Investigating Changes in Pharmacokinetics of Steroidal Alkaloids from a Hydroethanolic Fritillariae thunbergii Bulbus Extract in 2,4-Dinitrobenzene Sulfonic Acid-Induced Colitis Rats
Abstract
:1. Introduction
2. Results
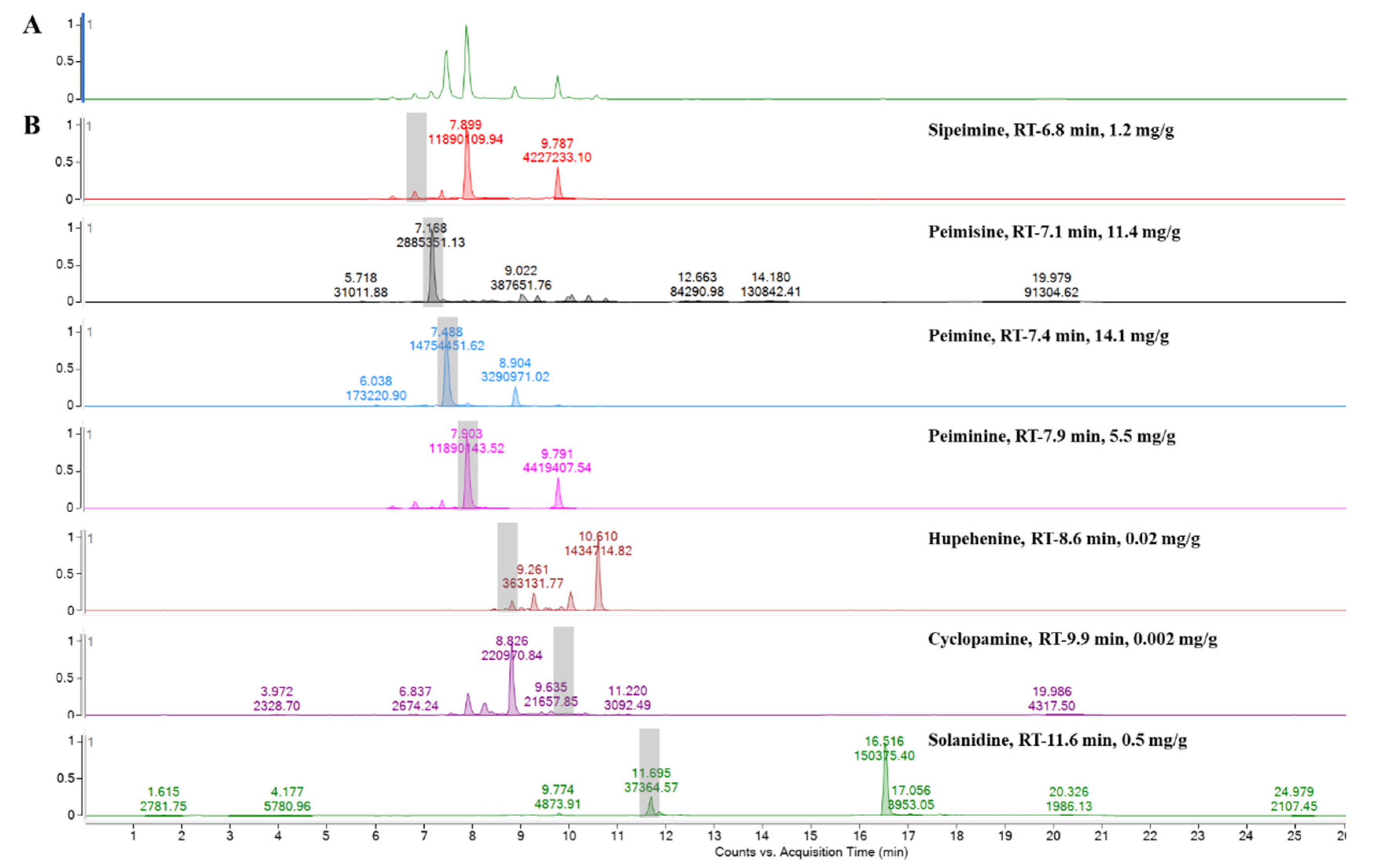
2.1. Steroidal Alkaloids Contents in FTB
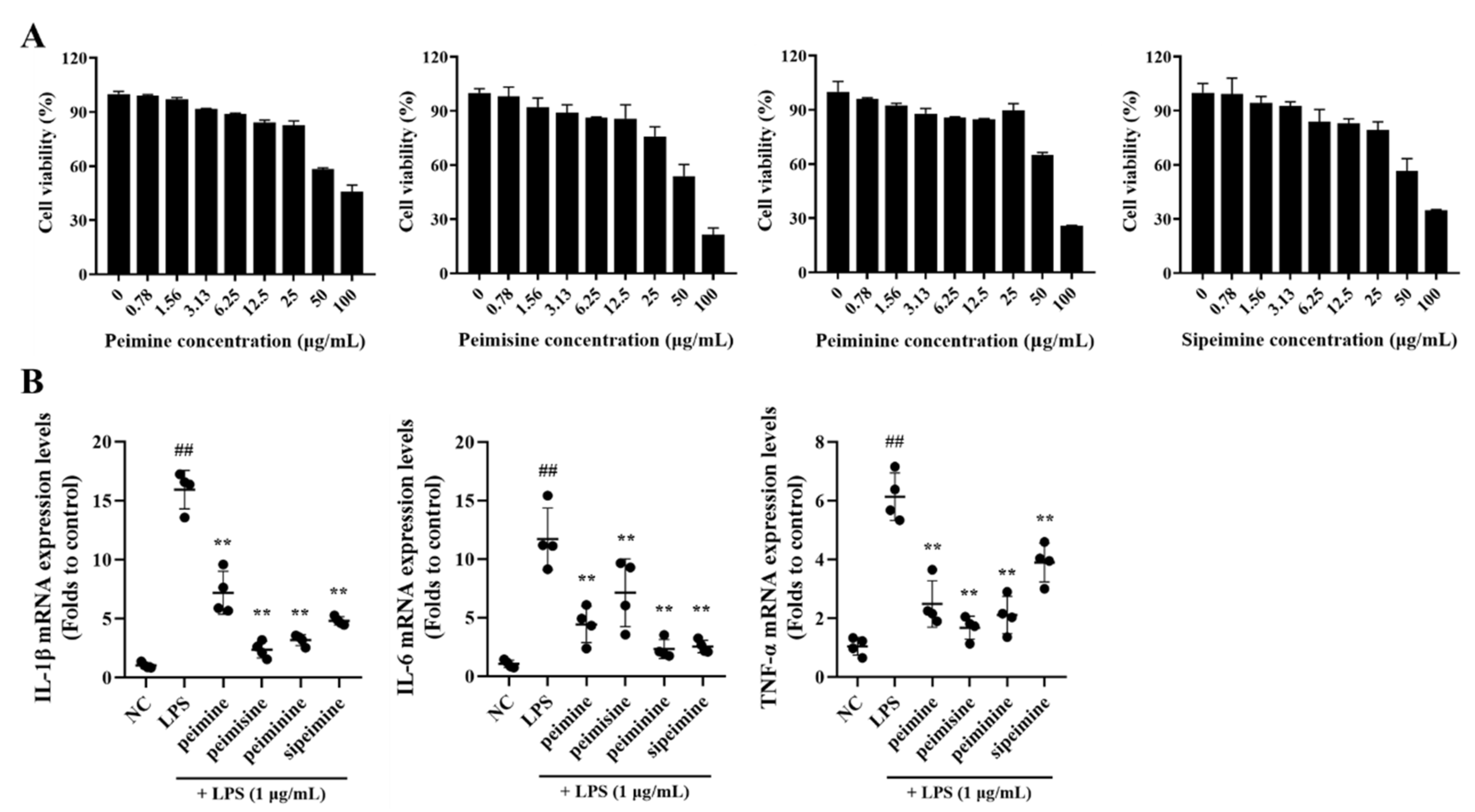
2.2. The Anti-Inflammatory Effect of Active Ingredients in FTB on Lipopolysaccharides (LPS) Stimulated RAW 264.7 Cells
2.3. Comparison of Symptoms and Colon Histological Damage to Normal in 2,4-Dinitrobenzene Sulfonic Acid (DNBS)-Induced Colitis in Rats
2.4. Differences in Pharmacokinetic Parameters of FTB Steroidal Alkaloids between Normal and DNBS-Treated Rats
3. Discussion
4. Materials and Methods
4.1. FTB Analysis
4.2. In Vitro Analysis of Anti-Inflammatory Activity of Steroidal Alkaloids
4.3. Pharmacokinetics of FTB in Normal and Colitis-Induced Rats
4.3.1. DNBS-Induced Colitis Rat Model
4.3.2. Sample Preparation and UPLC-MS/MS Analysis
4.3.3. Pharmacokinetic Data and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiang, M.L.; Hu, B.Y.; Qi, Z.H.; Wang, X.N.; Xie, T.Z.; Wang, Z.J.; Ma, D.Y.; Zeng, Q.; Luo, X.D. Chemistry and bioactivities of natural steroidal alkaloids. Nat. Prod. Bioprospect. 2022, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.L.; Chang, W.S.; Lu, W.C.; Wei, G.J.; Wang, Y.; Ho, C.T.; Hwang, L.S. Pharmacokinetics, bioavailability, tissue distribution and excretion of tangeretin in rat. J. Food Drug Anal. 2018, 26, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Su, J.; Wu, D.; Wang, L.; Hu, J.; Sieniawska, E.; Kai, G. Fritillaria thunbergii Miq. (Zhe Beimu): A review on its traditional uses, phytochemical profile and pharmacological properties. Food Chem. Toxicol. 2021, 153, 112289. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chan, S.W.; Ma, J.; Li, P.; Shaw, P.C.; Lin, G. Investigation of association of chemical profiles with the tracheobronchial relaxant activity of Chinese medicinal herb Beimu derived from various Fritillaria species. J. Ethnopharmacol. 2018, 210, 39–46. [Google Scholar] [CrossRef]
- Zhou, Y.; Ji, H.; Li, P.; Jiang, Y. Antimuscarinic function of five fritillaria alkaloids on guinea pig tracheal strips. J. China Pharmaceut. Univ. 2003, 34, 58–60. [Google Scholar]
- Kim, E.J.; Uoon, Y.P.; Woo, K.W.; Kim, J.H.; Min, S.Y.; Lee, H.J.; Lee, S.K.; Hong, J.H.; Lee, K.R.; Lee, C.J. Verticine, ebeiedine and suchengbeisine isolated from the bulbs of Fritillaria thunbergii Miq. inhibited the gene expression and production of MUC5AC mucin from human airway epithelial cells. Phytomedicine 2016, 23, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Yang, L.; Cui, W.X.; Zhang, M.X.; Li, Z.H.; Liu, B.; Wang, Q. Optimization of supercritical fluid extraction of total alkaloids, peimisine, peimine and peiminine from the Bulb of Fritillaria thunbergii Miq, and evaluation of antioxidant activities of the extracts. Materials 2016, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ma, X.; Ding, G.; Wang, Z.; Liu, D.; Tong, Y.; Zhou, H.; Gao, J.; Hou, Y.; Jiang, M.; et al. Comparison and evaluation of antimuscarinic and anti-inflammatory effects of five Bulbus fritillariae species based on UPLC-Q/TOF integrated dual-luciferase reporter assay, PCA and ANN analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1041–1042, 60–69. [Google Scholar] [CrossRef]
- Suh, W.S.; Lee, S.Y.; Park, J.E.; Kim, D.H.; Kim, S.; Lee, K.R. Two new steroidal alkaloids from the bulbs of fritillaria thunbergii. Heterocycles 2018, 96, 921–930. [Google Scholar]
- Li, H.; Hung, A.; Li, M.; Yang, A.W.H. Fritillariae thunbergii bulbus: Traditional uses, phytochemistry, pharmacodynamics, pharmacokinetics and toxicity. Int. J. Mol. Sci. 2019, 20, 1667. [Google Scholar] [CrossRef]
- Lee, A.; Chung, Y.C.; Kim, K.Y.; Jang, C.H.; Song, K.H.; Hwang, Y.H. Hydroethanolic extract of Fritillariae thunbergii Bulbus alleviates dextran sulfate sodium-induced ulcerative colitis by enhancing intestinal barrier integrity. Nutrients 2023, 15, 2810. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Mekjaruskul, C.; Jay, M.; Sripanidkulchai, B. Pharmacokinetics, bioavailability, tissue distribution, excretion, and metabolite identification of methoxyflavones in Kaempferia parviflora extract in rats. Drug Metab. Dispos. 2012, 40, 2342–2353. [Google Scholar] [CrossRef]
- Effinger, A.; O’Driscoll, C.M.; McAllister, M.; Fotaki, N. Impact of gastrointestinal disease states on oral drug absorption—Implications for formulation design—A PEARRL review. J. Pharm. Pharmacol. 2019, 71, 674–698. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Yang, Y.; Chen, Y. Pharmacokinetics of Chinese medicines: Strategies and perspectives. Chin. Med. 2018, 13, 24. [Google Scholar] [CrossRef]
- Liu, C.; Zhen, D.; Du, H.; Gong, G.; Wu, Y.; Ma, Q.; Quan, Z.S. Synergistic anti-inflammatory effects of peimine, peiminine, and forsythoside a combination on LPS-induced acute lung injury by inhibition of the IL-17-NF-κB/MAPK pathway activation. J. Ethnopharmacol. 2022, 295, 115343. [Google Scholar] [CrossRef]
- Liu, S.; Yang, T.; Ming, T.W.; Gaun, T.K.W.; Zhou, T.; Wang, S.; Ye, B. Isosteroid alkaloids with different chemical structures from Fritillariae cirrhosae bulbus alleviate LPS-induced inflammatory response in RAW 264.7 cells by MAPK signaling pathway. Int. Immunopharmacol. 2020, 78, 106047. [Google Scholar] [CrossRef] [PubMed]
- Morampudi, V.; Bhinder, G.; Wu, X.; Dai, C.; Sham, H.P.; Vallance, B.A.; Jacobson, K. DNBS/TNBS colitis models: Providing insights into inflammatory bowel disease and effects of dietary fat. J. Vis. Exp. 2014, 84, e51297. [Google Scholar]
- Fischer, M.; Siva, S.; Wo, J.M.; Fadda, H.M. Assessment of small intestinal transit times in ulcerative colitis and crohn’s disease patients with different disease activity using video capsule endoscopy. AAPS. PharmSciTech. 2017, 18, 404–409. [Google Scholar] [CrossRef]
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.G.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M.; et al. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur. J. Pharm. Sci. 2021, 162, 105812. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, L.; Wang, S.; Wu, J.; Shi, J.; Yan, T.; Xie, C.; Li, Q.; Hu, M.; Liu, Z. Oral absorption basics: Pathways and physicochemical and biological factors affecting absorption. In Developing Solid oral Dosage Forms; Academic Press: Cambridge, MA, USA, 2017; pp. 297–329. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 12 September 2023).
- Hwang, Y.H.; Yang, H.J.; Ma, J.Y. Simultaneous determination of three furanocoumarins by UPLC/MS/MS: Application to pharmacokinetic study of Angelica dahurica Radix after oral administration to normal and experimental colitis-induced rats. Molecules 2017, 22, 416. [Google Scholar] [CrossRef] [PubMed]
- Guideline on Bioanalytical Method Validation. Available online: https://www.mfds.go.kr/brd/m_1060/view.do?seq=15366 (accessed on 27 October 2023).


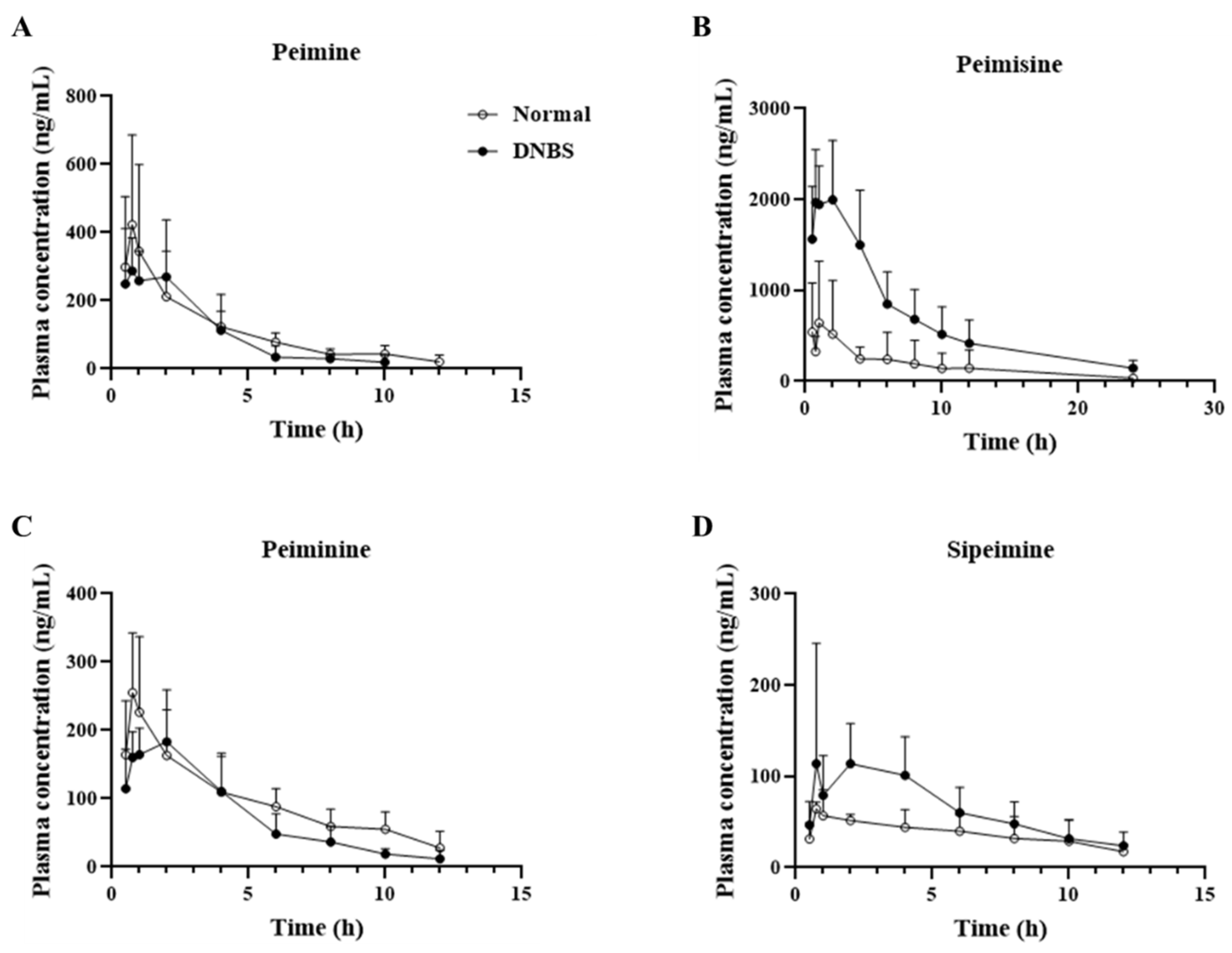

| Parameter | Peimine | Peimisine | ||
| Normal | DNBS | Normal | DNBS | |
| T1/2 (h) | 4.0 ± 2.5 | 1.9 ± 0.7 # | 3.9 ± 1.7 | 7.7 ± 4.9 |
| Tmax (h) | 0.7 ± 0.2 | 1.0 ± 0.7 | 0.9 ± 0.2 | 1.5 ± 0.8 |
| Cmax (ng/mL) | 356.2 ± 253.1 | 351.2 ± 149.5 | 655.5 ± 671.9 | 2330.5 ± 363.2 # |
| AUClast (h∗ng/mL) | 1311.8 ± 762.6 | 1076.9 ± 444.1 | 3959.3 ± 4738.6 | 16,895.4 ± 5782.1 # |
| AUC_%Extrap_obs (%) | 6.7 ± 5.5 | 6.6 ± 6.6 | 5.3 ± 4.0 | 8.9 ± 11.6 |
| AUMClast (h∗h∗ng/mL) | 4511.4 ± 2253.8 | 2819.7 ± 1408.9 | 23,522.8 ± 33,482.7 | 126,755.7 ± 54,141.2 # |
| MRTlast (h) | 3.7 ± 0.8 | 2.7 ± 0.6 # | 5.0 ± 2.0 | 7.3 ± 1.3 # |
| Parameter | Peiminine | Sipeimine | ||
| Normal | DNBS | Normal | DNBS | |
| T1/2 (h) | 4.5 ± 2.0 | 1.8 ± 0.9 # | 6.9 ± 5.6 | 3.2 ± 1.6 |
| Tmax (h) | 1.0 ± 0.2 | 1.3 ± 0.8 | 1.3 ± 0.5 | 2.4 ± 1.2 |
| Cmax (ng/mL) | 227.9 ± 109.5 | 209.0 ± 51.1 | 64.7 ± 13.4 | 162.3 ± 97.0 |
| AUClast (h∗ng/mL) | 1172.4 ± 392.3 | 888.8 ± 286.0 | 463.5 ± 232.8 | 764.0 ± 285.5 |
| AUC_%Extrap_obs (%) | 14.5 ± 15.7 | 3.3 ± 2.9 | 10.4 ± 8.6 | 11.0 ± 8.3 |
| AUMClast (h∗h∗ng/mL) | 5302.0 ± 1844.3 | 3149.7 ± 1242.0 # | 2675.8 ± 2026.3 | 3588.3 ± 1486.0 |
| MRTlast (h) | 4.7 ± 1.1 | 3.6 ± 0.6 # | 5.4 ± 1.7 | 4.7 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-S.; Kim, J.-W.; Kim, J.-H.; Chung, E.-H.; Ko, J.-W.; Hwang, Y.-H.; Kim, T.-W. Investigating Changes in Pharmacokinetics of Steroidal Alkaloids from a Hydroethanolic Fritillariae thunbergii Bulbus Extract in 2,4-Dinitrobenzene Sulfonic Acid-Induced Colitis Rats. Pharmaceuticals 2024, 17, 1001. https://doi.org/10.3390/ph17081001
Jeong J-S, Kim J-W, Kim J-H, Chung E-H, Ko J-W, Hwang Y-H, Kim T-W. Investigating Changes in Pharmacokinetics of Steroidal Alkaloids from a Hydroethanolic Fritillariae thunbergii Bulbus Extract in 2,4-Dinitrobenzene Sulfonic Acid-Induced Colitis Rats. Pharmaceuticals. 2024; 17(8):1001. https://doi.org/10.3390/ph17081001
Chicago/Turabian StyleJeong, Ji-Soo, Jeong-Won Kim, Jin-Hwa Kim, Eun-Hye Chung, Je-Won Ko, Youn-Hwan Hwang, and Tae-Won Kim. 2024. "Investigating Changes in Pharmacokinetics of Steroidal Alkaloids from a Hydroethanolic Fritillariae thunbergii Bulbus Extract in 2,4-Dinitrobenzene Sulfonic Acid-Induced Colitis Rats" Pharmaceuticals 17, no. 8: 1001. https://doi.org/10.3390/ph17081001





