Abstract
Background: Literature on the preferred anticoagulant for treating left ventricular thrombus (LVT) is lacking. Thus, our objective was to compare the efficacy of DOACs versus warfarin in treating LVT. Methods: Databases were searched for RCTs and adjusted observational studies that compared DOAC versus warfarin through March 2024. The primary efficacy outcomes of interest were LVT resolution, systemic embolism, composite of stroke, and TIA. The primary safety outcomes encompassed all-cause mortality and bleeding events. Results: Our meta-analysis including 31 studies demonstrated that DOAC use was associated with higher odds of thrombus resolution (OR: 1.08, 95% CI: 0.86–1.31, p: 0.46). A statistically significant reduction in the risk of stroke/TIA was observed in the DOAC group versus the warfarin group (OR: 0.65, 95% CI: 0.48–0.89, p: 0.007). Furthermore, statistically significant reduced risks of all-cause mortality (OR: 0.68, 95% CI: 0.47–0.98, p: 0.04) and bleeding events (OR: 0.70, 95% CI: 0.55–0.89, p: 0.004) were observed with DOAC use as compared to warfarin use. Conclusion: Compared to VKAs, DOACs are noninferior as the anticoagulant of choice for LVT treatment. However, further studies are warranted to confirm these findings.
1. Introduction
Left ventricular thrombus (LVT) is a dreaded complication in patients with myocardial infarction (MI) and dilated cardiomyopathy (DCM). Despite notable progress in managing these conditions, the occurrence of LVT persists at a considerable rate, varying between 4 and 39% in patients with acute MI [1] and 11–44% in those with DCM [2,3]. Depending on thrombus size and progression, LVT carries a risk of embolization of up to 22% [3,4,5,6] and a 37% risk of major adverse cardiovascular events (MACEs) [7].
To reduce the risk of thromboembolic (TE) events, clinical guidelines recommend anticoagulation for a duration of 3–6 months in patients with LVT. However, there seems to lack consensus among different societies regarding the choice of anticoagulation regimen. The 2013 American College of Cardiology/American Heart Association (ACC/AHA) ST segment elevation MI (STEMI) guideline recommends consideration of vitamin K antagonist (VKA) therapy for 3 months in patients with or at risk of LVT (e.g., those with anteroapical akinesis or dyskinesis) (Class IIb indication, level of evidence C) [8]. The 2023 European Society of Cardiology (ESC) guideline states that “the choice of (anticoagulant) therapy should be tailored to the patient’s clinical status and the results of follow-up investigations” but does not comment on the specific type of anticoagulant [9].
VKAs, predominantly warfarin, have been traditionally used for the prevention and treatment of LVT. However, difficulty in monitoring INR, drug–food and drug–drug interactions, and suboptimal times in therapeutic range (TTR) make warfarin a challenging therapeutic option for both providers and patients. Direct oral anticoagulant (DOAC) therapy, on the other hand, seems like an attractive option with fewer side effects while providing a more predictable and steady state of anticoagulation with enhanced patient compliance and fewer drug–drug interactions. Moreover, since inception, the cost of these drugs has fallen considerably. The 2022 AHA statement on the management of LVT indicates that DOAC therapy as a reasonable alternative to VKAs but does not comment on whether either anticoagulant is preferred [10]. In this context, our meta-analysis (meta-analysis) aimed to pool results from randomized clinical trials (RCTs) and observational studies to provide a more comprehensive understanding of the safety and efficacy of DOACs in LVT patients.
2. Methods
Our meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guideline [11]. This study was registered with PROSPERO database (registration ID 550050) [12].
2.1. Data Sources and Searches
We conducted a literature search using the following Medical Subject Headings (MeSH) terms: “Direct Oral Anticoagulants”, “warfarin”, “Vitamin K antagonist”, and “Left ventricular thrombus”. PubMed, Cochrane, Google scholar, and ClinicalTrials.gov databases were systematically queried for all RCTs and observational studies comparing DOACs versus warfarin in patients with LVT and published between 1 January 1990 and 1 March 2024. Additionally, two investigators (MV and DK) independently reviewed the reference lists of identified studies and relevant reviews to identify additional pertinent studies.
2.2. Study Selection
Our meta-analysis encompassed all RCTs and adjusted observational studies comparing DOACs with warfarin in patients diagnosed with LVT. The following criteria were employed for study inclusion: confirmation of LVT diagnosis via cardiac imaging modalities such as transthoracic echocardiography (TTE) or cardiac magnetic resonance imaging (CMRi), a median follow-up period of at least 1 month, and the reporting of at least one clinical endpoint related to treatment approach. Excluded from our analysis were case reports, case series, cross-sectional studies, and single-arm investigations. Additionally, studies involving patients with intracardiac, ventricular mural, and right ventricular thrombus were excluded from our analysis.
2.3. Outcome Measures and Quality Assessment
The primary efficacy outcomes in our study included LVT resolution, systemic embolism, composite of stroke, and transient ischemic attack (TIA). Primary safety outcomes encompassed all-cause mortality and bleeding events. Additionally, major bleeding as defined by categories 3–5 according to The Bleeding Academic Research Consortium (BARC) [13] criteria or moderate–severe bleeding according to the Global Use of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO) criteria were also included in the safety outcome [13].
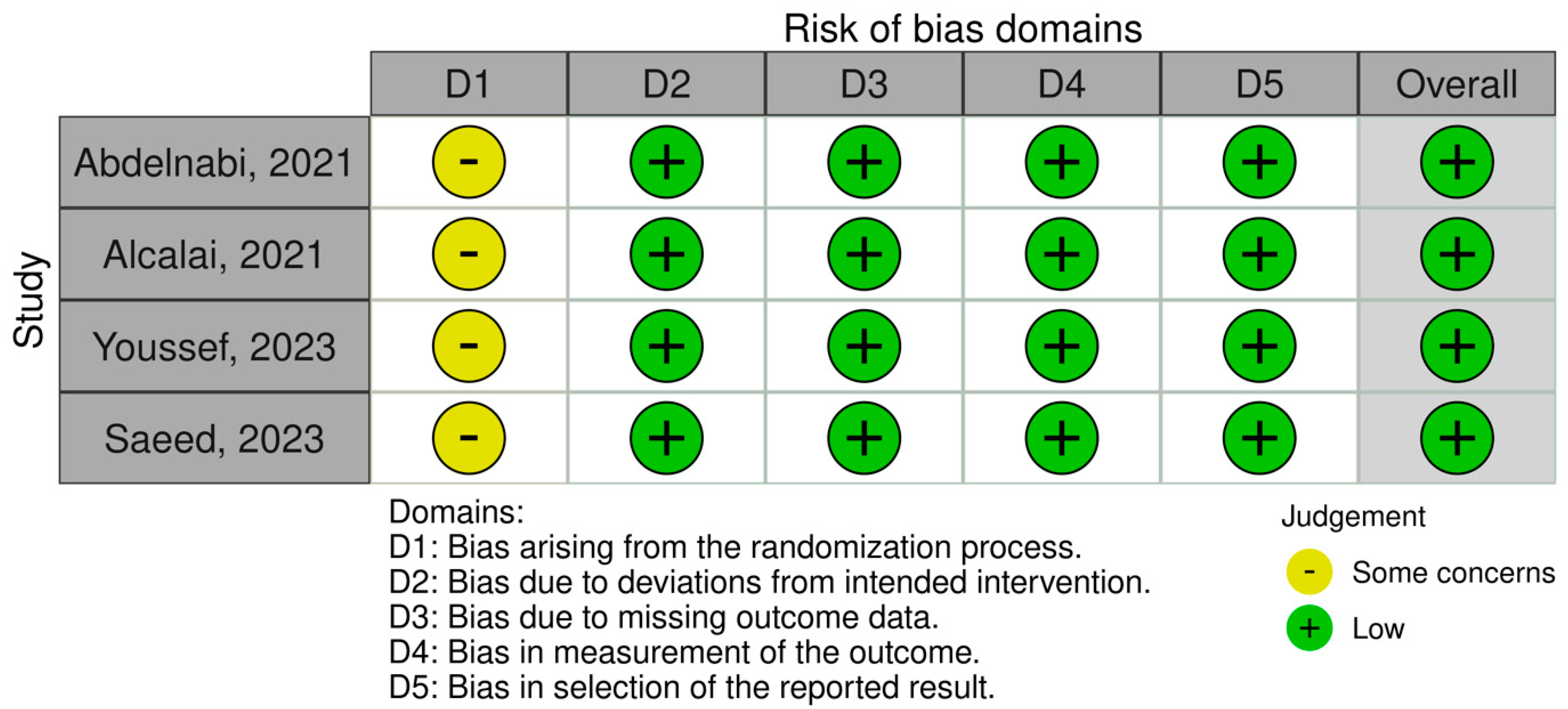
To assess the quality of included observational studies and RCTs, we employed the Newcastle–Ottawa Scale (NOS) [14] and the Cochrane Collaboration Risk-of-Bias 2 (RoB 2) [15] tools. The NOS is a 9-point scoring system comprising f variables such as study selection, comparability of groups, ascertainment of exposure, and outcome measurement in observational studies, each allocated individual scores. Scores ranging from 0 to 3 indicate a very high risk of bias, 4 to 6 indicate a high risk of bias, and 7 to 9 indicate a low risk of bias (Table 1). On the other hand, the RoB 2 is a web-based tool developed in collaboration with Cochrane to assess the overall quality of RCTs based on variables such as randomization, deviation from intended intervention, outcome measurement, and selection of reported results (Figure 1).

Table 1.
Assessing the risk of bias using Newcastle–Ottawa scale in observational studies.
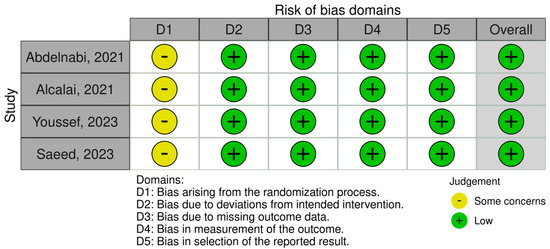
Figure 1.
Assessing the risk of bias in randomized clinical trials.
2.4. Data Synthesis and Statistical Analysis
Individual study-level data extraction was independently conducted by two reviewers (MV and DK) using a predefined form, which included information on study characteristics, baseline patient characteristics, and endpoint event rates.
Our meta-analysis was conducted according to the recommendations from Cochrane Collaboration using Review Manager, version 5.3 [43]. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using random-effects models with the Mantel–Haenszel method [44]. A p-value of less than 0.05 was deemed statistically significant for each clinical endpoint. The extent of heterogeneity among studies was assessed using the I2 statistic, with values exceeding 50% indicating significant heterogeneity. Forest plots were generated to visually depict the relative effect size of DOAC versus warfarin for individual clinical endpoints.
3. Results
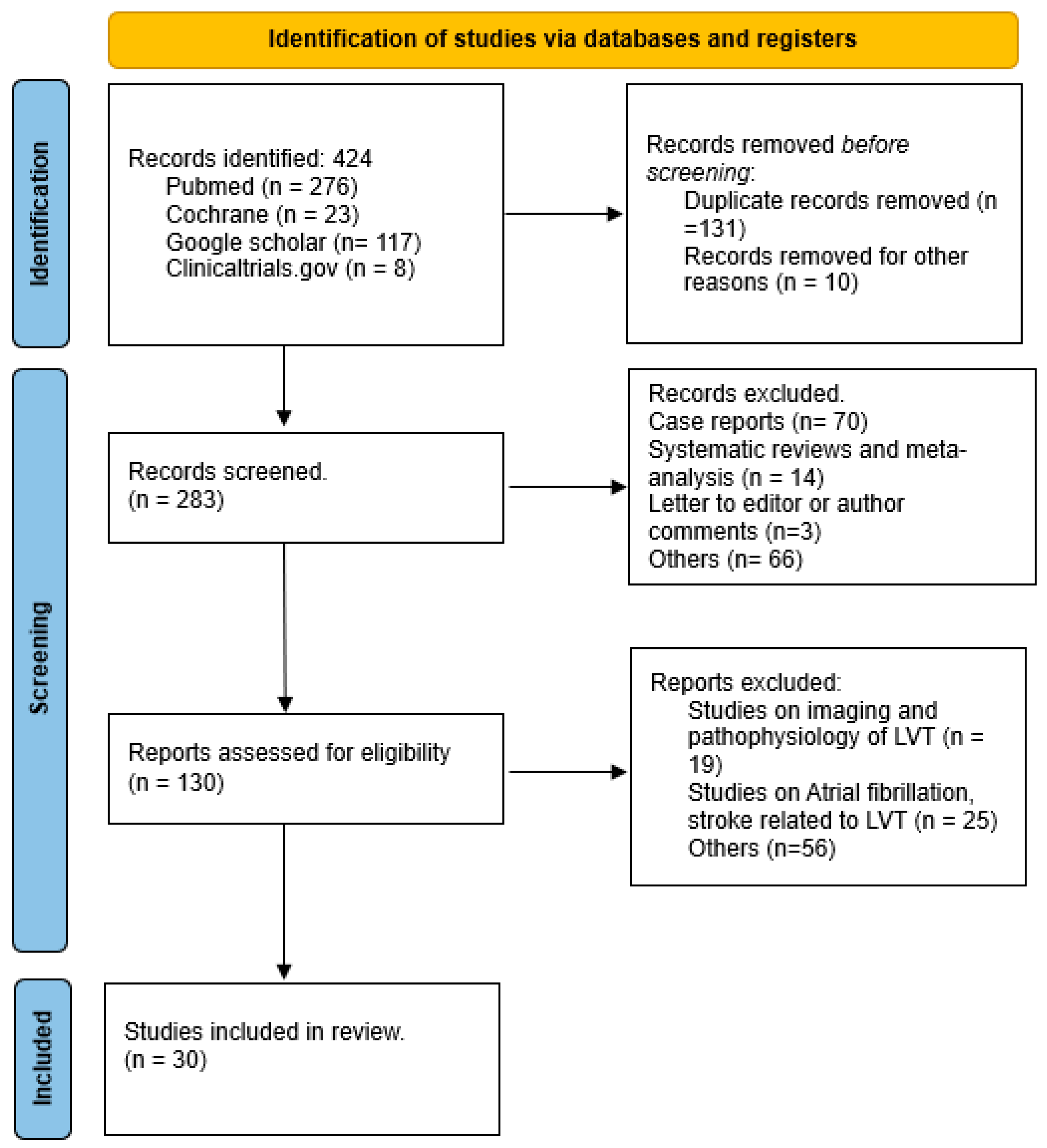
As depicted in Figure 2 the initial search yielded 424 publications. After reviewing titles and abstracts, 141 studies were excluded for lack of relevance. The remaining 283 articles underwent a comprehensive review and assessment to determine if they met the inclusion and exclusion criteria. Following a full-text review, 31 studies were included in the final analysis.
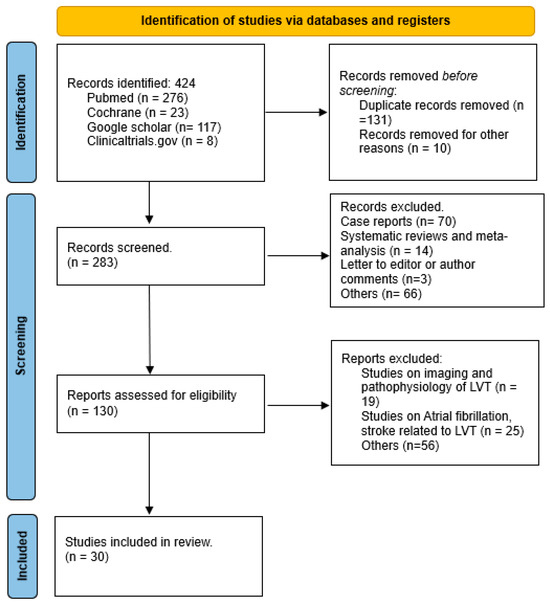
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-analyses flow sheet.
The included studies were homogeneous regarding the inclusion and exclusion criteria. Among these, 27 were observational studies, and 4 were RCTs [45,46,47,48]. Patients were followed for an average period of 16.9 months. The baseline characteristics of the patients in the included studies are summarized in Table 2. The mean age of the patients was 59 years. Of the study participants, 33% were treated with direct oral anticoagulants (DOACs) and 67% with warfarin. All studies included in the final analysis were deemed to have a low-to-intermediate risk of bias, as assessed using the Newcastle–Ottawa Scale (NOS) and Cochrane metrics for quality assessment.

Table 2.
Baseline characteristics of studies included.
3.1. Efficacy Outcomes
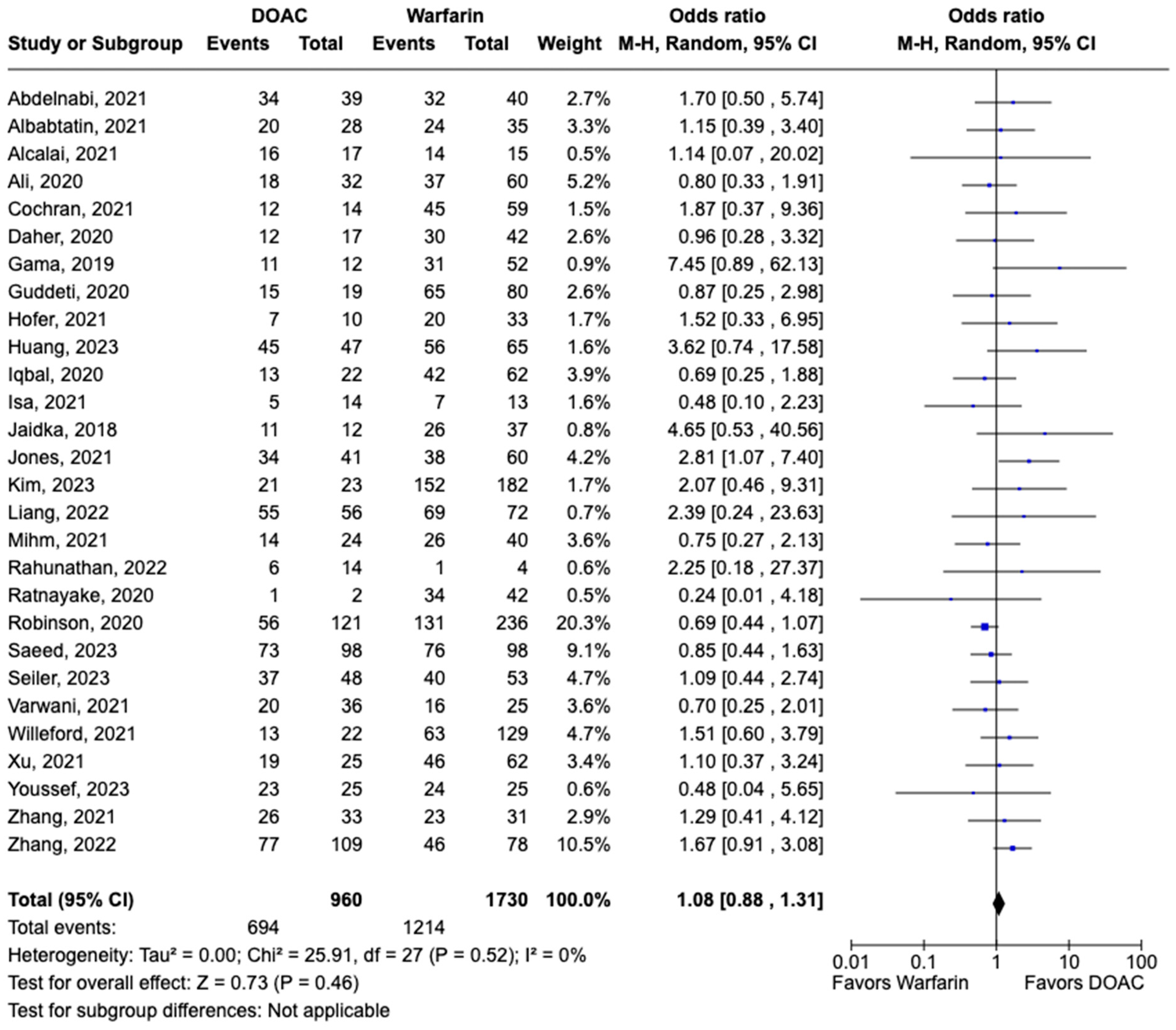
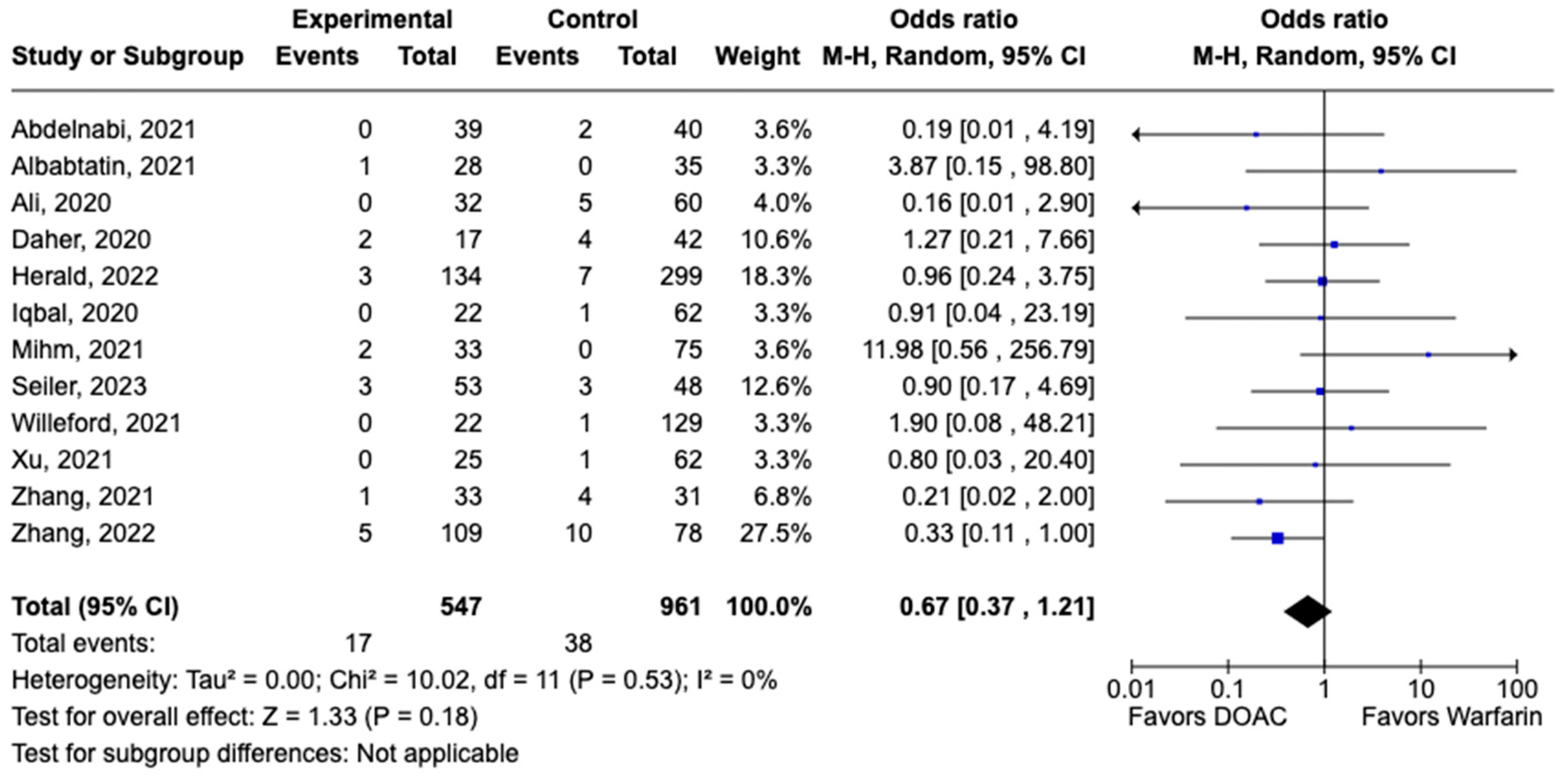
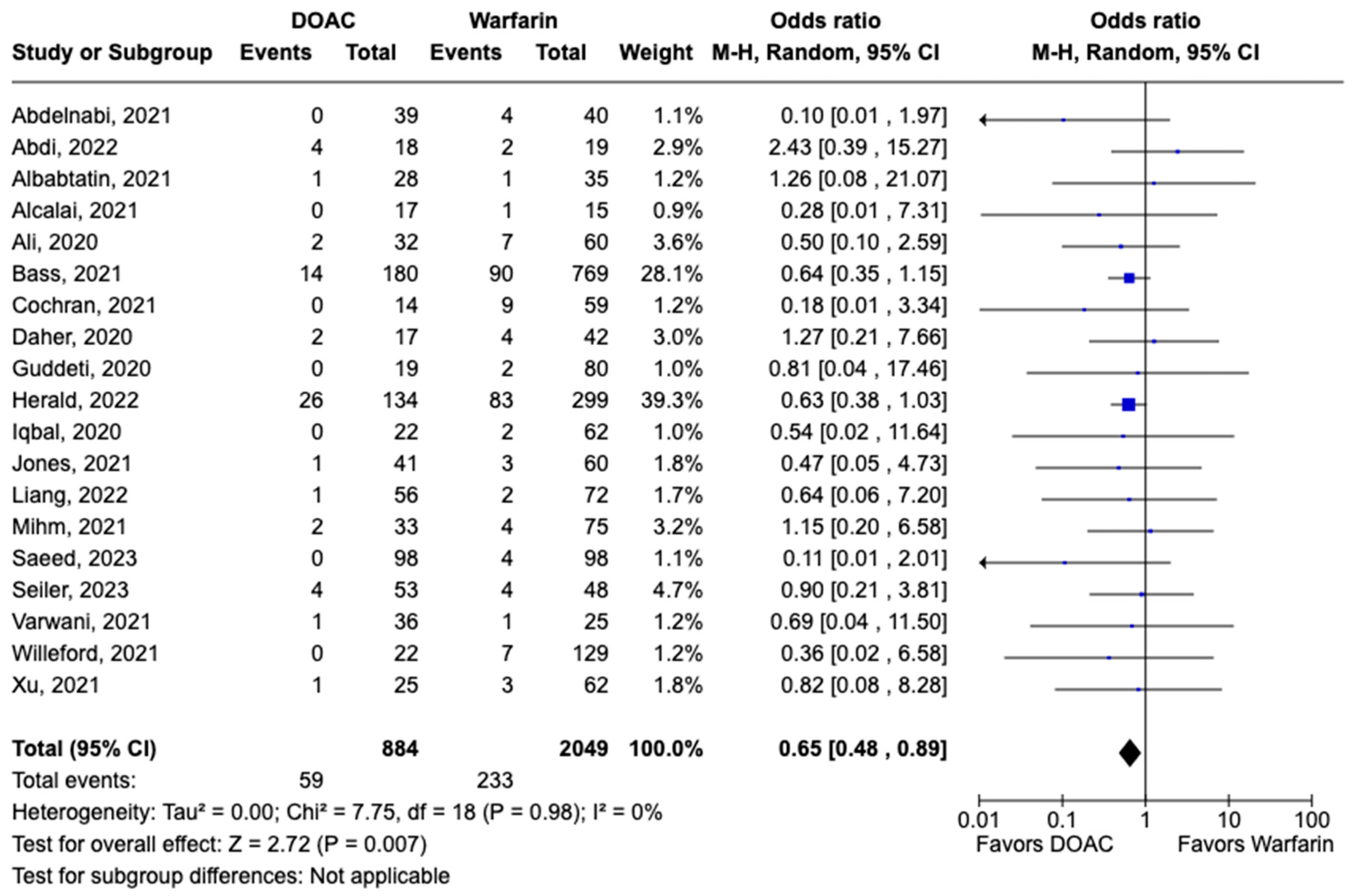
LVT resolution was reported in 28 studies including 2690 patients. Compared with warfarin, DOAC use showed a trend toward higher odds of thrombus resolution (OR: 1.08, 95% CI: 0.86–1.31, p: 0.46) (Figure 3). The occurrence of systemic embolism was reported in 12 studies including 1508 participants. Although not statistically significant, DOAC use was associated with lowered risk of systemic embolism as compared to warfarin (OR: 0.67, 95% CI: 0.37–1.21, p: 0.18) (Figure 4). Additionally, 19 studies involving 2933 participants reported stroke/TIA. A statistically significant lower risk of stroke/TIA was observed in the DOAC group versus the warfarin group (OR: 0.65, 95% CI: 0.48–0.89, p: 0.007), with no heterogeneity (I2-0%) among the studies included in our analysis (Figure 5).
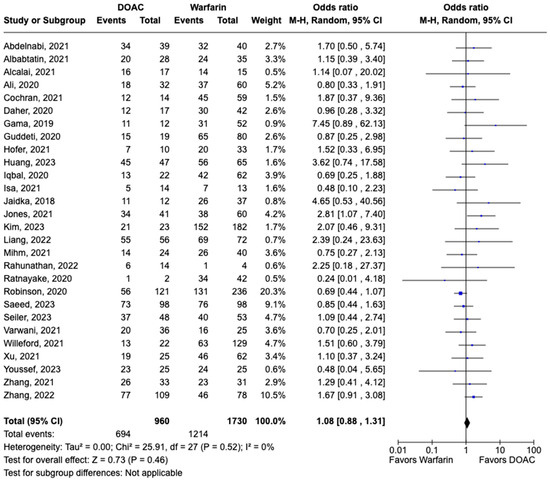
Figure 3.
Forest plot of LVT resolution in trials, comparing DOAC vs. warfarin treatment groups.
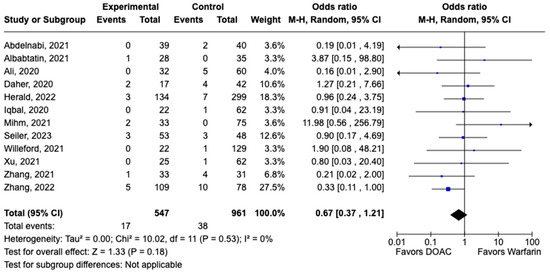
Figure 4.
Forest plot of systemic embolism in trials comparing DOAC vs. warfarin treatment groups.
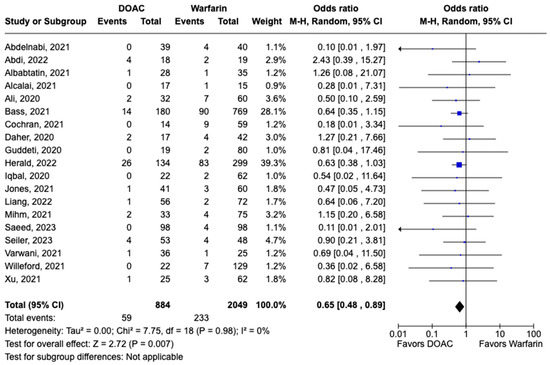
Figure 5.
Forest plot comparing the occurrence of stroke/TIA in DOAC and warfarin groups.
3.2. Safety Outcomes
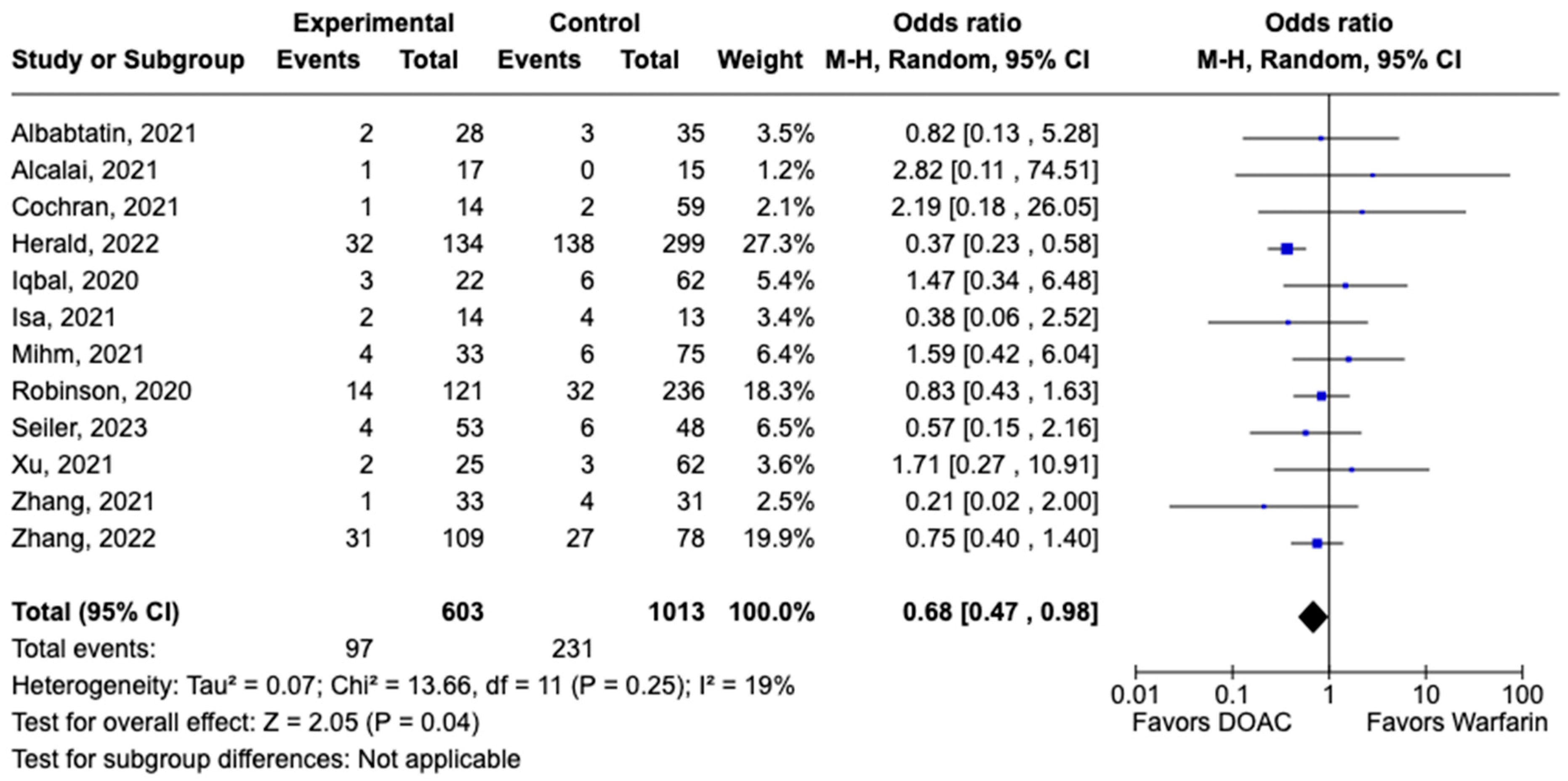
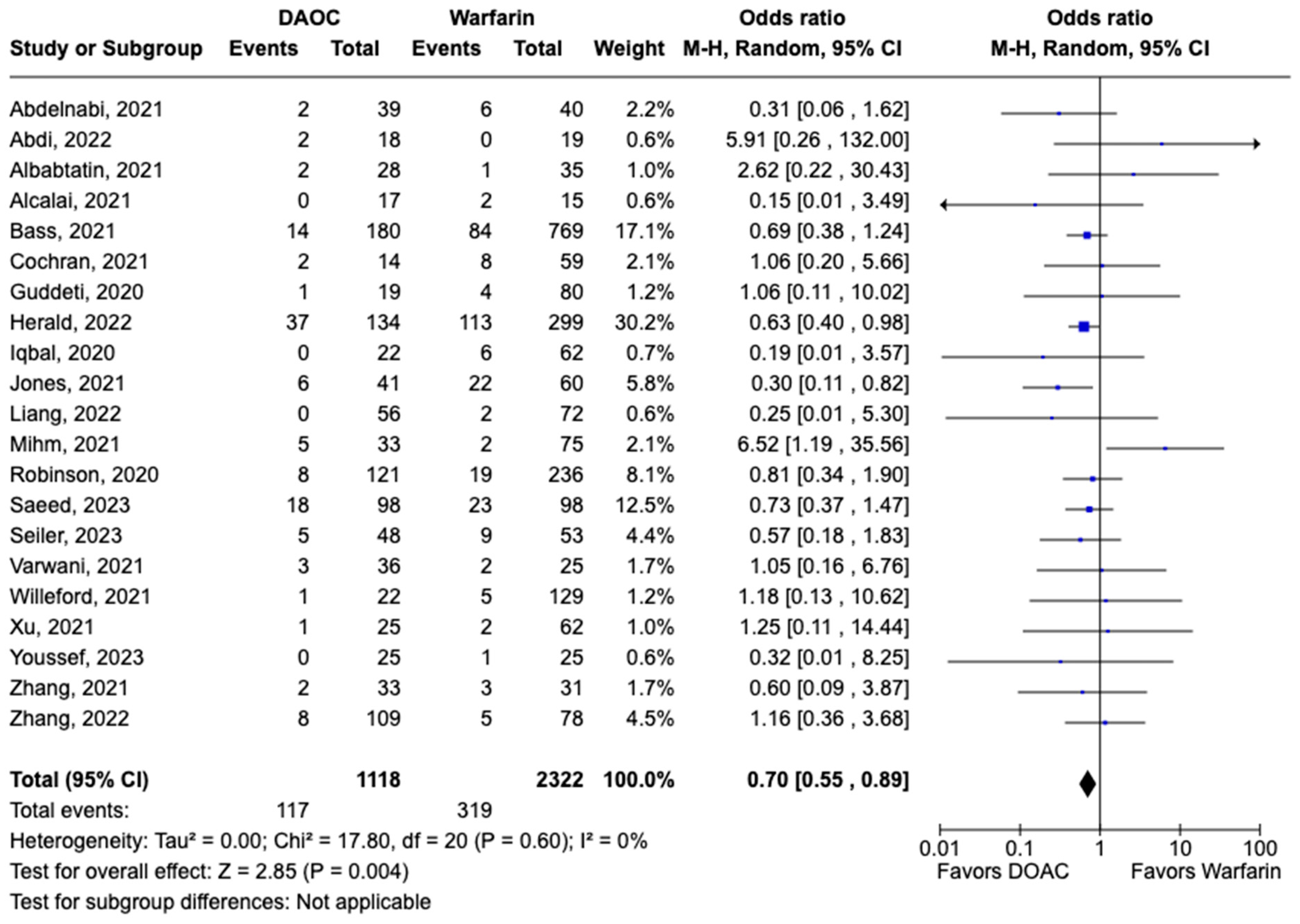
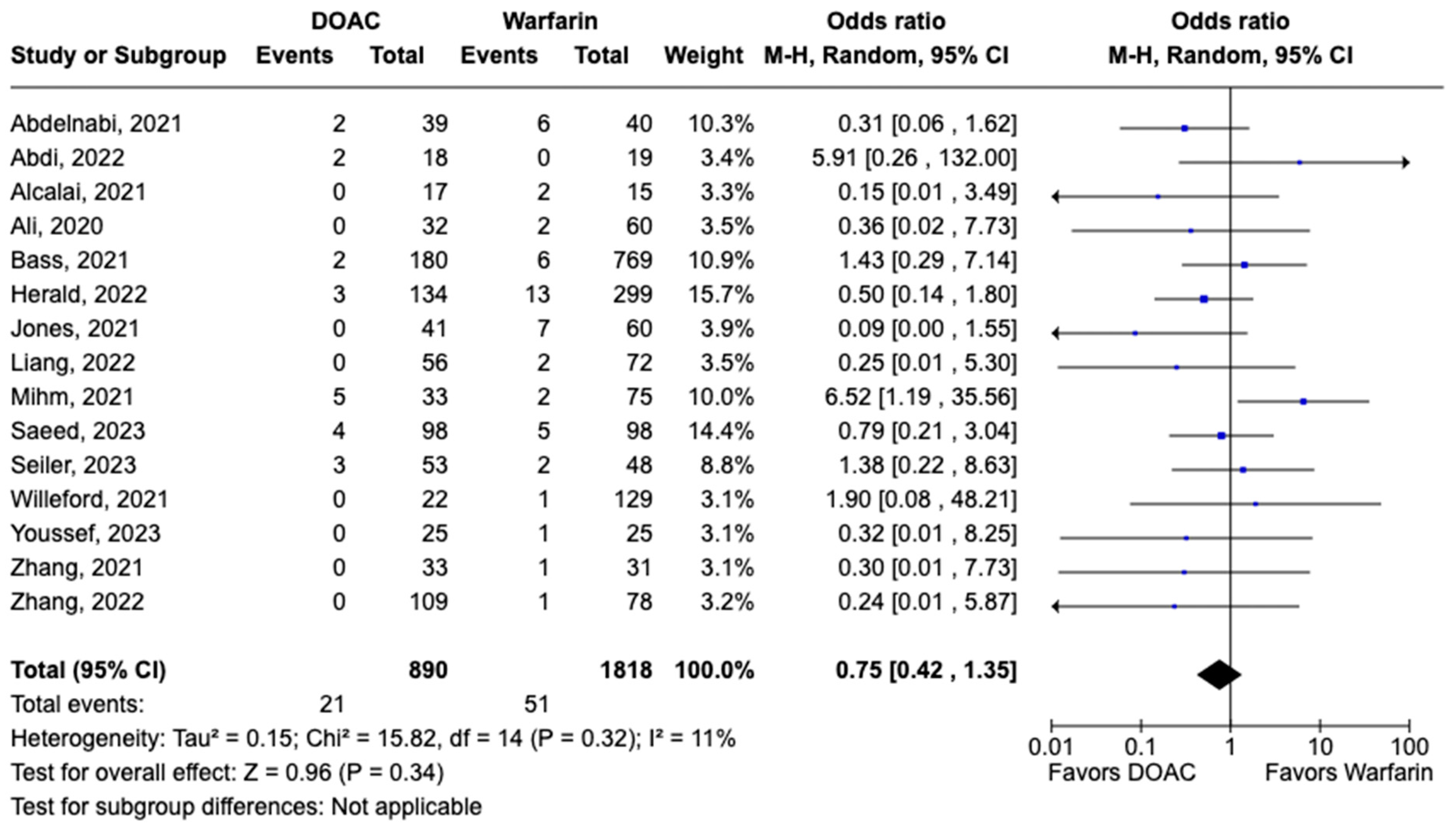
All-cause mortality was reported in 12 studies including 1616 patients. There was a statistically significant reduced risk of all-cause mortality with DOAC use when compared with warfarin use (OR: 0.68, 95% CI: 0.47–0.98, p: 0.04), with mild heterogeneity (I2-19%) among the included studies (Figure 6). Bleeding events were reported in 21 studies including 3440 participants. DOAC use was associated with statistically significant lower odds of bleeding when compared with warfarin use (OR: 0.70, 95% CI: 0.55–0.89, p: 0.004), with no heterogeneity (I2-0%) among the studies included for analysis (Figure 7). Although not statistically significant, the risk of major bleeding was also lower in the DOAC group versus warfarin group (OR: 0.75, 95% CI: 0.42–1.35, p: 0.34) (Figure 8).
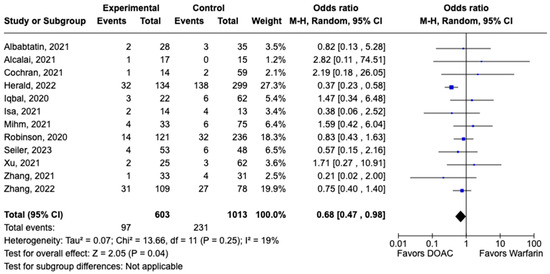
Figure 6.
Forest plot comparing the occurrence of all-cause mortality in DOAC and warfarin groups.
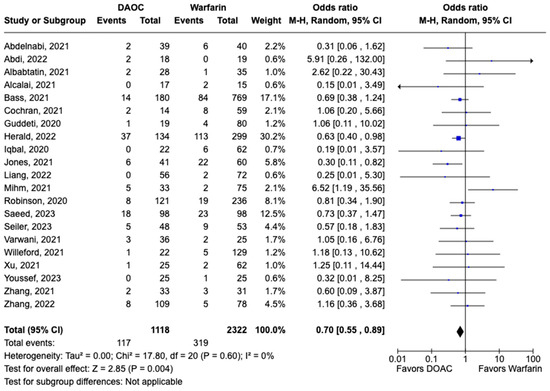
Figure 7.
Forest plot comparing the occurrence of bleeding events in DOAC and warfarin groups.
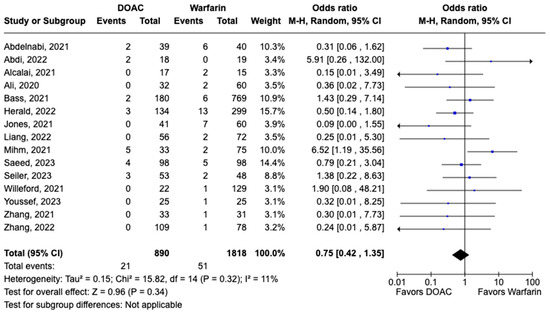
Figure 8.
Forest plot comparing the occurrence of major bleeding in DOAC and warfarin groups.
4. Discussion
LVT represents a concerning complication following acute MI, with an incidence of 3.5–8% [49,50,51] in the postpercutaneous coronary intervention PCI era. Likewise, incidences as high as 36–44% [2,3] and 68.5% [10] have been reported in anatomic pathology studies involving patients with DCM and heart failure (HF), respectively. Because of the heightened risk of TE complications, anticoagulation is imperative for preventing stroke and systemic embolism in patients with LVT. However, due to the scarcity of robust data, DOACs are merely recommended as alternatives to warfarin in patients with LVT requiring anticoagulation. Our meta-analysis including 31 studies is the most extensive comparison to date of DOACs vs. warfarin in patients with LVT. Random-effects analysis showed that DOACs are noninferior to warfarin for pharmacological anticoagulation in patients with LVT. In fact, DOAC use was associated with a significantly lowered risk of stroke/TIA, all-cause mortality, and bleeding when compared with warfarin use.
Our meta-analysis results corroborate those of previous studies comparing DOACs vs. warfarin in patients with LVT. A subgroup analysis of seven studies [25,29,35,41,46,47] investigating the effect of DOACs with warfarin in patients after MI favored DOACs for LVT resolution (OR: 1.70, 95% CI: 0.94–3.07, p: 0.08). Similarly, two studies evaluated the effect of DOACs in patients with HF [42] and DCM [26]. The rates of LVT resolution were comparable between the groups but did not reach statistical significance. Given the distinct pathophysiological mechanisms in those after MI (including endocardial injury, inflammation, and blood stasis) and with HF/DCM (involving blood stasis, endothelial dysfunction, and hypercoagulability), further research exploring the impact of DOACs in different etiological contexts is warranted. Furthermore, the effect of concurrent antiplatelet therapy on LVT resolution and safety events needs investigation.
The efficacy of rivaroxaban for LVT resolution has been evaluated in five studies [17,41,42,45,52]. Similarly, apixaban was assessed in three studies [28,46,47]. LVT resolution has occurred in 75% and 79% of patients treated with rivaroxaban and apixaban, respectively. Subgroup analysis favored rivaroxaban vs. warfarin; however, this difference did not reach statistical significance (OR: 1.26, 95% CI: 0.87–1.82, p: 0.23). Interestingly, the subgroup analysis of studies assessing apixaban for LVT resolution favored warfarin (OR: 0.55, 95% CI: 0.17–1.82, p: 0.33). The difference in outcomes between rivaroxaban and apixaban could be explained by different sample sizes and study design. Additionally, differences in thrombosis based on LVT etiology may reasonably translate into differences in anticoagulant responsiveness. Therefore, further research investigating the effect of different DOACs in patients with LVT is warranted.
Finally, our study demonstrated that DOACs are no-inferior to warfarin as an anticoagulant of choice in patients with LVT. However, our study has a few limitations: (1) Most included studies are observational and nonblinded, raising concerns regarding missing data and selection bias. (2) With respect to meta-analyses, there is always the possibility of residual confounding and publication bias. (3) The imaging modality used to diagnose LVT (e.g., TTE vs. CMR) was not uniform across different studies. (4) There are no studies to date comparing the relative efficacy of different classes of DOACs (apixaban, rivaroxaban, dabigatran, etc.) with warfarin in patients with LVT. (5) We could not obtain data on adherence to DOACs or the time in the therapeutic range of warfarin treatment. (6) Finally, we were unable to standardize the dose of anticoagulants, but this also reflects the current dilemma of anticoagulation management in the LVT population in the real world.
5. Conclusions
Since their introduction for treating venous TE and atrial fibrillation, DOACs have emerged as an appealing alternative to VKAs for both patients and clinicians. They offer advantages such as reduced need for monitoring, absence of dietary restrictions, and a lower risk of bleeding. Nevertheless, adequate data are lacking regarding the efficacy and safety of DOACs in managing LVT. Our meta-analysis demonstrates that that DOACs are comparable to warfarin in terms of efficacy (LVT resolution) and are associated with a decreased incidence of adverse events (bleeding). However, dedicated randomized clinical trials will be necessary to validate our findings and inform practice guidelines.
Author Contributions
Conceptualization, M.V. and D.K.K.; methodology, M.V.; formal analysis, M.V.; data curation, M.V. and D.K.K.; writing—original draft preparation, M.V.; writing—review and editing, D.K.K.; supervision, D.K.K.; project administration, M.V. and D.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACC | American College of Cardiology |
| AHA | American Heart Association |
| BARC | Bleeding Academic Research Consortium |
| CI | Confidence interval |
| CMRi | Cardiac magnetic resonance imaging |
| DCM | Dilated cardiomyopathy |
| DOAC | Direct oral anticoagulant |
| ESC | European Society of Cardiology |
| GUSTO | Global Use of Streptokinase and t-PA for Occluded Coronary Arteries |
| HF | Heart failure |
| LVT | Left ventricular thrombus |
| meta-analysis | Meta-analysis |
| MACE | Major adverse cardiovascular events |
| MeSH | Medical Subject Headings |
| MI | Myocardial infarction |
| NOS | Newcastle–Ottawa Scale |
| OR | Odds ratio |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | Randomized clinical trial |
| RoB 2 | Risk-of-Bias 2 |
| STEMI | ST segment elevation myocardial infarction |
| TE | Thromboembolic events |
| TIA | Transient ischemic attack |
| TTE | Transthoracic echocardiography |
| TTR | Time to achieve therapeutic range |
| VKA | Vitamin K antagonist |
References
- McCarthy, C.P.; Vaduganathan, M.; McCarthy, K.J.; Januzzi, J.L.; Bhatt, D.L.; McEvoy, J.W. Left Ventricular Thrombus After Acute Myocardial Infarction: Screening, Prevention, and Treatment. JAMA Cardiol. 2018, 3, 642–649. [Google Scholar] [CrossRef]
- Falk, R.H.; Foster, E.; Coats, M.H. Ventricular Thrombi and Thromboembolism in Dilated Cardiomyopathy: A Prospective Follow-up Study. Am. Heart J. 1992, 123, 136–142. [Google Scholar] [CrossRef]
- Gottdiener, J.S.; Gay, J.A.; VanVoorhees, L.; DiBianco, R.; Fletcher, R.D. Frequency and Embolic Potential of Left Ventricular Thrombus in Dilated Cardiomyopathy: Assessment by 2-Dimensional Echocardiography. Am. J. Cardiol. 1983, 52, 1281–1285. [Google Scholar] [CrossRef]
- Cruz Rodriguez, J.B.; Okajima, K.; Greenberg, B.H. Management of Left Ventricular Thrombus: A Narrative Review. Ann. Transl. Med. 2021, 9, 520. [Google Scholar] [CrossRef]
- Massussi, M.; Scotti, A.; Lip, G.Y.H.; Proietti, R. Left Ventricular Thrombosis: New Perspectives on an Old Problem. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 158–167. [Google Scholar] [CrossRef]
- Visser, C.A.; Kan, G.; Meltzer, R.S.; Dunning, A.J.; Roelandt, J. Embolic Potential of Left Ventricular Thrombus after Myocardial Infarction: A Two-Dimensional Echocardiographic Study of 119 Patients. J. Am. Coll. Cardiol. 1985, 5, 1276–1280. [Google Scholar] [CrossRef]
- Lattuca, B.; Bouziri, N.; Kerneis, M.; Portal, J.-J.; Zhou, J.; Hauguel-Moreau, M.; Mameri, A.; Zeitouni, M.; Guedeney, P.; Hammoudi, N.; et al. Antithrombotic Therapy for Patients with Left Ventricular Mural Thrombus. J. Am. Coll. Cardiol. 2020, 75, 1676–1685. [Google Scholar] [CrossRef]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes: Developed by the Task Force on the Management of Acute Coronary Syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Levine, G.N.; McEvoy, J.W.; Fang, J.C.; Ibeh, C.; McCarthy, C.P.; Misra, A.; Shah, Z.I.; Shenoy, C.; Spinler, S.A.; Vallurupalli, S.; et al. Management of Patients at Risk for and With Left Ventricular Thrombus: A Scientific Statement From the American Heart Association. Circulation 2022, 146, e205–e223. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The Nuts and Bolts of PROSPERO: An International Prospective Register of Systematic Reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials: A Consensus Report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef]
- Ottawa Hospital Research Institute. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 31 March 2024).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Abdi, I.A.; Karataş, M.; Öcal, L.; Elmi Abdi, A.; Farah Yusuf Mohamud, M. Retrospective Analysis of Left Ventricular Thrombus Among Heart Failure Patients with Reduced Ejection Fraction at a Single Tertiary Care Hospital in Somalia. Open Access Emerg. Med. 2022, 14, 591–597. [Google Scholar] [CrossRef]
- Albabtain, M.A.; Alhebaishi, Y.; Al-Yafi, O.; Kheirallah, H.; Othman, A.; Alghosoon, H.; Arafat, A.A.; Alfagih, A. Rivaroxaban versus Warfarin for the Management of Left Ventricle Thrombus. Egypt. Heart J. 2021, 73, 41. [Google Scholar] [CrossRef]
- Ali, Z.; Isom, N.; Dalia, T.; Sami, F.; Mahmood, U.; Shah, Z.; Gupta, K. Direct Oral Anticoagulant Use in Left Ventricular Thrombus. Thrombosis J. 2020, 18, 29. [Google Scholar] [CrossRef]
- Bass, M.E.; Kiser, T.H.; Page, R.L.; McIlvennan, C.K.; Allen, L.A.; Wright, G.; Shakowski, C. Comparative Effectiveness of Direct Oral Anticoagulants and Warfarin for the Treatment of Left Ventricular Thrombus. J. Thromb. Thrombolysis 2021, 52, 517–522. [Google Scholar] [CrossRef]
- Cochran, J.M.; Jia, X.; Kaczmarek, J.; Staggers, K.A.; Rifai, M.A.; Hamzeh, I.R.; Birnbaum, Y. Direct Oral Anticoagulants in the Treatment of Left Ventricular Thrombus: A Retrospective, Multicenter Study and Meta-Analysis of Existing Data. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 173–178. [Google Scholar] [CrossRef]
- Daher, J.; Da Costa, A.; Hilaire, C.; Ferreira, T.; Pierrard, R.; Guichard, J.B.; Romeyer, C.; Isaaz, K. Management of Left Ventricular Thrombi with Direct Oral Anticoagulants: Retrospective Comparative Study with Vitamin K Antagonists. Arch. Cardiovasc. Dis. Suppl. 2021, 13, 74. [Google Scholar] [CrossRef]
- Gama, F.; Freitas, P.; Trabulo, M.; Ferreira, A.; Andrade, M.J.; Matos, D.; Strong, C.; Ribeiras, R.; Ferreira, J.; Mendes, M. 459 Direct Oral Anticoagulants Are an Effective Therapy for Left Ventricular Thrombus Formation. Eur. Heart J. 2019, 40, ehz747.0118. [Google Scholar] [CrossRef]
- Guddeti, R.R.; Anwar, M.; Walters, R.W.; Apala, D.; Pajjuru, V.; Kousa, O.; Gujjula, N.R.; Alla, V.M. Treatment of Left Ventricular Thrombus With Direct Oral Anticoagulants: A Retrospective Observational Study. Am. J. Med. 2020, 133, 1488–1491. [Google Scholar] [CrossRef]
- Herald, J.; Goitia, J.; Duan, L.; Chen, A.; Lee, M.-S. Safety and Effectiveness of Direct Oral Anticoagulants Versus Warfarin for Treating Left Ventricular Thrombus. Am. J. Cardiovasc. Drugs 2022, 22, 437–444. [Google Scholar] [CrossRef]
- Hofer, F.; Kazem, N.; Schweitzer, R.; Horvat, P.; Winter, M.-P.; Koller, L.; Hengstenberg, C.; Sulzgruber, P.; Niessner, A. The Prognostic Impact of Left Ventricular Thrombus Resolution after Acute Coronary Syndrome and Risk Modulation via Antithrombotic Treatment Strategies. Clin. Cardiol. 2021, 44, 1692–1699. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, X.; Wang, J.; Liang, L.; Tian, P.; Chen, Y.; Zhai, M.; Huang, Y.; Zhou, Q.; Xin, A.; et al. Clinical Profile, Treatment, and Prognosis of Left Ventricular Thrombus in Dilated Cardiomyopathy. Clin. Appl. Thromb. Hemost. 2023, 29, 10760296231179683. [Google Scholar] [CrossRef]
- Iqbal, H.; Straw, S.; Craven, T.P.; Stirling, K.; Wheatcroft, S.B.; Witte, K.K. Direct Oral Anticoagulants Compared to Vitamin K Antagonist for the Management of Left Ventricular Thrombus. ESC Heart Fail. 2020, 7, 2032–2041. [Google Scholar] [CrossRef]
- Isa, W.Y.H.W.; Hwong, N.; Mohamed Yusof, A.K.; Yusof, Z.; Loong, N.S.; Wan-Arfah, N.; Naing, N.N. Apixaban versus Warfarin in Patients with Left Ventricular Thrombus: A Pilot Prospective Randomized Outcome Blinded Study Investigating Size Reduction or Resolution of Left Ventricular Thrombus. J. Clin. Prev. Cardiol. 2020, 9, 150. [Google Scholar] [CrossRef]
- Jaidka, A.; Zhu, T.; Lavi, S.; Johri, A. Treatment of Left Ventricular Thrombus Using Warfarin versus Direct Oral Anticoagulants Following Anterior Myocardial Infarction. Can. J. Cardiol. 2018, 34, S143. [Google Scholar] [CrossRef]
- Jones, D.A.; Wright, P.; Alizadeh, M.A.; Fhadil, S.; Rathod, K.S.; Guttmann, O.; Knight, C.; Timmis, A.; Baumbach, A.; Wragg, A.; et al. The Use of Novel Oral Anticoagulants Compared to Vitamin K Antagonists (Warfarin) in Patients with Left Ventricular Thrombus after Acute Myocardial Infarction. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 398–404. [Google Scholar] [CrossRef]
- Kim, S.-E.; Lee, C.J.; Oh, J.; Kang, S.-M. Factors Influencing Left Ventricular Thrombus Resolution and Its Significance on Clinical Outcomes. ESC Heart Fail. 2023, 10, 1987–1995. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Z.; Zhou, Y.; Shen, H.; Chai, M.; Ma, X.; Han, H.; Shao, Q.; Li, Q. Efficacy and Safety of Direct Oral Anticoagulants in the Treatment of Left Ventricular Thrombus After Acute Anterior Myocardial Infarction in Patients Who Underwent Percutaneous Coronary Intervention. Curr. Vasc. Pharmacol. 2022, 20, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Mihm, A.E.; Hicklin, H.E.; Cunha, A.L.; Nisly, S.A.; Davis, K.A. Direct Oral Anticoagulants versus Warfarin for the Treatment of Left Ventricular Thrombosis. Intern. Emerg. Med. 2021, 16, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Rahunathan, N.; Hurdus, B.; Straw, S.; Iqbal, H.; Witte, K.; Wheatcroft, S. Improving the Management of Left Ventricular Thrombus in a Tertiary Cardiology Centre: A Quality Improvement Project. BMJ Open Qual. 2023, 12, e002111. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, C.; Liu, B.; Benatar, J.; Stewart, R.A.H.; Somaratne, J.B. Left Ventricular Thrombus after ST Segment Elevation Myocardial Infarction: A Single-Centre Observational Study. N. Z. Med. J. 2020, 133, 45–54. [Google Scholar]
- Robinson, A.A.; Trankle, C.R.; Eubanks, G.; Schumann, C.; Thompson, P.; Wallace, R.L.; Gottiparthi, S.; Ruth, B.; Kramer, C.M.; Salerno, M.; et al. Off-Label Use of Direct Oral Anticoagulants Compared With Warfarin for Left Ventricular Thrombi. JAMA Cardiol. 2020, 5, 685. [Google Scholar] [CrossRef]
- Seiler, T.; Vasiliauskaite, E.; Grüter, D.; Young, M.; Attinger-Toller, A.; Madanchi, M.; Cioffi, G.M.; Tersalvi, G.; Müller, G.; Stämpfli, S.F.; et al. Direct Oral Anticoagulants Versus Vitamin K Antagonists for the Treatment of Left Ventricular Thrombi—Insights from a Swiss Multicenter Registry. Am. J. Cardiol. 2023, 194, 113–121. [Google Scholar] [CrossRef]
- Varwani, M.H.; Shah, J.; Ngunga, M.; Jeilan, M. Treatment and Outcomes in Patients with Left Ventricular Thrombus—Experiences from the Aga Khan University Hospital, Nairobi-Kenya. Pan Afr. Med. J. 2021, 39, 212. [Google Scholar] [CrossRef]
- Willeford, A.; Zhu, W.; Stevens, C.; Thomas, I.C. Direct Oral Anticoagulants Versus Warfarin in the Treatment of Left Ventricular Thrombus. Ann. Pharmacother. 2021, 55, 839–845. [Google Scholar] [CrossRef]
- Xu, Z.; Li, X.; Li, X.; Gao, Y.; Mi, X. Direct Oral Anticoagulants versus Vitamin K Antagonists for Patients with Left Ventricular Thrombus. Ann. Palliat. Med. 2021, 10, 9427–9434. [Google Scholar] [CrossRef]
- Zhang, Z.; Si, D.; Zhang, Q.; Qu, M.; Yu, M.; Jiang, Z.; Li, D.; Yang, P.; Zhang, W. Rivaroxaban versus Vitamin K Antagonists (Warfarin) Based on the Triple Therapy for Left Ventricular Thrombus after ST-Elevation Myocardial Infarction. Heart Vessel. 2022, 37, 374–384. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Zheng, H.; Qu, M.; Li, S.; Yang, P.; Si, D.; Zhang, W. Rivaroxaban in Heart Failure Patients with Left Ventricular Thrombus: A Retrospective Study. Front. Pharmacol. 2022, 13, 1008031. [Google Scholar] [CrossRef] [PubMed]
- Cochrane RevMan. RevMan: Systematic Review and Meta-Analysis Tool for Researchers Worldwide. Available online: https://revman.cochrane.org/info (accessed on 13 January 2024).
- ScienceDirect Topics. Mantel Haenszel Test—An Overview. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/mantel-haenszel-test (accessed on 31 March 2024).
- Abdelnabi, M.; Saleh, Y.; Fareed, A.; Nossikof, A.; Wang, L.; Morsi, M.; Eshak, N.; Abdelkarim, O.; Badran, H.; Almaghraby, A. Comparative Study of Oral Anticoagulation in Left Ventricular Thrombi (No-LVT Trial). J. Am. Coll. Cardiol. 2021, 77, 1590–1592. [Google Scholar] [CrossRef] [PubMed]
- Alcalai, R.; Butnaru, A.; Moravsky, G.; Yagel, O.; Rashad, R.; Ibrahimli, M.; Planer, D.; Amir, O.; Elbaz-Greener, G.; Leibowitz, D. Apixaban vs. Warfarin in Patients with Left Ventricular Thrombus: A Prospective Multicentre Randomized Clinical Trial. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.A.; Alrefae, M.A.; Khalil, H.H.; Abdullah, H.I.; Khalifa, Z.S.; Al Shaban, A.A.; Wali, H.A.; AlRajab, M.R.; Saleh, O.M.; Nashy, B.N. Apixaban in Patients With Post-Myocardial Infarction Left Ventricular Thrombus: A Randomized Clinical Trial. CJC Open 2023, 5, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Use of XARELTO in Ventricular Thrombus. Available online: https://www.janssenscience.com/products/xarelto/medical-content/use-of-xarelto-in-ventricular-thrombus (accessed on 15 April 2024).
- Rehan, A.; Kanwar, M.; Rosman, H.; Ahmed, S.; Ali, A.; Gardin, J.; Cohen, G. Incidence of Post Myocardial Infarction Left Ventricular Thrombus Formation in the Era of Primary Percutaneous Intervention and Glycoprotein IIb/IIIa Inhibitors. A Prospective Observational Study. Cardiovasc. Ultrasound 2006, 4, 20. [Google Scholar] [CrossRef]
- Phan, J.; Nguyen, T.; French, J.; Moses, D.; Schlaphoff, G.; Lo, S.; Juergens, C.; Dimitri, H.; Richards, D.; Thomas, L. Incidence and Predictors of Left Ventricular Thrombus Formation Following Acute ST-Segment Elevation Myocardial Infarction: A Serial Cardiac MRI Study. Int. J. Cardiol. Heart Vasc. 2019, 24, 100395. [Google Scholar] [CrossRef] [PubMed]
- Gianstefani, S.; Douiri, A.; Delithanasis, I.; Rogers, T.; Sen, A.; Kalra, S.; Charangwa, L.; Reiken, J.; Monaghan, M.; MacCarthy, P. Incidence and Predictors of Early Left Ventricular Thrombus after ST-Elevation Myocardial Infarction in the Contemporary Era of Primary Percutaneous Coronary Intervention. Am. J. Cardiol. 2014, 113, 1111–1116. [Google Scholar] [CrossRef]
- Saeedi, R.; Johns, K.; Frohlich, J.; Bennett, M.T.; Bondy, G. Lipid Lowering Efficacy and Safety of Ezetimibe Combined with Rosuvastatin Compared with Titrating Rosuvastatin Monotherapy in HIV-Positive Patients. Lipids Health Dis. 2015, 14, 57. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).