Evaluation of Cytotoxicity and Acute Oral Toxicity of Saline Extract and Protein-Rich Fraction from Moringa oleifera Lam. Leaves
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Leaf Extract and Protein-Rich Fraction
4.2. Determination of Protein Concentration and the Presence of Trypsin Inhibitor and Lectin
4.3. Thin-Layer Chromatography (TLC)
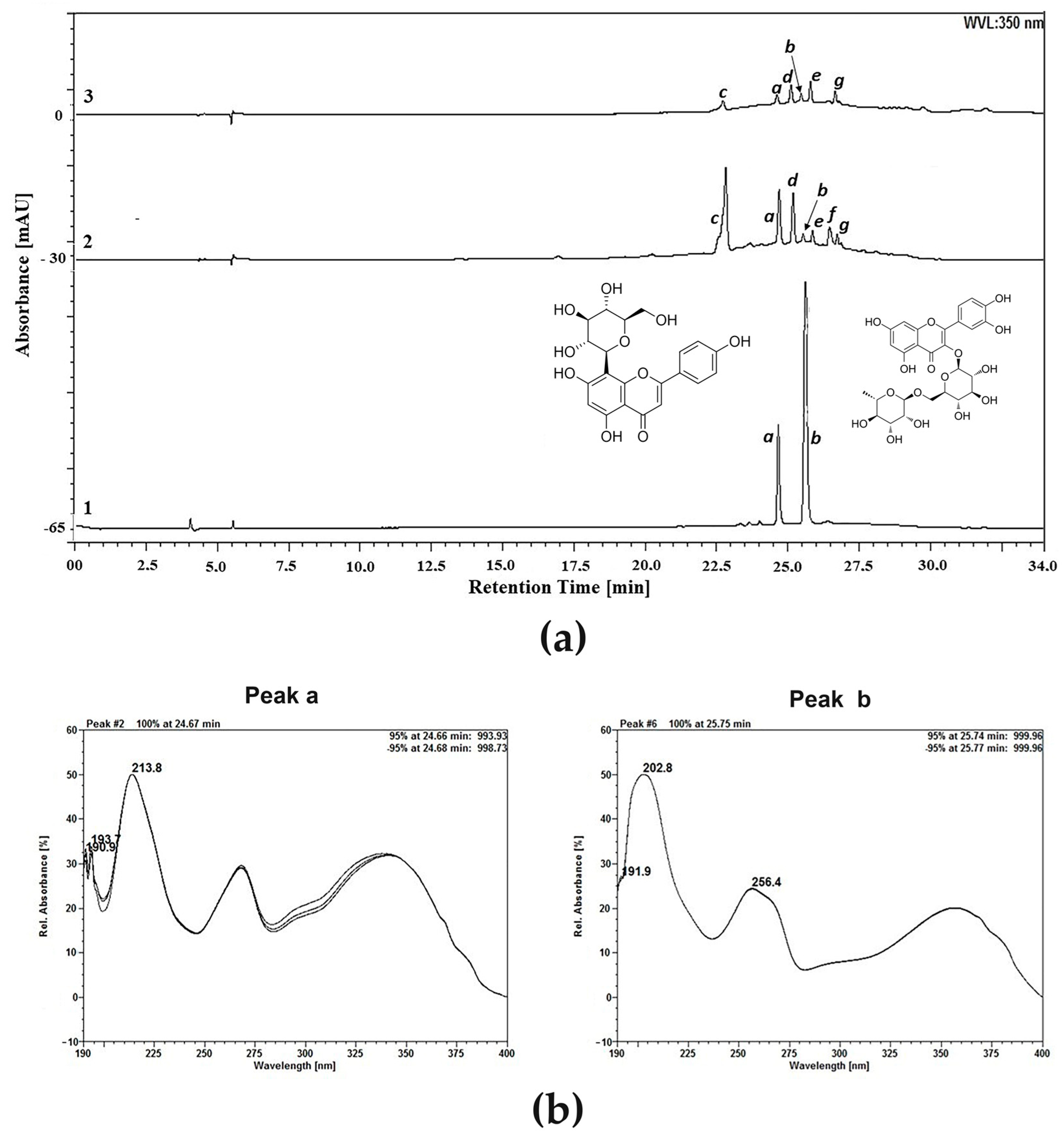
4.4. High-Performance Liquid Chromatography (HPLC) Analysis
4.5. Cytotoxicity to Human Peripheral Blood Mononuclear Cells (PBMCs)
4.6. Hemolytic Assay
4.7. Animals
4.8. Acute Oral Toxicity
4.8.1. Treatments
4.8.2. Biochemical and Hematological Analysis
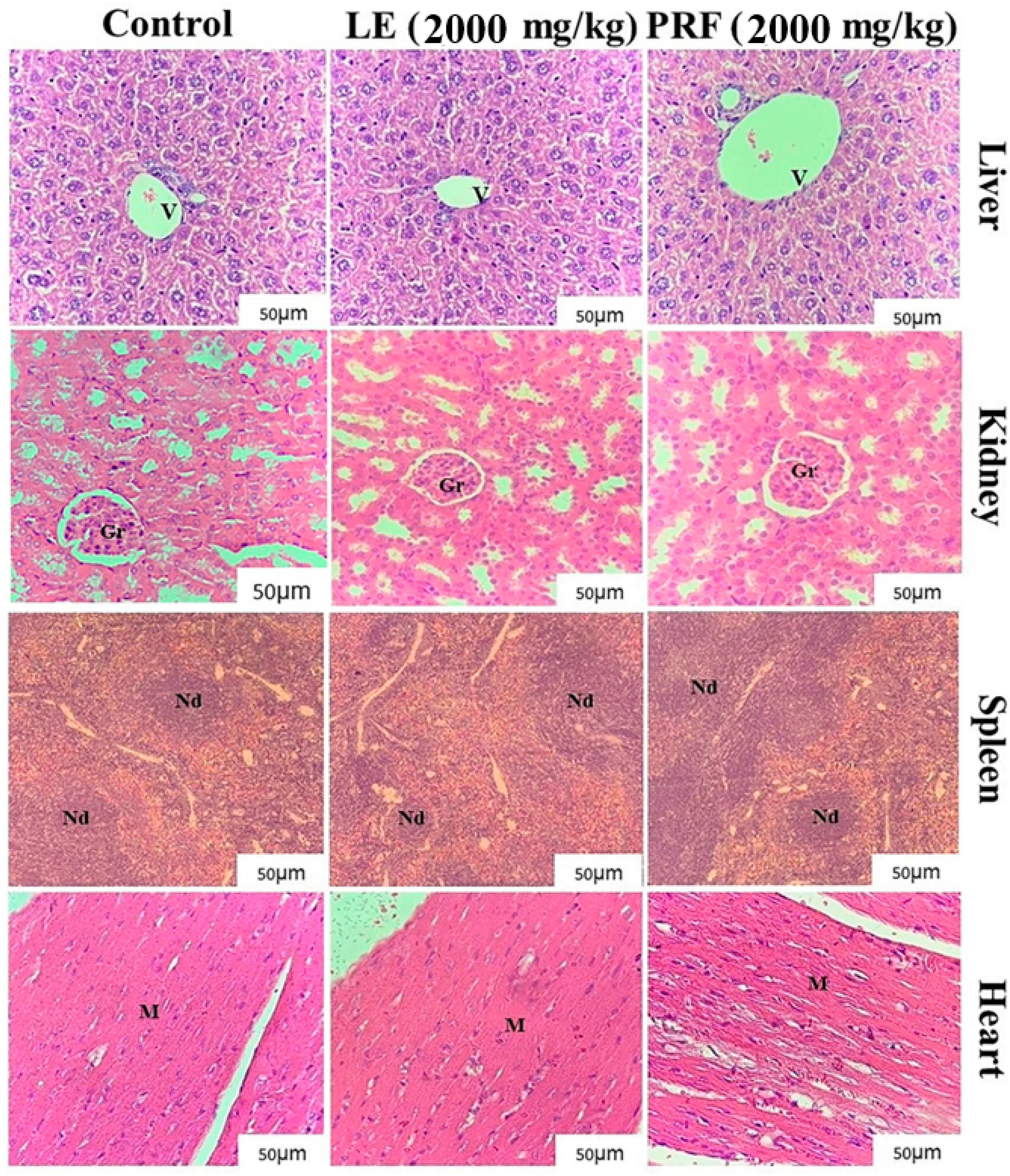
4.8.3. Histopathological Evaluation
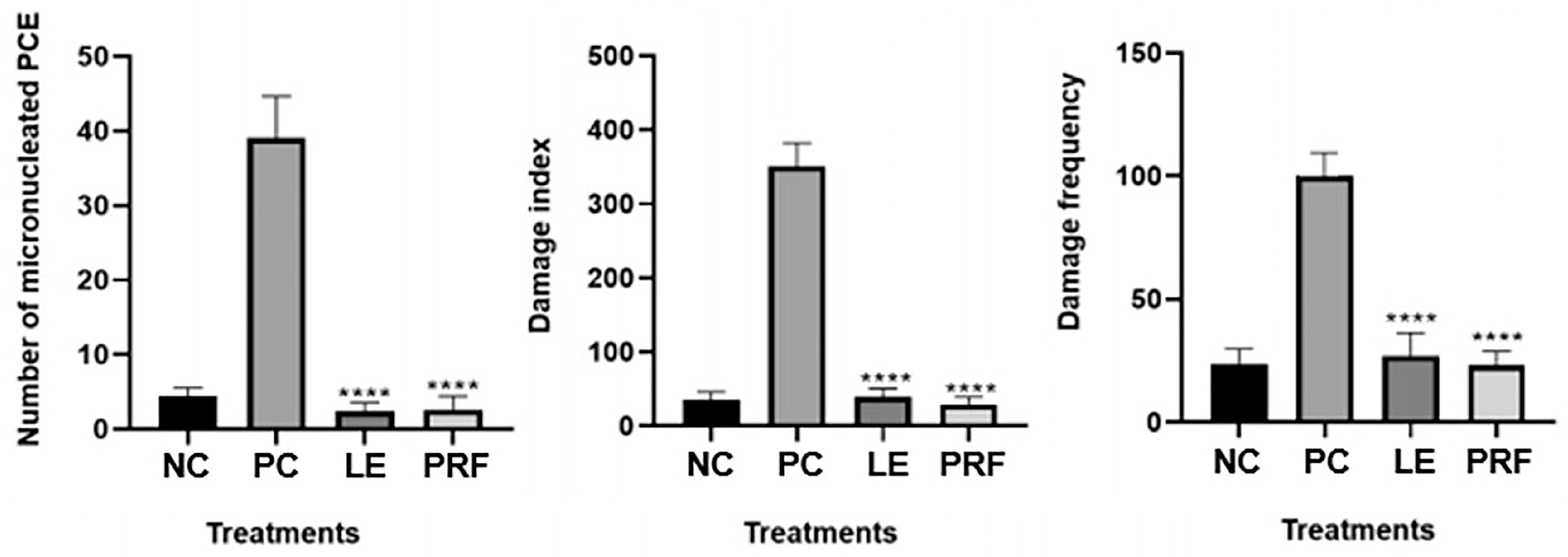
4.9. Genotoxicity Assays
4.9.1. Experimental Groups and Blood Collection
4.9.2. Comet Assay
4.9.3. Micronucleus Test
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adewale, O.B.; Onasanya, A.; Anadozie, S.O.; Abu, M.F.; Akintan, I.A.; Ogbole, C.J.; Olayide, I.I.; Afolabi, O.B.; Jaiyesimi, K.F.; Ajiboye, B.O.; et al. Evaluation of acute and subacute toxicity of aqueous extract of Crassocephalum rubens leaves in rats. J. Ethnopharmacol. 2016, 188, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.B.D.; Mezzomo, T.R.; Moraes, E.F.D. Conhecimento e uso de plantas medicinais por usuários de unidades básicas de saúde na região de Colombo, PR. Rev. Bras. Ciênc. Saúde 2018, 22, 57–64. [Google Scholar]
- Oliveira, A.M.; Nascimento, M.F.; Ferreira, M.R.A.; Moura, D.F.; Souza, T.G.S.; Silva, G.C.; Ramos, E.H.S.; Paiva, P.M.G.; Medeiros, P.L.; Silva, T.G.; et al. Evaluation of acute toxicity, genotoxicity and inhibitory effect on acute inflammation of an ethanol extract of Morus alba L. (Moraceae) in mice. J. Ethnopharmacol. 2016, 194, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.A.C.D.; Carvalho, R.B.F.D.; Sousa, D.P.D.; Souza, G.F.D.; Oliveira, J.D.S.; Freitas, R.M.D. Avaliação da toxicidade aguda do epóxilimoneno em camundongos adultos. Rev. Cub. Pl. Med. 2014, 19, 160–171. [Google Scholar]
- Maracajá, P.; Silveira, D.; Coelho, D.; Leite, D.; Freire, M.; Cavalcanti, M. Efeito tóxico do extrato de flores de Moringa oleifera L. para abelhas Apis melifera africanizadas. Agropecu. Cient. Semi-Árido 2010, 6, 33–37. [Google Scholar]
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or pharmacological potential of Moringa oleifera Lam. Nutrients 2018, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Manuwa, S.I.; Sedara, A.M.; Tola, F.A. Design, fabrication and performance evaluation of moringa (oleifera) dried leaves pulverizer. J. Agric. Food Res. 2020, 2, 100034. [Google Scholar] [CrossRef]
- Ohta, T.; Nakamura, S.; Nakashima, S.; Shimakawa, H.; Emi, Y.; Matsumoto, T.; Ogawa, K.; Fukaya, M.; Oda, Y.; Matsuda, H. Stimulators of acylated ghrelin secretion from Moringa oleifera leaves. Phytochem. Lett. 2017, 21, 1–5. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Sulastri, E.; Zubair, M.S.; Anas, N.I.; Abidin, S.; Hardani, R.; Yulianti, R. Aliyah Total phenolic, total flavonoid, quercetin content and antioxidant activity of standardized extract of Moringa oleifera leaf from regions with different elevation. Pharmacogn. J. 2018, 10, s104–s108. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, J.; Chen, X. Bioactive flavonoids in Moringa oleifera and their health-promoting properties. J. Funct. Foods 2018, 47, 469–479. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Owens, F.S., III; Dada, O.; Cyrus, J.W.; Adedoyin, O.O.; Adunlin, G. The effects of Moringa oleifera on blood glucose levels: A scoping review of the literature. Compl. Ther. Med. 2020, 50, 102362. [Google Scholar] [CrossRef] [PubMed]
- Muzumbukilwa, W.T.; Nlooto, M.; Owira, P.M.O. Hepatoprotective effects of Moringa oleifera Lam (Moringaceae) leaf extracts in streptozotocin-induced diabetes in rats. J. Funct. Foods 2019, 57, 75–82. [Google Scholar] [CrossRef]
- Berkovich, L.; Earon, G.; Ron, I.; Rimmon, A.; Vexler, A.; Lev-Ari, S. Moringa oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Compl. Altern. Med. 2013, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Tiloke, C.; Anand, K.; Gengan, R.M.; Chuturgoon, A.A. Moringa oleifera and their phytonanoparticles: Potential antiproliferative agents against cancer. Biomed. Pharmacother. 2018, 108, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.F.; Ahmad, J.; Zhang, H.; Khan, I.; Muhammad, S. Evaluation of phytochemical and medicinal properties of Moringa (Moringa oleifera) as a potential functional food. S. Afr. J. Bot. 2020, 129, 40–46. [Google Scholar] [CrossRef]
- Khatun, S.; Khan, M.M.H.; Ashraduzzaman, M.; Pervin, F.; Bari, L.; Absar, N. Antibacterial activity and cytotoxicity of three lectins purified from drumstick (Moringa oleifera Lam.) Leaves. J. Bio-Sci. 2009, 17, 89–94. [Google Scholar] [CrossRef]
- Teixeira, C.M.L.L.; Kirsten, F.V.; Teixeira, P.C.N. Evaluation of Moringa oleifera seed flour as a flocculating agent for potential biodiesel producer microalgae. J. Appl. Phycol. 2012, 24, 557–563. [Google Scholar] [CrossRef]
- Teixeira, E.M.B.; Carvalho, M.R.B.; Neves, V.A.; Silva, M.A.; Arantes-Pereira, L. Chemical characteristics and fractionation of proteins from Moringa oleifera Lam. leaves. Food Chem. 2014, 147, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Kala, B.K.; Mohan, V.R. Effect of microwave treatment on the antinutritional factors of two accessions of velvet bean, Mucuna pruriens (L.) DC. var. utilis (Wall. ex Wight) Bak. ex Burck. Int. Food Res. J. 2012, 19, 961. [Google Scholar]
- Alatorre-Cruz, J.M.; Pita-López, W.; López-Reyes, R.G.; Ferriz-Martínez, R.A.; Cervantes-Jiménez, R.; Carrillo, M.D.J.G.; Vargas, P.J.; López-Herrera, G.; Méndez, A.J.R.; Zamora-Arroyo, A.; et al. Effects of intragastrically-administered Tepary bean lectins on digestive and immune organs: Preclinical evaluation. Toxicol. Rep. 2018, 5, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Asare, G.A.; Gyan, B.; Bugyei, K.; Adjei, S.; Mahama, R.; Addo, P.; Otu-Nyarko, L.; Wiredu, E.K.; Nyarko, A. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. J. Ethnopharmacol. 2012, 139, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Adedapo, A.A.; Mogbojuri, O.M.; Emikpe, B.O. Safety evaluations of the aqueous extract of the leaves of Moringa oleifera in rats. J. Med. Plant. Res. 2009, 3, 586–591. [Google Scholar]
- Awodele, O.; Oreagba, I.A.; Odoma, S.; Silva, J.A.T.; Osunkalu, V.O. Toxicological evaluation of the aqueous leaf extract of Moringa oleifera Lam. (Moringaceae). J. Ethnopharmacol. 2012, 139, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; Van Damme, E.J. Toxic proteins in plants. Phytochemistry 2015, 117, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, K.; Bavithra, S.; Ganesh, L.; Krishnamoorthy, G.; Venkataraman, P.; Arunakaran, J. Polychlorinated biphenyls induced oxidative stress mediated neurodegeneration in hippocampus and behavioral changes of adult rats: Anxiolytic-like effects of quercetin. Toxicol. Lett. 2013, 222, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.O.; Sales, V.S.; Rodrigues, C.K.S.; Oliveira, L.R.; Lemos, I.C.S.; Delmondes, G.A.; Monteiro, A.B.; Nascimento, E.P.; Figueiredo, F.R.S.D.N.; Costa, J.G.M.; et al. Phytochemical analysis and central effects of Annona muricata Linnaeus: Possible involvement of the gabaergic and monoaminergic systems. Iran. J. Pharm. Res. 2018, 17, 1306. [Google Scholar] [PubMed]
- Krishnan, V.G.M.; Murugan, K. Acute and subchronic toxicological evaluation of the purified protease inhibitor from the fruits of Solanum aculeatissimum Jacq. on Wistar rats. Cogent Biol. 2016, 2, 1191588. [Google Scholar] [CrossRef]
- Madingou, N.O.K.; Traore, A.; Souza, A.; Mounanga, M.M.B.; Samseny, R.R.A.; Ouedraogo, S.; Traore, A.S. Preliminary studies of acute and sub-chronic toxicity of the aqueous extract of Guibourtia tessmannii (Harms) J. Leonard stem barks (Caesalpiniaceae) in mice and rats. Asian Pac. J. Trop. Biomed. 2016, 6, 506–510. [Google Scholar] [CrossRef]
- Ouédraogo, O.; Amyot, M. Effects of various cooking methods and food components on bioaccessibility of mercury from fish. Environ. Res. 2011, 111, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Nahar, S.; Faisal, F.M.; Iqbal, J.; Rahman, M.M.; Yusuf, M.A. Antiobesity activity of Moringa oleifera leaves against high fat diet-induced obesity in rats. Int. J. Basic Clin. Pharmacol. 2016, 10, 1263–1268. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Mesquita, M.S.; Silva, G.C.; Lima, E.O.; Medeiros, P.L.; Paiva, P.M.G.; Souza, I.A.; Napoleão, T.H. Evaluation of toxicity and antimicrobial activity of an ethanolic extract from leaves of Morus alba L.(Moraceae). Evid. Bas. Complem. Altern. Med. 2015, 2015, 513978. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Majumdar, S.; Singh, A.K.; Ghosh, B.; Ghosh, N.; Manna, S.; Chakraborty, T.; Mallick, S. Synthesis, characterization, antioxidant status, and toxicity study of vanadium–rutin complex in Balb/c mice. Biol. Trace Elem. Res. 2015, 166, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, J.D.C.; Neves, B.J.; Vasconcelos, F.G.; Napolitano, H.B.; Barbalho, M.G.D.S.; Rosseto, L.P. Flavonoids from Brazilian Cerrado: Biosynthesis, Chemical and Biological Profile. Molecules 2019, 24, 2891. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, C.; Kandemir, F.M.; Yildirim, S.; Kucukler, S.; Eser, G. Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. J. Trace Elem. Med. Biol. 2019, 54, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Ramteke, R.; Doneria, R.; Gendley, M.K. Antinutritional factors in feed and fodder used for livestock and poultry feeding. Act. Sci. Nutr. Health 2019, 3, 39–48. [Google Scholar]
- Patriota, L.L.S.; Ramos, D.B.M.; Santos, A.C.L.A.; Silva, Y.A.; Silva, M.G.; Torres, D.J.L.; Procópio, T.F.; Oliveira, A.M.; Coelho, L.C.B.B.; Pontual, E.V.; et al. Antitumor activity of Moringa oleifera (drumstick tree) flower trypsin inhibitor (MoFTI) in sarcoma 180-bearing mice. Food Chem. Toxicol. 2020, 145, 111691. [Google Scholar] [CrossRef] [PubMed]
- Ramos, D.B.M.; Araújo, M.T.M.F.; Araújo, T.C.L.; Silva, Y.A.; Santos, A.C.L.A.; Silva, M.G.; Paiva, P.M.G.; Mendes, R.L.; Napoleão, T.H. Antinociceptive activity of Schinus terebinthifolia leaf lectin (SteLL) in sarcoma 180-bearing mice. J. Ethnopharmacol. 2020, 259, 112952. [Google Scholar] [CrossRef] [PubMed]
- Green, A.A.; Hughes, W.L. Protein fractionation on the basis of solubility in aqueous solution of salts and organic solvents. In Methods in Enzymology; Colowick, S., Kaplan, N., Eds.; Academic Press: New York, NY, USA, 1955; pp. 67–90. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Bing, D.H.; Weyand, J.G.M.; Stavitsky, A.B. Hemagglutination with aldehyde-fixed erythrocytes for assay of antigens and antibodies. Proc. Soc. Exp. Biol. Med. 1967, 124, 1166–1170. [Google Scholar] [CrossRef]
- OECD. Test No. 423: Acute Oral toxicity—Acute Toxic Class Method; OECD: Paris, France, 2001. [Google Scholar]
- Sinha, K.; Khare, V. Review on: Antinutritional factors in vegetable crops. Pharma Innov. J. 2017, 6, 353–358. [Google Scholar]
- Pontual, E.V.; Napoleão, T.H.; Assis, C.R.D.; Bezerra, R.S.; Xavier, H.S.; Navarro, D.M.A.F.; Coelho, L.C.B.B.; Paiva, P.M.G. Effect of Moringa oleifera flower extract on larval trypsin and acetylcholinesterase activities in Aedes aegypti. Arch. Insect Biochem. Physiol. 2012, 79, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Procópio, T.F.; Patriota, L.L.S.; Moura, M.C.; Silva, P.M.; Oliveira, A.P.S.; Carvalho, L.V.N.; Lima, T.A.; Soares, T.; Silva, T.D.; Coelho, L.C.B.B.; et al. CasuL: A new lectin isolated from Calliandra surinamensis leaf pinnulae with cytotoxicity to cancer cells, antimicrobial activity and antibiofilm effect. Int. J. Biol. Macromol. 2017, 98, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.E.; Appelqvist, I.A.M.; Norton, I.T. Oral behaviour of food hydrocolloids and emulsions. Part 1. Lubrication and deposition considerations. Food Hydrocoll. 2003, 17, 763–773. [Google Scholar] [CrossRef]
- Sousa-Coelho, I.D.D.; Neto, C.J.C.L.; Souza, T.G.S.; Silva, M.A.; Chagas, C.A.; Santos, K.R.P.; Teixeira, V.W.; Teixeira, Á.A.C. Protective effect of exogenous melatonin in rats and their offspring on the genotoxic response induced by the chronic consumption of alcohol during pregnancy. Mut. Res. Gen. Toxicol. Environ. Mutagen. 2018, 832, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Oscoz, A.A.; Brunborg, G.; Gaivao, I.; Giovannelli, L.; Kruszewski, M.; Smith, C.C.; Štětina, R. The comet assay: Topical issues. Mutagenesis 2008, 23, 143–151. [Google Scholar] [CrossRef]
- Hayashi, M.; Morita, T.; Kodama, Y.; Sofuni, T.; Ishidate Jr, M. The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides. Mut. Res. Lett. 1990, 245, 245–249. [Google Scholar] [CrossRef]
- OECD. Test No. 474: Mammalian Erythrocyte Micronucleus Test; OECD: Paris, France, 2016. [Google Scholar]
| Cytotoxicity Evaluation | Hemolytic Activity | ||
|---|---|---|---|
| Concentration (µg/mL) | PBMC Viability (%) | Concentration (mg/mL) | Hemolysis (%) |
| LE | LE | ||
| 6.25 | 97.4 ± 0.16 a | 0.12 | 0.00 ± 0.00 a |
| 12.50 | 96.5 ± 0.31 a | 0.25 | 0.00 ± 0.00 a |
| 25.00 | 98.0 ± 0.08 a | 0.50 | 0.30 ± 0.02 b |
| 50.00 | 97.3 ± 0.18 a | 1.00 | 0.78 ± 0.09 c |
| 100.00 | 97.0 ± 0.30 a | 2.00 | 2.08 ± 0.15 d |
| PRF | PRF | ||
| 6.25 | 94.4 ± 1.8 a | 0.12 | 0.00 ± 0.00 a |
| 12.50 | 96.7 ± 0.7 a | 0.25 | 0.00 ± 0.00 a |
| 25.00 | 97.1 ± 0.2 a | 0.50 | 0.00 ± 0.00 a |
| 50.00 | 96.0 ± 1.3 a | 1.00 | 0.46 ± 0.11 b |
| 100.00 | 95.8 ± 0.9 a | 2.00 | 1.22 ± 0.02 e |
| Negative control | 96.6 ± 1.0 a | Negative control | 0.51 ± 0.14 b |
| Parameter | Control | LE | PRF |
|---|---|---|---|
| Water consumed (mL) | 25.46 ± 0.80 | 23.55 ± 0.66 | 24.71 ± 0.33 |
| Food consumed (g) | 17.64 ± 1.21 | 13.14 ± 1.05 * | 17.85 ± 1.65 |
| Weight gain (g) | 38.85 ± 3.09 | 40.01 ± 3.54 | 40.12 ± 3.47 |
| Parameter | Control | LE | PRF |
|---|---|---|---|
| Erythrocytes (106/mm3) | 5.00 ± 0.12 | 4.99 ± 1.04 | 5.02 ± 0.32 |
| Hematocrit (%) | 38.85 ± 0.12 | 38.96 ± 0.35 | 39.07 ± 0.27 |
| Hemoglobin (g/dL) | 13.22 ± 0.11 | 13.22 ± 0.28 | 13.41 ± 0.20 |
| MCV (fL) | 84.00 ± 0.51 | 83.67 ± 0.61 | 84.21 ± 0.24 |
| MCH (pg) | 28.17 ± 0.32 | 28.20 ± 0.20 | 28.01 ± 0.35 |
| MCHC (%) | 35.22 ± 0.43 | 35.02 ± 0.34 | 35.47 ± 0.61 |
| Leucocytes (103/mm3) | 6.26 ± 0.18 | 6.17 ± 0.42 | 6.28 ± 0.55 |
| Segmented (%) | 53.79 ± 0.66 | 54.74 ± 0.67 | 53.81 ± 0.79 |
| Lymphocytes (%) | 29.22 ± 0.32 | 30.08 ± 0.35 | 29.28 ± 0.40 |
| Monocytes (%) | 15.33 ± 0.55 | 15.09 ± 0.22 | 15.29 ± 0.42 |
| Parameter | Control | LE | PRF |
|---|---|---|---|
| Albumin (g/dL) | 1.99 ± 0.09 | 2.10 ± 0.14 | 2.00 ± 0.11 |
| ALT (U/L) | 68.20 ± 0.22 | 72.95 ± 0.45 * | 67.95 ± 0.45 |
| AST (U/L) | 92.09 ± 1.02 | 90.07 ± 0.93 | 91.07 ± 1.01 |
| Total protein (g/dL) | 6.20 ± 0.10 | 6.12 ± 0.22 | 6.19 ± 0.24 |
| Alkaline phosphatase (IU/L) | 12.61 ± 0.08 | 12.38 ± 0.24 | 12.43 ± 0.16 |
| GGT (U/L) | 9.60 ± 0.08 | 9.48 ± 0.38 | 9.54 ± 0.06 |
| Creatinine (mg/dL) | 0.23 ± 0.08 | 0.22 ± 0.07 | 0.20 ± 0.09 |
| BUN | 3.67 ± 0.12 | 3.52 ± 0.51 | 3.66 ± 0.14 |
| Total Cholesterol (mg/dL) | 78.45 ± 0.21 | 78.44 ± 0.76 | 79.00 ± 0.09 |
| Triglycerides (mg/dL) | 100.08 ± 0.91 | 101.32 ± 1.09 | 99.56 ± 0.75 |
| Parameter | Control | LE | PRF |
|---|---|---|---|
| Heart (g) | 0.18 ± 0.02 | 0.18 ± 0.02 | 0.18 ± 0.00 |
| Liver (g) | 2.03 ± 0.14 | 2.50 ± 0.26 | 2.05 ± 0.22 |
| Kidney (g) | 0.57 ± 0.02 | 0.56 ± 0.04 | 0.58 ± 0.05 |
| Spleen (g) | 0.18 ± 0.00 | 0.19 ± 0.04 | 0.18 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, R.R.d.V.; de Oliveira, A.M.; dos Prazeres, G.B.; da Silva, A.R.; Costa, F.F.; Barros, B.R.d.S.; Souza, T.G.d.S.; Coelho, L.C.B.B.; de Melo, C.M.L.; Ferreira, M.R.A.; et al. Evaluation of Cytotoxicity and Acute Oral Toxicity of Saline Extract and Protein-Rich Fraction from Moringa oleifera Lam. Leaves. Pharmaceuticals 2024, 17, 1045. https://doi.org/10.3390/ph17081045
Alves RRdV, de Oliveira AM, dos Prazeres GB, da Silva AR, Costa FF, Barros BRdS, Souza TGdS, Coelho LCBB, de Melo CML, Ferreira MRA, et al. Evaluation of Cytotoxicity and Acute Oral Toxicity of Saline Extract and Protein-Rich Fraction from Moringa oleifera Lam. Leaves. Pharmaceuticals. 2024; 17(8):1045. https://doi.org/10.3390/ph17081045
Chicago/Turabian StyleAlves, Robson Raion de Vasconcelos, Alisson Macário de Oliveira, Gabryella Borges dos Prazeres, Abdênego Rodrigues da Silva, Franciele Florencio Costa, Bárbara Rafaela da Silva Barros, Talita Giselly dos Santos Souza, Luana Cassandra Breintenbach Barroso Coelho, Cristiane Moutinho Lagos de Melo, Magda Rhayanny Assunção Ferreira, and et al. 2024. "Evaluation of Cytotoxicity and Acute Oral Toxicity of Saline Extract and Protein-Rich Fraction from Moringa oleifera Lam. Leaves" Pharmaceuticals 17, no. 8: 1045. https://doi.org/10.3390/ph17081045
APA StyleAlves, R. R. d. V., de Oliveira, A. M., dos Prazeres, G. B., da Silva, A. R., Costa, F. F., Barros, B. R. d. S., Souza, T. G. d. S., Coelho, L. C. B. B., de Melo, C. M. L., Ferreira, M. R. A., Soares, L. A. L., Chagas, C. A., Macedo, M. L. R., Napoleão, T. H., Fernandes, M. P., & Paiva, P. M. G. (2024). Evaluation of Cytotoxicity and Acute Oral Toxicity of Saline Extract and Protein-Rich Fraction from Moringa oleifera Lam. Leaves. Pharmaceuticals, 17(8), 1045. https://doi.org/10.3390/ph17081045








