In Vitro Antibiofilm Activity of Fosfomycin Alone and in Combination with Other Antibiotics against Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Results
2.1. Antibiotic Susceptibility
2.2. Detection of Biofilm Formation
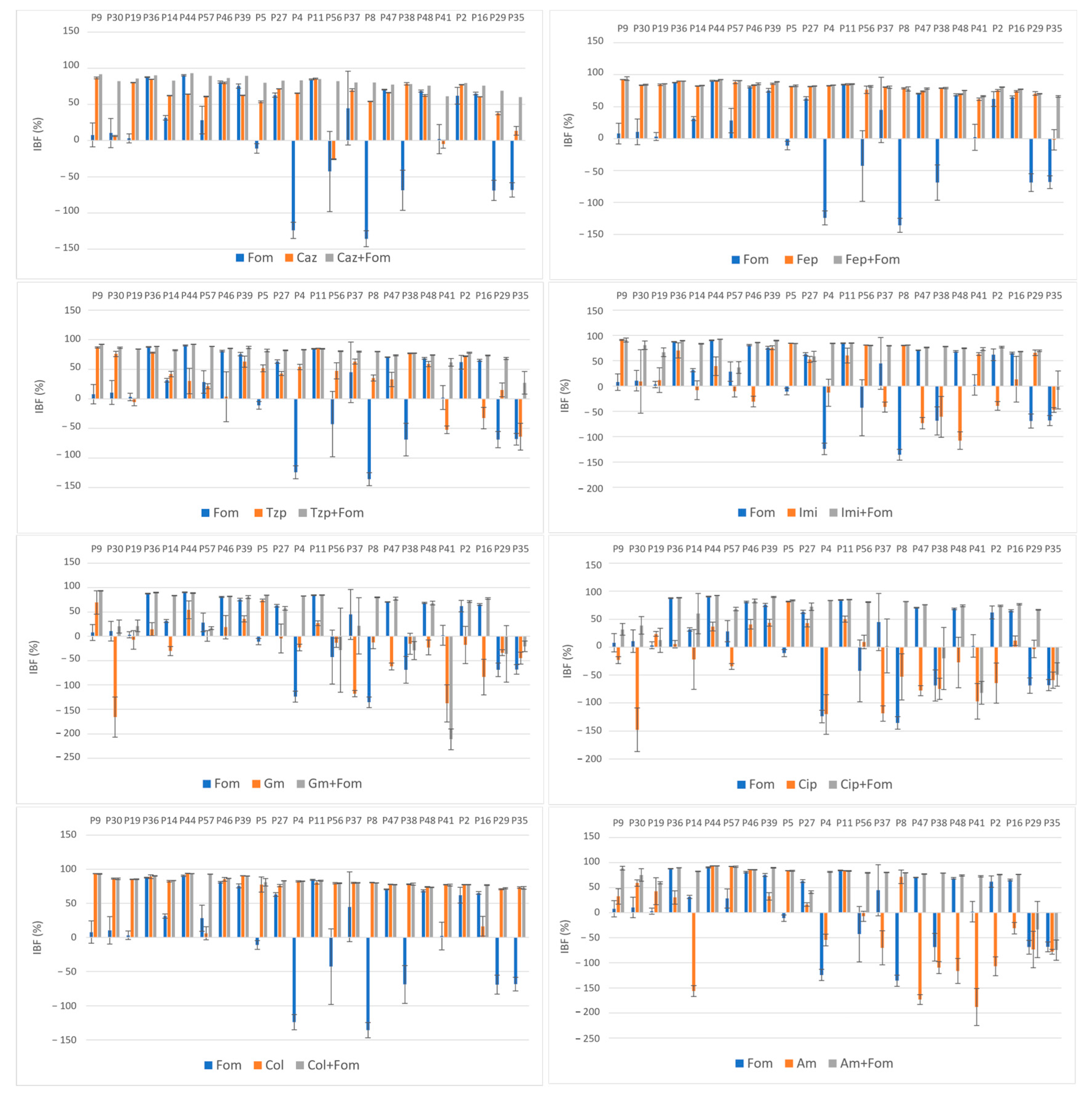
2.3. Inhibition of Biofilm Formation
2.4. Disruption of Preformed Biofilms
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
4.2. Quantitative Absorbance-Based Biofilm Measurement
4.2.1. The Microtiter Plate Assay
4.2.2. Inhibition of the Formation of and Eradication/Disruption of P. aeruginosa Biofilms
4.2.3. Quantification of Biofilm Inhibition
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zakhour, J.; Sharara, S.L.; Hindy, J.R.; Haddad, S.F.; Kanj, S.S. Antimicrobial Treatment of Pseudomonas aeruginosa Severe Sepsis. Antibiotics 2022, 11, 1432. [Google Scholar] [CrossRef]
- Olivares, E.; Badel-Berchoux, S.; Provot, C.; Prévost, G.; Bernardi, T.; Jehl, F. Clinical Impact of Antibiotics for the Treatment of Pseudomonas aeruginosa Biofilm Infections. Front. Microbiol. 2020, 10, 2894. [Google Scholar] [CrossRef] [PubMed]
- Yousef Memar, M.; Adibkia, K.; Farajnia, S.; Kafil, H.S.; Khalili, Y.; Azargun, R.; Ghotaslou, R. In-vitro Effect of Imipenem, Fosfomycin, Colistin, and Gentamicin Combination against Carbapenem-resistant and Biofilm-forming Pseudomonas aeruginosa Isolated from Burn Patients. Iran. J. Pharm. Res. 2021, 20, 286–296. [Google Scholar]
- Černohorská, L.; Votava, M. Antibiotic synergy against biofilm-forming Pseudomonas aeruginosa. Folia Microbiol. 2008, 53, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, H.; Memar, M.Y.; Sefidan, F.Y.; Yekani, M.; Ghotaslou, R. In vitro synergy of antibiotic combinations against planktonic and biofilm Pseudomonas aeruginosa. GMS Hyg. Infect. Control 2017, 12, Doc17. [Google Scholar] [PubMed]
- Costa, G.A.; Rossatto, F.C.P.; Medeiros, A.W.; Paula, A.; Correa, F.; Brandelli, A.; Frazzon, A.P.G.; Motta, A.D.S.D. Evaluation antibacterial and antibiofilm activity of the antimicrobial peptide P34 against Staphylococcus aureus and Enterococcus faecalis. An. Acad. Bras. Cienc. 2018, 90, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Molinero, E.; Macià, M.D.; Rubio, R.; Moyà, B.; Cabot, G.; López-Causapé, C.; Oliver, A. Sequential Treatment of Biofilms with Aztreonam and Tobramycin Is a Novel Strategy for Combating Pseudomonas aeruginosa Chronic Respiratory Infections. Antimicrob. Agents Chemother. 2016, 60, 2912–2922. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. Apmis 2017, 125, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Penesyan, A.; Paulsen, I.T.; Gillings, M.R.; Kjelleberg, S.; Manefield, M.J. Secondary Effects of Antibiotics on Microbial Biofilms. Front. Microbiol. 2020, 11, 2109. [Google Scholar] [CrossRef]
- Raz, R.; Raz, P.R. Fosfomycin: An old—New antibiotic. Clin. Microbiol. Infect. 2012, 18, 4–7. [Google Scholar] [CrossRef]
- Silver, L.L. Fosfomycin: Mechanism and Resistance. Cold Spring Harb. Perspect. Med. 2017, 7, a025262. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.C.; Landersdorfer, C.B.; McIntosh, M.P.; Peleg, A.Y.; Hirsch, E.B.; Kirkpatrick, C.M.; Bergen, P.J. Clinically relevant concentrations of fosfomycin combined with polymyxin, B.; tobramycin or ciprofloxacin enhance bacterial killing of Pseudomonas aeruginosa, but do not suppress the emergence of fosfomycin resistance. J. Antimicrob. Chemother. 2016, 71, 2218–2229. [Google Scholar] [CrossRef] [PubMed]
- Díez-Aguilar, M.; Cantón, R. New microbiological aspects of fosfomycin. Rev. Española Quimioter. 2019, 32, 8–18. [Google Scholar]
- Roussos, N.; Karageorgopoulos, D.E.; Samonis, G.; Falagas, M.E. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections Clinical signifi-cance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int. J. Antimicrob. Agents 2009, 34, 506–515. [Google Scholar] [PubMed]
- Michalopoulos, A.S.; Falagas, M.E. Colistin: Recent data on pharmacodynamics properties and clinical efficacy in critically ill patients. Ann. Intensive Care 2011, 1, 30. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.G.; Kenney, T.F.; MacLeod, D.L.; Henig, N.R.; O’Toole, G.A. Eradication of Pseudomonas aeruginosa biofilms on cultured airway cells by a fosfomycin/tobramycin antibiotic combination. Pathog. Dis. 2013, 67, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Chai, D.; Liu, X.; Wang, R.; Bai, Y.; Cai, Y. Efficacy of Linezolid and Fosfomycin in Catheter-Related Biofilm Infection Caused by Methicillin-Resistant Staphylococcus aureus. Biomed. Res. Int. 2016, 2016, 6413982. [Google Scholar] [CrossRef] [PubMed]
- González, M.J.; Da Cunda, P.; Notejane, M.; Zunino, P.; Scavone, P.; Robino, L. Fosfomycin tromethamine activity on biofilm and intracellular bacterial communities produced by uropathogenic Escherichia coli isolated from patients with urinary tract infection. Pathog. Dis. 2019, 77, ftz022. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.C.; McIntosh, M.P.; Peleg, A.Y.; Kirkpatrick, C.M.; Bergen, P.J. In vitro pharmacodynamics of fosfomycin against clinical isolates of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2015, 70, 3042–3050. [Google Scholar] [CrossRef]
- Samonis, G.; Maraki, S.; Karageorgopoulos, D.E.; Vouloumanou, E.K.; Falagas, M.E. Synergy of fosfomycin with carbapenems, colistin, netilmicin, and tigecycline against multidrug-resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa clinical isolates. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 695–701. [Google Scholar] [CrossRef]
- Wang, L.; Di Luca, M.; Tkhilaishvili, T.; Trampuz, A.; Gonzalez Moreno, M. Synergistic Activity of Fosfomycin, Ciprofloxacin, and Gentamicin Against Escherichia coli and Pseudomonas aeruginosa Biofilms. Front. Microbiol. 2019, 10, 2522. [Google Scholar] [CrossRef]
- Tré-Hardy, M.; Nagant, C.; El Manssouri, N.; Vanderbist, F.; Traore, H.; Vaneechoutte, M.; Dehaye, J.P. Efficacy of the Combination of Tobramycin and a Macrolide in an In Vitro Pseudomonas aeruginosa Mature Biofilm Model. Antimicrob. Agents Chemother. 2010, 54, 4409–4415. [Google Scholar] [CrossRef]
- Díez-Aguilar, M.; Morosini, M.I.; Köksal, E.; Oliver, A.; Ekkelenkamp, M.; Cantón, R. Use of Calgary and Microfluidic BioFlux Systems To Test the Activity of Fosfomycin and Tobramycin Alone and in Combination against Cystic Fibrosis Pseudomonas aeruginosa Biofilms. Antimicrob. Agents Chemother. 2018, 62, e01650-17. [Google Scholar] [CrossRef]
- Cai, Y.; Fan, Y.; Wang, R.; An, M.M.; Liang, B.B. Synergistic effects of aminoglycosides and fosfomycin on Pseudomonas aeruginosa in vitro and biofilm infections in a rat model. J. Antimicrob. Chemother. 2009, 64, 563–566. [Google Scholar] [CrossRef]
- Mikuniya, T.; Kato, Y.; Kariyama, R.; Monden, K.; Hikida, M.; Kumon, H. Synergistic effect of fosfomycin and fluoroquinolones against Pseudomonas aeruginosa growing in a biofilm. Acta Med. Okayama 2005, 59, 209–216. [Google Scholar]
- Mikuniya, T.; Kato, Y.; Ida, T.; Maebashi, K.; Monden, K.; Kariyama, R.; Kumon, H. Treatment of Pseudomonas aeruginosa biofilms with a combination of fluoroquinolones and fosfomycin in a rat urinary tract infection model. J. Infect. Chemother. 2007, 13, 285–290. [Google Scholar] [CrossRef]
- Monden, K.; Ando, E.; Iida, M.; Kumon, H. Role of fosfomycin in a synergistic combination with ofloxacin against Pseudomonas aeruginosa growing in a biofilm. J. Infect. Chemother. 2002, 8, 218–226. [Google Scholar] [CrossRef]
- Kumon, H.; Ono, N.; Iida, M.; Nickel, J.C. Combination Effect of Fosfomycin and Ofloxacin against Pseudomonas aeruginosa Growing in a Biofilm. Antimicrob. Agents Chemother. 1995, 39, 1038–1044. [Google Scholar] [CrossRef]
- Su, T.; He, J.; Li, N.; Liu, S.; Xu, S.; Gu, L. A Rational Designed PslG with Normal Biofilm Hydrolysis and Enhanced Resistance to Trypsin-Like Protease Digestion. Front. Microbiol. 2020, 11, 760. [Google Scholar] [CrossRef]
- Boncompagni, S.R.; Micieli, M.; Di Maggio, T.; Aiezza, N.; Antonelli, A.; Giani, T.; Rossolini, G.M. Activity of fosfomycin/colistin combinations against planktonic and biofilm Gram-negative pathogens. J. Antimicrob. Chemother. 2022, 77, 2199–2208. [Google Scholar] [CrossRef]
- Agyeman, A.A.; López-Causapé, C.; Rogers, K.E.; Lucas, D.D.; Cortés-Lara, S.; Gomis-Font, M.A.; Landersdorfer, C.B. Ceftolozane/tazobactam plus tobramycin against free-floating and biofilm bacteria of hypermutable Pseudomonas aeruginosa epidemic strains: Resistance mechanisms and synergistic activity. Int. J. Antimicrob. Agents 2023, 62, 106887. [Google Scholar] [CrossRef]
- Habash, M.B.; Park, A.J.; Vis, E.C.; Harris, R.J.; Khursigara, C.M. Synergy of silver nanoparticles and aztreonam against Pseudomonas aeruginosa PAO1 biofilms. Antimicrob. Agents Chemother. 2014, 58, 5818–5830. [Google Scholar] [CrossRef]
- Lebeaux, D.; Chauhan, A.; Rendueles, O.; Beloin, C. From in vitro to in vivo Models of Bacterial Biofilm-Related Infections. Pathogens 2013, 2, 288–356. [Google Scholar] [CrossRef]
- Slade-Vitković, M.; Bedenić, B.; Bielen, L.; Batarilo, I.; Kibel, S.; Maravić-Vlahoviček, G. In vitro killing of multidrug/extensively drug-resistant Pseudomonas aeruginosa by fosfomycin alone or in combination with antipseudomonal antibiotics. J. Chemother. 2023, 35, 219–230. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Monnet, D.L. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- López-Montesinos, I.; Horcajada, J.P. Oral and intravenous fosfomycin in complicated urinary tract infections. Rev. Esp. Quimioter. 2019, 32 (Suppl. S1), 37–44. [Google Scholar]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, L.; Han, J.; Dong, P.; Luo, X.; Zhang, Y.; Zhu, L. Inhibition of Biofilm Formation and Related Gene Expression of Listeria monocytogenes in Response to Four Natural Antimicrobial Compounds and Sodium Hypochlorite. Front. Microbiol. 2020, 11, 617473. [Google Scholar] [CrossRef]
- Warraich, A.A.; Mohammed, A.R.; Perrie, Y.; Hussain, M.; Gibson, H.; Rahman, A. Evaluation of anti-biofilm activity of acidic amino acids and synergy with ciprofloxacin on Staphylococcus aureus biofilms. Sci. Rep. 2020, 10, 9021. [Google Scholar] [CrossRef]
- Bernal-Mercado, A.; Vazquez-Armenta, F.; Tapia-Rodriguez, M.; Islas-Osuna, M.; Mata-Haro, V.; Gonzalez-Aguilar, G.; Ayala-Zavala, J.F. Comparison of single and combined use of catechin, protocatechuic, and vanillic acids as antioxidant and antibacterial agents against uropathogenic Escherichia coli at planktonic and biofilm levels. Molecules 2018, 23, 2813. [Google Scholar] [CrossRef]
- Wickremasinghe, H.; Yu, H.H.; Azad, M.A.K.; Zhao, J.; Bergen, P.J.; Velkov, T.; Li, J. Clinically relevant concentrations of polymyxin B and meropenem synergistically kill multidrug-resistant Pseudomonas aeruginosa and minimize biofilm formation. Antibiotics 2021, 10, 405. [Google Scholar] [CrossRef]
- Monogue, M.L.; Nicolau, D.P. Antibacterial activity of ceftolozane/tazobactam alone and in combination with other antimicrobial agents against MDR Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2018, 73, 942–952. [Google Scholar] [CrossRef]
- FDA. Ceptaz (Ceftazidime for Injection) [Internet]; England, GlaxoSmithKline: Research Triangle Park, NC, USA, 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/050646s014lbl.pdf (accessed on 20 December 2021).
- FDA. Primaxin (Imipenem and Cilastatin) for Injection, for Intravenous Use; Merck&Co., Inc.: Whitehouse Station, NJ, USA, 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/050587s074lbl.pdf (accessed on 20 December 2021).
- FDA. Fresenius Kabi USA, LCC. Gentamicin Injection. 2013. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/062366s033lbl.pdf (accessed on 20 December 2021).
- FDA. Amikacin Sulfate [Internet]; Abbott Laboratories: North Chicago, IL, USA, 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/97/64146AP.PDF (accessed on 20 December 2021).
- Car, H. In Vitro Synergy and Postantibiotic Effect of Colistin Combinations with Meropenem and Vancomycin against Gram Negative Bacteria with Multiple Carbapenem Resistance Mechanisms. Ph.D. Thesis, The Josip Juraj Strossmayer University of Osijek, Osijek, Croatia, 2020. [Google Scholar]
- Markou, N.; Markantonis, S.L.; Dimitrakis, E.; Panidis, D.; Boutzouka, E.; Karatzas, S.; Baltopoulos, G. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, gram-negative bacilli infections: A prospective, open-label, uncontrolled study. Clin. Ther. 2008, 30, 143–151. [Google Scholar] [CrossRef]
- Moni, M.; Sudhir, S.; Dipu, T.S.; Mohamed, Z.; Prabhu, B.P.; Edathadathil, F.; Menon, V.P. Clinical efficacy and pharmacokinetics of colistimethate sodium and colistin in critically ill patients in an Indian hospital with high endemic rates of multidrug-resistant Gram-negative bacterial infections: A prospective observational study. Int. J. Infect. Dis. 2020, 100, 497–506. [Google Scholar] [CrossRef]
- Das, M.C.; Sandhu, P.; Gupta, P.; Rudrapaul, P.; De, U.C.; Tribedi, P.; Bhattacharjee, S. Attenuation of Pseudomonas aeruginosa biofilm formation by Vitexin: A combinatorial study with azithromycin and gentamicin. Sci. Rep. 2016, 6, 23347. [Google Scholar] [CrossRef]
- Abu El-Wafa, W.M.; Ahmed, R.H.; Ramadan, M.A.H. Synergistic effects of pomegranate and rosemary extracts in combination with antibiotics against antibiotic resistance and biofilm formation of Pseudomonas aeruginosa. Braz. J. Microbiol. 2020, 51, 1079–1092. [Google Scholar] [CrossRef]
- Shinde, S.; Lee, L.H.; Chu, T. Inhibition of Biofilm Formation by the Synergistic Action of EGCG-S and Antibiotics. Antibiotics 2021, 10, 102. [Google Scholar] [CrossRef]

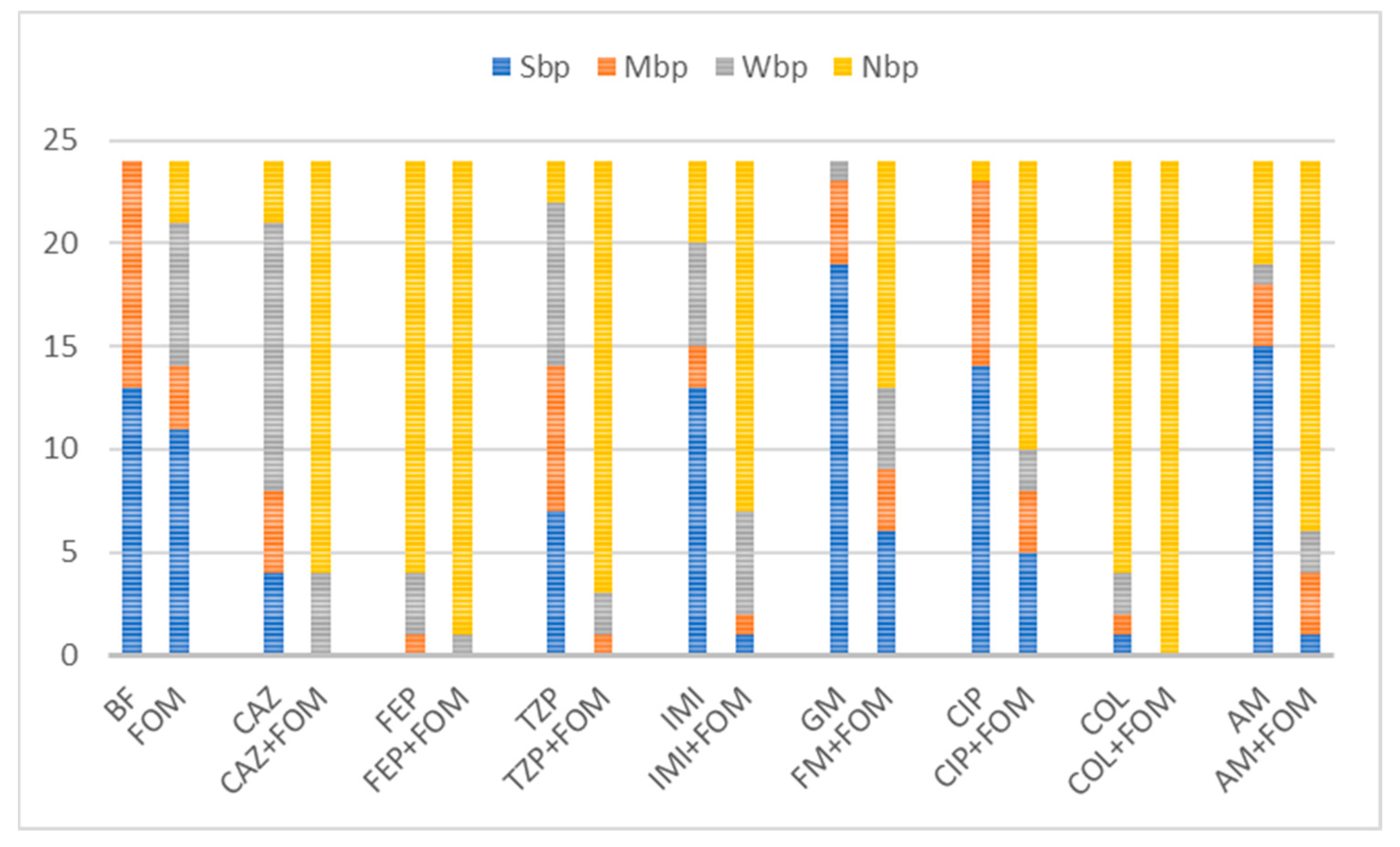
| Isolate | MIC (µg/mL) a | Category b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FOM | CAZ | FEP | TZP | IMI | MEM | GM | AM | CIP | COL | ||
| P1 | 128 | 64 | 32 | 128 | 128 | >128 | >128 | 8 | 32 | 1 | XDR |
| P2 | >256 | >128 | >128 | >128 | 64 | 32 | >128 | 128 | 64 | 2 | XDR |
| P3 | 64 | >128 | >128 | >128 | >128 | >128 | >128 | 32 | 64 | 2 | XDR |
| P4 | 128 | 128 | 32 | 32 | 128 | >128 | >128 | 128 | 32 | 1 | XDR |
| P5 | 128 | 128 | 16 | 64 | 128 | >128 | 1 | 2 | 2 | 1 | XDR |
| P6 | >256 | 64 | 32 | 64 | 128 | >128 | 32 | 128 | 64 | 1 | XDR |
| P7 | 256 | 64 | 32 | 128 | 128 | >128 | 32 | 128 | 32 | 1 | XDR |
| P8 | 64 | 32 | 16 | 64 | 128 | >128 | 32 | 32 | 32 | 1 | XDR |
| P9 | 128 | 64 | 32 | 32 | 32 | >128 | 32 | 128 | 32 | 2 | XDR |
| P11 | 8 | >128 | 32 | >128 | >128 | >128 | >128 | 2 | >128 | 1 | MDR |
| P12 | 32 | >128 | 32 | 32 | 128 | >128 | 16 | 64 | 64 | 1 | XDR |
| P14 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | 64 | >128 | 2 | XDR |
| P15 | 128 | >128 | 64 | >128 | >128 | >128 | >128 | 128 | >128 | 2 | XDR |
| P16 | 16 | 64 | 32 | 64 | >128 | >128 | 8 | 32 | >128 | 2 | XDR |
| P17 | 128 | 32 | 16 | 16 | 32 | 16 | >128 | 16 | 2 | 2 | MDR |
| P18 | >128 | 16 | 16 | 32 | >128 | 16 | 8 | 4 | 2 | 4 | XDR |
| P19 | 32 | >128 | 32 | >128 | >128 | 16 | >128 | 4 | 16 | 2 | MDR |
| P27 | 128 | 16 | 32 | 16 | 8 | 1 | 256 | 8 | 0.5 | 2 | MDR |
| P28 | 128 | 64 | 4 | 8 | 4 | 4 | 4 | 8 | 0.25 | 2 | MDR |
| P29 | 128 | 8 | 16 | 16 | 2 | 1 | >128 | 128 | >128 | 2 | MDR |
| P30 | 64 | 32 | 32 | 16 | 16 | 16 | >128 | 4 | >128 | 2 | MDR |
| P33 | 128 | 16 | 16 | 16 | 32 | 16 | 8 | 4 | 2 | 2 | MDR |
| P35 | 64 | 128 | 64 | 32 | >128 | 64 | >128 | 128 | 128 | 2 | XDR |
| P36 | 32 | >128 | 32 | 16 | 4 | 64 | 2 | 16 | 32 | 2 | MDR |
| P37 | 64 | 32 | 8 | 16 | 16 | 16 | 32 | 32 | 32 | 4 | MDR |
| P38 | 64 | >128 | 32 | 128 | 16 | 16 | >128 | 32 | 32 | 2 | XDR |
| P39 | 128 | >128 | 32 | 16 | 16 | 16 | 32 | 64 | 64 | 2 | MDR |
| P40 | 128 | 64 | 32 | 16 | 16 | 16 | >128 | 64 | 64 | 2 | MDR |
| P41 | >128 | 64 | 16 | 16 | 8 | 16 | 64 | 32 | 64 | 2 | MDR |
| P43 | 4 | >128 | >128 | >128 | 16 | 16 | >128 | 64 | 64 | 4 | XDR |
| P44 | 4 | >128 | 64 | >128 | 64 | 16 | >128 | 32 | 64 | 4 | XDR |
| P45 | 64 | 64 | 16 | 32 | 16 | 16 | 8 | 8 | 0.5 | 2 | MDR |
| P46 | 2 | 128 | 64 | 256 | 16 | 16 | >128 | 64 | 64 | 4 | XDR |
| P47 | 4 | >128 | >128 | >128 | 128 | 32 | >128 | 64 | 128 | 1 | XDR |
| P48 | 2 | >128 | 64 | >128 | 64 | 8 | >128 | 64 | 128 | 2 | XDR |
| P49 | 64 | 16 | 16 | 32 | 32 | 8 | 4 | 8 | 0.5 | 1 | MDR |
| P50 | 4 | >128 | >128 | >128 | 64 | 16 | >128 | 64 | 64 | 2 | XDR |
| P51 | 4 | >128 | >128 | 256 | 64 | 32 | >128 | 128 | 64 | 4 | XDR |
| P52 | 16 | >128 | 64 | >128 | 32 | 16 | >128 | 32 | 64 | 2 | XDR |
| P53 | >128 | 64 | 32 | 16 | 32 | 16 | >128 | 16 | 2 | 2 | MDR |
| P54 | >128 | 16 | 8 | 8 | 16 | 16 | 4 | 16 | 0.125 | 2 | MDR |
| P56 | >128 | >256 | >256 | 64 | 0.5 | 1 | >256 | 64 | 64 | 2 | MDR |
| P57 | >128 | >256 | 128 | 64 | 128 | 64 | >256 | 128 | 64 | 2 | XDR |
| Atb | Average IBF | ||||
|---|---|---|---|---|---|
| Single Use % | SD | Combination % | SD | p | |
| FOM | 14.9 | ±67.75 | |||
| CAZ | 56.5 | ±29.96 | 81.0 | ±8.39 | <0.001 |
| FEP | 77.1 | ±18.35 | 81.8 | ±7.35 | <0.001 |
| TZP | 36.8 | ±41.78 | 78.8 | ±13.19 | <0.001 |
| IMI | 15.2 | ±58.14 | 74.1 | ±21.16 | <0.001 |
| GM | −20.8 | ±59.99 | 39.9 | ±68.82 | <0.001 |
| CIP | −24.2 | ±61.78 | 52.8 | ±47.19 | <0.001 |
| COL | 75.6 | ±20.73 | 82.3 | ±6.41 | <0.001 |
| AM | −18.3 | ±91.26 | 68.1 | ±39.66 | <0.001 |
| ODatb | ODcomb | BD % | SD | BDcomb % | SD | |
|---|---|---|---|---|---|---|
| CAZ | 0.607594 | 0.521313 | 4.75 | ±14.80 | 18.81 | ±9.72 |
| FEP | 0.491531 | 0.495109 | 21.00 | ±12.42 | 22.35 | ±12.15 |
| TZP | 0.648563 | 0.552984 | −4.64 | ±12.71 | 13.24 | ±13.23 |
| IMI | 0.490469 | 0.467781 | 23.20 | ±9.57 | 27.59 | ±12.48 |
| GM | 0.55825 | 0.539375 | 11.93 | ±9.69 | 14.85 | ±15.81 |
| CIP | 0.644125 | 0.572797 | −4.15 | ±10.95 | 10.26 | ±14.79 |
| COL | 0.465239 | 0.495609 | 26.72 | ±17.43 | 23.94 | ±12.14 |
| AM | 0.580547 | 0.526031 | 10.77 | ±10.17 | 19.48 | ±13.24 |
| FOM | 0.562453 | 11.51 | ±11.28 |
| ODatb | ODcomb | BD % | SD | BDcomb % | SD | |
|---|---|---|---|---|---|---|
| CAZ | 0.625203 | 0.670141 | 8.49 | ±16.87 | 2.71 | ±19.91 |
| FEP | 0.593656 | 0.651391 | 11.91 | ±12.37 | 2.77 | ±12.91 |
| TZP | 0.561375 | 0.648823 | 16.70 | ±11.89 | 3.95 | ±10.97 |
| IMI | 0.590844 | 0.568 | 10.82 | ±11.63 | 14.02 | ±13.31 |
| GM | 0.586094 | 0.640443 | 13.68 | ±6.44 | 4.01 | ±9.45 |
| CIP | 0.601026 | 0.597516 | 10.70 | ±8.51 | 9.94 | ±17.19 |
| COL | 0.625568 | 0.62611 | 8.24 | ±8.52 | 7.54 | ±12.06 |
| AM | 0.636359 | 0.572573 | 6.61 | ±13.19 | 15.38 | ±11.91 |
| FOM | 0.678063 | −3.81 | ±23.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slade-Vitković, M.; Batarilo, I.; Bielen, L.; Maravić-Vlahoviček, G.; Bedenić, B. In Vitro Antibiofilm Activity of Fosfomycin Alone and in Combination with Other Antibiotics against Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa. Pharmaceuticals 2024, 17, 769. https://doi.org/10.3390/ph17060769
Slade-Vitković M, Batarilo I, Bielen L, Maravić-Vlahoviček G, Bedenić B. In Vitro Antibiofilm Activity of Fosfomycin Alone and in Combination with Other Antibiotics against Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa. Pharmaceuticals. 2024; 17(6):769. https://doi.org/10.3390/ph17060769
Chicago/Turabian StyleSlade-Vitković, Mia, Ivanka Batarilo, Luka Bielen, Gordana Maravić-Vlahoviček, and Branka Bedenić. 2024. "In Vitro Antibiofilm Activity of Fosfomycin Alone and in Combination with Other Antibiotics against Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa" Pharmaceuticals 17, no. 6: 769. https://doi.org/10.3390/ph17060769
APA StyleSlade-Vitković, M., Batarilo, I., Bielen, L., Maravić-Vlahoviček, G., & Bedenić, B. (2024). In Vitro Antibiofilm Activity of Fosfomycin Alone and in Combination with Other Antibiotics against Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa. Pharmaceuticals, 17(6), 769. https://doi.org/10.3390/ph17060769





