Anthocyanin-Rich Fraction of Black Rice Bran Extract Protects against Amyloid β-Induced Oxidative Stress, Endoplasmic Reticulum Stress, and Neuronal Apoptosis in SK-N-SH Cells
Abstract
:1. Introduction
2. Results
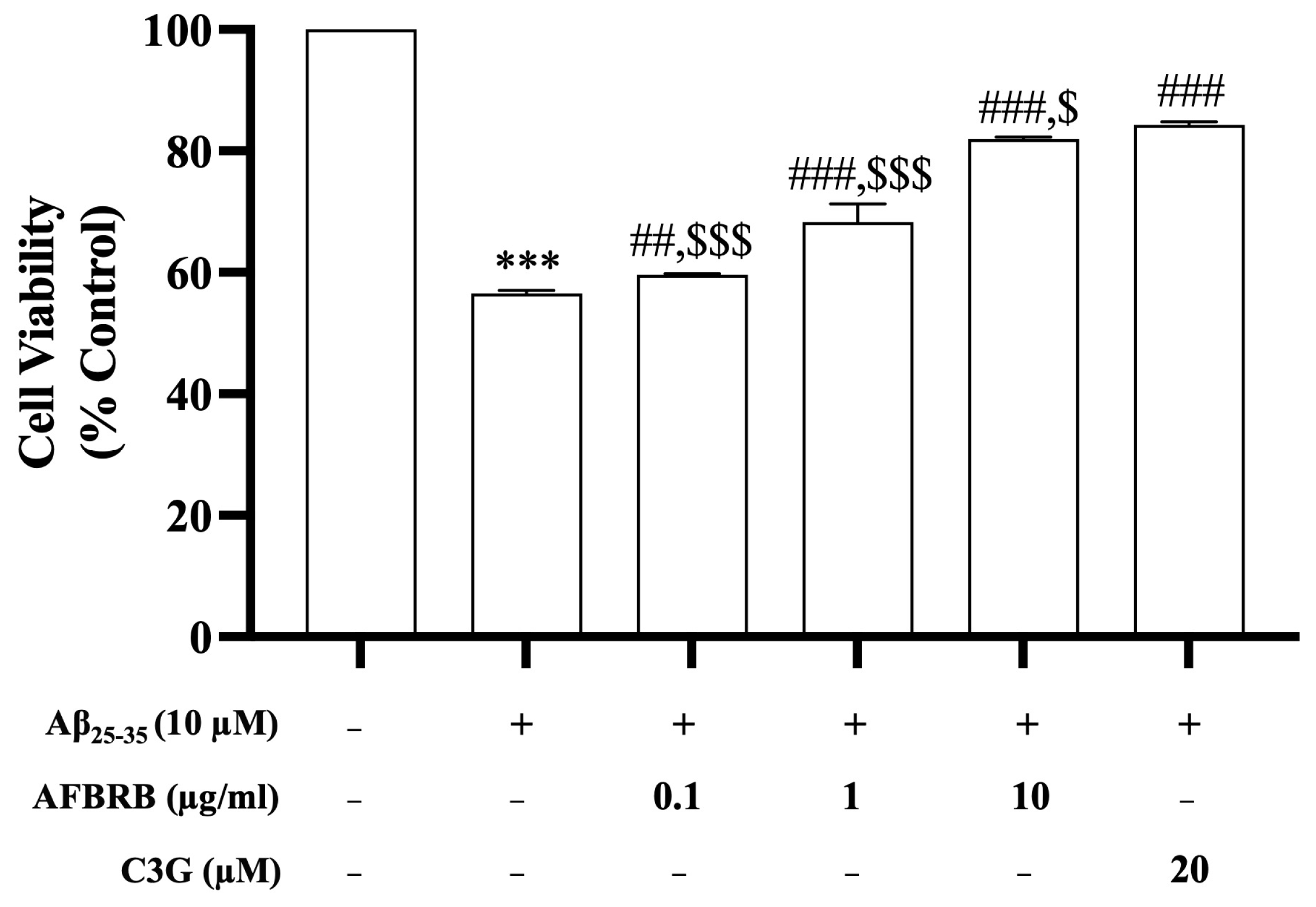
2.1. Effects of AFBRB on Cell Viability Induced by Aβ25–35 in SK-N-SH Cells
2.2. Effect of AFBRB on Intracellular ROS Production Induced by Aβ25–35 in SK-N-SH Cells
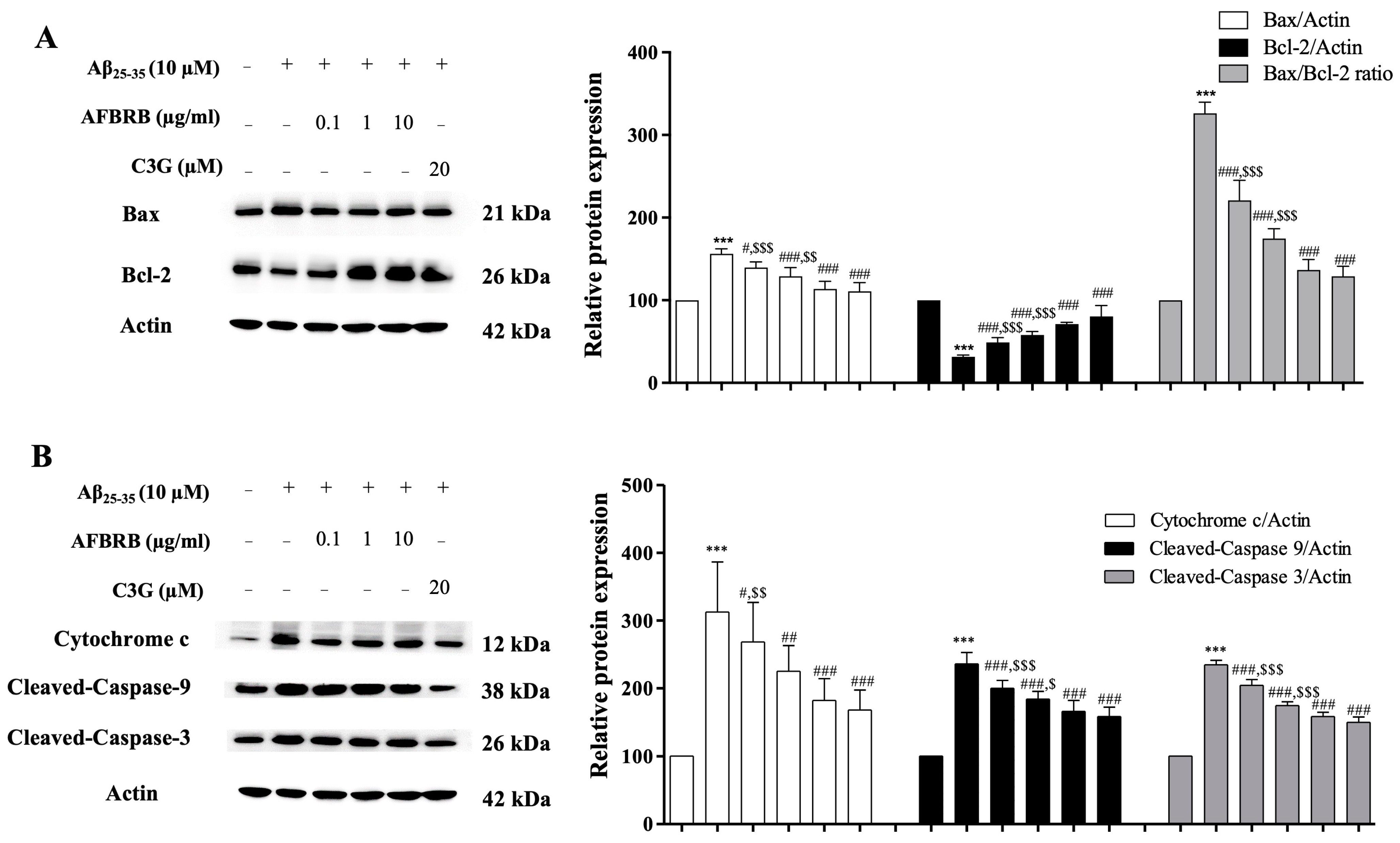
2.3. Effect of AFBRB on Aβ25–35-Induced Apoptosis via the Mitochondrial Death Pathway in SK-N-SH Cells
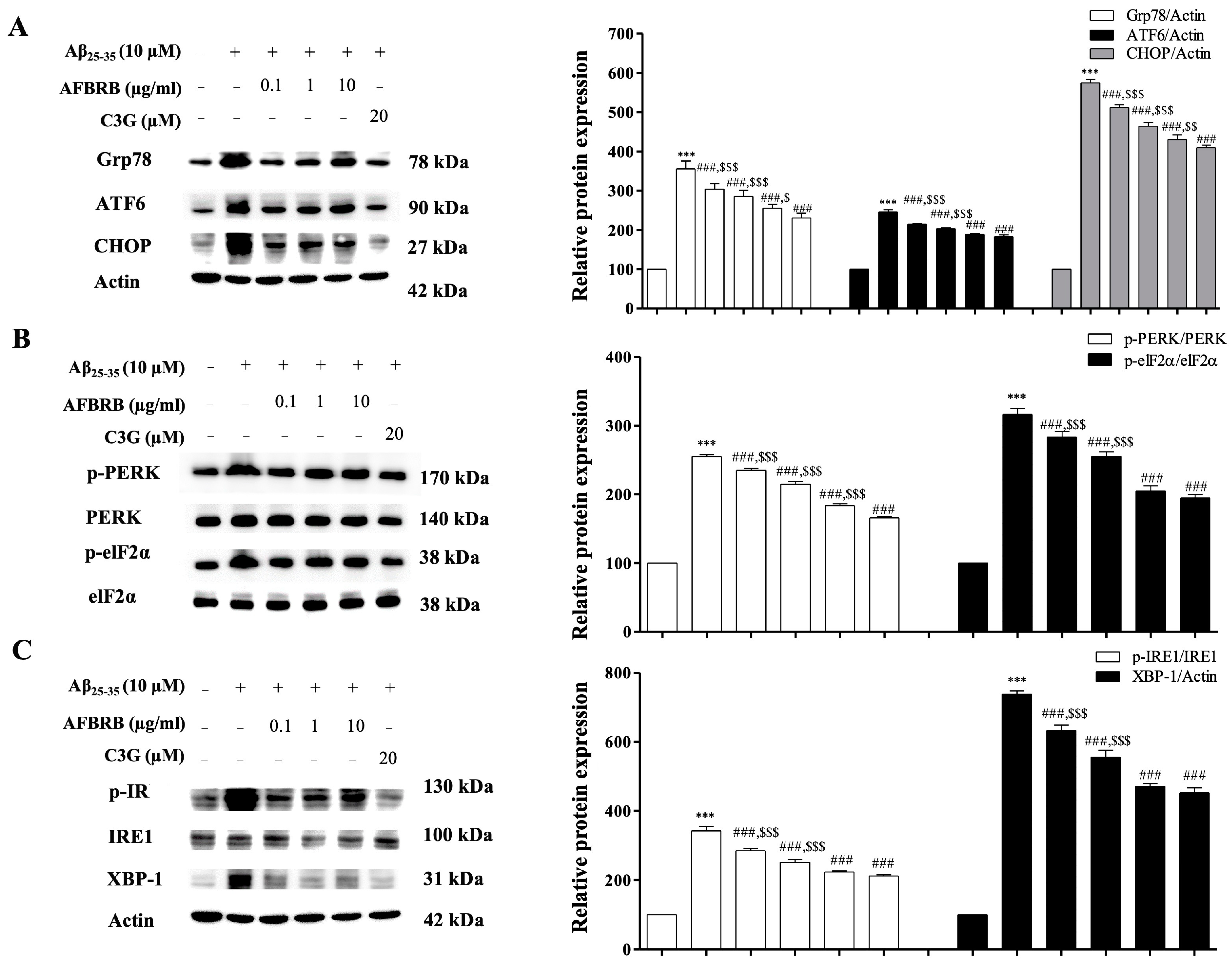
2.4. Effect of AFBRB on ER Stress in SK-N-SH Induced by Aβ25–35
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Chemicals
4.3. Preparation of Aggregated Aβ25–35
4.4. Preparation of Anthocyanin-Rich Fraction of Black Rice Bran (AFBRB)
4.5. Measurement of Cell Viability
4.6. Measurement of Reactive Oxygen Species (ROS)
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez, J.A.S.; González, H.M.; Léger, G.C. Alzheimer’s disease. Handb. Clin. Neurol. 2019, 167, 231–255. [Google Scholar] [CrossRef]
- Santiago, J.A.; Potashkin, J.A. The impact of disease comorbidities in Alzheimer’s disease. Front. Aging Neurosci. 2021, 13, 631770. [Google Scholar] [CrossRef] [PubMed]
- Sehar, U.; Rawat, P.; Reddy, A.P.; Kopel, J.; Reddy, P.H. Amyloid beta in aging and Alzheimer’s disease. Int. J. Mol. Sci. 2022, 21, 12924. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, P.R.; Dubey, A.K.; Masters, C.L.; Martins, R.N.; Macreadie, I.G. Aβ Aggregation and possible implications in Alzheimer’s disease pathogenesis. J. Cell Mol. Med. 2009, 13, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Asik, R.M.; Suganthy, N.; Aarifa, M.A.; Kumar, A.; Szigeti, K.; Mathe, D.; Gulyás, B.; Archunan, G.; Padmanabhan, P. Alzheimer’s disease: A molecular view of β-Amyloid induced morbific events. Biomedicines 2021, 9, 1126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Leuner, K.; Schütt, T.; Kurz, C.; Eckert, S.H.; Schiller, C.; Occhipinti, A.; Mai, S.; Jendrach, M.; Eckert, G.P.; Kruse, S.E.; et al. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxid. Redox Signal 2012, 16, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Sharma, C.; Kim, S.R. Linking oxidative stress and proteinopathy in Alzheimer’s disease. Antioxidants 2021, 10, 1231. [Google Scholar] [CrossRef]
- Karlnoski, R.; Wilcock, D.; Dickey, C.; Ronan, V.; Gordon, M.N.; Zhang, W.; Morgan, D.; Taglialatela, G. Up-regulation of Bcl-2 in APP transgenic mice is associated with neuroprotection. Neurobiol. Dis. 2007, 25, 179–188. [Google Scholar] [CrossRef]
- Thummayot, S.; Tocharus, C.; Pinkaew, D.; Viwatpinyo, K.; Sringarm, K.; Tocharus, J. Neuroprotective effect of purple rice extract and its constituent against amyloid beta-induced neuronal cell death in SK-N-SH cells. Neurotoxicology 2014, 45, 149–158. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef] [PubMed]
- Hussar, P. Apoptosis regulators Bcl-2 and caspase-3. Encyclopedia 2022, 2, 1624–1636. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wei, S.; Nguyen, T.H.; Jo, Y.; Zhang, Y.; Park, W.; Gariani, K.; Oh, C.-M.; Kim, H.H.; Ha, K.-T.; et al. Mitochondria-associated programmed cell death as a therapeutic target for age-related disease. Exp. Mol. Med. 2023, 5, 1595–1619. [Google Scholar] [CrossRef]
- Fonseca, A.C.R.G.; Ferreiro, E.; Oliveira, C.R.; Cardoso, S.M.; Pereira, C.F. Activation of the endoplasmic reticulum stress response by the amyloid-beta 1-40 peptide in brain endothelial cells. Biochim. Biophys. Acta 2013, 1832, 2191–2203. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, B.; Hedskog, L.; Wiehager, B.; Ankarcrona, M. Amyloid-β peptides are generated in mitochondria-associated endoplasmic reticulum membranes. J. Alzheimers Dis. 2015, 43, 369–374. [Google Scholar] [CrossRef]
- Volgyi, K.; Juhász, G.; Kovacs, Z.; Penke, B. Dysfunction of endoplasmic reticulum (ER) and mitochondria (MT) in Alzheimer’s disease: The role of the ER-MT cross-talk. Curr. Alzheimer Res. 2015, 12, 655–672. [Google Scholar] [CrossRef]
- Hashimoto, S.; Ishii, A.; Kamano, N.; Watamura, N.; Saito, T.; Ohshima, T.; Yokosuka, M.; Saido, T.C. Endoplasmic reticulum stress responses in mouse models of Alzheimer’s disease: Overexpression paradigm versus knockin paradigm. J. Biol. Chem. 2018, 293, 3118–3125. [Google Scholar] [CrossRef]
- Sharma, R.B.; Landa-Galván, H.V.; Alonso, L.C. Living dangerously: Protective and harmful ER stress responses in pancreatic β-cells. Diabetes 2021, 70, 2431–2443. [Google Scholar] [CrossRef]
- Abramov, A.; Canevari, L.; Duchen, M.R. Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim. Biophys. Acta 2004, 742, 81–87. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Hernando-Perez, E.; Nuñez, L.; Villalobos, C. Amyloid β oligomers increase ER-mitochondria Ca2+ cross talk in young hippocampal neurons and exacerbate aging-induced intracellular Ca2+ remodeling. Front. Cell Neurosci. 2019, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Zhang, J.; Chen, S.; Huang, Y.; Chen, W.; He, L.; Zhang, Y. Role of calcium homeostasis in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2022, 18, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Webber, E.K.; Fivaz, M.; Stutzmann, G.; Griffioen, G. Cytosolic calcium: Judge, jury and executioner of neurodegeneration in Alzheimer’s disease and beyond. Alzheimers Dement. 2023, 19, 3701–3717. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Dash, U.; Mahanand, S.S.; Nayak, P.K.; Kesavan, R.K. Black rice: A comprehensive review on its bioactive compounds, potential health benefits and food applications. Food Chem. Adv. 2023, 3, 100462. [Google Scholar] [CrossRef]
- Mackon, E.; Mackon, G.C.J.D.E.; Ma, Y.; Kashif, M.H.; Ali, N.; Usman, B.; Liu, P. Recent Insights into Anthocyanin Pigmentation, Synthesis, Trafficking, and Regulatory Mechanisms in Rice (Oryza sativa L.) Caryopsis. Biomolecules 2021, 11, 394. [Google Scholar] [CrossRef]
- Laorodphun, P.; Arjinajarn, P.; Thongnak, L.; Promsan, S.; Swe, M.T.; Thitisut, P.; Mahatheeranont, S.; Jaturasitha, S.; Lungkaphin, A. Anthocyanin-rich fraction from black rice, Oryza sativa L. var. indica “Luem Pua”, bran extract attenuates kidney injury induced by high-fat diet involving oxidative stress and apoptosis in obese rats. Phytother. Res. 2021, 35, 5189–5202. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mims, P.N.; Roman, R.J.; Fan, F. Is Beta-amyloid accumulation a cause or consequence of Alzheimer’s disease? J. Alzheimers Park. Dement. 2016, 1, 007. [Google Scholar]
- Zhang, Y.; Chen, D.; Tian, R.; Yan, X.; Zhou, Y. Resveratrol alleviates amyloid β-induced neuronal apoptosis, inflammation, and oxidative and endoplasmic reticulum stress by circ_0050263/miR-361-3p/PDE4A axis during Alzheimer’s disease. Chem. Biol. Drug Des. 2023, 102, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Naldi, M.; Fiori, J.; Pistolozzi, M.; Drake, A.F.; Bertucci, C.; Wu, R.; Mlynarczyk, K.; Filipek, S.; Simone, A.D.; Andrisano, V. Amyloid β-peptide 25–35 self-assembly and its inhibition: A model undecapeptide system to gain atomistic and secondary structure details of the Alzheimer’s disease process and treatment. ACS Chem. Neurosci. 2012, 3, 952–962. [Google Scholar] [CrossRef]
- Tang, Z.; Motoyoshi, K.; Honda, T.; Nakamura, H.; Murayama, T. Amyloid beta-peptide 25-35 (Aβ25-35) induces cytotoxicity via multiple mechanisms: Roles of the inhibition of glucosylceramide synthase by Aβ25-35 and its protection by D609. Biol. Pharm. Bull. 2021, 44, 1419–1426. [Google Scholar] [CrossRef]
- Canet, G.; Zussy, C.; Hernandez, C.; Maurice, T.; Desrumaux, C.; Givalois, L. The pathomimetic oAβ25-35 model of Alzheimer’s disease: Potential for screening of new therapeutic agents. Pharmacol. Ther. 2023, 245, 108398. [Google Scholar] [CrossRef]
- Nakamura, R.; Konishi, M.; Higashi, Y.; Saito, M.; Akizawa, T. Five-mer peptides prevent short-term spatial memory deficits in Aβ25-35-induced Alzheimer’s model mouse by suppressing Aβ25-35 aggregation and resolving its aggregate form. Alzheimers Res. Ther. 2023, 15, 83. [Google Scholar] [CrossRef]
- Thummayot, S.; Tocharus, C.; Suksamrarn, A.; Tocharus, J. Neuroprotective effects of cyanidin against Aβ-induced oxidative and ER stress in SK-N-SH cells. Neurochem. Int. 2016, 101, 15–21. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, L.; Zhu, X.; Zhang, K.; Huang, B.; Zhang, J.; Zhang, Y.; Zhu, L.; Zhou, B.; Zhou, F. Protective effect of paeoniflorin on Aβ25-35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell Mol. Neurobiol. 2014, 34, 227–234. [Google Scholar] [CrossRef]
- Zeng, M.; Feng, A.; Zhao, C.; Zhang, B.; Guo, P.; Liu, M.; Zhang, Q.; Zhang, Y.; Fan, R.; Lyu, J.; et al. Adenosine ameliorated Aβ25-35-induced brain injury through the inhibition of apoptosis and oxidative stress via an ERα pathway. Brain Res. 2022, 1788, 147944. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhang, J.; Qin, M. Protective effect of cyanidin 3-O-glucoside on beta-amyloid peptide-induced cognitive impairment in rats. Neurosci. Lett. 2013, 534, 285–288. [Google Scholar] [CrossRef]
- Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Roy, S.L.; Tambe, M.A.; Ferruzzi, M.G.; Wu, Q.-L.; Simon, J.E.; Lila, M.A.; Rochet, J.-C. Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinson’s disease. Brain Res. 2014, 155, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Part, K.; Künnis-Beres, K.; Poska, H.; Land, T.; Shimmo, R.; Fernaeus, S.Z. Amyloid β25-35 induced ROS-burst through NADPH oxidase is sensitive to iron chelation in microglial Bv2 cells. Brain Res. 2015, 629, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-F.; Li, J.-J.; Tao, Y.-G.; Wang, X.-Q.; Zhang, R.-L.; Zhang, J.-L.; Su, Z.-Q.; Huang, Q.-H.; Deng, Y.-H. Geniposide attenuates Aβ25–35-induced neurotoxicity via the TLR4/NF-κB pathway in HT22 cells. RSC Adv. 2018, 8, 18926–18937. [Google Scholar] [CrossRef]
- Paixão, J.; Dinis, T.C.P.; Almeida, L.M. Dietary anthocyanins protect endothelial cells against peroxynitrite-induced mitochondrial apoptosis pathway and Bax nuclear translocation: An in vitro approach. Apoptosis 2011, 16, 976–989. [Google Scholar] [CrossRef]
- Gahl, R.F.; He, Y.; Yu, S.; Tjandra, N. Conformational Rearrangements in the Pro-apoptotic Protein, Bax, as It Inserts into Mitochondria. J. Biol. Chem. 2014, 289, 32871–32882. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, K.; García-Sáez, A.J. Bax and Bak pores: Are we closing the circle? Trends Cell Biol. 2017, 27, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, T.; Wang, T.; Song, J.; Zhou, Z. Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on cerebral ischemia rats. Biomed. Rep. 2013, 1, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.-M.; Niu, C.-S. Effects of CDNF on 6-OHDA-induced apoptosis in PC12 cells via modulation of Bcl-2/Bax and caspase-3 activation. Neurol. Sci. 2014, 35, 1275–1280. [Google Scholar] [CrossRef]
- Pinkaew, D.; Changtam, C.; Tocharus, C.; Thummayot, S.; Suksamrarn, A.; Tocharus, J. Di-O-demethylcurcumin protects SK-N-SH cells against mitochondrial and endoplasmic reticulum-mediated apoptotic cell death induced by Aβ25-35. Neurochem. Int. 2015, 80, 110–119. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Suuronen, T.; Kaarniranta, K.; Ojala, J. ER stress in Alzheimer’s disease: A novel neuronal trigger for inflammation and Alzheimer’s pathology. J. Neuroinflammation 2009, 6, 41. [Google Scholar] [CrossRef]
- Huang, H.-C.; Tang, D.T.; Lu, S.-Y.; Jiang, Z.-F. Endoplasmic reticulum stress as a novel neuronal mediator in Alzheimer’s disease. Neurol. Res. 2015, 37, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Ba, M. Protective effects of diazoxide against Aβ25–35-induced PC12 cell apoptosis due to prevention of endoplasmic reticulum stress. Neuroreport 2012, 23, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Pei, D.; Shi, L.; Cui, Y.; Hong, Z. Ephrin-B2 inhibits Aβ25–35-induced apoptosis by alleviating endoplasmic reticulum stress and promoting autophagy in HT22 cells. Neurosci. Lett. 2019, 704, 50–56. [Google Scholar] [CrossRef]
- Li, G.; Liang, R.; Lian, Y.; Zhou, Y. Circ_0002945 functions as a competing endogenous RNA to promote Aβ25-35-induced endoplasmic reticulum stress and apoptosis in SK-N-SH cells and human primary neurons. Brain Res. 2022, 1785, 147878. [Google Scholar] [CrossRef]
- Hata, S.; Sorimachi, H.; Nakagawa, K.; Maeda, T.; Abe, K.; Suzuki, K. Domain II of m-calpain is a Ca(2+)-dependent cysteine protease. FEBS Lett. 2001, 501, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Sorimachi, H. Calpains: An elaborate proteolytic system. Biochim. Biophys. Acta 2012, 1824, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Yuan, J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J. Cell Biol. 2000, 150, 887–894. [Google Scholar] [CrossRef]
- Kerbiriou, M.; Teng, L.; Benz, N.; Trouvé, P.; Férec, C. The Calpain, caspase 12, caspase 3 cascade leading to apoptosis is altered in F508del-CFTR expressing cells. PLoS ONE 2009, 4, e8436. [Google Scholar] [CrossRef] [PubMed]
- Mapoung, S.; Semmarath, W.; Arjsri, P.; Thippraphan, P.; Srisawad, K.; Umsumarng, S.; Phromnoi, K.; Jamjod, S.; Prom-u-Thai, C.; Dejkriengkraiku, P. Comparative analysis of bioactive-phytochemical characteristics, antioxidants activities, and anti-inflammatory properties of selected black rice germ and bran (Oryza sativa L.) varieties. Eur. Food Res. Technol. 2023, 249, 451–464. [Google Scholar] [CrossRef]
- Jumnongprakhon, P.; Chokchaisiri, R.; Thummayot, S.; Suksamrarn, A.; Tocharus, C.; Tocharus, J. 5,6,7,4′-Tetramethoxyflavanone attenuates NADPH oxidase 1/4 and promotes sirtuin-1 to inhibit cell stress, senescence and apoptosis in Aß25–35-mediated SK-N-SH dysfunction. EXCLI J. 2021, 20, 1346–1362. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivasinprasasn, S.; Tocharus, J.; Mahatheeranont, S.; Nakrat, S.; Tocharus, C. Anthocyanin-Rich Fraction of Black Rice Bran Extract Protects against Amyloid β-Induced Oxidative Stress, Endoplasmic Reticulum Stress, and Neuronal Apoptosis in SK-N-SH Cells. Pharmaceuticals 2024, 17, 1039. https://doi.org/10.3390/ph17081039
Sivasinprasasn S, Tocharus J, Mahatheeranont S, Nakrat S, Tocharus C. Anthocyanin-Rich Fraction of Black Rice Bran Extract Protects against Amyloid β-Induced Oxidative Stress, Endoplasmic Reticulum Stress, and Neuronal Apoptosis in SK-N-SH Cells. Pharmaceuticals. 2024; 17(8):1039. https://doi.org/10.3390/ph17081039
Chicago/Turabian StyleSivasinprasasn, Sivanan, Jiraporn Tocharus, Sugunya Mahatheeranont, Sarun Nakrat, and Chainarong Tocharus. 2024. "Anthocyanin-Rich Fraction of Black Rice Bran Extract Protects against Amyloid β-Induced Oxidative Stress, Endoplasmic Reticulum Stress, and Neuronal Apoptosis in SK-N-SH Cells" Pharmaceuticals 17, no. 8: 1039. https://doi.org/10.3390/ph17081039




