What’s New in Ocular Drug Delivery: Advances in Suprachoroidal Injection since 2023
Abstract
:1. Introduction
2. Anatomy and Pathophysiology
3. Drug Delivery to the Suprachoroidal Space
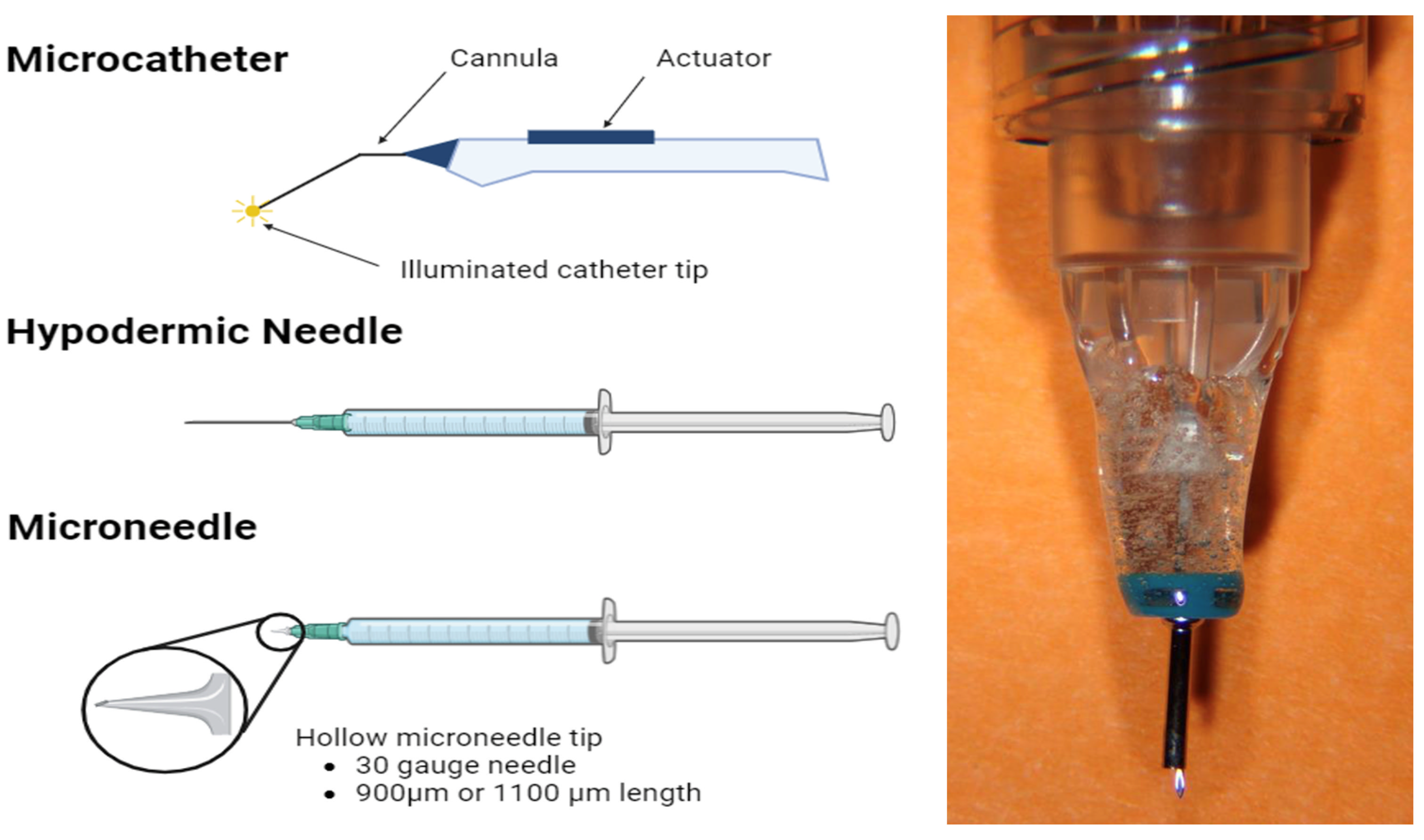
3.1. Microcatheters
3.2. Hypodermic Needles
3.3. Microneedles
4. Ocular Administration Methods
4.1. Intravitreal Administration
4.2. Subretinal Administration
4.3. Rationale for Suprachoroidal Administration
5. Nanotechnology
5.1. Nanomicelles
5.2. Nanoparticles
5.2.1. Polymer-Based Nanoparticles
Natural Polymers
Synthetic Polymers
5.2.2. Lipid-Based Nanoparticles
5.2.3. Metal-Based Nanoparticles
5.3. Liposomes
5.4. Hydrogels
6. Gene Delivery to the Suprachoroidal Space
6.1. Viral Gene Therapy
6.2. Non-Viral Gene Therapy
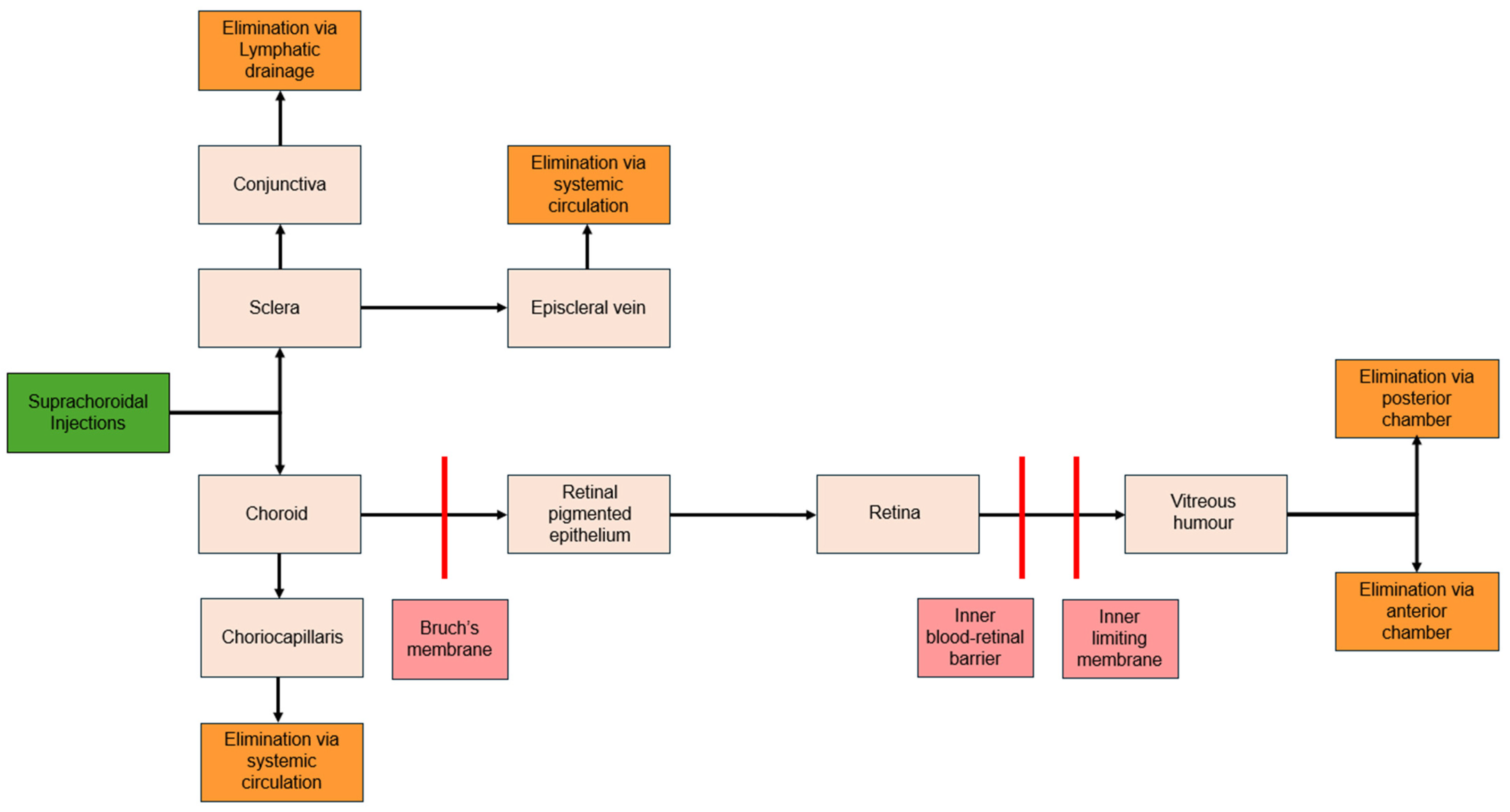
7. Pharmacokinetics
7.1. Distribution
7.2. Clearance
8. Clinical Applications
8.1. Macular Edema
8.1.1. Macular Edema Due to Uveitis
8.1.2. Diabetic Macular Edema
8.1.3. Macular Edema Due to Retinal Vein Occlusion
8.2. Age-Related Macular Degeneration
8.3. Choroidal Melanoma
8.4. Glaucoma
8.5. Regulatory Status of Suprachoroidal Technologies
8.6. Limitations of Clinical Applications
9. Suprachoroidal Delivery Advancements: A 2023–2024 Overview
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.K.; Hao, J.; Liu, H.; Lee, J. MRI Study of Subconjunctival and Intravitreal Injections. J. Pharm. Sci. 2012, 101, 2353–2363. [Google Scholar] [CrossRef]
- Ghasemi Falavarjani, K.; Nguyen, Q.D. Adverse Events and Complications Associated with Intravitreal Injection of Anti-VEGF Agents: A Review of Literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef]
- Tasman, W.; Jaeger, E.A. Duane’s Ophthalmology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; ISBN 0-7817-6855-1. [Google Scholar]
- Vurgese, S.; Panda-Jonas, S.; Jonas, J.B. Scleral Thickness in Human Eyes. PLoS ONE 2012, 7, e29692. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.E.; Flanagan, J.G.; Rausch, S.M.K.; Sigal, I.A.; Tertinegg, I.; Eilaghi, A.; Portnoy, S.; Sled, J.G.; Ethier, C.R. Dimensions of the Human Sclera: Thickness Measurement and Regional Changes with Axial Length. Exp. Eye Res. 2010, 90, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Tello, C.; Liebmann, J.; Ritch, R. Central Corneal Thickness Is Not Related to Anterior Scleral Thickness or Axial Length. J. Glaucoma 2006, 15, 190–194. [Google Scholar] [CrossRef]
- Worthington, K.S.; Wiley, L.A.; Bartlett, A.M.; Stone, E.M.; Mullins, R.F.; Salem, A.K.; Guymon, C.A.; Tucker, B.A. Mechanical Properties of Murine and Porcine Ocular Tissues in Compression. Exp. Eye Res. 2014, 121, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Copete, S.; Flores-Moreno, I.; Montero, J.A.; Duker, J.S.; Ruiz-Moreno, J.M. Direct Comparison of Spectral-Domain and Swept-Source OCT in the Measurement of Choroidal Thickness in Normal Eyes. Br. J. Ophthalmol. 2014, 98, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Koizumi, H.; Pozonni, M.C. Enhanced Depth Imaging Spectral-Domain Optical Coherence Tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Hanhart, J.; Yaacov, R. Optimized Imaging of the Suprachoroidal Space with Swept-Source OCT. Asian J. Ophthalmol. 2019, 16, 323–328. [Google Scholar] [CrossRef]
- Olsen, T.W.; Feng, X.; Wabner, K.; Conston, S.R.; Sierra, D.H.; Folden, D.V.; Smith, M.E.; Cameron, J.D. Cannulation of the Suprachoroidal Space: A Novel Drug Delivery Methodology to the Posterior Segment. Am. J. Ophthalmol. 2006, 142, 777–787.e2. [Google Scholar] [CrossRef]
- Olsen, T.W.; Feng, X.; Wabner, K.; Csaky, K.; Pambuccian, S.; Cameron, J.D. Pharmacokinetics of Pars Plana Intravitreal Injections versus Microcannula Suprachoroidal Injections of Bevacizumab in a Porcine Model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4749–4756. [Google Scholar] [CrossRef] [PubMed]
- Wellik, S.R.; Dale, E.A. A Review of the iStent® Trabecular Micro-Bypass Stent: Safety and Efficacy. Clin. Ophthalmol. 2015, 9, 677–684. [Google Scholar] [CrossRef]
- Fea, A.M.; Belda, J.I.; Rękas, M.; Jünemann, A.; Chang, L.; Pablo, L.; Voskanyan, L.; Katz, L.J. Prospective Unmasked Randomized Evaluation of the iStent Inject® versus Two Ocular Hypotensive Agents in Patients with Primary Open-Angle Glaucoma. Clin. Ophthalmol. 2014, 8, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Shen, J.; Hafiz, Z.; Hackett, S.F.; e Silva, R.L.; Khan, M.; Lorenc, V.E.; Chen, D.; Chadha, R.; Zhang, M.; et al. AAV8-Vectored Suprachoroidal Gene Transfer Produces Widespread Ocular Transgene Expression. J. Clin. Investig. 2019, 129, 4901–4911. [Google Scholar] [CrossRef]
- Shen, J.; Kim, J.; Tzeng, S.Y.; Ding, K.; Hafiz, Z.; Long, D.; Wang, J.; Green, J.J.; Campochiaro, P.A. Suprachoroidal Gene Transfer with Nonviral Nanoparticles. Sci. Adv. 2020, 6, eaba1606. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.W.; Aaberg, S.Y.; Geroski, D.H.; Edelhauser, H.F. Human Sclera: Thickness and Surface Area. Am. J. Ophthalmol. 1998, 125, 237–241. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.; Liu, J.; Han, Y.; Cheng, L. Safety and Pharmacodynamics of Suprachoroidal Injection of Triamcinolone Acetonide as a Controlled Ocular Drug Release Model. J. Control. Release 2015, 203, 109–117. [Google Scholar] [CrossRef]
- Patel, S.R.; Lin, A.S.P.; Edelhauser, H.F.; Prausnitz, M.R. Suprachoroidal Drug Delivery to the Back of the Eye Using Hollow Microneedles. Pharm. Res. 2011, 28, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Berezovsky, D.E.; McCarey, B.E.; Zarnitsyn, V.; Edelhauser, H.F.; Prausnitz, M.R. Targeted Administration into the Suprachoroidal Space Using a Microneedle for Drug Delivery to the Posterior Segment of the Eye. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4433–4441. [Google Scholar] [CrossRef]
- Wan, C.-R.; Muya, L.; Kansara, V.; Ciulla, T.A. Suprachoroidal Delivery of Small Molecules, Nanoparticles, Gene and Cell Therapies for Ocular Diseases. Pharmaceutics 2021, 13, 288. [Google Scholar] [CrossRef]
- Chiang, B.; Jang, K.; Goldberg, J.; Myung, D. Design and Ex Vivo Development of a Suprachoroidal Spacer Implant to Treat Glaucoma; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Everads Therapy. Everads Injector in Suprachoroidal Administration of TA Suspension, for Treatment of Patients with DME. Available online: https://clinicaltrials.gov/study/NCT06314217 (accessed on 31 December 2023).
- Katz, A.; KIM, C.; Duncan, N.; Potter, H.A.D.; Kim, C.B.Y.; Rasmussen, C.; Murphy, C.J.; Nork, T.M. Laboratory-Made Needle for Suprachoroidal Injections in Living Animals and Human Eye Bank Eyes. Investig. Ophthalmol. Vis. Sci. 2023, 64, 2623. [Google Scholar]
- Ahmed, S.; Amin, M.M.; Sayed, S. Ocular Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 66. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.M.; Ciulla, T.A.; Hariprasad, S.M. Treatment Burden in Neovascular AMD:Visual Acuity Outcomes Are Associated with Anti-VEGF Injection Frequency. Ophthalmic. Surg. Lasers Imaging Retin. 2017, 48, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Kansara, V.; Muya, L.; Wan, C.-R.; Ciulla, T.A. Suprachoroidal Delivery of Viral and Nonviral Gene Therapy for Retinal Diseases. J. Ocul. Pharmacol. Ther. Off J. Assoc. Ocul. Pharmacol. Ther. 2020, 36, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Peden, M.C.; Min, J.; Meyers, C.; Lukowski, Z.; Li, Q.; Boye, S.L.; Levine, M.; Hauswirth, W.W.; Ratnakaram, R.; Dawson, W.; et al. Ab-Externo AAV-Mediated Gene Delivery to the Suprachoroidal Space Using a 250 Micron Flexible Microcatheter. PLoS ONE 2011, 6, e17140. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.E.; Wan, C.-R.; Fisher, N.E.; Andino, R.V.; Ciulla, T.A. Biomechanics of Suprachoroidal Drug Delivery: From Benchtop to Clinical Investigation in Ocular Therapies. Expert Opin. Drug Deliv. 2021, 18, 777–788. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Hariprasad, S.M.; Albini, T.A.; Dutta, S.K.; John, D.; Padula, W.V.; Harrison, D.; Joseph, G. Suprachoroidal Injection of Triamcinolone Acetonide Injectable Suspension for the Treatment of Macular Edema Associated with Uveitis in the United States: A Cost-Effectiveness Analysis. Value Health 2022, 25, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Hariprasad, S.M.; Albini, T.A.; John, D.; Dutta, S.K.; Cohen, B.; Dashputre, A.A.; Harrison, D.; Joseph, G. EE105 Triamcinolone Acetonide Injectable Suspension, for Suprachoroidal Use, for the Treatment of Macular Edema Associated with Uveitis in the United States: A Budget Impact Analysis. Value Health 2022, 25, S355. [Google Scholar] [CrossRef]
- DailyMed—XIPERE—Triamcinolone Acetonide Injection, Suspension. Available online: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ea6ad429-967f-4f75-a33d-e06f849bee80 (accessed on 21 June 2024).
- Wykoff, C.C.; Avery, R.L.; Barakat, M.R.; Boyer, D.S.; Brown, D.M.; Brucker, A.J.; Cunningham, E.T.J.; Heier, J.S.; Holekamp, N.M.; Kaiser, P.K.; et al. Suprachoroidal Space Injection Technique: Expert Panel Guidance. Retina 2024, 44, 939. [Google Scholar] [CrossRef]
- Sharma, S.; Hu, A.; Hall, C.; Kapik, B.; Ciulla, T.A. Safety of Suprachoroidal Injection Procedure Utilizing a Microinjector across Three Retinal Disorders. Investig. Ophthalmol. Vis. Sci. 2021, 62, 1209. [Google Scholar]
- Joszt, L. Xipere for Macular Edema Associated with Uveitis Launches in United States. Available online: https://www.ajmc.com/view/xipere-for-macular-edema-associated-with-uveitis-launches-in-united-states (accessed on 28 May 2024).
- Tyagi, P.; Kadam, R.S.; Kompella, U.B. Comparison of Suprachoroidal Drug Delivery with Subconjunctival and Intravitreal Routes Using Noninvasive Fluorophotometry. PLoS ONE 2012, 7, e48188. [Google Scholar] [CrossRef] [PubMed]
- Yiu, G.; Chung, S.H.; Mollhoff, I.N.; Nguyen, U.T.; Thomasy, S.M.; Yoo, J.; Taraborelli, D.; Noronha, G. Suprachoroidal and Subretinal Injections of AAV Using Transscleral Microneedles for Retinal Gene Delivery in Nonhuman Primates. Mol. Ther. Methods Clin. Dev. 2020, 16, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Glassman, A.R.; Beck, R.W.; Bressler, N.M.; Fish, G.E.; Ferris, F.L.; Kinyoun, J.L. Diabetic Retinopathy Clinical Research Network Ocular Side Effects Associated with Peribulbar Injections of Triamcinolone Acetonide for Diabetic Macular Edema. Retina 2011, 31, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Cholkar, K.; Patel, A.; Vadlapudi, A.D.; Mitra, A.K. Novel Nanomicellar Formulation Approaches for Anterior and Posterior Segment Ocular Drug Delivery. Recent Pat. Nanomed. 2012, 2, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Seah, I.; Xue, K.; Wong, W.; Tan, Q.S.W.; Ma, X.; Lin, Q.; Lim, J.Y.C.; Liu, Z.; Parikh, B.H.; et al. Antiangiogenic Nanomicelles for the Topical Delivery of Aflibercept to Treat Retinal Neovascular Disease. Adv. Mater. 2022, 34, e2108360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiao, J.; Niu, M.; Gao, X.; Zhang, G.; Yu, H.; Yang, X.; Liu, L. Ten Years of Knowledge of Nano-Carrier Based Drug Delivery Systems in Ophthalmology: Current Evidence, Challenges, and Future Prospective. Int. J. Nanomed. 2021, 16, 6497–6530. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Pharmaceutical Applications of Chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A. The Use of Mucoadhesive Polymers in Ocular Drug Delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Pandit, J.; Sultana, Y.; Aqil, M. Chitosan Coated Nanoparticles for Efficient Delivery of Bevacizumab in the Posterior Ocular Tissues via Subconjunctival Administration. Carbohydr. Polym. 2021, 267, 118217. [Google Scholar] [CrossRef]
- Lyu, Q.; Peng, L.; Hong, X.; Fan, T.; Li, J.; Cui, Y.; Zhang, H.; Zhao, J. Smart Nano-Micro Platforms for Ophthalmological Applications: The State-of-the-Art and Future Perspectives. Biomaterials 2021, 270, 120682. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Sun, J.-G.; Yao, J.; Shan, K.; Liu, B.-H.; Yao, M.-D.; Ge, H.-M.; Jiang, Q.; Zhao, C.; Yan, B. Effect of Nanoencapsulation Using Poly (Lactide-Co-Glycolide) (PLGA) on Anti-Angiogenic Activity of Bevacizumab for Ocular Angiogenesis Therapy. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 107, 1056–1063. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Li, G.; Li, X.; Yu, C.; Fu, Z.; Li, X.; Teng, L.; Li, Y.; Sun, F. Highly Bioactive, Bevacizumab-Loaded, Sustained-Release PLGA/PCADK Microspheres for Intravitreal Therapy in Ocular Diseases. Int. J. Pharm. 2019, 563, 228–236. [Google Scholar] [CrossRef]
- Prieto, E.; Cardiel, M.J.; Vispe, E.; Idoipe, M.; Garcia-Martin, E.; Fraile, J.M.; Polo, V.; Mayoral, J.A.; Pablo, L.E.; Rodrigo, M.J. Dexamethasone Delivery to the Ocular Posterior Segment by Sustained-Release Laponite Formulation. Biomed. Mater. 2020, 15, 065021. [Google Scholar] [CrossRef] [PubMed]
- Arta, A.; Su, Y.; Rossato, F.A.; Gnanaguru, G.; D’Amore, P.A.; Ng, Y.S.E.; Urquhart, A.J. Solid Lipid Nanoparticles for the Delivery of a Sustained-Release Small Molecule Antioxidant for RPE Protection in Dry AMD. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1705. [Google Scholar]
- Tzameret, A.; Ketter-Katz, H.; Edelshtain, V.; Sher, I.; Corem-Salkmon, E.; Levy, I.; Last, D.; Guez, D.; Mardor, Y.; Margel, S.; et al. In Vivo MRI Assessment of Bioactive Magnetic Iron Oxide/Human Serum Albumin Nanoparticle Delivery into the Posterior Segment of the Eye in a Rat Model of Retinal Degeneration. J. Nanobiotechnol. 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, B.; Kalishwaralal, K.; Sheikpranbabu, S.; Deepak, V.; Haribalaganesh, R.; Gurunathan, S. Gold Nanoparticles Downregulate VEGF-and IL-1β-Induced Cell Proliferation through Src Kinase in Retinal Pigment Epithelial Cells. Exp. Eye Res. 2010, 91, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.V.; Petronilho, F.; Pereira, H.R.S.B.; Vuolo, F.; Mina, F.; Possato, J.C.; Vitto, M.F.; de Souza, D.R.; da Silva, L.; da Silva Paula, M.M.; et al. Effects of Gold Nanoparticles on Endotoxin-Induced Uveitis in Rats. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8036–8041. [Google Scholar] [CrossRef]
- Barenholz, Y. (Chezy) Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Bressler, N.M.; Bressler, S.B. Photodynamic Therapy with Verteporfin (Visudyne): Impact on Ophthalmology and Visual Sciences. Investig. Ophthalmol. Vis. Sci. 2000, 41, 624–628. [Google Scholar]
- Altamirano-Vallejo, J.C.; Navarro-Partida, J.; Gonzalez-De la Rosa, A.; Hsiao, J.H.; Olguín-Gutierrez, J.S.; Gonzalez-Villegas, A.C.; Keller, B.C.; Bouzo-Lopez, L.; Santos, A. Characterization and Pharmacokinetics of Triamcinolone Acetonide-Loaded Liposomes Topical Formulations for Vitreoretinal Drug Delivery. J. Ocul. Pharmacol. Ther. Off J. Assoc. Ocul. Pharmacol. Ther. 2018, 34, 416–425. [Google Scholar] [CrossRef]
- Li, J.; Cheng, T.; Tian, Q.; Cheng, Y.; Zhao, L.; Zhang, X.; Qu, Y. A More Efficient Ocular Delivery System of Triamcinolone Acetonide as Eye Drop to the Posterior Segment of the Eye. Drug Deliv. 2019, 26, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, M.; Wang, Q.; Bai, J.; McAlinden, C.; Skiadaresi, E.; Zhang, J.; Pan, L.; Mei, C.; Zeng, Z.; et al. Hydrogel Eye Drops as a Non-Invasive Drug Carrier for Topical Enhanced Adalimumab Permeation and Highly Efficient Uveitis Treatment. Carbohydr. Polym. 2021, 253, 117216. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Lin, D.; Ding, X.; Wang, Y.; Hu, Y.; Shi, H.; Chen, L.; Chu, B.; Lei, L.; Wen, C.; et al. Multifunctional Supramolecular Filament Hydrogel Boosts Anti-Inflammatory Efficacy In Vitro and In Vivo. Adv. Funct. Mater. 2022, 32, 2109173. [Google Scholar] [CrossRef]
- Tyagi, P.; Barros, M.; Stansbury, J.W.; Kompella, U.B. Light-Activated, In Situ Forming Gel for Sustained Suprachoroidal Delivery of Bevacizumab. Mol. Pharm. 2013, 10, 2858–2867. [Google Scholar] [CrossRef] [PubMed]
- Hackett, S.F.; Fu, J.; Kim, Y.C.; Tsujinaka, H.; Shen, J.; Lima, E.; Silva, R.; Khan, M.; Hafiz, Z.; Wang, T.; et al. Sustained Delivery of Acriflavine from the Suprachoroidal Space Provides Long Term Suppression of Choroidal Neovascularization. Biomaterials 2020, 243, 119935. [Google Scholar] [CrossRef] [PubMed]
- Moore, N.A.; Morral, N.; Ciulla, T.A.; Bracha, P. Gene Therapy for Inherited Retinal and Optic Nerve Degenerations. Expert Opin. Biol. Ther. 2018, 18, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Touchard, E.; Benard, R.; Bigot, K.; Laffitte, J.-D.; Buggage, R.; Bordet, T.; Behar-Cohen, F. Non-Viral Ocular Gene Therapy, pEYS606, for the Treatment of Non-Infectious Uveitis: Preclinical Evaluation of the Medicinal Product. J. Control. Release 2018, 285, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Liu, S.; Ou, L. rAAV Immunogenicity, Toxicity, and Durability in 255 Clinical Trials: A Meta-Analysis. Front. Immunol. 2022, 13, 1001263. [Google Scholar] [CrossRef]
- Gregory, S.M.; Nazir, S.A.; Metcalf, J.P. Implications of the Innate Immune Response to Adenovirus and Adenoviral Vectors. Future Virol. 2011, 6, 357–374. [Google Scholar] [CrossRef]
- Mendonça, S.A.; Lorincz, R.; Boucher, P.; Curiel, D.T. Adenoviral Vector Vaccine Platforms in the SARS-CoV-2 Pandemic. npj Vaccines 2021, 6, 97. [Google Scholar] [CrossRef]
- Balaggan, K.S.; Binley, K.; Esapa, M.; Iqball, S.; Askham, Z.; Kan, O.; Tschernutter, M.; Bainbridge, J.W.B.; Naylor, S.; Ali, R.R. Stable and Efficient Intraocular Gene Transfer Using Pseudotyped EIAV Lentiviral Vectors. J. Gene Med. 2006, 8, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L.; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2017, 31, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Mollhoff, I.N.; Mishra, A.; Sin, T.-N.; Ngo, T.; Ciulla, T.; Sieving, P.; Thomasy, S.M.; Yiu, G. Host Immune Responses after Suprachoroidal Delivery of AAV8 in Nonhuman Primate Eyes. Hum. Gene Ther. 2021, 32, 682–693. [Google Scholar] [CrossRef]
- AbbVie. RGX-314 Gene Therapy Administered in the Suprachoroidal Space for Participants with Neovascular Age-Related Macular Degeneration (nAMD) (AAVIATE). Available online: https://clinicaltrials.gov/study/NCT04514653 (accessed on 31 December 2023).
- Khanani, A.M. Suprachoroidal Delivery of RGX-314 Gene Therapy for Neovascular AMD: The Phase II AAVIATETM Study. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1497. [Google Scholar]
- AbbVie. RGX-314 Gene Therapy Administered in the Suprachoroidal Space for Participants with Diabetic Retinopathy (DR) without Center Involved-Diabetic Macular Edema (CI-DME) (ALTITUDE). Available online: https://clinicaltrials.gov/study/NCT04567550 (accessed on 31 December 2023).
- REGENXBIO. REGENXBIO Presents Positive Interim Data from Phase II ALTITUDETM Trial of RGX-314 for the Treatment of Diabetic Retinopathy Using Suprachoroidal Delivery. Available online: https://regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-presents-positive-interim-data-phase-ii-altitudetm/ (accessed on 30 May 2024).
- Luo, S.; Jiang, H.; Li, Q.; Qin, Y.; Yang, S.; Li, J.; Xu, L.; Gou, Y.; Zhang, Y.; Liu, F.; et al. An Adeno-Associated Virus Variant Enabling Efficient Ocular-Directed Gene Delivery across Species. Nat. Commun. 2024, 15, 3780. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, D.M.; Barnard, A.R.; Charbel Issa, P.; Singh, M.S.; De Silva, S.R.; Trabalza, A.; Eleftheriadou, I.; Ellison, S.M.; Mazarakis, N.D.; MacLaren, R.E. Vesicular Stomatitis Virus Glycoprotein- and Venezuelan Equine Encephalitis Virus-Derived Glycoprotein-Pseudotyped Lentivirus Vectors Differentially Transduce Corneal Endothelium, Trabecular Meshwork, and Human Photoreceptors. Hum. Gene Ther. 2014, 25, 50–62. [Google Scholar] [CrossRef]
- Bemelmans, A.-P.; Bonnel, S.; Houhou, L.; Dufour, N.; Nandrot, E.; Helmlinger, D.; Sarkis, C.; Abitbol, M.; Mallet, J. Retinal Cell Type Expression Specificity of HIV-1-Derived Gene Transfer Vectors upon Subretinal Injection in the Adult Rat: Influence of Pseudotyping and Promoter. J. Gene Med. 2005, 7, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Grüter, O.; Kostic, C.; Crippa, S.V.; Perez, M.-T.R.; Zografos, L.; Schorderet, D.F.; Munier, F.L.; Arsenijevic, Y. Lentiviral Vector-Mediated Gene Transfer in Adult Mouse Photoreceptors Is Impaired by the Presence of a Physical Barrier. Gene Ther. 2005, 12, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Gibbs, D.; Lillo, C.; Azarian, S.M.; Legacki, E.; Zhang, X.-M.; Yang, X.-J.; Williams, D.S. Lentiviral Gene Replacement Therapy of Retinas in a Mouse Model for Usher Syndrome Type 1B. Gene Ther. 2007, 14, 584–594. [Google Scholar] [CrossRef]
- Shanghai BDgene Co., Ltd. VEGFA-Targeting Gene Therapy to Treat Retinal and Choroidal Neovascularization Diseases. Available online: https://classic.clinicaltrials.gov/ct2/show/study/NCT05099094 (accessed on 1 June 2024).
- Picanço-Castro, V.; Pereira, C.G.; Covas, D.T.; Porto, G.S.; Athanassiadou, A.; Figueiredo, M.L. Emerging Patent Landscape for Non-Viral Vectors Used for Gene Therapy. Nat. Biotechnol. 2020, 38, 151–157. [Google Scholar] [CrossRef]
- Young, J.L.; Dean, D.A. Electroporation-Mediated Gene Delivery. Adv. Genet. 2015, 89, 49–88. [Google Scholar] [CrossRef] [PubMed]
- Al-Dosari, M.S.; Gao, X. Nonviral Gene Delivery: Principle, Limitations, and Recent Progress. AAPS J. 2009, 11, 671. [Google Scholar] [CrossRef]
- Masri, F.; Cheeseman, E.; Ansorge, S. Viral Vector Manufacturing: How to Address Current and Future Demands? Cell Gene Ther. Insights 2019, 5, 949–970. [Google Scholar] [CrossRef]
- Sainz-Ramos, M.; Gallego, I.; Villate-Beitia, I.; Zarate, J.; Maldonado, I.; Puras, G.; Pedraz, J.L. How Far Are Non-Viral Vectors to Come of Age and Reach Clinical Translation in Gene Therapy? Int. J. Mol. Sci. 2021, 22, 7545. [Google Scholar] [CrossRef]
- Zulliger, R.; Conley, S.M.; Naash, M.I. Non-Viral Therapeutic Approaches to Ocular Diseases: An Overview and Future Directions. J. Control. Release 2015, 219, 471–487. [Google Scholar] [CrossRef]
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef]
- Koirala, A.; Conley, S.M.; Naash, M.I. Episomal Maintenance of S/MAR-Containing Non-Viral Vectors for RPE-Based Diseases. Adv. Exp. Med. Biol. 2014, 801, 703–709. [Google Scholar] [CrossRef]
- Balazs, D.A.; Godbey, W.T. Liposomes for Use in Gene Delivery. J. Drug Deliv. 2010, 2011, e326497. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Sahu, B.; Gao, S.; Schur, R.M.; Vaidya, A.M.; Maeda, A.; Palczewski, K.; Lu, Z.-R. Targeted Multifunctional Lipid ECO Plasmid DNA Nanoparticles as Efficient Non-Viral Gene Therapy for Leber’s Congenital Amaurosis. Mol. Ther. Nucleic Acids 2017, 7, 42–52. [Google Scholar] [CrossRef]
- Kansara, V.; Yoo, J.; Cooper, M.J.; Laird, O.S.; Taraborelli, D.; Moen, R.; Noronha, G. Suprachoroidally Delivered Non-Viral DNA Nanoparticles Transfect Chorioretinal Cells in Non-Human Primates and Rabbits. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2909. [Google Scholar]
- Kansara, V.S.; Cooper, M.; Sesenoglu-Laird, O.; Muya, L.; Moen, R.; Ciulla, T.A. Suprachoroidally Delivered DNA Nanoparticles Transfect Retina and Retinal Pigment Epithelium/Choroid in Rabbits. Transl. Vis. Sci. Technol. 2020, 9, 21. [Google Scholar] [CrossRef]
- Kansara, V.; Cooper, M.J.; Muya, L.; Moen, R.; Ciulla, T.A. Suprachoroidally Delivered DNA Nanoparticles Produce Human Myosin7A Protein in RPE-Choroid in Rabbits. Investig. Ophthalmol. Vis. Sci. 2021, 62, 1200. [Google Scholar]
- Jaijo, T.; Aller, E.; Beneyto, M.; Najera, C.; Graziano, C.; Turchetti, D.; Seri, M.; Ayuso, C.; Baiget, M.; Moreno, F.; et al. MYO7A Mutation Screening in Usher Syndrome Type I Patients from Diverse Origins. J. Med. Genet. 2007, 44, e71. [Google Scholar] [CrossRef] [PubMed]
- Emi, K.; Pederson, J.E.; Toris, C.B. Hydrostatic Pressure of the Suprachoroidal Space. Investig. Ophthalmol. Vis. Sci. 1989, 30, 233–238. [Google Scholar]
- Edelhauser, H.F.; Verhoeven, R.S.; Burke, B.; Struble, C.B.; Patel, S.R. Intraocular Distribution and Targeting of Triamcinolone Acetonide Suspension Administered Into the Suprachoroidal Space. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5259. [Google Scholar]
- Kaiser, P.K.; Ciulla, T.; Kansara, V. Suprachoroidal CLS-AX (Axitinib Injectable Suspension), as a Potential Long-Acting Therapy for Neovascular Age-Related Macular Degeneration (nAMD). Investig. Ophthalmol. Vis. Sci. 2020, 61, 3977. [Google Scholar]
- Chiang, B.; Venugopal, N.; Grossniklaus, H.E.; Jung, J.H.; Edelhauser, H.F.; Prausnitz, M.R. Thickness and Closure Kinetics of the Suprachoroidal Space Following Microneedle Injection of Liquid Formulations. Investig. Ophthalmol. Vis. Sci. 2017, 58, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Nork, T.M.; Katz, A.W.; Kim, C.B.Y.; Rasmussen, C.A.; Wabers, H.D.; Bentley, E.; Struble, C.B.; Savinainen, A. Distribution of Aqueous Solutions Injected Suprachoroidally (SC) in Rabbits. Investig. Ophthalmol. Vis. Sci. 2020, 61, 320. [Google Scholar]
- Gu, B.; Liu, J.; Li, X.; Ma, Q.; Shen, M.; Cheng, L. Real-Time Monitoring of Suprachoroidal Space (SCS) Following SCS Injection Using Ultra-High Resolution Optical Coherence Tomography in Guinea Pig Eyes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3623–3634. [Google Scholar] [CrossRef]
- Chiang, B.; Wang, K.; Ethier, C.R.; Prausnitz, M.R. Clearance Kinetics and Clearance Routes of Molecules From the Suprachoroidal Space After Microneedle Injection. Investig. Ophthalmol. Vis. Sci. 2017, 58, 545–554. [Google Scholar] [CrossRef]
- Kim, Y.C.; Oh, K.H.; Edelhauser, H.F.; Prausnitz, M.R. Formulation to Target Delivery to the Ciliary Body and Choroid via the Suprachoroidal Space of the Eye Using Microneedles. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2015, 95, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Chiang, B.; Venugopal, N.; Edelhauser, H.F.; Prausnitz, M.R. Distribution of Particles, Small Molecules and Polymeric Formulation Excipients in the Suprachoroidal Space after Microneedle Injection. Exp. Eye Res. 2016, 153, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Tran, J.; Craven, C.; Wabner, K.; Schmit, J.; Matter, B.; Kompella, U.; Grossniklaus, H.E.; Olsen, T.W. A Pharmacodynamic Analysis of Choroidal Neovascularization in a Porcine Model Using Three Targeted Drugs. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3732–3740. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H.; Li, S.; Liang, L.; Xu, X.-D.; Zhang, X.-Z.; Jiang, F.-G. Evaluation of RGD Peptide Hydrogel in the Posterior Segment of the Rabbit Eye. J. Biomater. Sci. Polym. Ed. 2013, 24, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, W.; Lu, Q.; Zeng, H.; Liu, S.; Yue, Y.; Cheng, H.; Liu, Y.; Xue, M. Pharmacokinetic Comparison of Ketorolac after Intracameral, Intravitreal, and Suprachoroidal Administration in Rabbits. Retina 2012, 32, 2158. [Google Scholar] [CrossRef] [PubMed]
- Merrill, P.T.; Henry, C.R.; Nguyen, Q.D.; Reddy, A.; Kapik, B.; Ciulla, T.A. Suprachoroidal CLS-TA with and without Systemic Corticosteroid and/or Steroid-Sparing Therapy: A Post-Hoc Analysis of the Phase 3 PEACHTREE Clinical Trial. Ocul. Immunol. Inflamm. 2023, 31, 1579–1586. [Google Scholar] [CrossRef]
- Shah, M.; Albini, T.; Nguyen, Q.; Wykoff, C.; Barakat, M.; Khurana, R.N.; Kapik, B.; Ciulla, T.A. Safety and Efficacy of CLS-TA by Anatomic Location of Inflammation: Results from the Phase 3 PEACHTREE Clinical Trial. Ocul. Immunol. Inflamm. 2024, 2, 1–8. [Google Scholar] [CrossRef]
- Zhou, A.; Philip, A.; Babiker, F.; Chang, P.Y. Efficacy of Suprachoroidal Triamcinolone Acetonide for Uveitic Cystoid Macular Edema. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3561. [Google Scholar]
- Yeh, S.; Henry, C.R.; Kapik, B.; Ciulla, T.A. Triamcinolone Acetonide Suprachoroidal Injectable Suspension for Uveitic Macular Edema: Integrated Analysis of Two Phase 3 Studies. Ophthalmol. Ther. 2023, 12, 577–591. [Google Scholar] [CrossRef]
- Wan, C.; Kapik, B.; Wykoff, C.C.; Henry, C.R.; Barakat, M.R.; Shah, M.; Andino, R.V.; Ciulla, T.A. Clinical Characterization of Suprachoroidal Injection Procedure Utilizing a Microinjector across Three Retinal Disorders. Transl. Vis. Sci. Technol. 2020, 9, 27. [Google Scholar] [CrossRef]
- Fazel, F.; Malekahmadi, M.; Feizi, A.; Oliya, B.; Tavakoli, M.; Fazel, M. Suprachoroidal Injection of Triamcinolone Acetonide plus Intravitreal Bevacizumab in Diabetic Macular Edema: A Randomized Pilot Trial. BMC Ophthalmol. 2023, 23, 40. [Google Scholar] [CrossRef]
- Oxular Limited. Oxulumis® Suprachoroidal Microcatherization of Triesence® in Diabetic Macular Edema (CAPE). Available online: https://clinicaltrials.gov/study/NCT05512962 (accessed on 31 December 2023).
- Cerrada-Gimenez, M.; Vähätupa, M.; Lappeteläinen, B.; Tähtivaara, L.; Jokinen, V.; Haapaniemi, A.M.; Vergun, O.; Kvietkauskiene, N.; Bijeikis, S.; Jarutis, J.; et al. Suprachoroidal Administration of Aflibercept Protects against Neovascularization in the Mouse CNV Model. Investig. Ophthalmol. Vis. Sci. 2023, 64, 2233. [Google Scholar]
- Oxular Limited. Suprachoroidal Sustained-Release OXU-001 Compared to Intravitreal Ozurdex® in the Treatment of Diabetic Macular Edema (OXEYE). Available online: https://clinicaltrials.gov/study/NCT05697809 (accessed on 31 December 2023).
- El Rayes, E.N.; Leila, M. Visual Function and Retinal Morphological Changes after Single Suprachoroidal Delivery of Fluocinolone Acetonide (Iluvien®) Implant in Eyes with Chronic Diabetic Macular Edema. Int. J. Retin. Vitr. 2023, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Kansara, V.; Muya, L.; Ciulla, T.A. Suprachoroidal Delivery of CLS-301, a Potent Small Molecule Integrin Antagonist, Offers Multimonth Durability and High Bioavailability in the Chorioretina. Investig. Ophthalmol. Vis. Sci. 2023, 64, 2611. [Google Scholar]
- Muya, L.; El-Kattan, Y.; Kansara, V.; Babu, Y.; Ciulla, T.A. Long-Acting Potential of Suprachoroidally Delivered BCX4161, a Selective Plasma Kallikrein Inhibitor, for Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2021, 62, 2194. [Google Scholar]
- Ali, B.M.; Azmeh, A.M.; Alhalabi, N.M. Suprachoroidal Triamcinolone Acetonide for the Treatment of Macular Edema Associated with Retinal Vein Occlusion: A Pilot Study. BMC Ophthalmol. 2023, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Muslim, I.; Chaudhry, N.; Javed, R.M.M. Effect of Supra-Choroidal Triamcinolone Injection on Best-Corrected Visual Acuity and Central Retinal Thickness in Patients with Macular Edema Secondary to Retinal Vein Occlusion. Pak. J. Ophthalmol. 2022, 38, 45–50. [Google Scholar] [CrossRef]
- Clearside Biomedical. Extension Study to Evaluate the Long-Term Outcomes of Subjects Following CLS-AX Administration for Age-Related Macular Degeneration in the CLS-AX CLS1002-101 Study. Available online: https://clinicaltrials.gov/study/NCT05131646 (accessed on 31 December 2023).
- Clearside Biomedical. Study to Evaluate Suprachoroidally Administered CLS-AX in the Treatment of Neovascular Age-Related Macular Degeneration (ODYSSEY). Available online: https://clinicaltrials.gov/study/NCT05891548 (accessed on 31 December 2023).
- Shen, J.; Lima e Silva, R.; Zhang, M.; Luly, K.M.; Hackett, S.F.; Tzeng, S.Y.; Lowmaster, S.M.; Shannon, S.R.; Wilson, D.R.; Green, J.J. Suprachoroidal Gene Transfer with Nonviral Nanoparticles in Large Animal Eyes. Sci. Adv. 2024, 10, eadl3576. [Google Scholar] [CrossRef] [PubMed]
- Aura Biosciences. A Phase 3 Randomized, Masked, Controlled Trial to Evaluate Efficacy and Safety of Belzupacap Sarotalocan (AU-011) Treatment Compared to Sham Control in Subjects with Primary Indeterminate Lesions or Small Choroidal Melanoma (CoMpass). Available online: https://clinicaltrials.gov/study/NCT06007690 (accessed on 31 December 2023).
- Aura Biosciences Presents Final Phase 1b/2 Data for Its First Virus-Like Drug Conjugate, AU-011, in Patients with Choroidal Melanoma at the American Academy of Ophthalmology 2021 Annual Meeting—Aura Biosciences. Available online: https://ir.aurabiosciences.com/news-releases/news-release-details/aura-biosciences-presents-final-phase-1b2-data-its-first-virus/ (accessed on 27 May 2024).
- Aura Biosciences. Study in Subjects with Small Primary Choroidal Melanoma. Available online: https://clinicaltrials.gov/study/NCT03052127 (accessed on 31 December 2023).
- Aura Biosciences. A Prospective Group-Matched Study of Visual Outcomes in Subjects Treated with Belzupacap Sarotalocan (AU-011) or Plaque Radiotherapy for Primary Indeterminate Lesions or Choroidal Melanoma (IL/CM). Available online: https://clinicaltrials.gov (accessed on 17 March 2024).
- Massa, H.; Pipis, S.Y.; Adewoyin, T.; Vergados, A.; Patra, S.; Panos, G.D. Macular Edema Associated with Non-Infectious Uveitis: Pathophysiology, Etiology, Prevalence, Impact and Management Challenges. Clin. Ophthalmol. 2019, 13, 1761–1777. [Google Scholar] [CrossRef]
- Suhler, E.B.; Lloyd, M.J.; Choi, D.; Rosenbaum, J.T.; Austin, D.F. Incidence and Prevalence of Uveitis in Veterans Affairs Medical Centers of the Pacific Northwest. Am. J. Ophthalmol. 2008, 146, 890–896.e8. [Google Scholar] [CrossRef]
- Koronis, S.; Stavrakas, P.; Balidis, M.; Kozeis, N.; Tranos, P.G. Update in Treatment of Uveitic Macular Edema. Drug Des. Devel. Ther. 2019, 13, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.; Khurana, R.N.; Shah, M.; Henry, C.R.; Wang, R.C.; Kissner, J.M.; Ciulla, T.A.; Noronha, G. PEACHTREE Study Investigators Efficacy and Safety of Suprachoroidal CLS-TA for Macular Edema Secondary to Noninfectious Uveitis: Phase 3 Randomized Trial. Ophthalmology 2020, 127, 948–955. [Google Scholar] [CrossRef]
- Henry, C.R.; Kapik, B.; Ciulla, T.A. Suprachoroidal Triamcinolone Acetonide Injectable Suspension for Macular Edema Associated with Uveitis: Visual and Anatomic Outcomes by Age. Investig. Ophthalmol. Vis. Sci. 2022, 63, 3206–A0432. [Google Scholar]
- Khurana, R.N.; Merrill, P.; Yeh, S.; Suhler, E.; Barakat, M.R.; Uchiyama, E.; Henry, C.R.; Shah, M.; Wang, R.C.; Kapik, B.; et al. Extension Study of the Safety and Efficacy of CLS-TA for Treatment of Macular Oedema Associated with Non-Infectious Uveitis (MAGNOLIA). Br. J. Ophthalmol. 2022, 106, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.R.; Shah, M.; Barakat, M.R.; Dayani, P.; Wang, R.C.; Khurana, R.N.; Rifkin, L.; Yeh, S.; Hall, C.; Ciulla, T. Suprachoroidal CLS-TA for Non-Infectious Uveitis: An Open-Label, Safety Trial (AZALEA). Br. J. Ophthalmol. 2022, 106, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Thorne, J.E.; Sugar, E.A.; Holbrook, J.T.; Burke, A.E.; Altaweel, M.M.; Vitale, A.T.; Acharya, N.R.; Kempen, J.H.; Jabs, D.A.; Multicenter Uveitis Steroid Treatment Trial Research Group Periocular Triamcinolone vs. Intravitreal Triamcinolone vs. Intravitreal Dexamethasone Implant for the Treatment of Uveitic Macular Edema: The PeriOcular vs. INTravitreal Corticosteroids for Uveitic Macular Edema (POINT) Trial. Ophthalmology 2019, 126, 283–295. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Ophthalmology. Diabetic Macular Edema: Diagnosis and Management. Available online: https://www.aao.org/eyenet/article/diabetic-macular-edema-diagnosis-and-management (accessed on 28 May 2024).
- Maturi, R.K.; Glassman, A.R.; Liu, D.; Beck, R.W.; Bhavsar, A.R.; Bressler, N.M.; Jampol, L.M.; Melia, M.; Punjabi, O.S.; Salehi-Had, H.; et al. Effect of Adding Dexamethasone to Continued Ranibizumab Treatment in Patients with Persistent Diabetic Macular Edema: A DRCR Network Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 29–38. [Google Scholar] [CrossRef]
- Bressler, S.B.; Odia, I.; Glassman, A.R.; Danis, R.P.; Grover, S.; Hampton, G.R.; Jampol, L.M.; Maguire, M.G.; Melia, M. Changes In Diabetic Retinopathy Severity When Treating Diabetic Macular Edema with Ranibizumab: DRCR.Net Protocol I 5-Year Report. Retina 2018, 38, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Khurana, R.N.; Lampen, S.I.R.; Noronha, G.; Brown, D.M.; Ou, W.C.; Sadda, S.R. HULK Study Group Suprachoroidal Triamcinolone Acetonide for Diabetic Macular Edema: The HULK Trial. Ophthalmol. Retin. 2018, 2, 874–877. [Google Scholar] [CrossRef]
- Barakat, M.R.; Wykoff, C.C.; Gonzalez, V.; Hu, A.; Marcus, D.; Zavaleta, E.; Ciulla, T.A. Suprachoroidal CLS-TA plus Intravitreal Aflibercept for Diabetic Macular Edema: A Randomized, Double-Masked, Parallel-Design, Controlled Study. Ophthalmol. Retin. 2021, 5, 60–70. [Google Scholar] [CrossRef]
- Anwar, F.; Khan, A.A.; Majhu, T.M.; Javaid, R.M.M.; Ghaffar, M.T.; Bokhari, M.H. Comparison of Suprachoroidal Injection of Triamcinolone Acetonide Versus Intravitreal Bevacizumab in Primary Diabetic Macular Odema. Pak. J. Med. Health Sci. 2022, 16, 304. [Google Scholar] [CrossRef]
- Oxular Limited. Oxular Doses First Patients in OXEYE Phase 2 Clinical Trial of Suprachoroidal OXU-001 as a Long-Acting Treatment for Diabetic Macular Edema. Available online: https://www.biospace.com/article/oxular-doses-first-patients-in-oxeye-phase-2-clinical-trial-of-suprachoroidal-oxu-001-as-a-long-acting-treatment-for-diabetic-macular-edema/ (accessed on 30 May 2024).
- Campochiaro, P.A.; Brown, D.M.; Pearson, A.; Ciulla, T.; Boyer, D.; Holz, F.G.; Tolentino, M.; Gupta, A.; Duarte, L.; Madreperla, S.; et al. Long-Term Benefit of Sustained-Delivery Fluocinolone Acetonide Vitreous Inserts for Diabetic Macular Edema. Ophthalmology 2011, 118, 626–635.e2. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.A.; Sheth, V.; Mansour, S.E.; Coughlin, B.; Gonzalez, V.H. Three-Year Safety and Efficacy of the 0.19-Mg Fluocinolone Acetonide Intravitreal Implant for Diabetic Macular Edema: The PALADIN Study. Ophthalmology 2022, 129, 605–613. [Google Scholar] [CrossRef]
- Figueira, J.; Henriques, J.; Amaro, M.; Rosas, V.; Alves, D.; Cunha-Vaz, J. A Nonrandomized, Open-Label, Multicenter, Phase 4 Pilot Study on the Effect and Safety of ILUVIEN® in Chronic Diabetic Macular Edema Patients Considered Insufficiently Responsive to Available Therapies (RESPOND). Ophthalmic. Res. 2017, 57, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Bhatwadekar, A.D.; Kansara, V.; Luo, Q.; Ciulla, T. Anti-Integrin Therapy for Retinovascular Diseases. Expert Opin. Investig. Drugs 2020, 29, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Shearer, T.R.; Azuma, M. Increased Expression of Integrin α Chains and Their Ligands during Laser-Induced Neovascularization of Rat Choroid. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3010. [Google Scholar]
- Clermont, A.; Chilcote, T.J.; Kita, T.; Liu, J.; Riva, P.; Sinha, S.; Feener, E.P. Plasma Kallikrein Mediates Retinal Vascular Dysfunction and Induces Retinal Thickening in Diabetic Rats. Diabetes 2011, 60, 1590–1598. [Google Scholar] [CrossRef]
- Rhoades, W.; Dickson, D.; Nguyen, Q.D.; Do, D.V. Management of Macular Edema Due to Central Retinal Vein Occlusion—The Role of Aflibercept. Taiwan J. Ophthalmol. 2017, 7, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Wykoff, C.C.; Brown, D.M.; Boyer, D.S.; Barakat, M.; Taraborelli, D.; Noronha, G. Tanzanite Study Group Suprachoroidal Triamcinolone Acetonide for Retinal Vein Occlusion: Results of the Tanzanite Study. Ophthalmol. Retin. 2018, 2, 320–328. [Google Scholar] [CrossRef]
- Clearside Biomedical. Suprachoroidal Injection of Triamcinolone Acetonide with IVT Anti-VEGF in Subjects with Macular Edema Following RVO (TOPAZ). Available online: https://clinicaltrials.gov/study/NCT03203447 (accessed on 31 December 2023).
- Nawar, A.E. Modified Microneedle for Suprachoroidal Injection of Triamcinolone Acetonide Combined with Intravitreal Injection of Ranibizumab in Branch Retinal Vein Occlusion Patients. Clin. Ophthalmol. 2022, 16, 1139–1151. [Google Scholar] [CrossRef]
- Kawasaki, R.; Yasuda, M.; Song, S.J.; Chen, S.-J.; Jonas, J.B.; Wang, J.J.; Mitchell, P.; Wong, T.Y. The Prevalence of Age-Related Macular Degeneration in Asians: A Systematic Review and Meta-Analysis. Ophthalmology 2010, 117, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Bakri, S.J.; Bektas, M.; Sharp, D.; Luo, R.; Sarda, S.P.; Khan, S. Geographic Atrophy: Mechanism of Disease, Pathophysiology, and Role of the Complement System. J. Manag. Care Spec. Pharm. 2023, 29, S2–S11. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Chong, V.; Loewenstein, A.; Larsen, M.; Souied, E.; Schlingemann, R.; Eldem, B.; Monés, J.; Richard, G.; Bandello, F. Guidelines for the Management of Neovascular Age-Related Macular Degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 2014, 98, 1144–1167. [Google Scholar] [CrossRef]
- Wu, K.Y.; Fujioka, J.K.; Gholamian, T.; Zaharia, M.; Tran, S.D. Suprachoroidal Injection: A Novel Approach for Targeted Drug Delivery. Pharmaceuticals 2023, 16, 1241. [Google Scholar] [CrossRef] [PubMed]
- Muya, L.; Kansara, V.; Cavet, M.E.; Ciulla, T. Suprachoroidal Injection of Triamcinolone Acetonide Suspension: Ocular Pharmacokinetics and Distribution in Rabbits Demonstrates High and Durable Levels in the Chorioretina. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2022, 38, 459–467. [Google Scholar]
- Clearside Biomedical. Clearside Biomedical Announces Plans for ODYSSEY Phase 2b Clinical Trial of CLS-AX (Axitinib Injectable Suspension) in Wet AMD|Clearside Biomedical, Inc.—IR Site. Available online: https://ir.clearsidebio.com/news-releases/news-release-details/clearside-biomedical-announces-plans-odyssey-phase-2b-clinical (accessed on 28 May 2024).
- Clearside Biomedical. Safety and Tolerability Study of Suprachoroidal Injection of CLS-AX Following Anti-VEGF Therapy in Neovascular AMD (OASIS). Available online: https://clinicaltrials.gov/study/NCT04626128 (accessed on 31 December 2023).
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.-H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal Melanoma. Nat. Rev. Dis. Primers 2020, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Branisteanu, D.C.; Bogdanici, C.M.; Branisteanu, D.E.; Maranduca, M.A.; Zemba, M.; Balta, F.; Branisteanu, C.I.; Moraru, A.D. Uveal Melanoma Diagnosis and Current Treatment Options (Review). Exp. Ther. Med. 2021, 22, 1428. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.A.; Salama, A.K. New NCCN Guidelines for Uveal Melanoma and Treatment of Recurrent or Progressive Distant Metastatic Melanoma. J. Natl. Compr. Cancer Netw. 2018, 16, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Sayan, M.; Mamidanna, S.; Oncel, D.; Jan, I.; Vergalasova, I.; Weiner, J.; Ohri, N.; Acikalin, B.; Chundury, A. Clinical Management of Uveal Melanoma: A Comprehensive Review with a Treatment Algorithm. Radiat. Oncol. J. 2020, 38, 162–169. [Google Scholar] [CrossRef]
- Savinainen, A.; Grossniklaus, H.; Kang, S.; Rasmussen, C.; Bentley, E.; Krakova, Y.; Struble, C.B.; Rich, C. Ocular Distribution and Efficacy after Suprachoroidal Injection of AU-011 for Treatment of Ocular Melanoma. Investig. Ophthalmol. Vis. Sci. 2020, 61, 3615. [Google Scholar]
- Kang, S.J.; Patel, S.R.; Berezovsky, D.E.; Zhang, Q.; Yang, H.; Grossniklaus, H.E. Suprachoroidal Injection of Microspheres with Microcatheter in a Rabbit Model of Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1459. [Google Scholar]
- Demirci, H.; Narvekar, A.; Murray, C.; Rich, C. A Phase II Trial of AU-011, an Investigational, Virus-like Drug Conjugate (VDC) for the Treatment of Primary Indeterminate Lesions and Small Choroidal Melanoma (IL/CM) Using Suprachoroidal Administration. Ann. Oncol. 2022, 33, S924. [Google Scholar] [CrossRef]
- Demirci, H.; Narvekar, A.; Murray, C.C.; Rich, C.C. A Phase 2 Trial of Belzupacap Sarotalocan (AU-011), An Investigational, Virus-like Drug Conjugate (VDC) for the Treatment of Primary Indeterminate Lesions and Small Choroidal Melanoma (IL/CM) Using Suprachoroidal Administration—(NCT04417530); Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Ghate, D.; Edelhauser, H.F. Barriers to Glaucoma Drug Delivery. J. Glaucoma 2008, 17, 147–156. [Google Scholar] [CrossRef]
- Pederson, J.E.; Gaasterland, D.E.; MacLellan, H.M. Experimental Ciliochoroidal Detachment. Effect on Intraocular Pressure and Aqueous Humor Flow. Arch. Ophthalmol. 1960 1979, 97, 536–541. [Google Scholar] [CrossRef]
- Hao, H.; He, B.; Yu, B.; Yang, J.; Xing, X.; Liu, W. Suprachoroidal Injection of Polyzwitterion Hydrogel for Treating Glaucoma. Biomater. Adv. 2022, 142, 213162. [Google Scholar] [CrossRef]
- Chae, J.J.; Jung, J.H.; Zhu, W.; Gerberich, B.G.; Bahrani Fard, M.R.; Grossniklaus, H.E.; Ethier, C.R.; Prausnitz, M.R. Drug-Free, Nonsurgical Reduction of Intraocular Pressure for Four Months after Suprachoroidal Injection of Hyaluronic Acid Hydrogel. Adv. Sci. 2021, 8, 2001908. [Google Scholar] [CrossRef]
- Chiang, B.; Kim, Y.C.; Doty, A.C.; Grossniklaus, H.E.; Schwendeman, S.P.; Prausnitz, M.R. Sustained Reduction of Intraocular Pressure by Supraciliary Delivery of Brimonidine-Loaded Poly(Lactic Acid) Microspheres for the Treatment of Glaucoma. J. Control. Release 2016, 228, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Priyan, V. Arctic Vision Starts Dosing with ARVN001 in Macular Oedema Trial. Clin. Trials Arena 2021. [Google Scholar]
- Arctic Vision Arctic Vision Announces First Patient Dosed in Phase 1 Clinical Trial of ARVN001 for the Treatment of Diabetic Macular Edema (DME) in China. Available online: https://www.arcticvision.com/newsdetail/id/57.html (accessed on 7 July 2024).
- Barakat, M.; Wan, C.; Kapik, B. Post Hoc Analysis of Clinical Suprachoroidal Injection Experience across Indications. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4954. [Google Scholar]



| Administration Method | Advantages | Disadvantages |
|---|---|---|
| Intravitreal Injection [36] | Office-based, outpatient procedure | Requires frequent injections |
| High bioavailability (Bypasses corneal and blood–retinal barriers) | Limited drug penetration to the retina and choroid | |
| Widely used and well-established procedure | Potential for complications such as endophthalmitis, increased intraocular pressure, and cataracts | |
| Subretinal Injection [37] | Direct delivery to target retinal cells, ensuring high drug concentration | Limited distribution of the injectate |
| Reduced immunogenicity | Higher risk of complications such as retinal detachment and infection. | |
| Invasive surgical procedure that needs a hospital setting | ||
| Suprachoroidal Injection | Targeted delivery to the retina and choroid | Potential for injection site discomfort and transient IOP changes |
| High bioavailability (Bypasses corneal and blood–retinal barriers) | Newer and less-established procedure | |
| Office-based, outpatient procedure | Requires specialized equipment |
| Examples | Advantages | Disadvantages | |
|---|---|---|---|
| Tissue-specific tropisms | Potential immunogenic responses | |
| Viral Vectors [15,37] | Improved specificity | Limited size | |
| Increased transduction | Risk of mutagenesis | ||
| Expensive production | |||
| Non-Viral Vectors [27,62] |
| Lower risks of toxicity and immunogenicity | Low specificity |
| Larger sizes | Less stable than viral vectors | ||
| Cheaper production |
| Drug | Treatment | Key Findings | Study Design | Study |
|---|---|---|---|---|
| Macular Edema (ME) Secondary to Macular Edema (ME) | ||||
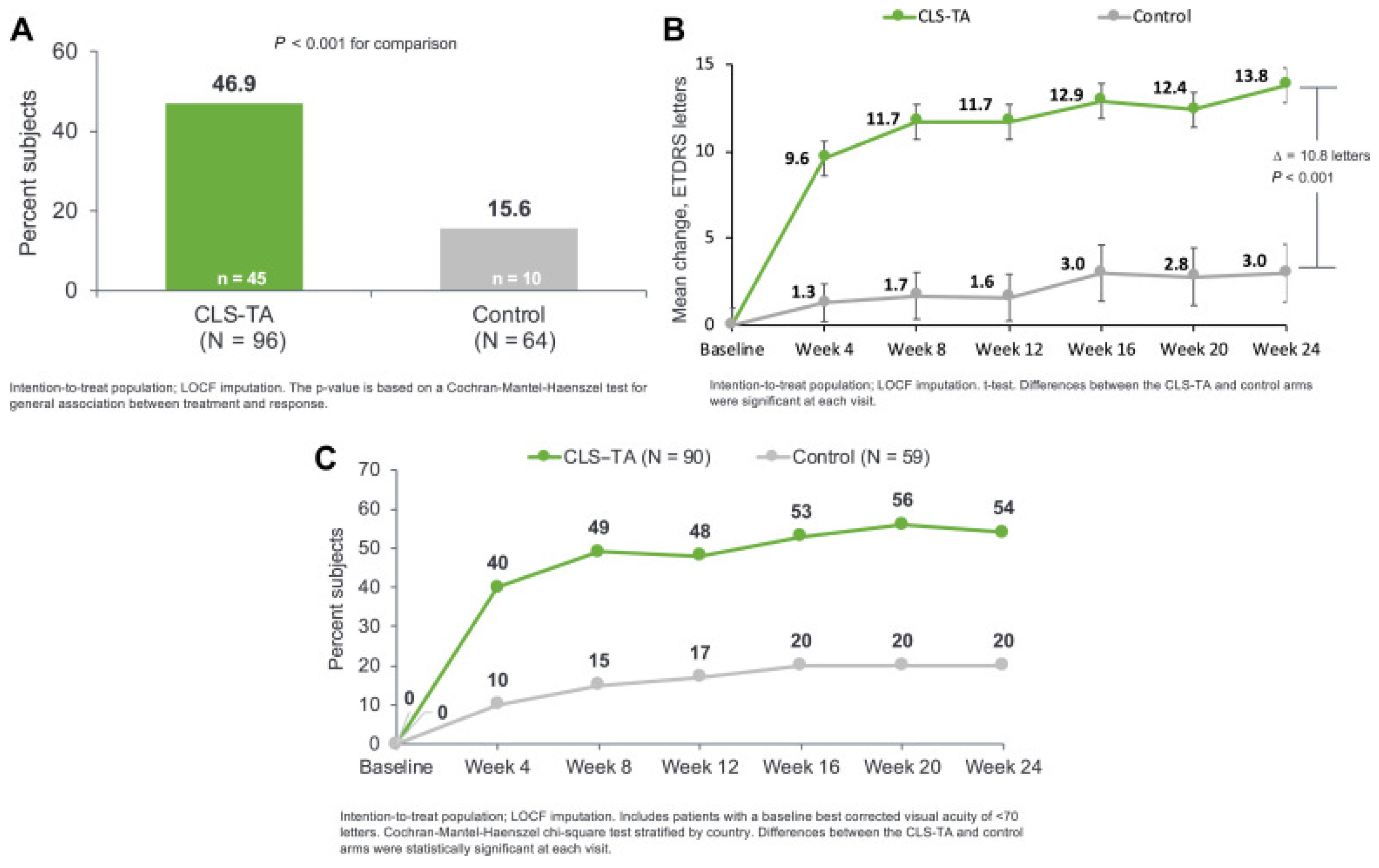
| TA | SCTA, 4 mg (0.1 mL of 40 mg/mL) at Day 0 and Week 12 vs. SC sham injection at Day 0 and Week 12 In patients with concurrent systemic corticosteroid or steroid-sparing therapy vs. no use | Patients with no steroid-sparing therapy, at Week 24: - ETDRS letter change: + 15.6 (SCTA) vs. + 4.9 (control) (p < 0.001). - CST change: -169.8 µm (SCTA) vs. - 10.3 µm (control) (p < 0.001). - Need for rescue therapy: 14.7% (SCTA) vs. 69.4% (control.) Among patients receiving steroid therapy, at Week 24: - ETDRS letter change: + 9.4 (SCTA) vs. - 3.2 (control) (p = 0.019). - CST change: -108.3 µm (SCTA) vs. - 43.5 µm (control) (p = 0.190). - Need for rescue therapy: 10.7% (SCTA) vs. 80% (control). - AE: no serious AEs in either group. | Post hoc analysis of phase III clinical trial (PEACHTREE) | Merrill, 2023 [105] |
| TA | SCTA, 4 mg (0.1 mL of 40 mg/mL) at Day 0 and Week 12 vs. SC sham injection at Day 0 and Week 12 In patients with different anatomic subtypes of uveitis | At Week 24: - BCVA: Similar gains (+ 12.1 to + 15.9 letters) across all anatomic subtypes (anterior, intermediate, posterior, and panuveitis). - CST: Change from baseline (− 120.1µm to − 189.0 µm) favored the treatment arm and achieved statistical significance for all subgroups except for Pts with anterior NIU. - Mean IOP: Similar increases (0.5 to + 3.1 mmHg) across all anatomic subtypes. - AE: Similar incidence among subtypes. | Post hoc analysis of phase III clinical trial (PEACHTREE) | Shah, 2024 [106] |
| TA | SCTA, 4 mg (0.1 mL of 40 mg/m) at Day 0 and Week 12 In patients with a CST ≥ 300 µm since 1/1/2022 | At 36 d: - BCVA: Improved from 0.57 ± 0.48 to 0.47 ± 0.22 logMAR. - CST: Mean reduction in CME by 74.9% (range 32–100%). - IOP: Increased by 2.9 ± 3.9 mmHg; 1 Pt had sustained IOP that required therapy. - AEs: Minimal IOP elevation even in steroid-responsive patients overall. - Follow-Up: 36.0 ± 26.7 d after injection; 8 non-responders, 2 partial responders, 15 complete responders. | Observational, retrospective study | Zhou, 2023 [107] |
| TA | SCT, 4 mg (0.1 mL of 40 mg/m) at Day 0 and Week 12 vs. SC sham injection at Day 0 and Week 12 Patients with CST ≥ 300 µm and BCVA of ≥ 5 and ≤ 70 ETDRS who received ≥ 1 study treatment in either PEACHTREE or AZALEA | At 24 weeks: - BCVA improvement: 47.4% SCTA Pts (n = 95) gained ≥ 15 ETDRS letters from baseline vs. 16.7% of control group Pts (n = 60; p < 0.001) - +9.6 EDTRS letter gain in the SCTA group at Week 4 and + 13.9 letters at Week 24. - CST reduction: − 163.9 µm (SCTA) vs. − 19.3 µm (control) (p < 0.001). - AE: No treatment-related serious AEs. - Incidence of elevated IOP: 12.6% (SCTA) and 15.0% (control). - Incidence of cataract formation/worsening: 7.4% (SCTA) and 6.7% (control). | Post hoc analysis of 2 phase III trials (PEACHTREE and AZALEA) | Yeh, 2023 [108] |
| TA | SCTA, 4 mg using a hollow microneedle | - Looked at NIU, DME, and RVO. - 84% of physicians did not find the SC injections more challenging than other ocular injections. - Needle Length: 71% of baseline injections used the 900 μm needle, 29% used the 1100 μm needle. - Gender: 76% of female Pts used 900 μm needles compared to 66% for male patients (p = 0.006). - Quadrant: 78% of injections in the superotemporal quadrant used 900 μm needles compared to 65% in the inferotemporal quadrant (p < 0.001). - No correlation between needle lengths and BCVA, CST, and IOP. - AE: Lower incidences of IOP elevation, exacerbation of glaucoma, and cataract development compared to intravitreal or periocular corticosteroid injections. | Retrospective analysis of 6 clinical trials: AZALEA, PEACHTREE, TYBEE, TANZANITE, SAPPHIRE, and TOPAZ. | Wan, 2020 [109] |
| Diabetic Macular Edema | ||||
| TA + bevacizumab | SCTA, (0.1 mL; 40 mg/mL) + IVTB (1.25 mg of 0.05 mL) vs. SC sham injection of TA + IVTB (1.25 mg of 0.05 mL) | - BCVA change: −0.37 ± 0.24 letters (p < 0.001) (combination) vs. −0.20 ± 0.20 (monotherapy) (p = 0.004) at 12 weeks (between-group analysis (p = 0.014)). - Combination group showed improvements from baseline at Week 4 (p = 0.046) and 24 weeks later (p < 0.001). - Mean CST changes: Monotherapy: -76 μm (95% CI 15 to 138, p = 0.015) after 4 weeks and 108 μm (95% CI 51 to 164, p < 0.001) after twelve weeks. Combination: 337 ± 107 μm (p < 0.001) after 4 weeks and 348 ± 132 μm (p < 0.001) after completing the study. - Higher CST reduction in combination group vs. monotherapy group (p = 0.019). - AEs: No serious AEs related to treatment in either group. | Clinical trial (Phase II/III) (IRCT20200314046761N1) | Fazel, 2023 [110] |
| TA + aflibercept | SCTA, 2.4 mg (2.4 mg/60 µl) via microcatheter vs. SCTA, 4 mg (4.0 mg/100 µl) via microcatheter | - Ongoing study - 24-week randomized, two-arm, single-masked study to evaluate the safety and efficacy of two doses of SCTA administered through a novel proprietary microcatheter device in patients with previously treated DME. | Clinical trial (phase II) (NCT05512962) | Oxular Limited, 2024 [111] |
| TA + aflibercept | Single SC injection of 4 mg (100 µl) of TA using a proprietary Everads injector | - Ongoing study - 6-week, open-label study aiming to evaluate the safety and performance of the Everads injector. | Pilot device study (NCT06314217) | Everads Therapy, 2024 [23] |
| Aflibercept | SC injection Aflibercept (80 µg/eye; 2 µl injection) or sterile PBS (control) vs. IVT Aflibercept (80 µg/eye; 2 µl injection) vs. sterile PBS (control) | - SC injections were feasible in a mouse CNV model. - Significant suppression of leaky CNV lesions in both delivery methods. FA Leakage area reduction: - Day 3: IVT 58.6% (p < 0.01) vs. SC 49.8% (p < 0.05) - Day 7: IVT 54.2% (p < 0.05) vs. SC 76.8% (p = 0.014) | Preclinical animal study | Cerrada-Gimenez, 2023 [112] |
| Dexamethasone | SC injection of OXU-001 (dexamethasone formulation) vs. IVT dexamethasone implant | - Ongoing study - 52-week 2-part trial. Part A: Open-label randomized, single dose 2 treatment arm comparing 2 dose levels of SC OXU-011. Part B: Randomized, masked, active comparator, single dose, 3-treatment-arm study of 2 dose levels of OXU-001 and IVT dexamethasone implant. - Primary outcome: Treatment-emergent adverse event, mean change in BCVA, CST, time until follow-up rescue treatment needed. | Clinical trial (phase II) (NCT05697809) | Oxular Limited, 2024 [113] |
| Fluocinolone Acetonide (Iluvien®) | Single SC delivery of a 0.19 mg fluocinolone acetonide implant. (releases 0.25 µg/day) | - BCVA: Significant median increase of 3 lines; from pre-operative median BCVA 0.07 to post-operative BCVA of 0.15 (p = 0.02). - Median CMT: 25% reduction (544 to 404 microns, p = 0.4). - IOP: 50% of Pts had a transient rise in IOP (< 10 mmHg) that resolved within 3 weeks with anti-glaucoma drops. - AE: 2/10 phakic Pts developed nuclear sclerosis grade I. None of the 3 Pts with existing sclerosis showed progression through the end of the follow-up period. | Retrospective interventional non-comparative case series | El Rayes, 2023 [114] |
| CLS-301 | Single bilateral SC injection of 100 µL of CLS-301 (4 mg/eye) in rabbit models at 16 weeks. (n=12 rabbits) | - Safety: Well-tolerated with no overt signs of toxicity or intraocular inflammation. - Drug distribution: Mean CLS-301 levels in the central retina were maintained 30- to 550-fold higher than the in-vitro IC50 values (0.5–5 ng/mL for blocking cell adhesion to vitronectin) for up to 4 months. - At day 112, mean drug levels in the central and peripheral retina were 1–2 orders of magnitude higher than IVT values (674 ng/gm and 955 ng/gm, respectively). - Sustained and high drug levels were observed in the RPE/choroid/scleral area and retina. - Compartmentalization: Minimal systemic and anterior segment exposure with low and sporadic CLS-301 levels were detected in the plasma, vitreous humor, and aqueous humor. | Preclinical animal study | Kansara, 2023 [115] |
| BCX- 4161 | Single bilateral SC injection of 100 µL of BCX4161 (0.5 mg/eye) in rabbit models up to 12 weeks. | - Safety: Well-tolerated with no overt signs of toxicity. - Distribution: Sustained and high exposure of BCX4161 was observed in the chorioretinal area. - The retina had 1 to 2 orders of magnitude higher mean concentration than the vitreous humor. - Low levels of BCX 4161 were observed in aqueous humour and plasma samples. - Cmax: Peripheral retina: 75 µg/gm at 24 hrs post-dose, Central retina: 13 µg/gm at 24 hrs post-dose. - Concentration at Day 84: Retinal concentration levels were 1–2 orders of magnitude higher than the in-vitro IC99 (100 nM) levels. Central retina: 30 µg/gm, Peripheral retina: 21 µg/gm. | Preclinical Animal experimental study | Muya, 2021 [116] |
| Macular Edema due to Retinal Vein Occlusion | ||||
| TA | SCTA, 4 mg (0.1 mL of 40 mg/mL) | - Mean BCVA gain: 68.7% participants had a >15 letter gain at Week 1, 62.5% at Month 1, 50% at Months 2 and 3. - >70 ETDRS letter score: 81.25% at Week 1, 75% at Months 1, 2, and 3. - Mean CST reduction: Associated with improvements in BCVA. - Mean IOP: No significant changes, with an increase ranging from 0.75 mmHg at Week 1 (p = 0.09) and 0.5 mmHg at 3 months (p = 0.72). | Clinical trial (Phase I/II) | Ali, 2023 [117] |
| TA | SCTA, 4 mg (0.1 mL of 40 mg/mL) | - Mean BCVA gain: significant improvement from baseline at 3 months (p = 0.003) - Mean central retinal thickness reduction: significantly decreased from 342.2 ± 40.2 µm to 289 ± 47.5 µm at 3 months (p = 0.002). | Single-center, case series | Muslim, 2022 [118] |
| Neovascular Age-related Macular Degeneration | ||||
| Axitinib | Subjects who received SC 0.1 mg, 0.5 mg, and 1.0 mg Axitinib in the parent study will be followed for an additional 12 weeks | - AEs: No serious AEs, no treatment emergent AEs related to study treatment, no dose limiting toxicities, and no adverse events related to inflammation, vasculitis or vascular occlusion. - 90% reduction in treatment burden from the average monthly injections before CLS-AX - Injection-free rate (Cohorts 3 and 4): 88% up to Month 5 and 75% up to Month 6. - Mean BCVA and CST remained stable at the 6-month mark | Observational, prospective cohort of a phase II clinical trial (OASIS) (NCT05131646) | Clearside Biomedical, 2024 [119] |
| Axitinib | SC injection of 1.0 mg axitinib with a flexible dosing regiment Vs. IVT injection of aflibercept Q8W | - Study Ongoing - Randomized, double-masked, parallel-group, active-controlled study - Outcomes: BVCA, CNV lesion size on fundus fluorescein angiography, number of study drug injections and supplemental therapies, serious AEs and treatment-emergent AEs. | Clinical trial (phase II) ODYSSEY (NCT05891548) | Clearside Biomedical, 2024 [120] |
| RGX-314 | Cohort 1: SC 2.5 × 1011 RGX gc/eye Vs. Cohort 2: SC 5 × 1011 RGX gc/eye Vs. Cohorts 1 and 2 control: Monthly 0.5 mg SC Ranibizumab Cohort 3: SC 5 × 1011 RGX gc/eye vs. SC 5 × 1011 RGX gc/eye in Pts with positive neutralizing antibody | - Pending full results - As of Nov 2021, RGX-314 was well tolerated in 50 patients dosed in Cohorts 1–3. - 4 serious non-treatment-related adverse events were reported in 4 patients. - For Cohorts 1 and 2, all common treatment emergent AEs were mild at 6 months through the study. - 23% (7/20) patients had mild intraocular inflammation, which all resolved with topical corticosteroids. - Stable BCVA and CRT at 6 months in RGX314-dosed patients. - 29% (4/14) of Cohort 1 and 40% (6/15) of Cohort 2 patients received no further anti-VEGF injections after RGX-314 administration at 6 months. | Clinical trial (phase II) AAVIATE (NCT04514653) | AbbVie, 2023 [69] Khanani, 2022 [70] |
| AAV-v128, AAV8 vector | Single SC injection of 100 μL of AAV8 or AAVv128 (3.5 × 1013 vg/mL) (n = 2 monkeys) Single SC injection of 100 μL of AAV8 or AAVv128 (1 × 1012 vg/mL) (n = 4 rabbits) | - CNV lesions: Absence of Grade IV lesions in the AAVv128-treated group at 35-days and 49-days. AAV8 group had 31.25% and 41.67% of Grade IV lesions at 35- and 49-days respectively. - Transduction: AAVv128 had a 4-fold increase in photoreceptors, RPE, and horizontal cells compared to AAV8 in mice. - eGFP fluorescence: Significantly increased with a smaller AAVv128 vector dose (3.5 × 1012 vg/eye) compared to AAV8 (7 × 1012 vg/eye). - Anti-VEGF: Protein levels in the aqueous humor were higher AAVv128-treated NHPs compared to AAV8-treated NHPs. | Preclinical animal study | Luo, 2024 [73] |
| Poly (β-amino ester) nanoparticles | SC injections of 50 µL (19.2 μg), 100 µL (38.4 µg), and 200 μL (76.8 µg) of pCAG-GFP-Z1 n = 25 human-sized minipig eyes | - GFP expression: Widespread in photoreceptors and RPE cells after a single SC injection. Increasing the volume of the injectate did not significantly improve the uniformity of GFP expression. Multiple injections at different locations reduced variability in GFP expression. - 2 weeks after a single SC injection, GFP expression was detected in all parts of the retina with considerable within-eye and between-eye variability. - 12 weeks after injection, GFP levels were comparable to those at two weeks. - Tolerance: No signs of retinal toxicity. Histological analysis showed normal retinal appearance with no inflammatory cells. | Preclinical animal study | Shen, 2024 [121] |
| BD311 | Single SC injection of integration deficient lentiviral vector expressing VEGFA antibody, 500 uL | - Looking at choroidal neovascularization associated diseases such as nAMD, DME, and RVO. - Recruiting participants, results pending study completion. | Clinical trial (Phase I) | Shanghai BDGene, 2022 [78] |
| Choroidal Melanoma | ||||
| AU-011 | SC high-dose belzupacap sarotalocan (bel-sar) treatment and laser vs. SC low-dose bel-sar treatment and laser vs. Sham injection and sham laser | - Currently recruiting patients as of May 2024, target enrollment = 100. - Outcomes: tumor progression, visual acuity at 52 weeks. | Clinical trial (phase III) (NCT06007690) | Aura Biosciences, 2024 [122] |
| AU-011 | Up to 2 cycles of 1 of 3 dose levels and repeat dose regimens of SC injections of AU-011 treatment with 2 laser applications | - Statistically significant reduction in the tumor growth rate (−0.483 mm/yr, p = 0.018) - Visual acuity preservation rate of 71%. - Favorable safety and tolerability profile with most AEs being transient and without clinical sequelae. - 2 patients had treatment-related serious AEs of vision loss (baseline 43/56 patients had juxta-foveal tumors) | Clinical trial (phase I/II) (NCT03052127) | Aura Biosciences, 2021 [123,124] |
| AU-011 | 3 cycles of 3 weekly SC AU-011 injections (max. dose 80 µg) and 2 laser applications vs. Plaque radiotherapy | - Terminated due to low enrollment. - This trial was based on patients who had received Belzupacap Sarotalocan in a previous clinical trial from the same industry sponsor. - The study intended to assess visual acuity at 5 years of time. | Case-Control (NCT05266430) | Aura Biosciences, 2023 [125] |
| Miscellaneous | ||||
| SC implant for Glaucoma | SC spacer implant made of photo-crosslinked polyethylene glycol (PEG) delivered via a custom-designed microneedle. | - Successful delivery and placement of PEG hydrogel implant within the SCS - Distribution: The implants of different lengths (15, 30, 37.5, and 45 mm) showed proportional increases in SCS thickness, cross-sectional area, and volume occupied. - No adverse events reported | Chiang, 2024 [22] | |
| AAV8 vector | Comparison of 3 different doses of SC RGX-314 (AAV8), one of which will be infused with topical steroid | - Investigating diabetic retinopathy - Recruitment ongoing Preliminary results (3 months): - Diabetic retinopathy severity: 33% improvement in treatment arm (≥2 improvement in diabetic retinopathy severity score) vs. 0% in in control arm. - AEs: no intraocular inflammation, commonly observed AEs were not considered treatment-related. | Clinical trial (Phase II) ALTITUDE (NCT04567550) | Abbvie, 2023 [71] |
| Microneedle device trial | 50-100 µl of sodium fluorescein (0.1%), indocyanine green (0.0074%), or 1.0 µm fluorescent (645 nm) polystyrene microspheres | - 0.2 × 0.9 mm needles with a sharp tip beveled at 12.5o was used. - A 25-gauge microvitrectomy cannula (5 mm long) was slid over the 34-gauge needle. The metal end of the microvitrectomy cannula was adjusted to expose either 700 or 900 µm of the 34-gauge needle tip exposed. - Epoxy resin connected the valved end of the cannula to the hub of the 34-gauge needle. - The SC needles were used to inject NaF, indocyanine green or fluorescent polystyrene microspheres into 40 rabbits, and 40 monkeys, and enucleated pig and human eyes. - Only 2 or 3 occasions where the SC needle needed to be slightly repositioned - No instances of inadvertent IVT injection. | Preclinical animal study | Katz, 2023 [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.Y.; Gao, A.; Giunta, M.; Tran, S.D. What’s New in Ocular Drug Delivery: Advances in Suprachoroidal Injection since 2023. Pharmaceuticals 2024, 17, 1007. https://doi.org/10.3390/ph17081007
Wu KY, Gao A, Giunta M, Tran SD. What’s New in Ocular Drug Delivery: Advances in Suprachoroidal Injection since 2023. Pharmaceuticals. 2024; 17(8):1007. https://doi.org/10.3390/ph17081007
Chicago/Turabian StyleWu, Kevin Y., Angel Gao, Michel Giunta, and Simon D. Tran. 2024. "What’s New in Ocular Drug Delivery: Advances in Suprachoroidal Injection since 2023" Pharmaceuticals 17, no. 8: 1007. https://doi.org/10.3390/ph17081007







