Ethanolic Extracts of Cupressaceae Species Conifers Provide Rapid Protection against Barium Chloride-Induced Cardiac Arrhythmia
Abstract
1. Introduction
2. Results
2.1. A Survey of the Medicinal Value of Various Conifer Plants
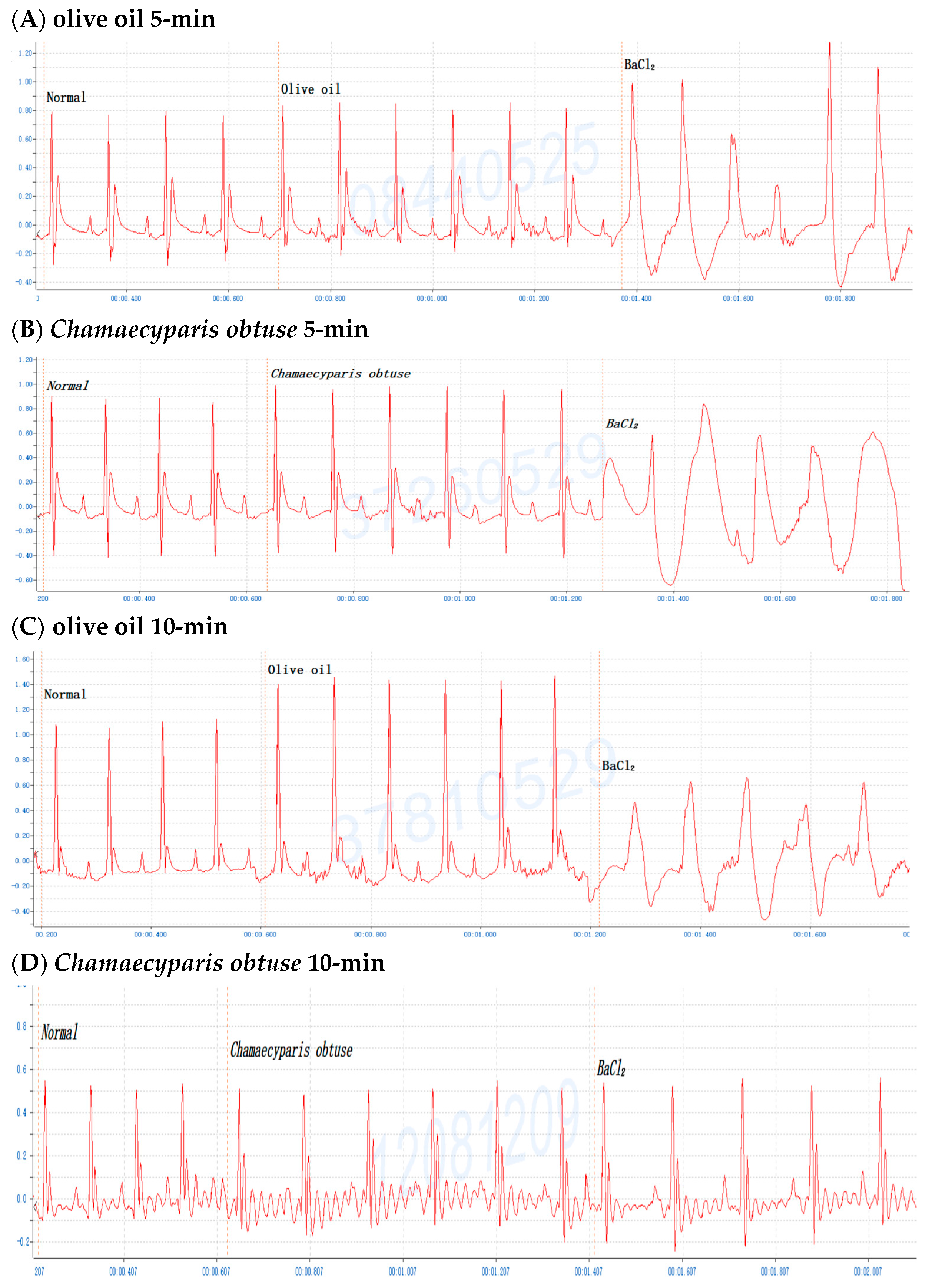
2.2. Screening of Various Conifer Plant Extracts in Mice
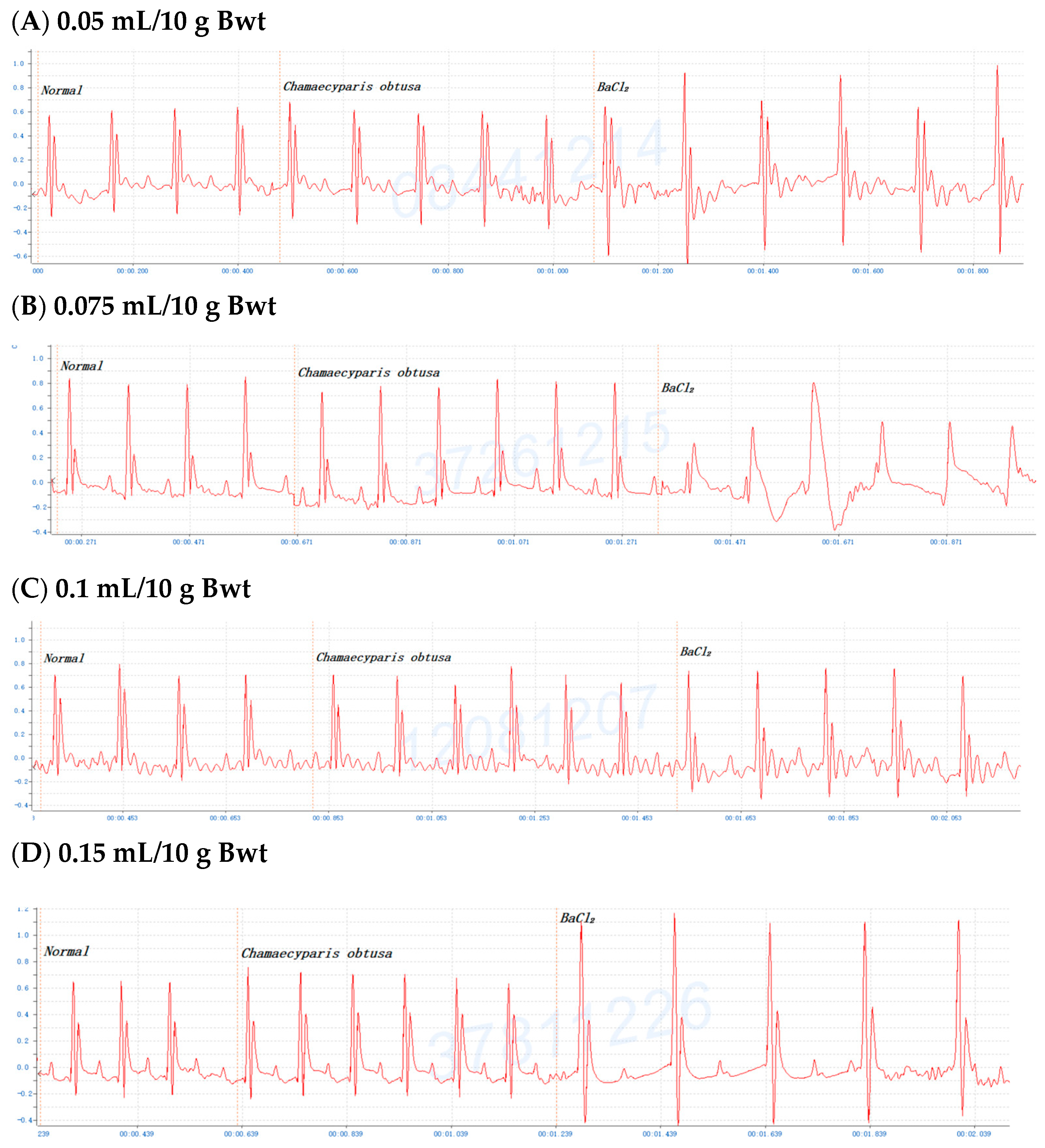
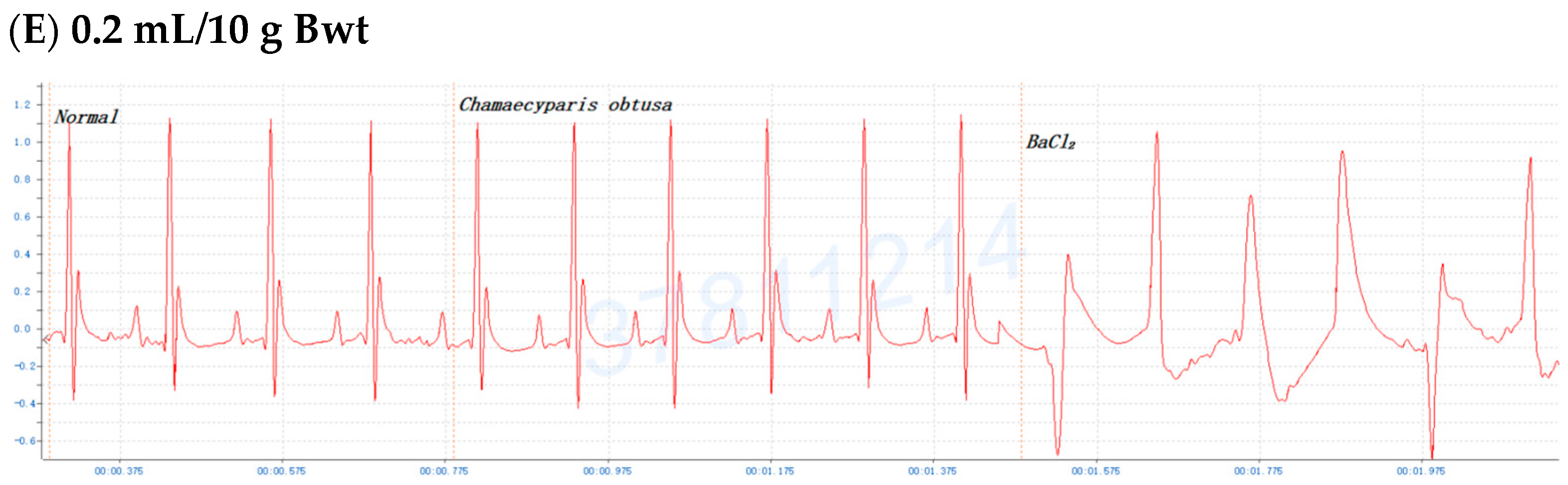
2.3. Dose-Dependent Protective Effects of C. obtusa, P. orientalis, and J. sabina Extracts on BaCl2-Induced Arrhythmia in Mice
2.4. Efficacy of C. obtusa, P. orientalis, and J. sabina Extracts in Protection against BaCl2-Induced Arrhythmia in Mice
2.5. Treated Efficacy of C. obtusa, P. orientalis, and J. sabina Extracts in BaCl2-Induced Arrhythmia in Rats
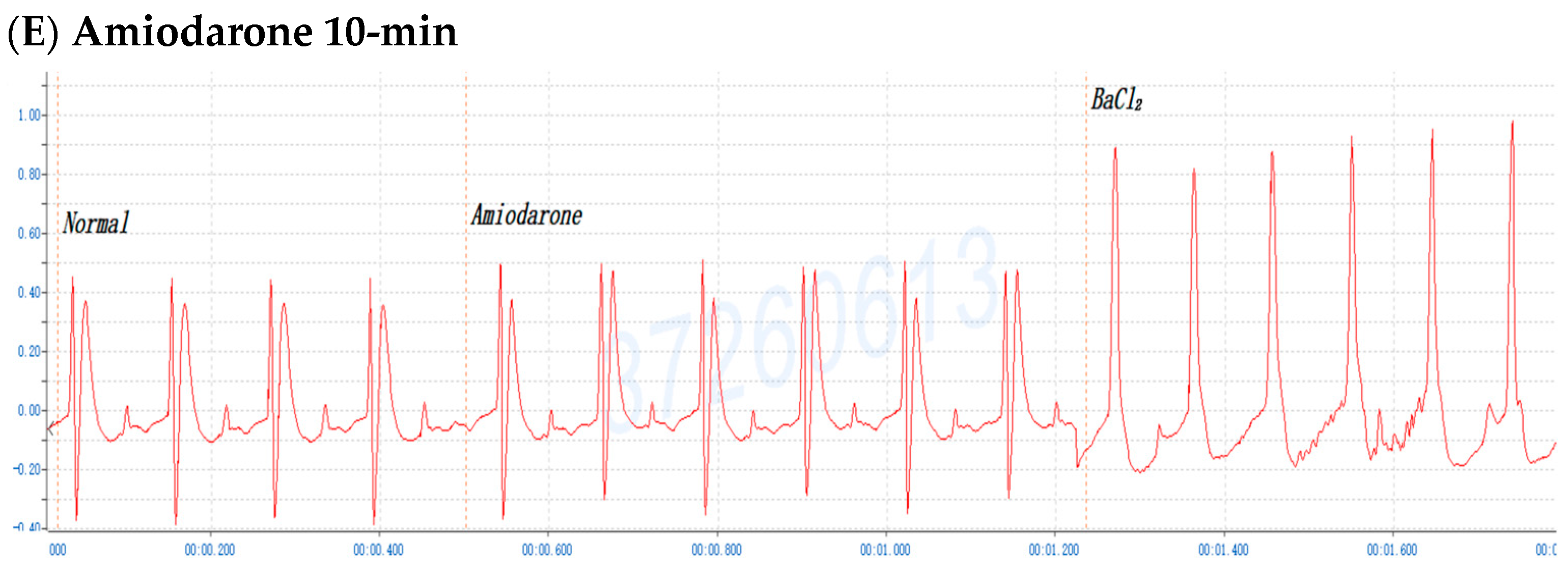
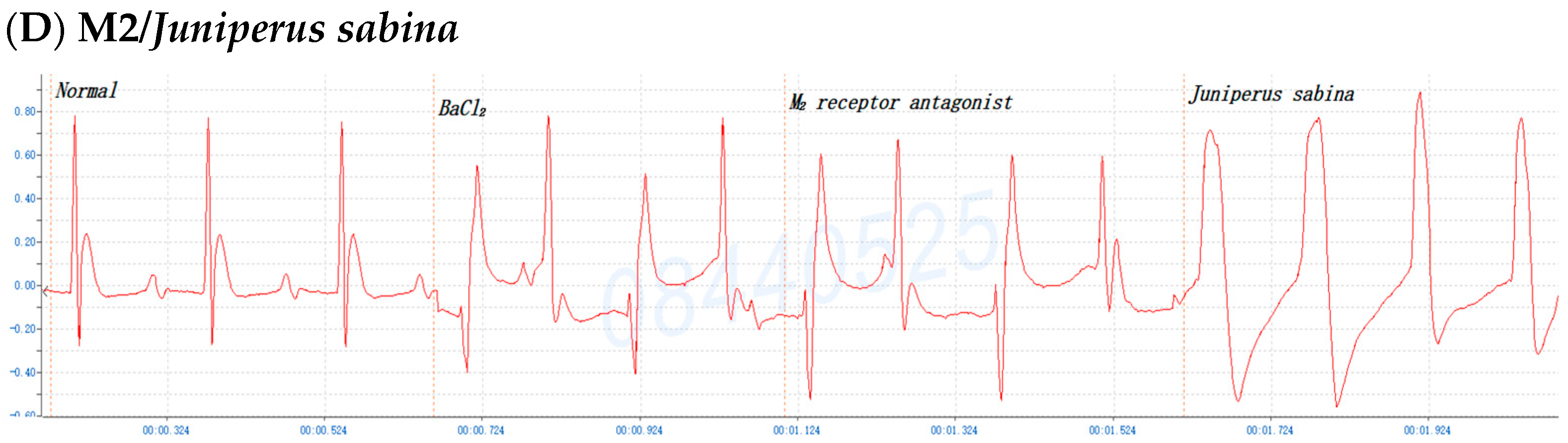
2.6. The Involvement of the M Receptor in the Antiarrhythmic Effects of C. obtusa, P. orientalis, and J. sabina Extracts Compared with That of Amiodarone in Mice
3. Discussion
4. Materials and Methods
4.1. Plant Source and Reagents
4.2. Extraction of Cypress Leaves
4.3. Animal Care
4.4. Antiarrhythmic Activity of the Extract in a Mouse Model of BaCl2-Induced Arrhythmia Prior to (Protection) BaCl2 Induction
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Krittayaphong, R.; Rangsin, R.; Thinkhamrop, B.; Hurst, C.; Rattanamongkolgul, S.; Sripaiboonkij, N.; Yindeengam, A. Prevalence and associating factors of atrial fibrillation in patients with hypertension: A nation-wide study. BMC Cardiovasc. Disord. 2016, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Shi, C.; Zhang, Z.; Xiao, J. Advance for Cardiovascular Health in China. J. Cardiovasc. Transl. Res. 2019, 12, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, F.M.; Bailey, K.R.; Chaouki, A.S.; Deshmukh, A.J.; Gautam, S.; Kim, R.J.; Kramer, D.B.; Lambrakos, L.K.; Nasser, N.H.; Sorajja, D. Systematic review for the 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018, 15, e253–e274. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cadrin-Tourigny, J.; Bhonsale, A.; Tichnell, C.; Murray, B.; Monfredi, O.; Chrispin, J.; Crosson, J.; Tandri, H.; James, C.A.; et al. Arrhythmic outcome of arrhythmogenic right ventricular cardiomyopathy patients without implantable defibrillators. J. Cardiovasc. Electrophysiol. 2018, 29, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Berger, R.D.; Calkins, H. Radiofrequency ablation: Technological trends, challenges, and opportunities. Europace 2021, 23, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.; Levy, A.; Varosy, P.D.; Matlock, D. Implantable cardioverter defibrillators and cardiac resynchronization therapy in older adults with heart failure. J. Am. Geriatr. Soc. 2019, 67, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Kusumoto, F.M. Implantable cardioverter-defibrillators have stood the test of time! Circulation 2019, 140, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
- Honarbakhsh, S.; Hunter, L.; Chow, A.; Hunter, R.J. Bradyarrhythmias and pacemakers. BMJ 2018, 360, k642. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Schibilsky, D.; Zeh, W.; Berchtold-Herz, M.; Beyersdorf, F.; Siepe, M. Does the heart transplant have a future? Eur. J. Cardio-Thorac. Surg. 2019, 55, I38–I48. [Google Scholar] [CrossRef]
- Sutanto, H.; Laudy, L.; Clerx, M.; Dobrev, D.; Crijns, H.J.G.M.; Heijman, J. Maastricht antiarrhythmic drug evaluator (MANTA): A computational tool for better understanding of antiarrhythmic drugs. Pharmacol. Res. 2019, 148, 104444. [Google Scholar] [CrossRef]
- Lu, J.; Jones, A.D.; Harkema, J.R.; Roth, R.A.; Ganey, P.E. Amiodarone exposure during modest inflammation induces idiosyncrasy-like liver injury in rats: Role of tumor necrosis factor-alpha. Toxicol. Sci. 2012, 125, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Mahavadi, P.; Henneke, I.; Ruppert, C.; Knudsen, L.; Venkatesan, S.; Liebisch, G.; Chambers, R.C.; Ochs, M.; Schmitz, G.; Vancheri, C.; et al. Altered surfactant homeostasis and alveolar epithelial cell stress in amiodarone-induced lung fibrosis. Toxicol. Sci. 2014, 142, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Brugada, J.; Katritsis, D.G.; Arbelo, E.; Arribas, F.; Bax, J.J.; Blomström-Lundqvist, C.; Calkins, H.; Corrado, D.; Deftereos, S.G.; Diller, G.P.; et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia. The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 655–720. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.J.; Liu, Y.R.; Tang, Z.S.; Duan, J.A.; Yan, Y.F.; Song, Z.X.; Wang, M.G.; Zhang, Y.R.; Chang, B.J.; Zhao, M.L.; et al. Poria cum Radix Pini Rescues Barium Chloride-Induced Arrhythmia by Regulating the cGMP-PKG Signalling Pathway Involving ADORA1 in Zebrafish. Front. Pharmacol. 2021, 12, 688746. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Huyan, T.; Li, Q.; Wang, Y.L.; Li, J.; Zhang, J.Y.; Liu, Y.X.; Shahid, M.R.; Yang, H.; Li, H.Q. Anti-tumor effect of hot aqueous extracts from Sonchus oleraceus (L.) L. and Juniperus sabina L—Two traditional medicinal plants in China. J. Ethnopharmacol. 2016, 185, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kader, M.S.; Soliman, G.A.; Alqarni, M.H.; Hamad, A.M.; Foudah, A.I.; Alqasoumi, S.I. Chemical composition and protective effect of Juniperus sabina, L. essential oil against CCl4 induced hepatotoxicity. Saudi Pharm. J. 2019, 27, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, X.; Liu, S.; Tian, P.; Li, D. Juniperus sabina, L. as a Source of Podophyllotoxins: Extraction Optimization and Anticholinesterase Activities. Int. J. Mol. Sci. 2022, 23, 10205. [Google Scholar] [CrossRef]
- Orhan, N.; Deliorman Orhan, D.; Gökbulut, A.; Aslan, M.; Ergun, F. Comparative Analysis of Chemical Profile, Antioxidant, In-vitro and In-vivo Antidiabetic Activities of Juniperus foetidissima Willd. and Juniperus sabina L. Iran. J. Pharm. Res. 2017, 16, 64–74. [Google Scholar]
- Shi, R.; Li, J. Study on the mechanism of Sabina przewalskii on chronic obstructive pulmonary disease based on network pharmacology. J. Qinghai Norm. Univ. 2022, 38, 44–52. [Google Scholar]
- Park, S.A.; Jegal, J.; Chung, K.W.; Jung, H.J.; Noh, S.G.; Chung, H.Y.; Ahn, J.; Kim, J.; Yang, M.H. Isolation of tyrosinase and melanogenesis inhibitory flavonoids from Juniperus chinensis fruits. Biosci. Biotechnol. Biochem. 2018, 82, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Nan, P.; Lin, M. Main Volatile Components in the Leaves of Sabina chinensis L. Ant. and Sabina chinensis L. Ant. Cv. Kaizuca and Their Effects on Bacteria. J. Environ. Health 2006, 1, 63–65. [Google Scholar] [CrossRef]
- Andersen, F.A. Final report on the safety assessment of Juniperus communis Extract, Juniperus oxycedrus Extract, Juniperus oxycedrus Tar, Juniperus phoenicea Extract, and Juniperus virginiana Extract. Int. J. Toxicol. 2001, 20 (Suppl. S2), 41–56. [Google Scholar] [CrossRef]
- Li, R.L.; Wu, P.L.; Li, C.R. Study on the chemical constituents of the branches and leaves of Juniperus formosana. West China J. Pharm. 2019, 34, 5–9. [Google Scholar]
- Wu, S.; Lin, Y. Advances in Chinese Juniper leaves. Chin. J. Ethn. Med. 2014, 20, 55–58. [Google Scholar]
- Jiang, J.H.; Li, X.C.; Gao, T.H.; He, D.N.; Chen, F.M.; Zhang, Y.P.; Huang, L.B. Volatile Constituents from the Cupressaceae Plants and Their Antitumor Activities. J. Fujian For. Sci. Technol. 2006, 2, 52–57. [Google Scholar] [CrossRef]
- Wu, X.D.; He, J.; Li, X.Y.; Dong, L.B.; Gong, X.; Song, L.D.; Li, Y.; Peng, L.Y.; Zhao, Q.S. Diterpenoids from the twigs and leaves of Fokienia hodginsii. J. Nat. Prod. 2013, 76, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.D.; Zhong, W.W.; Ding, L.F.; Tu, W.C.; Yang, H.; Gong, X.; Peng, L.Y.; Li, Y.; Xu, Z.Z.; Zhao, Q.S. Sesquiterpenoids from the twigs and leaves of Fokienia hodginsii. J. Asian Nat. Prod. Res. 2017, 19, 666–672. [Google Scholar] [CrossRef]
- Hau, D.V.; Sa, N.H.; Tam, N.T.; Diep, N.T.; Hoang Anh, N.T.; Thuy Linh, N.T.; Ngoc Ni, H.T.; Adorisio, S.; Delfino, D.V.; Thuy, T.T. Pro-apoptoticeffect of diterpenoids from Fokienia hodginsii on acute myeloid leukemia cells. Nat. Prod. Res. 2021, 35, 4685–4689. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Cao, Q.; Zhang, W.; Chen, F. Study on the Chemical Constituents and Biological Activities of Volatile Oil from Fokienia hodgisii. Anhui Agric. Sci. 2008, 17, 7290–7291, 7298. [Google Scholar]
- Manimaran, S.; Kumar, B.S.; Khan, S.; Patel, D.; Suresh, B. Anti inflammatory activity of cone vola tile oil of Cupressus funebris endl. Anc. Sci. Life. 2005, 25, 1–3. [Google Scholar] [PubMed]
- Liu, Y.X. Analysis of Chemical Constituents of Essential Oil Extracted from Leaves and Seeds of Sabina chinensis L. J. Hubei Univ. Natl. 2013, 31, 414–418, 441. [Google Scholar]
- Gu, D.; Fang, C.; Yang, J.; Li, M.; Liu, H.; Yang, Y. Chemical composition and α-amylase inhibitory activity of the essential oil from Sabina chinensis cv. Kaizuca leaves. Nat. Prod. Res. 2018, 32, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Z.; Liang, W.; Bi, J.; Zheng, Y.; Gu, X.; Fang, H. Essential oil from Sabina chinensis leaves: A promising green control agent against Fusarium sp. Front. Plant Sci. 2022, 13, 1006303. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.A.; El-Seedi, H.R.; Mohammed, M.M. Phytochemical investigation and hepatoprotective activity of Cupressus sempervirens, L. leaves growing in Egypt. Nat. Prod. Res. 2007, 21, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Leigh-de Rapper, S.; Viljoen, A.; van Vuuren, S. Essential Oil Blends: The Potential of Combined Use for Respiratory Tract Infections. Antibiotics 2021, 10, 1517. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Zhang, Y.; Dai, H.; Wang, Y. Analysis of volatile constituents in leaves of three cypress species by gas chromatography/mass spectrometry. Se Pu = Chin. J. Chromatogr. 2006, 24, 185–187. (In Chinese) [Google Scholar]
- Dai, S.; Chan, M.Y.; Lee, S.S.; Ogle, C.W. The antiarrhythmic effects of Sophora flavescens Ait. in rats and mice. Am. J. Chin. Med. 1986, 14, 119–123. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Han, G.; Li, X.; Lan, H.; Li, Y.; Dou, X.; Guo, Y.; Zhang, M.; Liu, H. Efficacy and Safety of Chinese Medicine in Treating Arrhythmia: Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Alternat Med. 2021, 2021, 9960471. [Google Scholar] [CrossRef]
- Yang, Y.; Ge, F.L.; Huang, Q.; Zeng, R.; Zhang, X.Y.; Liu, P.; Luo, G.; Yang, S.J.; Sun, Q. Randomized Controlled Trials of Zhigancao Decoction Combined with Metoprolol in the Treatment of Arrhythmia: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 795903. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Bryzgalov, A.O.; Tolstikova, T.G.; Shults, E.E.; Petrova, K.O. Natural Products as a Source of Antiarrhythmic Drugs. Mini Rev. Med. Chem. 2018, 18, 345–362. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, X.F.; Wei, W.S.; Zhang, J.; Li, Z.Z. The cardiovascular protective effect and mechanism of calycosin and its derivatives. Chin. J. Nat. Med. 2020, 18, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.J.; El-Shazly, M.; Yang, Y.H.; Tsai, Y.H.; Csupor, D.; Hohmann, J.; Wu, Y.C.; Tseng, T.G.; Chang, F.R.; Wang, H.C. The effectiveness of Fuzi in combination with routine heart failure treatment on chronic heart failure patients. J. Ethnopharmacol. 2022, 289, 115040. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Sun, Y.; Liu, S.; Li, C.; Chen, S.; Qiu, R.; Zhang, X.; Li, Y.; Li, M.; Shang, H. Therapeutic Effects of Wenxin Keli in Cardiovascular Diseases: An Experimental and Mechanism Overview. Front. Pharmacol. 2018, 9, 1005. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, Y.; Zeng, Z.; Zhao, Y.; Li, T.; Chen, R.; Zhang, R. Identification of Target Genes of Antiarrhythmic Traditional Chinese Medicine Wenxin Keli. Cardiovasc. Ther. 2020, 2020, 3480276. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Kirchner, D.; Kunze, F.; Chrétien, E.M.; Michel-Reher, M.B.; Voigt, N.; Knaut, M.; Michel, M.C.; Ravens, U.; Dobrev, D. Muscarinic type-1 receptors contribute to IKACh in human atrial cardiomyocytes and are upregulated in patients with chronic atrial fibrillation. Int. J. Cardiol. 2018, 255, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, J.; Zhang, L.; Xu, Y.; Wu, B.; Cao, J. The Agonist of Inward Rectifier Potassium Channel (IK1) Attenuates Rat Reperfusion Arrhythmias Linked to CaMKII Signaling. Int. Heart J. 2021, 62, 1348–1357. [Google Scholar] [CrossRef]
- de Araújo, R.B.; Azevedo, B.M.S.; Andrade, T.S.; Abalem, M.F.; Monteiro, M.L.R.; Carricondo, P.C. Subconjunctival 0.1% epinephrine versus placebo in maintenance of mydriasis during vitrectomy: A randomized controlled trial. Int. J. Retin. Vitr. 2018, 4, 38. [Google Scholar] [CrossRef]
- Janssens, U.; Michels, G. Adrenalin bei Patienten mit prähospitalem Herz-Kreislauf-Stillstand: PARAMEDIC2-Studie Adrenaline in patients with out-of-hospital cardiac arrest: PARAMEDIC2 trial. Med. Klin. Intensivmed. Notfmed. 2019, 114, 63–67. [Google Scholar] [CrossRef]
- Torrente, A.G.; Mesirca, P.; Bidaud, I.; Mangoni, M.E. Channelopathies of voltage-gated L-type Cav1.3/α1D and T-type Cav3.1/α1G Ca2+ channels in dysfunction of heart automaticity. Pflugers Arch. 2020, 472, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Escobar, G.J.; LaGuardia, J.C.; Turk, B.J.; Ragins, A.; Kipnis, P.; Draper, D. Early detection of impending physiologic deterioration among patients who are not in intensive care: Development of predictive models using data from an automated electronic medical record. J. Hosp. Med. 2012, 7, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Suita, K.; Fujita, T.; Hasegawa, N.; Cai, W.; Jin, H.; Hidaka, Y.; Prajapati, R.; Umemura, M.; Yokoyama, U.; Sato, M.; et al. Norepinephrine-Induced Adrenergic Activation Strikingly Increased the Atrial Fibrillation Duration through β1- and α1-Adrenergic Receptor-Mediated Signaling in Mice. PLoS ONE. 2015, 10, e0133664. [Google Scholar] [CrossRef] [PubMed]
- Sapa, J.; Kubacka, M. The possible mechanism of hypotensive activity of some pyrrolidin-2-one derivatives with antagonist properties at alpha1-adrenoceptors. Eur. J. Pharmacol. 2011, 673, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, M.; Mogilski, S.; Filipek, B.; Marona, H. The hypotensive activity and alpha1-adrenoceptor antagonistic properties of some aroxyalkyl derivatives of 2-methoxyphenylpiperazine. Eur. J. Pharmacol. 2013, 698, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Rapacz, A.; Sapa, J.; Nowiński, L.; Mogilski, S.; Pytka, K.; Filipek, B.; Siwek, A.; Szkaradek, N.; Marona, H. Biofunctional studies of new 2-methoxyphenylpiperazine xanthone derivatives with α₁-adrenolytic properties. Pharmacol. Rep. 2015, 67, 267–274. [Google Scholar] [CrossRef]
- Pytka, K.; Rapacz, A.; Zygmunt, M.; Olczyk, A.; Waszkielewicz, A.; Sapa, J.; Filipek, B. Antidepressant-like activity of a new piperazine derivative of xanthone in the forced swim test in mice: The involvement of serotonergic system. Pharmacol. Rep. 2015, 67, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Pytka, K.; Lustyk, K.; Żmudzka, E.; Kotańska, M.; Siwek, A.; Zygmunt, M.; Dziedziczak, A.; Śniecikowska, J.; Olczyk, A.; Gałuszka, A.; et al. Chemically Homogenous Compounds with Antagonistic Properties at All α1-Adrenoceptor Subtypes but not β1-Adrenoceptor Attenuate Adrenaline-Induced Arrhythmia in Rats. Front. Pharmacol. 2016, 7, 229. [Google Scholar] [CrossRef]
- van Zwieten, P.A.; Doods, H.N. Muscarinic receptors and drugs in cardiovascular medicine. Cardiovasc. Drugs Ther. 1995, 9, 159–167. [Google Scholar] [CrossRef]
- Kosmachev, A.B.; Fil’ko, O.A.; Petrov, V.V. Izuchenie roli otdel’nykh podtipov M-kholinoretseptorov v narushenii serdechnogo ritma razlichnoĭ étiologii the role of M-cholinoreceptor subtypes in heart rhythm disturbances of various etiology. Eksp. Klin. Farmakol. 2002, 65, 34–36. (In Russian) [Google Scholar]
- Hardouin, S.N.; Richmond, K.N.; Zimmerman, A.; Hamilton, S.E.; Feigl, E.O.; Nathanson, N.M. Altered cardiovascular responses in mice lacking the M(1) muscarinic acetylcholine receptor. J. Pharmacol. Exp. Ther. 2002, 301, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Fajardo, P.D.; Aréchiga-Figueroa, I.A.; López-Serrano, A.L.; Rodriguez-Elias, J.C.; Alamilla, J.; Sánchez-Chapula, J.A.; Tristani-Firouzi, M.; Navarro-Polanco, R.A.; Moreno-Galindo, E.G. The voltage-sensitive cardiac M2 muscarinic receptor modulates the inward rectification of the G protein-coupled, ACh-gated K+ current. Pflugers Arch. 2018, 470, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, H.; Li, D.; Nattel, S.; Wang, Z. Differential alterations of receptor densities of three muscarinic acetylcholine receptor subtypes and current densities of the corresponding K+ channels in canine atria with atrial fibrillation induced by experimental congestive heart failure. Cell Physiol. Biochem. 2004, 14, 31–40. [Google Scholar] [CrossRef] [PubMed]
- James, A.F.; Hancox, J.C. More types than one: Multiple muscarinic receptor coupled K+ currents undergo remodelling in an experimental model of atrial fibrillation. Br. J. Pharmacol. 2007, 152, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Uemura, H.; Hara, Y.; Endou, M.; Mori, K.; Nakaya, H. Interaction of class III antiarrhythmic drugs with muscarinic M2 and M3 receptors: Radioligand binding and functional studies. Naunyn Schmiedebergs Arch. Pharmacol. 1995, 353, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Su, Y.; Zhang, Y.; Pan, Z.; Yang, L.; Chen, X.; Liu, Y.; Lu, Y.; Du, Z.; Yang, B. Activation of cardiac muscarinic M3 receptors induces delayed cardioprotection by preserving phosphorylated connexin43 and up-regulating cyclooxygenase-2 expression. Br. J. Pharmacol. 2010, 159, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Rauch, B.; Niroomand, F. Specific M2-receptor activation: An alternative to treatment with beta-receptor blockers? Eur. Heart J. 1991, 12 (Suppl. F), 76–82. [Google Scholar] [CrossRef]
- Anderson, A.; Kulkarni, K.; Marron Fernandez de Velasco, E.; Carlblom, N.; Xia, Z.; Nakano, A.; Martemyanov, K.A.; Tolkacheva, E.G.; Wickman, K. Expression and relevance of the G protein-gated K+ channel in the mouse ventricle. Sci. Rep. 2018, 8, 1192. [Google Scholar] [CrossRef]
- Yang, B.; Lin, H.; Xu, C.; Liu, Y.; Wang, H.; Han, H.; Wang, Z. Choline produces cytoprotective effects against ischemic myocardial injuries: Evidence for the role of cardiac m3 subtype muscarinic acetylcholine receptors. Cell Physiol. Biochem. 2005, 16, 163–174. [Google Scholar] [CrossRef]
- Ramesh, P.; Palaniappan, A. Terminalia arjuna, a Cardioprotective Herbal Medicine–Relevancy in the Modern Era of Pharmaceuticals and Green Nanomedicine—A Review. Pharmaceuticals 2023, 16, 126. [Google Scholar] [CrossRef]
- Hashem-Dabaghian, F.; Ziaee, M.; Ghaffari, S.; Nabati, F.; Kianbakht, S. A systematic review on the cardiovascular pharmacology of Emblica officinalis Gaertn. J. Cardiovasc. Thorac. Res. 2018, 10, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Nehdi, I.A. Cupressus sempervirens var. horizentalis seed oil: Chemical composition, physicochemical characteristics, and utilizations. Ind. Crops Prod. 2013, 41, 381–385. [Google Scholar] [CrossRef]
- Hasaballah, A.; Shehata, A.; Fouda, M.; Hassan, M.; Gad, M. The biological activity of Cupressus sempervirens extracts against Musca domestica. Asian J. Biol. 2018, 5, 1–12. [Google Scholar] [CrossRef]
- Mohamed, A. Biological applications of Cupressus sempervirens biomass extracted at various levels of pressure using different critical fluid extraction protocol. Biomass Conv. Bioref. 2024. [Google Scholar] [CrossRef]
- Zhao, J.; Gu, Z.; Liu, T.; Xu, F.; You, S.; Li, C. Anti-arthritic effects of total flavonoids from Juniperus sabina on complete freund’s adjuvant induced arthritis in rats. Pharmacogn. Mag. 2016, 12, 178–183. [Google Scholar] [CrossRef]

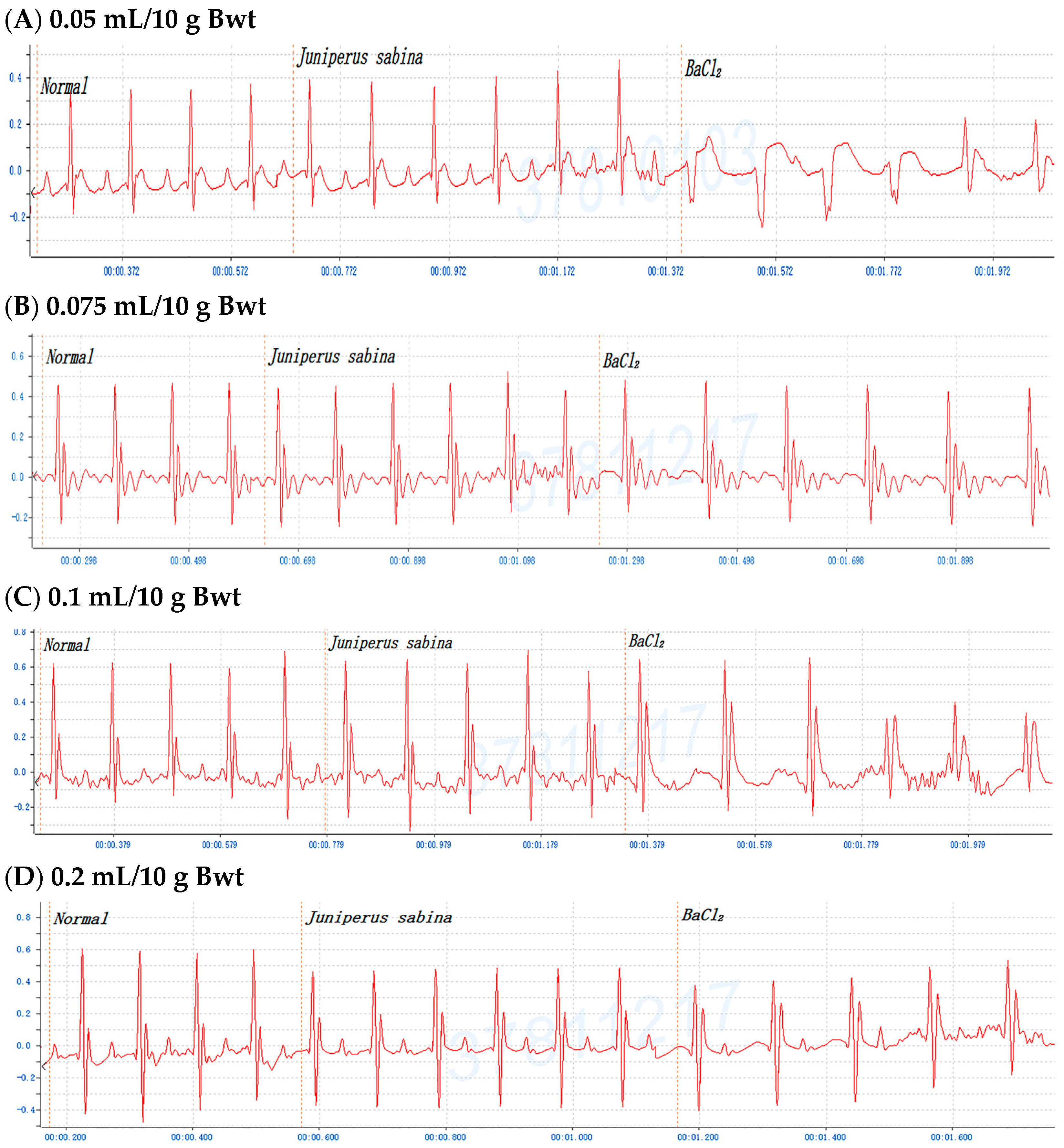


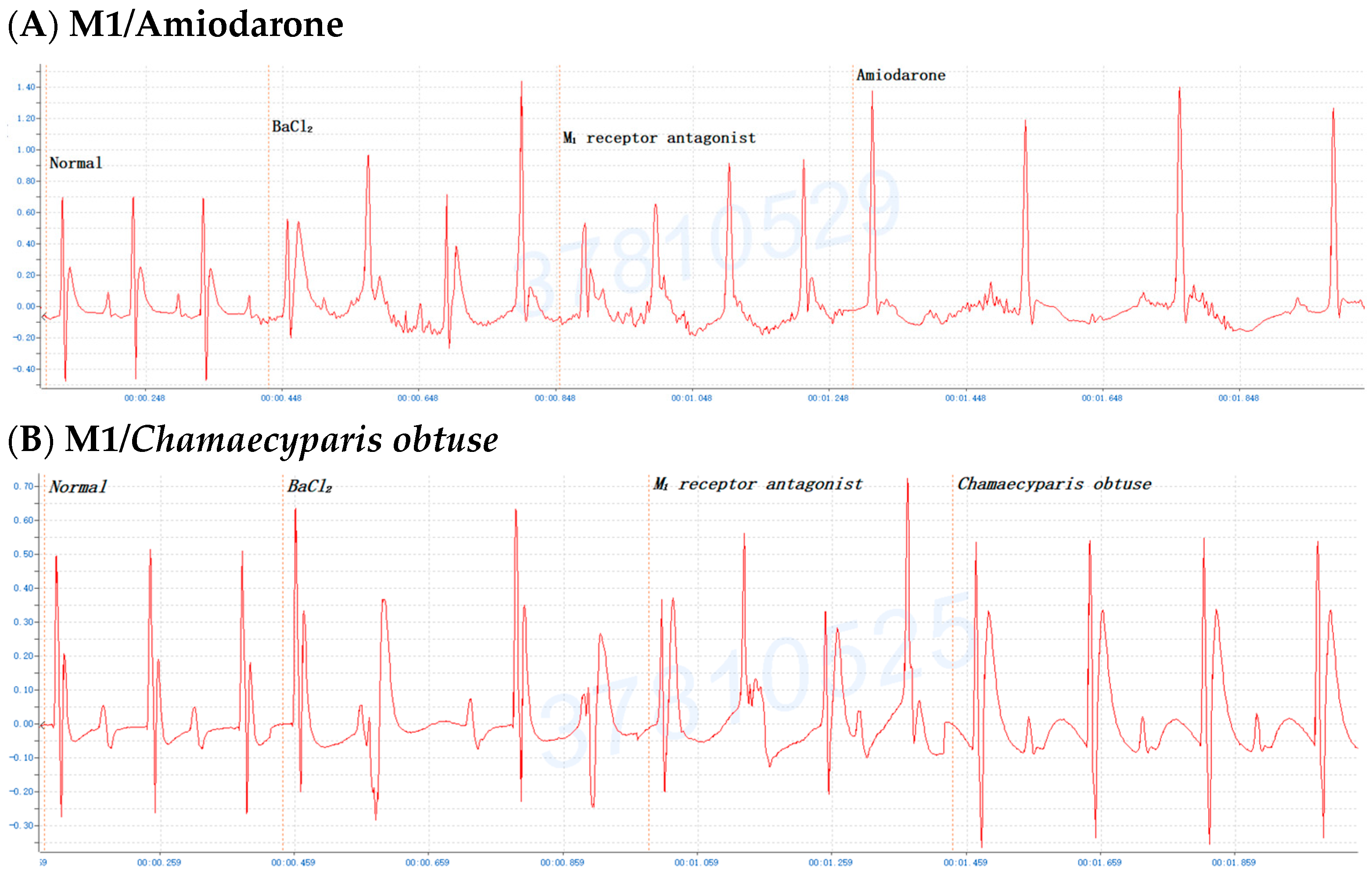
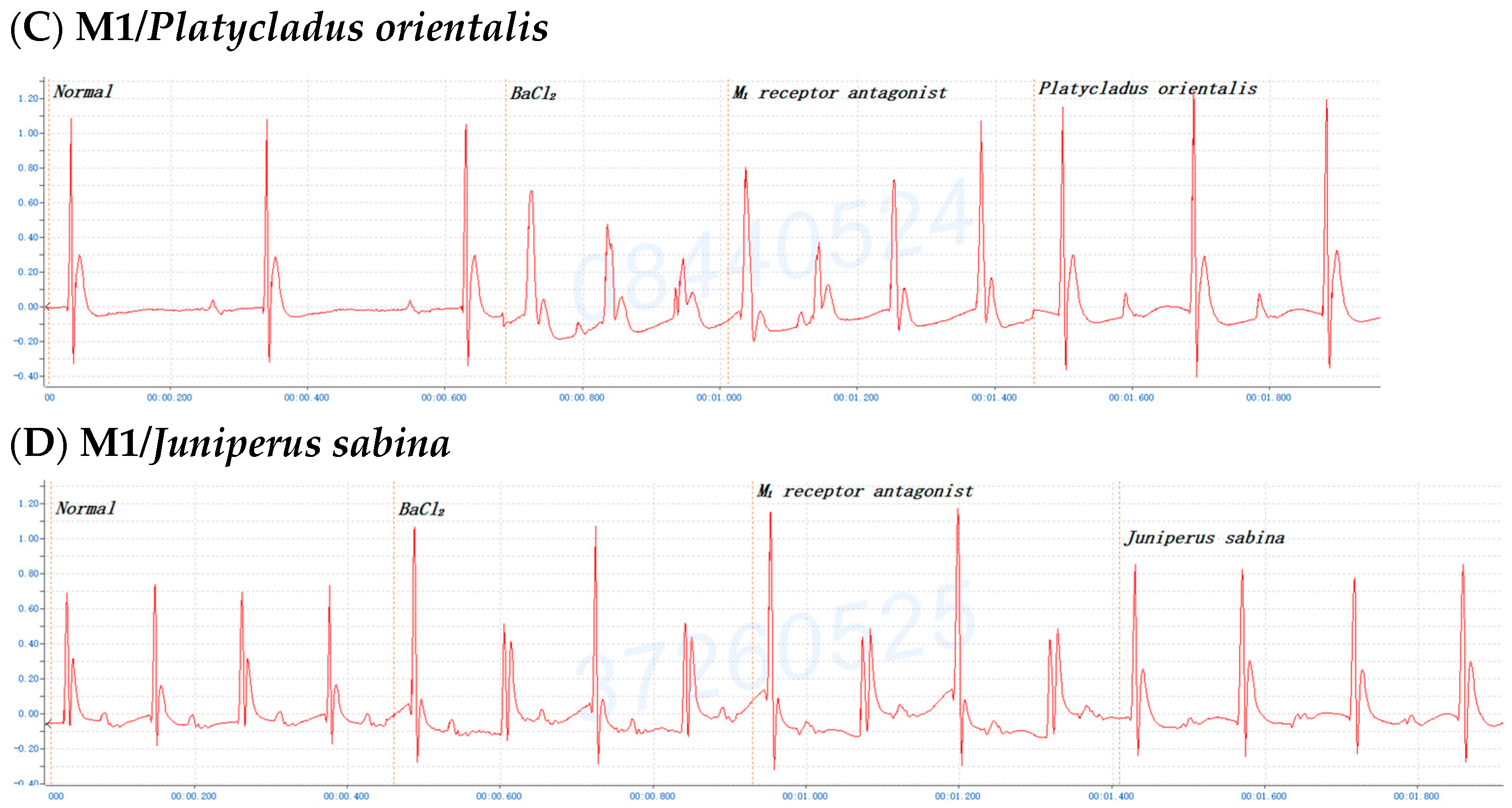
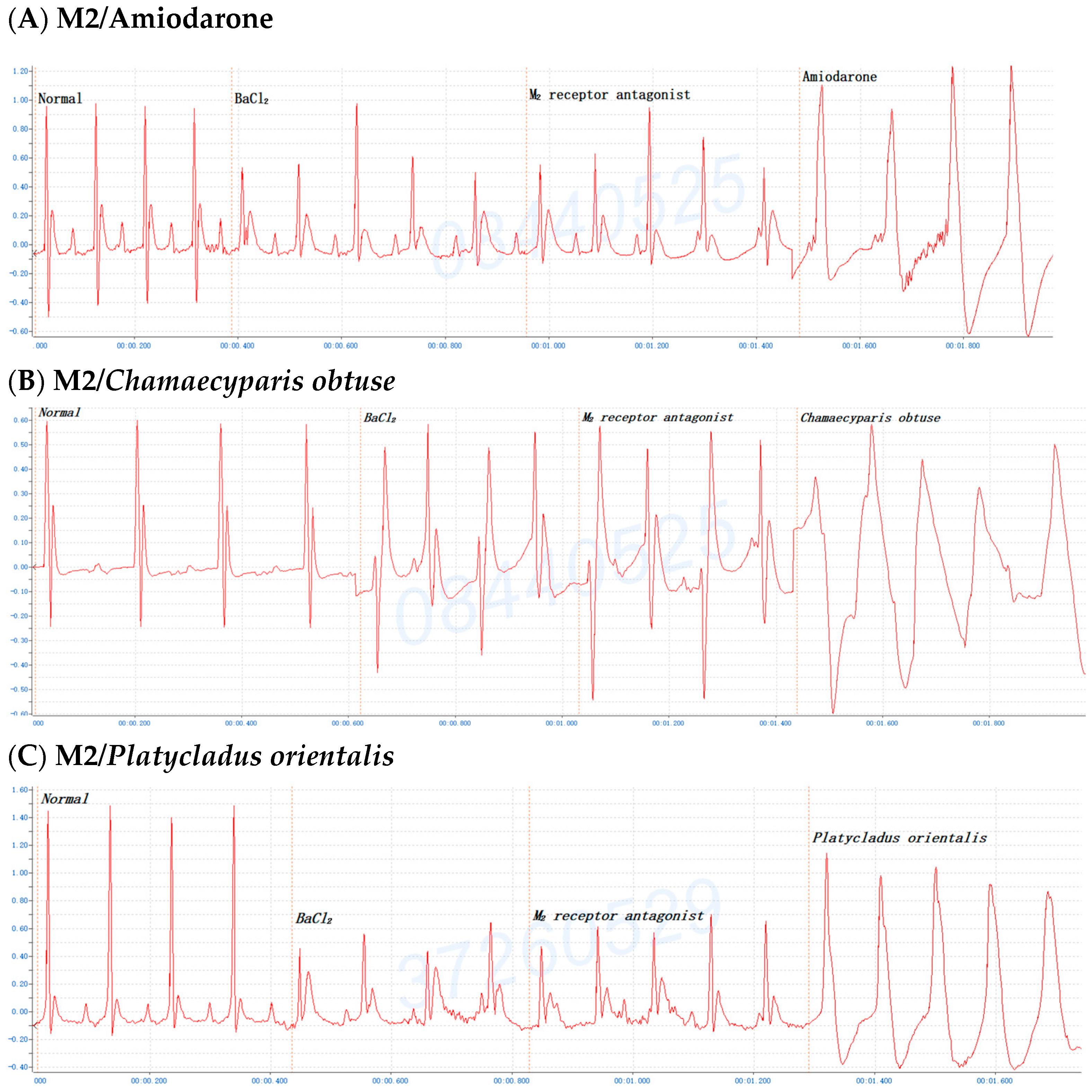

| Species | 0.05 mL/10 g Bwt | 0.075 mL/10 g Bwt | 0.10 mL/10 g Bwt | 0.15/0.20 mL/10 g Bwt |
|---|---|---|---|---|
| Chamaecyparis obtusa | 0.5 ± 0.2 min | 4.0 ± 1.1 min ** | 5.0 ± 1.1 min ** | 15.0 ± 2.9 min *** (0.15 mL/10 g) |
| Platycladus orientalis | 1.0 ± 0.4 min | 5.0 ± 1.4 min ** | 5.0 ± 1.7 min ** | 9.0 ± 2.1 min ** |
| Juniperus sabina | 0.5 ± 0.3 min | 4.5 ± 1.9 min ** | 5.0 ± 1.5 min ** | 10.0 ± 2.3 min *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, M.-T.; Huang, L.-Y.; Zheng, X.-H.; Fu, Y.-Q.; Weng, C.-F. Ethanolic Extracts of Cupressaceae Species Conifers Provide Rapid Protection against Barium Chloride-Induced Cardiac Arrhythmia. Pharmaceuticals 2024, 17, 1003. https://doi.org/10.3390/ph17081003
Zeng M-T, Huang L-Y, Zheng X-H, Fu Y-Q, Weng C-F. Ethanolic Extracts of Cupressaceae Species Conifers Provide Rapid Protection against Barium Chloride-Induced Cardiac Arrhythmia. Pharmaceuticals. 2024; 17(8):1003. https://doi.org/10.3390/ph17081003
Chicago/Turabian StyleZeng, Meng-Ting, Li-Yue Huang, Xiao-Hui Zheng, Yan-Qi Fu, and Ching-Feng Weng. 2024. "Ethanolic Extracts of Cupressaceae Species Conifers Provide Rapid Protection against Barium Chloride-Induced Cardiac Arrhythmia" Pharmaceuticals 17, no. 8: 1003. https://doi.org/10.3390/ph17081003
APA StyleZeng, M.-T., Huang, L.-Y., Zheng, X.-H., Fu, Y.-Q., & Weng, C.-F. (2024). Ethanolic Extracts of Cupressaceae Species Conifers Provide Rapid Protection against Barium Chloride-Induced Cardiac Arrhythmia. Pharmaceuticals, 17(8), 1003. https://doi.org/10.3390/ph17081003










