Electrospun Poly-ε-Caprolactone Nanofibers Incorporating Keratin Hydrolysates as Innovative Antioxidant Scaffolds
Abstract
1. Introduction
2. Results and Discussion
2.1. Thermogravimetric Analysis
2.2. Differential Scanning Calorimetry
2.3. Fourier-Transform Infrared Spectroscopy
2.4. Mechanical Properties
2.5. Scanning Electron Microscopy
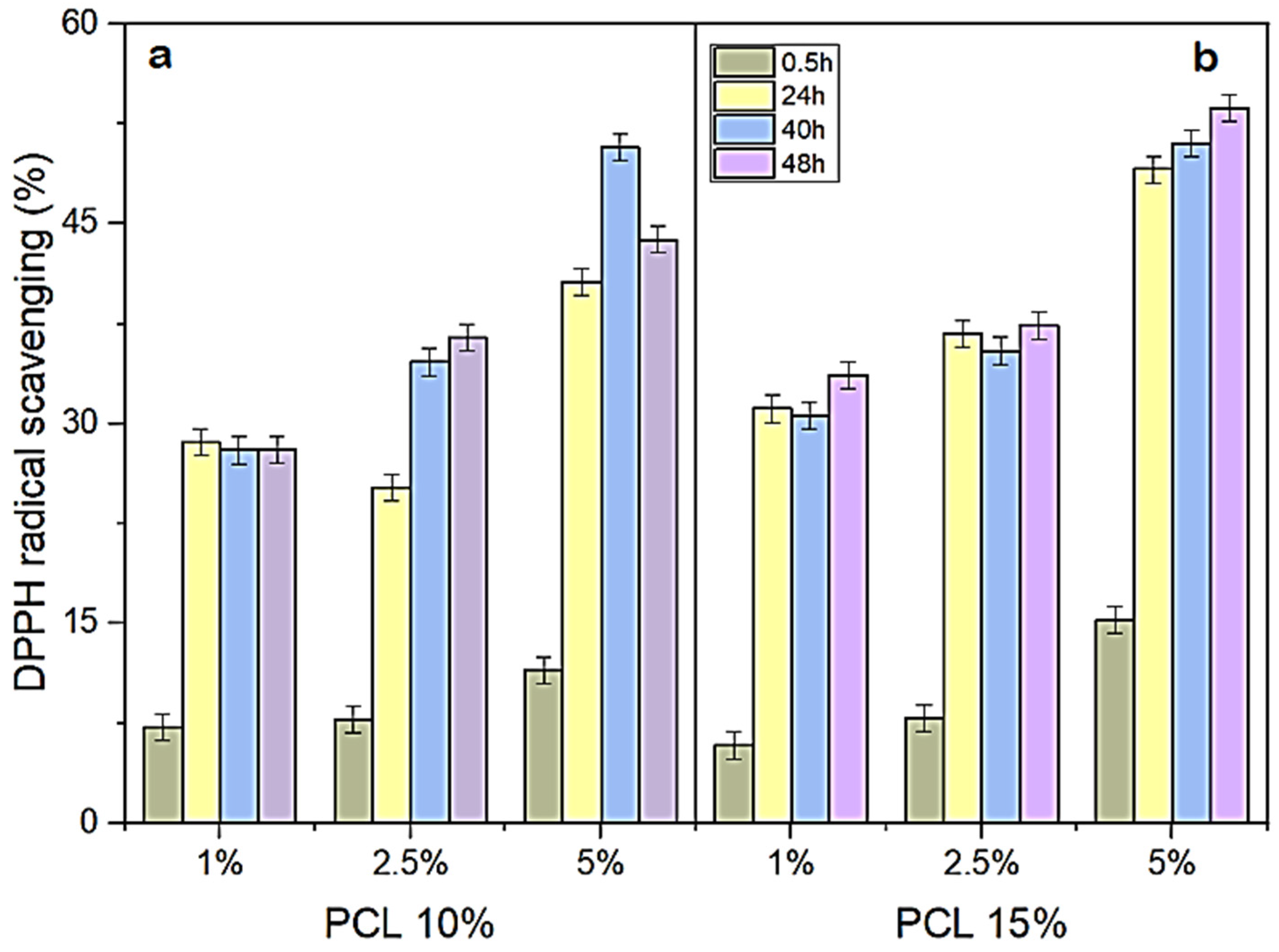
2.6. Antioxidant Activities
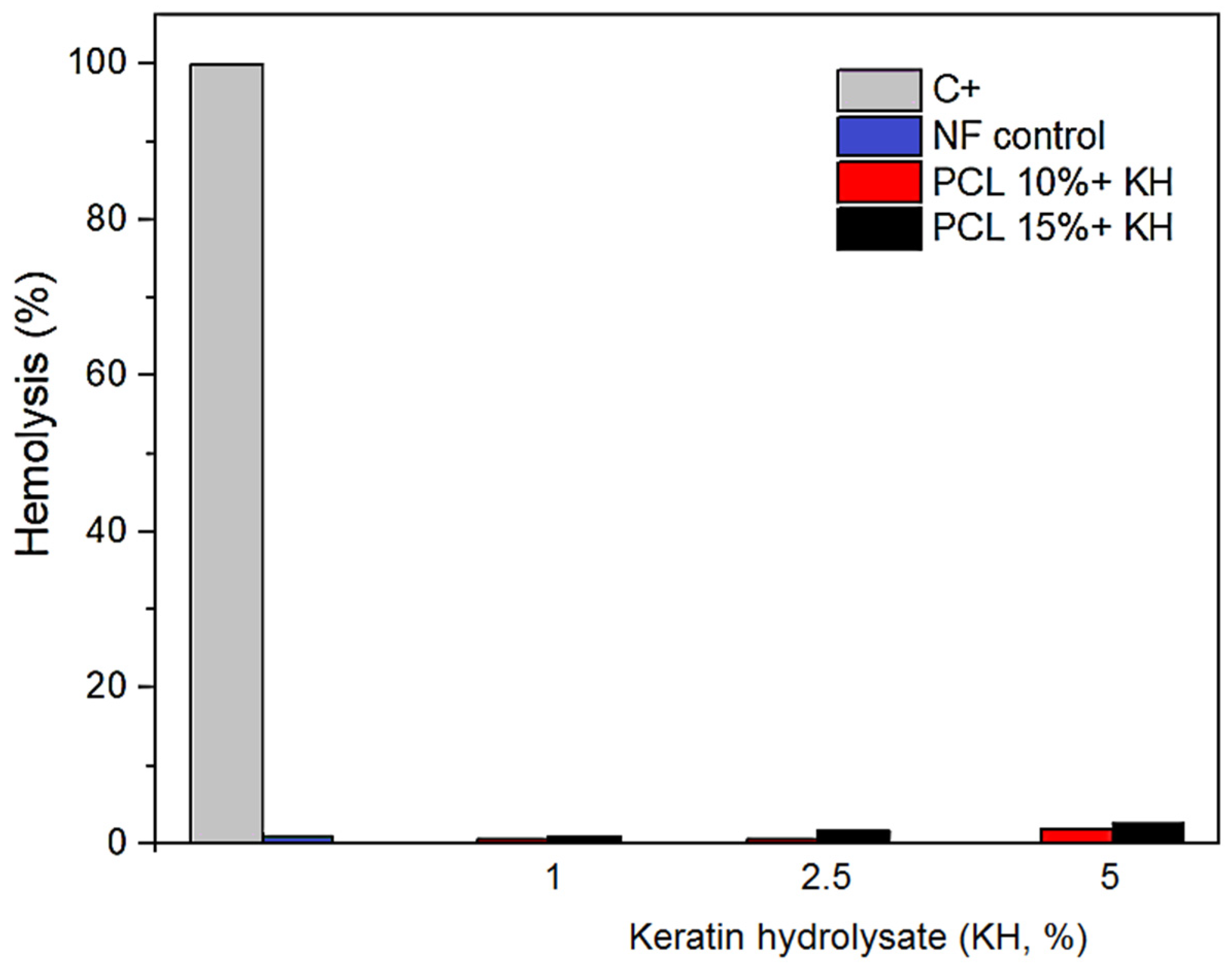
2.7. Hemolytic Activity
3. Materials and Methods
3.1. Chemicals
3.2. Microorganism
3.3. Production of Keratin Hydrolysate (KH)
3.4. Nanofiber Manufacturing
3.5. Thermogravimetric Analysis (TGA)
3.6. Differential Scanning Calorimetry (DSC)
3.7. Scanning Electron Microscopy (SEM)
3.8. Fourier-Transform Infrared Spectroscopy (FTIR)
3.9. Mechanical Analysis and Rheological Measurements
3.10. Antioxidant Activity
3.10.1. DPPH Assays
3.10.2. ABTS Assays
3.11. Hemolysis Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Datta, L.P.; Manchineella, S.; Govindaraju, T. Biomolecules-derived biomaterials. Biomaterials 2020, 230, 119633. [Google Scholar] [CrossRef]
- He, M.; Zhang, B.; Dou, Y.; Yin, G.; Cui, Y.; Chen, X. Fabrication and characterization of electrospun feather keratin/poly(vinyl alcohol) composite nanofibers. RSC Adv. 2017, 7, 9854–9861. [Google Scholar] [CrossRef]
- Callegaro, K.; Brandelli, A.; Daroit, D.J. Beyond plucking: Feathers bioprocessing into valuable protein hydrolysates. Waste Manag. 2019, 95, 399–415. [Google Scholar] [CrossRef]
- Brandelli, A.; Sala, L.; Kalil, S.J. Microbial enzymes for bioconversion of poultry waste into added-value products. Food Res. Int. 2015, 73, 3–12. [Google Scholar] [CrossRef]
- Lemes, A.C.; Álvares, G.T.; Egea, M.B.; Brandelli, A.; Kalil, S.J. Simultaneous production of proteases and antioxidant compounds from agro-industrial by-products. Bioresour. Technol. 2016, 222, 210–216. [Google Scholar] [CrossRef]
- Tasaki, K. A novel thermal hydrolysis process for extraction of keratin from hog hair for commercial applications. Waste Manag. 2020, 104, 33–41. [Google Scholar] [CrossRef]
- Su, S.; Bedir, T.; Kalkandelen, C.; Başar, A.O.; Şaşmazel, H.T.; Ustundag, C.B.; Sengor, M.; Gunduz, O. Coaxial and emulsion electrospinning of extracted hyaluronic acid and keratin based nanofibers for wound healing applications. Eur. Polym. J. 2021, 142, 110158. [Google Scholar] [CrossRef]
- Wan, X.; Liu, P.; Jin, X.; Xin, X.; Li, P.; Yuan, J.; Shen, J. Electrospun PCL/keratin/AuNPs mats with the catalytic generation of nitric oxide for potential of vascular tissue engineering. J. Biomed. Mater. Res. A 2018, 106, 3239–3247. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Arab-Bafrani, Z.; Karimi, F.; Javid, N. Designing hybrid nanofibers based on keratin-poly (vinyl alcohol) and poly (Ɛ-caprolactone) for application as wound dressing. J. Ind. Text. 2022, 51, 1729S–1949S. [Google Scholar] [CrossRef]
- Baker, S.R.; Banerjee, S.; Bonin, K.; Guthold, M. Determining the mechanical properties of electrospun poly-ε-caprolactone (PCL) nanofibers using AFM and a novel fiber anchoring technique. Mater. Sci. Eng C 2016, 59, 203–212. [Google Scholar] [CrossRef]
- Islam, M.T.; Laing, R.M.; Wilson, C.A.; McConnell, M.; Ali, M.A. Fabrication and characterization of 3-dimensional electrospun poly(vinyl alcohol)/keratin/chitosan nanofibrous scaffold. Carbohydr. Polym. 2022, 275, 118682. [Google Scholar] [CrossRef]
- Cruz-Maya, I.; Guarino, V.; Almaguer-Flores, A.; Alvarez-Perez, M.A.; Varesano, A.; Vineis, C. Highly polydisperse keratin rich nanofibers: Scaffold design and in vitro characterization. J. Biomed. Mater. Res. A 2019, 107, 1803–1813. [Google Scholar] [CrossRef]
- Schifino, G.; Gasparini, C.; Drudi, S.; Giannelli, M.; Sotgiu, G.; Posati, T.; Zamboni, R.; Treossi, E.; Maccaferri, E.; Giorgini, L.; et al. Keratin/polylactic acid/graphene oxide composite nanofibers for drug delivery. Int. J. Pharm. 2022, 623, 121888. [Google Scholar] [CrossRef]
- Fortunato, G.M.; Da Ros, F.; Bisconti, S.; De Acutis, A.; Biagini, F.; Lapomarda, A.; Magliaro, C.; De Maria, C.; Montemurro, F.; Bizzotto, D.; et al. Electrospun structures made of a hydrolyzed keratin-based biomaterial for development of in vitro tissue models. Front. Bioeng. Biotechnol. 2019, 7, 174. [Google Scholar] [CrossRef]
- Zhang, S.; Campagne, C.; Salaün, F. Influence of solvent selection in the electrospraying process of polycaprolactone. Appl. Sci. 2019, 9, 402. [Google Scholar] [CrossRef]
- Ghazalian, M.; Afshar, S.; Rostami, A.; Rashedi, S.; Bahrami, S.H. Fabrication and characterization of chitosan-polycaprolactone core-shell nanofibers containing tetracycline hydrochloride. Colloids Surf. A 2022, 636, 128163. [Google Scholar] [CrossRef]
- Souza, S.O.L.; Cotrim, M.A.P.; Oréfice, R.L.; Carvalho, S.G.; Dutra, J.A.P.; de Paula Careta, F.; Resende, J.A.; Villanova, J.C.O. Electrospun poly(ε-caprolactone) matrices containing silver sulfadiazine complexed with β-cyclodextrin as a new pharmaceutical dosage form to wound healing: Preliminary physicochemical and biological evaluation. J. Mater. Sci. Mater. Med. 2018, 29, 67. [Google Scholar] [CrossRef]
- Goksen, G.; Demir, D.; Echegaray, N.; Bangar, S.P.; Cruz, A.G.; Shao, P.; Lin, Y.; Lorenzo, J.M. New insights of active and smart natural-based electrospun mats for food safety in meat and meat products. Food Biosci. 2024, 59, 104159. [Google Scholar] [CrossRef]
- Yavari Maroufi, L.; PourvatanDoust, S.; Naeijian, F.; Ghorbani, M. Fabrication of electrospun polycaprolactone/casein nanofibers containing green tea essential oils: Applicable for active food packaging. Food Bioprocess. Technol. 2022, 15, 2601–2615. [Google Scholar] [CrossRef]
- Irani, M.; Abadi, P.G.; Ahmadian-Attari, M.M.; Rezaee, A.; Kordbacheh, H.; Goleij, P. In vitro and in vivo studies of Dragon’s blood plant (D. cinnabari)-loaded electrospun chitosan/PCL nanofibers: Cytotoxicity, antibacterial, and wound healing activities. Int. J. Biol. Macromol. 2024, 257, 128634. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, S.; Seyedabadi, S.; Alemzadeh, E. Anticancer efficiency of doxorubicin and berberine-loaded PCL nanofibers in preventing local breast cancer recurrence. J. Drug Deliv. Sci. Technol. 2022, 67, 102984. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Solouk, A.; Saber-Samandari, S.; Akbari, S.; Ghanbari, H.; Brycki, B.E. Capsaicin-loaded alginate nanoparticles embedded polycaprolactone-chitosan nanofibers as a controlled drug delivery nanoplatform for anticancer activity. J. Colloid Interface Sci. 2023, 638, 616–628. [Google Scholar] [CrossRef]
- Huang, K.; Si, Y.; Guo, C.; Hu, J. Recent advances of electrospun strategies in topical products encompassing skincare and dermatological treatments. Adv. Colloid Interface Sci. 2024, 103236. [Google Scholar] [CrossRef] [PubMed]
- Muratoglu, S.; Inal, M.; Akdag, Y.; Gulsun, T.; Sahin, S. Electrospun nanofiber drug delivery systems and recent applications: An overview. J. Drug Deliv. Sci. Technol. 2024, 92, 105342. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, Y.; Dong, Y.; Li, X.; Hao, L.; Sun, L.; Zhou, L.; Jiang, R.; Liu, W. Intelligent electrospinning nanofibrous membranes for monitoring and promotion of the wound healing. Mater. Today Bio 2024, 26, 101093. [Google Scholar] [CrossRef]
- Pedro, A.C.; Paniz, O.G.; Fernandes, I.d.A.A.; Bortolini, D.G.; Rubio, F.T.V.; Haminiuk, C.W.I.; Maciel, G.M.; Magalhães, W.L.E. The importance of antioxidant biomaterials in human health and technological innovation: A review. Antioxidants 2022, 11, 1644. [Google Scholar] [CrossRef]
- Shahrousvand, M.; Haddadi-Asl, V.; Shahrousvand, M. Step-by-step design of poly (ε-caprolactone)/chitosan/Melilotus officinalis extract electrospun nanofibers for wound dressing applications. Int. J. Biol. Macromol. 2021, 180, 36–50. [Google Scholar] [CrossRef]
- Abdulhussain, R.; Adevisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun nanofibers: Exploring process parameters, polymer selection, and recent applications in pharmaceuticals and drug delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Daroit, D.J.; Corrêa, A.P.F.; Brandelli, A. Keratinolytic potential of a novel Bacillus sp. P45 isolated from the Amazon basin fish Piaractus mesopotamicus. Int. Biodeter. Biodegrad. 2009, 63, 358–363. [Google Scholar] [CrossRef]
- Liao, N.; Unnithan, A.R.; Joshi, M.K.; Tiwari, A.P.; Hong, S.T.; Park, C.-H.; Kim, C.S. Electrospun bioactive poly (ɛ-caprolactone)–cellulose acetate–dextran antibacterial composite mats for wound dressing applications. Colloids Surf. A 2015, 469, 194–201. [Google Scholar] [CrossRef]
- Giri Dev, V.R.; Hemamalini, T. Porous electrospun starch rich polycaprolactone blend nanofibers for severe hemorrhage. Int. J. Biol. Macromol. 2018, 118, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Deng, Z.-A.; Shen, C.; Yang, Z.; Cai, Z.; Wu, D.; Chen, K. Fabrication of antimicrobial PCL/EC nanofibrous films containing natamycin and trans-cinnamic acid by microfluidic blow spinning for fruit preservation. Food Chem. 2024, 442, 138436. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Sheng, D.; Jiang, L.; Shafiq, M.; Khan, A.R.; Hashim, R.; Chen, Y.; Li, B.; Xie, X.; Chen, J.; et al. Vascular endothelial growth factor-capturing aligned electrospun polycaprolactone/gelatin nanofibers promote patellar ligament regeneration. Acta Biomater. 2022, 140, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, R.; Saravanan, S.; Babu, V.P.S.; Dharmalingam, S. Fabrication and evaluation of docetaxel doped ZnO nanoparticles incorporated PCL nanofibers for its hemocompatibility, cytotoxicity and apoptotic effects against A549. Materialia 2022, 21, 101278. [Google Scholar] [CrossRef]
- Reshmi, C.R.; Suja, P.S.; Manaf, O.; Sanu, P.P.; Sujith, A. Nanochitosan enriched poly ε-caprolactone electrospun wound dressing membranes: A fine tuning of physicochemical properties, hemocompatibility and curcumin release profile. Int. J. Biol. Macromol. 2018, 108, 1261–1272. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, C.; Wang, P.; Zhang, Y.; Zhang, H. Electrospun chitosan/polycaprolactone nanofibers containing chlorogenic acid-loaded halloysite nanotube for active food packaging. Carbohydr. Polym. 2020, 247, 116711. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Jahed-khaniki, G.; Molaee-aghaee, E.; Shariatifar, N.; Sani, M.A.; Azami, M.; Rezvantalab, S.; Ramezani, S.; Ghorbani, M. Polycaprolactone/polyacrylic acid/graphene oxide composite nanofibers as a highly efficient sorbent to remove lead toxic metal from drinking water and apple juice. Sci. Rep. 2024, 14, 4372. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodríguez, L.C.; Quintanilla-Carvajal, M.X.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Jiménez-Junca, C. Preparation and characterization of an electrospun whey protein/polycaprolactone nanofiber membrane for chromium removal from water. Nanomaterials 2022, 12, 2744. [Google Scholar] [CrossRef] [PubMed]
- Darshan, T.G.; Chen, C.-H.; Kuo, C.-Y.; Shalumon, K.T.; Chien, Y.-M.; Kao, H.-H.; Chen, J.-P. Development of high resilience spiral wound suture-embedded gelatin/PCL/heparin nanofiber membrane scaffolds for tendon tissue engineering. Int. J. Biol. Macromol. 2022, 221, 314–333. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; Luo, B.; Ramakrishna, S.; Kai, D.; Loh, X.J.; Yang, I.H.; Deen, G.R.; Mo, X. Engineering PCL/lignin nanofibers as an antioxidant scaffold for the growth of neuron and Schwann cell. Colloids Surf. B 2018, 169, 356–365. [Google Scholar] [CrossRef]
- Reshmi, C.R.; Sagitha, P.; Sheeja; Sujith, A. Poly Ɛ-caprolactone/nanostarch composite nanofibrous wound dressing with antibacterial property and pH stimulus drug release. Cellulose 2022, 29, 427–443. [Google Scholar] [CrossRef]
- Zehetmeyer, G.; Meira, S.M.M.; Scheibel, J.M.; Silva, C.; Rodembusch, F.S.; Brandelli, A.; Soares, R.M.D. Biodegradable and antimicrobial films based on poly(butylene adipate-co-terephthalate) electrospun fibers. Polym. Bull. 2017, 74, 3243–3268. [Google Scholar] [CrossRef]
- Khasteband, M.; Sharifi, Y.; Akbari, A. Chrysin loaded polycaprolactone-chitosan electrospun nanofibers as potential antimicrobial wound dressing. Int. J. Biol. Macromol. 2024, 263, 130250. [Google Scholar] [CrossRef] [PubMed]
- Yaseri, R.; Fadaie, M.; Mirzaei, E.; Samadian, H.; Ebrahiminezhad, A. Surface modification of polycaprolactone nanofibers through hydrolysis and aminolysis: A comparative study on structural characteristics, mechanical properties, and cellular performance. Sci. Rep. 2023, 13, 9434. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Cui, C.; Sun, S.; Wu, S.; Chen, S.; Ma, J.; Li, C.M. Electrospun ZnO-loaded chitosan/PCL bilayer membranes with spatially designed structure for accelerated wound healing. Carbohydr. Polym. 2022, 282, 119131. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.K.; Kharaghani, D.; Sun, L.; Ullah, S.; Sarwar, M.N.; Ullah, A.; Khatri, M.; Yoshiko, Y.; Gopiraman, M.; Kim, I.S. Synthesized bioactive lignin nanoparticles/polycaprolactone nanofibers: A novel nanobiocomposite for bone tissue engineering. Biomater. Adv. 2023, 144, 213203. [Google Scholar] [CrossRef] [PubMed]
- Osanloo, M.; Noori, F.; Tavassoli, A.; Ataollahi, M.R.; Davoodi, A.; Seifalah-Zade, M.; Taghinezhad, A.; Fereydouni, N.; Goodarzi, A. Effect of PCL nanofiber mats coated with chitosan microcapsules containing cinnamon essential oil for wound healing. BMC Complement. Med. Ther. 2023, 23, 84. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Adhikari, J.; Ghosh, A.; Chowdhury, S.R.; Thomas, S.; Datta, P.; Saha, P. Electrospun chitosan/polycaprolactone-hyaluronic acid bilayered scaffold for potential wound healing applications. Int. J. Biol. Macromol. 2018, 116, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, A.; Vineis, C.; Varesano, A.; Mazzuchetti, G.; Ferrero, F.; Tonin, C. Structure and properties of keratin/PEO blend nanofibres. Eur. Polym. J. 2008, 44, 2465–2475. [Google Scholar] [CrossRef]
- Phillipson, K.; Hay, J.N.; Jenkins, M.J. Thermal analysis FTIR spectroscopy of poly(ε-caprolactone). Thermochim. Acta 2014, 595, 74–82. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Bondi, E.; Posati, T.; Sotgiu, G.; Zamboni, R.; Torreggiani, A.; Corticelli, F.; Lotti, N.; Aluigi, A. Effects of the blending ratio on the design of keratin/poly(butylene succinate) nanofibers for drug delivery applications. Biomolecules 2021, 11, 1194. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Chen, Y.-K.; Tang, K.-C.; Yang, K.-C.; Cheng, N.-C.; Yu, J. Keratin scaffolds with human adipose stem cells: Physical and biological effects toward wound healing. J. Tissue Eng. Regen. Med. 2019, 13, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.; Daraei, A.; Lee, H.; Guthold, M. The Effect of molecular weight and fiber diameter on the mechanical properties of single, electrospun PCL nanofibers. Mater. Today Commun. 2023, 35, 105773. [Google Scholar] [CrossRef]
- Parın, F.N.; Parın, U. Spirulina biomass-loaded thermoplastic polyurethane/polycaprolacton (TPU/PCL) nanofibrous mats: Fabrication, characterization, and antibacterial activity as potential wound healing. ChemistrySelect 2022, 7, e202104148. [Google Scholar] [CrossRef]
- Kamalipooya, S.; Fahimirad, S.; Abtahi, H.; Golmohammadi, M.; Satari, M.; Dadashpour, M.; Nasrabadi, D. Diabetic wound healing function of PCL/cellulose acetate nanofiber engineered with chitosan/cerium oxide nanoparticles. Int. J. Pharm. 2024, 653, 123880. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Luo, F.; Han, J.; Li, J.; Zhou, C.; Yang, C.; Wang, Z.; Yang, W.; Hu, Y. EGCG/HP-β-CD inclusion complexes integrated into PCL/chitosan oligosaccharide nanofiber membranes developed by ELS for fruit packaging. Food Hydrocoll. 2023, 144, 108992. [Google Scholar] [CrossRef]
- Li, X.; Sun, S.; Yang, A.; Li, X.; Jiang, Z.; Wu, S.; Zhou, F. Dual-crosslinked methacrylamide chitosan/poly(ε-caprolactone) nanofibers sequential releasing of tannic acid and curcumin drugs for accelerating wound healing. Int. J. Biol. Macromol. 2023, 253, 127601. [Google Scholar] [CrossRef] [PubMed]
- Ardekani-Zadeh, A.H.; Hosseini, S.F. Electrospun essential oil-doped chitosan/poly(ε-caprolactone) hybrid nanofibrous mats for antimicrobial food biopackaging exploits. Carbohydr. Polym. 2019, 223, 115108. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.R.; Granja, P.L.; Bártolo, P.J. Advances in electrospun skin substitutes. Progr. Mater. Sci. 2016, 84, 314–334. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Electrospun PCL membranes incorporated with biosynthesized silver nanoparticles as antibacterial wound dressings. Appl. Nanosci. 2016, 6, 337–344. [Google Scholar] [CrossRef]
- Abel, S.B.; Liverani, L.; Boccaccini, A.R.; Abraham, G.A. Effect of benign solvents composition on poly(ε-caprolactone) electrospun fiber properties. Mater. Lett. 2019, 245, 86–89. [Google Scholar] [CrossRef]
- Jirkovec, R.; Kalous, T.; Chvojka, J. The modification of the wetting of polycaprolactone nanofibre layers via alternating current spinning. Mater. Des. 2021, 210, 110096. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun curcumin loaded poly(ε-caprolactone)/gum tragacanth nanofibers for biomedical application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef]
- Rad, Z.P.; Mokhtari, J.; Abbasi, M. Fabrication and characterization of PCL/zein/gum Arabic electrospun nanocomposite scaffold for skin tissue engineering. Mater. Sci. Eng. C 2018, 93, 356–366. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Electrospun polycaprolactone/aloe vera_chitosan nanofibrous asymmetric membranes aimed for wound healing applications. Polymers 2017, 9, 183. [Google Scholar] [CrossRef]
- Joseph, B.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Seantier, B.; Grohens, Y. Recent advances in electrospun polycaprolactone based scaffolds for wound healing and skin bioengineering applications. Mater. Today Commun. 2019, 19, 319–335. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, L.; Zhang, Y.; Ren, X. Preparation of antibacterial biocompatible polycaprolactone/keratin nanofibrous mats by electrospinning. J. Appl. Polym. Sci. 2021, 138, 49862. [Google Scholar] [CrossRef]
- Keirouz, A.; Wang, Z.; Reddy, V.S.; Nagy, Z.K.; Vass, P.; Buzgo, M.; Ramakrishna, S.; Radacsi, N. The history of electrospinning: Past, present, and future developments. Adv. Mater. Technol. 2023, 8, 2201723. [Google Scholar] [CrossRef]
- Selvaraj, S.; Fathima, N.N. Fenugreek incorporated silk fibroin nanofibers—A potential antioxidant scaffold for enhanced wound healing. ACS Appl. Mater. Interfaces 2017, 9, 5916–5926. [Google Scholar] [CrossRef]
- Rastegar, A.; Mahmoodi, M.; Mirjalili, M.; Nasirizadeh, N. Platelet-rich fibrin-loaded PCL/chitosan core-shell fibers scaffold for enhanced osteogenic differentiation of mesenchymal stem cells. Carbohydr. Polym. 2021, 269, 118351. [Google Scholar] [CrossRef]
- Liang, R.; Zhao, J.; Li, B.; Cai, P.; Loh, X.J.; Xu, C.; Chen, P.; Kai, D.; Zheng, L. Implantable and degradable antioxidant poly(ε-caprolactone)-lignin nanofiber membrane for effective osteoarthritis treatment. Biomaterials 2020, 230, 119601. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Isaia, H.A.; Pinilla, C.M.B.; Brandelli, A. Evidence that protein corona reduces the release of antimicrobial peptides from polymeric nanocapsules in milk. Food Res. Int. 2021, 140, 110074. [Google Scholar] [CrossRef] [PubMed]
- Mosayebi, V.; Fathi, M.; Shahedi, M.; Soltanizadeh, N.; Emam-Djomeh, Z. Fast-dissolving antioxidant nanofibers based on Spirulina protein concentrate and gelatin developed using needleless electrospinning. Food Biosci. 2022, 47, 101759. [Google Scholar] [CrossRef]
- Wang, F.; Xie, C.; Tang, H.; Hao, W.; Wu, J.; Sun, Y.; Sun, J.; Liu, Y.; Jiang, L. Development, characterization and application of intelligent/active packaging of chitosan/chitin nanofibers films containing eggplant anthocyanins. Food Hydrocoll. 2023, 139, 108496. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, W.; Li, Y.; Zhang, S.; Liang, H.; Ji, C.; Lin, X. High-speed electrospinning of phycocyanin and probiotics complex nanofibrous with higher probiotic activity and antioxidation. Food Res. Int. 2023, 167, 112715. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.B.; Lim, L.-T.; Zavareze, E.d.R.; Dias, A.R.G.; Costa, J.A.V.; Morais, M.G. Antioxidant ultrafine fibers developed with microalga compounds using a free surface electrospinning. Food Hydrocoll. 2019, 93, 131–136. [Google Scholar] [CrossRef]
- Uebel, L.S.; Schmatz, D.A.; Kuntzler, S.G.; Dora, C.L.; Muccillo-Baisch, A.L.; Costa, J.A.V.; De Morais, M.G. Quercetin and curcumin in nanofibers of polycaprolactone and poly(hydroxybutyrate--co--hydroxyvalerate): Assessment of in vitro antioxidant activity. J. Appl. Polym. Sci. 2016, 133, app.43712. [Google Scholar] [CrossRef]
- Ajmal, G.; Bonde, G.V.; Mittal, P.; Khan, G.; Pandey, V.K.; Bakade, B.V.; Mishra, B. Biomimetic PCL-gelatin based nanofibers loaded with ciprofloxacin hydrochloride and quercetin: A potential antibacterial and anti-oxidant dressing material for accelerated healing of a full thickness wound. Int. J. Pharm. 2019, 567, 118480. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.-K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef]
- He, J.; Liang, Y.; Shi, M.; Guo, B. Anti-oxidant electroactive and antibacterial nanofibrous wound dressings based on poly(ε-caprolactone)/quaternized chitosan-graft-polyaniline for full-thickness skin wound healing. Chem. Eng. J. 2020, 385, 123464. [Google Scholar] [CrossRef]
- Callegaro, K.; Welter, N.; Daroit, D.J. Feathers as bioresource: Microbial conversion into bioactive protein hydrolysates. Process Biochem. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Hao, S.; Luo, T.; Zhou, T.; Yang, X.; Wang, B. Keratose/poly (vinyl alcohol) blended nanofibers: Fabrication and biocompatibility assessment. Mater. Sci. Eng. C 2017, 72, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Dorneles, M.S.; de Azevedo, E.S.; Noreña, C.P.Z. Effect of heat treatment at low moisture on the increase of resistant starch content in Araucaria angustifolia seed starch. Food Hydrocoll. 2024, 150, 109639. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Veras, F.F.; Ritter, A.C.; Roggia, I.; Pranke, P.; Pereira, C.N.; Brandelli, A. Natamycin-loaded electrospun poly(ε-caprolactone) nanofibers as an innovative platform for antifungal applications. SN Appl. Sci. 2020, 2, 1105. [Google Scholar] [CrossRef]

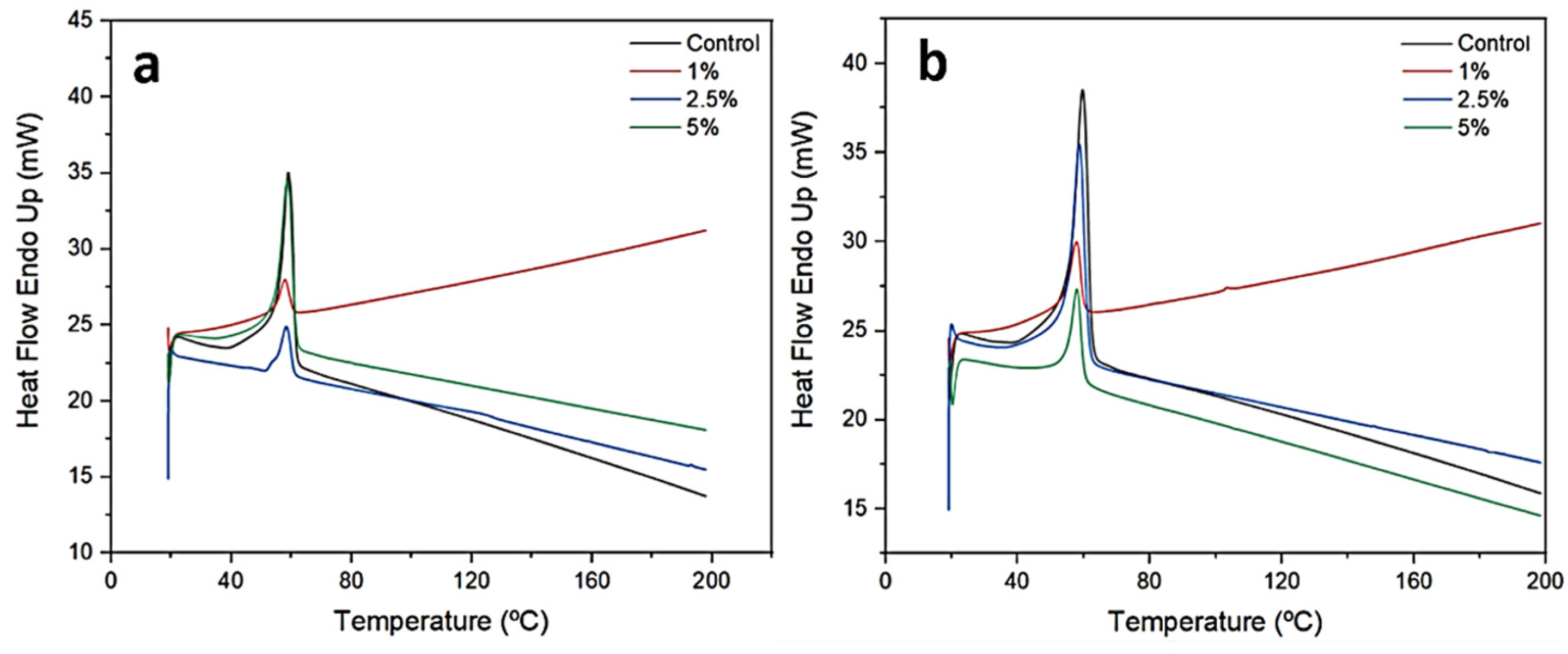
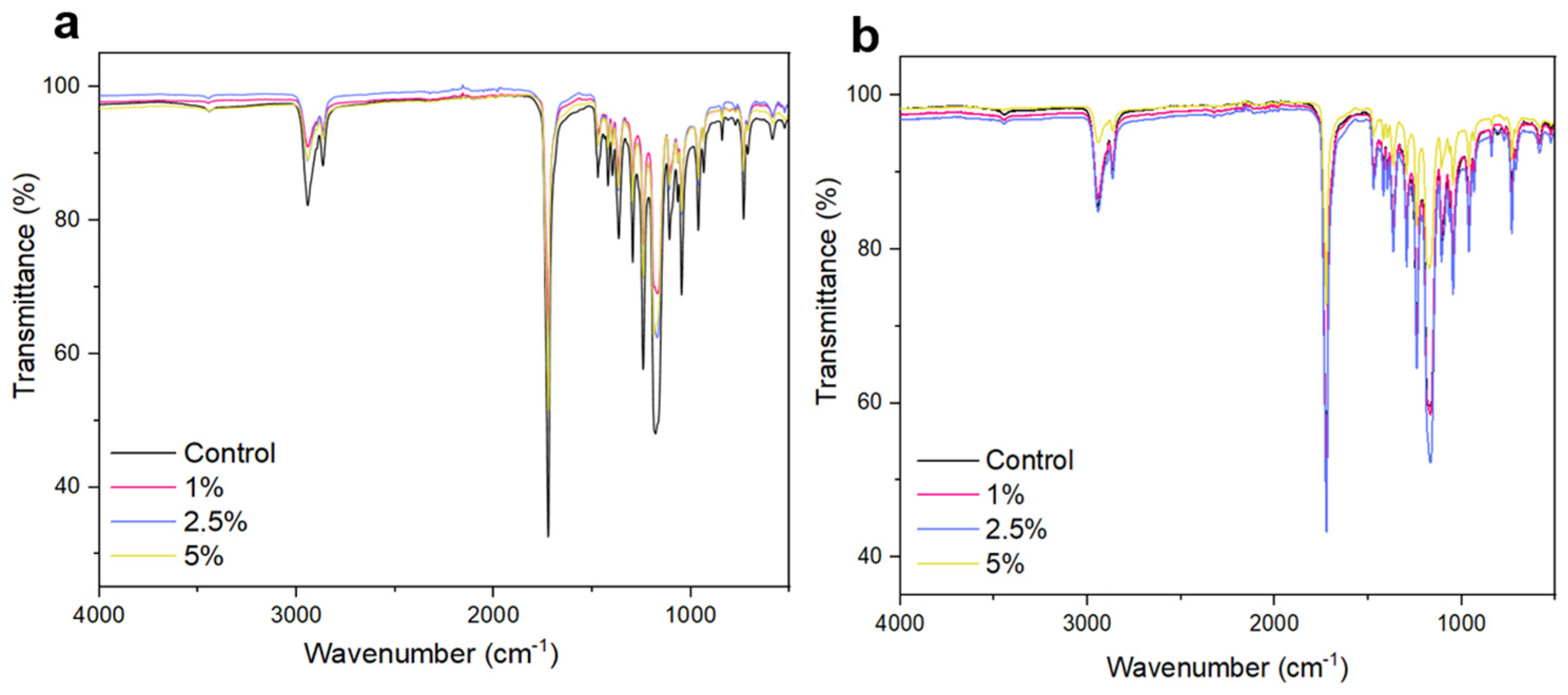
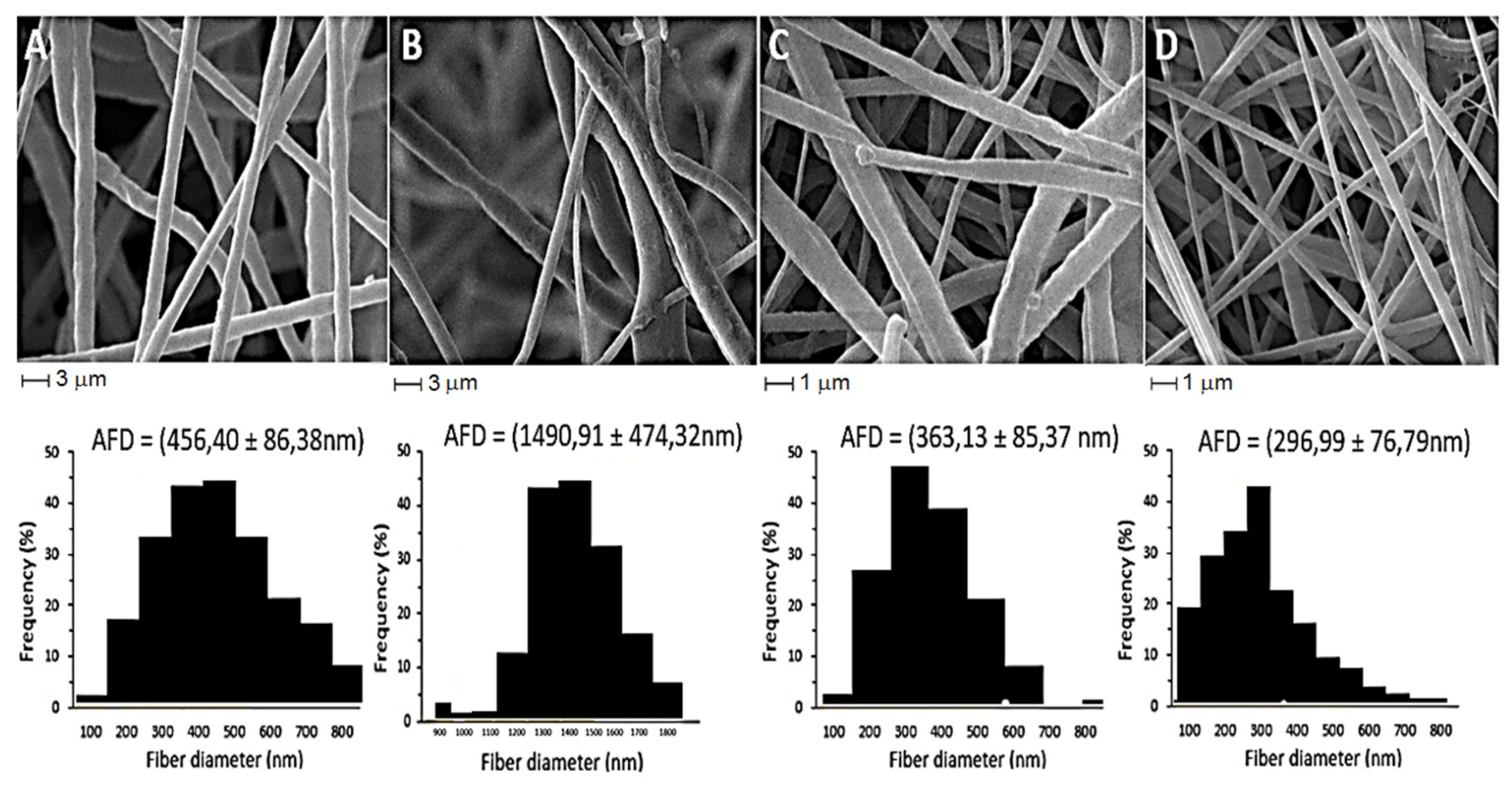
| Nanofiber | Tm (°C) | ΔHm (J/g) | Tonset (°C) | Xc (%) |
|---|---|---|---|---|
| PCL 10% (control) | 58.99 | 71.7060 | 54.89 | 87.87 |
| PCL10 + 1% KH | 57.92 | 47.7180 | 54.22 | 58.48 |
| PCL10 + 2.5% KH | 58.40 | 33.8602 | 54.40 | 41.49 |
| PCL10 + 5% KH | 58.74 | 66.3929 | 54.33 | 81.36 |
| PCL 15% (control) | 59.69 | 70.9667 | 55.08 | 86.97 |
| PCL15 + 1% KH | 57.88 | 45.5125 | 53.79 | 55.77 |
| PCL15 + 2.5% KH | 58.72 | 67.6502 | 54.48 | 82.90 |
| PCL15 + 5% KH | 58.09 | 51.8343 | 54.62 | 63.52 |
| Nanofiber | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| PCL 10% (control) | 42.64 ± 5.68 | 5.76 ± 0.17 | 138 ± 0.029 |
| PCL10 + 1% KH | 27.96 ± 2.81 | 1.43 ± 0.36 | 132 ± 0.054 |
| PCL10 + 2.5% KH | 25.93 ± 2.23 | 1.98 ± 0.40 | 142 ± 0.126 |
| PCL10 + 5% KH | 25.85 ± 3.69 | 0.84 ± 0.20 | 122 ± 0.026 |
| PCL 15% (control) | 24.81 ± 2.12 | 1.82 ± 0.44 | 183 ± 0.23 |
| PCL15 + 1% KH | 20.57 ± 1.23 | 1.59 ± 0.18 | 179 ± 0.052 |
| PCL15 + 2.5% KH | 18.99 ± 0.18 | 1.34 ± 0.059 | 199 ± 0.050 |
| PCL15 + 5% KH | 19.96 ± 0.59 | 1.30 ± 0.20 | 186 ± 0.038 |
| Nanofiber | Absolute Viscosity η (mPas) | |
|---|---|---|
| Control | 5% KH | |
| PCL 10% | 25.82 | 184.89 |
| PCL 15% | 26.20 | 234.36 |
| Nanofiber | DPPH (%) | DPPH (μM) 2 | ABTS (%) | ABTS (μM) 2 |
|---|---|---|---|---|
| PCL (control) | nd 3 | nd | nd | nd |
| PCL + 1% KH | 49.03 ± 0.02 | 972.2 ± 3.0 | 44.19 ± 0.02 | 972.0 ± 7.4 |
| PCL + 2.5% KH | 49.24 ± 0.07 | 982.2 ± 13.5 | 49.61 ± 0.01 | 1153.3 ± 44.4 |
| PCL + 5% KH | 49.07 ± 0.01 | 974.7 ± 1.5 | 56.21 ± 0.006 | 1228.7 ± 18.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clerici, N.J.; Vencato, A.A.; Helm Júnior, R.; Daroit, D.J.; Brandelli, A. Electrospun Poly-ε-Caprolactone Nanofibers Incorporating Keratin Hydrolysates as Innovative Antioxidant Scaffolds. Pharmaceuticals 2024, 17, 1016. https://doi.org/10.3390/ph17081016
Clerici NJ, Vencato AA, Helm Júnior R, Daroit DJ, Brandelli A. Electrospun Poly-ε-Caprolactone Nanofibers Incorporating Keratin Hydrolysates as Innovative Antioxidant Scaffolds. Pharmaceuticals. 2024; 17(8):1016. https://doi.org/10.3390/ph17081016
Chicago/Turabian StyleClerici, Naiara Jacinta, Aline Aniele Vencato, Rafael Helm Júnior, Daniel Joner Daroit, and Adriano Brandelli. 2024. "Electrospun Poly-ε-Caprolactone Nanofibers Incorporating Keratin Hydrolysates as Innovative Antioxidant Scaffolds" Pharmaceuticals 17, no. 8: 1016. https://doi.org/10.3390/ph17081016
APA StyleClerici, N. J., Vencato, A. A., Helm Júnior, R., Daroit, D. J., & Brandelli, A. (2024). Electrospun Poly-ε-Caprolactone Nanofibers Incorporating Keratin Hydrolysates as Innovative Antioxidant Scaffolds. Pharmaceuticals, 17(8), 1016. https://doi.org/10.3390/ph17081016







