Potential Role of Phytochemicals as Glucagon-like Peptide 1 Receptor (GLP-1R) Agonists in the Treatment of Diabetes Mellitus
Abstract
:1. Introduction
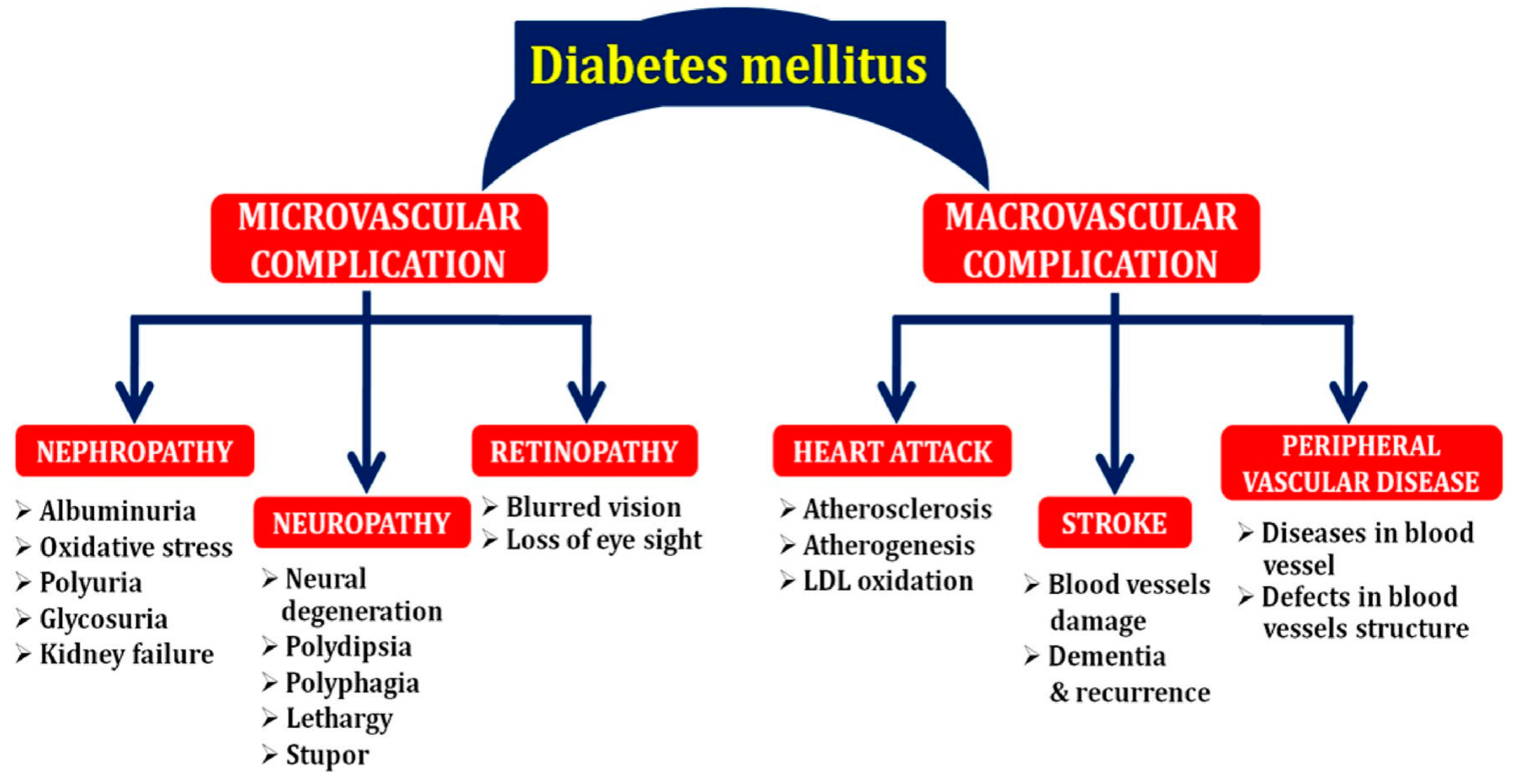
2. Complications Associated with Diabetes Mellitus (DM)
2.1. Nephropathy
2.2. Neuropathy
2.3. Cardiovascular Disease
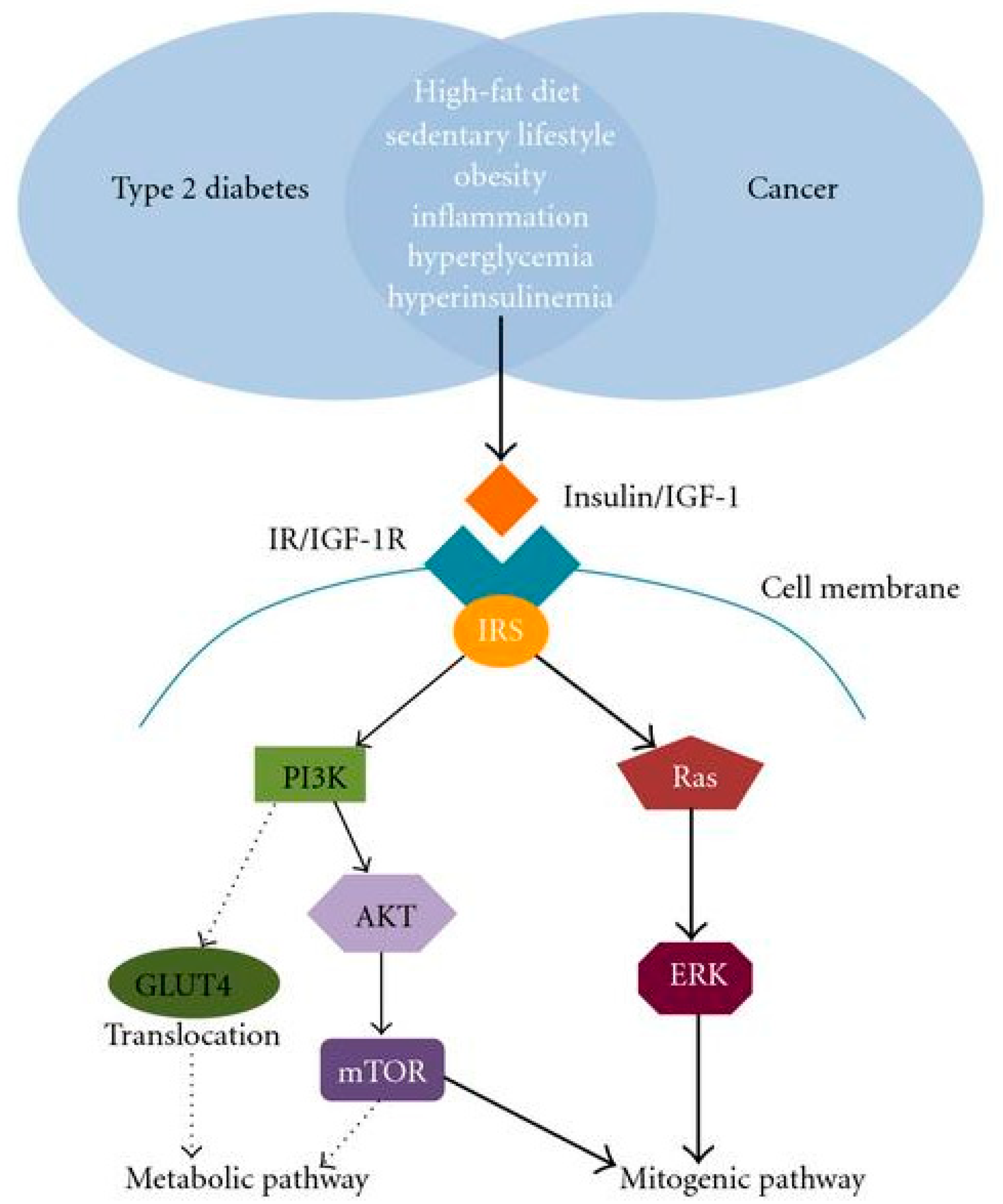
2.4. Diabetes Mellitus and Cancer
3. Management of Diabetes Mellitus by GLP-1
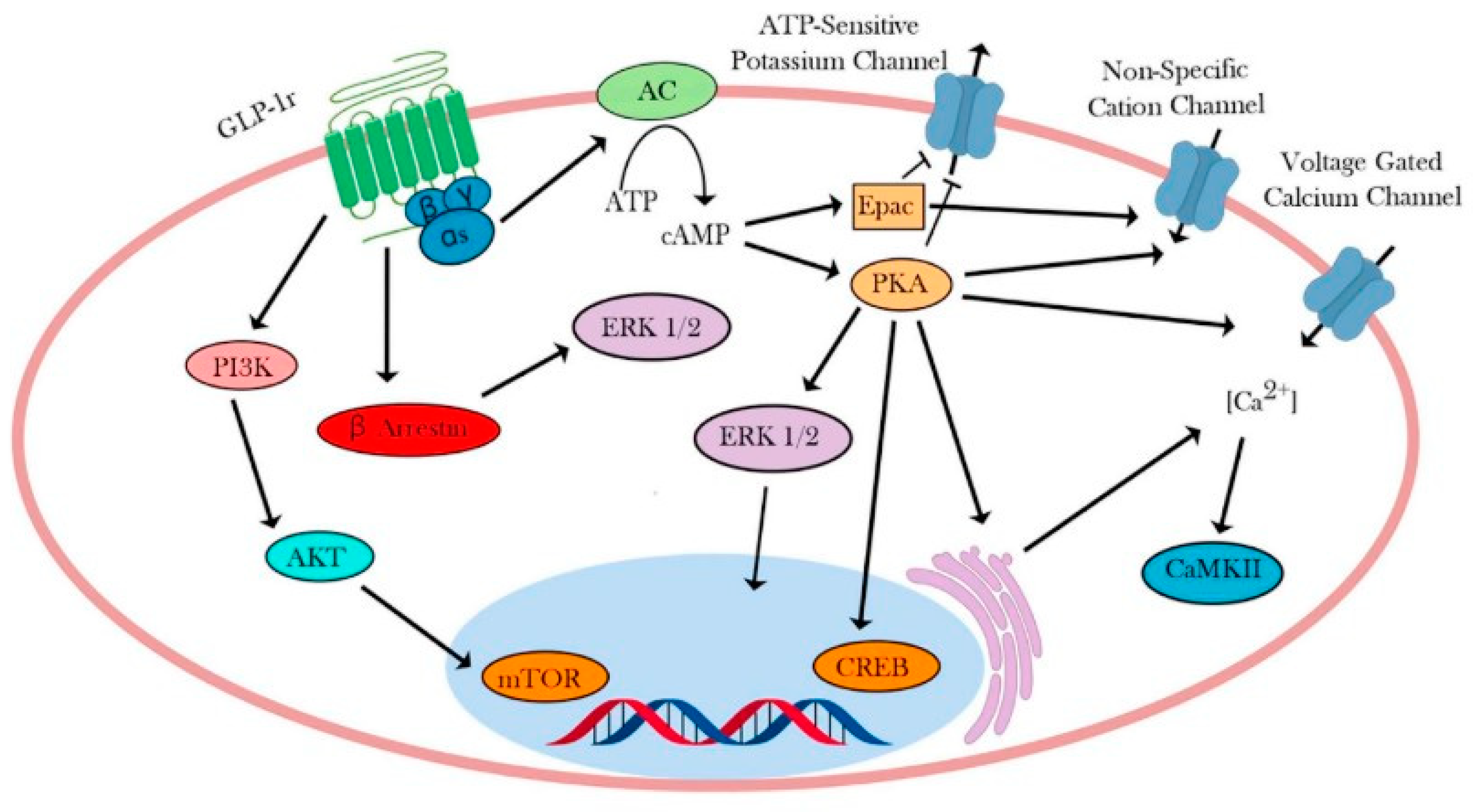
4. Intracellular Signaling Pathway of GLP-1R
5. Agonists of GLP-1R
5.1. GLP-R1 Agonists and Their Importance in Managing Diabetes
5.1.1. Brain and Heart Protection
5.1.2. Kidney Protection
5.1.3. Effect on Weight
6. Medicinal Plants
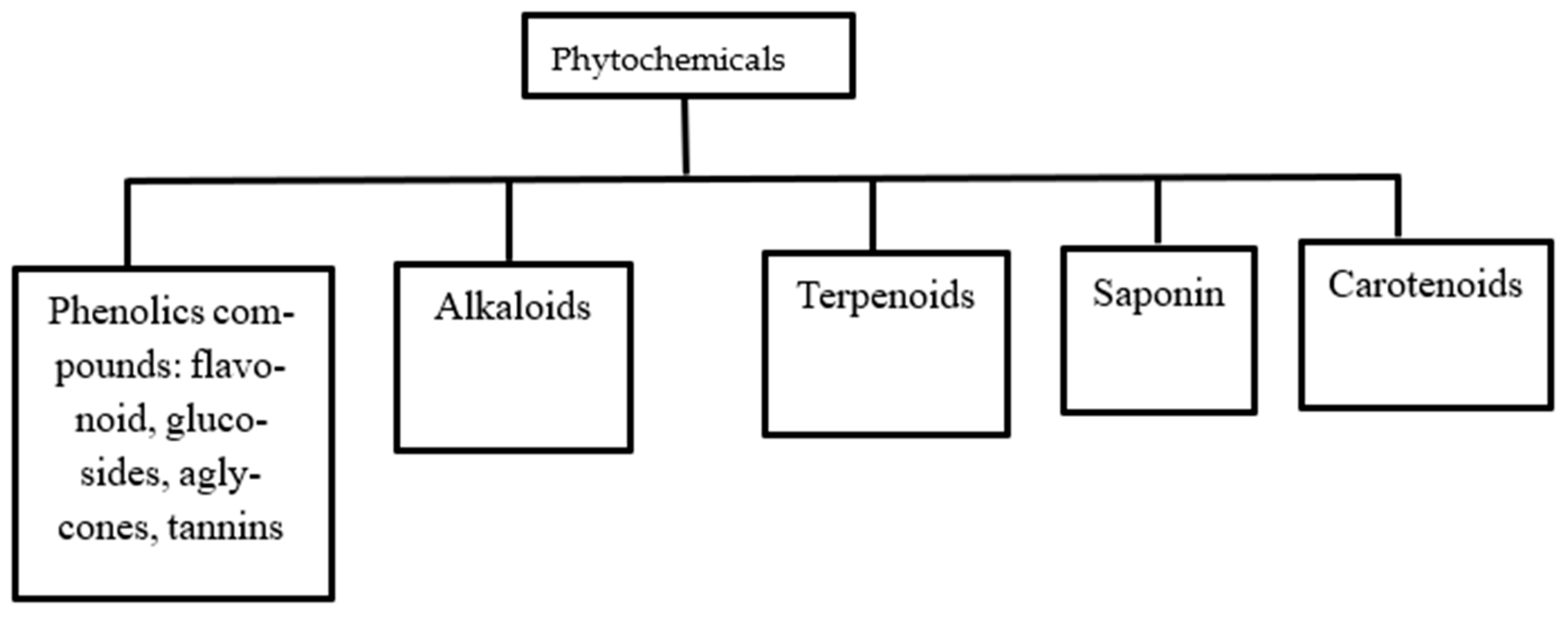
6.1. Class of Plant Phytochemicals
6.1.1. Phenolic Compounds
6.1.2. Alkaloids
6.1.3. Terpenoids
6.1.4. Saponin
6.1.5. Carotenoids
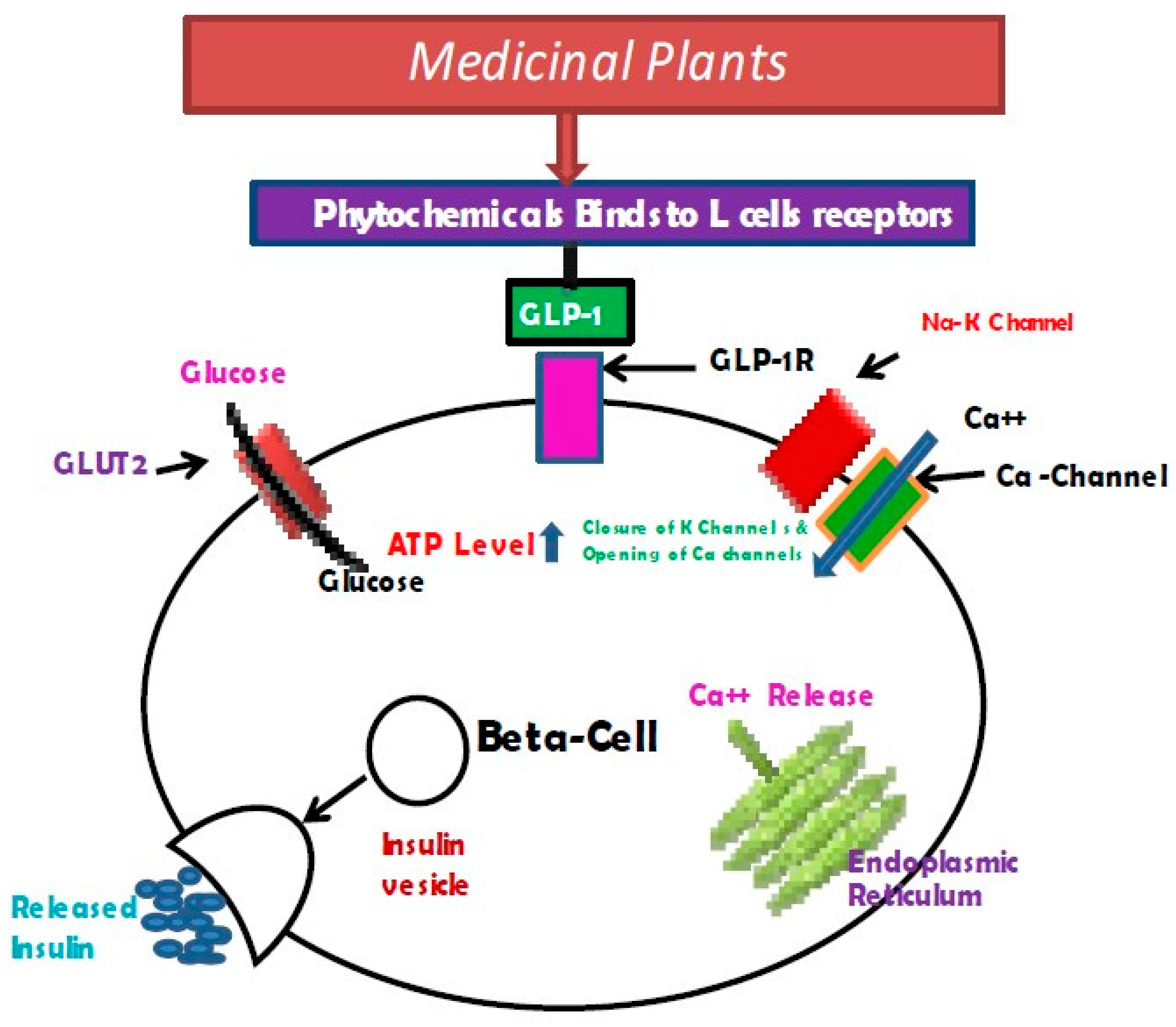
6.2. Possible GLP-1-Inducing Mechanism by Phytochemicals
6.3. Role of Medicinal Plants in Enhancing GLP-1 Level
6.4. Phytochemicals and GLP-1
6.5. Flavonoids as GLP-1R Agonists
6.6. Alkaloids as GLP-1R Agonists
Berberine
7. Survival Proteins of β-Cells Revealed by GLP-1RAs
8. Traditional Medicine and GLP-1R Agonists
8.1. Ayurvedic Antidiabetic Plants with GLP-1R Agonism
8.2. Traditional Chinese Medicine Antidiabetics and GLP-1 Receptor Agonist
8.3. African Traditional Treatments of Diabetes
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karamanou, M.; Protogerou, A.; Tsoucalas, G.; Androutsos, G.; Poulakou-Rebelakou, E. Milestones in the history of diabetes mellitus: The main contributors. World J. Diabetes 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Magliano, D.J.; Stein, C.; Basit, A.; Chan, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Uloko, A.E.; Musa, B.M.; Ramalan, M.A.; Gezawa, I.D.; Puepet, F.H.; Uloko, A.T.; Borodo, M.M.; Sada, K.B. Prevalence and risk factors for diabetes mellitus in Nigeria: A systematic review and meta-analysis. Diabetes Ther. 2018, 9, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Solis-Herrera, C.; Triplitt, C.; Reasner, C.; DeFronzo, R.A.; Cersosimo, E. Classification of Diabetes Mellitus. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2018. [Google Scholar]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Naveen, J.; Baskaran, V. Antidiabetic plant-derived nutraceuticals: A critical review. Eur. J. Nutr. 2018, 57, 1275–1299. [Google Scholar] [CrossRef] [PubMed]
- Pecoits-Filho, R.; Abensur, H.; Betônico, C.C.; Machado, A.D.; Parente, E.B.; Queiroz, M.; Salles, J.E.N.; Titan, S.; Vencio, S. Interactions between kidney disease and diabetes: Dangerous liaisons. Diabetol. Metab. Syndr. 2016, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Gronda, E.; Jessup, M.; Iacoviello, M.; Palazzuoli, A.; Napoli, C. Glucose metabolism in the kidney: Neurohormonal activation and heart failure development. J. Am. Heart Assoc. 2020, 9, e018889. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Stumvoll, M.; Nadkarni, V.; Dostou, J.; Mitrakou, A.; Gerich, J. Abnormal renal and hepatic glucose metabolism in type 2 diabetes mellitus. J. Clin. Investig. 1998, 102, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Eastman, D.M.; Dreyer, M.A. Neuropathic Ulcer. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559214/ (accessed on 10 January 2024).
- Bondar, A.; Popa, A.R.; Papanas, N.; Popoviciu, M.; Vesa, C.M.; Sabau, M.; Daina, C.; Stoica, R.A.; Katsiki, N.; Stoian, A.P. Diabetic neuropathy: A narrative review of risk factors, classification, screening and current pathogenic treatment options. Exp. Ther. Med. 2021, 22, 690. [Google Scholar] [CrossRef]
- Watkins, P.J. Progression of diabetic autonomic neuropathy. Diabet. Med. 1993, 10, 77S–78S. [Google Scholar] [CrossRef]
- Edmonds, M.; Manu, C.; Vas, P. The current burden of diabetic foot disease. J. Clin. Orthop. Trauma 2021, 17, 88–93. [Google Scholar] [CrossRef]
- Urso, B.; Ghias, M.; John, A.; Khachemoune, A. Neuropathic ulcers: A focused review. Int. J. Dermatol. 2021, 60, e383–e389. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef]
- Ma, C.X.; Ma, X.N.; Guan, C.H.; Li, Y.D.; Mauricio, D.; Fu, S.B. Cardiovascular disease in type 2 diabetes mellitus: Progress toward personalized management. Cardiovasc. Diabetol. 2022, 21, 74. [Google Scholar] [CrossRef]
- Sharma, A.; Mittal, S.; Aggarwal, R.; Chauhan, M.K. Diabetes and cardiovascular disease: Inter-relation of risk factors and treatment. Future J. Pharm. Sci. 2020, 6, 130. [Google Scholar] [CrossRef]
- Bjornsdottir, H.H.; Rawshani, A.; Rawshani, A.; Franzén, S.; Svensson, A.M.; Sattar, N.; Gudbjörnsdottir, S. A national observation study of cancer incidence and mortality risks in type 2 diabetes compared to the background population over time. Sci. Rep. 2020, 10, 17376. [Google Scholar] [CrossRef]
- Ballotari, P.; Vicentini, M.; Manicardi, V.; Gallo, M.; Chiatamone Ranieri, S.; Greci, M.; Giorgi Rossi, P. Diabetes and risk of cancer incidence: Results from a population-based cohort study in northern Italy. BMC Cancer 2017, 17, 703. [Google Scholar] [CrossRef]
- Yee, L.D.; Mortimer, J.E.; Natarajan, R.; Dietze, E.C.; Seewaldt, V.L. Metabolic health, insulin, and breast cancer: Why oncologists should care about insulin. Front. Endocrinol. 2020, 11, 58. [Google Scholar] [CrossRef]
- Haeusler, R.A.; McGraw, T.E.; Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018, 19, 31–44. [Google Scholar] [CrossRef]
- Sun, G.; Kashyap, S.R. Cancer risk in type 2 diabetes mellitus: Metabolic links and therapeutic complications. J. Nutr. Metab. 2011, 2011, 708183. [Google Scholar] [CrossRef]
- Jin, F.; Wu, Z.; Hu, X.; Zhang, J.; Gao, Z.; Han, X.; Qin, J.; Li, C.; Wang, Y. The PI3K/Akt/GSK-3β/ROS/eIF2B pathway promotes breast cancer growth and metastasis via suppression of NK cell cytotoxicity and tumor cell susceptibility. Cancer Biol. Med. 2019, 16, 38. [Google Scholar]
- Mirzaei, H.; Hamblin, M.R. Regulation of glycolysis by non-coding RNAs in cancer: Switching on the Warburg effect. Mol. Ther.-Oncolytics 2020, 19, 218–239. [Google Scholar] [CrossRef]
- Yu, H.; Zhong, X.; Gao, P.; Shi, J.; Wu, Z.; Guo, Z.; Wang, Z.; Song, Y. The potential effect of metformin on cancer: An umbrella review. Front. Endocrinol. 2019, 10, 617. [Google Scholar] [CrossRef]
- Wu, H.; Huang, D.; Zhou, H.; Sima, X.; Wu, Z.; Sun, Y.; Wang, L.; Ruan, Y.; Wu, Q.; Wu, F.; et al. Metformin: A promising drug for human cancers. Oncol. Lett. 2022, 24, 204. [Google Scholar] [CrossRef]
- Zhang, R.; Cai, X.L.; Liu, L.; Han, X.Y.; Ji, L.N. Type 1 diabetes induced by immune checkpoint inhibitors. Chin. Med. J. 2020, 133, 2595–2598. [Google Scholar] [CrossRef]
- Singh, R.; Bhat, G.A.; Sharma, P. GLP-1 secretagogues potential of medicinal plants in management of diabetes. J. Pharmacogn. Phytochem. 2015, 4, 197–202. [Google Scholar]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Medical management of hyperglycaemia in type 2 diabetes mellitus: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009, 52, 17–30. [Google Scholar] [CrossRef]
- Amori, R.E.; Lau, J.; Pittas, A.G. Efficacy and safety of incretin therapy in type 2 diabetes: Systematic review and meta-analysis. JAMA 2007, 298, 194–206. [Google Scholar] [CrossRef]
- Phung, O.J.; Scholle, J.M.; Talwar, M.; Coleman, C.I. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA 2010, 303, 1410–1418. [Google Scholar] [CrossRef]
- Pratley, R.E.; Nauck, M.; Bailey, T.; Montanya, E.; Cuddihy, R.; Filetti, S.; Thomsen, A.B.; Søndergaard, R.E.; Davies, M. Liraglutide vs sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: A 26-week, randomised, parallelgroup, open-label trial. Lancet 2010, 375, 1447–1456. [Google Scholar] [CrossRef]
- Flint, A.; Nazzal, K.; Jagielski, P.; Segel, S.; Zdravkovic, M. Influence of hepatic impairment on pharmacokinetics of the long-acting human GLP-1 analogue liraglutide. Diabetes 2007, 56, A145. [Google Scholar]
- Jacobsen, L.V.; Hindsberger, C.; Robson, R.; Zdravkovic, M. Pharmacokinetics of the long-acting human GLP-1 analogue liraglutide in subjects with renal impairment. In Proceedings of the Program and Abstracts of the American Diabetes Association 67th Sessions, Chicago, IL, USA, 22–26 June 2007; p. 513. [Google Scholar]
- Mannucci, E.; Ognibene, A.; Cremasco, F.; Bardini, G.; Mencucci, A.; Pierazzuoli, E.; Ciani, S.; Messeri, G.; Rotella, C.M. Effect of Metformin on Glucagon-Like Peptide 1 (GLP-1) and Leptin Levels in Obese Nondiabetic Subjects. Diabetes Care 2001, 24, 489–494. [Google Scholar] [CrossRef]
- Maida, A.; Lamont, B.J.; Cao, X.; Drucker, D.J. Metformin regulates the incretin receptor axis via a peroxisome proliferatoractivated receptor alpha-dependent pathway in mice. Diabetologia 2011, 54, 339–349. [Google Scholar] [CrossRef]
- Yao, X.; Chen, L.; McIntyre, M.S.; Danger, D.P.; Nystrom, C.C.; Smith, C.D.; Young, A.A. Biguanide antidiabetic agents increase fecal bile acids via inhibition of apical sodium dependent bile acid transporter. Diabetes 2010, 59, 611. [Google Scholar]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef]
- Lenhard, J.M.; Croom, D.K.; Minnick, D.T. Reduced serum dipeptidyl peptidase-IV after metformin and pioglitazone treatments. Biochem. Biophys. Res. Commun. 2004, 324, 92–97. [Google Scholar] [CrossRef]
- Lindsay, J.R.; Duffy, N.A.; McKillop, A.M.; Ardill, J.; O’Harte, F.P.M.; Flatt, P.R.; Bell, P.M. Inhibition of dipeptidyl peptidase IV activity by oral metformin in type 2 diabetes. Diabet. Med. 2005, 22, 654–657. [Google Scholar] [CrossRef]
- Kieffer, T.J.; Habener, J.F. The Glucagon-Like Peptides. Endocr. Rev. 1999, 20, 876–913. [Google Scholar] [CrossRef]
- Barnett, A. Exenatide. Expert Opin. Pharmacother. 2007, 8, 2593–2608. [Google Scholar] [CrossRef]
- Nielsen, L.L.; Young, A.A.; Parkes, D.G. Pharmacology of exenatide (synthetic exendin-4): A potential therapeutic for improved glycemic control of type 2 diabetes. Regul. Pept. 2004, 117, 77–88. [Google Scholar] [CrossRef]
- Anderson, S.L.; Trujillo, J.M. Association of pancreatitis with glucagon-like peptide-1 agonist use. Ann. Pharmacother. 2010, 44, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.K.; Hackett, T.A.; Galli, A.; Flynn, C.R. GLP-1, Molecular mechanisms and outcomes of a complex signaling system. Neurochem. Int. 2019, 128, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Philippe, J.; Mojsov, S.; Chick, W.L.; Habener, J.F. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc. Natl. Acad. Sci. USA 1987, 84, 3434–3438. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.M.; Fujita, Y.; Kieffer, T.J. Glucagon-like peptide-1, glucose homeostasis and beyond. Annu. Rev. Physiol. 2014, 76, 535–559. [Google Scholar] [CrossRef]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.E.; El-kholy, W.; Riedel, M.J.; Salapatek, A.M.F.; Light, P.E.; Wheeler, M.B. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 2002, 51, S434–S442. [Google Scholar] [CrossRef]
- Seino, S.; Shibasaki, T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol. Rev. 2005, 85, 1303–1342. [Google Scholar] [CrossRef]
- Kang, G.; Chepurny, O.G.; Malester, B.; Rindler, M.J.; Rehmann, H.; Bos, J.L.; Schwede, F.; Coetzee, W.A.; Holz, G.G. cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic β cells and rat INS-1 cells. J. Physiol. 2006, 573, 595–609. [Google Scholar] [CrossRef]
- Light, P.E.; Manning Fox, J.E.; Riedel, M.J.; Wheeler, M.B. Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A-and ADP-dependent mechanism. Mol. Endocrinol. 2002, 16, 2135–2144. [Google Scholar] [CrossRef]
- Nakazaki, M.; Crane, A.; Hu, M.; Seghers, V.; Ullrich, S.; Aguilar-Bryan, L.; Bryan, J. cAMP-activated protein kinase-independent potentiation of insulin secretion by cAMP is impaired in SUR1 null islets. Diabetes 2002, 51, 3440–3449. [Google Scholar] [CrossRef]
- Shiota, C.; Larsson, O.; Shelton, K.D.; Shiota, M.; Efanov, A.M.; Høy, M.; Lindner, J.; Kooptiwut, S.; Juntti-Berggren, L.; Gromada, J. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J. Biol. Chem. 2002, 277, 37176–37183. [Google Scholar] [CrossRef] [PubMed]
- Britsch, S.; Krippeitdrews, P.; Lang, F.; Gregor, M.; Drews, G. Glucagon-like peptide-1 modulates Ca2+ current but not K+ ATP current in intact mouse pancreatic B-cells. Biochem. Biophys. Res. Commun. 1995, 207, 33–39. [Google Scholar] [CrossRef]
- Yada, T.; Itoh, K.; Nakata, M. Glucagon-like peptide-1-(7–36) amide and a rise in cyclic adenosine 3′, 5′-monophosphate increase cytosolic free Ca2+ in rat pancreatic beta-cells by enhancing Ca2+ channel activity. Endocrinology 1993, 133, 1685–1692. [Google Scholar] [CrossRef]
- Leech, C.A.; Habener, J.F. Insulinotropic glucagon-like peptide-1-mediated activation of non-selective cation currents in insulinoma cells is mimicked by maitotoxin. J. Biol. Chem. 1997, 272, 17987–17993. [Google Scholar] [CrossRef]
- Holz, G.G.; Leech, C.A.; Habener, J.F. Activation of a cAMP-regulated Ca-Signaling Pathway in Pancreatic β-Cells by the Insulinotropic Hormone Glucagon-like Peptide-1. J. Biol. Chem. 1995, 270, 17749–17757. [Google Scholar] [CrossRef] [PubMed]
- Holz IV IV, G.G.; Kiihtreiber, W.M.; Habener, J.F. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1 (7–37). Nature 1993, 361, 362. [Google Scholar] [CrossRef]
- Beak, S.A.; Heath, M.M.; Small, C.J.; Morgan, D.G.A.; Ghatei, M.A.; Taylor, A.D.; Buckingham, J.C.; Bloom, S.R.; Smith, D.M. Glucagon-like peptide-1 stimulates luteinizing hormone-releasing hormone secretion in a rodent hypothalamic neuronal cell line. J. Clin. Investig. 1998, 101, 1334. [Google Scholar] [CrossRef]
- Hayes, M.R.; Leichner, T.M.; Zhao, S.; Lee, G.S.; Chowansky, A.; Zimmer, D.; De Jonghe, B.C.; Kanoski, S.E.; Grill, H.J.; Bence, K.K. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 2011, 13, 320–330. [Google Scholar] [CrossRef]
- Gilman, C.P.; Perry, T.; Furukawa, K.; Grieg, N.H.; Egan, J.M.; Mattson, M.P. Glucagon-like peptide 1 modulates calcium responses to glutamate and membrane depolarization in hippocampal neurons. J. Neurochem. 2003, 87, 1137–1144. [Google Scholar] [CrossRef]
- Collins, L.; Costello, R.A. Glucagon-like peptide-1 receptor agonists. In StartPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551568/ (accessed on 10 January 2024).
- Hunt, B.; Malkin, S.J.P.; Moes, R.G.J.; Huisman, E.L.; Vandebrouck, T.; Wolffenbuttel, B.H.R. Once-weekly semaglutide for patients with type 2 diabetes: A cost-effectiveness analysis in the Netherlands. BMJ Open Diabetes Res. Care 2019, 7, e000705. [Google Scholar] [CrossRef]
- Burcelin, R.; Gourdy, P. Harnessing glucagon-like peptide-1 receptor agonists for the pharmacological treatment of overweight and obesity. Obes Rev. 2017, 18, 86–98. [Google Scholar] [CrossRef]
- Gourgari, E.; Wilhelm, E.E.; Hassanzadeh, H.; Aroda, V.R.; Shoulson, I. A comprehensive review of the FDA-approved labels of diabetes drugs: Indications, safety, and emerging cardiovascular safety data. J. Diabetes Complicat. 2017, 31, 1719–1727. [Google Scholar] [CrossRef]
- Janzen, K.M.; Steuber, T.D.; Nisly, S.A. GLP-1 Agonists in Type 1 Diabetes Mellitus. Ann. Pharmacother. 2016, 50, 656–665. [Google Scholar] [CrossRef]
- Sanford, M. Dulaglutide: First global approval. Drugs 2014, 74, 2097–2103. [Google Scholar] [CrossRef]
- Hinnen, D. Glucagon-Like Peptide 1 Receptor Agonists for Type 2 Diabetes. Diabetes Spectr. 2017, 30, 202–210. [Google Scholar] [CrossRef]
- Madsbad, S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes. Metab. 2016, 18, 317–332. [Google Scholar] [CrossRef]
- Ku, H.C.; Chen, W.P.; Su, M.J. DPP4 deficiency exerts protective effect against H2O2 induced oxidative stress in isolated cardiomyocytes. PLoS ONE 2013, 8, e54518. [Google Scholar] [CrossRef]
- Barale, C.; Buracco, S.; Cavalot, F.; Frascaroli, C.; Guerrasio, A.; Russo, I. Glucagon-like peptide 1-related peptides increase nitric oxide effects to reduce platelet activation. Thromb. Haemost. 2017, 117, 1115–1128. [Google Scholar] [CrossRef]
- Tang, S.T.; Tang, H.Q.; Su, H.; Wang, Y.; Zhou, Q.; Zhang, Q.; Wang, Y.; Zhu, H. Glucagon-like peptide-1 attenuates endothelial barrier injury in diabetes via cAMP/PKA mediated down-regulation of MLC phosphorylation. Biomed. Pharmacother. 2019, 113, 108667. [Google Scholar] [CrossRef]
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE−/− and LDLr−/− mice by a mechanism that includes inflammatory pathways. JACC Basic Transl. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef]
- Secher, A.; Jelsing, J.; Baquero, A.F.; Hecksher-Sørensen, J.; Cowley, M.A.; Dalbøge, L.S.; Hansen, G.; Grove, K.L.; Pyke, C.; Raun, K.; et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Investig. 2014, 124, 4473–4488. [Google Scholar] [CrossRef]
- Kadouh, H.; Chedid, V.; Halawi, H.; Burton, D.D.; Clark, M.M.; Khemani, D.; Vella, A.; Acosta, A.; Camilleri, M. GLP-1 analog modulates appetite, taste preference, gut hormones, and regional body fat stores in adults with obesity. J. Clin. Endocrinol. Metab. 2020, 105, 1552–1563. [Google Scholar] [CrossRef]
- Schlögl, H.; Kabisch, S.; Horstmann, A.; Lohmann, G.; Müller, K.; Lepsien, J.; Busse-Voigt, F.; Kratzsch, J.; Pleger, B.; Villringer, A.; et al. Exenatide-induced reduction in energy intake is associated with increase in hypothalamic connectivity. Diabetes Care 2013, 36, 1933–1940. [Google Scholar] [CrossRef]
- Haj-Zaroubi, M.; Mattar, N.; Awabdeh, S.; Sweidan, R.; Markovics, A.; Klein, J.D.; Azaizeh, H. Willow (Salix acmophylla Boiss.) Leaf and Branch Extracts Inhibit In Vitro Sporulation of Coccidia (Eimeria spp.) from Goats. Agriculture 2024, 14, 648. [Google Scholar] [CrossRef]
- Carmona, F.; Pereira AM, S. Herbal medicines: Old and new concepts, truths and misunderstandings. Rev. Bras. Farmacogn. 2013, 23, 379–385. [Google Scholar] [CrossRef]
- Rabizadeh, F.; Mirian, M.S.; Doosti, R.; Kiani-Anbouhi, R.; Eftekhari, E. Phytochemical Classification of Medicinal Plants Used in the Treatment of Kidney Disease Based on Traditional Persian Medicine. Evid.-Based Complement. Altern. Med. 2022, 2022, 8022599. [Google Scholar] [CrossRef]
- Ahmed, M.N. Medicinal plant-based functional foods for the management of neurological health. Preprints 2020, 2020060311. [Google Scholar] [CrossRef]
- AlAli, M.; Alqubaisy, M.; Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.S.; Lim, S.H.E. Nutraceuticals: Transformation of conventional foods into health promoters/disease preventers and safety considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef]
- Oz, A.T.; Kafkas, E. Phytochemicals in fruits and vegetables. In Superfood and Functional Food; Waisundara, V., Ed.; IntechOpen: London, UK, 2017; pp. 175–184. [Google Scholar]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From theory to practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 283. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Şahin, T.Ö.; Yılmaz, B.; Ekenci, K.D.; Duyar Özer, Ş.; Capasso, R. Cruciferous vegetables and their bioactive metabolites: From prevention to novel therapies of colorectal cancer. Evid.-Based Complement. Altern. Med. 2022, 2022, 1534083. [Google Scholar] [CrossRef]
- Koche, D.; Shirsat, R.; Kawale, M.V. An overerview of major classes of phytochemicals: Their types and role in disease prevention. Hislopia J. 2016, 9, 1–11. [Google Scholar]
- Mani, V.; Park, S.; Kim, J.A.; Lee, S.I.; Lee, K. Metabolic perturbation and synthetic biology strategies for plant terpenoid production—An updated overview. Plants 2021, 10, 2179. [Google Scholar] [CrossRef]
- Hussain, M.; Debnath, B.; Qasim, M.; Bamisile, B.S.; Islam, W.; Hameed, M.S.; Wang, L.; Qiu, D. Role of saponins in plant defense against specialist herbivores. Molecules 2019, 24, 2067. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Kibe, M.N.; Konyole, S.; Nguka, G.; Oloo, M.O.; Kathure, D.; Wangari, P.M. The role of phytochemicals in prevention and control of chronic diseases. Int. J. Curr. Res. 2017, 9, 62540–62543. [Google Scholar]
- Hosseini, S.A.; Zand, H.; Cheraghpour, M. The influence of curcumin on the downregulation of MYC, insulin and IGF-1 receptors: A possible mechanism underlying the anti-growth and anti-migration in chemoresistant colorectal cancer cells. Medicina 2019, 55, 90. [Google Scholar] [CrossRef]
- Banik, U.; Parasuraman, S.; Adhikary, A.K.; Othman, N.H. Curcumin: The spicy modulator of breast carcinogenesis. J. Exp. Clin. Cancer Res. 2017, 36, 98. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin attenuates oxidative stress in RAW264. 7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar]
- Shin, S.A.; Joo, B.J.; Lee, J.S.; Ryu, G.; Han, M.; Kim, W.Y.; Park, H.H.; Lee, J.H.; Lee, C.S. Phytochemicals as anti-inflammatory agents in animal models of prevalent inflammatory diseases. Molecules 2020, 25, 5932. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Kan, L.; Wu, L.; Zhu, Y.; Wang, Q. Effect of baicalin on renal function in patients with diabetic nephropathy and its therapeutic mechanism. Exp. Ther. Med. 2019, 17, 2071–2076. [Google Scholar] [CrossRef]
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethanopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.K.; Doble, M. Mechanism of Action of Natural Products Used in the Treatment of Diabetes Mellitus. Chin. J. Int. Med. 2011, 17, 563–574. [Google Scholar] [CrossRef]
- Hussein, G.M.; Matsuda, H.; Nakamura, S.; Hamao, M.; Akiyama, T.; Tamura, K.; Yoshikawa, M. Mate tea (Ilex paraguariensis) promotes satiety and body weight lowering in mice: Involvement of glucagon-like peptide-1. Biol. Pharm. Bull. 2011, 34, 1849–1855. [Google Scholar] [CrossRef]
- Akawa, A.B.; Oyinloye, B.E.; Ajiboye, B.O. Computer-aided Identification of Bioactive Compounds from Brachystegia eurycoma with Therapeutic Potential against Drug Targets of Type 2 Diabetes mellitus. Biointerface Res. Appl. Chem. 2022, 13, 454. [Google Scholar]
- Ajiboye, B.O.; Iwaloye, O.; Owolabi, O.V.; Ejeje, J.N.; Okerewa, A.; Johnson, O.O.; Udebor, A.E.; Oyinloye, B.E. Screening of potential antidiabetic phytochemicals from Gongronema latifolium leaf against therapeutic targets of type 2 diabetes mellitus: Multi-targets drug design. SN Appl. Sci. 2022, 4, 14. [Google Scholar] [CrossRef]
- Liu, J.; Yin, F.; Xiao, H.; Guo, L.; Gao, X. Glucagon-like peptide 1 receptor plays an essential role in geniposide attenuating lipotoxicity-induced β-cell apoptosis. Toxicol. Vitr. 2012, 26, 1093–1097. [Google Scholar] [CrossRef]
- Hlebowicz, J.; Hlebowicz, A.; Lindstedt, S.; Björgell, O.; Höglund, P.; Holst, J.J.; Darwiche, G.; Almer, L.O. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. Am. J. Clin. Nutr. 2009, 89, 815–821. [Google Scholar] [CrossRef]
- Ribnicky, D.M.; Poulev, A.; Watford, M.; Cefalu, W.T.; Raskin, I. Antihyperglycemic activity of Tarralin™, an ethanolic extract of Artemisia dracunculus L. Phytomedicine 2006, 3, 550–557. [Google Scholar] [CrossRef]
- Park, S.; Ahn, I.S.; Kim, J.H.; Lee, M.R.; Kim, J.S.; Kim, H.J. Glyceollins, one of the phytoalexins derived from soybeans under fungal stress, enhance insulin sensitivity and exert insulinotropic actions. J. Agric. Food Chem. 2010, 58, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Urías-Silvas, J.E.; Cani, P.D.; Delmée, E.; Neyrinck, A.; López, M.G.; Delzenne, N.M. Physiological effects of dietary fructans extracted from Agave tequilana Gto. and Dasylirion spp. Br. J. Nutr. 2008, 99, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.C.; Honoré, S.M.; Genta, S.B.; Sánchez, S.S. Hypolipidemic effect of Smallanthus sonchifolius (yacon) roots on diabetic rats. Chem. Biol. Interact. 2011, 194, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Suleman, K.Y. The Effects of a Kenyan Antidiabetic Plant on Insulin Homeostasis. Ph.D. Dissertation, Nelson Mandela Metropolitan University, Port Elizabeth, South Africa, 2009. Chapter IV: Insulin release. [Google Scholar]
- Huang, T.; Lu, K.N.; Pai, Y.P.; Hsu, C.; Huang, C.J. Role of GLP1 in the Hypoglycemic Effects of Wild Bitter Gourd. Evid.-Based Complement. Altern. Med. 2013, 2013, 625892. [Google Scholar] [CrossRef] [PubMed]
- Freeland, K.R.; Wilson, C.; Wolever, T.M. Adaptation of colonic fermentation and glucagon-like peptide-1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br. J. Nutr. 2010, 103, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Yogisha, S.; Raveesha, K.A. Dipeptidyl Peptidase IV inhibitory activity of Mangifera indica. J. Nat. Prod. 2010, 3, 76–79. [Google Scholar]
- Pasman, W.J.; Heimerikx, J.; Rubingh, C.M.; van den Berg, R.; O’Shea, M.; Gambelli, L.; Hendriks, H.F.J.; Einerhand, A.W.C.; Scott, C.; Keizer, H.G.; et al. The effect of Korean pine nut oil on in vitro CCK release, on appetite sensations and on gut hormones in post-menopausal overweight women. Lipids Health Dis. 2008, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Tartagni, E. Antidiabetic properties of berberine: From cellular pharmacology to clinical effects. Hosp. Pract. 2012, 40, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, J.; Ruan, H.; Pi, H.; Wu, J. Antihyperglycemic Activity of Kinsenoside, a High Yielding Constituent from Anoectochilus roxburghii in Streptozotocin Diabetic Rats. J. Ethnopharmacol. 2007, 114, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Maity, T.K.; Singh, J. Antihyperglycemic activity of bacosine, a triterpene from Bacopa monnieri, in alloxan-induced diabetic rats. Planta Medica 2011, 77, 804–808. [Google Scholar] [CrossRef]
- Potdar, D.; Hirwani, R.R.; Dhulap, S. Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia 2012, 83, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Ruhil, S.; Balhara, M.; Dhankhar, S.; Chhillar, A.K. Aegle marmelos (Linn.) Correa: A potential source of Phytomedicine. J. Med. Plant Res. 2011, 5, 1497–1507. [Google Scholar]
- Samad, M.B.; Mohsin, M.N.A.B.; Razu, B.A.; Hossain, M.T.; Mahzabeen, S.; Unnoor, N.; Muna, I.A.; Akhter, F.; Kabir, A.U.; Hannan, J.M.A. [6]-Gingerol, from Zingiber officinale, potentiates GLP-1 mediated glucose-stimulated insulin secretion pathway in pancreatic β-cells and increases RAB8/RAB10-regulated membrane presentation of GLUT4 transporters in skeletal muscle to improve hyperglycemia in Leprdb/db type 2 diabetic mice. BMC Complement. Altern. Med. 2017, 17, 395. [Google Scholar]
- Naik, S.R.; Barbosa Filho, J.M.; Dhuley, J.N.; Deshmukh, V. Probable Mechanism of Hypoglycemic Activity of Bassic Acid, a Natural Product Isolated from Bumelia sartorum. J. Ethnopharmacol. 1991, 33, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Benhaddou-Andaloussi, A.; Martineau, L.C.; Spoor, D.; Vuong, T.; Leduc, C.; Joly, E.; Burt, A.; Meddah, B.; Settaf, A.; Arnason, J.T.; et al. Antidiabetic activity of Nigella sativa. Seed extract in cultured pancreatic β-cells, skeletal muscle cells, and adipocytes. Pharm. Biol. 2008, 46, 96–104. [Google Scholar] [CrossRef]
- Takikawa, M.; Kurimoto, Y.; Tsuda, T. Curcumin stimulates glucagon-like peptide- 1 secretion in GLUTag cells via Ca2+/calmodulin-dependent kinase II activation. Biochem. Biophys. Res. Commun. 2013, 435, 165–170. [Google Scholar] [CrossRef]
- Tsoukalas, M.; Muller, C.D.; Lobstein, A.; Urbain, A. Pregnane glycosides from Cynanchum marnierianum stimulate GLP-1 secretion in STC-1 cells. Planta Medica 2016, 82, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Kartinah, N.T.; Fadilah, F.; Ibrahim, E.I.; Suryati, Y. The potential of Hibiscus sabdariffa Linn in inducing glucagon-like peptide-1 via SGLT-1 and GLPR in DM Rats. BioMed Res. Int. 2019, 2019, 8724824. [Google Scholar] [CrossRef] [PubMed]
- Dans, A.M.L.; Villarruz, M.V.C.; Jimeno, C.A.; Javelosa, M.A.U.; Chua, J.; Bautista, R.; Velez, G.G.B. The effect of Momordica charantia capsule preparation on glycemic control in type 2 diabetes mellitus needs further studies. J. Clin. Epidemiol. 2007, 60, 554–559. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, A.; AlKhalidy, H.; Luo, J.; Moomaw, E.; Neilson, A.P.; Liu, D. Flavone Hispidulin Stimulates Glucagon-Like Peptide-1 Secretion and Ameliorates Hyperglycemia in Streptozotocin-Induced Diabetic Mice. Mol. Nutr. Food Res. 2020, 64, e1900978. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Hu, Q.; Xiao, X.; Ou, L.; Chen, Y.; Luo, S.; Cheng, Y.; Jiang, Y.; Ma, X.; et al. The emerging possibility of the use of geniposide in the treatment of cerebral diseases: A review. Chin. Med. 2021, 16, 86. [Google Scholar] [CrossRef]
- Kato, M.; Tani, T.; Terahara, N.; Tsuda, T. The Anthocyanin Delphinidin 3-Rutinoside Stimulates Glucagon-Like Peptide-1 Secretion in Murine GLUTag Cell Line via the Ca2+/Calmodulin-Dependent Kinase II Pathway. PLoS ONE 2015, 10, e0126157. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Yang, H.J.; Lee, I.-S.; Kim, K.-H.; Park, J.; Jeong, H.-S.; Kim, Y.; Ahn, K.S.; Na, Y.-C.; Jang, H.-J. The aglycone of ginsenoside Rg3 enables glucagon-like peptide-1 secretion in enteroendocrine cells and alleviates hyperglycemia in type 2 diabetic mice. Sci. Rep. 2015, 5, 18325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.-T.; Yin, Y.-C.; Xing, S.; Li, W.-N.; Fu, X.-Q. Hypoglycemic effect and mechanism of isoquercitrin as an inhibitor of dipeptidyl peptidase-4 in type 2 diabetic mice. RSC Adv. 2018, 8, 14967–14974. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-Y.; Aihara, Y.; Hashimoto, T.; Kanazawa, K.; Mizuno, M. (−)-Epigallocatechin-3-gallate induces secretion of anorexigenic gut hormones. J. Clin. Biochem. Nutr. 2015, 57, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Nishikawa, S.; Ikehata, A.; Dochi, K.; Tani, T.; Takahashi, T.; Imaizumi, A.; Tsuda, T. Curcumin improves glucose tolerance via stimulation of glucagon-like peptide-1 secretion. Mol. Nutr. Food Res. 2017, 61, 1600471. [Google Scholar] [CrossRef] [PubMed]
- Kalivarathan, J.; Kalaivanan, K.; Chandrasekaran, S.P.; Nanda, D.; Ramachandran, V.; Venkatraman, A.C. Apigenin modulates hippocampal CREB-BDNF signaling in high fat, high fructose diet-fed rats. J. Funct. Foods 2020, 68, 103898. [Google Scholar] [CrossRef]
- Dao, T.M.; Waget, A.; Klopp, P.; Serino, M.; Vachoux, C.; Pechere, L.; Drucker, D.J.; Champion, S.; Barthélemy, S.; Barra, Y.; et al. Resveratrol Increases Glucose Induced GLP-1 Secretion in Mice: A Mechanism which Contributes to the Glycemic Control. PLoS ONE 2011, 6, e20700. [Google Scholar] [CrossRef]
- Casanova-Martí, À.; Serrano, J.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. Acute selective bioactivity of grape seed proanthocyanidins on enteroendocrine secretions in the gastrointestinal tract. Food Nutr. Res. 2017, 61, 1321347. [Google Scholar] [CrossRef]
- Rehman, K.; Ali, M.B.; Akash, M.S.H. Genistein enhances the secretion of glucagon-like peptide-1 (GLP-1) via downregulation of inflammatory responses. Biomed. Pharmacother. 2019, 112, 108670. [Google Scholar] [CrossRef]
- González-Abuín, N.; Martínez-Micaelo, N.; Blay, M.; Ardévol, A.; Pinent, M. Grape-Seed Procyanidins Prevent the Cafeteria-DietInduced Decrease of Glucagon-Like Peptide-1 Production. J. Agric. Food Chem. 2014, 62, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, N.; Sadashivaiah, B.; Ramaprasad, T.R.; Singh, S.A. Anti-hyperglycemic activity of myricetin, through inhibition of DPP-4 and enhanced GLP-1 levels, is attenuated by co-ingestion with lectin-rich protein. PLoS ONE 2020, 15, e0231543. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.-Y.; Choi, M.-S. Luteolin Targets the Toll-Like Receptor Signaling Pathway in Prevention of Hepatic and Adipocyte Fibrosis and Insulin Resistance in Diet-Induced Obese Mice. Nutrients 2018, 10, 1415. [Google Scholar] [CrossRef] [PubMed]
- Araj-Khodaei, M.; Ayati, M.H.; Zeinalhajlou, A.A.; Novinbahador, T.; Yousefi, M.; Shiri, M.; Mahmoodpoor, A.; Shamekh, A.; Namazi, N.; Sanaie, S. Berberine-induced glucagon-like peptide-1 and its mechanism for controlling types 2 diabetes mellitus: A comprehensive pathway review. Arch. Physiol. Biochem. 2023, 3, 1–8. [Google Scholar]
- Yusta, B.; Baggio, L.L.; Estall, J.L.; Koehler, J.A.; Holland, D.P.; Li, H.; Pipeleers, D.; Ling, Z.; Drucker, D.J. GLP-1 Receptor Activation Improves Beta Cell Function and Survival Following Induction of Endoplasmic Reticulum Stress. Cell Metab. 2006, 4, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Jun, H.-S. Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling. Int. J. Mol. Sci. 2017, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Zummo, F.P.; Cullen, K.S.; Honkanen-Scott, M.; Shaw, J.A.M.; Lovat, P.E.; Arden, C. Glucagon-Like Peptide 1 Protects Pancreatic β-Cells from Death by Increasing Autophagic Flux and Restoring Lysosomal Function. Diabetes 2017, 66, 1272–1285. [Google Scholar] [CrossRef]
- Ezanno, H.; Pawlowski, V.; Abdelli, S.; Boutry, R.; Gmyr, V.; Kerr-Conte, J.; Bonny, C.; Pattou, F.; Abderrahmani, A. JNK3 Is Required for the Cytoprotective Effect of Exendin 4. J. Diabetes Res. 2014, 2014, 814854. [Google Scholar] [CrossRef]
- Camaya, I.; Donnelly, S.; O’Brien, B. Targeting the PI3K/Akt Signaling Pathway in Pancreatic Β-cells to Enhance Their Survival and Function: An Emerging Therapeutic Strategy for Type 1 Diabetes. J. Diabetes 2022, 14, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Quoyer, J.; Longuet, C.; Broca, C.; Linck, N.; Costes, S.; Varin, E.; Bockaert, J.; Bertrand, G.; Dalle, S. GLP-1 Mediates Antiapoptotic Effect by Phosphorylating Bad through a Beta-Arrestin 1-Mediated ERK1/2 Activation in Pancreatic Beta-Cells. J. Biol. Chem. 2010, 285, 1989–2002. [Google Scholar] [CrossRef]
- Lee, W.-Y. New Potential Targets of Glucagon-Like Peptide 1 Receptor Agonists in Pancreatic β-Cells and Hepatocytes. Endocrinol. Metab. 2017, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Kono, T.; Anderson-Baucum, E.K.; Yamamoto, W.; Gilon, P.; Lebeche, D.; Day, R.N.; Shull, G.E.; Evans-Molina, C. SERCA2 Deficiency Impairs Pancreatic β-Cell Function in Response to Diet-Induced Obesity. Diabetes. 2016, 65, 3039–3052. [Google Scholar] [CrossRef] [PubMed]
- Jhala, U.S.; Canettieri, G.; Screaton, R.A.; Kulkarni, R.N.; Krajewski, S.; Reed, J.; Walker, J.; Lin, X.; White, M.; Montminy, M. CAMP Promotes Pancreatic Beta-Cell Survival via CREB-Mediated Induction of IRS2. Genes. Dev. 2003, 17, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, M.; Plaisance, V.; Boutry, R.; Pawlowski, V.; Jacovetti, C.; Sanchez-Parra, C.; Ezanno, H.; Bourry, J.; Beeler, N.; Pasquetti, G.; et al. The Map3k12 (Dlk)/JNK3 Signaling Pathway Is Required for Pancreatic Beta-Cell Proliferation during Postnatal Development. Cell. Mol. Life Sci. 2021, 78, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Malgaonkar, M.; Shirolkar, A.; Murthy, S.N.; Pawar, S. Ayurvedic Plants with Antidiabetic Potential. In Medicinal Plants-Recent. Advances in Research and Development; Springer: Singapore, 2016; pp. 439–468. [Google Scholar]
- Tzeng, T.F.; Liou, S.S.; Liu, I.M. The selected traditional chinese medicinal formulas for treating diabetic nephropathy: Perspective of modern science. J. Tradit. Complement. Med. 2013, 3, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wu, H.Y.; Liu, H.; Gong, N.; Wang, Y.R.; Wang, Y.X. Morroniside, a secoiridoid glycoside from Cornus officinalis, attenuates neuropathic pain by activation of spinal glucagon-like peptide-1 receptors. Br. J. Pharmacol. 2017, 174, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Noh, J.S.; Kim, J.H.; Tanaka, T.; Zhao, Q.; Matsumoto, K.; Shibahara, N.; Yokozawa, T. Evaluation of morroniside, iridoid glycoside from Corni Fructus, on diabetes-induced alterations such as oxidative stress, inflammation, and apoptosis in the liver of type 2 diabetic db/db mice. Biol. Pharm. Bull. 2011, 34, 1559–1565. [Google Scholar] [CrossRef]
- Tang, H.C.; Chen, C.Y.C. Design of Glucagon-like peptide-1 receptor agonist for diabetes mellitus from traditional Chinese medicine. Evid.-Based Complement. Altern. Med. 2014, 2014, 385120. [Google Scholar] [CrossRef]
- Hu, D.; Gao, J.; Yang, X.; Liang, Y. A Comprehensive Mini-Review of Curcumae Radix: Ethnopharmacology, Phytochemistry, and Pharmacology. Nat. Prod. Commun. 2021, 16, 1934578X211020628. [Google Scholar] [CrossRef]
- Han, L.K.; Zheng, Y.N.; Yoshikawa, M.; Okuda, H.; Kimura, Y. Anti-obesity effects of chikusetsusaponins isolated from Panax japonicus rhizomes. BMC Complement. Altern. Med. 2005, 5, 9. [Google Scholar] [CrossRef]
- Mohammed, A.; Tajuddeen, N. Antidiabetic compounds from medicinal plants traditionally used for the treatment of diabetes in Africa: A review update (2015–2020). S. Afr. J. Bot. 2022, 146, 585–602. [Google Scholar] [CrossRef]
| Common Name | Used Part | Phytochemicals | Mechanism | References |
|---|---|---|---|---|
| Gardenia | Fruit | Geniposide | Geniposide has been shown to protect against neuronal apoptosis induced by oxidative stress and enhance glucose-stimulated insulin secretion via activation of the glucagon-like peptide 1 receptor (GLP-1R) in INS-1 cells. | Liu et al. [103] |
| Cinnamon tree | Bark | Cinnamon | Consuming 3 g of cinnamon resulted in decreased postprandial serum insulin levels and increased concentrations of GLP-1, with no significant impact on blood glucose levels. | Hlebowicz et al. [104] |
| Mate tea | Leaves | Mate-saponin 2, matesaponin, 3,5-O-dicaffeoyl-D-quinic acid | The acute administration of key components of mate led to notable GLP-1 increased levels. Specifically, compounds such as 3,5-O-dicaffeoyl-D-quinic acid and matesaponin 2, along with alpha-linolenic acid, demonstrated significant enhancements in GLP-1 levels. | Hussein et al. [100] |
| Little dragon | Leaves | Tarralin | The extract demonstrated enhancement in the binding of glucagon-like peptide (GLP-1) to its receptor in in vitro studies. | Ribnicky et al. [105] |
| Soybean | Roots | Glyceollins | Glyceollins were found to enhance GLP-1 secretion, thereby amplifying insulinotropic effects in enteroendocrine cells. | Park et al. [106] |
| Agave | Roots | Agave fructans | Agave fructans has been shown to increase GLP-1 levels and enhance the concentration of its precursors. | Urias-Silvas et al. [107] |
| Yacon | Roots | Fructooligosaccharides | Diabetic rats treated with a yacon flour-supplemented diet exhibited a significant increase in glucagon-like peptide-1 (GLP-1) content compared to diabetic control rats. | Habib et al. [108] |
| Pygeum | Bark | This plant is concluded to enhance insulin secretion by reducing DPP-4 activity, thereby prolonging the half-life of GLP-1. | Suleiman [109] | |
| Bitter melon | Fruit | Karavilagenine E | Mice that received a single oral dose of WES for 30 min exhibited higher serum levels of GLP-1 and insulin, along with lower glucose levels. This suggests that WES stimulates GLP-1 secretion in vivo. | Huang et al. [110] |
| Wheat | Fibers | Consuming more wheat fiber over an extended period leads to an eventual increase in the production of short-chain fatty acids (SCFA) and the secretion of glucagon-like peptide-1 (GLP-1). | Freeland et al. [111] | |
| Mango | Leaves | Mangifera indica inhibits DPP-4 and enhances GLP-1 levels in individuals with type 2 diabetes mellitus (T2DM). | Yogisha and Raveesha [112] | |
| Korean pine | Seed | Triglyceride and free fatty acids | GLP-1 levels were observed to be higher 60 min after the introduction of pine nuts. | Pasman et al. [113] |
| Barberry | Roots, rhizomes | Berberine | The antidiabetic effect of berberine is attributed to its ability to increase insulin secretion, promote glycolysis, and elevate levels of glucose transporter-4 (GLUT-4) and glucagon-like peptide-1 (GLP-1). | Cicero and Tartagni [114] |
| Plants | Activities | Methods | Name of Compound | References | |
|---|---|---|---|---|---|
| 1. | Anoectochilus roxburghii | In vivo | Restoration of damaged β cells in pancreas | Kinsenoside | Zhang et al. [115] |
| 2. | Bacopa monnieri (L.) Wettst. | In vivo | Consumption of peripheral glucose and protection against oxidative damage | Bacosine | Ghosh et al. [116] |
| 3. | Berberis aristata | In vivo | Regulates glucose homeostasis by reducing gluconeogenesis and oxidative stress | Berberine | Potdar et al. [117] |
| 4. | Berberis vulgaris | In vivo | Increases insulin secretion and stimulates glycolysis | Berberine | Cicero and Tartagni [114] |
| 5. | Gardenia jasminoides J. Ellis | Phosphorylation of Akt and FOXO1 in INS-1 cells | Geniposide | Liu et al. [103] | |
| 6. | Artemisia dracunculus L. | In vitro | Lessens the secretion of glucagon | Tarralin | Ribnicky et al. [105] |
| 7. | Aegle marmelos Correa | In vivo | Stimulates insulin secretion from β cells | Coumarins | Ruhil et al. [118] |
| 8. | Zingiber officinale Roscoe | In vivo | Enhanced ability to withstand glucose and support the release of insulin induced by glucose | 6-gingerol | Samad et al., [119] |
| 9. | Bumelia sartorum Mart. | In vivo | Increase in glucose uptake and glycogen synthesis. Increase in the amount of insulin secreted by pancreatic beta-cells | Bassic acid | Naik et al. [120] |
| 10 | Nigella sativa L. | In vivo | Potential stimulation in pancreatic β-cells causing insulin secretion, reduced hepatic gluconeogenesis | Thymoquinone, Dithymoquinone | Benhaddou-Andaloussi et al. [121] |
| 11 | Agave tequilana F.A.C. Weber | In vivo | Improved lipid glucose metabolism by inducing proglucagon | Fructans | Urias-Silvas et al. [107] |
| 12 | Panax ginseng C.A. Mey | In vitro and in vivo | Upregulation of proglucagon gene expression and glucose induced GLP-1 | Saponins and Ginsenoside | Liu et al. [103] |
| 13 | Cinnamomum verum J. Presl. | Increased blood GLP-1 concentration | Cinnamon | Hlebowicz et al. [104] | |
| 14 | Curcuma longa L. | In vitro | Increased GLP-1 secretion | Curcumin | Takikawa et al. [122] |
| 15 | Cynanchum marnierianum Rauh | In vitro | Stimulates GLP-1 secretion | Pregnane glycoside | Tsoukalas et al. [123] |
| 16 | Glycine max (L.) | In vitro | Dose-dependent increase in GLP-1 secretion | Glyceollins | Park et al. [106] |
| 17 | Hibiscus sabdariffa L. | Elevation of GLP-1 in ileum and pancreas | Delphinidin | Kartinah et al. [124] | |
| 18 | Hoodia gordonii (Masson) | In vivo | Induces the release of GLP-1 via GPR119 | Gordonoside F | Zhang et al. [91] |
| 19 | Momordica charantia | In vivo | Induces plasma GLP-1 and stimulates insulin release | Cucurbiracin | Dans et al. [125] |
| 20 | Rheum palmatum L. | In vivo and in vitro | Increased plasma GLP-1 secretion | Emodin | Wang et al. [126] |
| Compound | Models | Effect | Reference |
|---|---|---|---|
| Curcumin | Rats | Elevated GLP-1 in plasma, elevated tolerance to glucose | Kato et al. [132] |
| Epigallocatechin-3-gallate | Murine ileal tissue and caco-2 cells | Elevated secretion of GLP-1 | Song et al. [131] |
| Delphinidin 3-rutinoside | GLUTag cells | Elevated secretion of GLP-1 | Kato et al. [128] |
| Apigenin | High-fructose and -fat diet rats | Elevated GLP-1 in plasma | Kalivarathan et al. [133] |
| Curcumin | GLUTag cells | Elevated secretion of GLP-1 | Takikawa et al. [122] |
| Resveratrol | High-fat diet mice | Elevated GLP-1 in plasma, elevated tolerance to glucose | Dao et al. [134] |
| Hispidulin | STZ-treated mice | Elevated GLP-1 in plasma, elevated tolerance to glucose | Wang et al. [126] |
| Gallic acid | Ileal segment of rat | Elevated secretion of GLP-1 | Casanova-Marti et al. [135] |
| Genistein-metformin | Alloxan-induced diabetic rats | Elevated intestinal and serum GLP-1 | Rehman et al. [136] |
| Isoquercitrin | High fat diet; streptozotocin-administered rats | Elevated plasma glucose and GLP-1; reduced plasma DPP-4 | Zhang et al. [130] |
| Procyanidin | Cafeteria-diet rats | Elevated intestinal GLP-1 | Gonzalez-Abuin et al. [137] |
| Ginsenoside metabolite | NCI-H716 cells | Elevated secretion of GLP-1; elevated plasma GLP-1; elevated tolerance to glucose | Kim et al. [129] |
| Myricetin | streptozotocin-administered rats; high-fat diet rats | Elevated plasma GLP-1; reduced tissue and plasma DPP-4 | Lalitha et al. [138] |
| Luteolin | High-fat diet mice | Elevated GLP-1 in plasma, elevated tolerance to glucose | Kwon and Choi [139] |
| Name of Protein | Role of Protein | Reference |
|---|---|---|
| Protein kinase B | AKT, a serine/threonine kinase, exerts its effects by activating CREB, PDX1, and the mammalian target of rapamycin (mTOR) complex 1. Additionally, it inhibits glycogen synthase kinase 3 (GSK3β), caspase-9, FoxO1, and the Bcl-2-associated death promoter (Bad) | Camaya et al. [145] |
| ERK1/2 | The Ras-dependent extracellular signal-regulated kinase 1 (ERK1)/2 mitogen-activated protein (MAP) kinase pathway plays a role in regulating cell survival. | Quoyer et al. [146] |
| MAPK10/JNK3 | Anti-apoptotic mechanisms involving unidentified targets are present. JNK3 is regulated by MAP8IP1/JIP-1/IB1 | Ezanno et al. [144] |
| SERCA2b | A P-type ATPase that regulates endoplasmic reticulum (ER) Ca2+ stores is responsible for maintaining calcium ion levels within the ER | Lee [147]; Tong et al. [148] |
| CREB | A transcription factor that enhances the expression of insulin receptor substrate 2 (IRS2), which is essential for IGF-1 and insulin receptor signaling, ultimately resulting in AKT activation. | Jhala et al. [149] |
| MAK8IP1 also called Islet Brain 1/JIP1 | MAPK8IP1 functions as a scaffold protein that anchors MAP3K/MAP2K/JNK in the anti-apoptotic JNK signaling pathway. This scaffold protein plays a crucial role in coordinating and facilitating the signaling cascade to prevent apoptosis. | Tenenbaum et al. [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abiola, J.O.; Oluyemi, A.A.; Idowu, O.T.; Oyinloye, O.M.; Ubah, C.S.; Owolabi, O.V.; Somade, O.T.; Onikanni, S.A.; Ajiboye, B.O.; Osunsanmi, F.O.; et al. Potential Role of Phytochemicals as Glucagon-like Peptide 1 Receptor (GLP-1R) Agonists in the Treatment of Diabetes Mellitus. Pharmaceuticals 2024, 17, 736. https://doi.org/10.3390/ph17060736
Abiola JO, Oluyemi AA, Idowu OT, Oyinloye OM, Ubah CS, Owolabi OV, Somade OT, Onikanni SA, Ajiboye BO, Osunsanmi FO, et al. Potential Role of Phytochemicals as Glucagon-like Peptide 1 Receptor (GLP-1R) Agonists in the Treatment of Diabetes Mellitus. Pharmaceuticals. 2024; 17(6):736. https://doi.org/10.3390/ph17060736
Chicago/Turabian StyleAbiola, Julianah Ore, Ayoola Abidemi Oluyemi, Olajumoke Tolulope Idowu, Oluwatoyin Mary Oyinloye, Chukwudi Sunday Ubah, Olutunmise Victoria Owolabi, Oluwatobi T. Somade, Sunday Amos Onikanni, Basiru Olaitan Ajiboye, Foluso Oluwagbemiga Osunsanmi, and et al. 2024. "Potential Role of Phytochemicals as Glucagon-like Peptide 1 Receptor (GLP-1R) Agonists in the Treatment of Diabetes Mellitus" Pharmaceuticals 17, no. 6: 736. https://doi.org/10.3390/ph17060736
APA StyleAbiola, J. O., Oluyemi, A. A., Idowu, O. T., Oyinloye, O. M., Ubah, C. S., Owolabi, O. V., Somade, O. T., Onikanni, S. A., Ajiboye, B. O., Osunsanmi, F. O., Nash, O., Omotuyi, O. I., & Oyinloye, B. E. (2024). Potential Role of Phytochemicals as Glucagon-like Peptide 1 Receptor (GLP-1R) Agonists in the Treatment of Diabetes Mellitus. Pharmaceuticals, 17(6), 736. https://doi.org/10.3390/ph17060736





