Abstract
Azorella compacta (A. compacta) is a shrub of the Andean Altiplano of Bolivia, Chile and Peru, consumed by local communities as a traditional medicine for several maladies such as diabetes, hepatic and inflammatory diseases. A. compacta is rich in mulinane- and azorellane-type diterpenoids. For two of these, acute hypoglycemic effects have been described, but the impact of A. compacta diterpenoids on fatty liver disease has not been investigated. Therefore, A. compacta organic fractions were prepared using petroleum ether, dichloromethane and methanol. Their content was characterized by UHPLC/MS, revealing the presence of ten diterpenoids, mainly mulinic acid, azorellanol and mulin-11,13-diene. Next, mice fed with a high-fat diet (HFD), a model of metabolic dysfunction-associated fatty liver disease (MAFLD), received one of the fractions in drinking water for two weeks. After this treatment, hepatic parameters were evaluated. The A. compacta fractions did not reduce hyperglycemia or body weight in the HFD-fed mice but increased the serum levels of hepatic transaminases (AST and ALT), reduced albumin and increased bilirubin, indicating hepatic damage, while histopathological alterations such as steatosis, inflammation and necrosis generated by the HFD were, overall, not ameliorated by the fractions. These results suggest that organic A. compacta extracts may generate hepatic complications in patients with MAFLD.
1. Introduction
Azorella compacta (Phil. Apiaceae) is a native shrub that grows in the Andean region of the north part of Chile, south Peru, Bolivia and north Argentina at high altitudes (above 4000 mt above sea level) [1]. It is a cushion plant which presents green woody mounds, and it is able to survive in geographical conditions where very few other plants grow [2]. Known by the locals as “yareta”, Azorella compacta (A. compacta) is used in this part of the Andean Altiplano (highlands) as a medicinal plant for the treatment of inflammatory diseases, diabetes and renal and hepatic conditions, among others [3]. It is consumed as an infusion made from its roots and resins for topical use [4]. Currently, A. compacta is sold as a herbal medicine over the rest of the continent as a dry plant.
A. compacta is rich in terpenoids, particularly diterpenoids of the types mulinane and azorellane [5,6]. Mulinanes present a tricyclic skeleton of 21 carbon atoms [6,7,8], while azorellanes have a tetracyclic structure of 20 carbon atoms [9,10]. To date, 27 diterpenoids from A. compacta have been isolated and identified: 11 mulinanes and 6 azorellanes [10].
Several biological activities have been attributed to these A. compacta-derived secondary metabolites. For instance, mulinanes and azorellanes exhibit antimicrobial and cytotoxic activity [10]. At a systemic level, molecules that belong to these families of terpenoids have been reported to show anti-ulcer and anti-inflammatory activities, consistent with the use of A. compacta in folk medicine [10]. Interestingly, mulinolic acid and azorellanol isolated from A. compacta presented anti-diabetic activity when evaluated acutely in rats [11,12]. Nevertheless, neither these compounds nor A. compacta extracts have been tested as a chronic treatment.
Non-alcoholic fatty liver disease (NAFLD) is a complex syndrome characterized by triglyceride accumulation in hepatocytes (steatosis), developed in the absence of excessive alcohol consumption [13]. NAFLD is the hepatic manifestation of metabolic syndrome, which includes hyperglycemia, obesity, hypertension and dyslipidemia [14]. Hence, it is usually observed in a range of conditions that include overweight and obesity. Recently, an international panel of experts modified the NAFLD nomenclature to “metabolic dysfunction-associated fatty liver disease” (MAFLD), incorporating a set of medical/biochemical criteria to enable an easier diagnosis [15]. The main criteria for NAFLD/MALFLD are a hepatic steatosis of ≥5% hepatocytes without current liver disease and two metabolic risks (metabolic deregulatory factors) such as increased waist circumference, high blood pressure, increased plasma triglycerides, HDL and insulin resistance, among others [16]. On the other hand, type 2 diabetes mellitus (T2DM) and/or overweight/obesity are classically involved in liver fat deposition and have been associated with disease progression and hepatic and extra-hepatic complications [15]. MAFLD affects approximately 30% of adults in the general population and up to 70% of patients with T2DM [17]. It includes a spectrum of progressive conditions, such as simple steatosis (a benign and reversible liver disorder), steatohepatitis (NASH), varied severity degrees of fibrosis and eventually cirrhosis and hepatocellular carcinoma [13,18].
Currently, MAFLD is a condition that has no established treatment. The most suitable approaches to reducing steatosis include lifestyle changes and drug therapy as a second strategy [19]. Treatments such as lifestyle modifications and body weight loss associated with bariatric surgery show modest benefits, and the adverse effects of the treatments complicate their use [14]. There are studies based on natural supplements and derivatives indicating that chronic liver disease complications could be reversed and improved by these products [20,21,22,23]. Interestingly, terpenoids, which are abundant in A. compacta, have been proposed for the treatment of NAFLD [22]. Therefore, here, we aimed to evaluate the impact of the administration of A. compacta in a chronic manner to mice fed a high-fat diet as a model of metabolic disease.
2. Results
2.1. Metabolomic Characterization of Azorella compacta Extracts
Methanol, dichloromethane and petroleum ether fractions of A. compacta were characterized. For this, aliquots of each fraction were subjected to Ultrahigh-Performance Liquid Chromatography (UHPLC)–mass spectrometry identification. With this methodology, a total of ten diterpenoids were identified (Table 1 and Figure 1). In addition, four different secondary metabolites were identified: 5.7-dihydroxychromone (11), biochanin A (12), azelaic acid (13) and homoorientin (14).

Table 1.
Identification of compounds present in organic extracts of Azorella compacta by UHLPC/MS.
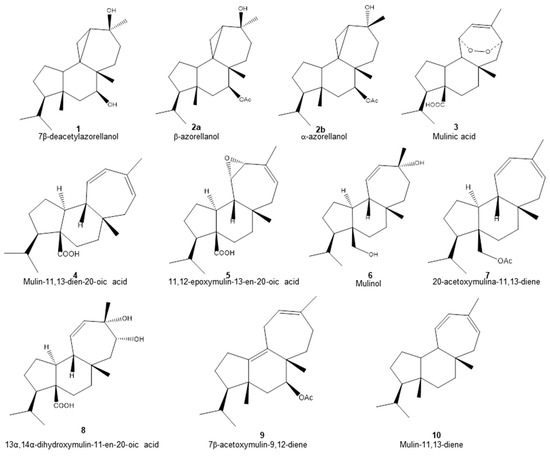
Figure 1.
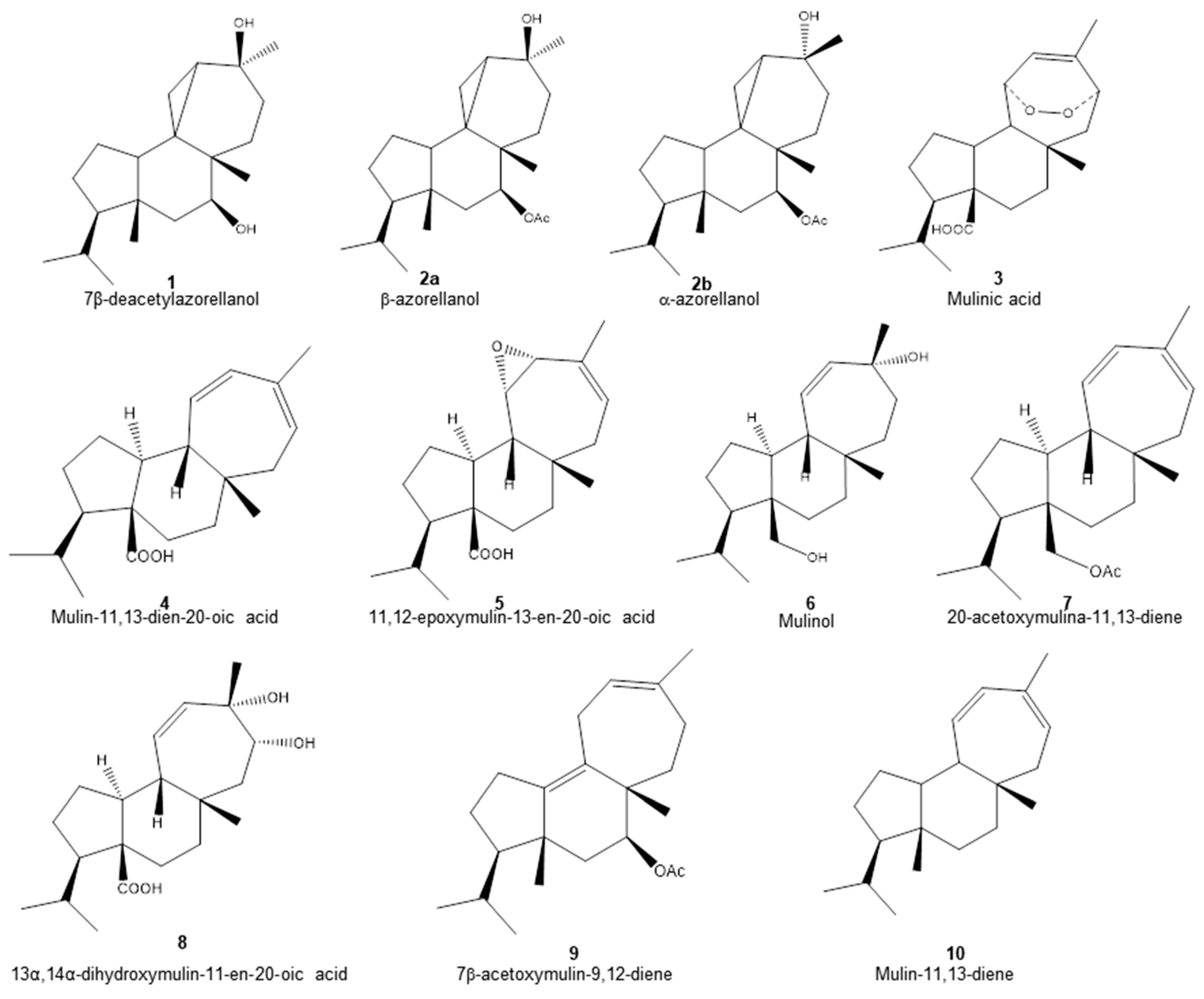
Chemical structure of diterpenoids identified in the organic fractions obtained from Azorella compacta by UHPLC-MS.
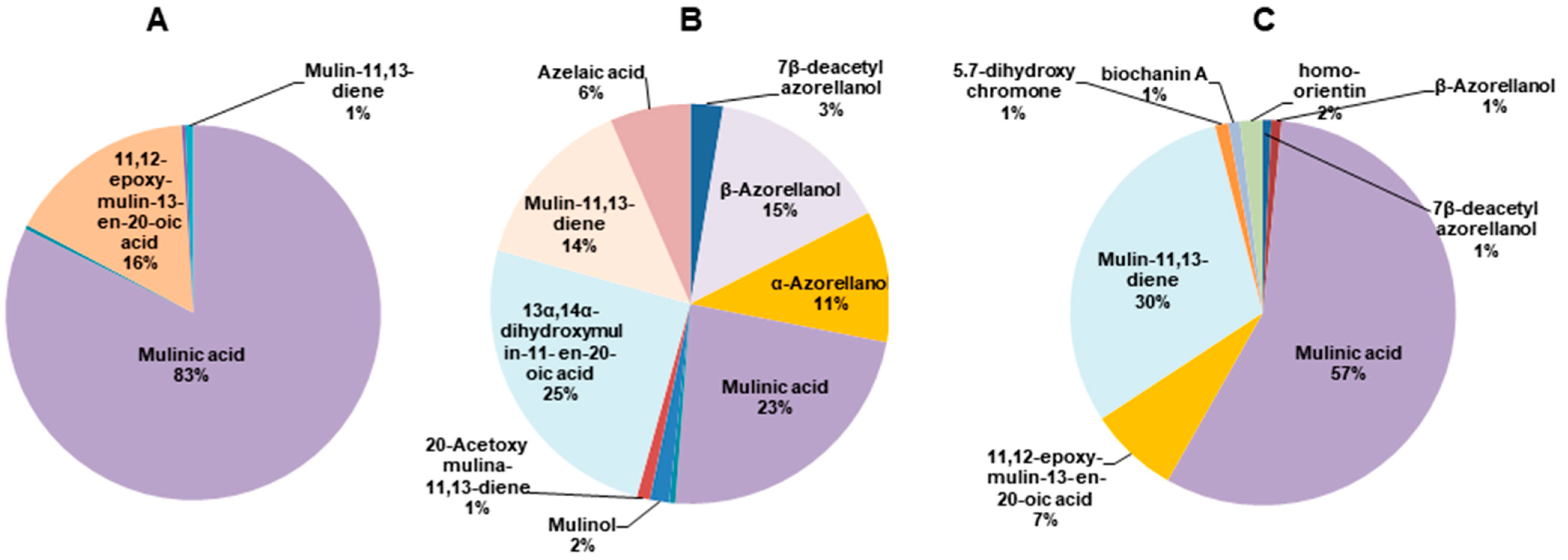
The structures of the diterpenoids identified are shown in Figure 1. Of these, compounds 1, 2a and 2b correspond to azorellanes, and compounds 3–10 are mulinane-type diterpenoids. The relative abundance of each fraction is shown in Figure 2. In the petroleum ether fraction, mulinic acid (3) and 11,12-epoxymulin-13-en-20-oic acid (5) dominated. The content of the dichloromethane fraction was more heterogeneous (nine compounds). It had high concentrations of azorellanes (1–3), mainly α and β azorellanol, but also included a significant amount of mulinic acid (23%), while the methanol fraction (the most polar) was also dominated by mulinic acid (57%), but also contained 30% mulin 11,13 diene (10).
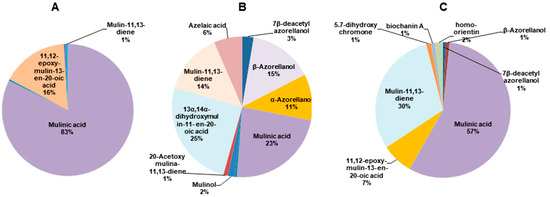
Figure 2.
Pie charts of the relative abundance of diterpenoids identified by UHPLC-MS in organic fractions obtained from Azorella compacta. (A) Petroleum ether fraction; (B) dichloromethane fraction and (C) methanol fraction from Azorella compacta. Compounds with less than 1% abundance in each fraction are not shown.
2.2. Impact of Azorella compacta Organic Fractions on Mice Fed a High-Fat Diet
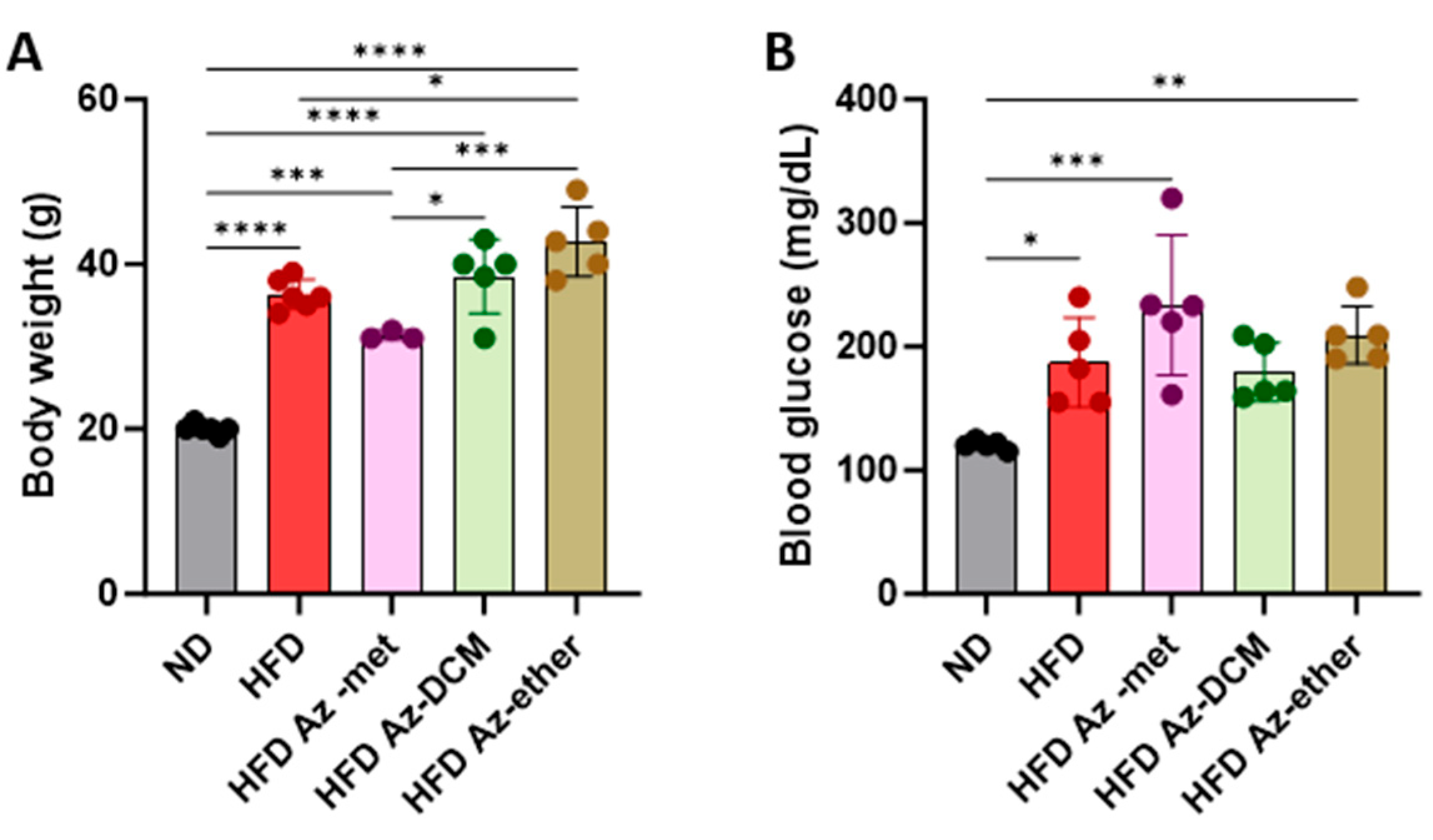
Next, the impact of the three organic extracts from A. compacta was analyzed in a model of metabolic syndrome since A. compacta compounds have been described to reduce hyperglycemia in diabetic rats. For this purpose, mice were fed a high-fat diet for eight weeks. Then, hyperglycemic mice received one of the A. compacta fractions, HFD Az-met (methanol extract), HFD Az-DCM (dichloromethane extract), and HFD Az-ether, (petroleum ether extract) or a vehicle in drinking water for two weeks while maintaining the high-fat diet regime. During this period, none of the animals that received the extracts died. As shown in Figure 3, at the end of the protocol, the HFD-fed mice presented an increase in body weight (Figure 3A) and blood glucose (Figure 3B). The HFD Az-DCM- and HFD Az-met-treated mice did not present changes in body weight compared to the HFD control animals, while the Az-ether group had increased body weight by 17% compared to the HFD control (36.3 g to 42.7 g, p = 0.0132). A similar situation was observed for the analysis of glycemia (Figure 3B). None of the extracts reduced the hyperglycemia observed in the HFD mice: 187.4 ± 36.1, 233.5 ± 56.8, 179.6 ± 23.9 and 209.5 ± 23.5 mg/dL for the HFD-, Az-met-, Az-DCM- and Az-ether-treated groups, respectively. Notably, the mice that received Az-met presented an increase in blood glucose of 93% and 24% compared to the animals that received the normal and HFD diets, respectively.
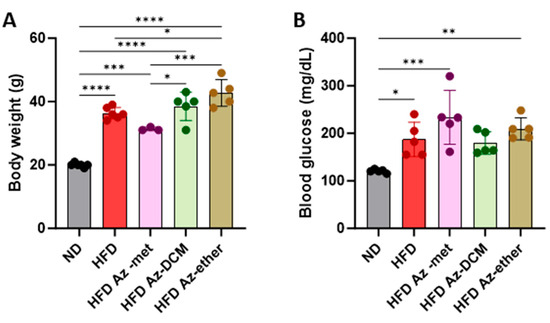
Figure 3.
Body weight (A) and blood glucose (B) levels in animals treated with Azorella compacta organic extracts. ND, normal diet; HFD, high-fat diet; HFD Az-met, HFD-fed mice treated with the methanol fraction of A. compacta; HFD Az-DCM, HFD-fed mice treated with the dichloromethane fraction of A. compacta; and HFD Az-ether, HFD-fed mice treated with the petroleum ether fraction of A. compacta. * p < 0. 05; ** p < 0.005; *** p < 0.0005; **** p < 0.0001; n = 5 each group.
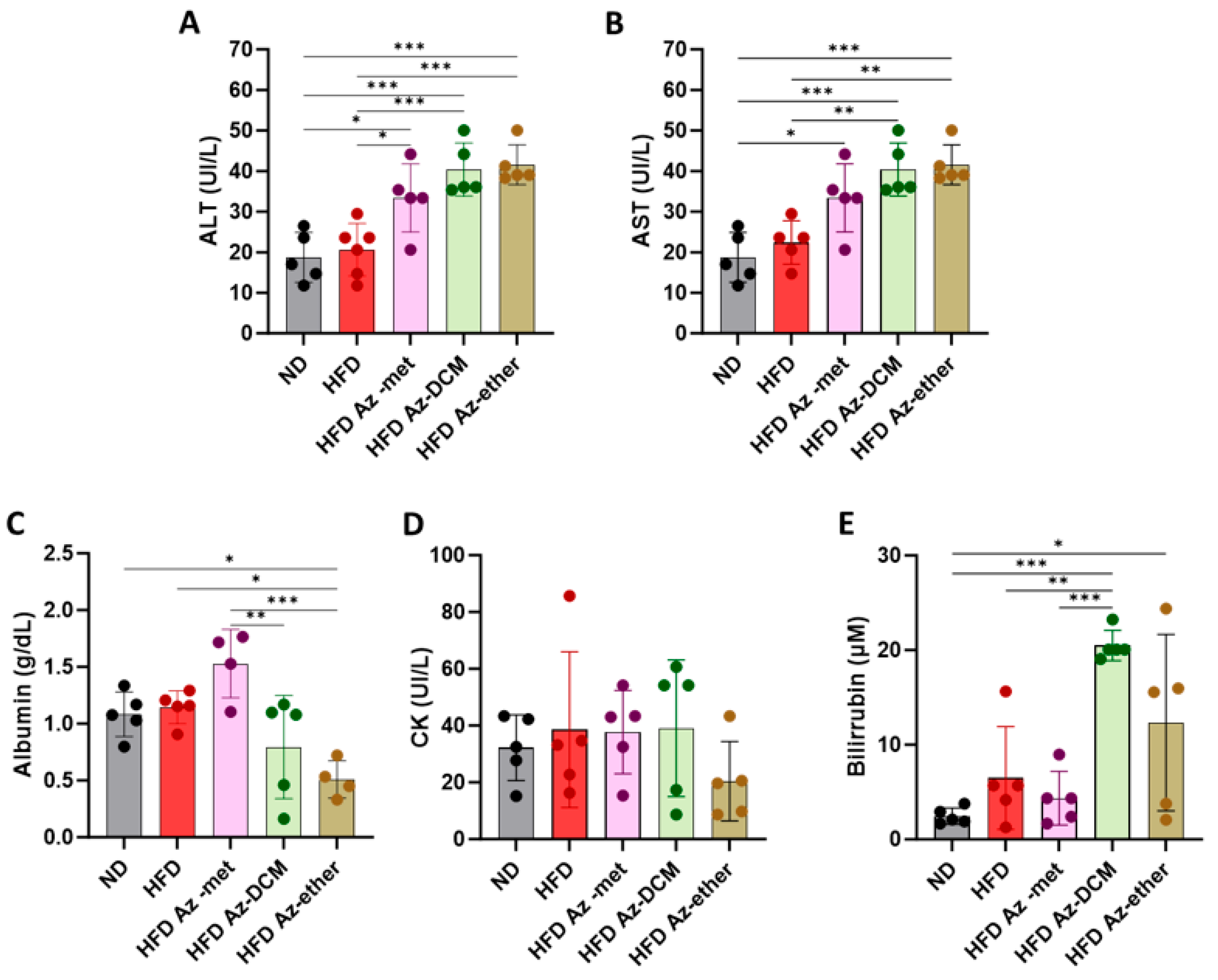
Next, hepatic biochemical parameters were analyzed in the serum of the treated animals (Figure 4). The enzymatic activity of hepatic transaminases ALT and AST was augmented in the serum of the animals that received any of the fractions, with respect to the control and HFD-alone-treated mice (Figure 4A,B). Regarding other parameters of liver function, albumin was decreased in the Az-ether group by 55 and 44.5% with respect to the control and HFD alone groups, respectively (Figure 4C). Serum creatine kinase (CK) did not present statistical differences among the groups, and all levels remained within normal laboratory ranges, suggesting no impact on the heart or skeletal muscle (Figure 4D). Total bilirubin levels presented significant increases in the serum of all the groups treated with the A. compacta organic fractions (Figure 4E). The averages observed for total bilirubin (µM) were 2.5 ± 0.9, 6.5 ± 5.4, 4.3 ± 2.8, 20.5 ± 1.6 and 12.3 ± 9.3 for the control, HFD, Az-met, Az-DCM and Az-ether groups, respectively. The highest levels were observed in the Az-DCM and Az-ether groups, with an enhancement of 4.7 and 2.8 times with respect to the HFD alone group.
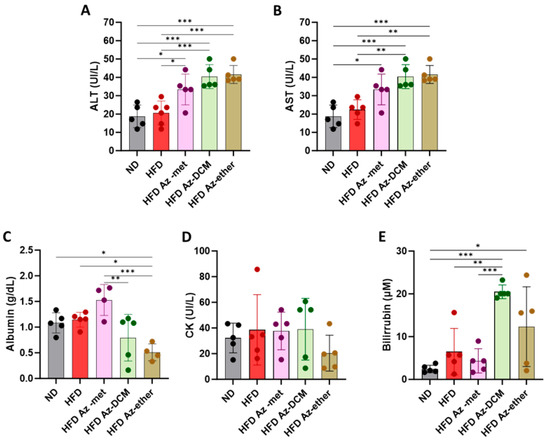
Figure 4.
Biochemical parameters of hepatic damage in the serum of mice treated with Azorella compacta (A. compacta) organic extracts. (A,B) Serum levels of hepatic enzymes: ALT, alanine aminotransferase (A); AST, aspartate aminotransferase (B). (C) Serum levels of albumin. (D) Serum levels of CK, creatine kinase. (E) Serum levels of bilirubin. ND, normal diet; HFD, high-fat diet; HFD Az-met, HFD-fed mice treated with the methanol fraction of A. compacta; HFD Az-DCM, HFD-fed mice treated with the dichloromethane fraction of A. compacta; and HFD Az-ether, HFD-fed mice treated with the petroleum ether fraction of A. compacta. * p < 0.05; ** p < 0.001; *** p < 0.0005; n = 5 each group.
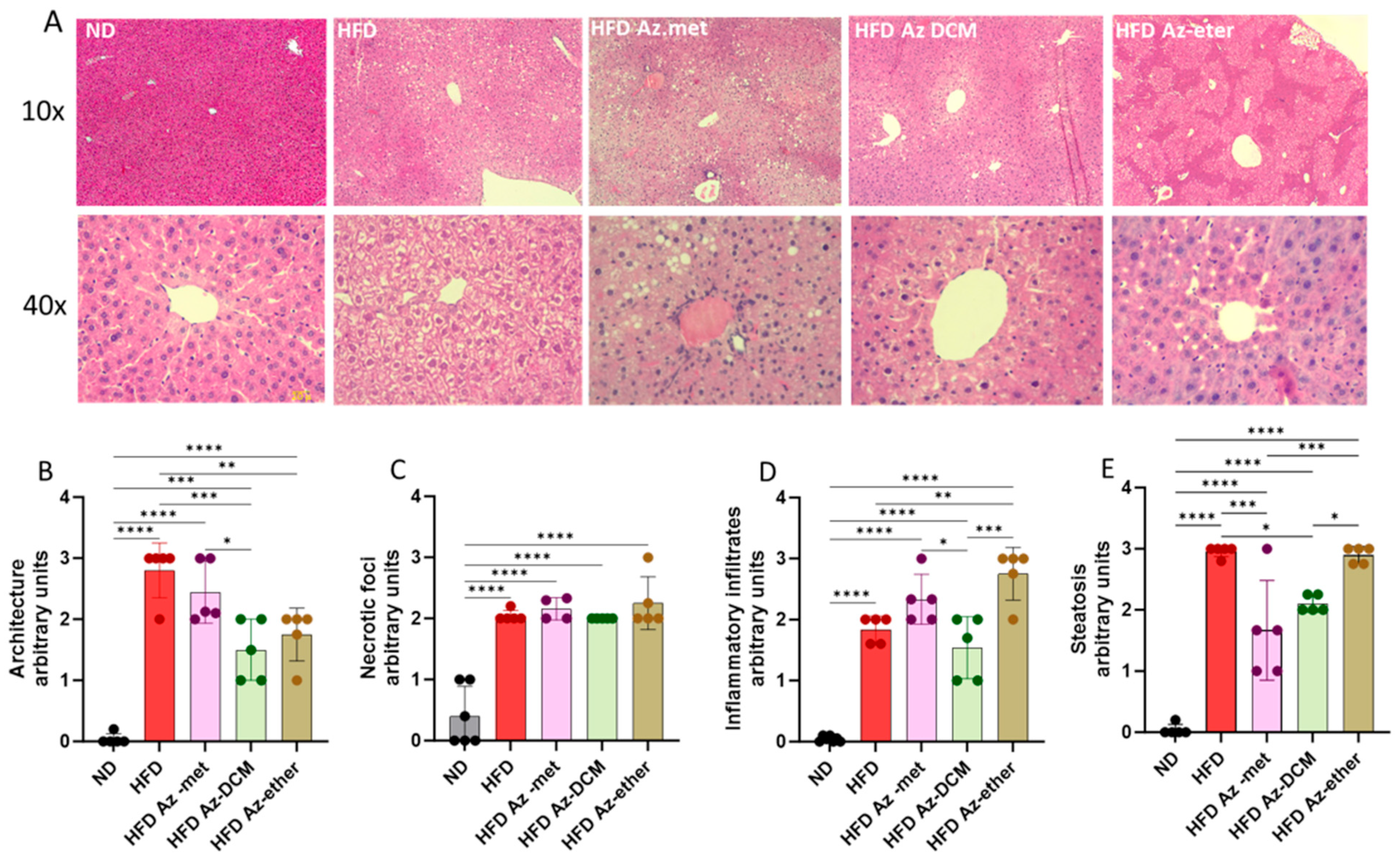
2.3. Effect of A. compacta Organic Extract Treatment on Hepatic Pathology
Hepatic damage in the HFD-treated animals and the impact of the treatment with the A. compacta organic fractions were evaluated through a histopathology analysis of liver sections (Figure 5). Hematoxylin/eosin-stained sections from control mice showed normal liver histoarchitecture, with the absence of necrotic foci, inflammatory infiltrates and lipid drops/steatosis, whereas the HFD mice’s liver sections displayed deterioration in these four parameters with appreciable degenerative changes, especially liver steatosis (more than 30% lipid droplet accumulation) compared to the normal diet-treated animals. The A. compacta extract-treated groups showed variable degrees of liver damage, which is apparent in the analysis of cytoarchitecture, inflammation, necrosis and steatosis (Figure 5B–E). Regarding hepatic cytoarchitecture damage (4B), the Az-DCM- and Az-ether-treated groups showed a 37.5 and 46% decrease compared to the HFD group (p = 0.0009 and 0.007, respectively), with no difference observed for the Az-met group. In the case of the necrotic foci analysis (5C), none of the groups showed differences in damage compared to the HFD group. When inflammatory cell infiltration was evaluated, the Az-ether group presented an increase in inflammatory foci (149% increase, p = 0.0035), while the other groups showed no differences compared to the HFD-treated mice. For steatosis, the Az-met group presented a 44% decrease (p = 0.002) and the Az-DCM-treated mice a 29% reduction (p = 0.0142) compared to the HFD alone group, while the Az-ether group presented no changes in this parameter.
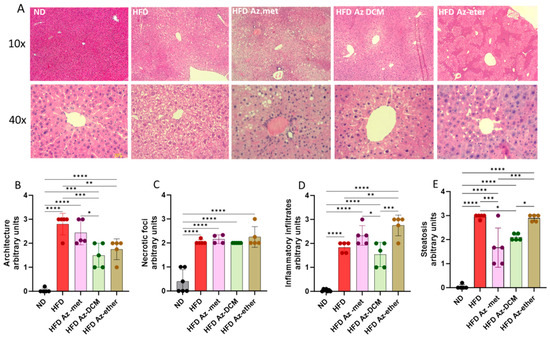
Figure 5.
Histopathological analysis of liver sections of animals treated with organic fractions from Azorella compacta (A. compacta). (A) Representative images of hematoxylin/eosin staining of liver sections from animals treated with ND, normal diet; HFD, high-fat diet; HFD Az-met, HFD-fed mice treated with the methanol fraction of A. compacta; HFD Az-DCM, HFD-fed mice treated with the dichloromethane fraction of A. compacta; and HFD Az-ether, HFD-fed mice treated with the petroleum ether fraction of A. compacta. (B) Analysis of tissue architecture. (C) Analysis of necrotic foci. (D) Analysis of inflammatory infiltration. (E) Analysis of steatosis. * p < 0.05; ** p < 0.001; *** p < 0.0005; **** p < 0.0001; n = 5 each group.
In summary, the impact of the organic fractions of A. compacta on the pathology parameters evaluated is heterogeneous, with both positive and negative effects. Overall, a mild positive effect was observed with the Az-DCM fraction, but a slight worsening one was observed with the Az-ether fraction.
2.4. In Silico Analysis of Toxicity and Metabolism of A. compacta Diterpenoids
Given the potential for hepatic damage under the conditions studied, we used an in silico analysis for the toxicological and metabolic parameters of the ten diterpenoids found in the organic fractions using the ADMET 2.0 platform (Table 2). For this, we first focused on parameters with the potential to induce hepatotoxicity in humans (H-HT) and drug-induced liver injury (DILI). For these parameters, compound 4 exhibited an intermediate risk for HILI, while compound 9 presented a high probability of inducing DILI. To evaluate parameters of biotransformation, we focused on the potential of the terpenoids as being substrates or inhibitors of the CYPA2/2C19/2C9/2D6/3A4 isoforms of the human cytochrome p450 family, which produce phase I (oxidative) reactions mainly in the liver. In general, all the compounds analyzed presented a very high potential to be substrates of CYPAC19; half of them (to a lesser extent) had the potential to be substrates and/or inhibitors of CYP3A4; and almost none were likely to be substrates or inhibitors of CYP2D6. They also presented, in general, a significant probability (although to a lesser extent) of being substrates of CYP3A4. None of the compounds presented significant probabilities of being inhibitors of CYP2C9. In addition, several potential toxicological targets were also analyzed in silico. Of these, the most prominent profiles found included a stress response (SR) to activate p53 and affect the mitochondrial membrane potential (MMP) in eight and nine of the compounds studied, respectively. P53 is a tumor suppressor activated by cell and DNA damage, which, under cell injury, induces cell cycle arrest, apoptosis or cellular senescence. Nine of the compounds presented a high probability of affecting MMP, and eight showed potential as activating molecules of p53, most notably compounds 2a and 2b (β and α azorellanol).

Table 2.
Hepatic toxicity and metabolism parameters obtained in silico for the diterpenoids identified from the organic fractions of Azorella compacta.
3. Discussion
A. compacta is used as a herbal medicine in Andes altiplano communities, mainly as infusions to treat a variety of maladies such as diabetes and inflammatory conditions [3]. Several diterpenoids have been isolated from this plant and tested for pharmacological properties, such as microbicide, anti-hyperglycemic, gastro-protective, anti-inflammatory and analgesic activities [10], but the impact of A. compacta in a chronic model in vivo has not been fully assessed. Here, we evaluated the effect of organic extracts from A. compacta in a model of metabolic disease that presents hyperglycemia, obesity and hepatic alterations. These extracts (petroleum ether, dichloromethane and methanol) contained ten diterpenoids distributed differentially among the fractions. Interestingly, the petroleum ether and methanol fractions were mainly rich in mulinic acid, whereas the dichloromethane fraction included azorellanol, mulinic acid and 13α,14α-dihydroxymulin-11-en-20-oic acid as its main components. The treatment consisted of a two-week administration of a high dose of the extracts (~50 mg per day). Notably, the treatment with these extracts did not change the hyperglycemic state or increase weight due to the HFD diet, suggesting that A. compacta organic fractions are not able to reduce increased glycemic levels in a model more closely resembling T2DM. Previous results in a model of T1D (rats treated with streptozotocin) showed an acute reduction in blood glucose induced by mulinolic acid and azorellanol administration but not by mulin-11,13-dien-20-oic acid [11]. Interestingly, our fractions did not contain mulinolic acid, and azorellanol was abundant mainly in the DCM fraction, but as a racemic mixture. These conditions might explain the lack of effect on hyperglycemia of the organic fractions. Importantly, in the mentioned study, rats treated with streptozotocin reached glycemia values of ~500 mg/dL. Under those conditions, azorellanol acted as an insulin secretagogue, a mechanism that might not be relevant in the present HFD model, which essentially generates insulin resistance, with blood sugar levels of approximately 200 mg/dL.
Furthermore, when we evaluated hepatic damage parameters, an exacerbation of the alterations generated by the HFD was observed. This was evident mainly in the serum levels of the transaminases AST and ALT, which were increased in the animals treated with any of the three organic fractions. Furthermore, the ether and DCM fractions reduced plasma albumin and increased bilirubin levels, both clear signs of hepatic damage.
The histopathology analyses indicated that, in general, the fractions failed to normalize the parameters of liver damage, especially necrotic foci, which correlates with the increased activity of ALT and AST found in the serum of the treated animals. Although, in terms of liver cytoarchitecture, the DCM and petroleum ether extracts reduced the damage observed, the petroleum ether fraction enhanced the inflammatory infiltrates in liver tissue. Hepatic inflammatory infiltration is a cardinal sign of liver disease [20].
The A. compacta organic fractions used in this study contained ten diterpenoids. Some diterpenoids have been reported to be hepatoprotective by exerting antioxidant, anti-inflammatory, hypolipidemic and anti-fibrotic effects [24]. For example, it was recently reported that ruebellapenes extracted from Callicarpa rubella decreased the levels of transaminases in an in vitro model of BRL/hepatocyte damage [25]. Another study showed that carnosic acid, a diterpenoid derived from rosemary leaves (Rosmarinus officinalis), attenuated obesity, insulin levels and liver adiposity and modulated genes associated with lipid metabolism in ob/ob mice [26]. The same positive results were found with Ginkgolide A and B in HFD-induced NAFLD mice, where these diterpenoids derived from Ginkgo biloba L ameliorated hepatic steatosis through the inhibition of lipid accumulation and lipoapoptosis and exerted anti-inflammatory effects [22,27,28].
However, the use of diterpenoids is controversial due to their potential toxic effects. For example, diterpenoids from Aconitum presented anti-inflammatory and anti-cancer activities [29] but also presented toxic effects on the nervous and cardiovascular systems [30]. Also, diterpenoids derived from Delphinium and Aconitum species have been described as cytotoxic to HepG2 cells, an activity that could be useful in hepatocarcinogenesis [31]. In addition, tripolide, a component of Triptergium wilfordii Hook used in the treatment of autoimmune diseases, has been described as a hepatotoxic agent [32]. Tripolide acts on hepatic influx and efflux transporters and causes severe jaundice, drug-induced hepatitis (DILI) and abnormal liver function, which obstacles to its clinical use [33,34]. Other diterpenoids that generate hepatotoxicity in humans include Paclitaxel (isolated from the Western Yew tree) and the protein kinase inhibitors Lapatinib and germander, isolated from Teucrium lamiaceae [24].
Analogous to DILI, an adverse toxic drug reaction resulting in liver injury, there has been established herb-induced liver injury (HILI) [35]. The incidence of HILI has increased as the use of phytotherapy has gained popularity in Western countries, considering that the most promoted herbal products lack scientific evidence of efficacy and safety [36,37]. Terpenes have been reported as HILI-inducing products. For instance, citral, a monoterpene obtained from myrtle trees, lemons, and limes, causes dose-dependent liver necrosis [38]. The sesquiterpene seredone (from Curcuma elata) causes hepatocyte degeneration, centrolobulillar necrosis and immune cell infiltration, increasing ALT and total bilirubin [39]. Dioscorea bulbifera (used in goiters and tumors) is rich in furanoterpenoids and generates hepatotoxicity associated with their oxidation by CYP3A [40].
The use of A. compacta in folk medicine is mainly as an aqueous infusion of dried areal parts and roots [3,4]. Indeed, those preparations may not be abundant in diterpenoids but are rich in water-soluble antioxidants, as has been shown in a study on immune cell activation by an Azorella infusion. In that study, the infusion of A. compacta included chlorogenic acid, apigenin, isoorientin (homoorientin), orientin, dicaffeoylquinic acid, biochanin A and licoisoflavone A, but not diterpenoids [41]. Our fractions contained small amounts of 5.7-dihydroxychromone, biochanin A and homoorientin as potential sources of antioxidants. On the contrary, methanol fractions of A. compacta have been shown to induce apoptosis in HL60 cells, a cell line of neutrophils [42], although the composition of those extracts was not described. In MCF-7 cells, a breast cancer cell line, several A. compacta diterpenoids exhibited cytotoxic activity [43], including 7β-deacetylazorellanol, azorellanol and mulin-11,13-dien-20-oic acid. These terpenoids (compounds 1, 2a/2b and 4 in our study) are abundant in our fractions, which is consistent with the hepatotoxic effect observed. These results are supported by the in silico ADMET analysis, which assigned compound 4 as having an intermediate probability of inducing HILI, with mitochondrial membrane potential as a target, and compounds 1 and 2a/b as having a high probability of altering mitochondrial membrane potential and activating p53, both converging signals for cell death.
From our results, it can be speculated that A. compacta preparations that include terpenoids have the potential to exacerbate hepatic damage, especially in patients with established metabolic diseases such as diabetes and obesity. Particularly, the increase in total bilirubin levels should be considered a warning for potential hepatotoxic effects. Therefore, it is necessary to further evaluate the hepatotoxicity of the A. compacta diterpenoids.
4. Materials and Methods
4.1. Plant Material
Azorella compacta Phil. Apiaceae was collected in May 2013 in the El Tatio geysers area (Atacama Desert) at an elevation of 4200 m in the Region of Antofagasta, Chile. A sample specimen was deposited at the Herbarium of Natural Products Laboratory of the Faculty of Science, Universidad de Chile, under the code n° 0513 [44]. Leaves and stems were separated and dried at ambient temperature, avoiding direct sun exposure, over three days. After this period, areal parts (127 g) were macerated and extracted, first with petroleum ether (three times), then with dichloromethane and finally with methanol. Then, the extracts were filtered and dried under reduced pressure. All chemical reagents used were of analytical grade, obtained from Merck (Merck KGaA, Darmstadt, Germany)
4.2. Metabolomic Analysis
An aliquot of each dried extract was prepared for the metabolomics analysis by UltraHigh-Performance Liquid Chromatography (UHPLC)–mass spectrometry (Compact QTOF MS + Elute UHPLC, Bruker Daltonik GmbH, Bremen, Germany). Petroleum ether, dichloromethane and methanol fractions were solubilized in 500 µL of hexane, dichloromethane and ethanol, respectively, and transferred to HPLC vials. The chromatographic separation of 5 μL of each extract was carried out in a Kinetex C18 (Phenomenex, Torrence, CA, USA) (2.1 mm × 100 mm, particle size 1.7 µm) at 0.5 mL/min and 35 °C using 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) as mobile phases, according to the following gradient: 0 min, 85% A; 18 min, 10% A; 20 min, 10% A; 30 min, 85% A; 35 min, 85% A. The total separation time was 35 min. Mass spectrometry data were acquired over a range of m/z 120–1800 in the negative and positive ion modes of the electrospray ionization (ESI) source: sheath gas: 30 psi; auxiliary gas: 13; spray voltage: 3 kV; capillary temperature: 350 °C; S-Lens RF level: 50; heater temperature: 150 °C. Mass spectrometry was performed in positive and negative modes. Data were analyzed using Metaboscape 4.0 software (Bruker, Billerica, MA, USA), using the MassBank of North America (Mona, Davis, CA, USA) spectra library for metabolite identification.
4.3. Mice and Diets
Adult male C57Bl6 mice (6–8 weeks old), obtained from the animal facility of the Universidad de Talca, were used. The study protocol (N° 2015-04) was approved by the Institutional Animal Care and Use Committee (CIECUAL) of Universidad de Talca. Animals were kept at 21–25 °C in cycles of 12 h light/darkness with water and chow ad libitum. Animals were randomly selected to receive normal (Prolab rat/mouse/hamster 3000) or high-fat diets (DIO rodent purified diet, 45% energy from fat), both from LabDiet (St. Louis, MO, USA) for eight weeks. After this period, animals on the DIO diet were checked for glycemia (obtained from a tail puncture using a Hemoglucotest device). Those that presented values above 150 mg/dL were randomly selected to receive either a vehicle (n = 5) or one of the A. compacta extracts (n = 5 each group). The fractions (13.4 g each) were reconstituted in 2 mL of the vehicle (12% Tween 80 in NaCl 0.9%) and administrated in drinking water (final concentration: 13.4 mg/mL). Since mice drink, on average, 4–6 mL/day, each animal received approximately 50 mg/day. The animals received the extracts for two weeks in drinking water, maintaining the high-fat diet regime. At the end of the experimental period, the animals were euthanized through the administration of 3% isoflurane to induce anesthesia, then ketamine (Ketoshop, Drag Pharma Invetec S.A, Santiago, Chile) at 80 mg/kg and xylazine (Xylavet, Agroland, Santiago, Chile) at 12 mg/kg, both intraperitoneally. Under deep anesthesia, the animals were exsanguinated by cardiac puncture for plasma and organ extraction.
4.4. Determination of Biochemical Parameters
Serum was obtained by blood clotting for three hours at room temperature and centrifugation at 2000× g for 5 min. Alanine aminotransferase (ALT), aspartate aminotransferase (AST) and creatine kinase (CK) activities were measured using specific diagnostic kits (ALT, AST and Valtek® Diagnostic Kits, Ñuñoa, Chile). Albumin and bilirubin were measured using kits from LiquidColor Human™ (Wiesbaden, Germany), and Stanbio Total & Direct Bilirrubin® (StanBio, Boerne, TX, USA), respectively. Adequate two-level controls, normal and pathological, were used. All the serum assays were evaluated in triplicate.
4.5. Liver Histopathology
After extraction, the livers were immersed in Bouin solution for 12–24 h. After this period, pieces of the tissues were dehydrated in alcohol solutions and included in Paraplast. After inclusion, 5 µm sections were obtained using a microtome and mounted on 0.1% polylysine-treated coverslips. After this, sections were stained with hematoxylin/eosin in an automatized tissue processor, Leica TP1020 (Leica Microsystems Inc. (Schweiz) AG, Heerbrugg, Switzerland) and Leica EG11504 H (Leica Microsystems Inc.).
The examination was performed by two investigators in a blinded fashion. Twenty random fields were assessed for (i) necrosis, which was evaluated according to the Korourian score [45]: none = 1, occasional (1%) necrotic hepatocyte = 1, frequent (5–10%) necrotic hepatocytes = 2, small foci of necrosis (clusters greater than 10 necrotic hepatocytes) = 3, and extensive areas of necrosis (over 25%) = 4. (ii) Focal and portal inflammation was assessed according to Goodman’s adapted Ishack score [46,47]: none = 0, one focus per 10× objective or less and/or mild inflammation in portal area = 1, two to four foci per 10× objective and/or moderate, some or all portal areas = 2, five to ten foci per 10× objective and/or moderate/marked inflammation in all portal areas = 3, and more than 10 foci per 10× objective and/or marked inflammation in all portal areas = 4. (iii) Histoarchitecture criteria were analyzed according to Goodman’s schematic diagram [46]: no changes = 1; mild interphase hepatitis and parenchymal injury but central vein conserved = 2; moderate interface hepatitis with necroinflammatory foci and moderate parenchymal injury and more than 50% of the central vein integrity = 3; and marked interface hepatitis, stromal collapse with indicators of parenchymal injury and loss of more than 50% of the central vein integrity = 4. Steatosis was evaluated as a percentage of lipid droplets, where 1 <5%, 2 <30%, 3 <50% and 4 >50%. The histological examination was carried out using a Leica DM500 microscope (Leica Microsystems) with a high-definition digital camera, Leica ICC50W (Leica Microsystems), connected to LAS EZ software (Leica Application Suite version 3.4.0., Heerbrugg, Switzerland).
4.6. In Silico Analysis of Metabolism and Toxicity
Parameters of the metabolism and toxicity of the diterpenoids identified in the metabolomics analysis were evaluated using the ADMETLAB 2.0 (https://admetmesh.scbdd.com/ accessed on 2, 3, 5, 6 and 7 of January 2014) online platform, where SMILES strings were employed throughout the generation process [48].
4.7. Statistical Analyses
Data are presented as the average ± standard deviation (SD). A one way analysis of variance (ANOVA) with multiple comparisons and Tukey’s post hoc test were used. A value of p < 0.05 was considered significant. Analyses were performed using Graphpad Prism 9.1.0. (Boston, MA, USA).
5. Conclusions
The results of the present study showed that the mice fed with a high-fat diet that received a two-week treatment with organic extracts (methanol, dichloromethane and petroleum ether) from Azorella compacta had neither reduced hyperglycemia nor body weight. Furthermore, our findings indicate that these fractions exacerbated the liver alterations in the HFD-fed mice, such as the serum levels of hepatic enzymes. These findings suggest that high doses of A. compacta products may induce liver damage in patients with metabolic diseases.
Author Contributions
Conceptualization, D.R.G.; Methodology, A.S.-M.; Validation, A.S.-M.; Formal analysis, J.Z.-H., M.Q.S.M. and D.R.G.; Investigation, J.Z.-H., M.Q.S.M., U.N., F.A.M., B.F. and D.R.G.; Resources, Francisco Monsalve Abaca and A.S.-M.; Data curation, J.Z.-H. and M.B.; Writing—original draft, J.Z.-H. and D.R.G.; Writing—review & editing, D.R.G.; Supervision, A.S.-M.; Project administration, D.R.G.; Funding acquisition, J.Z.-H. and D.R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Proyecto Fondecyt Regular (ANID Chile) 1150662 (D.R.G.) and Fondequip EQM 210006.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (CIECUAL) of Universidad de Talca (protocol code 2015-04, approved on 23 May 2016).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Metabolomic analysis was performed thanks to Fondequip EQM170172.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kleier, C.; Trenary, T.; Graham, E.A.; Stenzel, W.; Rundel, P.W. Size class structure, growth rates, and orientation of the central Andean cushion Azorella compacta. PeerJ 2015, 3, e843. [Google Scholar] [CrossRef] [PubMed]
- Pugnaire, F.I.; Morillo, J.A.; Armas, C.; Rodríguez-Echeverría, S.; Gaxiola, A. Azorella compacta: Survival champions in extreme, high-elevation environments. Ecosphere 2020, 11, e03031. [Google Scholar] [CrossRef]
- Wickens, G.E. Llareta (Azorella compacta, Umbelliferae): A review. Econ. Bot. 1995, 49, 207–212. [Google Scholar] [CrossRef]
- Fatima, C.; Ignazio, P.; Spadaro, V. Evaluación etnobotanica de la Yareta (Azorella compacta) en Arequipa (Perú) y sus posibles aplicaciones. Quad. Bot. Amb. Appl. 2013, 23, 15–30. [Google Scholar]
- Delporte, C.; Backhouse, N.; Salinas, P.; San-Martin, A.; Borquez, J.; Loyola, A. Pharmaco-toxicological study of diterpenoids. Bioorganic Med. Chem. 2003, 11, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Areche, C.; Rojas-Alvarez, F.; Campos-Briones, C.; Lima, C.; Perez, E.G.; Sepulveda, B. Further mulinane diterpenoids from Azorella compacta. J. Pharm. Pharmacol. 2013, 65, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Borquez, J.; Ardiles, A.; Loyola, L.A.; Pena-Rodriguez, L.M.; Molina-Salinas, G.M.; Vallejos, J.; Collado, I.G.; Simirgiotis, M.J. Further mulinane and azorellane diterpenoids isolated from Mulinum crassifolium and Azorella compacta. Molecules 2014, 19, 3898–3908. [Google Scholar] [CrossRef] [PubMed]
- Salgado, F.; Areche, C.; Sepulveda, B.; Simirgiotis, M.J.; Caceres, F.; Quispe, C.; Quispe, L.; Cano, T. A new mulinane diterpenoid from the cushion shrub Azorella compacta growing in Peru. Pharmacogn. Mag. 2014, 10, S543–S548. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Oros, D.R.; Jaffe, R.; Didyk-Pena, A.; Areche, C.; Sepulveda, B.; Didyk, B.M. Mulinane and Azorellane Diterpenoid Biomarkers by GC-MS from a Representative Apiaceae (Umbelliferae) Species of the Andes. Molecules 2019, 24, 684. [Google Scholar] [CrossRef] [PubMed]
- Dzul-Beh, A.J.; Uc-Cachon, A.H.; Borquez, J.; Loyola, L.A.; Pena-Rodriguez, L.M.; Molina-Salinas, G.M. Mulinane- and Azorellane-Type Diterpenoids: A Systematic Review of Their Biosynthesis, Chemistry, and Pharmacology. Biomolecules 2020, 10, 1333. [Google Scholar] [CrossRef]
- Fuentes, N.L.; Sagua, H.; Morales, G.; Borquez, J.; San Martin, A.; Soto, J.; Loyola, L.A. Experimental antihyperglycemic effect of diterpenoids of llareta Azorella compacta (Umbelliferae) Phil in rats. Phytother. Res. 2005, 19, 713–716. [Google Scholar] [CrossRef] [PubMed]
- San-Martín, A.; Bacho, M.; Cretton, S.; Christen, P.; Olea, A.; Muñoz, D.; Guillen, A.; Balcazar, N. Evaluation of the hypoglycemic effects of extracts and diterpenoids from Azorella compacta (llareta). Planta Med. 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Pafili, K.; Roden, M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol. Metab. 2021, 50, 101122. [Google Scholar] [CrossRef] [PubMed]
- Gofton, C.; Upendran, Y.; Zheng, M.H.; George, J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol. 2023, 29, S17–S31. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Scorletti, E.; Mosca, A.; Alisi, A.; Byrne, C.D.; Targher, G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism 2020, 111, 154170. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rodas, M.C.; Valenzuela, R.; Videla, L.A. Relevant Aspects of Nutritional and Dietary Interventions in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2015, 16, 25168–25198. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Sabaj, M.; Tolosa, G.; Herrera Vielma, F.; Zuniga, M.J.; Gonzalez, D.R.; Zuniga-Hernandez, J. Maresin-1 Prevents Liver Fibrosis by Targeting Nrf2 and NF-kappaB, Reducing Oxidative Stress and Inflammation. Cells 2021, 10, 3406. [Google Scholar] [CrossRef]
- Videla, L.A.; Valenzuela, R.; Del Campo, A.; Zuniga-Hernandez, J. Omega-3 Lipid Mediators: Modulation of the M1/M2 Macrophage Phenotype and Its Protective Role in Chronic Liver Diseases. Int. J. Mol. Sci. 2023, 24, 15528. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Liu, Y. Terpenoids: Natural Compounds for Non-Alcoholic Fatty Liver Disease (NAFLD) Therapy. Molecules 2022, 28, 272. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ou, Y.; Hu, G.; Wen, C.; Yue, S.; Chen, C.; Xu, L.; Xie, J.; Dai, H.; Xiao, H.; et al. Naringenin attenuates non-alcoholic fatty liver disease by down-regulating the NLRP3/NF-κB pathway in mice. Br. J. Pharmacol. 2020, 177, 1806–1821. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.; Islam, M.T.; Tabrez, S.; Firoz, C.K.; Jabir, N.R.; Kamal, M.A.; Melo-Cavalcante, A.A.C.; Almeida, F.R.C. Effect of Diterpenes on Hepatic System. Curr. Pharm. Des. 2018, 24, 4093–4100. [Google Scholar] [CrossRef]
- Chen, P.; Huang, Y.; Zhang, X.; Zhao, Z.; Sun, Z.; Cui, H.; Lin, C.; Peng, G.; Wu, A.; Zhu, C. New chemical structures and liver-protective activity of the diterpenoids from Callicarpa rubella. Fitoterapia 2023, 165, 105394. [Google Scholar] [CrossRef]
- Park, M.Y.; Sung, M.K. Carnosic acid attenuates obesity-induced glucose intolerance and hepatic fat accumulation by modulating genes of lipid metabolism in C57BL/6J-ob/ob mice. J. Sci. Food Agric. 2015, 95, 828–835. [Google Scholar] [CrossRef]
- Jeong, H.S.; Kim, K.H.; Lee, I.S.; Park, J.Y.; Kim, Y.; Kim, K.S.; Jang, H.J. Ginkgolide A ameliorates non-alcoholic fatty liver diseases on high fat diet mice. Biomed. Pharmacother. 2017, 88, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, Y.; Wang, D.; Zhao, Y.; Wang, Y.; Li, F.; Fang, J.; Chen, H.; Fan, S.; Huang, C. Ginkgolide B lowers body weight and ameliorates hepatic steatosis in high-fat diet-induced obese mice correlated with pregnane X receptor activation. RSC Adv. 2017, 7, 37858–37866. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Thawabteh, A.; Lelario, F.; Bufo, S.A.; Scrano, L. Classification, Toxicity and Bioactivity of Natural Diterpenoid Alkaloids. Molecules 2021, 26, 4103. [Google Scholar] [CrossRef]
- Chan, T.Y. Incidence and Causes of Aconitum Alkaloid Poisoning in Hong Kong from 1989 to 2010. Phytother. Res. 2015, 29, 1107–1111. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, J.-L.; Li, X.; Guo, M.-Q.; Leung, E.L.-H.; Zhou, H.; Liu, L.; Li, N. Anti-cancer and anti-inflammatory new vakognavine-type alkaloid from the roots of Aconitum carmichaelii. Tetrahedron Lett. 2016, 57, 5881–5884. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, C.; Wang, K.; Takahashi, S.; Krausz, K.W.; Lu, D.; Wang, Q.; Luo, Y.; Gong, X.; Mu, X.; et al. Comprehensive analysis of transcriptomics and metabolomics to understand triptolide-induced liver injury in mice. Toxicol. Lett. 2020, 333, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Chen, L.; Fang, P.; Cai, H.; Tang, H.; Peng, Y.; Deng, Y.; Cao, L.; Li, H.; Zhang, B.; et al. Mechanisms of Triptolide-Induced Hepatotoxicity and Protective Effect of Combined Use of Isoliquiritigenin: Possible Roles of Nrf2 and Hepatic Transporters. Front. Pharmacol. 2018, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Guo, Q.; Shen, J.; Zheng, K.; Lu, C.; Zhang, G.; Lu, A.; He, X. The Effect of Triptolide in Rheumatoid Arthritis: From Basic Research towards Clinical Translation. Int. J. Mol. Sci. 2018, 19, 376. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.; Monteiro, C.S.J.; Dos Santos, J.L. Herb-Induced Liver Injury—A Challenging Diagnosis. Healthcare 2022, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.K.-S.; Tujios, S.R. Hidden Dangers: Herbal and Dietary Supplement Induced Hepatotoxicity. Livers 2023, 3, 618–636. [Google Scholar] [CrossRef]
- Ballotin, V.R.; Bigarella, L.G.; Brandao, A.B.M.; Balbinot, R.A.; Balbinot, S.S.; Soldera, J. Herb-induced liver injury: Systematic review and meta-analysis. World J. Clin. Cases 2021, 9, 5490–5513. [Google Scholar] [CrossRef] [PubMed]
- Fandohan, P.; Gnonlonfin, B.; Laleye, A.; Gbenou, J.D.; Darboux, R.; Moudachirou, M. Toxicity and gastric tolerance of essential oils from Cymbopogon citratus, Ocimum gratissimum and Ocimum basilicum in Wistar rats. Food Chem. Toxicol. 2008, 46, 2493–2497. [Google Scholar] [CrossRef]
- Pimkaew, P.; Suksen, K.; Somkid, K.; Chokchaisiri, R.; Jariyawat, S.; Chuncharunee, A.; Suksamrarn, A.; Piyachaturawat, P. Zederone, a sesquiterpene from Curcuma elata Roxb, is hepatotoxic in mice. Int. J. Toxicol. 2013, 32, 454–462. [Google Scholar] [CrossRef]
- Li, H.; Peng, Y.; Zheng, J. Dioscorea bulbifera L.-induced hepatotoxicity and involvement of metabolic activation of furanoterpenoids. Drug Metab. Rev. 2020, 52, 568–584. [Google Scholar] [CrossRef]
- Tumova, L.; Ducaiova, Z.; Cheel, J.; Vokral, I.; Sepulveda, B.; Vokurkova, D. Azorella compacta Infusion Activates Human Immune Cells and Scavenges Free Radicals In vitro. Pharmacogn. Mag. 2017, 13, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.H.; Kwon, O.K.; Oh, S.R.; Lee, J.; Park, S.H.; Han, S.B.; Ahn, K.S. Azorella compacta methanolic extract induces apoptosis via activation of mitogen-activated protein kinase. Mol. Med. Rep. 2015, 12, 6821–6828. [Google Scholar] [CrossRef] [PubMed]
- Borquez, J.; Bartolucci, N.L.; Echiburu-Chau, C.; Winterhalter, P.; Vallejos, J.; Jerz, G.; Simirgiotis, M.J. Isolation of cytotoxic diterpenoids from the Chilean medicinal plant Azorella compacta Phil from the Atacama Desert by high-speed counter-current chromatography. J. Sci. Food Agric. 2016, 96, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- San-Martín, A.; Bacho, M.; Núñez, S.; Rovirosa, J.; Soler, A.; Blanc, V.; León, R.; Olea, A.F. A novel normulinane isolated from Azorella compacta and assessment of its antibacterial activity. J. Chil. Chem. Soc. 2018, 63, 4082–4085. [Google Scholar] [CrossRef]
- Korourian, S.; Hakkak, R.; Ronis, M.J.; Shelnutt, S.R.; Waldron, J.; Ingelman-Sundberg, M.; Badger, T.M. Diet and risk of ethanol-induced hepatotoxicity: Carbohydrate-fat relationships in rats. Toxicol. Sci. 1999, 47, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Goodman, Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007, 47, 598–607. [Google Scholar] [CrossRef]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).