Green-Synthesized Characterization, Antioxidant and Antibacterial Applications of CtAC/MNPs-Ag Nanocomposites
Abstract
:1. Introduction
2. Result and Discussion
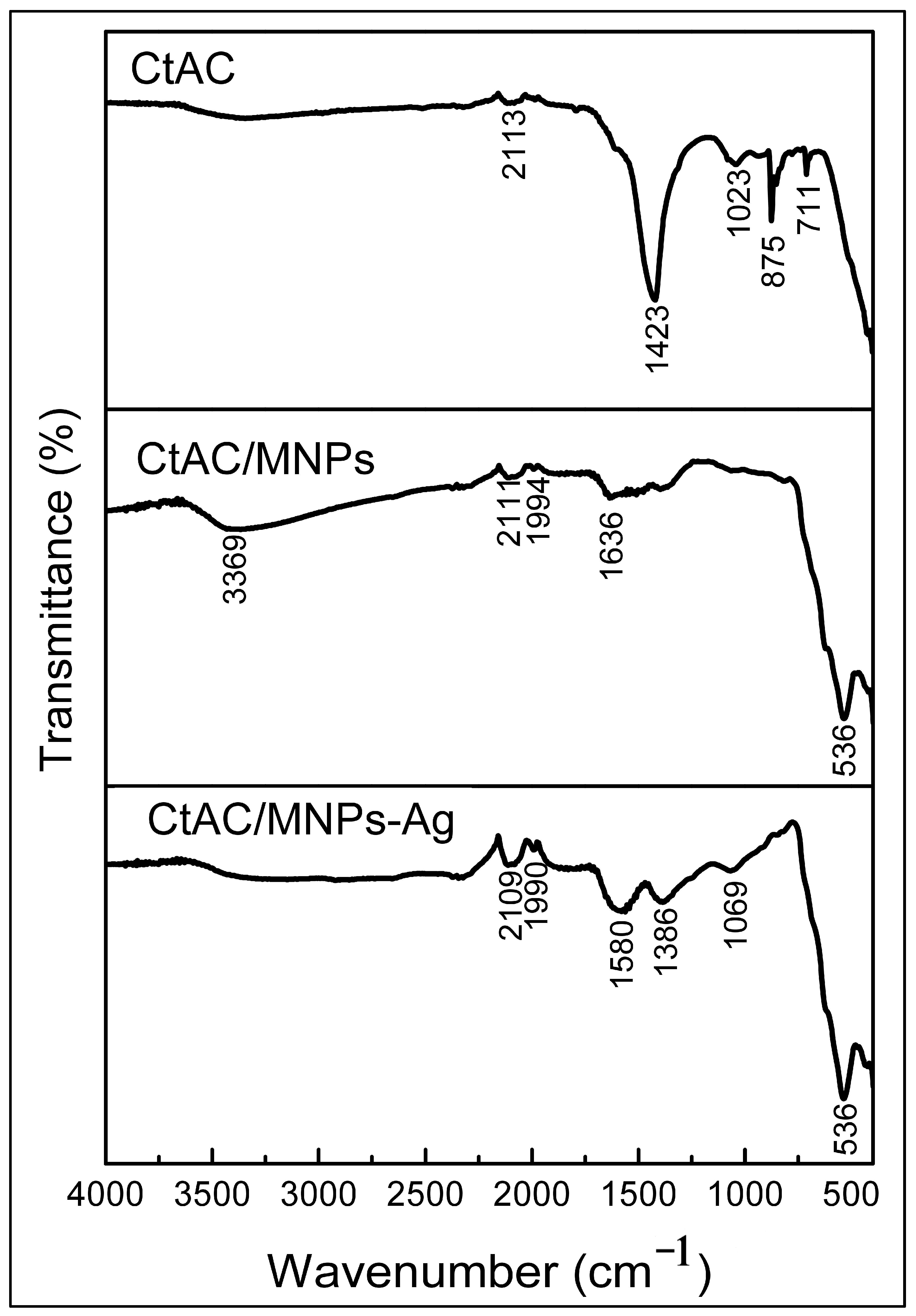
2.1. FT-IR Analysis
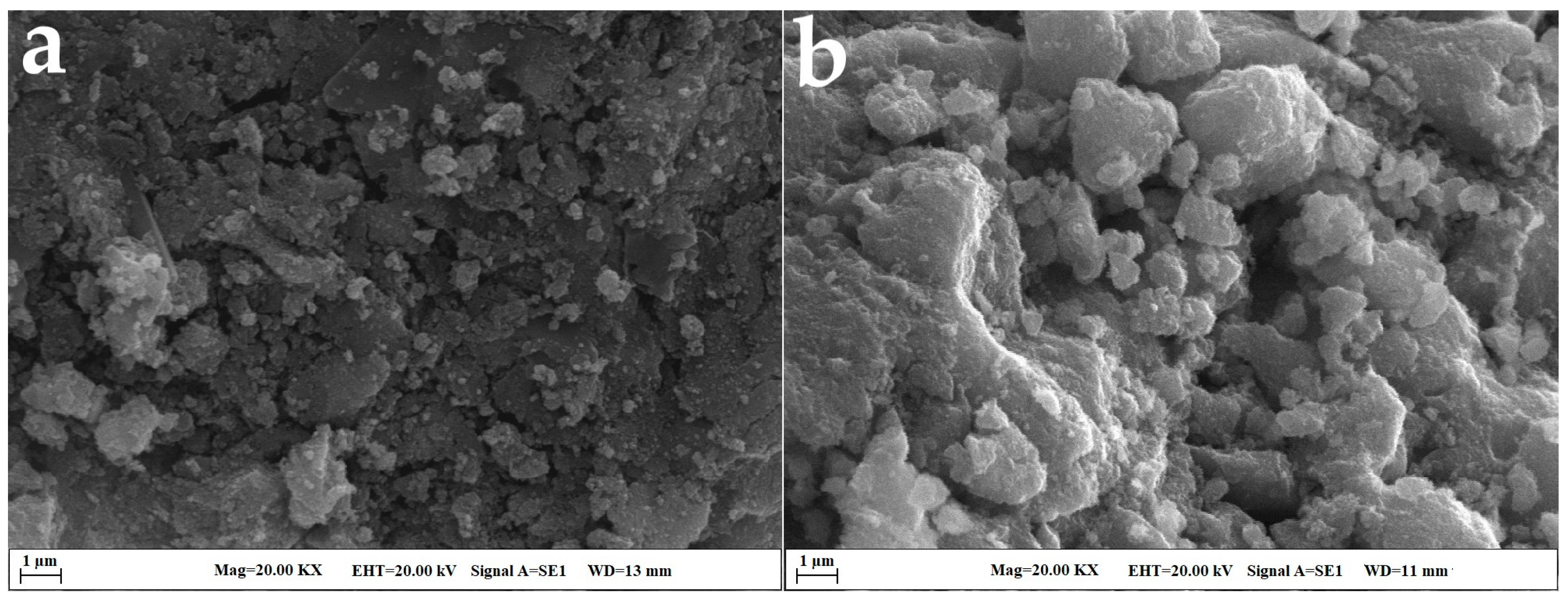
2.2. SEM and EDX Analysis
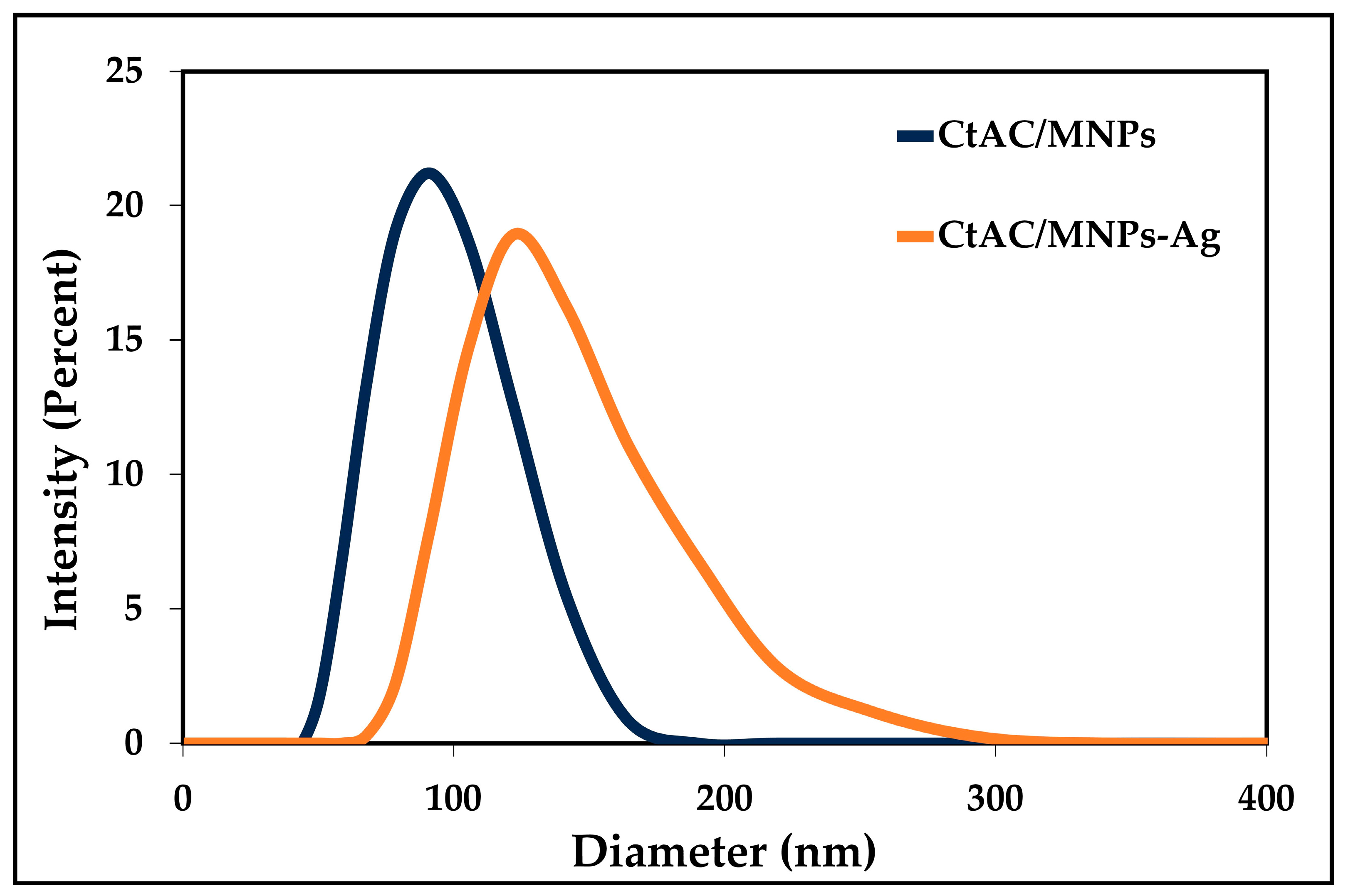
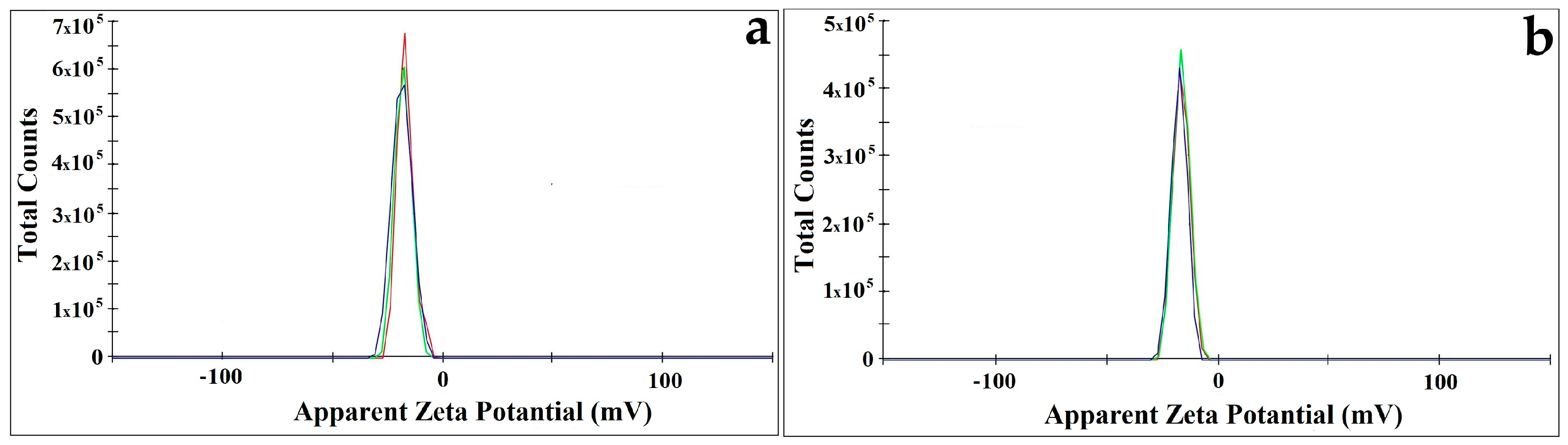
2.3. Particle Size and Zeta Potential Analysis
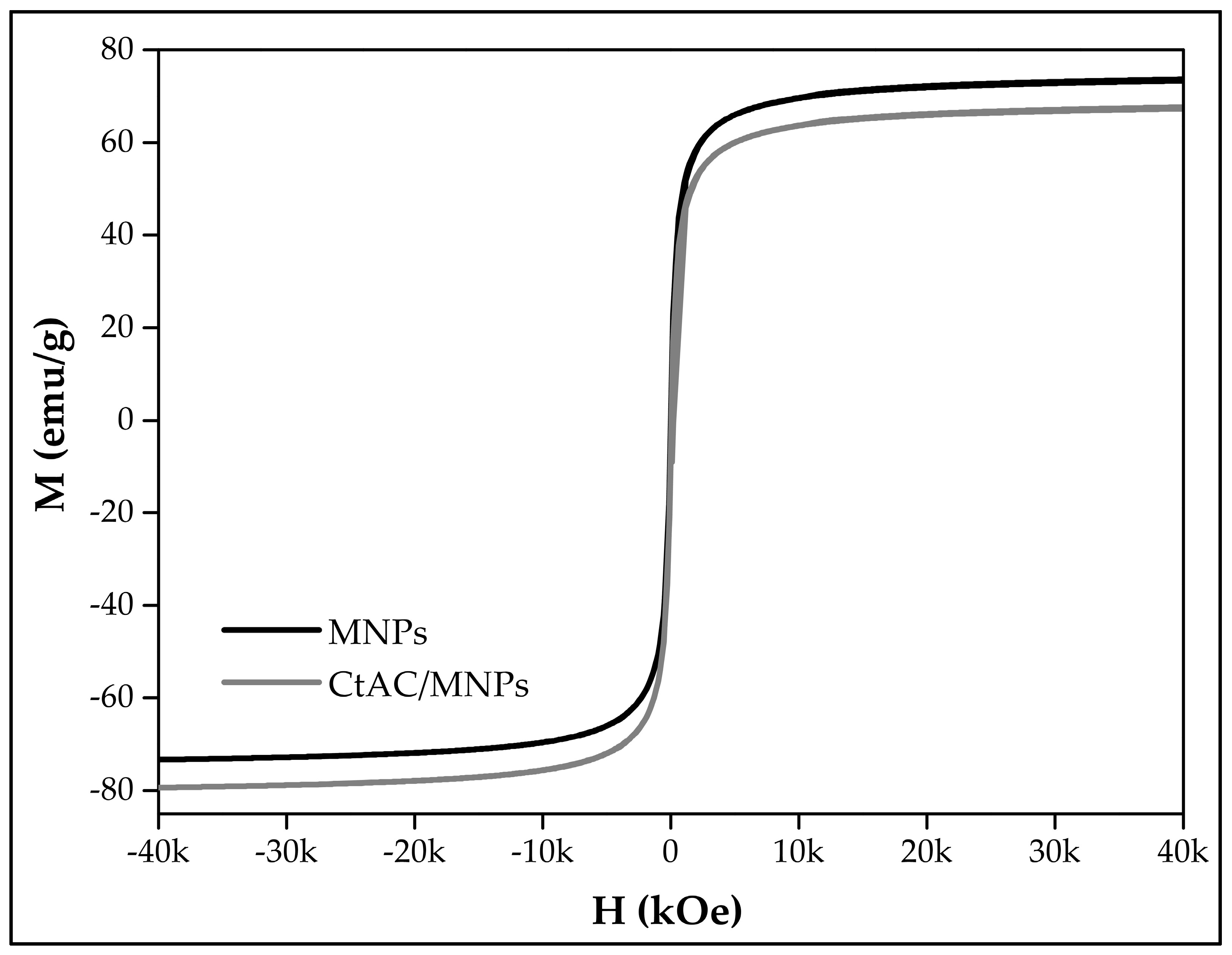
2.4. VSM Analysis
2.5. XRD Analysis CtAC/MNPs and CtAC/MNPs-Ag Nanocomposites
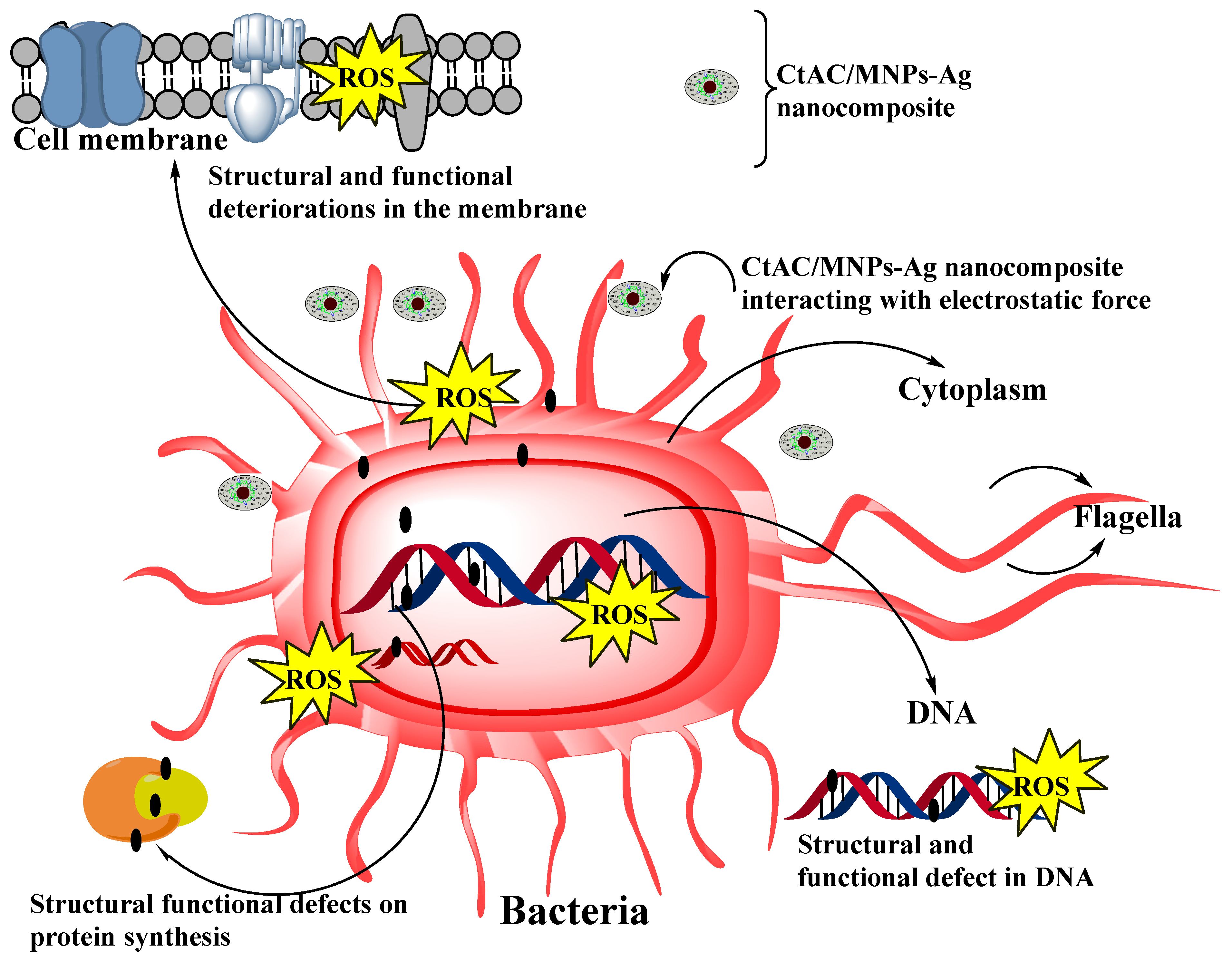
2.6. CtAC/MNPs and CtAC/MNPs-Ag Nanocomposites Antibacterial Effect
2.7. The Total Phenolic Content
2.8. Antioxidant Capacity
2.9. Comparison of Antimicrobial Effect of Various Silver Nanoparticles
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of CtAC
3.3. Synthesis of CtAC/MNPs Nanocomposite
3.4. Synthesis of CtAC/MNPs-Ag Nanocomposite
3.5. Characterization
3.6. CtAC/MNPs-Ag and CtAC/MNPs Nanocomposites Antibacterial Effect
3.7. Determination of Total Phenol Content
3.8. Determination of the Efficacy of CtAC/MNPs-Ag Nanocomposite to Scavenge DPPH Radicals
3.9. Determination of Reduction Force According to the Cuprac Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalilov, R.; Bakishzade, A.; Nasibova, A. Future prospects of biomaterials in nanomedicine. Adv. Biol. Earth Sci. 2024, 9, 5–10. [Google Scholar] [CrossRef]
- Erdil, N. Cardiovascular disease, signaling, gene/cell therapy and advanced nanobiomaterials. Adv. Biol. Earth Sci. 2024, 9, 58–80. [Google Scholar] [CrossRef]
- Salahshour, P.; Abdolmaleki, S.; Monemizadeh, S.; Gholizadeh, S.; Khaksar, S. Nanobiomaterials/bioinks based scaffolds in 3d bioprinting for tissue engineering and artificial human organs. Adv. Biol. Earth Sci. 2024, 9, 97–104. [Google Scholar] [CrossRef]
- Babanli, S.; Ahmadov, I.; Khalilov, R. Effect of nano zinc oxide on the chlorophyll content of bean plants under drought stress. Adv. Biol. Earth Sci. 2024, 9, 40–52. [Google Scholar] [CrossRef]
- Li, B.; Wang, W.; Zhao, L.; Li, M.; Yan, D.; Li, X.; Zhang, J.; Gao, Q.; Feng, Y.; Zheng, J.; et al. Aggregation-Induced Emission-Based Macrophage-Like Nanoparticles for Targeted Photothermal Therapy and Virus Transmission Blockage in Monkeypox. Adv. Mater. 2023, 36, e2305378. [Google Scholar] [CrossRef]
- Jiang, Z.; Shi, H.; Tang, X.; Qin, J. Recent advances in droplet microfluidics for single-cell analysis. TrAC Trends Anal. Chem. 2023, 159, 116932. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.; Tewari, D.; Yadav, A.B.; Sharma, N.; Bawarig, S.; García-Betancourt, M.-L.; Karatutlu, A.; Bechelany, M.; Barhoum, A. Cutting-edge advances in tailoring size, shape, and functionality of nanoparticles and nanostructures: A review. J. Taiwan Inst. Chem. Eng. 2023, 149, 105010. [Google Scholar] [CrossRef]
- Prasad, R.B.; Prasad, S.R.; Singha, S.B.; Sinha, P.; Saxena, S.; Vaidya, A.K.; Teli, S.B.B.; Saxena, U.R.; Harale, A.; Deshmukh, M.B.; et al. A Review on Modern Characterization Techniques for Analysis of Nanomaterials and Biomaterials. ES Energy Environ. 2024, 23, 1087. [Google Scholar] [CrossRef]
- Gawai, A.A.; Sarode, R.J.; Biyani, K.R. A Review on nanoparticles, their preparation and applications. Int. J. Pharma Sci. 2020, 11, 6540. [Google Scholar]
- Salem, S.S.; Hammad, E.N.; Mohamed, A.A.; El-Dougdoug, W. A comprehensive review of nanomaterials: Types, synthesis, characterization, and applications. Biointerface Res. Appl. Chem. 2022, 13, 41. [Google Scholar]
- Tadic, M.; Kopanja, L.; Panjan, M.; Lazovic, J.; Tadic, B.V.; Stanojevic, B.; Motte, L. Rhombohedron and plate-like hematite (α-Fe2O3) nanoparticles: Synthesis, structure, morphology, magnetic properties and potential biomedical applications for MRI. Mater. Res. Bull. 2021, 133, 111055. [Google Scholar] [CrossRef]
- Zhang, W.; Taheri-Ledari, R.; Ganjali, F.; Mirmohammadi, S.S.; Qazi, F.S.; Saeidirad, M.; KashtiAray, A.; Zarei-Shokat, S.; Tian, Y.; Maleki, A. Effects of morphology and size of nanoscale drug carriers on cellular uptake and internalization process: A review. RSC Adv. 2022, 13, 80–114. [Google Scholar] [CrossRef] [PubMed]
- Nair, G.M.; Sajini, T.; Mathew, B. Advanced green approaches for metal and metal oxide nanoparticles synthesis and their environmental applications. Talanta Open 2022, 5, 100080. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Ibrahim, M.N.M. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A. Silver nanoparticles: Synthesis, characterisation and biomedical applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef] [PubMed]
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. J. Nanomater. 2021, 2021, 1–23. [Google Scholar] [CrossRef]
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Uddin, F.; Kamran, A.W.; Kallistova, A.; Pimenov, N.; et al. A Review on Silver Nanoparticles: Classification, Various Methods of Synthesis, and Their Potential Roles in Biomedical Applications and Water Treatment. Water 2021, 13, 2216. [Google Scholar] [CrossRef]
- Rosic, G.; Selakovic, D.; Omarova, S. Cancer signaling, cell/gene therapy, diagnosis and role of nanobiomaterials. Adv. Biol. Earth Sci. 2024, 9, 11–34. [Google Scholar] [CrossRef]
- Chew, T.W.; H’ng, P.S.; Abdullah, B.C.T.G.L.C.; Chin, K.L.; Lee, C.L.; Hafizuddin, B.M.S.M.N.; TaungMai, L. A Review of Bio-Based Activated Carbon Properties Produced from Different Activating Chemicals during Chemicals Activation Process on Biomass and Its Potential for Malaysia. Materials 2023, 16, 7365. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Ahmadpour, A. Using of activated carbon adsorption in wastewater industries. J. Chem. Lett. 2022, 3, 2–9. [Google Scholar]
- Luceri, A.; Francese, R.; Lembo, D.; Ferraris, M.; Balagna, C. Silver Nanoparticles: Review of Antiviral Properties, Mechanism of Action and Applications. Microorganisms 2023, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Jiang, Z.; Akakuru, O.U.; Li, J.; Wu, A. Nanoscale covalent organic frameworks: From controlled synthesis to cancer therapy. Chem. Commun. 2021, 57, 12417–12435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Tao, Y.; Chen, S.; Lu, Y. Preparation and properties of the silver loaded activated carbon fibers. Fibers Polym. 2015, 16, 2003–2010. [Google Scholar] [CrossRef]
- Kazmi, S.J.; Shehzad, M.; Mehmood, S.; Yasar, M.; Naeem, A.; Bhatti, A. Effect of varied Ag nanoparticles functionalized CNTs on its anti-bacterial activity against E. coli. Sens. Actuators A Phys. 2014, 216, 287–294. [Google Scholar] [CrossRef]
- Chingombe, P.; Saha, B.; Wakeman, R.J. Sorption of atrazine on conventional and surface modified activated carbons. J. Colloid Interface Sci. 2006, 302, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Li, L.; Yin, Z.-J.; Quan, Y.-Y.; Tan, R.-R.; Chen, S.-L.; Lang, J.-R.; Li, J.; Zeng, J.; Li, Y.; et al. Polypeptide from Moschus Suppresses Lipopolysaccharide-Induced Inflammation by Inhibiting NF-κ B-ROS/NLRP3 Pathway. Chin. J. Integr. Med. 2023, 29, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Fatima, T.; Mushtaq, A. Efficacy and challenges of carbon-based nanomaterials in water treatment: A review. Int. J. Chem. Biochem. Sci. 2023, 23, 232–248. [Google Scholar]
- Baabu, P.R.S.; Kumar, H.K.; Gumpu, M.B.; Babu, K.J.; Kulandaisamy, A.J.; Rayappan, J.B.B. Iron Oxide Nanoparticles: A Review on the Province of Its Compounds, Properties and Biological Applications. Materials 2022, 16, 59. [Google Scholar] [CrossRef]
- Baran, A.; Keskin, C.; Kandemir, S.İ. Rapid biosynthesis of silver nanoparticles by Celtis tournefortii LAM. leaf extract; investigation of antimicrobial and anticancer activities. KSÜ Tarım Doğa Derg. 2022, 25, 72–84. [Google Scholar] [CrossRef]
- Baran, A.; Keskin, C. Determination of Constituents of Extract of Celtis tournefortii Lam. by LC-MS/MS, Investigation of Enzyme Inhibition, Antimicrobial and Anticancer Effects. Int. J. Pure Appl. Sci. 2023, 9, 56–65. [Google Scholar] [CrossRef]
- Hamat, N.A.B.A.C.; Phaik-Ching, A.; Ibrahim, M.N.M.; Abu Bakar, N.H.H.; Tan, T.-W.; Amin, N.F.B.M.; Garba, Z.N.; Perumal, V.; Raja, P.B. Cocos Nucifera’s lignin mediated silver nanoparticle and their photocatalytic activity. Inorg. Chem. Commun. 2024, 160, 111845. [Google Scholar] [CrossRef]
- Maniom, M.; Gunasegaran, Y.R. Performance of Waste Tire Activated Carbon on Methylene Blue. Multidiscip. Appl. Res. Innov. 2023, 4, 83–90. [Google Scholar]
- Veerakumar, P.; Muthuselvam, I.P.; Hung, C.-T.; Lin, K.-C.; Chou, F.-C.; Liu, S.-B. Biomass-Derived Activated Carbon Supported Fe3O4 Nanoparticles as Recyclable Catalysts for Reduction of Nitroarenes. ACS Sustain. Chem. Eng. 2016, 4, 6772–6782. [Google Scholar] [CrossRef]
- Islam, M.A.; Tan IA, W.; Benhouria, A.; Asif, M.; Hameed, B.H. Mesoporous and adsorptive properties of palm date seed activated carbon prepared via sequential hydrothermal carbonization and sodium hydroxide activation. Chem. Eng. J. 2015, 270, 187–195. [Google Scholar] [CrossRef]
- Anyika, C.; Asri, N.A.M.; Majid, Z.A.; Yahya, A.; Jaafar, J. Synthesis and characterization of magnetic activated carbon developed from palm kernel shells. Nanotechnol. Environ. Eng. 2017, 2, 16. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Wang, Z.; Tahir, M.H.; Wang, Z.; Wang, X.; Wang, C. Full recycling of high-value resources from cabbage waste by multi-stage utilization. Sci. Total Environ. 2022, 804, 149951. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Zhang, W.; Wu, Z.; Liu, R.; Yang, C.; Hui, A.; Huang, X.; Xian, Z. Preparation, characterization, antioxidant and anti-inflammatory activities of acid-soluble pectin from okra (Abelmoschus esculentus L.). Int. J. Biol. Macromol. 2021, 181, 824–834. [Google Scholar] [CrossRef]
- Kristianto, H.; Arie, A.A.; Susanti, R.F.; Halim, M.; Lee, J.K. The effect of activated carbon support surface modification on characteristics of carbon nanospheres prepared by deposition precipitation of Fe-catalyst. IOP Conf. Ser. Mater. Sci. Eng. 2016, 162, 012034. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Tran, H.N.; Juang, R.-S.; Dat, N.D.; Tomul, F.; Ivanets, A.; Woo, S.H.; Hosseini-Bandegharaei, A.; Nguyen, V.P.; Chao, H.-P. Adsorption process and mechanism of acetaminophen onto commercial activated carbon. J. Environ. Chem. Eng. 2020, 8, 104408. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Mahvi, A.H.; Khatibi, A.D.; Balarak, D. Effective adsorption of ciprofloxacin antibiotic using powdered activated carbon magnetized by iron(III) oxide magnetic nanoparticles. J. Porous Mater. 2021, 28, 835–852. [Google Scholar] [CrossRef]
- Kakavandi, B.; Jonidi, A.; Rezaei, R.; Nasseri, S.; Ameri, A.; Esrafily, A. Synthesis and properties of Fe3O4-activated carbon magnetic nanoparticles for removal of aniline from aqueous solution: Equilibrium, kinetic and thermodynamic studies. Iran. J. Environ. Health Sci. Eng. 2013, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Chishti, A.N.; Guo, F.; Aftab, A.; Ma, Z.; Liu, Y.; Chen, M.; Diao, G. Synthesis of silver doped Fe3O4/C nanoparticles and its catalytic activities for the degradation and reduction of methylene blue and 4-nitrophenol. Appl. Surf. Sci. 2021, 546, 149070. [Google Scholar] [CrossRef]
- Amiri, O.; Beshkar, F.; Ahmed, S.S.; Hamad, B.W.; Mahmood, P.H.; Dezaye, A.A. Magnetically-driven Ag/Fe3O4/graphene ternary nanocomposite as efficient photocatalyst for desulfurization of thiophene under visible-light irradiation. Int. J. Hydrog. Energy 2021, 46, 19913–19925. [Google Scholar] [CrossRef]
- Huang, T.; AlSalem, H.S.; Binkadem, M.S.; Al-Goul, S.T.; El-Kott, A.F.; Alsayegh, A.A.; Majdou, G.J.; Batiha, G.E.-S.; Karmakar, B. Green synthesis of Ag/Fe3O4 nanoparticles using Mentha extract: Preparation, characterization and investigation of its anti-human lung cancer application. J. Saudi Chem. Soc. 2022, 26, 101505. [Google Scholar] [CrossRef]
- Ravichandran, R.; Annamalai, K.; Annamalai, A.; Elumalai, S. Solid state–Green construction of starch- beaded Fe3O4@Ag nanocomposite as superior redox catalyst. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131117. [Google Scholar] [CrossRef]
- Pop, D.; Buzatu, R.; Moacă, E.-A.; Watz, C.G.; Pînzaru, S.C.; Tudoran, L.B.; Nekvapil, F.; Avram, Ș.; Dehelean, C.A.; Crețu, M.O.; et al. Development and Characterization of Fe3O4@Carbon Nanoparticles and Their Biological Screening Related to Oral Administration. Materials 2021, 14, 3556. [Google Scholar] [CrossRef] [PubMed]
- Lopes, K.L.; de Oliveira, H.L.; Serpa, J.A.S.; Torres, J.A.; Nogueira, F.G.E.; de Freitas, V.A.A.; Borges, K.B.; Silva, M.C. Nanomagnets based on activated carbon/magnetite nanocomposite for determination of endocrine disruptors in environmental water samples. Microchem. J. 2021, 168, 106366. [Google Scholar] [CrossRef]
- Rodrigues, S.C.; Silva, M.C.; Torres, J.A.; Bianchi, M.L. Use of Magnetic Activated Carbon in a Solid Phase Extraction Procedure for Analysis of 2,4-dichlorophenol in Water Samples. Water Air Soil Pollut. 2020, 231, 294. [Google Scholar] [CrossRef]
- Forest, V.; Pourchez, J. Preferential binding of positive nanoparticles on cell membranes is due to electrostatic interactions: A too simplistic explanation that does not take into account the nanoparticle protein corona. Mater. Sci. Eng. C 2017, 70, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-Z.; Zheng, D.-F.; Mo, Z.-Y.; Dong, R.-J.; Qiu, X.-Q. Magnetic lignin-based carbon nanoparticles and the adsorption for removal of methyl orange. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 226–234. [Google Scholar] [CrossRef]
- Patil, M.P.; Singh, R.D.; Koli, P.B.; Patil, K.T.; Jagdale, B.S.; Tipare, A.R.; Kim, G.-D. Antibacterial potential of silver nanoparticles synthesized using Madhuca longifolia flower extract as a green resource. Microb. Pathog. 2018, 121, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Reith, J.; Mayer, C. Peptidoglycan turnover and recycling in Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2011, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Anbalagan, K.; Manosathyadevan, M.; Lee, K.-J.; Cho, M.; Lee, S.-M.; Park, J.-H.; Oh, S.-G.; Bang, K.-S.; Oh, B.-T. Green synthesis of silver and gold nanoparticles using Zingiber officinale root extract and antibacterial activity of silver nanoparticles against food pathogens. Bioprocess Biosyst. Eng. 2014, 37, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Hashmi, A.; Khan, M.S.; Musarrat, J. ROS mediated destruction of cell membrane, growth and biofilms of human bacterial pathogens by stable metallic AgNPs functionalized from bell pepper extract and quercetin. Adv. Powder Technol. 2018, 29, 1601–1616. [Google Scholar] [CrossRef]
- Hatipoğlu, A.; Baran, A.; Keskin, C.; Baran, M.F.; Eftekhari, A.; Omarova, S.; Janas, D.; Khalilov, R.; Adican, M.T.; Kandemir, S.I. Green synthesis of silver nanoparticles based on the Raphanus sativus leaf aqueous extract and their toxicological/microbiological activities. Environ. Sci. Pollut. Res. 2023, 1–13. [Google Scholar] [CrossRef]
- Susanti, D.; Haris, M.S.; Taher, M.; Khotib, J. Natural Products-Based Metallic Nanoparticles as Antimicrobial Agents. Front. Pharmacol. 2022, 13, 895616. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Alzahrani, F.M.; Amari, A.; Osman, H.; Harharah, H.N.; Elboughdiri, N.; Tahoon, M.A. Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives. Molecules 2023, 28, 463. [Google Scholar] [CrossRef]
- Keskin, C.; Ölçekçi, A.; Baran, A.; Baran, M.F.; Eftekhari, A.; Omarova, S.; Khalilov, R.; Aliyev, E.; Sufianov, A.; Beilerli, A.; et al. Green synthesis of silver nanoparticles mediated Diospyros kaki L. (Persimmon): Determination of chemical composition and evaluation of their antimicrobials and anticancer activities. Front. Chem. 2023, 11, 1187808. [Google Scholar] [CrossRef]
- Anand, K.K.H.; Mandal, B.K. Activity study of biogenic spherical silver nanoparticles towards microbes and oxidants. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothi, P.; Sreekanth, T.; Lee, J.-I.; Lee, K.D. Mycosynthesis: Antibacterial, antioxidant and antiproliferative activities of silver nanoparticles synthesized from Inonotus obliquus (Chaga mushroom) extract. J. Photochem. Photobiol. B Biol. 2014, 130, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Geng, Y.; Wan, H.; Xu, Y.; Zhou, Y.; Kong, X.; Wang, J.; Qi, Y.; Yao, B.; Gao, Z. Facile preparation of Fe3O4/Ag/RGO reusable ternary nanocomposite and its versatile application as catalyst and antibacterial agent. J. Alloys Compd. 2021, 876, 160153. [Google Scholar] [CrossRef]
- Furlan, P.Y.; Fisher, A.J.; Furlan, A.Y.; Melcer, M.E.; Shinn, D.W.; Warren, J.B. Magnetically Recoverable and Reusable Antimicrobial Nanocomposite Based on Activated Carbon, Magnetite Nanoparticles, and Silver Nanoparticles for Water Disinfection. Inventions 2017, 2, 10. [Google Scholar] [CrossRef]
- Qamar, M.A.; Javed, M.; Shahid, S. Designing and investigation of enhanced photocatalytic and antibacterial properties of 3d (Fe, Co, Ni, Mn and Cr) metal-doped zinc oxide nanoparticles. Opt. Mater. 2022, 126, 112211. [Google Scholar] [CrossRef]
- Elakremi, M.; Sillero, L.; Ayed, L.; Mannai, F.; BEN Salem, R.; Labidi, J.; Moussaoui, Y. Chemical composition and biological activity of Pistacia vera L. leaves: Beneficial effects of female leaves extract on food products. Cellul. Chem. Technol. 2022, 56, 309–319. [Google Scholar] [CrossRef]
- Senthilkumar, S.R.; Sivakumar, T.; Arulmozhi, K.T.; Mythili, N. FT-IR analysis and correlation studies on the antioxidant activity, total phenolics and total flavonoids of Indian commercial teas (Camellia sinensis L.)-A novel approach. Int. Res. J. Biol. Sci. 2017, 6, 1–7. [Google Scholar]
- Shah, A.T.; Din, M.I.; Bashir, S.; Qadir, M.A.; Rashid, F. Green Synthesis and Characterization of Silver Nanoparticles Using Ferocactus echidne Extract as a Reducing Agent. Anal. Lett. 2015, 48, 1180–1189. [Google Scholar] [CrossRef]
- Abdelkader, H.; Alzahrani, H.; Al-Ayafi, A.; Al-Mulah, H.; Al-Zubaidi, S. Green synthesis, characterization and antimicrobial activity of biosynthesized silver nanoparticles using Ziziphus spina-christi leaf extracts. Adv. Microbiol. Res. 2019, 3, 10. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Mohamed, B.S.; Zayed, M.; El-Sabbagh, S.M. Antibacterial, antibiofilm, and anticancer activity of silver-nanoparticles synthesized from the cell-filtrate of Streptomyces enissocaesilis. BMC Biotechnol. 2024, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Choi, H.-J.; Lim, J.-A.; Woo, M.-A.; Chang, H.-J.; Lee, N.; Lim, M.-C. Biosynthesis of Silver Nanoparticles from Duchesnea indica Extracts Using Different Solvents and Their Antibacterial Activity. Microorganisms 2023, 11, 1539. [Google Scholar] [CrossRef] [PubMed]
- Ramasundaram, S.; Manikandan, V.; Vijayalakshmi, P.; Devanesan, S.; Bin Salah, M.; Babu, A.R.; Priyadharsan, A.; Oh, T.H.; Ragupathy, S. Synthesis and investigation on synergetic effect of activated carbon loaded silver nanoparticles with enhanced photocatalytic and antibacterial activities. Environ. Res. 2023, 233, 116431. [Google Scholar] [CrossRef] [PubMed]
- Alzubaidi, A.K.; Al-Kaabi, W.J.; Al Ali, A.; Albukhaty, S.; Al-Karagoly, H.; Sulaiman, G.M.; Asiri, M.; Khane, Y. Green Synthesis and Characterization of Silver Nanoparticles Using Flaxseed Extract and Evaluation of Their Antibacterial and Antioxidant Activities. Appl. Sci. 2023, 13, 2182. [Google Scholar] [CrossRef]
- Habib, U.; Ahmad Khan, A.; Rahman, T.U.; Zeb, M.A.; Liaqat, W. Green synthesis, characterization, and antibacterial activity of silver nanoparticles using stem extract of Zanthoxylum armatum. Microsc. Res. Tech. 2022, 85, 3830–3837. [Google Scholar] [CrossRef] [PubMed]
- Jamaludin, N.F.M.; Jamali, N.S.; Abdullah, L.C.; Idrus, S.; Engliman, N.S.; Abdul, P.M. Biohydrogen production with utilisation of magnetite nanoparticles embedded in granular activated carbon from coconut shell. Int. J. Hydrog. Energy 2023, 48, 11695–11708. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Peighambardoust, S.H.; Pateiro, M.; Lorenzo, J.M. Adsorption of crystal violet dye using activated carbon of lemon wood and activated carbon/Fe3O4 magnetic nanocomposite from aqueous solutions: A kinetic, equilibrium and thermodynamic study. Molecules 2021, 26, 2241. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Asthana, A.; Chakraborty, R.; Jain, B.; Singh, A.K.; Carabineiro, S.A.C.; Susan, A.B.H. Cationic Dye Removal Using Novel Magnetic/Activated Charcoal/β-Cyclodextrin/Alginate Polymer Nanocomposite. Nanomaterials 2020, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Tomke, P.D.; Rathod, V.K. Facile fabrication of silver on magnetic nanocomposite (Fe3O4@ Chitosan–AgNP nanocomposite) for catalytic reduction of anthropogenic pollutant and agricultural pathogens. Int. J. Biol. Macromol. 2020, 149, 989–999. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, P.; Wei, Y.; Jin, Y.; Sun, S.; Wang, Z.; Jiang, Y. Silver nanoparticles decorated magnetic polymer composites (Fe3O4@PS@Ag) as highly efficient reusable catalyst for the degradation of 4-nitrophenol and organic dyes. J. Environ. Manag. 2021, 278, 111473. [Google Scholar] [CrossRef]
- Acay, H.; Baran, M.F.; Eren, A. Investigating antimicrobial activity of silver nanoparticles produced through green synthesis using leaf extract of common grape (Vitis vinifera). Appl. Ecol. Environ. Res. 2019, 17, 4539–4546. [Google Scholar] [CrossRef]
- Zapata, A.; Ramirez-Arcos, S. A Comparative Study of McFarland Turbidity Standards and the Densimat Photometer to Determine Bacterial Cell Density. Curr. Microbiol. 2015, 70, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Srećković, N.Z.; Nedić, Z.P.; Monti, D.M.; D’Elia, L.; Dimitrijević, S.B.; Mihailović, N.R.; Mihailović, V.B. Biosynthesis of silver nanoparticles using Salvia pratensis L. aerial part and root extracts: Bioactivity, biocompatibility, and catalytic potential. Molecules 2023, 28, 1387. [Google Scholar] [CrossRef] [PubMed]
- Nagalingam, M.; Rajeshkumar, S.; Balu, S.K.; Tharani, M.; Arunachalam, K. Anticancer and Antioxidant Activity of Morinda citrifolia Leaf Mediated Selenium Nanoparticles. J. Nanomater. 2022, 2022, 1–7. [Google Scholar] [CrossRef]
- Abdel-Aty, A.M.; Barakat, A.Z.; Bassuiny, R.I.; Mohamed, S.A. Statistical optimization, characterization, antioxidant and antibacterial properties of silver nanoparticle biosynthesized by saw palmetto seed phenolic extract. Sci. Rep. 2023, 13, 15605. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Çelik, S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 2007, 160, 413–419. [Google Scholar] [CrossRef]


| MICROORGANISM | MICRODILUTION METHOD (µg/mL) | DISC DIFFUSION METHOD Inhibition Zone (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Antibiotic | CtAC/MNPs | CtAC/MNPs-Ag | Control Disc | CtAC/MNPs-Ag | Image | |||
| DW | CtAC/MNPs | CtAC/MNPs | CtAC/MNPs-Ag | |||||
| S. aureus | 1.00 | 32 | 1.17 | 1.00 | 0.00 | 12.00 |  |  |
| E. coli | 2.00 | 128 | 2.34 | 2.00 | 0.00 | 11.00 |  |  |
| Samples | Total Phenolic Content (mg GAE/g) | DPPH (%) | CUPRAC (μM TE/100 mL) |
|---|---|---|---|
| CtAC/MNPs-Ag | 10.68 ± 0.435 | 70.37 ± 0.64 | 32.70 ± 1.035 |
| Samples | S.aureus | E. coli | References |
|---|---|---|---|
| AgNP (Ferocactus echidne) | 2.10 | 1.80 | [69] |
| AgNP (Ziziphus spina-christi) | 5.20 | 4.40 | [70] |
| AgNP (Streptomyces enissocaesilis) | 16.60 | 16.30 | [71] |
| AgNP (Duchesnea ındica) | 11.64 | 11.35 | [72] |
| Ag/CNSAC | 11.00 | 8.00 | [73] |
| AgNP (Linumusitatissimum L.) | 8.90 | 11.00 | [74] |
| AgNP (Zanthoxylum armatum) | 21.00 | 19.00 | [75] |
| CtAC/MNPs-Ag | 12.00 | 11.00 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baran, A.; Ertaş, E.; Baran, M.F.; Eftekhari, A.; Gunes, Z.; Keskin, C.; Usanov, S.A.; Khalilov, R. Green-Synthesized Characterization, Antioxidant and Antibacterial Applications of CtAC/MNPs-Ag Nanocomposites. Pharmaceuticals 2024, 17, 772. https://doi.org/10.3390/ph17060772
Baran A, Ertaş E, Baran MF, Eftekhari A, Gunes Z, Keskin C, Usanov SA, Khalilov R. Green-Synthesized Characterization, Antioxidant and Antibacterial Applications of CtAC/MNPs-Ag Nanocomposites. Pharmaceuticals. 2024; 17(6):772. https://doi.org/10.3390/ph17060772
Chicago/Turabian StyleBaran, Ayşe, Erdal Ertaş, Mehmet Fırat Baran, Aziz Eftekhari, Zübeyir Gunes, Cumali Keskin, Sergey A. Usanov, and Rovshan Khalilov. 2024. "Green-Synthesized Characterization, Antioxidant and Antibacterial Applications of CtAC/MNPs-Ag Nanocomposites" Pharmaceuticals 17, no. 6: 772. https://doi.org/10.3390/ph17060772






