A Double-edged Sword. Cancer Med. 2019, 8, 2252–2267. [Google Scholar] [CrossRef] [PubMed]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme Oxygenase-1 Signaling and Redox Homeostasis in Physiopathological Conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, B.; Nemeth, Z.; Correa-Costa, M.; Bulmer, A.C.; Otterbein, L.E. Heme Oxygenase-1: A Metabolic Nike. Antioxid. Redox Signal. 2014, 20, 1709–1722. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.; Daiber, A. Direct Antioxidant Properties of Bilirubin and Biliverdin. Is There a Role for Biliverdin Reductase? Front. Pharmacol. 2012, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Dutra, F.F.; Bozza, M.T. Heme on Innate Immunity and Inflammation. Front. Pharmacol. 2014, 5, 115. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.-O.; Chung, H.-T. Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases. Immune Netw. 2009, 9, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Rojo, A.I. Heme Oxygenase-1 as a Therapeutic Target in Neurodegenerative Diseases and Brain Infections. Curr. Pharm. Des. 2008, 14, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Neis, V.B.; Rosa, P.B.; Moretti, M.; Rodrigues, A.L.S. Involvement of Heme Oxygenase-1 in Neuropsychiatric and Neurodegenerative Diseases. Curr. Pharm. Des. 2018, 24, 2283–2302. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-L.; Ho, Y.-C.; Lin, C.-Y.; Yet, S.-F. Heme Oxygenase-1 in Inflammation and Cardiovascular Disease. Am. J. Cardiovasc. Dis. 2011, 1, 150–158. [Google Scholar] [PubMed]
- Wu, Y.-H.; Hsieh, H.-L. Roles of Heme Oxygenase-1 in Neuroinflammation and Brain Disorders. Antioxidants 2022, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.; Kareem, O.; Khushtar, M.; Akbar, M.; Haque, M.R.; Iqubal, A.; Haider, M.F.; Pottoo, F.H.; Abdulla, F.S.; Al-Haidar, M.B.; et al. Neuroinflammation: A Potential Risk for Dementia. Int. J. Mol. Sci. 2022, 23, 616. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The Devil Is in the Details. J. Neurochem. 2016, 139 (Suppl. S2), 136–153. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Chugh, H.; Sakharkar, M.K.; Dhawan, U.; Chidambaram, S.B.; Chandra, R. Neuroinflammation Mechanisms and Phytotherapeutic Intervention: A Systematic Review. ACS Chem. Neurosci. 2020, 11, 3707–3731. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Onyango, I.G.; Jauregui, G.V.; Čarná, M.; Bennett, J.P.; Stokin, G.B. Neuroinflammation in Alzheimer’s Disease. Biomedicines 2021, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation Pathways: A General Review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and Kaempferol Rhamnosides with Depigmenting and Anti-Inflammatory Properties. Molecules 2011, 16, 3338. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, H.J.; Choi, S.E.; Park, K.H.; Choi, H.K.; Lee, M.W. Anti-Oxidative and Inhibitory Activities on Nitric Oxide (NO) and Prostaglandin E2 (COX-2) Production of Flavonoids from Seeds of Prunus tomentosa Thunberg. Arch. Pharm. Res. 2008, 31, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Jung, E.; Kim, S.; Kim, J.-H.; Kim, E.-G.; Lee, J.; Park, D. Antagonizing Effects and Mechanisms of Afzelin against UVB-Induced Cell Damage. PLoS ONE 2013, 8, e0061971. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.-S.; Lee, H.; Liu, Z.; Lee, H.-K.; Lee, D.-S. Effects of Compounds Isolated from Lindera Erythrocarpa on Anti-Inflammatory and Anti-Neuroinflammatory Action in BV2 Microglia and RAW264.7 Macrophage. Int. J. Mol. Sci. 2022, 23, 7122. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Quilantang, N.G.; Kim, H.Y.; Lee, S.; Cho, E.J. Attenuation of Hydrogen Peroxide-Induced Oxidative Stress in SH-SY5Y Cells by Three Flavonoids from Acer Okamotoanum. Chem. Pap. 2019, 73, 1135–1144. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.; Cho, E.J. Acer Okamotoanum Protects SH-SY5Y Neuronal Cells against Hydrogen Peroxide-Induced Oxidative Stress. Food Sci. Biotechnol. 2019, 28, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Radziejewska, I.; Supruniuk, K.; Czarnomysy, R.; Buzun, K.; Bielawska, A. Anti-Cancer Potential of Afzelin towards AGS Gastric Cancer Cells. Pharmaceuticals 2021, 14, 973. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Xu, X.; Li, M.; Zhang, X.; Cao, F. Afzelin Induces Immunogenic Cell Death against Lung Cancer by Targeting NQO2. BMC Complement. Med. Ther. 2023, 23, 381. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.; Tomàs, M.; Santafé, M.M.; Besalduch, N.; Lanuza, M.A.; Tomàs, J. The Interaction between Tropomyosin-Related Kinase B Receptors and Presynaptic Muscarinic Receptors Modulates Transmitter Release in Adult Rodent Motor Nerve Terminals. J. Neurosci. 2010, 30, 16514–16522. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Yu, J.T.; Tan, L. Brain-Derived Neurotrophic Factor in Alzheimer’s Disease: Risk, Mechanisms, and Therapy. Mol. Neurobiol. 2015, 52, 1477–1493. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Dhankhar, S.; Chauhan, S.; Garg, N.; Bhattacharya, T.; Ali, M.; Chaudhary, A.A.; Rudayni, H.A.; Al-Zharani, M.; Ahmad, W.; et al. A Review on Natural Antioxidants for Their Role in the Treatment of Parkinson’s Disease. Pharmaceuticals 2023, 16, 908. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R. Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 6827. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-Y.; Jang, M.J.; Choi, Y.-H.; Hwang, H.; Rhim, H.; Lee, B.; Choi, C.W.; Kim, M.S. Central Administration of Afzelin Extracted from Ribes Fasciculatum Improves Cognitive and Memory Function in a Mouse Model of Dementia. Sci. Rep. 2021, 11, 9182. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.D.; Manadas, B.J.; Melo, C.V.; Gomes, J.R.; Mendes, C.S.; Grãos, M.M.; Carvalho, R.F.; Carvalho, A.P.; Duarte, C.B. Neuroprotection by BDNF against Glutamate-Induced Apoptotic Cell Death Is Mediated by ERK and PI3-Kinase Pathways. Cell Death Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-K.; Liu, C.-C.; Wang, S.; Cheng, H.-C.; Meadows, C.; Chang, K.-C. The Role of Aldose Reductase in Beta-Amyloid-Induced Microglia Activation. Int. J. Mol. Sci. 2022, 23, 15088. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Lee, J.S.; Quilantang, N.G.; Jacinto, S.D.; Lee, S. Determination of Afzelin and Astragalin from Lespedeza Cuneata on Aldose Reductase Inhibition. J. Chromatogr. Sci. 2021, 59, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-M.; Yu, Q.; Dong, X.; Yang, H.O.; Zeng, K.-W.; Li, J.; Tu, P.-F. Aldose Reductase Inhibitors Attenuate β-Amyloid-Induced TNF-α Production in Microlgia via ROS-PKC-Mediated NF-κB and MAPK Pathways. Int. Immunopharmacol. 2017, 50, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Iannitelli, A.F.; Segal, A.; Pare, J.-F.; Mulvey, B.; Liles, L.C.; Sloan, S.A.; McCann, K.E.; Dougherty, J.D.; Smith, Y.; Weinshenker, D. Tyrosinase-Induced Neuromelanin Accumulation Triggers Rapid Dysregulation and Degeneration of the Mouse Locus Coeruleus. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T.; Nakashima, A.; Watanabe, H.; Ito, S.; Wakamatsu, K. Neuromelanin in Parkinson’s Disease: Tyrosine Hydroxylase and Tyrosinase. Int. J. Mol. Sci. 2022, 23, 4176. [Google Scholar] [CrossRef]
- Rho, H.S.; Ahn, S.M.; Lee, B.C.; Kim, M.K.; Ghimeray, A.K.; Jin, C.W.; Cho, D.H. Changes in Flavonoid Content and Tyrosinase Inhibitory Activity in Kenaf Leaf Extract after Far-Infrared Treatment. Bioorg. Med. Chem. Lett. 2010, 20, 7534–7536. [Google Scholar] [CrossRef] [PubMed]
- Sayuti, N.H.; Zulkefli, N.; Tan, J.K.; Saad, N.; Baharum, S.N.; Hamezah, H.S.; Bunawan, H.; Ahmed, Q.U.; Parveen, H.; Mukhtar, S.; et al. Ethanolic Extract of Polygonum Minus Protects Differentiated Human Neuroblastoma Cells (SH-SY5Y) against H2O2-Induced Oxidative Stress. Molecules 2023, 28, 6726. [Google Scholar] [CrossRef] [PubMed]
- Işık, M.; Beydemir, Ş. AChE mRNA Expression as a Possible Novel Biomarker for the Diagnosis of Coronary Artery Disease and Alzheimer’s Disease, and Its Association with Oxidative Stress. Arch. Physiol. Biochem. 2022, 128, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Rachmi, E.; Purnomo, B.B.; Endharti, A.T.; Fitri, L.E. Afzelin Inhibits Migration of MDA-MB-231 Cells by Suppressing FAK Expression and Rac1 Activation. J. Appl. Pharm. Sci. 2020, 10, 77–82. [Google Scholar] [CrossRef]
- Rachmi, E.; Purnomo, B.B.; Endharti, A.T.; Fitri, L.E. Identification of Afzelin Potential Targets in Inhibiting Triple-Negative Breast Cancer Cell Migration Using Reverse Docking. Porto Biomed. J. 2020, 5, e095. [Google Scholar] [CrossRef]
- Diantini, A.; Subarnas, A.; Lestari, K.; Halimah, E.; Susilawati, Y.; Supriyatna, S.; Julaeha, E.; Achmad, T.H.; Suradji, E.W.; Yamazaki, C.; et al. Kaempferol-3-O-Rhamnoside Isolated from the Leaves of Schima wallichii Korth. Inhibits MCF-7 Breast Cancer Cell Proliferation through Activation of the Caspase Cascade Pathway. Oncol. Lett. 2012, 3, 1069–1072. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, Y.; Zhou, Z.; Xiang, W.; Gong, Z.; Chen, S.; Wang, Y.; Wang, A.; Lan, Y.; Li, Y.; et al. Comparative Pharmacokinetics of Quercitrin, Astragalin, Afzelin and Taxifolin in Plasma after Oral Administration of Polygonum Orientale Inflorescence in Sham-Operated and Myocardial Ischemia-Reperfusion Injury Rats. Xenobiotica 2020, 50, 822–830. [Google Scholar] [CrossRef]
- Alves, J.M.; Munari, C.C.; de Azevedo Bentes Monteiro Neto, M.; Furtado, R.A.; Senedese, J.M.; Bastos, J.K.; Tavares, D.C. In Vivo Protective Effect of Copaifera langsdorffii Hydroalcoholic Extract on Micronuclei Induction by Doxorubicin. J. Appl. Toxicol. 2013, 33, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Ozelin, S.D.; Senedese, J.M.; Alves, J.M.; Munari, C.C.; Costa, J.D.C.D.; Resende, F.A.; Campos, D.L.; Lima, I.M.D.S.; Andrade, A.F.; Varanda, E.A.; et al. Preventive Activity of Copaifera langsdorffii Desf. Leaves Extract and Its Major Compounds, Afzelin and Quercitrin, on DNA Damage in In Vitro and In Vivo Models. J. Toxicol. Environ. Health A 2021, 84, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.-O.; Lee, H.A.; Rhee, C.H.; Choung, S.-Y.; Lee, K.-W. Separation of the Antioxidant Compound Quercitrin from Lindera obtusiloba blume and Its Antimelanogenic Effect on B16F10 Melanoma Cells. Biosci. Biotechnol. Biochem. 2013, 77, 58–64. [Google Scholar] [CrossRef]
- Vellosa, J.C.R.; Regasini, L.O.; Belló, C.; Schemberger, J.A.; Khalil, N.M.; de Araújo Morandim-Giannetti, A.; da Silva Bolzani, V.; Brunetti, I.L.; de Faria Oliveira, O.M.M. Preliminary in Vitro and Ex Vivo Evaluation of Afzelin, Kaempferitrin and Pterogynoside Action over Free Radicals and Reactive Oxygen Species. Arch. Pharm. Res. 2015, 38, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Kumar, V.; Singh, S.K.; Dubey, A.K.; Kim, J.-J. Flavonoids: Potential Candidates for the Treatment of Neurodegenerative Disorders. Biomedicines 2021, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Alharthy, K.M.; Rashid, S.; Yusufoglu, H.S.; Alqasoumi, S.I.; Ganaie, M.A.; Alam, A. Neuroprotective Potential of Afzelin: A Novel Approach for Alleviating Catalepsy and Modulating Bcl-2 Expression in Parkinson’s Disease Therapy. Saudi Pharm. J. 2024, 32, 101928. [Google Scholar] [CrossRef] [PubMed]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A Double-Edged Sword in Cancer Treatment. Cancer Immunol. Immunother. 2022, 71, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Majidpoor, J.; Toolee, H.; Mortezaee, K. The Current Knowledge Concerning Solid Cancer and Therapy. J. Biochem. Mol. Toxicol. 2021, 35, e22900. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, W.; He, F.; Li, D.; Wang, D. Simultaneous Enrichment and Separation of Four Flavonoids from Zanthoxylum bungeanum Leaves by Ultrasound-Assisted Extraction and Macroporous Resins with Evaluation of Antioxidant Activities. J. Food Sci. 2018, 83, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, J.P.B.; Brancalion, A.P.S.; Júnior, M.G.; Bastos, J.K. A Validated Chromatographic Method for the Determination of Flavonoids in Copaifera langsdorffii by HPLC. Nat. Prod. Commun. 2012, 7, 25–28. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kciuk, M.; Garg, N.; Dhankhar, S.; Saini, M.; Mujwar, S.; Devi, S.; Chauhan, S.; Singh, T.G.; Singh, R.; Marciniak, B.; et al. Exploring the Comprehensive Neuroprotective and Anticancer Potential of Afzelin. Pharmaceuticals 2024, 17, 701. https://doi.org/10.3390/ph17060701
Kciuk M, Garg N, Dhankhar S, Saini M, Mujwar S, Devi S, Chauhan S, Singh TG, Singh R, Marciniak B, et al. Exploring the Comprehensive Neuroprotective and Anticancer Potential of Afzelin. Pharmaceuticals. 2024; 17(6):701. https://doi.org/10.3390/ph17060701
Chicago/Turabian StyleKciuk, Mateusz, Nitika Garg, Sanchit Dhankhar, Monika Saini, Somdutt Mujwar, Sushma Devi, Samrat Chauhan, Thakur Gurjeet Singh, Randhir Singh, Beata Marciniak, and et al. 2024. "Exploring the Comprehensive Neuroprotective and Anticancer Potential of Afzelin" Pharmaceuticals 17, no. 6: 701. https://doi.org/10.3390/ph17060701
APA StyleKciuk, M., Garg, N., Dhankhar, S., Saini, M., Mujwar, S., Devi, S., Chauhan, S., Singh, T. G., Singh, R., Marciniak, B., Gielecińska, A., & Kontek, R. (2024). Exploring the Comprehensive Neuroprotective and Anticancer Potential of Afzelin. Pharmaceuticals, 17(6), 701. https://doi.org/10.3390/ph17060701






