Pharmacovigilance in Vaccines: Importance, Main Aspects, Perspectives, and Challenges—A Narrative Review
Abstract
1. Introduction
2. Overview of Vaccines
3. Pharmacovigilance
3.1. Overview of Pharmacovigilance
- (i)
- Pharmacovigilance information systems: these systems allow the collection, storage, analysis, and communication of information on adverse drug events;
- (ii)
- Pharmacovigilance databases: these databases serve as reliable sources of information on adverse drug events;
- (iii)
- Adverse event tracking applications: these tools assist in monitoring and tracking adverse drug events;
- (iv)
- Statistical analysis: this technique is used to evaluate data collected on adverse drug-related events.
3.2. Pharmacovigilance of Vaccines
3.2.1. Legislation and Responsible Authorities
3.2.2. Monitoring Special Populations
4. Vaccines and Adverse Reactions after Immunization
- (i)
- (ii)
- Clinical Trials [163]: participants are closely monitored for vaccination-related adverse reactions;
- (iii)
- Vaccination registries [164]: systems that collect information on vaccine administration and associated adverse events;
- (iv)
- Public health research [165]: cohort studies and randomized controlled trials may offer insights into long-term adverse reactions related to vaccination.
4.1. COVID-19 Vaccines
4.1.1. Adverse Reactions
4.1.2. Special Populations
4.2. Polio Vaccine
4.2.1. Adverse Reactions
4.2.2. Special Populations
4.3. Influenza Vaccines
4.3.1. Adverse Reactions
4.3.2. Special Populations
4.4. Hepatitis B Vaccine
4.4.1. Adverse Reactions
4.4.2. Special Populations
5. New Vaccines
6. Perspectives and Challenges
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACIP | Advisory Committee on Immunization Practices |
| ADEM | Acute disseminated encephalomyelitis |
| ADR | Adverse drug reactions |
| AEFI | Adverse Event Following Immunization |
| AI | Artificial Intelligence |
| ANVISA | Brazilian Health Regulatory Agency |
| CDC | Centers for Disease Control and Prevention |
| CIFAVI | Interinstitutional Committee for Pharmacovigilance of Vaccines and other Immunobiologicals |
| CSM | Committee on the Safety of Medicines |
| DENV | Dengue virus |
| ECDC | European Centre for Disease Prevention and Control |
| EDCTP | European Developing countries Clinical Trials Partnership |
| EMA | European Medicines Agency |
| EU | European Union |
| FDA | Food and Drug Administration |
| GACVS | Global Advisory Committee on Vaccine Safety |
| GVP | Good practices in pharmacovigilance |
| HA | Hemagglutinin |
| HBV | Hepatitis B virus |
| HBsAg | Hepatitis B surface antigen |
| HIV | With human immunodeficiency virus |
| ICSR | Individual Case Safety Reports |
| ICH | International Conference on Harmonization |
| IIV | Inactivated influenza virus |
| IPV | Inactivated poliovirus vaccine |
| LAIV | Live-attenuated influenza vaccine |
| LMICs | Low- and middle-income countries |
| MIHARI | Medical Information for Risk Assessment Initiative |
| MMR | Measles, mumps, and rubella |
| MPX | Monkeypox |
| NA | Neuraminidase |
| NPS | National Pharmacovigilance System |
| OPV | Oral poliovirus vaccine |
| PAVIA | Pharmacovigilance Africa |
| PMDA | Pharmaceuticals and Medical Devices Agency |
| PVAE | Post-vaccination adverse events |
| QIV | Quadrivalent vaccines |
| QR | Quick response |
| TGA | Therapeutic Goods Administration |
| UMC | Uppsala Monitoring Centre |
| USFDA | US Food and Drug Administration |
| VAERS | Vaccine Adverse Event Reporting System |
| VDPV | Vaccine-derived poliovirus |
| VSV | Vesicular stomatitis virus |
| VZV | Varicella-zoster virus |
| WHO | World Health Organization |
References
- de Oliveira, M.M.M.; Wagner, G.A.; Gattás, V.L.; de Souza Arruda, L.; Taminato, M. Pharmacovigilance Quality System for Vaccine Monitoring (COVID-19) Using Quality Indicators: A Scoping Review. Int. J. Infect. Control 2021, 17. [Google Scholar] [CrossRef]
- Khalid Abbood, M.; Alaa Aldeen Khalaf, H.; Abudlqader, E.H.; Sagban Taghi, H.; Alaa Al-Temimi, A. Scope of Pharmacovigilance: Comprehensive Review. Chem. Sci. Int. J. 2022, 31, 29–39. [Google Scholar] [CrossRef]
- Garashi, H.Y.; Steinke, D.T.; Schafheutle, E.I. A Systematic Review of Pharmacovigilance Systems in Developing Countries Using the WHO Pharmacovigilance Indicators. Ther. Innov. Regul. Sci. 2022, 56, 717–743. [Google Scholar] [CrossRef]
- Asiamah, M.; Akuffo, K.O.; Nortey, P.; Donkor, N.; Danso-Appiah, A. Spontaneous Reporting of Adverse Drug Reaction among Health Professionals in Ghana. Arch. Public Health 2022, 80, 33. [Google Scholar] [CrossRef]
- Bellavite, P. Causality Assessment of Adverse Events Following Immunization: The Problem of Multifactorial Pathology. F1000Research 2020, 9, 170. [Google Scholar] [CrossRef]
- Silva, L.T.; Modesto, A.C.F.; Amaral, R.G.; Lopes, F.M. Hospitalizations and Deaths Related to Adverse Drug Events Worldwide: Systematic Review of Studies with National Coverage. Eur. J. Clin. Pharmacol. 2022, 78, 435–466. [Google Scholar] [CrossRef]
- Sharrar, R.G.; Dieck, G.S. Monitoring Product Safety in the Postmarketing Environment. Ther. Adv. Drug Saf. 2013, 4, 211–219. [Google Scholar] [CrossRef]
- Tizard, I.R. Adverse Consequences of Vaccination. In Vaccines for Veterinarians; Elsevier: Amsterdam, The Netherlands, 2021; pp. 115–130.e1. [Google Scholar]
- Mahdiabadi, S.; Rezaei, N. Anaphylaxis and Allergic Reactions to COVID-19 Vaccines: A Narrative Review of Characteristics and Potential Obstacles on Achieving Herd Immunity. Health Sci. Rep. 2022, 5, e787. [Google Scholar] [CrossRef]
- Hauben, M.; Bate, A. Decision Support Methods for the Detection of Adverse Events in Post-Marketing Data. Drug Discov. Today 2009, 14, 343–357. [Google Scholar] [CrossRef]
- Kuçuku, M. Role of Pharmacovigilance on Vaccines Control. J. Rural. Med. 2012, 7, 5. [Google Scholar]
- Shah, R.R. Importance of Publishing Adverse Drug Reaction Case Reports: Promoting Public Health and Advancing Pharmacology and Therapeutics. Drug Saf. Case Rep. 2017, 4, 11. [Google Scholar] [CrossRef]
- Azad, A.; Laidlaw, D.A.H.; Orlans, H.O. Using QR Smartphone Technology to Improve Patient Communication and Information Distribution. Eye 2022, 36, 1321–1322. [Google Scholar] [CrossRef]
- Cook, L.; Dermesropian, R.; Dulipsingh, L. Use of QR Codes to Provide Patient Education During the Coronavirus Disease 19 Pandemic. J. Diabetes Sci. Technol. 2021, 15, 1408–1409. [Google Scholar] [CrossRef]
- Alegre, J.C.; Sharma, S.; Cleghorn, F.; Avila, C. Strengthening Primary Health Care in Low-and Middle-Income Countries: Furthering Structural Changes in the Post-Pandemic Era. Front. Public Health 2024, 11, 1270510. [Google Scholar] [CrossRef]
- Rcg, Z.; Ara, F. Precarious Work at a Surgical Center: Implications for the Organization and for the Health of Nursing Workers. Rev. Bras. Enferm. 2023, 76, 20220120–20220127. [Google Scholar]
- Mota, D.M.; Vigo, Á.; de Souza Kuchenbecker, R. Evaluation of the Performance of the Brazilian Notification System for Health Surveillance: A Tool in Brazil’s Pharmacovigilance System. Cienc. Saude Coletiva 2020, 25, 1955–1966. [Google Scholar] [CrossRef]
- ElSawi, H.A.; Elborollosy, A. Immune-Mediated Adverse Events Post-COVID Vaccination and Types of Vaccines: A Systematic Review and Meta-Analysis. Egypt. J. Intern. Med. 2022, 34, 44. [Google Scholar] [CrossRef]
- Talbot, J.C.C.; Nilsson, B.S. Pharmacovigilance in the Pharmaceutical Industry. Br. J. Clin. Pharmacol. 1998, 45, 427. [Google Scholar] [CrossRef]
- Kim, M.S.; Jung, S.Y.; Ahn, J.G.; Park, S.J.; Shoenfeld, Y.; Kronbichler, A.; Koyanagi, A.; Dragioti, E.; Tizaoui, K.; Hong, S.H.; et al. Comparative Safety of MRNA COVID-19 Vaccines to Influenza Vaccines: A Pharmacovigilance Analysis Using WHO International Database. J. Med. Virol. 2022, 94, 1085–1095. [Google Scholar] [CrossRef]
- Lindquist, M. VigiBase, the WHO Global ICSR Database System: Basic Facts. Drug Inf. J. 2008, 42, 409–419. [Google Scholar] [CrossRef]
- Rudolph, A.; Mitchell, J.; Barrett, J.; Sköld, H.; Taavola, H.; Erlanson, N.; Melgarejo-González, C.; Yue, Q.Y. Global Safety Monitoring of COVID-19 Vaccines: How Pharmacovigilance Rose to the Challenge. Ther. Adv. Drug Saf. 2022, 13, 20420986221118972. [Google Scholar] [CrossRef]
- Amaro, C.; Monteiro, C.; Duarte, A.P. COVID-19 Vaccines Adverse Reactions Reported to the Pharmacovigilance Unit of Beira Interior in Portugal. J. Clin. Med. 2022, 11, 5591. [Google Scholar] [CrossRef]
- Ferrara, F.; Mancaniello, C.; Varriale, A.; Sorrentino, S.; Zovi, A.; Nava, E.; Trama, U.; Boccellino, M.; Vitiello, A. COVID-19 MRNA Vaccines: A Retrospective Observational Pharmacovigilance Study. Clin. Drug Investig. 2022, 42, 1065–1074. [Google Scholar] [CrossRef]
- Bates, D.W.; Evans, R.S.; Murff, H.; Stetson, P.D.; Pizzifferri, L.; Hripcsak, G. Detecting Adverse Events Using Information Technology. J. Am. Med. Inform. Assoc. 2003, 10, 115–128. [Google Scholar] [CrossRef]
- Silva, B.S.; De Azevedo Guimarães, E.A.; De Oliveira, V.C.; Cavalcante, R.B.; Pinheiro, M.M.E.K.; Gontijo, T.L.; Rodrigues, S.B.; Ferreira, A.P.; De Oliveira Quites, H.F.; Pinto, I.C. National Immunization Program Information System: Implementation Context Assessment. BMC Health Serv. Res. 2020, 20, 333. [Google Scholar] [CrossRef]
- Marzouk, M.; Omar, M.; Sirison, K.; Ananthakrishnan, A.; Durrance-Bagale, A.; Pheerapanyawaranun, C.; Porncharoen, C.; Pimsarn, N.; Lam, S.T.; Ung, M.; et al. Monitoring and Evaluation of National Vaccination Implementation: A Scoping Review of How Frameworks and Indicators Are Used in the Public Health Literature. Vaccines 2022, 10, 567. [Google Scholar] [CrossRef]
- Czochor, J.; Turchick, A. Focus: Vaccines. Yale J. Biol. Med. 2014, 87, 401–402. [Google Scholar]
- Nagy, A.; Alhatlani, B. An Overview of Current COVID-19 Vaccine Platforms. Comput. Struct. Biotechnol. J. 2021, 19, 2508–2517. [Google Scholar] [CrossRef]
- Greenwood, B. The Contribution of Vaccination to Global Health: Past, Present and Future. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef]
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef]
- Riedel, S. Edward Jenner and the History of Smallpox and Vaccination. In Baylor University Medical Center Proceedings; Taylor & Francis: Oxford, NY, USA, 2005. [Google Scholar]
- Stewart, A.J.; Devlin, P.M. The History of the Smallpox Vaccine. J. Infect. 2006, 52, 329–334. [Google Scholar] [CrossRef]
- Smith, K.A. Edward Jenner and the Small Pox Vaccine. Front. Immunol. 2011, 2, 21. [Google Scholar] [CrossRef]
- Tuells, J. Vaccinology: The Name, the Concept, the Adjectives. Vaccine 2012, 30, 5491–5495. [Google Scholar] [CrossRef]
- Sheppard, M.; Isaacs, D.; Fitzgerald, D.A. The Accumulating Consequences of COVID-19 in Children. Paediatr. Respir. Rev. 2021, 39, 1–2. [Google Scholar] [CrossRef]
- Saleh, A.; Qamar, S.; Tekin, A.; Singh, R.; Kashyap, R. Vaccine Development Throughout History. Cureus 2021, 13, e16635. [Google Scholar] [CrossRef]
- Mullard, A. COVID-19 Vaccine Development Pipeline Gears Up. Lancet 2020, 395, 1751–1752. [Google Scholar] [CrossRef]
- Krause, P.R.; Gruber, M.F. Emergency Use Authorization of Covid Vaccines—Safety and Efficacy Follow-up Considerations. N. Engl. J. Med. 2020, 383, e107. [Google Scholar] [CrossRef]
- Tran, A.; Witek, T.J. The Emergency Use Authorization of Pharmaceuticals: History and Utility During the COVID-19 Pandemic. Pharmaceut Med. 2021, 35, 203. [Google Scholar] [CrossRef]
- Rawat, K.; Kumari, P.; Saha, L. COVID-19 Vaccine: A Recent Update in Pipeline Vaccines, Their Design and Development Strategies. Eur. J. Pharmacol. 2021, 892, 173751. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 Vaccines in Development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Chakraborty, C.; Bhattacharya, M.; Dhama, K. SARS-CoV-2 Vaccines, Vaccine Development Technologies, and Significant Efforts in Vaccine Development during the Pandemic: The Lessons Learned Might Help to Fight against the Next Pandemic. Vaccines 2023, 11, 682. [Google Scholar] [CrossRef]
- Deyhimfar, R.; Izady, M.; Shoghi, M.; Kazazi, M.H.; Ghazvini, Z.F.; Nazari, H.; Fekrirad, Z.; Arefian, E. The Clinical Impact of MRNA Therapeutics in the Treatment of Cancers, Infections, Genetic Disorders, and Autoimmune Diseases. Heliyon 2024, 10, e26971. [Google Scholar] [CrossRef]
- WHO. Vaccine Efficacy, Effectiveness and Protection. Available online: https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection (accessed on 7 February 2024).
- Yao, B.; Zhu, L.; Jiang, Q.; Xia, H. Safety Monitoring in Clinical Trials. Pharmaceutics 2013, 5, 94–106. [Google Scholar] [CrossRef]
- Umscheid, C.A.; Margolis, D.J.; Grossman, C.E. Key Concepts of Clinical Trials: A Narrative Review. Postgrad. Med. 2011, 123, 194–204. [Google Scholar] [CrossRef]
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.-H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The Efficacy and Effectiveness of the COVID-19 Vaccines in Reducing Infection, Severity, Hospitalization, and Mortality: A Systematic Review. Hum. Vaccin. Immunother. 2022, 18, 2027160. [Google Scholar] [CrossRef]
- Brazete, C.; Pinto, M.; Sá, L.; Aguiar, A.; Alves, F.; Duarte, R. Evaluation of the Real-World Effectiveness of Vaccines against COVID-19 at a Local Level: Protocol for a Test-Negative Case–Control Study. Vaccines 2022, 10, 822. [Google Scholar] [CrossRef]
- Bruxvoort, K.J.; Sy, L.S.; Qian, L.; Ackerson, B.K.; Luo, Y.; Lee, G.S.; Tian, Y.; Florea, A.; Takhar, H.S.; Tubert, J.E.; et al. Real-World Effectiveness of the MRNA-1273 Vaccine against COVID-19: Interim Results from a Prospective Observational Cohort Study. Lancet Reg. Health 2022, 6, 100134. [Google Scholar] [CrossRef]
- Di Pasquale, A.; Bonanni, P.; Garçon, N.; Stanberry, L.R.; El-Hodhod, M.; Tavares Da Silva, F. Vaccine Safety Evaluation: Practical Aspects in Assessing Benefits and Risks. Vaccine 2016, 34, 6672–6680. [Google Scholar] [CrossRef]
- Younus, M.M.; Al-Jumaili, A.A. An Overview of COVID-19 Vaccine Safety and Post-Marketing Surveillance Systems. Innov. Pharm. 2021, 12, 8. [Google Scholar] [CrossRef]
- Cole, A.; Webster, P.; Van Liew, D.; Salas, M.; Aimer, O.; Malikova, M.A. Safety Surveillance and Challenges in Accelerated COVID-19 Vaccine Development. Ther. Adv. Drug Saf. 2022, 13, 20420986221116452. [Google Scholar] [CrossRef]
- Sharif, N.; Alzahrani, K.J.; Ahmed, S.N.; Dey, S.K. Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 714170. [Google Scholar] [CrossRef]
- Toubasi, A.A.; Al-Sayegh, T.N.; Obaid, Y.Y.; Al-Harasis, S.M.; AlRyalat, S.A.S. Efficacy and Safety of COVID-19 Vaccines: A Network Meta-analysis. J. Evid. Based Med. 2022, 15, 245–262. [Google Scholar] [CrossRef]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and Safety of COVID-19 Vaccines. Cochrane Database Syst. Rev. 2022, 2023, CD015477. [Google Scholar] [CrossRef]
- Beale, A.J. Efficacy and Safety of Oral Poliovirus Vaccine and Inactivated Poliovirus Vaccine. Pediatr. Infect. Dis. 1991, 10, 970–972. [Google Scholar] [CrossRef]
- Hawken, J.; Troy, S.B. Adjuvants and Inactivated Polio Vaccine: A Systematic Review. Vaccine 2012, 30, 6971–6979. [Google Scholar] [CrossRef]
- O’Grady, M.; Bruner, P.J. Polio Vaccine; StatPearls: Tampa, FL, USA, 2023. [Google Scholar]
- Minozzi, S.; Lytras, T.; Gianola, S.; Gonzalez-Lorenzo, M.; Castellini, G.; Galli, C.; Cereda, D.; Bonovas, S.; Pariani, E.; Moja, L. Comparative Efficacy and Safety of Vaccines to Prevent Seasonal Influenza: A Systematic Review and Network Meta-Analysis. EClinicalMedicine 2022, 46, 101331. [Google Scholar] [CrossRef]
- Jordan, K.; Murchu, E.O.; Comber, L.; Hawkshaw, S.; Marshall, L.; O’Neill, M.; Teljeur, C.; Harrington, P.; Carnahan, A.; Pérez-Martín, J.J.; et al. Systematic Review of the Efficacy, Effectiveness and Safety of Cell-based Seasonal Influenza Vaccines for the Prevention of Laboratory-confirmed Influenza in Individuals ≥18 Years of Age. Rev. Med. Virol. 2023, 33, e2332. [Google Scholar] [CrossRef]
- Comber, L.; O Murchu, E.; Jordan, K.; Hawkshaw, S.; Marshall, L.; O’Neill, M.; Teljeur, C.; Ryan, M.; Carnahan, A.; Pérez Martín, J.J.; et al. Systematic Review of the Efficacy, Effectiveness and Safety of High-dose Seasonal Influenza Vaccines for the Prevention of Laboratory-confirmed Influenza in Individuals ≥18 Years of Age. Rev. Med. Virol. 2023, 33, e2330. [Google Scholar] [CrossRef]
- Vesikari, T.; Finn, A.; van Damme, P.; Leroux-Roels, I.; Leroux-Roels, G.; Segall, N.; Toma, A.; Vallieres, G.; Aronson, R.; Reich, D.; et al. Immunogenicity and Safety of a 3-Antigen Hepatitis B Vaccine vs a Single-Antigen Hepatitis B Vaccine. JAMA Netw. Open 2021, 4, e2128652. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, S.; Feng, Y.; Su, X.; Cai, J.; Chen, S.; Liu, J.; Huang, S.; Huang, H.; Zhu, S.; et al. Efficacy and Safety of Hepatitis B Vaccine: An Umbrella Review of Meta-Analyses. Expert. Rev. Vaccines 2024, 23, 69–81. [Google Scholar] [CrossRef]
- Das, S.; Ramakrishnan, K.; Behera, S.K.; Ganesapandian, M.; Xavier, A.S.; Selvarajan, S. Hepatitis B Vaccine and Immunoglobulin: Key Concepts. J. Clin. Transl. Hepatol. 2019, 7, 165–171. [Google Scholar] [CrossRef]
- Chaves, S.S.; Haber, P.; Walton, K.; Wise, R.P.; Izurieta, H.S.; Schmid, D.S.; Seward, J.F. Safety of Varicella Vaccine after Licensure in the United States: Experience from Reports to the Vaccine Adverse Event Reporting System, 1995–2005. J. Infect. Dis. 2008, 197, S170–S177. [Google Scholar] [CrossRef]
- Moro, P.L.; Leung, J.; Marquez, P.; Kim, Y.; Wei, S.; Su, J.R.; Marin, M. Safety Surveillance of Varicella Vaccines in the Vaccine Adverse Event Reporting System, United States, 2006–2020. J. Infect. Dis. 2022, 226, S431–S440. [Google Scholar] [CrossRef]
- Liu, G.; Cao, W.; Salawudeen, A.; Zhu, W.; Emeterio, K.; Safronetz, D.; Banadyga, L. Vesicular Stomatitis Virus: From Agricultural Pathogen to Vaccine Vector. Pathogens 2021, 10, 1092. [Google Scholar] [CrossRef]
- Tomczyk, T.; Orzechowska, B. Vesicular Stomatitis Virus (VSV) as a Vaccine Vector for Immunization against Viral Infections. Postepy Hig. Med. Dosw. 2013, 67, 1345–1358. [Google Scholar] [CrossRef]
- Strebel, P.M.; Papania, M.J.; Gastañaduy, P.A.; Goodson, J.L. Measles Vaccines. In Plotkin’s Vaccines; Elsevier: Amsterdam, The Netherlands, 2018; pp. 579–618.e21. [Google Scholar]
- Tangy, F.; Naim, H.Y. Live Attenuated Measles Vaccine as a Potential Multivalent Pediatric Vaccination Vector. Viral Immunol. 2005, 18, 317–326. [Google Scholar] [CrossRef]
- Rappuoli, R. Vaccines: Science, Health, Longevity, and Wealth. Proc. Natl. Acad. Sci. USA 2014, 111, 12282. [Google Scholar] [CrossRef]
- Plotkin, S. History of Vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef]
- Lahariya, C. Vaccine Epidemiology: A Review. J. Family Med. Prim. Care 2016, 5, 7. [Google Scholar] [CrossRef]
- Du, L.; Wang, M.; Raposo, V.L. International Efforts and Next Steps to Advance COVID-19 Vaccines Research and Production in Low-and Middle-Income Countries. Vaccines 2022, 10, 42. [Google Scholar] [CrossRef]
- Miller, M.A.; Sentz, J.T. Vaccine-Preventable Diseases. In Disease and Mortality in Sub-Saharan Africa; Jamison, D.T., Feachem, R.G., Makgoba, M.W., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2006. [Google Scholar]
- Frenkel, L.D. The Global Burden of Vaccine-Preventable Infectious Diseases in Children Less than 5 Years of Age: Implications for COVID-19 Vaccination. How Can We Do Better? Allergy Asthma Proc. 2021, 42, 378–385. [Google Scholar] [CrossRef]
- Bull, J.J.; Smithson, M.W.; Nuismer, S.L. Transmissible Viral Vaccines. Trends Microbiol. 2018, 26, 6–15. [Google Scholar] [CrossRef]
- Plitnick, L.M. Global Regulatory Guidelines for Vaccines. In Nonclinical Development of Novel Biologics, Biosimilars, Vaccines and Specialty Biologics; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 225–241. ISBN 9780123948106. [Google Scholar]
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. Vaccines and Vaccination. In Fenner and White’s Medical Virology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 155–167. [Google Scholar]
- Deng, S.; Liang, H.; Chen, P.; Li, Y.; Li, Z.; Fan, S.; Wu, K.; Li, X.; Chen, W.; Qin, Y.; et al. Viral Vector Vaccine Development and Application during the COVID-19 Pandemic. Microorganisms 2022, 10, 11450. [Google Scholar] [CrossRef]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The Use of Viral Vectors in Vaccine Development. NPJ Vaccines 2022, 7, 75. [Google Scholar] [CrossRef]
- Francis, M.J. Recent Advances in Vaccine Technologies. Vet. Clin. N. Am.—Small Anim. Pract. 2018, 48, 231–241. [Google Scholar] [CrossRef]
- Qin, F.; Xia, F.; Chen, H.; Cui, B.; Feng, Y.; Zhang, P.; Chen, J.; Luo, M. A Guide to Nucleic Acid Vaccines in the Prevention and Treatment of Infectious Diseases and Cancers: From Basic Principles to Current Applications. Front. Cell Dev. Biol. 2021, 9, 633776. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Chevalier-Cottin, E.-P.; Ashbaugh, H.; Brooke, N.; Gavazzi, G.; Santillana, M.; Burlet, N.; Tin Tin Htar, M. Communicating Benefits from Vaccines Beyond Preventing Infectious Diseases. Infect. Dis. Ther. 2020, 9, 467–480. [Google Scholar] [CrossRef]
- Hotez, P.J.; Batista, C.; Amor, Y.B.; Ergonul, O.; Figueroa, J.P.; Gilbert, S.; Gursel, M.; Hassanain, M.; Kang, G.; Kaslow, D.C.; et al. Global Public Health Security and Justice for Vaccines and Therapeutics in the COVID-19 Pandemic. EClinicalMedicine 2021, 39. [Google Scholar] [CrossRef]
- Hammad, T.A.; Afsar, S.; Le-Louet, H.; Kugener, V.F. Navigating a Transforming Landscape: The Evolving Role of Pharmacovigilance Physicians in Drug Development and Implications for Future Challenges and Training Requirements. Front. Drug Saf. Regul. 2023, 3, 1257732. [Google Scholar] [CrossRef]
- Agmon-Levin, N.; Paz, Z.; Israeli, E.; Shoenfeld, Y. Vaccines and Autoimmunity. Nat. Rev. Rheumatol. 2009, 5, 648–652. [Google Scholar] [CrossRef]
- Lucas, S.; Ailani, J.; Smith, T.R.; Abdrabboh, A.; Xue, F.; Navetta, M.S. Pharmacovigilance: Reporting Requirements throughout a Product’s Lifecycle. Ther. Adv. Drug Saf. 2022, 13, 20420986221125006. [Google Scholar] [CrossRef]
- Santuccio, C.; Trotta, F.; Felicetti, P. Ongoing Pharmacovigilance on Vaccines. Pharmacol. Res. 2015, 92, 2–5. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H. A Research Framework for Pharmacovigilance in Health Social Media: Identification and Evaluation of Patient Adverse Drug Event Reports. J. Biomed. Inform. 2015, 58, 268–279. [Google Scholar] [CrossRef]
- Zuber, P.L.F.; Gruber, M.; Kaslow, D.C.; Chen, R.T.; Giersing, B.K.; Friede, M.H. Evolving Pharmacovigilance Requirements with Novel Vaccines and Vaccine Components. BMJ Glob. Health 2021, 6, e003403. [Google Scholar] [CrossRef]
- Hong, R.; Walker, R.; Hovan, G.; Henry, L.; Pescatore, R. The Power of Public Health Surveillance. Dela J. Public Health 2020, 6, 60–63. [Google Scholar] [CrossRef]
- Nsubuga, P.; White, M.E.; Thacker, S.B. Public Health Surveillance: A Tool for Targeting and Monitoring Interventions. In Disease Control Priorities in Developing Countries; Jamison, D., Breman, J., Measham, A., Eds.; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Institute of Medicine (US). Committee on a National Surveillance System for Cardiovascular and Select Chronic Diseases Existing Surveillance Data Sources and Systems. In A Nationwide Framework for Surveillance of Cardiovascular and Chronic Lung Diseases; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Jose, J.; Al Rubaie, M.H.; Al Ramimmy, H.; Varughese, S.S. Pharmacovigilance Basic Concepts and an Overview of the System in Oman. Sultan Qaboos Univ. Med. J. 2021, 21, e161–e163. [Google Scholar] [CrossRef]
- Sienkiewicz, K.; Burzynska, M.; Rydlewska-Liszkowska, I.; Sienkiewicz, J.; Gaszynska, E. The Importance of Direct Patient Reporting of Adverse Drug Reactions in the Safety Monitoring Process. Int. J. Environ. Res. Public Health 2022, 19, 413. [Google Scholar] [CrossRef]
- Nour, S.; Plourde, G. Pharmacovigilance. In Pharmacoepidemiology and Pharmacovigilance; Elsevier: Amsterdam, The Netherlands, 2019; pp. 7–23. [Google Scholar]
- Mayall, S.J.; Banerjee, A.K. Pharmacovigilance Planning. In Therapeutic Risk Management of Medicines; Elsevier: Amsterdam, The Netherlands, 2014; pp. 137–161. [Google Scholar]
- Thomas, D.; Klika, C. Pharmacovigilance Systems. In Clinical Pharmacy Education, Practice and Research: Clinical Pharmacy, Drug Information, Pharmacovigilance, Pharmacoeconomics and Clinical Research; Elsevier: Amsterdam, The Netherlands, 2018; pp. 215–225. ISBN 9780128142769. [Google Scholar]
- Pande, S. Causality or Relatedness Assessment in Adverse Drug Reaction and Its Relevance in Dermatology. Indian J. Dermatol. 2018, 63, 18. [Google Scholar] [CrossRef]
- Hammad, T.A.; Afsar, S.; McAvoy, L.B.; Le Louet, H. Aspects to Consider in Causality Assessment of Safety Signals: Broadening the Thought Process. Front. Drug Saf. Regul. 2023, 3, 1193413. [Google Scholar] [CrossRef]
- WHO-UMC. The Use of the WHO-UMC System for Standardised Case Causality Assessment; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Brown, E.G.; Wood, L.; Wood, S. The Medical Dictionary for Regulatory Activities (MedDRA). Drug Saf. 1999, 20, 109–117. [Google Scholar] [CrossRef]
- Khan, M.A.A.; Hamid, S.; Babar, Z.-U.-D. Pharmacovigilance in High-Income Countries: Current Developments and a Review of Literature. Pharmacy 2023, 11, 10. [Google Scholar] [CrossRef]
- Hussain, R.; Hassali, M.A.; Ur Rehman, A.; Muneswarao, J.; Hashmi, F. Physicians’ Understanding and Practices of Pharmacovigilance: Qualitative Experience from a Lower Middle-Income Country. Int. J. Environ. Res. Public Health 2020, 17, 2209. [Google Scholar] [CrossRef]
- Jeetu, G.; Anusha, G. Pharmacovigilance: A Worldwide Master Key for Drug Safety Monitoring. J. Young Pharm. 2010, 2, 315–320. [Google Scholar] [CrossRef]
- Anvisa VigiMed—Saiba Mais. Available online: https://www.gov.br/anvisa/pt-br/assuntos/fiscalizacao-e-monitoramento/notificacoes/vigimed/vigimed-saiba-mais (accessed on 29 November 2023).
- Johansson, K.; Olsson, S.; Hellman, B.; Meyboom, R.H.B. An analysis of Vigimed, a global e-mail system for the exchange of pharmacovigilance information. Drug Saf. 2007, 30, 883–889. [Google Scholar] [CrossRef]
- Rose, J. Critical Appraisal of VAERS Pharmacovigilance: Is the U.S. Vaccine Adverse Events Reporting System (VAERS) a Functioning Pharmacovigilance System? Sci. Public Health Policy Law 2021, 3, 100–129. [Google Scholar]
- Department of Health & Human Services (HHS). Background and Public Health Importance. Available online: https://vaers.hhs.gov (accessed on 12 March 2024).
- Postigo, R.; Brosch, S.; Slattery, J.; van Haren, A.; Dogné, J.M.; Kurz, X.; Candore, G.; Domergue, F.; Arlett, P. EudraVigilance Medicines Safety Database: Publicly Accessible Data for Research and Public Health Protection. Drug Saf. 2018, 41, 665–675. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). EudraVigilance. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/pharmacovigilance-research-development/eudravigilance (accessed on 12 March 2024).
- Ishiguro, C.; Takeuchi, Y.; Uyama, Y.; Tawaragi, T. The MIHARI Project: Establishing a New Framework for Pharmacoepidemiological Drug Safety Assessments by the Pharmaceuticals and Medical Devices Agency of Japan. Pharmacoepidemiol. Drug Saf. 2016, 25, 854–859. [Google Scholar] [CrossRef]
- Pharmaceuticals and Medical Devices Agency. MIHARI Project. Available online: https://www.pmda.go.jp/english/safety/surveillance-analysis/0001.html?print (accessed on 12 March 2024).
- Tiemersma, E.W.; Ali, I.; Alemu, A.; Avong, Y.K.; Duga, A.; Elagbaje, C.; Isah, A.; Kay, A.; Mmbaga, B.T.; Mmari, E.; et al. Baseline Assessment of Pharmacovigilance Activities in Four Sub-Saharan African Countries: A Perspective on Tuberculosis. BMC Health Serv. Res. 2021, 21, 1062. [Google Scholar] [CrossRef]
- PAVIA. About Us & What We Do. Available online: https://pavia-africa.net (accessed on 12 March 2024).
- Ghosh, D.; Skinner, M.; Ferguson, L.R. The Role of the Therapeutic Goods Administration and the Medicine and Medical Devices Safety Authority in Evaluating Complementary and Alternative Medicines in Australia and New Zealand. Toxicology 2006, 221, 88–94. [Google Scholar] [CrossRef]
- Australian Government Therapeutic Goods Administration (TGA). Available online: https://www.tga.gov.au/about-tga/what-we-do (accessed on 12 March 2024).
- Saldaña, A.; Rodríguez, M.; Roldán, J.; Lobos, C.; González, C.; Avendaño, M.; Villena, R.; González, M.; de Kartzow, R.V.; Vergara, N. Vaccine Pharmacovigilance and Its Application in Chile. Rev. Medica Clin. Las. Condes 2020, 31, 240–255. [Google Scholar] [CrossRef]
- Hussain, R.; Hassali, M.A.; Babar, Z.U.D. Medicines Safety in the Globalized Context. In Global Pharmaceutical Policy; Palgrave Macmillan: Basingstoke, UK, 2020; pp. 1–28. ISBN 9789811527241. [Google Scholar]
- Krska, J.; Anderson, C.; Murphy, E.; Avery, A.J. How Patient Reporters Identify Adverse Drug Reactions. Drug Saf. 2011, 34, 429–436. [Google Scholar] [CrossRef]
- Donzanti, B.A. Pharmacovigilance Is Everyone’s Concern: Let’s Work It Out Together. Clin. Ther. 2018, 40, 1967–1972. [Google Scholar] [CrossRef]
- Friede, A.; Reid, J.A.; Ory, H.W. CDC WONDER: A Comprehensive On-Line Public Health Information System of the Centers for Disease Control and Prevention. Am. J. Public Health 1993, 83, 1289–1294. [Google Scholar] [CrossRef]
- de Oliveira, P.M.N.; Lignani, L.K.; da Conceição, D.A.; de Mello Farias, P.M.C.; Takey, P.R.G.; de Sousa Maia, M.d.L.; Camacho, L.A.B. Surveillance of Adverse Events Following Immunization in the Late 2010s: An Overview of the Importance, Tools, and Challenges. Cad Saude Publica 2020, 36, e00182019. [Google Scholar] [CrossRef]
- Martins, R.d.M.; Pavão, A.L.B.; de Oliveira, P.M.N.; dos Santos, P.R.G.; Carvalho, S.M.D.; Mohrdieck, R.; Fernandes, A.R.; Sato, H.K.; de Figueiredo, P.M.; dos Reis von Doellinger, V.; et al. Adverse Events Following Yellow Fever Immunization: Report and Analysis of 67 Neurological Cases in Brazil. Vaccine 2014, 32, 6676–6682. [Google Scholar] [CrossRef]
- Santoro, A.; Genov, G.; Spooner, A.; Raine, J.; Arlett, P. Promoting and Protecting Public Health: How the European Union Pharmacovigilance System Works. Drug Saf. 2017, 40, 855–869. [Google Scholar] [CrossRef]
- Leal, M.M.; Sanz, M.M.; Ferrando, J.R.C.; Martinez-Martinez, F. A Comparative Analysis of the Pharmacovigilance Systems of Brazil, Spain, the European Union and the United States Based on the Information Provided by Their Regulatory Agency Websites. DARU J. Pharm. Sci. 2019, 27, 379–387. [Google Scholar] [CrossRef]
- European Medicines Agency. Good Pharmacovigilance Practices. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/post-authorisation/pharmacovigilance-post-authorisation/good-pharmacovigilance-practices (accessed on 4 December 2023).
- WHO. Guideline for AEFI Surveillance; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Sardella, M.; Costanzo, L. The 6th European Pharmacovigilance Congress: Speaker Abstracts. Ther. Adv. Drug Saf. 2023, 14, 204209862211445. [Google Scholar] [CrossRef]
- Nishioka, K.; Makimura, T.; Ishiguro, A.; Nonaka, T.; Yamaguchi, M.; Uyama, Y. Evolving Acceptance and Use of RWE for Regulatory Decision Making on the Benefit/Risk Assessment of a Drug in Japan. Clin. Pharmacol. Ther. 2022, 111, 35–43. [Google Scholar] [CrossRef]
- KNCV. Tuberculosis Foundation PAVIA. Available online: https://www.kncvtbc.org/en/pavia/ (accessed on 12 March 2024).
- PAVIA. Project A Blueprint for Strengthening Pharmacovigilance Systems in Resourcelimited Countries. 2023. Available online: http://www.efda.gov.et/wp-content/uploads/2023/06/A-blueprint-for-strengthening-pharmacovigilance-systems-in-resource-limited-countries.pdf (accessed on 3 February 2024).
- Australian Government. The Role of the TGA. Available online: https://www.tga.gov.au/role-tga (accessed on 12 March 2024).
- Menang, O.; Kuemmerle, A.; Maigetter, K.; Burri, C. Strategies and Interventions to Strengthen Pharmacovigilance Systems in Low-Income and Middle-Income Countries: A Scoping Review. BMJ Open 2023, 13, e071079. [Google Scholar] [CrossRef]
- Abiri, O.T.; Johnson, W.C.N. Pharmacovigilance Systems in Resource-Limited Settings: An Evaluative Case Study of Sierra Leone. J. Pharm. Policy Pract. 2019, 12, 13. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Jiang, T.; Xing, H.; Xu, J.; Li, C.; Ni, R.; Zhang, N.; Xiang, G.; Li, L.; et al. Opportunities and Challenges of Pharmacovigilance in Special Populations: A Narrative Review of the Literature. Ther. Adv. Drug Saf. 2023, 14, 20420986231200746. [Google Scholar] [CrossRef]
- Al-Worafi, Y.M. Safety of Medications in Special Population. In Drug Safety in Developing Countries: Achievements and Challenges; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–162. ISBN 9780128198377. [Google Scholar]
- Miller, M.A.; Rathore, M.H. Immunization in Special Populations. Adv. Pediatr. 2012, 59, 95–136. [Google Scholar] [CrossRef]
- Kantarcioglu, B.; Iqbal, O.; Lewis, J.; Carter, C.A.; Singh, M.; Lievano, F.; Ligocki, M.; Jeske, W.; Adiguzel, C.; Gerotziafas, G.T.; et al. An Update on the Status of Vaccine Development for SARS-CoV-2 Including Variants. Practical Considerations for COVID-19 Special Populations. Clin. Appl. Thromb./Hemost. 2022, 28, 10760296211056648. [Google Scholar] [CrossRef]
- Edwards, K.M.; Creech, C.B. Vaccine Development in Special Populations. In Human Vaccines: Emerging Technologies in Design and Development; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 163–185. ISBN 9780128023020. [Google Scholar]
- Doherty, M.; Schmidt-Ott, R.; Santos, J.I.; Stanberry, L.R.; Hofstetter, A.M.; Rosenthal, S.L.; Cunningham, A.L. Vaccination of Special Populations: Protecting the Vulnerable. Vaccine 2016, 34, 6681–6690. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Han, Y.; Gu, Z.; Sun, C. Immunosenescence, Aging and Successful Aging. Front. Immunol. 2022, 13, 942796. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Hirokawa, K.; Cohen, A.A.; Witkowski, J.M. Immunosenescence Is Both Functional/Adaptive and Dysfunctional/Maladaptive. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Understanding Immunosenescence to Improve Responses to Vaccines. Nat. Immunol. 2013, 14, 428–436. [Google Scholar] [CrossRef]
- Weinberger, B. Vaccines for the Elderly: Current Use and Future Challenges. Immun. Ageing 2018, 15, 3. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Tafuri, S. Vaccination of Elderly People Affected by Chronic Diseases: A Challenge for Public Health. Vaccines 2022, 10, 641. [Google Scholar] [CrossRef]
- Hajat, C.; Stein, E. The Global Burden of Multiple Chronic Conditions: A Narrative Review. Prev. Med. Rep. 2018, 12, 284–293. [Google Scholar] [CrossRef]
- Stephens, M.M.; Kavanaugh, E. Improving Immunization Coverage in Special Populations. Prim. Care—Clin. Off. Pract. 2020, 47, 453–465. [Google Scholar] [CrossRef]
- Shoham, S.; Batista, C.; Ben Amor, Y.; Ergonul, O.; Hassanain, M.; Hotez, P.; Kang, G.; Kim, J.H.; Lall, B.; Larson, H.J.; et al. Vaccines and Therapeutics for Immunocompromised Patients with COVID-19. EClinicalMedicine 2023, 59, 101965. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Travers, P.; Walport, M. Inherited Immunodeficiency Diseases. In Immunobiology: The Immune System in Health and Disease; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Raje, N.; Dinakar, C. Overview of Immunodeficiency Disorders. Immunol. Allergy Clin. N. Am. 2015, 35, 599–623. [Google Scholar] [CrossRef]
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. Executive Summary: 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host. Clin. Infect. Dis. 2014, 58, 309–318. [Google Scholar] [CrossRef]
- Mohme, S.; Schmalzing, M.; Müller, C.S.L.; Vogt, T.; Goebeler, M.; Stoevesandt, J. Immunizations in Immunocompromised Patients: A Guide for Dermatologists. J. Dtsch. Dermatol. Ges. 2020, 18, 699–723. [Google Scholar] [CrossRef]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events after COVID-19 MRNA Vaccination. JAMA—J. Am. Med. Assoc. 2021, 326, 1390–1399. [Google Scholar] [CrossRef]
- Araja, D.; Krumina, A.; Nora-Krukle, Z.; Berkis, U.; Murovska, M. Vaccine Vigilance System: Considerations on the Effectiveness of Vigilance Data Use in COVID-19 Vaccination. Vaccines 2022, 10, 2115. [Google Scholar] [CrossRef]
- Curlin, G.; Landry, S.; Bernstein, J.; Gorman, R.L.; Mulach, B.; Hackett, C.J.; Foster, S.; Miers, S.E.; Strickler-Dinglasan, P. Integrating Safety and Efficacy Evaluation throughout Vaccine Research and Development. Pediatrics 2011, 127, S9–S15. [Google Scholar] [CrossRef]
- Lloyd, P.C.; Hu, M.; Wong, H.L.; Shoaibi, A.; Ke Zhou, C.; Lo, A.C.; Amend, K.; Beachler, D.C.; McMahill-Walraven, C.N.; Smith, E.R.; et al. Near Real-Time Surveillance of Safety Outcomes in US COVID-19 Vaccine Recipients Aged 12 to 64 Years. Vaccine 2022, 40, 6481–6488. [Google Scholar] [CrossRef]
- Eland, I.A.; Belton, K.J.; van Grootheest, A.C.; Meiners, A.P.; Rawlins, M.D.; Ch Stricker, B.H. Attitudinal Survey of Voluntary Reporting of Adverse Drug Reactions. Br. J. Clin. Pharmacol. 1999, 48, 623–627. [Google Scholar] [CrossRef]
- Desai, M. Pharmacovigilance and Spontaneous Adverse Drug Reaction Reporting: Challenges and Opportunities. Perspect. Clin. Res. 2022, 13, 177–179. [Google Scholar] [CrossRef]
- Cheaib, N. Pharmacovigilance in Clinical Trials: Current Practice and Challenges. Account. Res. 2016, 23, 23–30. [Google Scholar] [CrossRef]
- Domingues, C.M.A.S.; Teixeira, A.M.d.S.; Carvalho, S.M.D. National Immunization Program: Vaccination, Compliance and Pharmacovigilance. Rev. Inst. Med. Trop. Sao Paulo 2012, 54, 22–27. [Google Scholar] [CrossRef]
- Kshirsagar, N.; Ferner, R.; Figueroa, B.A.A.; Ghalib, H.; Lazdin, J. Pharmacovigilance Methods in Public Health Programmes: The Example of Miltefosine and Visceral Leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 61–67. [Google Scholar] [CrossRef]
- Lopez-Gonzalez, E.; Herdeiro, M.T.; Figueiras, A. Determinants of Under-Reporting of Adverse Drug Reactions. Drug Saf. 2009, 32, 19–31. [Google Scholar] [CrossRef]
- Hazell, L.; Shakir, S.A.W. Under-Reporting of Adverse Drug Reactions. Drug Saf. 2006, 29, 385–396. [Google Scholar] [CrossRef]
- García-Abeijon, P.; Costa, C.; Taracido, M.; Herdeiro, M.T.; Torre, C.; Figueiras, A. Factors Associated with Underreporting of Adverse Drug Reactions by Health Care Professionals: A Systematic Review Update. Drug Saf. 2023, 46, 625–636. [Google Scholar] [CrossRef]
- Costa, C.; Abeijon, P.; Rodrigues, D.A.; Figueiras, A.; Herdeiro, M.T.; Torre, C. Factors Associated with Underreporting of Adverse Drug Reactions by Patients: A Systematic Review. Int. J. Clin. Pharm. 2023, 45, 1349–1358. [Google Scholar] [CrossRef]
- Al Meslamani, A.Z. Underreporting of Adverse Drug Events: A Look into the Extent, Causes, and Potential Solutions. Expert. Opin. Drug Saf. 2023, 22, 351–354. [Google Scholar] [CrossRef]
- International Society of Pharmacovigilance. Abstracts 9th ISoP Annual Meeting ‘From Pharmacovigilance to Risk Management’ Reims. Drug-Saf. 2009, 32, 875–993. [Google Scholar] [CrossRef]
- Salvador, M.R.; Monteiro, C.; Pereira, L.; Duarte, A.P. Quality of Spontaneous Reports of Adverse Drug Reactions Sent to a Regional Pharmacovigilance Unit. Int. J. Environ. Res. Public Health 2022, 19, 3754. [Google Scholar] [CrossRef]
- Celi, L.A.; Moseley, E.; Moses, C.; Ryan, P.; Somai, M.; Stone, D.; Tang, K. From Pharmacovigilance to Clinical Care Optimization. Big Data 2014, 2, 134–141. [Google Scholar] [CrossRef]
- Curtin, F.; Schulz, P. Assessing the Benefit:Risk Ratio of a Drug—Randomized and Naturalistic Evidence. Dialogues Clin. Neurosci. 2011, 13, 183–190. [Google Scholar] [CrossRef]
- Feehan, A.K.; Garcia-Diaz, J. Investigator Responsibilities in Clinical Research. Ochsner J. 2020, 20, 44–49. [Google Scholar] [CrossRef]
- Nikam, S.; Jambhulkar, A.; Kayande, K.; Ghule, A.; Inde, A. Pharmacovigilance Safety Monitoring in Clinical Trials. Int. J. Pharm. Sci. Rev. Res. 2021, 70, 9–16. [Google Scholar] [CrossRef]
- Vijayananthan, A.; Nawawi, O. The Importance of Good Clinical Practice Guidelines and Its Role in Clinical Trials. Biomed. Imaging Interv. J. 2008, 4, e5. [Google Scholar] [CrossRef]
- Brown, J.N.; Britnell, S.R.; Stivers, A.P.; Cruz, J.L. Medication Safety in Clinical Trials: Role of the Pharmacist in Optimizing Practice, Collaboration, and Education to Reduce Errors. Yale J. Biol. Med. 2017, 90, 125–133. [Google Scholar]
- Patel, C.; Rendell, N.; Sargent, G.M.; Ali, A.; Morgan, C.; Fields, R.; Sheel, M. Measuring National Immunization System Performance: A Systematic Assessment of Available Resources. Glob. Health Sci. Pract. 2023, 11, e220055. [Google Scholar] [CrossRef]
- Baum, U.; Sundman, J.; Jääskeläinen, S.; Nohynek, H.; Puumalainen, T.; Jokinen, J. Establishing and Maintaining the National Vaccination Register in Finland. Eurosurveillance 2017, 22, 30520. [Google Scholar] [CrossRef]
- Cutts, F.T.; Claquin, P.; Danovaro-Holliday, M.C.; Rhoda, D.A. Monitoring Vaccination Coverage: Defining the Role of Surveys. Vaccine 2016, 34, 4103–4109. [Google Scholar] [CrossRef]
- Nakayama, T. Causal Relationship between Immunological Responses and Adverse Reactions Following Vaccination. Vaccine 2019, 37, 366–371. [Google Scholar] [CrossRef]
- Gershwin, L.J. Adverse Reactions to Vaccination: From Anaphylaxis to Autoimmunity. Vet. Clin. N. Am.—Small Anim. Pract. 2018, 48, 279–290. [Google Scholar] [CrossRef]
- Schafer, I.; Oltrogge, J.H.; Nestoriuc, Y.; Warren, C.V.; Brassen, S.; Blattner, M.; Luhmann, D.; Tinnermann, A.; Scherer, M.; Buchel, C. Expectations and Prior Experiences Associated with Adverse Effects of COVID-19 Vaccination. JAMA Netw. Open 2023, 6, e234732. [Google Scholar] [CrossRef]
- Yi, S.; Cagkan Inkaya, A.; Jo, J.; Sitaresmi, M.N. Adverse Events Following Immunization of COVID-19 Vaccine among Children Aged 6–11 Years. Front. Public Health 2021, 10, 999354. [Google Scholar]
- Clair, L.A.S.; Chaulagain, S.; Klein, S.L.; Benn, C.S.; Flanagan, K.L. Sex-Differential and Non-Specific Effects of Vaccines Over the Life Course. Curr. Top. Microbiol. Immunol. 2023, 441, 225. [Google Scholar] [CrossRef]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of Immune Responses to Viral Vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- Janekrongtham, C.; Salazar, M.; Doung-ngern, P. Sex Differences in Serious Adverse Events Reported Following Booster Doses of COVID-19 Vaccination in Thailand: A Countrywide Nested Unmatched Case-Control Study. Vaccines 2023, 11, 1772. [Google Scholar] [CrossRef]
- Turner, P.J.; Ansotegui, I.J.; Campbell, D.E.; Cardona, V.; Ebisawa, M.; El-Gamal, Y.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; et al. COVID-19 Vaccine-Associated Anaphylaxis: A Statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ. J. 2021, 14, 100517. [Google Scholar] [CrossRef]
- Shah, R.H.; Kuder, M.M.; Lang, D.M. Anaphylaxis to Drugs, Biological Agents, and Vaccines. Immunol. Allergy Clin. N. Am. 2022, 42, 121–144. [Google Scholar] [CrossRef]
- Kuder, M.M.; Lang, D.M.; Patadia, D.D. Anaphylaxis to Vaccinations: A Review of the Literature and Evaluation of the COVID-19 MRNA Vaccinations. Clevel. Clin. J. Med. 2021. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 Vaccines Strategies: A Comprehensive Review of Phase 3 Candidates. NPJ Vaccines 2021, 6, 28. [Google Scholar] [CrossRef]
- WHO Number of COVID-19 Cases Reported to WHO. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 29 December 2023).
- Kiazand, A.; Luther, R.; Mårlind Würtele, J.; Southall, N.; Domalik, D.; Ysander, M. Pandemic Vaccines: A Formidable Challenge for Pharmacovigilance. Nat. Rev. Drug Discov. 2023, 22, 1–2. [Google Scholar] [CrossRef]
- Martínez-Flores, D.; Zepeda-Cervantes, J.; Cruz-Reséndiz, A.; Aguirre-Sampieri, S.; Sampieri, A.; Vaca, L. SARS-CoV-2 Vaccines Based on the Spike Glycoprotein and Implications of New Viral Variants. Front. Immunol. 2021, 12, 701501. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and Functional Properties of SARS-CoV-2 Spike Protein: Potential Antivirus Drug Development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Wu, N.; Joyal-Desmarais, K.; Ribeiro, P.A.B.; Vieira, A.M.; Stojanovic, J.; Sanuade, C.; Yip, D.; Bacon, S.L. Long-Term Effectiveness of COVID-19 Vaccines against Infections, Hospitalisations, and Mortality in Adults: Findings from a Rapid Living Systematic Evidence Synthesis and Meta-Analysis up to December 2022. Lancet Respir. Med. 2023, 11, 439–452. [Google Scholar] [CrossRef]
- El-Elimat, T.; AbuAlSamen, M.M.; Almomani, B.A.; Al-Sawalha, N.A.; Alali, F.Q. Acceptance and Attitudes toward COVID-19 Vaccines: A Cross-Sectional Study from Jordan. PLoS ONE 2021, 16, e0250555. [Google Scholar] [CrossRef]
- Sabbaghi, A.; Miri, S.M.; Keshavarz, M.; Zargar, M.; Ghaemi, A. Inactivation Methods for Whole Influenza Vaccine Production. Rev. Med. Virol. 2019, 29, e2074. [Google Scholar] [CrossRef]
- Ebenig, A.; Lange, M.V.; Mühlebach, M.D. Versatility of Live-Attenuated Measles Viruses as Platform Technology for Recombinant Vaccines. NPJ Vaccines 2022, 7, 119. [Google Scholar] [CrossRef]
- Akuzum, B.; Kim, S.; Nguyen, T.T.; Hong, J.; Lee, S.; Kim, E.; Kim, J.; Choi, Y.; Jhun, H.; Lee, Y.; et al. L1 Recombinant Proteins of HPV Tested for Antibody Forming Using Sera of HPV Quadrivalent Vaccine. Immune Netw. 2018, 18, e19. [Google Scholar] [CrossRef]
- Dolzhikova, I.V.; Tokarskaya, E.A.; Dzharullaeva, A.S.; Tukhvatulin, A.I.; Shcheblyakov, D.V.; Voronina, O.L.; Syromyatnikova, S.I.; Borisevich, S.V.; Pantyukhov, V.B.; Babira, V.F.; et al. Virus-Vectored Ebola Vaccines. Acta Naturae 2017, 9, 4–11. [Google Scholar] [CrossRef]
- Dolgin, E. The tangled history of mrna vaccines hundreds of scientists had worked on mrna vaccines for decades before the coronavirus pandemic brought a breakthrough. Nature 2021, 597, 318–324. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Hodel, K.V.S.; Fonseca, L.M.D.S.; Mascarenhas, L.A.B.; Andrade, L.P.C.d.S.; Rocha, V.P.C.; Soares, M.B.P.; Berglund, P.; Duthie, M.S.; Reed, S.G.; et al. The Importance of Rna-Based Vaccines in the Fight against COVID-19: An Overview. Vaccines 2021, 9, 1345. [Google Scholar] [CrossRef]
- Goyal, L.; Zapata, M.; Ajmera, K.; Chaurasia, P.; Pandit, R.; Pandit, T. A Hitchhiker’s Guide to Worldwide COVID-19 Vaccinations: A Detailed Review of Monovalent and Bivalent Vaccine Schedules, COVID-19 Vaccine Side Effects, and Effectiveness Against Omicron and Delta Variants. Cureus 2022, 14, e29837. [Google Scholar] [CrossRef]
- Callegaro, A.; Borleri, D.; Farina, C.; Napolitano, G.; Valenti, D.; Rizzi, M.; Maggiolo, F. Antibody Response to SARS-CoV-2 Vaccination Is Extremely Vivacious in Subjects with Previous SARS-CoV-2 Infection. J. Med. Virol. 2021, 93, 4612–4615. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Frankland, T.B.; Xie, F.; Ackerson, B.K.; Takhar, H.; Ogun, O.A.; Simmons, S.; et al. Analysis of MRNA COVID-19 Vaccine Uptake among Immunocompromised Individuals in a Large US Health System. JAMA Netw. Open 2023, 6, E2251833. [Google Scholar] [CrossRef]
- Anderson, E.J.; Creech, C.B.; Berthaud, V.; Piramzadian, A.; Johnson, K.A.; Zervos, M.; Garner, F.; Griffin, C.; Palanpurwala, K.; Turner, M.; et al. Evaluation of MRNA-1273 Vaccine in Children 6 Months to 5 Years of Age. N. Engl. J. Med. 2022, 387, 1673–1687. [Google Scholar] [CrossRef]
- Edwards, K.M.; Orenstein, W.A. COVID-19: Vaccines. Available online: https://www.uptodate.com/contents/image?imageKey=ID%2F130711&topicKey=ID%2F129849&search=COVID-19%20grupos%20vulneraveis%20vaccine&rank=4~150&source=see_link (accessed on 11 April 2023).
- Teijaro, J.R.; Farber, D.L. COVID-19 Vaccines: Modes of Immune Activation and Future Challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in Ensuring Global Access to COVID-19 Vaccines: Production, Affordability, Allocation, and Deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- Lin, T.C.; Fu, P.A.; Hsu, Y.T.; Chen, T.Y. Vaccine-Induced Immune Thrombotic Thrombocytopenia Following BNT162b2 MRNA COVID-19 Booster: A Case Report. Vaccines 2023, 11, 1115. [Google Scholar] [CrossRef]
- Ng, X.L.; Betzler, B.K.; Testi, I.; Ho, S.L.; Tien, M.; Ngo, W.K.; Zierhut, M.; Chee, S.P.; Gupta, V.; Pavesio, C.E.; et al. Ocular Adverse Events After COVID-19 Vaccination. Ocul. Immunol. Inflamm. 2021, 29, 1216–1224. [Google Scholar] [CrossRef]
- Gomes, D.A.; Santos, R.R.; Freitas, P.; Paiva, M.S.; Ferreira, J.; Trabulo, M. Acute Myocarditis Following MRNA COVID-19 Vaccine. Arq. Bras. Cardiol. 2022, 118, 783–788. [Google Scholar] [CrossRef]
- Alami, A.; Krewski, D.; Farhat, N.; Mattison, D.; Wilson, K.; Gravel, C.A.; Farrell, P.J.; Crispo, J.A.G.; Haddad, N.; Perez-Lloret, S.; et al. Risk of Myocarditis and Pericarditis in MRNA COVID-19-Vaccinated and Unvaccinated Populations: A Systematic Review and Meta-Analysis. BMJ Open 2023, 13, e065687. [Google Scholar] [CrossRef]
- Hilts, A.; Schreiber, A.; Singh, A. A Clinical Case of COVID-19 Vaccine-Associated Guillain-Barré Syndrome. Am. J. Case Rep. 2022, 23, e936896-1–e936896-4. [Google Scholar] [CrossRef]
- Boufidou, F.; Hatziantoniou, S.; Theodoridou, K.; Maltezou, H.C.; Vasileiou, K.; Anastassopoulou, C.; Medić, S.; Tsakris, A. Anaphylactic Reactions to COVID-19 Vaccines: An Updated Assessment Based on Pharmacovigilance Data. Vaccines 2023, 11, 613. [Google Scholar] [CrossRef]
- McNeil, M.M.; Weintraub, E.S.; Duffy, J.; Sukumaran, L.; Jacobsen, S.J.; Klein, N.P.; Hambidge, S.J.; Lee, G.M.; Jackson, L.A.; Irving, S.A.; et al. Risk of Anaphylaxis after Vaccination in Children and Adults. J. Allergy Clin. Immunol. 2016, 137, 868–878. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Cole, M.; Su, J.R. Reports of Anaphylaxis after Receipt of MRNA COVID-19 Vaccines in the US-December 14, 2020-January 18, 2021. JAMA—J. Am. Med. Assoc. 2021, 325, 1101–1102. [Google Scholar] [CrossRef]
- Jaggers, J.; Wolfson, A.R. MRNA COVID-19 Vaccine Anaphylaxis: Epidemiology, Risk Factors, and Evaluation. Curr. Allergy Asthma Rep. 2023, 23, 195–200. [Google Scholar] [CrossRef]
- McSweeney, M.D.; Mohan, M.; Commins, S.P.; Lai, S.K. Anaphylaxis to Pfizer/BioNTech MRNA COVID-19 Vaccine in a Patient with Clinically Confirmed PEG Allergy. Front. Allergy 2021, 2, 715844. [Google Scholar] [CrossRef]
- Mayfield, J.; Bandi, S.; Ganti, L.; Rubero, J. Anaphylaxis after Moderna COVID-19 Vaccine. Ther. Adv. Vaccines Immunother. 2021, 9, 25151355211048418. [Google Scholar] [CrossRef]
- Furqan, M.; Chawla, S.; Majid, M.; Mazumdar, S.; Mahalwar, G.; Harmon, E.; Klein, A. COVID-19 Vaccine–Related Myocardial and Pericardial Inflammation. Curr. Cardiol. Rep. 2022, 24, 2031–2041. [Google Scholar] [CrossRef]
- Karlstad, Ø.; Hovi, P.; Husby, A.; Härkänen, T.; Selmer, R.M.; Pihlström, N.; Hansen, J.V.; Nohynek, H.; Gunnes, N.; Sundström, A.; et al. SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiol. 2022, 7, 600–612. [Google Scholar] [CrossRef]
- Wassif, M.; Lo, P.; Satouris, P.; Swan, L.; Tardo, D.; Kovacic, J.C.; Muller, D.; Muthiah, K.; Kotlyar, E.; Bart, N.K. Acute Myocarditis and Pericarditis After MRNA COVID-19 Vaccinations—A Single-Centre Retrospective Analysis. Heart Lung Circ. 2023, 32, 467–479. [Google Scholar] [CrossRef]
- Macías Saint-Gerons, D.; Ibarz, M.T.; Castro, J.L.; Forés-Martos, J.; Tabarés-Seisdedos, R. Myopericarditis Associated with the Novavax COVID-19 Vaccine (NVX-CoV2373): A Retrospective Analysis of Individual Case Safety Reports from VigiBase. Drugs Real. World Outcomes 2023, 10, 263–270. [Google Scholar] [CrossRef]
- Thant, H.L.; Morgan, R.; Paese, M.M.; Persaud, T.; Diaz, J.; Hurtado, L. Guillain-Barré Syndrome After Ad26.COV2.S Vaccination. Am. J. Case Rep. 2022, 23, e935275-1–e935275-5. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Hatziantoniou, S.; Vlachopoulos, C.; Spanakis, N.; Tsioufis, C.; Tsakris, A.; Lazaros, G. Temporal Relationship of Myocarditis and Pericarditis Following COVID-19 Vaccination: A Pragmatic Approach. Int. J. Cardiol. 2022, 358, 136–139. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 NCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Yamamoto, J.; Awaya, T.; Nakagawa, T.; Tamura, A.; Hiroi, Y. Myocarditis with Ventricular Tachycardia Following Bivalent COVID-19 MRNA Vaccination. CJC Open 2023, 5, 654–657. [Google Scholar] [CrossRef]
- Andersson, N.W.; Thiesson, E.M.; Hansen, J.V.; Hviid, A. Safety of BA.4-5 or BA.1 Bivalent MRNA Booster Vaccines: Nationwide Cohort Study. BMJ 2023, 382, e075015. [Google Scholar] [CrossRef]
- Tutar, N.K.; EYIGÜRBÜZ, T.; YILDIRIM, Z.; KALE, N. SARS-CoV-2 Vaccine Inactivated Sinovac Biotech: Guillain-Barre Syndrome: Case Report. React. Wkly. 2021, 74, 286–288. [Google Scholar] [CrossRef]
- Laisuan, W.; Wongsa, C.; Chiewchalermsri, C.; Thongngarm, T.; Rerkpattanapipat, T.; Iamrahong, P.; Ruangwattanachok, C.; Nanthapisal, S.; Sompornrattanaphan, M. Coronavac Covid-19 Vaccine-Induced Anaphylaxis: Clinical Characteristics and Revaccination Outcomes. J. Asthma Allergy 2021, 14, 1209–1215. [Google Scholar] [CrossRef]
- Acharya, B.; Sabin, K.C.; Karki, S.; Thapa, P.; Pooja, K.C. Guillain-Barré Syndrome Following the Second Dose of COVID AstraZeneca Vaccine in a 78-Year-Old Male: A Case Report from Nepal. Ann. Med. Surg. 2023, 85, 498–501. [Google Scholar] [CrossRef]
- Oo, W.M.; Giri, P.; de Souza, A. AstraZeneca COVID-19 Vaccine and Guillain- Barré Syndrome in Tasmania: A Causal Link? J. Neuroimmunol. 2021, 360, 577719. [Google Scholar] [CrossRef]
- Turmukhambetova, A.; Yegorov, S.; Korshukov, I.; Barkhanskaya, V.; Kolesnichenko, S.; Klyuyev, D.; Zhumadilova, Z.; Pralieva, A.; Absaghit, L.; Belyaev, R.; et al. The Impact of Gam-COVID-Vac, an Adv5/Adv26 COVID-19 Vaccine, on the Biomarkers of Endothelial Function, Coagulation and Platelet Activation. PLoS ONE 2023, 18, e0293074. [Google Scholar] [CrossRef]
- Yi, P.; Yang, X.; Ding, C.; Chen, Y.; Xu, K.; Ni, Q.; Zhao, H.; Li, Y.; Zhang, X.; Liu, J.; et al. Risk Factors and Clinical Features of Deterioration in COVID-19 Patients in Zhejiang, China: A Single-Centre, Retrospective Study. BMC Infect. Dis. 2020, 20, 943. [Google Scholar] [CrossRef]
- Atluri, K.; Aimlin, I.; Arora, S. Current Effective Therapeutics in Management of COVID-19. J. Clin. Med. 2022, 11, 3838. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Qian, L.; Hong, V.; Wei, R.; Nadjafi, R.F.; Fischer, H.; Li, Z.; Shaw, S.F.; Caparosa, S.L.; Nau, C.L.; et al. Obesity and Mortality among Patients Diagnosed with COVID-19: Results from an Integrated Health Care Organization. Ann. Intern. Med. 2020, 173, 773–781. [Google Scholar] [CrossRef]
- Niu, S.; Tian, S.; Lou, J.; Kang, X.; Zhang, L.; Lian, H.; Zhang, J. Clinical Characteristics of Older Patients Infected with COVID-19: A Descriptive Study. Arch. Gerontol. Geriatr. 2020, 89, 104058. [Google Scholar] [CrossRef]
- Nagy, É.; Cseh, V.; Barcs, I.; Ludwig, E. The Impact of Comorbidities and Obesity on the Severity and Outcome of COVID-19 in Hospitalized Patients—A Retrospective Study in a Hungarian Hospital. Int. J. Environ. Res. Public Health 2023, 20, 1372. [Google Scholar] [CrossRef]
- Cevik, M.; Marcus, J.L.; Buckee, C.; Smith, T.C. Severe Acute Respiratory Syndrome Coronavirus 2 (SARSCoV-2) Transmission Dynamics Should Inform Policy. Clin. Infect. Dis. 2021, 73, S170–S176. [Google Scholar] [CrossRef]
- Poudel, K.M.; Shah, N.; Prakash, M.; Deo, S.K.; Bhandari, S.; Poudel, T.R. Determinants of Associated Events Following AZD1222 (Covishield) Vaccination in a High-Risk Population in Nepal. BMC Infect. Dis. 2022, 22, 422. [Google Scholar] [CrossRef]
- Alqahtani, S.; Jokhdar, H.; Al-Tawfiq, J.A.; Al-Otaibi, S.; Assiri, A.; Almudarra, S.; Alabdulkareem, K.; Haji, A. Adverse Events Following Administration of COVID-19 Vaccines in Saudi Arabia. Sci. Rep. 2022, 12, 19551. [Google Scholar] [CrossRef]
- Verity, R.; Okell, L.C.; Dorigatti, I.; Winskill, P.; Whittaker, C.; Imai, N.; Cuomo-Dannenburg, G.; Thompson, H.; Walker, P.G.T.; Fu, H.; et al. Estimates of the Severity of Coronavirus Disease 2019: A Model-Based Analysis. Lancet Infect. Dis. 2020, 20, 669–677. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors Associated with COVID-19-Related Death Using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Miyaji, K.T.; Itto, L.Y.U.; Jacintho, L.C.; Sales, A.C.R.; Hiratsuka, M.; Leonel, F.C.; Higa-Taniguchi, K.T.; Picone, C.M.; Lara, A.N.; Rodrigues, C.C.M.; et al. Adverse Events Following Immunization of Elderly with COVID-19 Inactivated Virus Vaccine (CoronaVac) in Southeastern Brazil: An Active Surveillance Study. Rev. Inst. Med. Trop. Sao Paulo 2022, 64, e56. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent Fatigue Following SARS-CoV-2 Infection Is Common and Independent of Severity of Initial Infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Anderson, M.R.; Geleris, J.; Anderson, D.R.; Zucker, J.; Nobel, Y.R.; Freedberg, D.; Small-Saunders, J.; Rajagopalan, K.N.; Greendyk, R.; Chae, S.R.; et al. Body Mass Index and Risk for Intubation or Death in SARS-CoV-2 Infection: A Retrospective Cohort Study. Ann. Intern. Med. 2020, 173, 782–790. [Google Scholar] [CrossRef]
- Holman, N.; Knighton, P.; Kar, P.; O’Keefe, J.; Curley, M.; Weaver, A.; Barron, E.; Bakhai, C.; Khunti, K.; Wareham, N.J.; et al. Risk Factors for COVID-19-Related Mortality in People with Type 1 and Type 2 Diabetes in England: A Population-Based Cohort Study. Lancet Diabetes Endocrinol. 2020, 8, 823–833. [Google Scholar] [CrossRef]
- Akhtar, S.M.; Gazzaz, Z.J.; Baig, M.; Majeed, R.; Hashmi, A.A. Association Between Pfizer COVID-19 Vaccine Adverse Effects and Diabetes Mellitus: A Prospective Multicenter Study. Cureus 2023, 15, e48263. [Google Scholar] [CrossRef]
- Cunningham, J.W.; Vaduganathan, M.; Claggett, B.L.; Jering, K.S.; Bhatt, A.S.; Rosenthal, N.; Solomon, S.D. Clinical Outcomes in Young US Adults Hospitalized with COVID-19. JAMA Intern. Med. 2021, 181, 379–381. [Google Scholar] [CrossRef]
- Henry, B.M.; Lippi, G. Chronic Kidney Disease Is Associated with Severe Coronavirus Disease 2019 (COVID-19) Infection. Int. Urol. Nephrol. 2020, 52, 1193–1194. [Google Scholar] [CrossRef]
- Bouhanick, B.; Montastruc, F.; Tessier, S.; Brusq, C.; Bongard, V.; Senard, J.M.; Montastruc, J.L.; Herin, F. Hypertension and Covid-19 Vaccines: Are There Any Differences between the Different Vaccines? A Safety Signal. Eur. J. Clin. Pharmacol. 2021, 77, 1937–1938. [Google Scholar] [CrossRef]
- WHO. Poliomyelitis. Available online: https://www.who.int/news-room/fact-sheets/detail/poliomyelitis (accessed on 10 December 2023).
- Louten, J. Poliovirus. In Essential Human Virology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 257–271. [Google Scholar]
- Garg, R.R.; Karst, S.M. Chapter 5.2—Interactions Between Enteric Viruses and the Gut Microbiota. In Viral Gastroenteritis; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Kerr, L. From Eradication to the Risk of Reintroduction of Poliomyelitis in Brazil. Cienc. Saude Coletiva 2023, 28, 328–329. [Google Scholar]
- Ruiz, S.I.; Zumbrun, E.E.; Nalca, A. Animal Models of Human Viral Diseases. In Animal Models for the Study of Human Disease; Elsevier: Amsterdam, The Netherlands, 2013; pp. 927–970. ISBN 9780124158948. [Google Scholar]
- Tan, S.Y.; Ponstein, N. Jonas Salk (1914–1995): A Vaccine against Polio. Singapore Med. J. 2019, 60, 9–10. [Google Scholar] [CrossRef]
- Shampo, M.A.; Kyle, R.A.; Steensma, D.P. Albert Sabin—Conqueror of Poliomyelitis. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2011; Volume 86. [Google Scholar]
- Nathanson, N. Eradication of Poliomyelitis in the United States. Rev. Infect. Dis. 1982, 4, 940–950. [Google Scholar] [CrossRef]
- Yeh, M.T.; Bujaki, E.; Dolan, P.T.; Smith, M.; Wahid, R.; Konz, J.; Weiner, A.J.; Bandyopadhyay, A.S.; Van Damme, P.; De Coster, I.; et al. Engineering the Live-Attenuated Polio Vaccine to Prevent Reversion to Virulence. Cell Host Microbe 2020, 27, 736–751.e8. [Google Scholar] [CrossRef]
- Tseha, S.T. Polio: The Disease That Reemerged after Six Years in Ethiopia. Ethiop. J. Health Sci. 2021, 31, 897–902. [Google Scholar]
- Van Damme, P.; De Coster, I.; Bandyopadhyay, A.S.; Revets, H.; Withanage, K.; De Smedt, P.; Suykens, L.; Oberste, M.S.; Weldon, W.C.; Costa-Clemens, S.A.; et al. The Safety and Immunogenicity of Two Novel Live Attenuated Monovalent (Serotype 2) Oral Poliovirus Vaccines in Healthy Adults: A Double-Blind, Single-Centre Phase 1 Study. Lancet 2019, 394, 148–158. [Google Scholar] [CrossRef]
- Yeh, M.T.; Smith, M.; Carlyle, S.; Konopka-Anstadt, J.L.; Burns, C.C.; Konz, J.; Andino, R.; Macadam, A. Genetic Stabilization of Attenuated Oral Vaccines against Poliovirus Types 1 and 3. Nature 2023, 619, 135–142. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.S.; Garon, J.; Seib, K.; Orenstein, W.A. Polio Vaccination: Past, Present and Future. Future Microbiol. 2015, 10, 791–808. [Google Scholar] [CrossRef]
- Global Polio Eradication Initiative (GPEI). Polio and the Introduction of IPV; Global Polio Eradication Initiative (GPEI): Geneva, Switzerland, 2014. [Google Scholar]
- Alfaro-Murillo, J.A.; Ávila-Agüero, M.L.; Fitzpatrick, M.C.; Crystal, C.J.; Falleiros-Arlant, L.H.; Galvani, A.P. The Case for Replacing Live Oral Polio Vaccine with Inactivated Vaccine in the Americas. Lancet 2020, 395, 1163–1166. [Google Scholar] [CrossRef]
- Vela Ramirez, J.E.; Sharpe, L.A.; Peppas, N.A. Current State and Challenges in Developing Oral Vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Andersen, A.; Fisker, A.B.; Nielsen, S.; Rodrigues, A.; Benn, C.S.; Aaby, P. National Immunization Campaigns with Oral Polio Vaccine May Reduce All-Cause Mortality: An Analysis of 13 Years of Demographic Surveillance Data from an Urban African Area. Clin. Infect. Dis. 2021, 72, E596–E603. [Google Scholar] [CrossRef]
- Mileno, M.D.; Lau, C.; Lonks, J.R.; Garland, J.M.; Sanchez, M.C.; Nau, G.J.; Larkin, J.M. Vaccines for Adult Travelers: When and Why? In Vaccinations; Elsevier: Amsterdam, The Netherlands, 2018; pp. 163–177. ISBN 9780323554350. [Google Scholar]
- Furesz, J. Developments in the Production and Quality Control of Poliovirus Vaccines—Historical Perspectives. Biologicals 2006, 34, 87–90. [Google Scholar] [CrossRef]
- Scherrer, M.A.R.; Abreu, É.P.; Rocha, V.B. Neomycin: Sources of Contact and Sensitization Evaluation in 1162 Patients Treated at a Tertiary Service. An. Bras. Dermatol. 2023, 98, 487–492. [Google Scholar] [CrossRef]
- Skibinski, D.A.G.; Baudner, B.C.; Singh, M.; O’hagan, D.T. Combination Vaccines. Proc. J. Glob. Infect. Dis. 2011, 3, 63–72. [Google Scholar] [CrossRef]
- Our World in Data Which Countries Have Mandatory Childhood Vaccination Policies? Available online: https://ourworldindata.org/grapher/mandatory-childhood-vaccination (accessed on 1 February 2024).
- Stratton, K.R.; Wilson, C.B.; McCormick, M.C.; Institute of Medicine (U.S.); Immunization Safety Review Committee; Board on Health Promotion and Disease Prevention. Immunization Safety Review: Multiple Immunizations and Immune Dysfunction; National Academies Press: Washington, DC, USA, 2004; ISBN 0309508665. [Google Scholar]
- Stone, C.A.; Rukasin, C.R.F.; Beachkofsky, T.M.; Phillips, E.J. Immune-Mediated Adverse Reactions to Vaccines. Br. J. Clin. Pharmacol. 2019, 85, 2694–2706. [Google Scholar] [CrossRef]
- Dreskin, S.C.; Halsey, N.A.; Kelso, J.M.; Wood, R.A.; Hummell, D.S.; Edwards, K.M.; Caubet, J.C.; Engler, R.J.M.; Gold, M.S.; Ponvert, C.; et al. International Consensus (ICON): Allergic Reactions to Vaccines. World Allergy Organ. J. 2016, 9, 32. [Google Scholar] [CrossRef]
- Gao, J.; Kang, G.; Hu, R.; Zhang, L.; Yu, J.; Wang, Z.; Tang, F. Adverse Events Following Immunization with Bivalent Oral Poliovirus Vaccine in Jiangsu, China. Br. J. Clin. Pharmacol. 2021, 87, 4831–4838. [Google Scholar] [CrossRef]
- Nzolo, D.; Aloni, M.N.; Ngamasata, T.M.; Luemba, B.M.; Marfeza, S.B.; Ekila, M.B.; Nsibu, C.N.; Tona, N.L. Adverse Events Following Immunization with Oral Poliovirus in Kinshasa, Democratic Republic of Congo: Preliminary Results. Pathog. Glob. Health 2013, 107, 381–384. [Google Scholar] [CrossRef][Green Version]
- Su, J.R.; Moro, P.L.; Ng, C.S.; Lewis, P.W.; Said, M.A.; Cano, M.V. Anaphylaxis after Vaccination Reported to the Vaccine Adverse Event Reporting System, 1990-2016. J. Allergy Clin. Immunol. 2019, 143, 1465–1473. [Google Scholar] [CrossRef]
- Kang, G.; Tang, F.; Wang, Z.; Hu, R.; Yu, J.; Gao, J. Surveillance of Adverse Events Following the Introduction of Inactivated Poliovirus Vaccine Made from Sabin Strains (SIPV) to the Chinese EPI and a Comparison with Adverse Events Following Inactivated Poliovirus Vaccine Made from Wild Strains (WIPV) in Jiangsu, China. Hum. Vaccin. Immunother. 2021, 17, 2568–2574. [Google Scholar] [CrossRef]
- Hervé, C.; Laupèze, B.; Del Giudice, G.; Didierlaurent, A.M.; Da Silva, F.T. The How’s and What’s of Vaccine Reactogenicity. NPJ Vaccines 2019, 4, 39. [Google Scholar] [CrossRef]
- Tang, X.; Xiao, Y.; Deng, X.; Zhou, Y.; Chen, H.; Yan, R.; Zhu, Y.; Wang, S.; Wang, H.; Zhu, X.; et al. Immuno-persistence of the different primary polio vaccine schedules and immunogenicity of the booster dose by sabin inactivated or bivalent oral poliovirus vaccine in children aged 4 years: An open-label, randomised, controlled phase 4 trial in China. Lancet Reg. Health–West. Pac. 2023, 34, 100725. [Google Scholar] [CrossRef]
- Lv, H.; Pan, X.; Liang, H.; Chen, Y.; Wang, Y.; Chen, F.; Shen, L.; Hu, Y. A Comparison with Adverse Events Following Immunization Associated with Sabin-Strains and Salk-Strains Inactivated Polio Vaccines in Zhejiang Province, China. Vaccines 2022, 10, 319. [Google Scholar] [CrossRef]
- Gumede, N.; Muthambi, V.; Schoub, B.D. Immunodeficiency-Associated Vaccine-Derived Poliovirus Type 3 in Infant, South Africa, 2011. Emerg. Infect. Dis. 2012, 18, 992–994. [Google Scholar] [CrossRef]
- Pöyhönen, L.; Bustamante, J.; Casanova, J.L.; Jouanguy, E.; Zhang, Q. Life-Threatening Infections Due to Live-Attenuated Vaccines: Early Manifestations of Inborn Errors of Immunity. J. Clin. Immunol. 2019, 39, 376–390. [Google Scholar] [CrossRef]
- Domingues, C.M.A.S.; De Fitima Pereira, S.; Marreiros, A.C.C.; Menezes, N.; Flannery, B. Introduction of Sequential Inactivated Polio Vaccine-Oral Polio Vaccine Schedule for Routine Infant Immunization in Brazil’s National Immunization Program. J. Infect. Dis. 2014, 210, S143–S151. [Google Scholar] [CrossRef]
- Wallace, G.S.; Seward, J.F.; Pallansch, M.A. Interim CDC Guidance for Polio Vaccination for Travel to and from Countries by Wild Poliovirus. Morb. Mortal. Wkly. Rep. 2014, 63, 591–594. [Google Scholar]
- Jong, E.C. Immunizations for Travelers. In The Travel and Tropical Medicine Manual, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 47–70. ISBN 9780323375061. [Google Scholar]
- Mittal, S.; Rawat, C.; Gupta, A.; Solanki, H.K.; Singh, R. Adverse Events Following Immunization Among Children Under Two Years of Age: A Prospective Observational Study from North India. Cureus 2023, 15, e38356. [Google Scholar] [CrossRef]
- Soofi, S.B.; Vadsaria, K.; Mannan, S.; Habib, M.A.; Tabassum, F.; Hussain, I.; Muhammad, S.; Feroz, K.; Ahmed, I.; Islam, M.; et al. Factors Associated with Vaccine Refusal (Polio and Routine Immunization) in High-Risk Areas of Pakistan: A Matched Case-Control Study. Vaccines 2023, 11, 947. [Google Scholar] [CrossRef]
- Zaman, K.; Bandyopadhyay, A.S.; Hoque, M.; Gast, C.; Yunus, M.; Jamil, K.M.; Mainou, B.A.; Konopka-Anstadt, J.L.; Hendley, W.S.; Vincent, A.; et al. Evaluation of the Safety, Immunogenicity, and Faecal Shedding of Novel Oral Polio Vaccine Type 2 in Healthy Newborn Infants in Bangladesh: A Randomised, Controlled, Phase 2 Clinical Trial. Lancet 2023, 401, 131–139. [Google Scholar] [CrossRef]
- Blümel, J.; Burger, R.; Drosten, C.; Gröner, A.; Gürtler, L.; Heiden, M.; Hildebrandt, M.; Jansen, B.; Klamm, H.; Montag-Lessing, T.; et al. Influenza Virus. Transfus. Med. Hemother. 2009, 36, 32–39. [Google Scholar]
- Petrova, V.N.; Russell, C.A. The Evolution of Seasonal Influenza Viruses. Nat. Rev. Microbiol. 2018, 16, 47–60. [Google Scholar] [CrossRef]
- Kalarikkal, S.M.; Jaishankar, G.B. Influenza Vaccine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537197/ (accessed on 11 December 2023).
- Peteranderl, C.; Herold, S.; Schmoldt, C. Human Influenza Virus Infections. Semin. Respir. Crit. Care Med. 2016, 37, 487–500. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Hui, D.S.; Zambon, M.; Wentworth, D.E.; Monto, A.S. Influenza. Lancet 2022, 400, 693–706. [Google Scholar] [CrossRef]
- Gomez Lorenzo, M.M.; Fenton, M.J. Immunobiology of Influenza Vaccines. Chest 2013, 143, 502–510. [Google Scholar] [CrossRef]
- Flerlage, T.; Boyd, D.F.; Meliopoulos, V.; Thomas, P.G.; Schultz-Cherry, S. Influenza Virus and SARS-CoV-2: Pathogenesis and Host Responses in the Respiratory Tract. Nat. Rev. Microbiol. 2021, 19, 425–441. [Google Scholar] [CrossRef]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 1–21. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Kistner, O.; Montomoli, E.; Viviani, S.; Marchi, S. Influenza Viruses and Vaccines: The Role of Vaccine Effectiveness Studies for Evaluation of the Benefits of Influenza Vaccines. Vaccines 2022, 10, 714. [Google Scholar] [CrossRef]
- Nuwarda, R.F.; Alharbi, A.A.; Kayser, V. An Overview of Influenza Viruses and Vaccines. Vaccines 2021, 9, 1032. [Google Scholar] [CrossRef]
- Hu, W.; Sjoberg, P.A.; Fries, A.C.; Demarcus, L.S.; Robbins, A.S. Waning Vaccine Protection against Influenza among Department of Defense Adult Beneficiaries in the United States, 2016–2017 through 2019–2020 Influenza Seasons. Vaccines 2022, 10, 888. [Google Scholar] [CrossRef]
- Esposito, S.; Montinaro, V.; Groppali, E.; Tenconi, R.; Semino, M.; Principi, N. Live Attenuated Intranasal Influenza Vaccine. Hum. Vaccin. Immunother. 2012, 8, 76–80. [Google Scholar] [CrossRef]
- Rodriguez, L.; Reedy, S.; Nogales, A.; Murcia, P.R.; Chambers, T.M.; Martinez-Sobrido, L. Development of a Novel Equine Influenza Virus Live-Attenuated Vaccine. Virology 2018, 516, 76–85. [Google Scholar] [CrossRef]
- Jang, Y.H.; Seong, B.L. Immune Responses Elicited by Live Attenuated Influenza Vaccines as Correlates of Universal Protection against Influenza Viruses. Vaccines 2021, 9, 353. [Google Scholar] [CrossRef]
- Tisa, V.; Barberis, I.; Faccio, V.; Paganino, C.; Trucchi, C.; Martini, M.; Ansaldi, F. Quadrivalent Influenza Vaccine: A New Opportunity to Reduce the Influenza Burden. J. Prev. Med. Hyg. 2016, 57, E28. [Google Scholar]
- Barberis, I.; Martini, M.; Iavarone, F.; Orsi, A. Available Influenza Vaccines: Immunization Strategies, History and New Tools for Fighting the Disease. J. Prev. Med. Hyg. 2016, 57, E41. [Google Scholar]
- Ray, R.; Dos Santos, G.; Buck, P.O.; Claeys, C.; Matias, G.; Innis, B.L.; Bekkat-Berkani, R. A Review of the Value of Quadrivalent Influenza Vaccines and Their Potential Contribution to Influenza Control. Hum. Vaccin. Immunother. 2017, 13, 1640–1652. [Google Scholar] [CrossRef]
- Rockman, S.; Laurie, K.L.; Parkes, S.; Wheatley, A.; Barr, I.G. New Technologies for Influenza Vaccines. Microorganisms 2020, 8, 1145. [Google Scholar] [CrossRef]
- Lin, Q.; Zhao, Q.; Lev, B. Influenza Vaccine Supply Chain Coordination under Uncertain Supply and Demand. Eur. J. Oper. Res. 2022, 297, 930–948. [Google Scholar] [CrossRef]
- Pérez-Rubio, A.; Ancochea, J.; Eiros Bouza, J.M. Quadrivalent Cell Culture Influenza Virus Vaccine. Comparison to Egg-Derived Vaccine. Hum. Vaccin. Immunother. 2020, 16, 1746–1752. [Google Scholar] [CrossRef]
- Ambrozaitis, A.; Groth, N.; Bugarini, R.; Sparacio, V.; Podda, A.; Lattanzi, M. A Novel Mammalian Cell-Culture Technique for Consistent Production of a Well-Tolerated and Immunogenic Trivalent Subunit Influenza Vaccine. Vaccine 2009, 27, 6022–6029. [Google Scholar] [CrossRef]
- Lopez, C.E.; Legge, K.L. Influenza a Virus Vaccination: Immunity, Protection, and Recent Advances toward a Universal Vaccine. Vaccines 2020, 8, 434. [Google Scholar] [CrossRef]
- Hansen, L.; Zhou, F.; Amdam, H.; Trieu, M.C.; Cox, R.J. Repeated Influenza Vaccination Boosts and Maintains H1N1pdm09 Neuraminidase Antibody Titers. Front. Immunol. 2021, 12, 748264. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Gianchecchi, E.; Montomoli, E. Influenza Vaccines: Evaluation of the Safety Profile. Hum. Vaccin. Immunother. 2018, 14, 657–670. [Google Scholar] [CrossRef]
- McNeil, M.M.; DeStefano, F. Vaccine-Associated Hypersensitivity. J. Allergy Clin. Immunol. 2018, 141, 463–472. [Google Scholar] [CrossRef]
- Kim, M.J.; Shim, D.H.; Cha, H.R.; Kim, C.B.; Kim, S.Y.; Park, J.H.; Sohn, M.H.; Lee, J.M.; Kim, K.W. Delayed-Onset Anaphylaxis Caused by Ige Response to Influenza Vaccination. Allergy Asthma Immunol. Res. 2020, 12, 359–363. [Google Scholar] [CrossRef]
- Izurieta, H.S.; Haber, P.; Wise, R.P.; Iskander, J.; Pratt, D.; Mink, C.; Chang, S.; Miles Braun, M.; Ball, R. Adverse Events Reported Following Live, Cold-Adapted, Intranasal Influenza Vaccine. JAMA 2005, 294, 2720–2725. [Google Scholar] [CrossRef]
- Institute of Medicine (U.S.); Committee to Review Adverse Effects of Vaccines; Stratton, K.R. Adverse Effects of Vaccines: Evidence and Causality; Stratton, K., Ford, A., Rusch, E., Clayton, E.W., Eds.; National Academies Press: Washington, DC, USA, 2012; ISBN 9780309214353. [Google Scholar]
- Machicado, J.D.; Bhagya-Rao, B.; Davogustto, G.; McKelvy, B.J. Acute Disseminated Encephalomyelitis Following Seasonal Influenza Vaccination in an Elderly Patient. Clin. Vaccine Immunol. 2013, 20, 1485–1486. [Google Scholar] [CrossRef]
- Ghaderi, S.; Størdal, K.; Gunnes, N.; Bakken, I.J.; Magnus, P.; Haberg, S.E. Encephalitis after Influenza and Vaccination: A Nationwide Population-Based Registry Study from Norway. Int. J. Epidemiol. 2017, 46, 1618–1626. [Google Scholar] [CrossRef]
- Baker, M.A.; Jankosky, C.; Yih, W.K.; Gruber, S.; Li, L.; Cocoros, N.M.; Lipowicz, H.; Coronel-Moreno, C.; DeLuccia, S.; Lin, N.D.; et al. The Risk of Febrile Seizures Following Influenza and 13-Valent Pneumococcal Conjugate Vaccines. Vaccine 2020, 38, 2166–2171. [Google Scholar] [CrossRef]
- Arcondo, M.F.; Wachs, A.; Zylberman, M. Transverse Myelitis Associated with Anti-Influenza A (H1N1) Vaccination. Medicina 2011, 71, 161–164. [Google Scholar]
- Akkad, W.; Salem, B.; Freeman, J.W.; Huntington, M.K. Longitudinally Extensive Transverse Myelitis Following Vaccination With Nasal Attenuated Novel Influenza A(H1N1) Vaccine. Arch. Neurol. 2010, 67, 1018–1020. [Google Scholar] [CrossRef]
- Jun, B.; Fraunfelder, F.W. Atypical Optic Neuritis After Inactivated Influenza Vaccination. Neuro-Ophthalmol. 2018, 42, 105–108. [Google Scholar] [CrossRef]
- Hull, T.P.; Bates, J.H. Optic Neuritis after Influenza Vaccination. Am. J. Ophthalmol. 1997, 124, 703–704. [Google Scholar] [CrossRef]
- Levison, L.S.; Thomsen, R.W.; Andersen, H. Guillain–Barré Syndrome Following Influenza Vaccination: A 15-Year Nationwide Population-Based Case–Control Study. Eur. J. Neurol. 2022, 29, 3389–3394. [Google Scholar] [CrossRef]
- Ferrarini, M.A.G.; Scattolin, M.A.; Rodrigues, M.M.; Resende, M.H.; dos Santos, I.C.L.; Iazzetti, A.V. Guillain-Barré Syndrome in Temporal Association with Influenza A Vaccine. Rev. Paul. Pediatr. 2011, 29, 685–693. [Google Scholar] [CrossRef][Green Version]
- Coop, C.A.; Balanon, S.K.; White, K.M.; Whisman, B.A.; Rathkopf, M.M. Anaphylaxis from the Influenza Virus Vaccine. Int. Arch. Allergy Immunol. 2008, 146, 85–88. [Google Scholar] [CrossRef]
- Nypaver, C.; Dehlinger, C.; Carter, C. Influenza and Influenza Vaccine: A Review. J. Midwifery Womens Health 2021, 66, 45–53. [Google Scholar] [CrossRef]
- Orrico-Sánchez, A.; Valls-Arévalo, Á.; Garcés-Sánchez, M.; Álvarez Aldeán, J.; Ortiz de Lejarazu Leonardo, R. Efficacy and Effectiveness of Influenza Vaccination in Healthy Children. A Review of Current Evidence. Enferm. Infecc. Microbiol. Clin. 2023, 41, 396–406. [Google Scholar] [CrossRef]
- Smetana, J.; Chlibek, R.; Shaw, J.; Splino, M.; Prymula, R. Influenza Vaccination in the Elderly. Hum. Vaccin. Immunother. 2018, 14, 540–549. [Google Scholar] [CrossRef]
- Buchy, P.; Badur, S. Who and When to Vaccinate against Influenza. Int. J. Infect. Dis. 2020, 93, 375–387. [Google Scholar] [CrossRef]
- Carreras, J.J.; Lluch, J.A.; Taboada, J.A.; Pastor-Villalba, E.; Nartallo-Penas, V.; Díez-Domingo, J. Acontecimientos Adversos En Embarazadas Con La Vacuna Tetravalente Contra La Gripe Obtenida de Cultivos Celulares. Enferm. Infecc. Microbiol. Clin. 2023, 41, 420–422. [Google Scholar] [CrossRef]
- Daley, M.F.; Clarke, C.L.; Glanz, J.M.; Xu, S.; Hambidge, S.J.; Donahue, J.G.; Nordin, J.D.; Klein, N.P.; Jacobsen, S.J.; Naleway, A.L.; et al. The Safety of Live Attenuated Influenza Vaccine in Children and Adolescents 2 through 17 Years of Age: A Vaccine Safety Datalink Study. Pharmacoepidemiol. Drug Saf. 2018, 27, 59–68. [Google Scholar] [CrossRef]
- Oliveira, D.S.; Lara, A.N.; Luiz, A.M.; Miyaji, K.T.; Sartori, A.M.C.; Lopes, M.H. Spontaneous Reporting of Adverse Events Following Pandemic Influenza A (H1N1) Immunization in a Reference Center in the State of Sao Paulo, Brazil. Rev. Soc. Bras. Med. Trop. 2013, 46, 348–351. [Google Scholar] [CrossRef][Green Version]
- Romanò, L.; Paladini, S.; Galli, C.; Raimondo, G.; Pollicino, T.; Zanetti, A.R. Hepatitis B Vaccination: Are Escape Mutant Viruses a Matter of Concern? In Human Vaccines and Immunotherapeutics; Landes Bioscience: Austin, TX, USA, 2015; Volume 11, pp. 53–57. [Google Scholar]
- Hodgens, A.; Marathi, R. Hepatitis B Vaccine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554400/ (accessed on 13 December 2023).
- Ho, J.K.T.; Jeevan-Raj, B.; Netter, H.J. Hepatitis b Virus (Hbv) Subviral Particles as Protective Vaccines and Vaccine Platforms. Viruses 2020, 12, 126. [Google Scholar] [CrossRef]
- Poovorawan, Y.; Chongsrisawat, V.; Theamboonlers, A.; Leroux-Roels, G.; Kuriyakose, S.; Leyssen, M.; Jacquet, J.M. Evidence of Protection against Clinical and Chronic Hepatitis B Infection 20 Years after Infant Vaccination in a High Endemicity Region. J. Viral Hepat. 2011, 18, 369–375. [Google Scholar] [CrossRef]
- Banatvala, J.; Van Damme, P.; Oehen, S. Lifelong Protection against Hepatitis B: The Role of Vaccine Immunogenicity in Immune Memory. Vaccine 2000, 19, 877–885. [Google Scholar]
- Matthews, P.C.; Maponga, T.; Ghosh, I.; Lemoine, M.; Ocama, P.; Abubakar, I.; Story, A.; Flanagan, S. Hepatitis B Virus: Infection, Liver Disease, Carcinogen or Syndemic Threat? Remodelling the Clinical and Public Health Response. PLoS Glob. Public Health 2022, 2, e0001359. [Google Scholar] [CrossRef]
- MacLachlan, J.H.; Cowie, B.C. Hepatitis B Virus Epidemiology. Cold Spring Harb. Perspect. Med. 2015, 5, a021410. [Google Scholar] [CrossRef]
- di Filippo Villa, D.; Navas, M.C. Vertical Transmission of Hepatitis B Virus—An Update. Microorganisms 2023, 11, 1140. [Google Scholar] [CrossRef]
- Meireles, L.C.; Marinho, R.T.; Van Damme, P. Three Decades of Hepatitis B Control with Vaccination. World J. Hepatol. 2015, 7, 2127–2132. [Google Scholar]
- Pattyn, J.; Hendrickx, G.; Vorsters, A.; Van Damme, P. Hepatitis B Vaccines. J. Infect. Dis. 2021, 224, S343–S351. [Google Scholar] [CrossRef]
- Moghadami, M.; Dadashpour, N.; Mokhtari, A.M.; Ebrahimi, M.; Mirahmadizadeh, A. The Effectiveness of the National Hepatitis B Vaccination Program 25 Years after Its Introduction in Iran: A Historical Cohort Study. Braz. J. Infect. Dis. 2019, 23, 419–426. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Huang, D.Q.; Nguyen, M.H. Global Burden of Hepatitis B Virus: Current Status, Missed Opportunities and a Call for Action. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 524–537. [Google Scholar] [CrossRef]
- WHO. Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 18 December 2023).
- Shouval, D. Hepatitis B Vaccines. J. Hepatol. 2003, 39, 70–76. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, X.; Zhou, Y.H. Hepatitis B Vaccine Development and Implementation. Hum. Vaccin. Immunother. 2020, 16, 1533–1544. [Google Scholar] [CrossRef]
- Leroux-Roels, G. Old and New Adjuvants for Hepatitis B Vaccines. Med. Microbiol. Immunol. 2015, 204, 69–78. [Google Scholar] [CrossRef]
- Anutebeh, E.N.; Tatah, L.; Feteh, V.F.; Aroke, D.; Assob, J.C.N.; Choukem, S.P. Immune Response to Hepatitis B Vaccine Following Complete Immunization of Children Attending Two Regional Hospitals in the Southwest Region of Cameroon: A Cross Sectional Study. BMC Infect. Dis. 2021, 21, 1205. [Google Scholar] [CrossRef]
- Bedford, H.; Elliman, D. Concerns about Immunisation. BMJ 2000, 320, 240–243. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Yang, M.; Chen, Q. Adverse Events of Vaccination against Hepatitis B Virus in Post-Marketing Surveillance from 2005 to 2017 in Guangdong Province, China. Vaccines 2022, 10, 1087. [Google Scholar] [CrossRef]
- Hartman, S. Convulsion Associated with Fever Following Hepatitis B Vaccination. J. Paediatr. Child. Health 1990, 26, 65. [Google Scholar] [CrossRef]
- Kaygusuz, S.; Kemal Erdemoglu, A.; Köksal, I. Afebrile Convulsion in an Adult after Recombinant Hepatitis B Vaccination. Scand. J. Infect. Dis. 2002, 34, 314–315. [Google Scholar] [CrossRef]
- Erguven, M.; Guven, S.; Akyuz, U.; Bilgiç, O.; Laloglu, F. Optic Neuritis Following Hepatitis B Vaccination in a 9-Year-Old Girl. J. Chin. Med. Assoc. 2009, 72, 594–597. [Google Scholar] [CrossRef]
- Voigt, U.; Baum, U.; Behrendt, W.; Hegemann, S.; Terborg, C.; Strobel, J. Neuritis of the Optic Nerve after Vaccinations against Hepatitis A, Hepatitis B and Yellow Fever. Klin. Monbl. Augenheilkd. 2001, 218, 688–690. [Google Scholar] [CrossRef]
- Khamaisi, M.; Shoenfeld, Y.; Orbach, H.; Khamaisi, M. Guillain-Barré Syndrome Following Hepatitis B Vaccination. Clin. Exp. Rheumatol. 2004, 22, 767–770. [Google Scholar]
- Kakar, A.; Sethi, P.K. Guillain Barre Syndrome Associated with Hepatitis B Vaccination. Indian J. Pediatr. 1997, 64, 710–712. [Google Scholar] [CrossRef]
- Bulbul, A.; Karadag, A.; Köklü, E.; Pamuk, U.; Umit Sarici, S. Anaphylactic Shock Due to Hepatitis B Immunoglobulin in a Newborn. J. Matern.-Fetal Neonatal Med. 2010, 23, 1257–1259. [Google Scholar] [CrossRef]
- Weng, M.K.; Doshani, M.; Khan, M.A.; Frey, S.; Ault, K.; Moore, K.L.; Hall, E.W.; Morgan, R.L.; Campos-Outcalt, D.; Wester, C.; et al. Universal Hepatitis B Vaccination in Adults Aged 19–59 Years: Updated of the Advisory Committee on Immunization Practices—United States. Morb. Mortal. Wkly. Rep. 2022, 71, 477–483. [Google Scholar] [CrossRef]
- Herrera-Restrepo, O.; Davis, K.; Sweeney, C.; Davenport, E.; Ghaswalla, P.; Buck, P.O. Hepatitis A and B Vaccination in Adults at Risk: A Survey of US Healthcare Providers’ Attitudes and Practices. Hum. Vaccin. Immunother. 2022, 18, 2123180. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Wong, G.; Gane, E.; Kao, J.-H.; Dusheiko, G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin. Microbiol. Rev. 2020, 33, e00046-19. [Google Scholar] [CrossRef]
- Xu, J.J.; Smith, M.K.; Chu, J.; Ding, G.W.; Chang, D.F.; Sharp, G.B.; Qian, H.Z.; Lu, L.; Bi, A.M.; Wang, N. Dynamics of the HIV Epidemic in Southern China: Sexual and Drug-Using Behaviours among Female Sex Workers and Male Clients in Yunnan. Int. J. STD AIDS 2012, 23, 670–675. [Google Scholar] [CrossRef][Green Version]
- Kwon, S.Y.; Lee, C.H. Epidemiology and Prevention of Hepatitis B Virus Infection. Korean J. Hepatol. 2011, 17, 87–95. [Google Scholar] [CrossRef]
- Kramvis, A.; Mammas, I.; Spandidos, D. Exploring the Optimal Vaccination Strategy against Hepatitis B Virus in Childhood (Review). Biomed. Rep. 2023, 19, 48. [Google Scholar] [CrossRef]
- Huang, C.-H.; Tsai, Y.-T.; Wang, S.-F.; Wang, W.-H.; Chen, Y.-H. Dengue Vaccine: An Update. Expert. Rev. Anti. Infect. Ther. 2021, 19, 1495–1502. [Google Scholar] [CrossRef]
- Khan, M.B.; Yang, Z.-S.; Lin, C.-Y.; Hsu, M.-C.; Urbina, A.N.; Assavalapsakul, W.; Wang, W.-H.; Chen, Y.-H.; Wang, S.-F. Dengue Overview: An Updated Systemic Review. J. Infect. Public Health 2023, 16, 1625–1642. [Google Scholar] [CrossRef]
- Harapan, H.; Michie, A.; Sasmono, R.T.; Imrie, A. Dengue: A Minireview. Viruses 2020, 12, 829. [Google Scholar] [CrossRef]
- Lessa, C.L.S.; Hodel, K.V.S.; Gonçalves, M.d.S.; Machado, B.A.S. Dengue as a Disease Threatening Global Health: A Narrative Review Focusing on Latin America and Brazil. Trop. Med. Infect. Dis. 2023, 8, 241. [Google Scholar] [CrossRef]
- Paz-Bailey, G.; Adams, L.; Wong, J.M.; Poehling, K.A.; Chen, W.H.; McNally, V.; Atmar, R.L.; Waterman, S.H. Dengue Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021. MMWR Recomm. Rep. 2021, 70, 1–16. [Google Scholar] [CrossRef]
- Kariyawasam, R.; Lachman, M.; Mansuri, S.; Chakrabarti, S.; Boggild, A.K. A Dengue Vaccine Whirlwind Update. Ther. Adv. Infect. Dis. 2023, 10, 204993612311672. [Google Scholar] [CrossRef]
- Angelin, M.; Sjölin, J.; Kahn, F.; Ljunghill Hedberg, A.; Rosdahl, A.; Skorup, P.; Werner, S.; Woxenius, S.; Askling, H.H. Qdenga®—A Promising Dengue Fever Vaccine; Can It Be Recommended to Non-Immune Travelers? Travel. Med. Infect. Dis. 2023, 54, 102598. [Google Scholar] [CrossRef]
- Walter, K.; John, C.C. Malaria. JAMA 2022, 327, 597. [Google Scholar] [CrossRef]
- Syed, Y.Y. RTS,S/AS01 Malaria Vaccine (Mosquirix®): A Profile of Its Use. Drugs Ther. Perspect. 2022, 38, 373–381. [Google Scholar] [CrossRef]
- Björkman, A.; Benn, C.S.; Aaby, P.; Schapira, A. RTS,S/AS01 Malaria Vaccine—Proven Safe and Effective? Lancet Infect. Dis. 2023, 23, e318–e322. [Google Scholar] [CrossRef]
- Zavala, F. RTS,S: The First Malaria Vaccine. J. Clin. Investig. 2022, 132, e156588. [Google Scholar] [CrossRef]
- Beeson, J.G.; Kurtovic, L.; Valim, C.; Asante, K.P.; Boyle, M.J.; Mathanga, D.; Dobano, C.; Moncunill, G. The RTS,S Malaria Vaccine: Current Impact and Foundation for the Future. Sci. Transl. Med. 2022, 14, eabo6646. [Google Scholar] [CrossRef]
- Chutiyami, M.; Saravanakumar, P.; Bello, U.M.; Salihu, D.; Adeleye, K.; Kolo, M.A.; Dawa, K.K.; Hamina, D.; Bhandari, P.; Sulaiman, S.K.; et al. Malaria Vaccine Efficacy, Safety, and Community Perception in Africa: A Scoping Review of Recent Empirical Studies. Infection 2024. [Google Scholar] [CrossRef]
- Karagoz, A.; Tombuloglu, H.; Alsaeed, M.; Tombuloglu, G.; AlRubaish, A.A.; Mahmoud, A.; Smajlović, S.; Ćordić, S.; Rabaan, A.A.; Alsuhaimi, E. Monkeypox (Mpox) Virus: Classification, Origin, Transmission, Genome Organization, Antiviral Drugs, and Molecular Diagnosis. J. Infect. Public Health 2023, 16, 531–541. [Google Scholar] [CrossRef]
- Singhal, T.; Kabra, S.K.; Lodha, R. Monkeypox: A Review. Indian J. Pediatr. 2022, 89, 955–960. [Google Scholar] [CrossRef]
- Rao, A.K.; Petersen, B.W.; Whitehill, F.; Razeq, J.H.; Isaacs, S.N.; Merchlinsky, M.J.; Campos-Outcalt, D.; Morgan, R.L.; Damon, I.; Sánchez, P.J.; et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 734–742. [Google Scholar] [CrossRef]
- Brentwood (TN); Organization of Teratology Information Specialists (OTIS). Mother to Baby|Fact Shetos. Available online: https://www.ncbi.nlm.nih.gov/books/NBK583255/ (accessed on 8 February 2024).
- Sah, R.; Paul, D.; Mohanty, A.; Shah, A.; Mohanasundaram, A.S.; Padhi, B.K. Monkeypox (Mpox) Vaccines and Their Side Effects: The Other Side of the Coin. Int. J. Surg. 2023, 109, 215–217. [Google Scholar] [CrossRef]
- Huang, Y.; Mu, L.; Wang, W. Monkeypox: Epidemiology, Pathogenesis, Treatment and Prevention. Signal Transduct. Target. Ther. 2022, 7, 373. [Google Scholar] [CrossRef]
- Sharma, D. Pharmacovigilance in Patient Safety: Challenges and Perspectives. Natl. J. Physiol. Pharm. Pharmacol. 2022, 12, 1975–1978. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Data Quality Monitoring and Surveillance System Evaluation: A Handbook of Methods and Applications; ECDC: Stockholm, Sweden, 2014; ISBN 9789291935925.
- Jayatilleke, K. Challenges in Implementing Surveillance Tools of High-Income Countries (HICs) in Low Middle Income Countries (LMICs). Curr. Treat. Options Infect. Dis. 2020, 12, 191–201. [Google Scholar] [CrossRef]
- Suvarna, V. Phase IV of Drug Development. Perspect. Clin. Res. 2010, 1, 57–60. [Google Scholar] [CrossRef]
- Asturias, E.J.; Wharton, M.; Pless, R.; MacDonald, N.E.; Chen, R.T.; Andrews, N.; Salisbury, D.; Dodoo, A.N.; Hartigan-Go, K.; Zuber, P.L.F. Contributions and Challenges for Worldwide Vaccine Safety: The Global Advisory Committee on Vaccine Safety at 15 Years. Vaccine 2016, 34, 3342–3349. [Google Scholar] [CrossRef]
- Kochhar, S.; Barreira, D.; Beattie, P.; Cavaleri, M.; Cravioto, A.; Frick, M.W.; Ginsberg, A.M.; Hudson, I.; Kaslow, D.C.; Kurtz, S.; et al. Building the Concept for WHO Evidence Considerations for Vaccine Policy (ECVP): Tuberculosis Vaccines Intended for Adults and Adolescents as a Test Case. In Vaccine; Elsevier Ltd.: Amsterdam, The Netherlands, 2022; Volume 40, pp. 1681–1690. [Google Scholar]
- Naniche, D.; Hotez, P.; Bottazzi, M.E.; Ergonul, O.; Figueroa, J.P.; Gilbert, S.; Gursel, M.; Hassanain, M.; Kang, G.; Kaslow, D.; et al. Beyond the Jab: A Need for Global Coordination of Pharmacovigilance for COVID-19 Vaccine Deployment. EClinicalMedicine 2021, 36, 100925. [Google Scholar] [CrossRef]
- Kompa, B.; Hakim, J.B.; Palepu, A.; Kompa, K.G.; Smith, M.; Bain, P.A.; Woloszynek, S.; Painter, J.L.; Bate, A.; Beam, A.L. Artificial Intelligence Based on Machine Learning in Pharmacovigilance: A Scoping Review. Drug Saf. 2022, 45, 477–491. [Google Scholar] [CrossRef]
- Salvo, F.; Micallef, J.; Lahouegue, A.; Chouchana, L.; Létinier, L.; Faillie, J.-L.; Pariente, A. Will the Future of Pharmacovigilance Be More Automated? Expert. Opin. Drug Saf. 2023, 22, 541–548. [Google Scholar] [CrossRef]
- Trifirò, G.; Crisafulli, S. A New Era of Pharmacovigilance: Future Challenges and Opportunities. Front. Drug Saf. Regul. 2022, 2, 866898. [Google Scholar] [CrossRef]
- Mah, P.M.; Skalna, I.; Muzam, J. Natural Language Processing and Artificial Intelligence for Enterprise Management in the Era of Industry 4.0. Appl. Sci. 2022, 12, 9207. [Google Scholar] [CrossRef]
- Davenport, T.; Kalakota, R. The Potential for Artificial Intelligence in Healthcare. Future Healthc. J. 2019, 6, 94–98. [Google Scholar] [CrossRef]
- Pilipiec, P.; Liwicki, M.; Bota, A. Using Machine Learning for Pharmacovigilance: A Systematic Review. Pharmaceutics 2022, 14, 266. [Google Scholar] [CrossRef]
- Spiker, J.; Kreimeyer, K.; Dang, O.; Boxwell, D.; Chan, V.; Cheng, C.; Gish, P.; Lardieri, A.; Wu, E.; De, S.; et al. Information Visualization Platform for Postmarket Surveillance Decision Support. Drug Saf. 2020, 43, 905–915. [Google Scholar] [CrossRef]
- Painter, J.L.; Kassekert, R.; Bate, A. An Industry Perspective on the Use of Machine Learning in Drug and Vaccine Safety. Front. Drug Saf. Regul. 2023, 3, 1110498. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]
- Arlett, P.; Straus, S.; Rasi, G. Pharmacovigilance 2030. Clin. Pharmacol. Ther. 2020, 107, 89–91. [Google Scholar] [CrossRef]
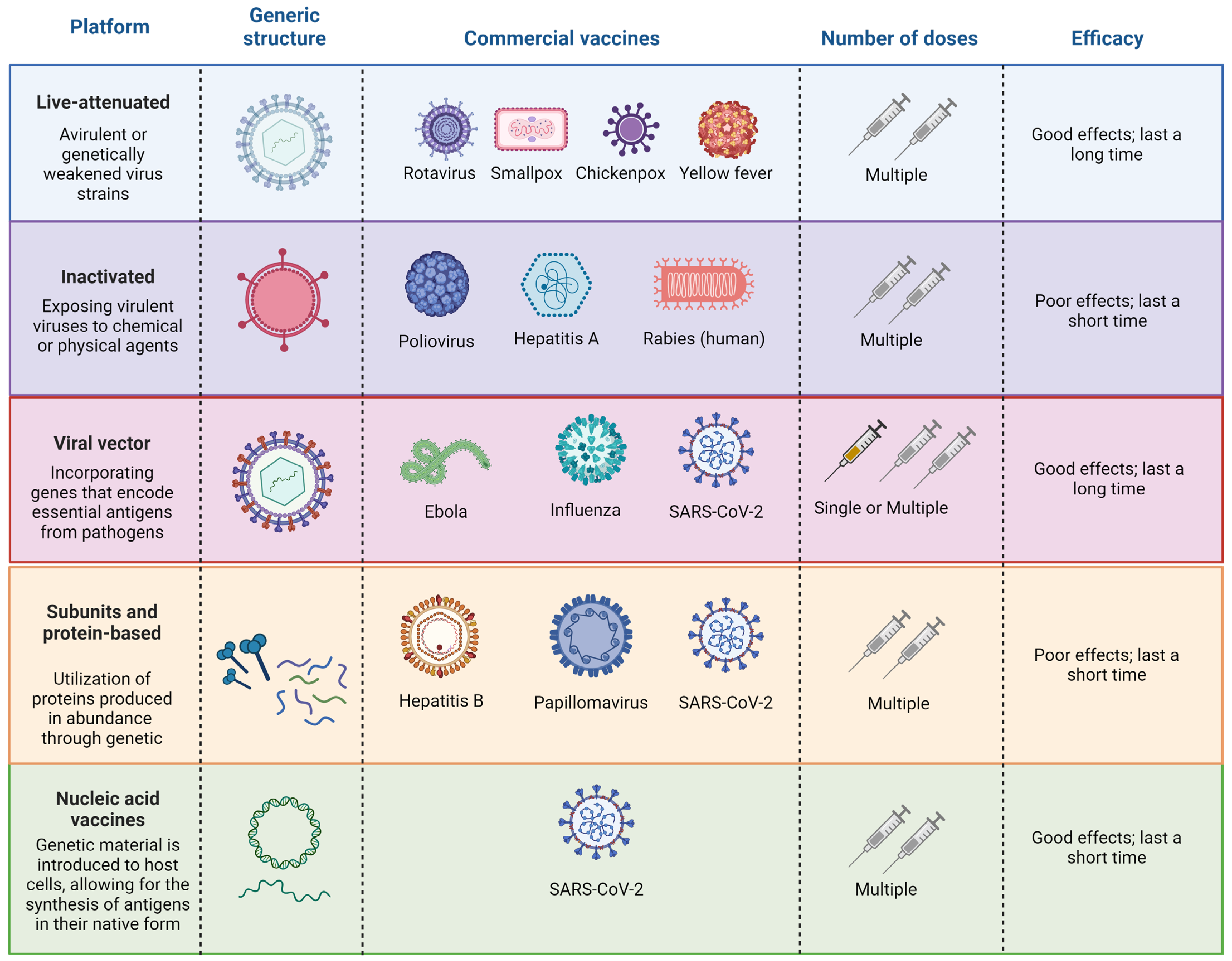
| Virus | Platform | Overview | Reference |
|---|---|---|---|
| SARS-CoV-2 | Nucleic acid, viral vector, inactivated virus, and subunit protein | Most vaccines either eliminate or probably lower the percentage of individuals who have COVID-19 symptoms that have been proven, and for some, there is strong evidence that the immunizations lessen serious or critical illness. | [54,55,56] |
| Poliovirus | Inactivated and live-attenuated | The efficacy of the vaccinations against the three different poliovirus types is quite good. However, there are notable variations in each vaccine’s safety and effectiveness profiles as well as how it functions. High levels of immunity are conferred by finishing the polio vaccination regimen. The usual four-dose series of inactivated polio vaccine has an effectiveness of 99% to 100% after three doses. | [57,58,59] |
| A(H1N1)pdm09 | Inactivated and live-attenuated | When combined, all vaccines significantly decreased the prevalence of influenza in adults, children, and the elderly that was confirmed by a laboratory. Many vaccination kinds are safe for use in adults and the elderly, even if the live-attenuated vaccine proved to be more effective than the inactivated version in children. | [60,61,62] |
| Hepatovirus A | Recombinant | Booster doses improve the hepatitis B vaccine’s effectiveness. Strategies for both targeted and universal vaccinations substantially lower the prevalence of hepatitis B. | [63,64,65] |
| Varicella-zoster virus (VZV) | Recombinant and live-attenuated | Adverse effects from varicella immunization are rare and should be considered alongside its substantial benefits. Varicella vaccination has had a remarkable positive effect on public health, it has led to a reduction of over 90% in cases of hospitalizations and deaths related to varicella. | [66,67] |
| Vesicular stomatitis virus (VSV) | Recombinant | The vesicular stomatitis virus (VSV) is being explored as a promising candidate for antiviral vaccine development. Extensive research aims to ensure satisfactory expression of viral antigens while maintaining vector safety. Multiple VSV-based vaccines have been developed, notably including a highly effective vaccine against the Ebola virus, which recently received clinical approval. | [68,69] |
| Measles virus (MV) | Live-attenuated | The measles virus (MV) vaccine that is currently available on the market is not only safe but also highly effective and reasonably priced. Administered to a significant number of children, it has consistently demonstrated its efficacy and safety. Just a single dose or two low-dose of the MV vaccine are ample to provide lifelong immunity. | [70,71] |
| Pharmacovigilance Tool | Country | Associated Agency | Description | References |
|---|---|---|---|---|
| Drug Vigilance Center (VigiMed) | Brazil | Brazilian Health Regulatory Agency (ANVISA) | Developed by The Uppsala Monitoring Centre (UMC), this is a web-based system for managing individual case safety reports (ICSR). | [109,110] |
| Vaccine Adverse Event Reporting System (VAERS) | US | Food and Drug Administration (FDA) | In 1990, the Vaccine Adverse Event Reporting System (VAERS) was established and implemented by the US FDA and Centers for Disease Control and Prevention (CDC) to collect reports related to adverse events associated with biological products, including vaccines. | [111,112] |
| EudraVigilance | European Union Countries | European Medicines Agency (EMA) | Pharmacovigilance database responsible for overseeing the collection and analysis of suspected adverse reactions to medicines authorized in the European Economic Area. | [113,114] |
| Medical Information for Risk Assessment Initiative (MIHARI) project | Japan | Pharmaceuticals and Medical Devices Agency (PMDA) | Objective of using large-scale electronic health information databases as innovative sources of information for pharmacoepidemiological drug safety assessments in Japan. | [115,116] |
| Pharmacovigilance Africa (PAVIA) | Ethiopia, Eswatini, Nigeria, and Tanzania | European Developing countries Clinical Trials Partnership (EDCTP) | Objective to enhance pharmacovigilance by leveraging collaborative support from multiple institutions both in Europe and Africa. | [117,118] |
| Therapeutic Goods Administration (TGA) | Australia | Australian Government Department of Health and Aged Care | Regulating medicines, medical devices, and biologicals to safeguard the health and well-being of the Australian population, ensuring that these products are both effective and safe for use. | [119,120] |
| Developer | Platform | Indicated Ages | Rare Adverse Effects | Reference |
|---|---|---|---|---|
| Pfizer/BioNTech (BNT162b2/Cominarty) | mRNA | 4 months to 12 years and older | Anaphylaxis; myocarditis/pericarditis | [220,223,224,225] |
| Moderna (mRNA-1273/Spikevax) | mRNA | 6 months to 18 years and older | [226,227,228] | |
| Novavax (NVX-CoV2373) | Recombinant protein, adjuvant | 12 years and older | Possible risk of myocarditis/pericarditis | [229] |
| Janssen/Johnson & Johnson (Ad26.COV2.S) | Replication-incompetent adenovirus 26 vector | 18 years and older | Thrombotic complications associated with thrombocytopenia; Guillain–Barre syndrome; possible risk of myocarditis/pericarditis | [230,231,232] |
| Pfizer/BioNTech (BA.4/BA.5 bivalent) | mRNA | 12 years and older | Myocarditis/pericarditis | [233,234] |
| Butantan/Sinovac (Coronavac) | Inactivated virus antigen | 3 years and older | Anaphylaxis; Guillain–Barre syndrome | [235,236] |
| Oxford/AstraZeneca (ChAdOx1 nCoV-19/Covishield) | Recombinant adenovirus vector | 18 years and older | Thrombosis with thrombocytopenia syndrome (TTS); Guillain–Barre syndrome | [237,238] |
| Gamaleya Research Institute Sputnik V (Gam-COVID-Vac) | Adenovirus D-26 D-5 | 18 years and older | Immune thrombocytopenia and thrombosis | [239] |
| Special Group | Clinical Considerations | References |
|---|---|---|
| Advanced age | Advanced age is associated with an increase in the rate of hospitalizations and mortality due to COVID-19. | [248,249,250] |
| Obesity | Patients with body mass index (BMI) ≥40 kg/m2 as a high-risk condition for severe COVID-19 (obesity was associated with an increased risk of intubation or death). | [251,252] |
| Diabetes mellitus | The risk of mortality was higher with glycated hemoglobin (A1C) levels of 7.6 to 8.9 percent compared to 6.5 to 7 percent. | [253,254] |
| Hypertension | Patients with history of hypertension is associated with an increase in the rate of hospitalizations and mortality due to COVID-19. | [255,256,257] |
| Platform | Strains | Rare Adverse Effects | References |
|---|---|---|---|
| bOPV | Sabin types 1 and 3 | Vaccine-associated paralytic poliomyelitis | [283] |
| OPV and bOPV | Type 1; Types 1 and 3 | Paralysis, asthma-like reaction | [284] |
| IPV | - | Anaphylaxis | [285] |
| IPV | Sabin | Thrombocytopenia, allergic purpura | [286] |
| IPV | Wild | Thrombocytopenia, epilepsy |
| Type | Platform | Rare Adverse Effects | References |
|---|---|---|---|
| Subtypes A and B | Inactivated | Acute disseminated encephalomyelitis (ADEM) | [326] |
| A(H1N1) pdm09 | Inactivated | Encephalitis | [327] |
| - | Inactivated | Febrile seizures | [328] |
| A (H1N1) | Inactivated/Live-attenuated | Transverse myelitis | [329,330] |
| - | Inactivated/Live-attenuated | Optic neurits | [331,332] |
| A (H1N1) | Inactivated | Guillain–Barré Syndrome | [333,334] |
| Tri and quadrivalent | Inactivated | Anaphylaxis | [323,335] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodel, K.V.S.; Fiuza, B.S.D.; Conceição, R.S.; Aleluia, A.C.M.; Pitanga, T.N.; Fonseca, L.M.d.S.; Valente, C.O.; Minafra-Rezende, C.S.; Machado, B.A.S. Pharmacovigilance in Vaccines: Importance, Main Aspects, Perspectives, and Challenges—A Narrative Review. Pharmaceuticals 2024, 17, 807. https://doi.org/10.3390/ph17060807
Hodel KVS, Fiuza BSD, Conceição RS, Aleluia ACM, Pitanga TN, Fonseca LMdS, Valente CO, Minafra-Rezende CS, Machado BAS. Pharmacovigilance in Vaccines: Importance, Main Aspects, Perspectives, and Challenges—A Narrative Review. Pharmaceuticals. 2024; 17(6):807. https://doi.org/10.3390/ph17060807
Chicago/Turabian StyleHodel, Katharine Valéria Saraiva, Bianca Sampaio Dotto Fiuza, Rodrigo Souza Conceição, Augusto Cezar Magalhães Aleluia, Thassila Nogueira Pitanga, Larissa Moraes dos Santos Fonseca, Camila Oliveira Valente, Cintia Silva Minafra-Rezende, and Bruna Aparecida Souza Machado. 2024. "Pharmacovigilance in Vaccines: Importance, Main Aspects, Perspectives, and Challenges—A Narrative Review" Pharmaceuticals 17, no. 6: 807. https://doi.org/10.3390/ph17060807
APA StyleHodel, K. V. S., Fiuza, B. S. D., Conceição, R. S., Aleluia, A. C. M., Pitanga, T. N., Fonseca, L. M. d. S., Valente, C. O., Minafra-Rezende, C. S., & Machado, B. A. S. (2024). Pharmacovigilance in Vaccines: Importance, Main Aspects, Perspectives, and Challenges—A Narrative Review. Pharmaceuticals, 17(6), 807. https://doi.org/10.3390/ph17060807







