Phytochemical Study of the Anthelminthic Potential of Guadeloupean Plant Biodiversity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Eco Extraction Efficiency
2.2. Chitin Extraction Yield
2.3. Quantification of Polyphenols
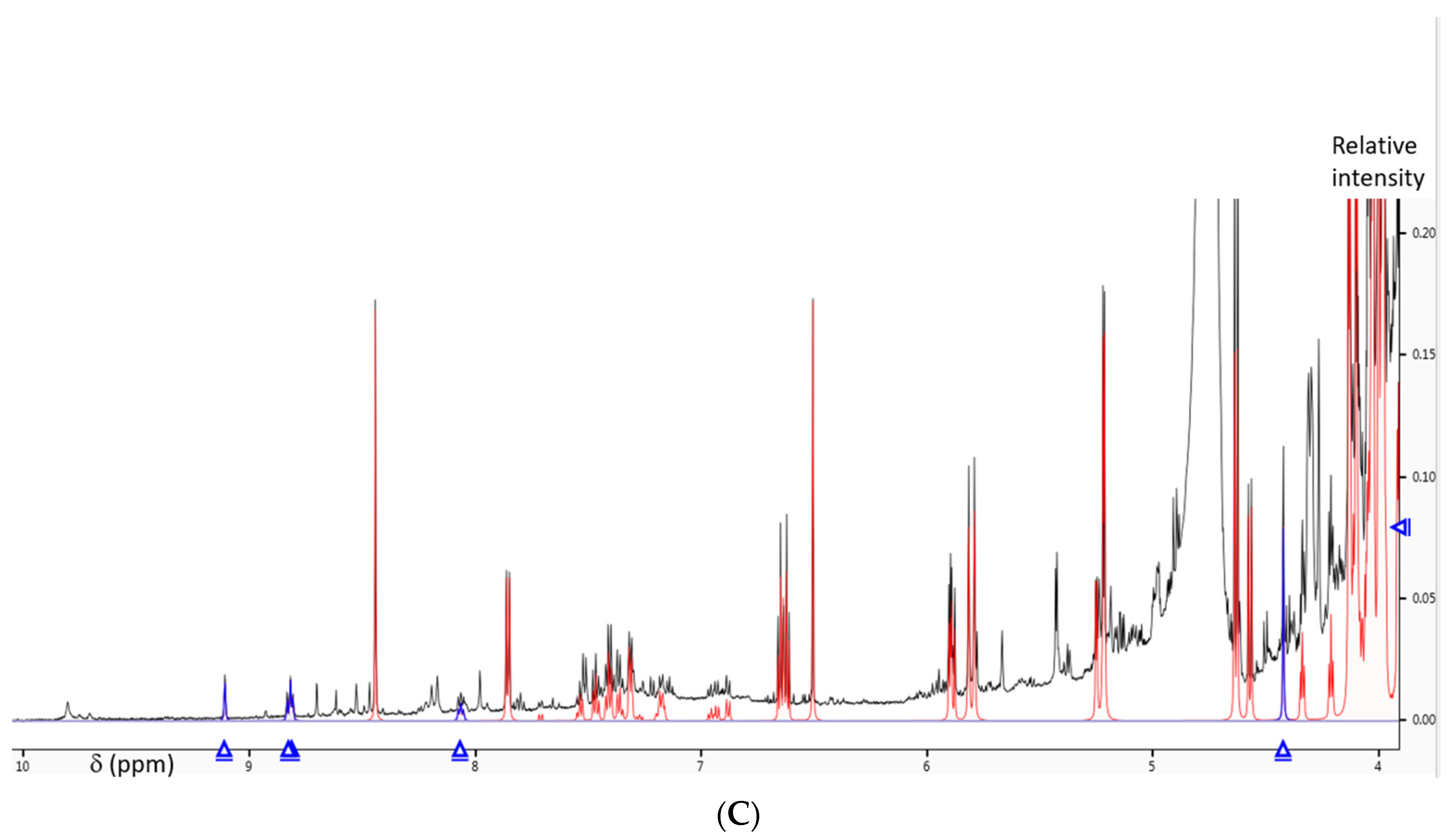
2.4. NMR-Based Structural Analysis and Metabolomics
2.5. Biological Effects of Extracts
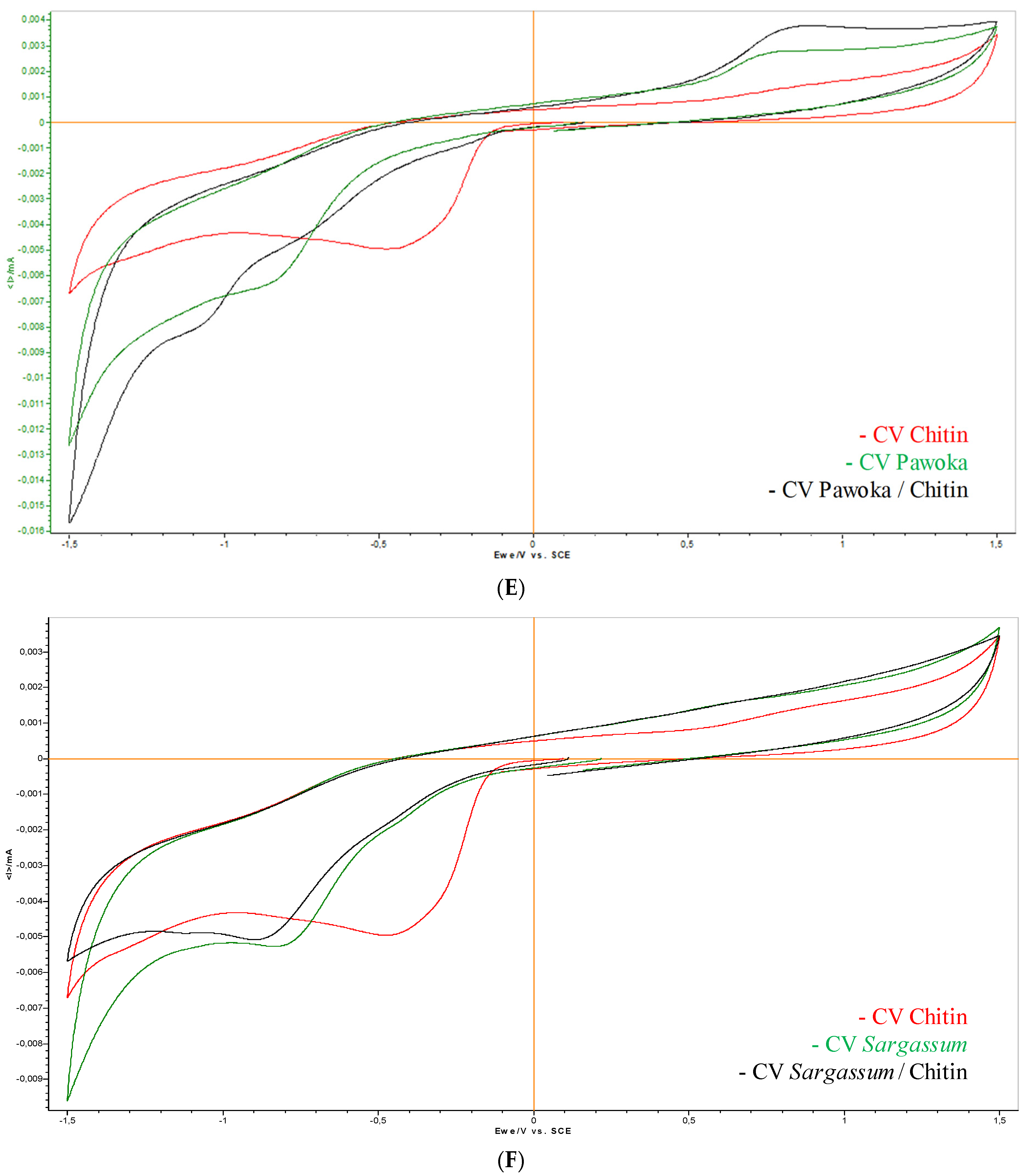
2.6. Electrochemical Screening
3. Materials and Methods
3.1. Eco Extraction
3.2. Extraction of Chitin
3.3. Quantification of Total Polyphenols
3.4. NMR-Based Structural Analysis and Metabolomics
3.5. Biological Assay
3.6. Electrochemical Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tayo, G.M.; Poné, J.W.; Komtangi, M.C.; Yondo, J.; Ngangout, A.M.; Mbida, M. Anthelminthic Activity of Moringa oleifera Leaf Extracts Evaluated in vitro on Four Developmental Stages of Haemonchus contortus from Goats. Am. J. Plant Sci. 2014, 5, 1702–1710. [Google Scholar] [CrossRef]
- Botura, M.B.; Silva, G.D.; Lima, H.G.; Oliveira, J.V.A.; Souza, T.S.; Santos, J.D.G.; Branco, A.; Moreira, E.L.T.; Almeida, M.A.O.; Batatinha, M.J.M. In vivo anthelmintic activity of an aqueous extract from sisal waste (Agave sisalana Perr.) against gastrointestinal nematodes in goats. Vet. Parasitol. 2011, 177, 104–110. [Google Scholar] [CrossRef]
- Cabardo, D.E.; Portugaliza, H.P. Anthelmintic activity of Moringa oleifera seed aqueous and ethanolic extracts against Haemonchus contortus eggs and third stage larvae. Int. J. Vet. Sci. Med. 2017, 5, 30–34. [Google Scholar] [CrossRef]
- del Carmen Acevedo-Ramírez, P.M.; Hallal-Calleros, C.; Flores-Pérez, I.; Alba-Hurtado, F.; Mendoza-Garfías, M.B.; Castro Del Campo, N.; Barajas, R. Anthelmintic effect and tissue alterations induced in vitro by hydrolysable tannins on the adult stage of the gastrointestinal nematode Haemonchus contortus. Vet. Parasitol. 2019, 266, 1–6. [Google Scholar] [CrossRef]
- Zenebe, S.; Feyera, T.; Assefa, S. In Vitro Anthelmintic Activity of Crude Extracts of Aerial Parts of Cissus quadrangularis L. and Leaves of Schinus molle L. against Haemonchus contortus. BioMed Res. Int. 2017, 2017, 1905987. [Google Scholar] [CrossRef]
- Lubega, G.W.; Prichard, R.K. Specific interaction of benzimidazole anthelmintics with tubulin from developing stages of thiabendazole-susceptible and-resistant Haemonchus contortus. Biochem. Pharmacol. 1991, 41, 93–101. [Google Scholar] [CrossRef]
- Zabré, G.; Kaboré, A.; Bayala, B.; Katiki, L.M.; Costa-Júnior, L.M.; Tamboura, H.H.; Belem, A.M.G.; Abdalla, A.L.; Niderkorn, V.; Hoste, H.; et al. Comparison of the in vitro anthelmintic effects of Acacia nilotica and Acacia raddiana. Parasite 2017, 24, 44. [Google Scholar] [CrossRef]
- Sangster, N.C.; Bjorn, H. Levamisole resistance of Haemonchus contortus Selected at Different Stages of Infection. Int. J. Parasitol. 1995, 25, 343–348. [Google Scholar] [CrossRef]
- Boulin, T.; Fauvin, A.; Charvet, C.L.; Cortet, J.; Cabaret, J.; Bessereau, J.-L.; Neveu, C. Functional reconstitution of Haemonchus contortus acetylcholine receptors in Xenopus oocytes provides mechanistic insights into levamisole resistance. Br. J. Pharmacol. 2011, 164, 1421–1432. [Google Scholar] [CrossRef]
- Chagas, A.C.S.; Figueiredo, A.; Politi, F.A.S.; Moro, I.J.; Esteves, S.N.; Bizzo, H.R.; Gama, P.E.; Chaves, F.C.M. Efficacy of essential oils from plants cultivated in the Amazonian Biome against gastrointestinal nematodes in sheep. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 2018, 42, 357–364. [Google Scholar] [CrossRef]
- Hoste, H.; Martinez-Ortiz-De-Montellano, C.; Manolaraki, F.; Brunet, S.; Ojeda-Robertos, N.; Fourquaux, I.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A. Direct and indirect effects of bioactive tannin-rich tropical and temperate legumes against nematode infections. Vet. Parasitol. 2012, 186, 18–27. [Google Scholar] [CrossRef]
- Joshi, B.; Panda, S.K.; Jouneghani, R.S.; Liu, M.; Parajuli, N.; Leyssen, P.; Neyts, J.; Luyten, W. Antibacterial, Antifungal, Antiviral, and Anthelmintic Activities of Medicinal Plants of Nepal Selected Based on Ethnobotanical Evidence. Evid.-Based Complement. Altern. Med. ECAM 2020, 2020, 1043471. [Google Scholar] [CrossRef]
- Kaplan, R.M. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004, 20, 477–481. [Google Scholar] [CrossRef]
- Arsenopoulos, K.V.; Fthenakis, G.C.; Katsarou, E.I.; Papadopoulos, E. Haemonchosis: A Challenging Parasitic Infection of Sheep and Goats. Animals 2021, 11, 363. [Google Scholar] [CrossRef]
- Mahieu, M.; Ferré, B.; Madassamy, M.; Mandonnet, N. Fifteen years later, anthelmintic resistances have dramatically spread over goat farms in Guadeloupe. Vet. Parasitol. 2014, 205, 379–384. [Google Scholar] [CrossRef]
- Birhan, M.; Gesses, T.; Kenubih, A.; Dejene, H.; Yayeh, M. Evaluation of Anthelminthic Activity of Tropical Taniferous Plant Extracts Against Haemonchus contortus. Vet. Med. Res. Rep. 2020, 11, 109–117. [Google Scholar] [CrossRef]
- Singh, G.; Singh, R.; Verma, P.K.; Singh, R.; Anand, A. Anthelmintic efficacy of aqueous extract of Butea monosperma (Lam.) Kuntze against Haemonchus contortus of sheep and goats. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 2015, 39, 200–205. [Google Scholar]
- Rochfort, S.; Parker, A.J.; Dunshea, F.R. Plant bioactives for ruminant health and productivity. Phytochemistry 2008, 69, 299–322. [Google Scholar] [CrossRef]
- Eguale, T.; Giday, M. In vitro anthelmintic activity of three medicinal plants against Haemonchus contortus. Int. J. Green Pharm. 2009, 3, 29–35. [Google Scholar] [CrossRef]
- Santos, F.O.; Cerqueira, A.P.M.; Branco, A.; Batatinha, M.J.M.; Botura, M.B. Anthelmintic activity of plants against gastrointestinal nematodes of goats: A review. Parasitology 2019, 146, 1233–1246. [Google Scholar] [CrossRef]
- Spiegler, V.; Liebau, E.; Hensel, A. Medicinal plant extracts and plant-derived polyphenols with anthelmintic activity against intestinal nematodes: Review. Nat. Prod. Rep. 2017, 34, 627–643. [Google Scholar] [CrossRef]
- Oliveira, A.F.; Costa Junior, L.M.; Lima, A.S.; Silva, C.R.; Ribeiro, M.N.S.; Mesquista, J.W.C.; Rocha, C.Q.; Tangerina, M.M.P.; Vilegas, W. Anthelmintic activity of plant extracts from Brazilian savanna. Vet. Parasitol. 2017, 236, 121–127. [Google Scholar] [CrossRef]
- Monteiro, M.V.B.; Bevilaqua, C.M.L.; Morais, S.M.; Machado, L.K.A.; Camurça-Vasconcelos, A.L.F.; Campello, C.C.; Ribeiro, W.L.C.; Mesquita, M.d.A. Anthelmintic activity of Jatropha curcas L. seeds on Haemonchus contortus. Vet. Parasitol. 2011, 182, 259–263. [Google Scholar] [CrossRef]
- Engström, M.T.; Karonen, M.; Ahern, J.R.; Baert, N.; Payré, B.; Hoste, H.; Salminen, J.-P. Chemical Structures of Plant Hydrolyzable Tannins Reveal Their in Vitro Activity against Egg Hatching and Motility of Haemonchus contortus Nematodes. J. Agric. Food Chem. 2016, 64, 840–851. [Google Scholar] [CrossRef]
- Mravčáková, D.; Komáromyová, M.; Babják, M.; Dolinská, M.U.; Königová, A.; Daniel Petrič, D.; Čobanová, K.; Ślusarczyk, S.; Cieslak, A.; Várady, M.; et al. Anthelmintic Activity of Wormwood (Artemisia absinthium L.) and Mallow (Malva sylvestris L.) against Haemonchus contortus in Sheep. Animals 2020, 10, 219. [Google Scholar] [CrossRef]
- Jato, J.; Waindok, P.; Ngnodandi Belga, F.; Orman, E.; Agyare, C.; Oppong Bekoe, E.; Strube, C.; Hensel, A.; Liebau, E.; Spiegler, V. Anthelmintic Activities of Extract and Ellagitannins from Phyllanthus urinaria against Caenorhabditis elegans and Zoonotic or Animal Parasitic Nematodes. Planta Med. 2023, 89, 1215–1228. [Google Scholar] [CrossRef]
- Escareño-Díaz, S.; Alonso-Díaz, M.A.; Mendoza de Gives, P.; Castillo-Gallegos, E.; von Son-de Fernex, E. Anthelmintic-like activity of polyphenolic compounds and their interactions against the cattle nematode Cooperia punctate. Vet Parasitol. 2019, 274, 108909. [Google Scholar] [CrossRef]
- Molla, S.H.; Bandyopadhyay, P.K. In vitro and in vivo anthelmintic activity of Murraya koenigii against gastro-intestinal nematodes of sheep. J. Parasit. Dis. 2016, 40, 362–368. [Google Scholar] [CrossRef]
- Baihaqi, Z.A.; Widiyono, I.; Nurcahyo, W. In vitro anthelmintic activity of aqueous and ethanol extracts of Paraserianthes falcataria bark waste against Haemonchus contortus obtained from a local slaughterhouse in Indonesia. Vet. World 2020, 13, 1549–1554. [Google Scholar]
- Oliveira, M.; Lima, C.S.; Ketavong, S.; Llorent-Martínez, E.J.; Hoste, H.; Custódio, L. Disclosing the bioactive metabolites involved in the in vitro anthelmintic effects of salt-tolerant plants through a combined approach using PVPP and HPLC-ESI-MS. Sci. Rep. 2021, 11, 24303. [Google Scholar] [CrossRef]
- Karonen, M.; Ahern, J.R.; Legroux, L.; Suvanto, J.; Engström, M.T.; Sinkkonen, J.; Salminen, J.-P.; Hoste, H. Ellagitannins Inhibit the Exsheathment of Haemonchus contortus and Trichostrongylus colubriformis Larvae: The Efficiency Increases Together with the Molecular Size. J. Agric. Food Chem. 2020, 68, 4176–4186. [Google Scholar] [CrossRef]
- Martínez-Ortiz-de-Montellano, C.; Torres-Acosta, J.F.J.; Fourquaux, I.; Sandoval-Castro, C.A.; Hoste, H. Ultrastructural study of adult Haemonchus contortus exposed to polyphenol-rich materials under in vivo conditions in goats. Parasite 2019, 26, 65. [Google Scholar] [CrossRef]
- Maestrini, M.; Tava, A.; Mancini, S.; Tedesco, D.; Perrucci, S. In Vitro Anthelmintic Activity of Saponins from Medicago spp. Against Sheep Gastrointestinal Nematodes. Molecules 2020, 25, 242. [Google Scholar] [CrossRef]
- Beloin, N.; Gbeassor, M.; Akpagana, K.; Hudson, J.; de Soussa, K.; Koumaglo, K.; Arnason, J.T. Ethnomedicinal uses of Momordica charantia (Cucurbitaceae) in Togo and relation to its phytochemistry and biological activity. J. Ethnopharmacol. 2005, 96, 49–55. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008, 110, 881–890. [Google Scholar] [CrossRef]
- Goku, P.E.; Orman, E.; Quartey, A.N.K.; Ansong, G.T.; Asare-Gyan, E.B. Comparative Evaluation of the In Vitro Anthelminthic Effects of the Leaves, Stem, and Seeds of Carica papaya (Linn) Using the Pheretima posthuma Model. Evid.-Based Complement. Altern. Med. 2020, 2020, 9717304. [Google Scholar] [CrossRef]
- Yap, J.Y.; Hii, C.L.; Ong, S.P.; Lim, K.H.; Abas, F.; Pin, K.Y. Effects of drying on total polyphenols content and antioxidant properties of Carica papaya leaves. J. Sci. Food Agric. 2020, 100, 2932–2937. [Google Scholar] [CrossRef]
- Abdel-Halim, S.; Ibrahim, M.; Mohsen, M.A.; Abou-Setta, L.; Sleem, A.; El-Missiry, M. The influence of the extraction method on polyphenols, flavonoids composition and anti-hyperlipidemic properties of papaya leaves (Carica papaya L.). Bull. Natl. Res. Cent. 2021, 45, 85–93. [Google Scholar] [CrossRef]
- Liu, J.; Luthuli, S.; Yang, Y.; Cheng, Y.; Zhang, Y.; Wu, M.; Choi, J.-I.; Tong, H. Therapeutic and nutraceutical potentials of a brown seaweed Sargassum fusiforme. Food Sci. Nutr. 2020, 8, 5195–5205. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Rodríguez, A.D.; Berlinck, R.G.S.; Fusetani, N. Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 191–222. [Google Scholar]
- Dashtiannasab, A.; Kakoolaki, S.; Sharif Rohani, M.; Yeganeh, V. In vitro effects of Sargassum latifolium (Agardeh, 1948) againstselected bacterial pathogens of shrimp. Iran. J. Fish. Sci. 2012, 11, 765–775. [Google Scholar]
- Chemat, F.; Cravotto, G. Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Mansfield, L.S.; Gamble, H.R.; Fetterer, R.H. Characterization of the eggshell of Haemonchus contortus—I. Structural components. Comp. Biochem. Physiol. B 1992, 103, 681–686. [Google Scholar] [CrossRef]
- Percot, A.; Viton, C.; Domard, A. Optimization of Chitin Extraction from Shrimp Shells. Biomacromolecules 2003, 4, 12–18. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Luis Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Bahuaud, D.; Martinez-ortiz de Montellano, C.; Chauveau, S.; Prevot, F.; Torres-Acosta, F.; Fouraste, I.; Hoste, H. Effects of four tanniferous plant extracts on the in vitro exsheathment of third-stage larvae of parasitic nematodes. Parasitology 2006, 132, 545–554. [Google Scholar] [CrossRef]
- Beane, R.D.; Hobbs, N.T. The Baermann technique for estimating Protostrongylus infection in bighorn sheep: Effect of laboratory procedures. J. Wildl. Dis. 1983, 19, 7–9. [Google Scholar] [CrossRef]
- Doménech-Carbό, A.; Maciuk, A.; Figadère, B.; Poupon, E.; Cebrián-Torrejón, G. Solid-State Electrochemical Assay of Heme-Binding Molecules for Screening of Drugs with Antimalarial Potential. Anal. Chem. 2013, 85, 4014–4021. [Google Scholar] [CrossRef]
- Minatchy, N.; Marie-Magdeleine, C.; Garin, M.; Nimir, F.; Romil-Granville, D.; Philibert, L.; Calif, V.; Bambou, J.-C.; Archimède, H. Nutraceutical properties of Leucaena leucocephala, Manihot esculenta, Cajanus cajan and a foliage blend in goat kids infected with Haemonchus contortus. Sci. Rep. 2020, 10, 9969. [Google Scholar] [CrossRef]
- Marie-Magdeleine, C.; Udino, L.; Philibert, L.; Bocage, B.; Archimede, H. In vitro effects of Cassava (Manihot esculenta) leaf extracts on four development stages of Haemonchus contortus. Vet. Parasitol. 2010, 173, 85–92. [Google Scholar] [CrossRef]
- Marie-Magdeleine, C.; Ceriac, S.; Barde, D.J.; Minatchy, N.; Periacarpin, F.; Pommier, F.; Calif, B.; Philibert, L.; Bambou, J.-C.; Archimède, H. Evaluation of nutraceutical properties of Leucaena leucocephala leaf pellets fed to goat kids infected with Haemonchus contortus. BMC Vet. Res. 2020, 16, 280. [Google Scholar] [CrossRef]





| Sample | Average OD (750 nm) | Gallic Acid Concentration (µg/mL) |
|---|---|---|
| S1 | 0 | 0 |
| S2 | 0.1500 | 25 |
| S3 | 0.2615 | 50 |
| S4 | 0.3325 | 100 |
| S5 | 0.8635 | 200 |
| S6 | 1.2100 | 300 |
| Sargassum (1/10) | 0.1080 | 22.125 |
| Papaya (1/10) | 0.6320 | 153.125 |
| Pawoka (1/10) | 0.3607 | 85.300 |
| A. | |
| Compound Name | Relative Concentration (mM) in Sargassum |
| Mannitol | 0.3474 |
| Alanine | 0.0096 |
| Glutamate | 0.0092 |
| Glutamine | 0.0090 |
| Aspartate | 0.0060 |
| Acetate | 0.0026 |
| Valine | 0.0024 |
| Lactate | 0.0011 |
| Formate | 0.0008 |
| B. | |
| Compound Name | Relative Concentration (mM) in Papaya |
| Glucose | 88.1664 |
| Malate | 35.2627 |
| Asparagine | 10.2431 |
| Alanine | 8.7825 |
| 4-aminobutyrate | 4.7034 |
| Chlorogenate | 4.1628 |
| Valine | 2.1308 |
| Trigonelline | 2.0848 |
| Leucine | 1.9908 |
| Lactate | 1.8929 |
| Isoleucine | 1.3825 |
| Formate | 1.3148 |
| Uridine | 0.9758 |
| S-Adenosylhomocysteine | 0.7200 |
| C. | |
| Compounds | Relative Concentration (mM) in Pawoka |
| Acetate | 6.5881 |
| Fructose | 5.5754 |
| Glucose | 3.6688 |
| 2-Octenoate | 2.8576 |
| Gluconate | 2.3584 |
| Galactose | 1.4728 |
| Aspartate | 1.3986 |
| Alanine | 1.3960 |
| Formate | 1.0814 |
| Succinate | 1.0653 |
| 2-Hydroxybutyrate | 1.0585 |
| Propionate | 0.9949 |
| 4-aminobutyrate | 0.9265 |
| Choline | 0.8408 |
| Glutamate | 0.6515 |
| Valine | 0.6485 |
| Methanol | 0.6405 |
| Lactate | 0.5504 |
| Leucine | 0.4479 |
| Asparagine | 0.4321 |
| Fumarate | 0.4159 |
| Isoleucine | 0.3571 |
| Uridine | 0.3504 |
| Phenylalanine | 0.1766 |
| Trigonelline | 0.1533 |
| Benzoate | 0.1301 |
| Tyrosine | 0.0534 |
| 2-Hydroxyphenylacetate | 0.0454 |
| Tryptophan | 0.0287 |
| Average Percentage of Exsheatment (%) | IC50 (mg/mL) | |
|---|---|---|
| Control PBS | ||
| PBS Pawoka | 94.04 | - |
| PBS Papaya | 97.69 | - |
| PBS Sargassum | 95.36 | - |
| Plant extracts | ||
| Pawoka | 40.21 | 1.148 |
| Papaya | 79.27 | 4.161 |
| Sargassum | 57.37 | 1.980 |
| Sample | Potential of Heme (mV) | Potential of Heme + Samples (mV) | Potential of Samples (Control, mV) |
|---|---|---|---|
| Ivermectin | −0.20 | −0.72 | −0.60 |
| Levamisole | −0.20 | −0.16 | −0.18 |
| Thiabendazole | −0.20 | −0.20 | −0.36 |
| Leucene tannins | −0.20 | −0.44 | −0.42 |
| Manioc tannins | −0.20 | −0.40 | −0.40 |
| Papaya | −0.20 | −0.52 | −0.60 |
| Pawoka | −0.20 | −0.28 | −0.60 |
| Sargassum | −0.20 | −0.36 | −0.56 |
| Sample | Potential of Chitin (mV) | Potentiel of Chitin + Samples (mV) | Potential of Samples (Control, mV) |
|---|---|---|---|
| Ivermectin | −0.40 | −0.70 | −0.65 |
| Levamisole | −0.40 | −0.65 | −0.25 |
| Thiabendazole | −0.40 | −0.90 | −0.40 |
| Leucene tannins | −0.40 | −0.50 | −0.80 |
| Manioc tannins | −0.40 | −0.75 | −0.75 |
| Papaya | −0.40 | −0.85 | −1 |
| Pawoka | −0.40 | −1.10 | −0.85 |
| Sargassum | −0.40 | −0.90 | −0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabald, T.; Marie-Magdeleine, C.; Philibert, L.; Caradeuc, C.; Bertho, G.; Giraud, N.; Cebrián-Torrejón, G.; Sylvestre, M. Phytochemical Study of the Anthelminthic Potential of Guadeloupean Plant Biodiversity. Pharmaceuticals 2024, 17, 774. https://doi.org/10.3390/ph17060774
Cabald T, Marie-Magdeleine C, Philibert L, Caradeuc C, Bertho G, Giraud N, Cebrián-Torrejón G, Sylvestre M. Phytochemical Study of the Anthelminthic Potential of Guadeloupean Plant Biodiversity. Pharmaceuticals. 2024; 17(6):774. https://doi.org/10.3390/ph17060774
Chicago/Turabian StyleCabald, Tressy, Carine Marie-Magdeleine, Lucien Philibert, Cédric Caradeuc, Gildas Bertho, Nicolas Giraud, Gerardo Cebrián-Torrejón, and Muriel Sylvestre. 2024. "Phytochemical Study of the Anthelminthic Potential of Guadeloupean Plant Biodiversity" Pharmaceuticals 17, no. 6: 774. https://doi.org/10.3390/ph17060774
APA StyleCabald, T., Marie-Magdeleine, C., Philibert, L., Caradeuc, C., Bertho, G., Giraud, N., Cebrián-Torrejón, G., & Sylvestre, M. (2024). Phytochemical Study of the Anthelminthic Potential of Guadeloupean Plant Biodiversity. Pharmaceuticals, 17(6), 774. https://doi.org/10.3390/ph17060774








