Characterization of Liquid Dosage Forms of Atenolol and Enalapril Maleate for Oral and Enteral Feeding Administration
Abstract
1. Introduction
2. Results
2.1. Preliminary Characterization of the SuspendIt® Vehicle and the Cardiovascular Oral Formulations
2.1.1. pH
2.1.2. Rheological Properties
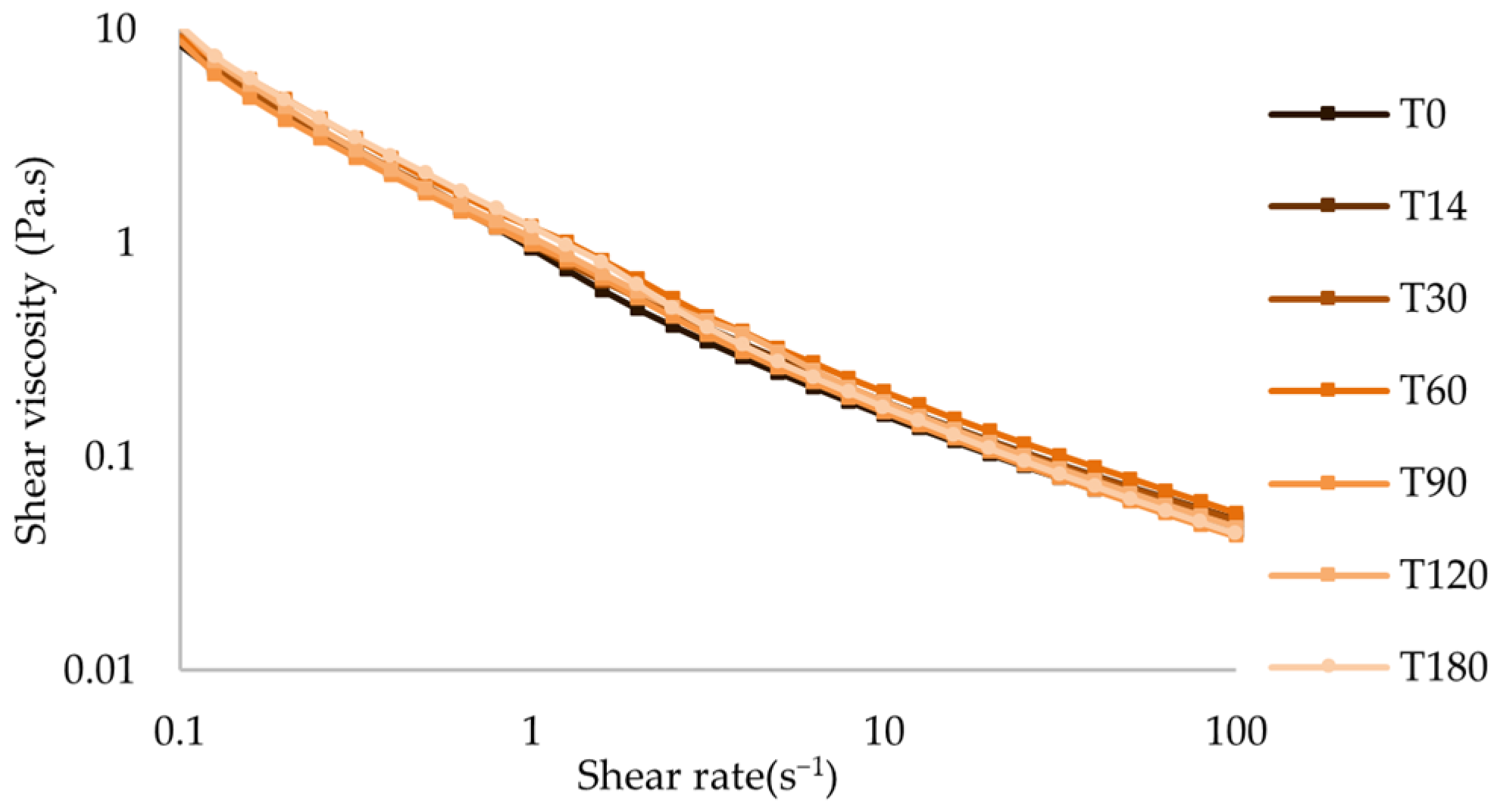
Flow Behavior
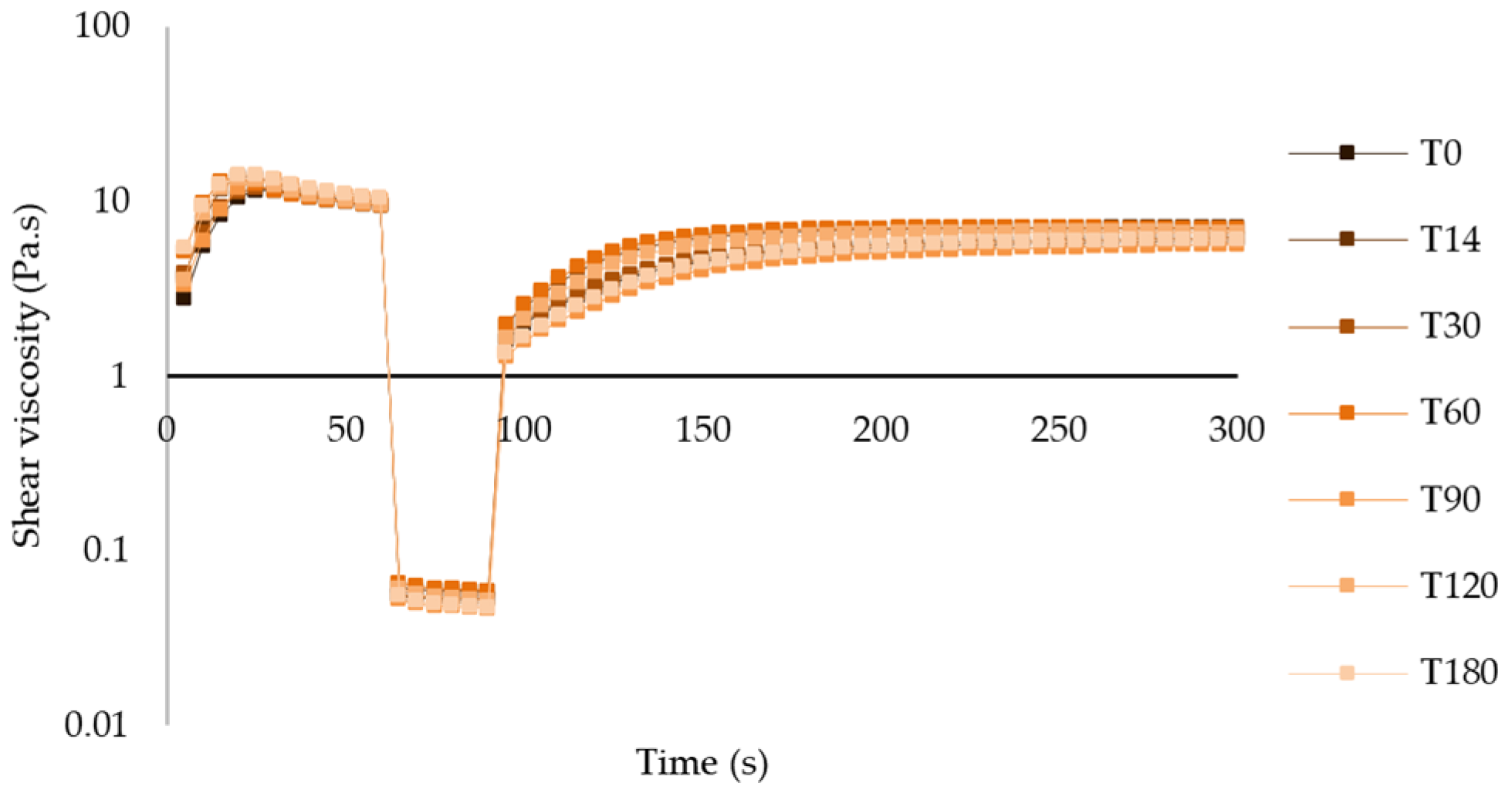
Thixotropy
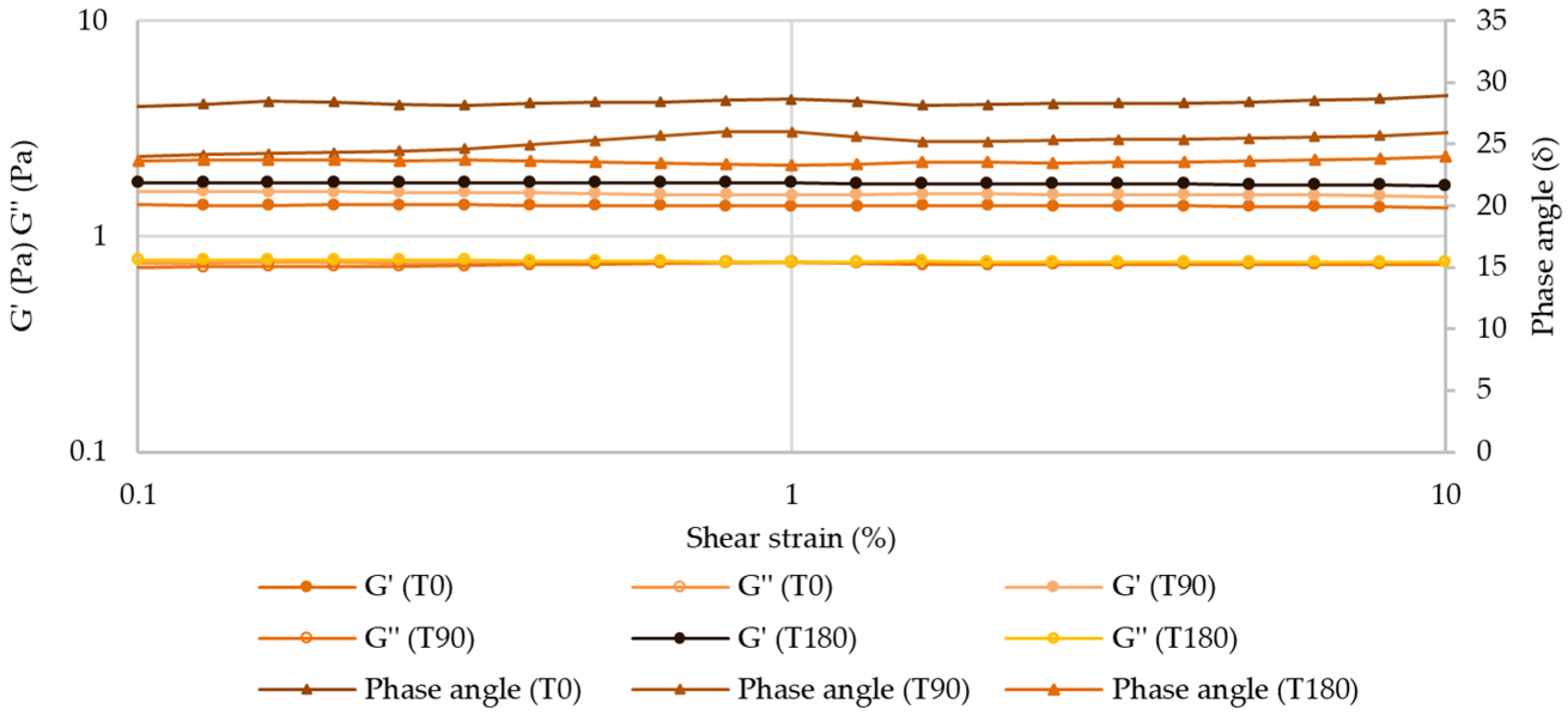
Amplitude Sweep—Linear Viscoelastic Region (LVER)
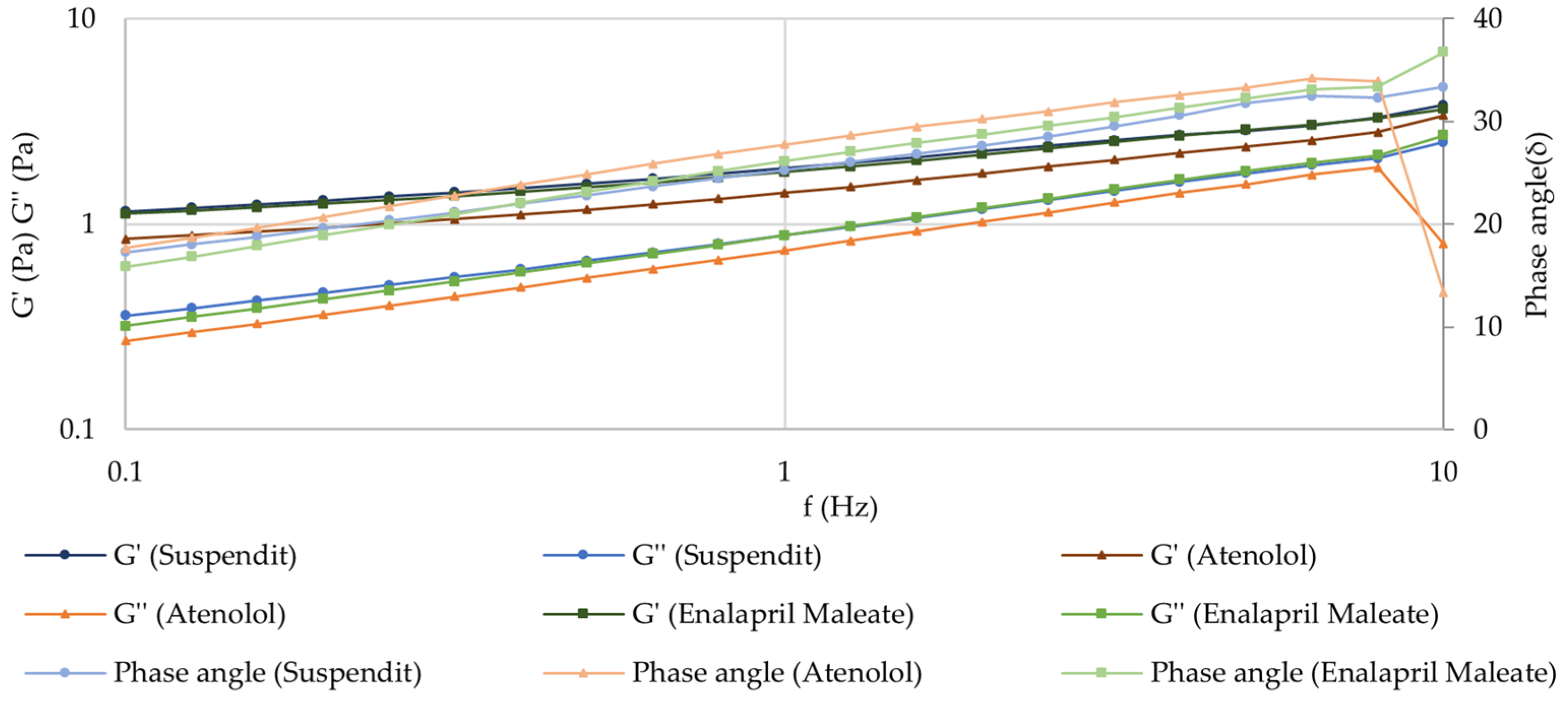
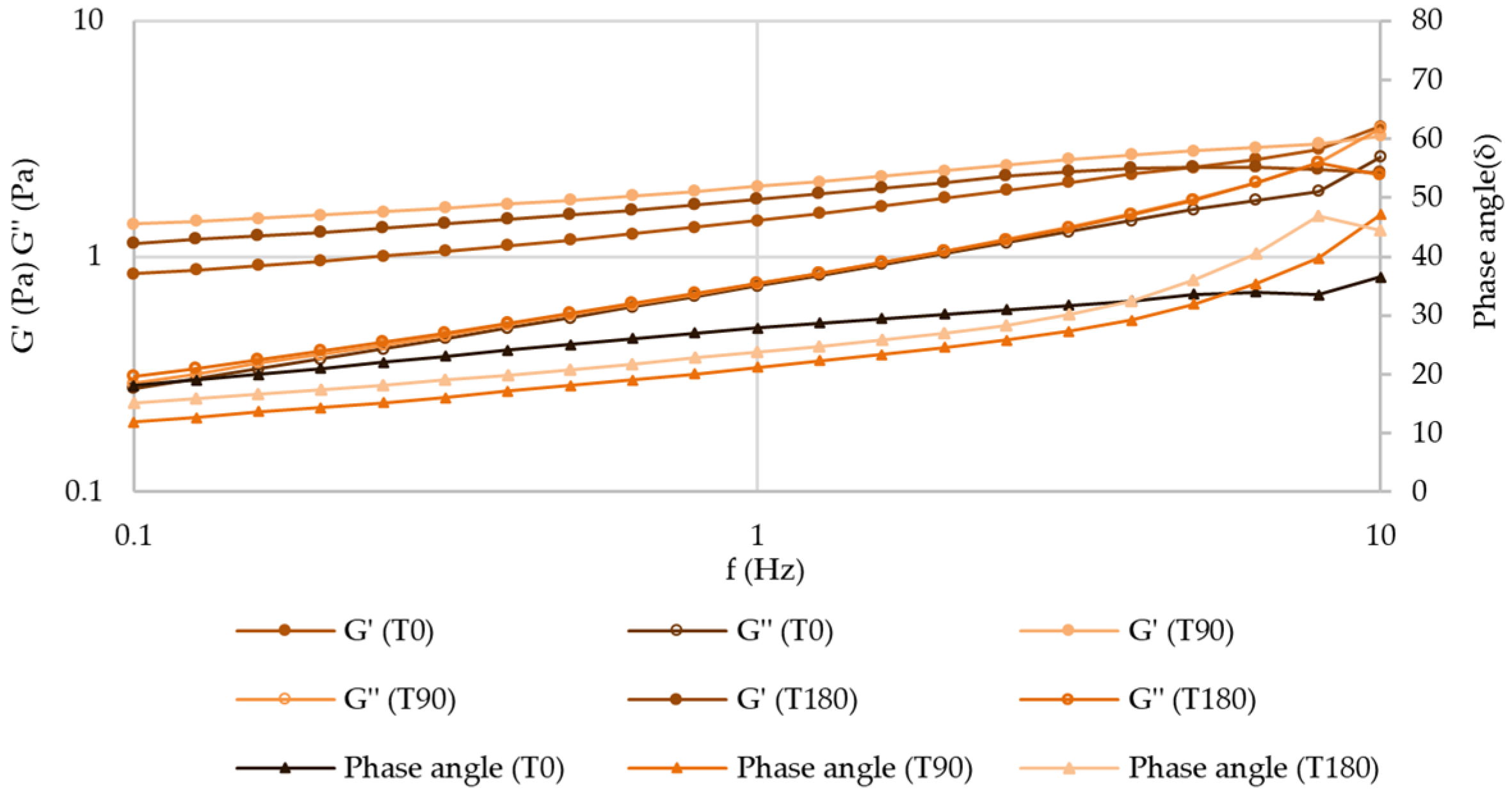
Frequency Sweep (Mechanical Spectrum)
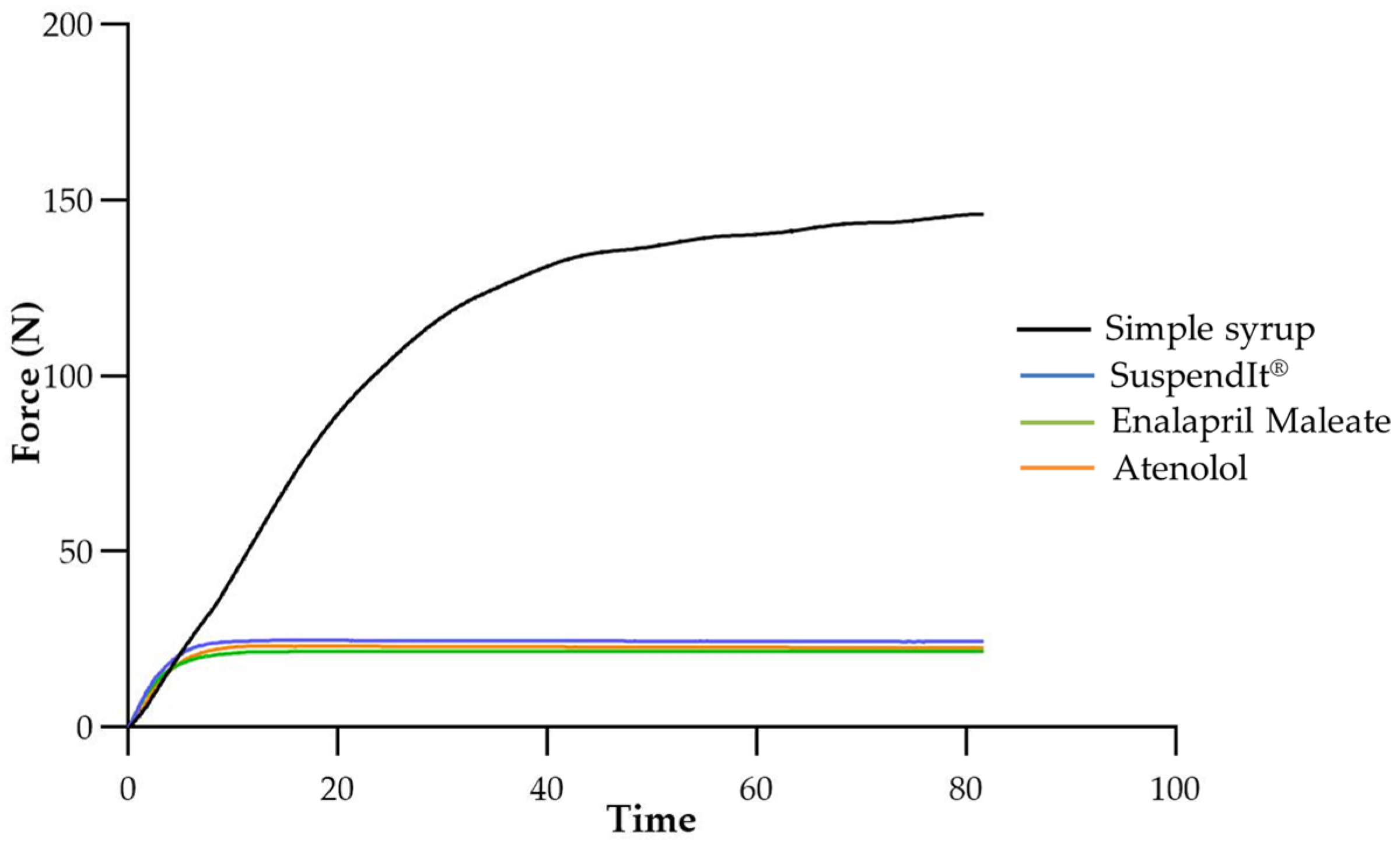
2.1.3. Injectability
2.1.4. Evaluation of the SuspendIt® Oral Suspending Vehicle as a Bitter Bloc for Atenolol and Enalapril Maleate
2.2. Stability Study of the Oral Formulations
2.2.1. Organoleptic Characteristics
2.2.2. pH
2.2.3. Rheological Properties
Flow Behavior
Thixotropy
Amplitude Sweep—Linear Viscoelastic Region (LVER)
Frequency Sweep (Mechanical Spectrum)
2.2.4. Preservative Effectiveness
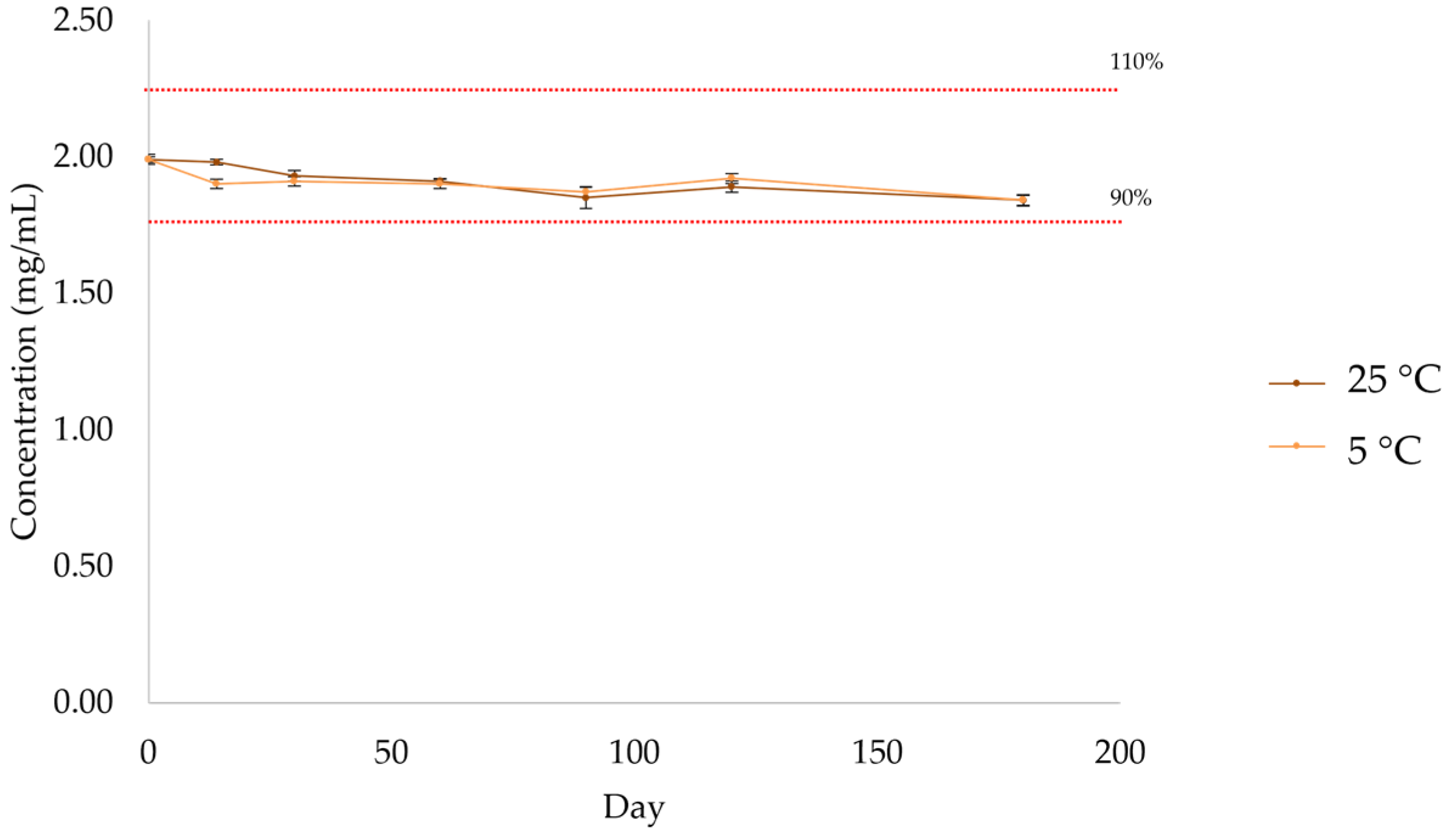
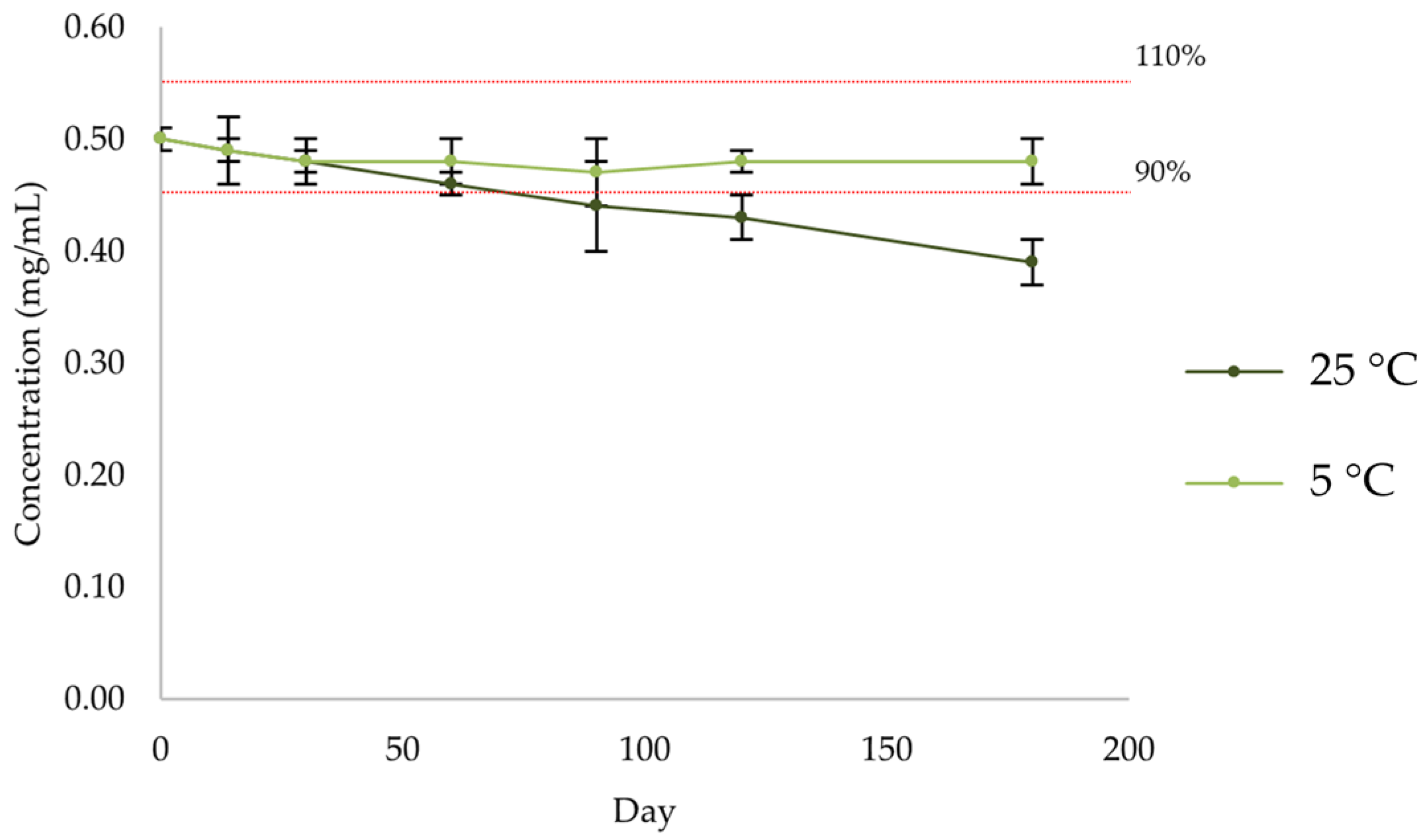
2.2.5. Active Substance Assay
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preliminary Characterization of SuspendIt® Vehicle and the Oral Suspensions
4.2.1. pH
4.2.2. Rheological Properties
4.2.3. Injectability
4.2.4. E-tongue Apparatus and Potentiometric Analysis of Atenolol and Enalapril Maleate Solutions
Statistical Analysis
4.3. Stability Study
4.3.1. Preservative Effectiveness
- Bacteria: not less than 1 log reduction from the initial count at 14 days, and no increase from the 14 days’ count at 28 days;
- Yeasts and molds: no increase from the initial calculated count at 14 days and 28 days.
4.3.2. Active Substance Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, M.; Almeida, I.F. The Role of Pharmaceutical Compounding in Promoting Medication Adherence. Pharmaceuticals 2022, 15, 1091. [Google Scholar] [CrossRef]
- Smeets, N.J.L.; Schreuder, M.F.; Dalinghaus, M.; Male, C.; Lagler, F.B.; Walsh, J.; Laer, S.; de Wildt, S.N. Pharmacology of enalapril in children: A review. Drug Discov. Today 2020, 25, 1957–1970. [Google Scholar] [CrossRef] [PubMed]
- Schoolwerth, A.C.; Sica, D.A.; Ballermann, B.J.; Wilcox, C.S. Renal considerations in angiotensin converting enzyme inhibitor therapy: A statement for healthcare professionals from the Council on the Kidney in Cardiovascular Disease and the Council for High Blood Pressure Research of the American Heart Association. Circulation 2001, 104, 1985–1991. [Google Scholar] [CrossRef]
- Heel, R.C.; Brogden, R.N.; Speight, T.M.; Avery, G.S. Atenolol: A Review of its Pharmacological Properties and Therapeutic Efficacy in Angina Pectoris and Hypertension. Drugs 1979, 17, 425–460. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.G. Beta-Blockers. In Encyclopedia of Heart Diseases; Khan, M.G., Ed.; Academic Press: Burlington, VT, USA, 2006; pp. 159–167. [Google Scholar]
- Gorre, F.; Vandekerckhove, H. Beta-blockers: Focus on mechanism of action Which beta-blocker, when and why? Acta Cardiol. 2010, 65, 565–570. [Google Scholar] [CrossRef] [PubMed]
- INFARMED, I.P. Resumo das características do medicamento: Enalapril. Infomed. Available online: https://extranet.infarmed.pt/INFOMED-fo/ (accessed on 5 August 2024).
- Medicamento, C.T.d. Formulário Galénico Português; Associação Nacional das Farmácias: Lisboa, Portugal, 2001. [Google Scholar]
- Siddiqi, N.; Shatat, I.F. Antihypertensive agents: A long way to safe drug prescribing in children. Pediatr. Nephrol. 2020, 35, 2049–2065. [Google Scholar] [CrossRef] [PubMed]
- INFARMED, I.P. Resumo das características do medicamento: Atenolol. Infomed. Available online: https://extranet.infarmed.pt/INFOMED-fo/ (accessed on 5 August 2024).
- Breitkreutz, J.; Boos, J. Paediatric and geriatric drug delivery. Expert Opin. Drug Deliv. 2007, 4, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.S. Extemporaneous compounding of oral liquid dosage formulations and alternative drug delivery methods for anticancer drugs. Pharmacotherapy 2011, 31, 164–192. [Google Scholar] [CrossRef] [PubMed]
- Stavroulakis, T.; McDermott, C.J. Enteral feeding in neurological disorders. Pract. Neurol. 2016, 16, 352–361. [Google Scholar] [CrossRef]
- Ferreira-Neto, C.J.B.; de Andrade, R.A.; Tonin, F.S.; Wiens, A. Solid Oral Dosage Forms Use in Adults with Neurological Disorders and Swallowing Difficulties: A Scoping Review. Dysphagia 2022, 37, 909–922. [Google Scholar] [CrossRef]
- Kurien, M.; Penny, H.; Sanders, D.S. Impact of direct drug delivery via gastric access devices. Expert Opin. Drug Deliv. 2015, 12, 455–463. [Google Scholar] [CrossRef]
- Malkawi, W.A.; AlRafayah, E.; AlHazabreh, M.; AbuLaila, S.; Al-Ghananeem, A.M. Formulation Challenges and Strategies to Develop Pediatric Dosage Forms. Children 2022, 9, 488. [Google Scholar] [CrossRef]
- Ogbonna, J.D.; Cunha, E.; Attama, A.A.; Ofokansi, K.C.; Ferreira, H.; Pinto, S.; Gomes, J.; Marx, Í.M.; Peres, A.M.; Lobo, J.M.; et al. Overcoming Challenges in Pediatric Formulation with a Patient-Centric Design Approach: A Proof-of-Concept Study on the Design of an Oral Solution of a Bitter Drug. Pharmaceuticals 2022, 15, 1331. [Google Scholar] [CrossRef]
- INFARMED–Instituto Nacional da Farmácia e do Medicamento. Farmacopeia Portuguesa 9: Edição Oficial; INFARMED, I.P.: Lisboa, Portugal, 2009. [Google Scholar]
- Leon Lachman, H.A.L.; Joseph, L.K. The Theory and Practice of Industrial Pharmacy, 3rd ed.; Lea & Febiger Philadelphia: Philadelphia, PA, USA, 1970. [Google Scholar]
- Loyd, V.; Allen, N.G.P.; Howard, C.A. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems, 9th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Cutaia, K.; Chablani, L.; Zhao, F. Basics of Compounding: Vehicles for Compounded Oral Liquid Medications: A Review. Int. J. Pharm. Compd. 2018, 22, 480–489. [Google Scholar] [PubMed]
- Freerks, L.; Sucher, W.; Tarnow, M.-J.; Eckert, C.; Klein, S. Vehicles for Drug Administration to Children: Results and Learnings from an In-Depth Screening of FDA-Recommended Liquids and Soft Foods for Product Quality Assessment. Pharm. Res. 2022, 39, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Blaszczyk, A.; Brandt, N.; Ashley, J.; Tuders, N.; Doles, H.; Stefanacci, R.G. Crushed Tablet Administration for Patients with Dysphagia and Enteral Feeding: Challenges and Considerations. Drugs Aging 2023, 40, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.S. Handbook of Drug Administration via Enteral Feeding Tubes on Behalf of the British Pharmaceutical Nutrition Group. Am. J. Pharm. Educ. 2007, 71, 99. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019. [Google Scholar]
- Vogelpoel, H.; Welink, J.; Amidon, G.L.; Junginger, H.E.; Midha, K.K.; Möller, H.; Olling, M.; Shah, V.P.; Barends, D.M. Biowaiver monographs for immediate release solid oral dosage forms based on biopharmaceutics classification system (BCS) literature data: Verapamil hydrochloride, propranolol hydrochloride, and atenolol. J. Pharm. Sci. 2004, 93, 1945–1956. [Google Scholar] [CrossRef]
- Bontha, L.; Sriramaneni, R.N. New Dissolution Method for the Evaluation of Enalapril Maleate Tablets using pH 7.2 Phosphate Buffer in vitro and Determination of its Content by Validated UV Spectrophotometric Method. JASA 2007, 2, 34–37. [Google Scholar]
- Professional Compounding Centers of America (PCCA). Similar Names. Different Products—SuspendIt® & SuspendIt® Anhydrous. Available online: https://www.pccarx.com/Blog/similar-names-different-products-suspendit-suspendit-anhydrous (accessed on 15 November 2023).
- Ip, K.; Shan, A.; Carvalho, M.; Baker, S.; Banov, D. Physicochemical Stability of Extemporaneously Prepared Oral Suspension of Fluconazole 50 mg/mL in SuspendIt™. Pharm. Technol. Hosp. Pharm. 2018, 3, 101–112. [Google Scholar] [CrossRef]
- Graves, R.; Phan, K.V.; Bostanian, L.A.; Mandal, T.K.; Pramar, Y.V. Stability of Spironolactone Oral Suspension in PCCA Base, SuspendIt. Int. J. Pharm. Compd. 2017, 21, 334–338. [Google Scholar] [PubMed]
- Graves, R.A.; Phan, K.V.; Bostanian, L.A.; Mandal, T.K.; Pramar, Y.V. Physicochemical Stability of an Oral Suspension of Trimethoprim 20 mg/mL in Combination with Sulfadiazine 200 mg/mL in PCCA Base SuspendIt. Int. J. Pharm. Compd. 2017, 21, 430–435. [Google Scholar] [PubMed]
- Pramar, Y.V.; Mandal, T.K.; Bostanian, L.A.; Le, G.; Morris, T.C.; Graves, R.A. Physicochemical and Microbiological Stability of Compounded Metronidazole Suspensions in PCCA SuspendIt. Int. J. Pharm. Compd. 2021, 25, 169–175. [Google Scholar]
- Pramar, Y.V.; Mandal, T.K.; Bostanian, L.A.; Nguyen, A.T.; Miller, V.; Morris, T.C.; Graves, R.A. Stability of Compounded Ursodiol Suspensions in PCCA Base, SuspendIt. Int. J. Pharm. Compd. 2019, 23, 70–76. [Google Scholar]
- Graves, R.A.; Mandal, T.K.; Bostanian, L.A.; Nguyen, A.T.; Swopes, D.; Morris, T.C.; Pramar, Y.V. Physicochemical Stability of Compounded Amlodipine Besylate Suspensions in PCCA Base, SuspendIt. Int. J. Pharm. Compd. 2019, 23, 519–527. [Google Scholar] [PubMed]
- Pramar, Y.V.; Mandal, T.K.; Bostanian, L.A.; Le, G.; Morris, T.C.; Graves, R.A. Physicochemical Stability of Compounded Allopurinol Suspensions in PCCA Base, SuspendIt. Int. J. Pharm. Compd. 2020, 24, 413–419. [Google Scholar] [PubMed]
- Pramar, Y.V.; Graves, R.A.; Ledet, G.A.; Phan, K.V.; Bostanian, L.A.; Mandal, T.K. Stability of Clindamycin Hydrochloride in PCCA Base SuspendIt. Int. J. Pharm. Compd. 2016, 20, 421–425. [Google Scholar] [PubMed]
- Pramar, Y.V.; Mandal, T.K.; Bostanian, L.A.; Nguyen, A.T.; Morris, T.C.; Graves, R.A. Physicochemical Stability of Compounded Naltrexone Hydrochloride Solutions in PCCA Base SuspendIt. Int. J. Pharm. Compd. 2019, 23, 157–162. [Google Scholar] [PubMed]
- TA Instruments. Understanding Rheology of Structured Fluids. Available online: https://www.tainstruments.com/pdf/literature/AAN016_V1_U_StructFluids.pdf (accessed on 27 May 2024).
- Peter Herh, J.T.; Wu, S.; Bernzen, M.; Rudolph, B. The rheology of pharmaceutical and cosmetic semisolids. Am. Lab. 1998, 30, 12–14. [Google Scholar]
- Paar, A. Basics of Thixotropy. Available online: https://wiki.anton-paar.com/en/basics-of-thixotropy/ (accessed on 27 May 2024).
- NETZSCH-Gerätebau GmbH. Rotational Rheology: Interpretation of Data by Application, 2nd ed.; NETZSCH-Gerätebau GmbH: Selb, Germany, 2022. [Google Scholar]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Medicamento, C.T.d. Xarope Simples, BP2000 (FGP B.7.). In Formulário Galénico Português; Associação Nacional de Farmácias: Lisboa, Portugal, 2007. [Google Scholar]
- Pharmacopeia, U.S. Antimicrobial Effectiveness Testing <51>. In USP–NF; United States Pharmacopeia: Rockville, MD, USA, 2020. [Google Scholar]
- Chiclana-Rodríguez, B.; Garcia-Montoya, E.; Romero-Obon, M.; Rouaz-El-Hajoui, K.; Nardi-Ricart, A.; Suñé-Pou, M.; Suñé-Negre, J.M.; Pérez-Lozano, P. Palatability and Stability Studies to Optimize a Carvedilol Oral Liquid Formulation for Pediatric Use. Pharmaceutics 2024, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Elgammal, A.; Ryan, J.; Bradley, C.; Crean, A.; Bermingham, M. The impact of drug palatability on prescribing and dispensing of antibiotic formulations for paediatric patients: A cross-sectional survey of general practitioners and pharmacists. Fam. Pract. 2023. [Google Scholar] [CrossRef]
- Mrokowska, M.M.; Krztoń-Maziopa, A. Viscoelastic and shear-thinning effects of aqueous exopolymer solution on disk and sphere settling. Sci. Rep. 2019, 9, 7897. [Google Scholar] [CrossRef] [PubMed]
- Ramli, H.; Zainal, N.F.A.; Hess, M.; Chan, C.H. Basic principle and good practices of rheology for polymers for teachers and beginners. Chem. Teach. Int. 2022, 4, 307–326. [Google Scholar] [CrossRef]
- Wang, C.; Xu, M.; Lv, W.-P.; Qiu, P.; Gong, Y.-Y.; Li, D.-S. Study on Rheological Behavior of Konjac Glucomannan. Phys. Procedia 2012, 33, 25–30. [Google Scholar] [CrossRef]
- Zhong, L.; Oostrom, M.; Truex, M.J.; Vermeul, V.R.; Szecsody, J.E. Rheological behavior of xanthan gum solution related to shear thinning fluid delivery for subsurface remediation. J. Hazard. Mater. 2013, 244–245, 160–170. [Google Scholar] [CrossRef]
- Al-Malah, K. Rheological properties of carbomer dispersions. Annu. Trans. Nord. Rheol. Soc. 2006, 14, 123. [Google Scholar]
- Miller, S.C.; Drabik, B.R. Rheological properties of poloxamer vehicles. Int. J. Pharm. 1984, 18, 269–276. [Google Scholar] [CrossRef]
- Ross, A.I.V.; Tyler, P.; Borgognone, M.G.; Eriksen, B.M. Relationships between shear rheology and sensory attributes of hydrocolloid-thickened fluids designed to compensate for impairments in oral manipulation and swallowing. J. Food Eng. 2019, 263, 123–131. [Google Scholar] [CrossRef]
- Chen, T.T. Rheological Techniques for Yield Stress Analysis; TA Instruments: New Castle, DE, USA, 2000. [Google Scholar]
- Banov, D.; Liu, Y.; Ip, K.; Shan, A.; Vu, C.; Zdoryk, O.; Bassani, A.S.; Carvalho, M. Analysis of the Physical Characteristics of an Anhydrous Vehicle for Compounded Pediatric Oral Liquids. Pharmaceutics 2023, 15, 2642. [Google Scholar] [CrossRef]
- Sangroniz, L.; Fernández, M.; Santamaria, A. Polymers and rheology: A tale of give and take. Polymer 2023, 271, 125811. [Google Scholar] [CrossRef]
- Öhrlund, Å. Evaluation of Rheometry Amplitude Sweep Cross-Over Point as an Index of Flexibility for HA Fillers. J. Cosmet. Dermatol. Sci. Appl. 2018, 8, 47–54. [Google Scholar] [CrossRef]
- Carmona, J.A.; Lucas, A.; Ramírez, P.; Calero, N.; Muñoz, J. Nonlinear and linear viscoelastic properties of a novel type of xanthan gum with industrial applications. Rheol. Acta 2015, 54, 993–1001. [Google Scholar] [CrossRef]
- Ghebremedhin, M.; Schreiber, C.; Zielbauer, B.; Dietz, N.; Vilgis, T. Interaction of xanthan gums with galacto- and glucomannans. Part II: Heat induced synergistic gelation mechanism and their interaction with salt. J. Phys. Mater. 2020, 3, 034014. [Google Scholar] [CrossRef]
- Paar, A. Frequency Sweeps. Available online: https://wiki.anton-paar.com/en/frequency-sweeps/ (accessed on 11 April 2024).
- European Medicines Agency. Use of Methyl- and Propylparaben as Excipients in Human Medicinal Products for Oral use—Scientific Guideline. Available online: https://www.ema.europa.eu/en/use-methyl-propylparaben-excipients-human-medicinal-products-oral-use-scientific-guideline (accessed on 28 May 2024).
- Saito, J.; Nadatani, N.; Setoguchi, M.; Nakao, M.; Kimura, H.; Sameshima, M.; Kobayashi, K.; Matsumoto, H.; Yoshikawa, N.; Yokoyama, T.; et al. Potentially harmful excipients in neonatal medications: A multicenter nationwide observational study in Japan. J. Pharm. Health Care Sci. 2021, 7, 23. [Google Scholar] [CrossRef]
- Álvaro-Alonso, E.A.; Lorenzo, M.P.; Gonzalez-Prieto, A.; Izquierdo-García, E.; Escobar-Rodríguez, I.; Aguilar-Ros, A. Physicochemical and Microbiological Stability of Two Oral Solutions of Methadone Hydrochloride 10 mg/mL. Molecules 2022, 27, 2812. [Google Scholar] [CrossRef] [PubMed]
- Binson, G.; Beuzit, K.; Migeot, V.; Marco, L.; Troussier, B.; Venisse, N.; Dupuis, A. Preparation and Physicochemical Stability of Liquid Oral Dosage Forms Free of Potentially Harmful Excipient Designed for Pediatric Patients. Pharmaceutics 2019, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Belayneh, A.; Tadese, E.; Molla, F. Safety and Biopharmaceutical Challenges of Excipients in Off-Label Pediatric Formulations. Int. J. Gen. Med. 2020, 13, 1051–1066. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Blanco, S.; González-Freire, L.; Dávila-Pousa, M.C.; Crespo-Diz, C. pH determination as a quality standard for the elaboration of oral liquid compounding formula. Farm. Hosp. 2018, 42, 221–227. [Google Scholar] [CrossRef]
- Foppa, T.; Murakami, F.S.; Silva, M.A. Development, validation and stability study of pediatric atenolol syrup. Pharmazie 2007, 62, 519–521. [Google Scholar]
- Garner, S.S.; Wiest, D.B.; Reynolds, E.R., Jr. Stability of atenolol in an extemporaneously compounded oral liquid. Am. J. Hosp. Pharm. 1994, 51, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Nahata, M.C.; Morosco, R.S.; Hipple, T.F. Stability of enalapril maleate in three extemporaneously prepared oral liquids. Am. J. Health Syst. Pharm. 1998, 55, 1155–1157. [Google Scholar] [CrossRef] [PubMed]
- Diego, M.; Godoy, G.; Mennickent, S.; Godoy, R. Chemical stability of enalapril maleate drug substance and tablets by a stability-indicating liquid chromatographic method. Química Nova 2010, 34, 450–454. [Google Scholar] [CrossRef]
- Bout, M.R.; Vromans, H. Influence of commonly used excipients on the chemical degradation of enalapril maleate in its solid state: The role of condensed water. Eur. J. Pharm. Sci. 2022, 171, 106121. [Google Scholar] [CrossRef] [PubMed]
- Hatem, O.; Suhail, F.; Juda, A. Determination of chemical potential for carvedilol, atenolol and Propranolol diffusion through SDS micelle solution. Der Pharma Chem. 2016, 8, 276–287. [Google Scholar]
- Rafik, K. Prodrugs for Masking the Bitter Taste of Drugs. In Application of Nanotechnology in Drug Delivery; Ali Demir, S., Ed.; IntechOpen: Rijeka, Croatia, 2014; p. 12. [Google Scholar]
- Georgieva, Y.; Kassarova, M.; Kokova, V.; Apostolova, E.; Pilicheva, B. Taste masking of enalapril maleate by microencapsulation in Eudragit EPO(®) microparticles. Pharmazie 2020, 75, 61–69. [Google Scholar] [PubMed]
- Patel, D.; Doshi, D.H.; Desai, A. Short-term stability of atenolol in oral liquid formulations. Int. J. Pharm. Compd. 1997, 1, 437–439. [Google Scholar]
- Allen, L.V., Jr.; Erickson, M.A., III. Stability of alprazolam, chloroquine phosphate, cisapride, enalapril maleate, and hydralazine hydrochloride in extemporaneously compounded oral liquids. Am. J. Health-Syst. Pharm. 1998, 55, 1915–1920. [Google Scholar] [CrossRef]
- Sosnowska, K.; Winnicka, K.; Czajkowska-Kośnik, A. Stability of extemporaneous enalapril maleate suspensions for pediatric use prepared from commercially available tablets. Acta Pol. Pharm. 2009, 66, 321–326. [Google Scholar]
- Vajravelu, K.; Sreenadh, S.; Devaki, P.; Prasad, K. Mathematical model for a Herschel-Bulkley fluid flow in an elastic tube. Open Phys. 2011, 9, 1357–1365. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, R.; Aier, I.; Semwal, R.; Tyagi, P.; Varadwaj, P. Sense of Smell: Structural, Functional, Mechanistic Advancements and Challenges in Human Olfactory Research. Curr. Neuropharmacol. 2019, 17, 891–911. [Google Scholar] [CrossRef]
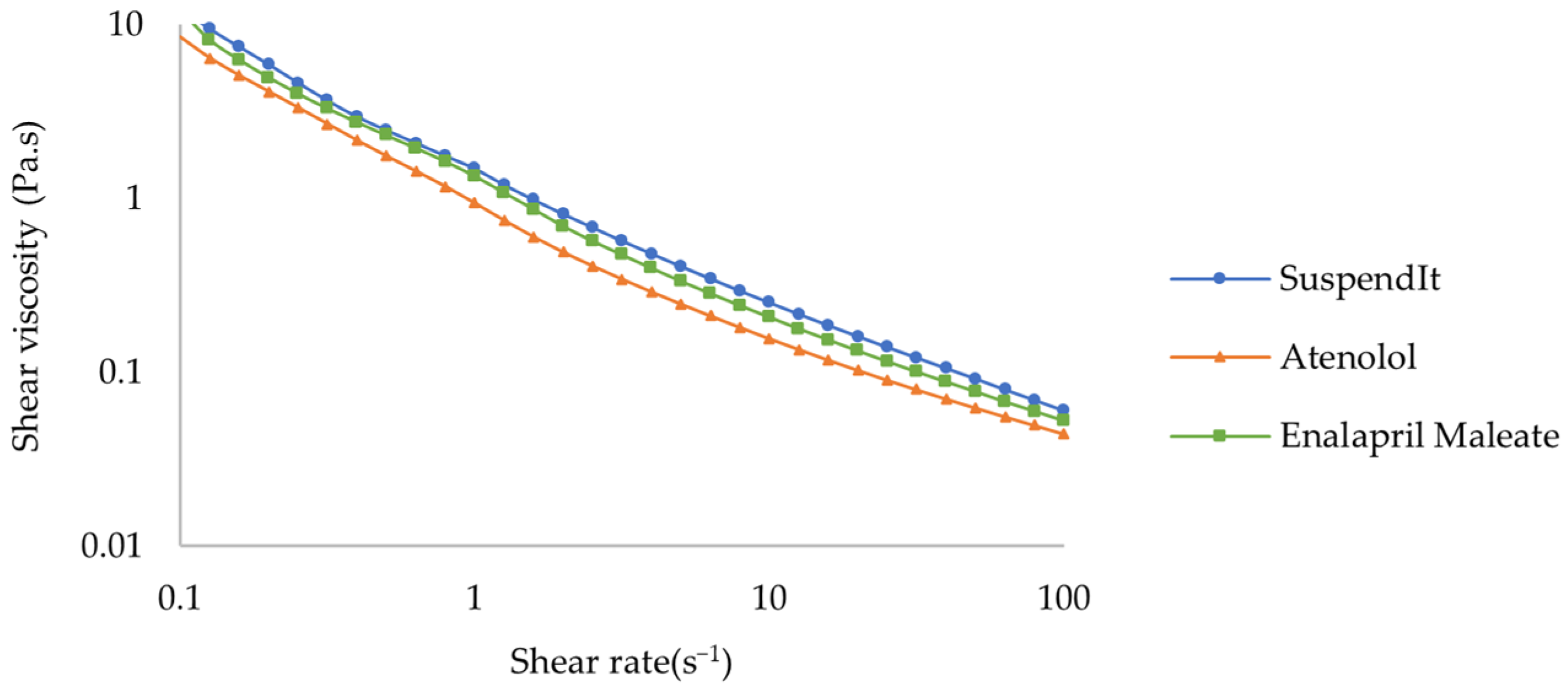
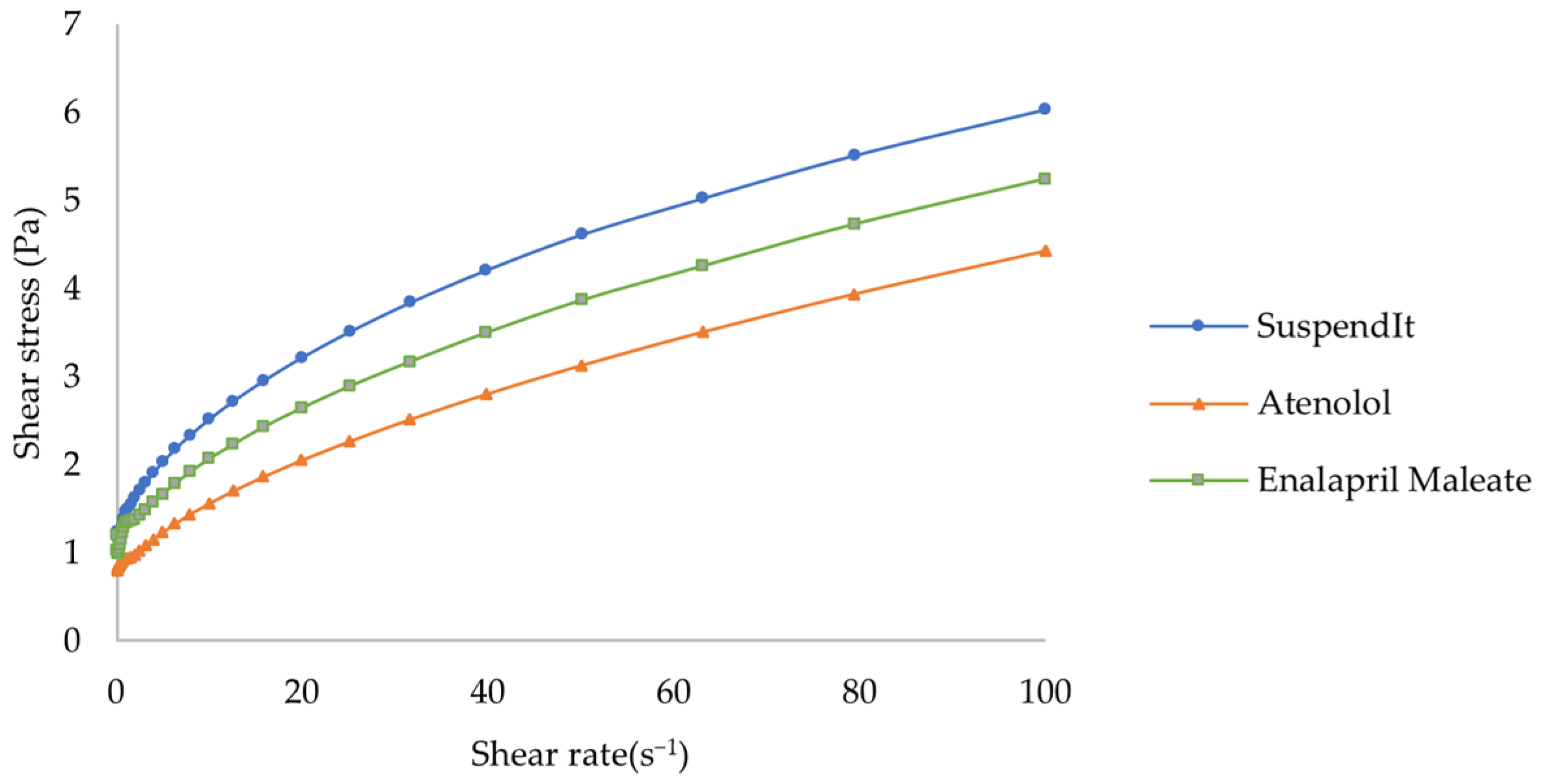
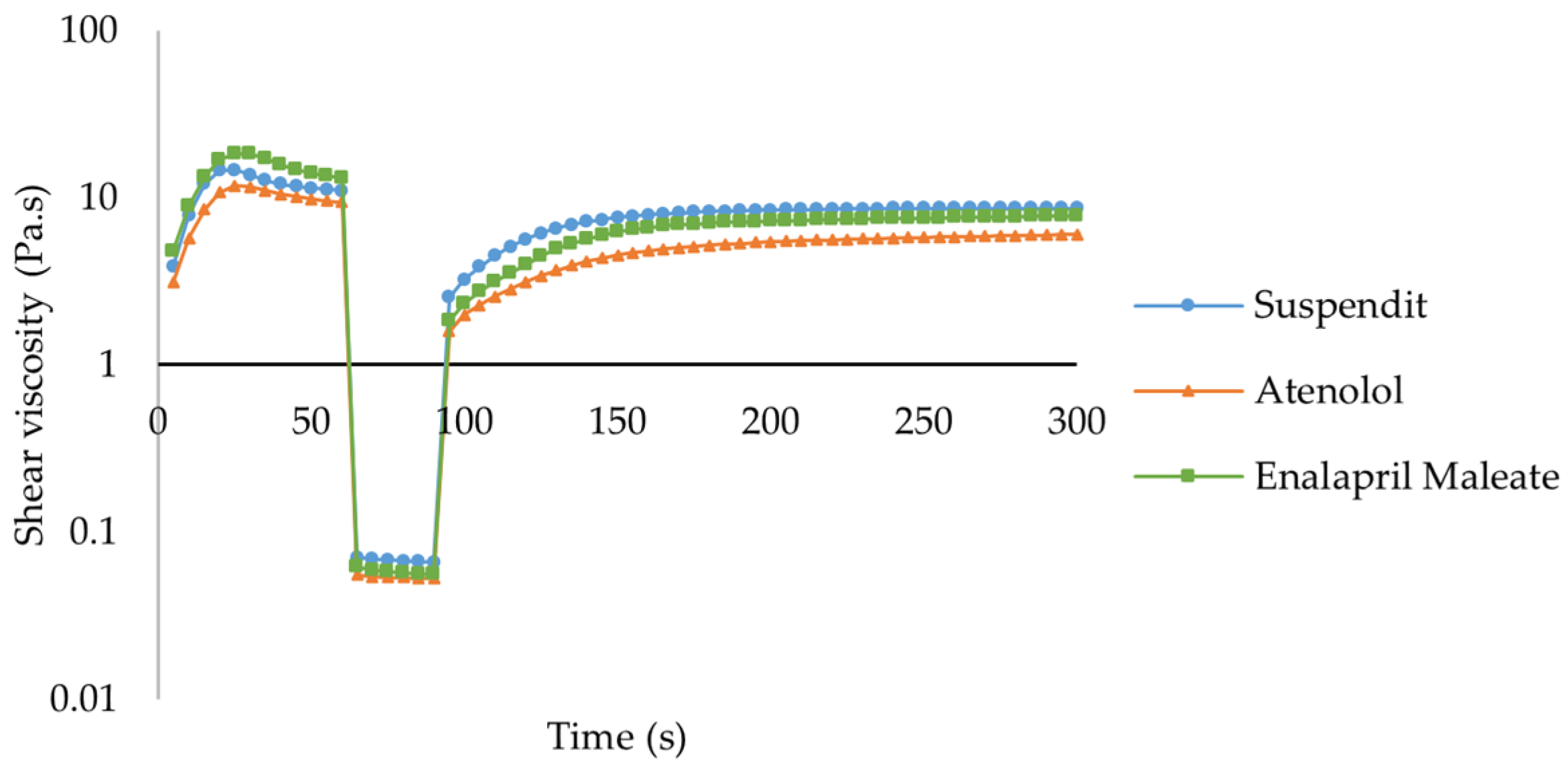
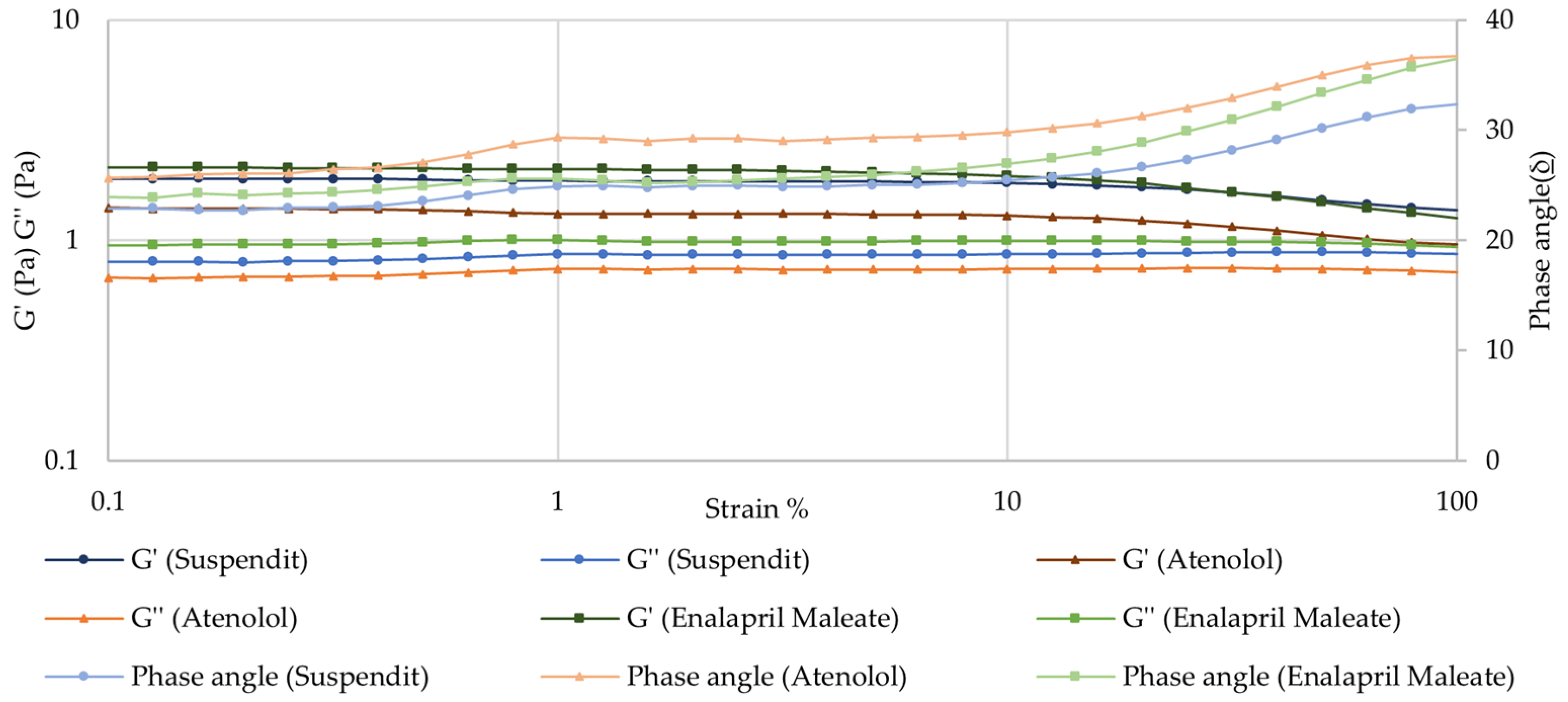



| Sample | K (Pa.sn) | n | τ0 (Pa) | R2 |
|---|---|---|---|---|
| Atenolol formulation | 0.238 ± 0.061 | 0.633 ± 0.030 | 0.756 ± 0.042 | 0.999 ± 0.000 |
| Enalapril Maleate formulation | 0.273 ± 0.028 | 0.594 ± 0.027 | 0.980 ± 0.068 | 0.998 ± 0.000 |
| SuspendIt® | 0.483 ± 0.007 | 0.516 ± 0.003 | 0.954 ± 0.003 | 0.999 ± 0.001 |
| Sample | Recovery Period (s) | Recovery Rate (%) |
|---|---|---|
| Atenolol formulation | 140.00 ± 13.64 | 69.12 ± 2.34 |
| Enalapril Maleate formulation | 150.56 ± 17.10 | 64.10 ± 6.99 |
| SuspendIt® | 120.0 ± 13.5 | 78.4 ± 3.1 |
| Sample | Mean Force ± Standard Deviation (N) |
|---|---|
| Atenolol formulation | 22.5 ± 0.2 |
| Enalapril Maleate formulation | 21.6 ± 1.5 |
| Simple syrup | 146.8 ± 2.0 |
| SuspendIt® | 22.8 ± 1.4 |
| Atenolol Formulation | Enalapril Maleate Formulation | |||
|---|---|---|---|---|
| 5 °C | 25 °C | 5 °C | 25 °C | |
| Day 0 | 4.65 ± 0.00 | 4.95 ± 0.01 | ||
| Day 14 | 4.67 ± 0.01 | 4.66 ± 0.03 | 4.97 ± 0.01 | 4.95 ± 0.01 |
| Day 30 | 4.73 ± 0.01 | 4.71 ± 0.01 | 4.95 ± 0.01 | 4.96 ± 0.01 |
| Day 60 | 4.73 ± 0.01 | 4.74 ± 0.00 | 4.93 ± 0.01 | 4.98 ± 0.02 |
| Day 90 | 4.69 ± 0.02 | 4.67 ± 0.02 | 4.90 ± 0.02 | 4.94 ± 0.06 |
| Day 120 | 4.76 ± 0.02 | 4.76 ± 0.02 | 4.96 ± 0.01 | 4.98 ± 0.02 |
| Day 180 | 4.72 ± 0.00 | 4.74 ± 0.02 | 4.94 ± 0.02 | 4.93 ± 0.03 |
| Day | 0 | 14 | 30 | 60 | 90 | 120 | 180 |
|---|---|---|---|---|---|---|---|
| K (Pa.sn) | 0.238 ± 0.061 | 0.245 ± 0.031 | 0.257 ± 0.017 | 0.255 ± 0.055 | 0.221 ± 0.019 | 0.221 ± 0.053 | 0.239 ± 0.052 |
| n | 0.633 ± 0.030 | 0.629 ± 0.023 | 0.620 ± 0.004 | 0.618 ± 0.032 | 0.609 ± 0.023 | 0.608 ± 0.035 | 0.584 ± 0.048 |
| τ0 (Pa) | 0.756 ± 0.042 | 0.811 ± 0.088 | 0.763 ± 0.009 | 0.835 ± 0.050 | 0.786 ± 0.085 | 0.822 ± 0.098 | 0.827 ± 0.100 |
| R2 | 0.999 ± 0.000 | 0.999 ± 0.001 | 1.000 ± 0.000 | 0.999 ± 0.001 | 0.999 ± 0.001 | 0.997 ± 0.002 | 0.995 ± 0.005 |
| Day | 14 | 30 | 60 | 90 | 120 | 180 |
|---|---|---|---|---|---|---|
| K (Pa.sn) | 0.241 ± 0.037 | 0.239 ± 0.032 | 0.225 ± 0.038 | 0.247 ± 0.007 | 0.229 ± 0.024 | 0.221 ± 0.011 |
| N | 0.635 ± 0.034 | 0.639 ± 0.022 | 0.645 ± 0.025 | 0.611 ± 0.008 | 0.628 ± 0.025 | 0.613 ± 0.012 |
| τ0 (Pa) | 0.817 ± 0.112 | 0.821 ± 0.068 | 0.857 ± 0.050 | 0.767 ± 0.036 | 0.826 ± 0.083 | 0.738 ± 0.050 |
| R2 | 0.999 ± 0.000 | 0.999 ± 0.000 | 0.999 ± 0.000 | 0.999 ± 0.000 | 0.999 ± 0.000 | 0.999 ± 0.000 |
| Day | 0 | 14 | 30 | 60 | 90 | 120 | 180 |
|---|---|---|---|---|---|---|---|
| K (Pa.sn) | 0.273 ± 0.028 | 0.298 ± 0.030 | 0.290 ± 0.075 | 0.265 ± 0.019 | 0.210 ± 0.040 | 0.216 ± 0.040 | 0.229 ± 0.002 |
| n | 0.594 ± 0.027 | 0.586 ± 0.018 | 0.584 ± 0.048 | 0.606 ± 0.014 | 0.614 ± 0.027 | 0.635 ± 0.036 | 0.586 ± 0.007 |
| τ0 (Pa) | 0.980 ± 0.068 | 1.086 ± 0.015 | 1.076 ± 0.052 | 1.116 ± 0.030 | 1.077 ± 0.038 | 1.209 ± 0.068 | 1.128 ± 0.089 |
| R2 | 0.998 ± 0.000 | 0.995 ± 0.002 | 0.997 ± 0.001 | 0.996 ± 0.004 | 0.993 ± 0.000 | 0.988 ± 0.005 | 0.985 ± 0.009 |
| Day | 14 | 30 | 60 | 90 | 120 | 180 |
|---|---|---|---|---|---|---|
| K (Pa.sn) | 0.226 ± 0.035 | 0.250 ± 0.118 | 0.203 ± 0.017 | 0.226 ± 0.029 | 0.262 ± 0.022 | 0.192 ± 0.026 |
| N | 0.641 ± 0.029 | 0.592 ± 0.064 | 0.655 ± 0.016 | 0.621 ± 0.022 | 0.593 ± 0.009 | 0.641 ± 0.024 |
| τ0 (Pa) | 1.115 ± 0.069 | 1.012 ± 0.092 | 1.143 ± 0.062 | 1.063 ± 0.026 | 1.003 ± 0.044 | 1.113 ± 0.107 |
| R2 | 0.998 ± 0.001 | 0.999 ± 0.001 | 0.997 ± 0.001 | 0.997 ± 0.002 | 0.998 ± 0.001 | 0.993 ± 0.006 |
| Challenge Microorganism | Day | 5 °C | 25 °C |
|---|---|---|---|
| Escherichia coli | 0 | Pass * | |
| Pseudomonas aeruginosa | Pass ** | ||
| Staphylococcus aureus | Pass * | ||
| Candida albicans | Pass ** | ||
| Aspergillus brasiliensis | Pass ** | ||
| Escherichia coli | 30 | Pass * | Pass * |
| Pseudomonas aeruginosa | Pass * | Pass * | |
| Staphylococcus aureus | Pass * | Pass * | |
| Candida albicans | Pass * | Pass * | |
| Aspergillus brasiliensis | Pass * | Pass * | |
| Escherichia coli | 90 | Pass * | Pass * |
| Pseudomonas aeruginosa | Pass * | Pass * | |
| Staphylococcus aureus | Pass * | Pass * | |
| Candida albicans | Pass * | Pass * | |
| Aspergillus brasiliensis | Pass * | Pass * | |
| Escherichia coli | 180 | Pass * | Pass * |
| Pseudomonas aeruginosa | Pass * | Pass * | |
| Staphylococcus aureus | Pass * | Pass * | |
| Candida albicans | Pass * | Pass * | |
| Aspergillus brasiliensis | Pass * | Pass * | |
| Day | 0 | 14 | 30 | 60 | 90 | 120 | 180 |
|---|---|---|---|---|---|---|---|
| Mean (mg/mL) | 1.99 | 1.98 | 1.93 | 1.91 | 1.85 | 1.89 | 1.84 |
| SD | 0.01 | 0.01 | 0.02 | 0.01 | 0.04 | 0.02 | 0.02 |
| % | 99 | 99 | 97 | 95 | 93 | 95 | 92 |
| Day | 0 | 14 | 30 | 60 | 90 | 120 | 180 |
|---|---|---|---|---|---|---|---|
| Mean (mg/mL) | 0.50 | 0.49 | 0.48 | 0.46 | 0.44 | 0.43 | 0.39 |
| SD | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 |
| % | 101 | 97 | 96 | 91 | 88 | 85 | 78 |
| Atenolol USP | Active Substance | 0.200 g | |
|---|---|---|---|
| Atenolol vehicle | Citric acid USP monohydrate | pH buffer/chelating agent | 0.935 g |
| Sodium citrate dihydrate USP | pH buffer/chelating agent | 1.632 g | |
| Acesulfame potassium FCC | Sweetener | 0.100 g | |
| Steviol glycosides 95% | Sweetener | 0.100 g | |
| SuspendIt® | Suspending agent | q.s. 100 mL |
| Enalapril Maleate USP | Active Substance | 0.05 g |
|---|---|---|
| SuspendIt® | Suspending agent | q.s. 100 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, S.; Torres, A.; Quintas, C.; Peres, A.M.; Ferreiro, N.; Cruz, R.; Ferreira, H.; Almeida, I.F.; Casal, S. Characterization of Liquid Dosage Forms of Atenolol and Enalapril Maleate for Oral and Enteral Feeding Administration. Pharmaceuticals 2024, 17, 1052. https://doi.org/10.3390/ph17081052
Mota S, Torres A, Quintas C, Peres AM, Ferreiro N, Cruz R, Ferreira H, Almeida IF, Casal S. Characterization of Liquid Dosage Forms of Atenolol and Enalapril Maleate for Oral and Enteral Feeding Administration. Pharmaceuticals. 2024; 17(8):1052. https://doi.org/10.3390/ph17081052
Chicago/Turabian StyleMota, Sandra, Ana Torres, Clara Quintas, António M. Peres, Nuno Ferreiro, Rebeca Cruz, Helena Ferreira, Isabel F. Almeida, and Susana Casal. 2024. "Characterization of Liquid Dosage Forms of Atenolol and Enalapril Maleate for Oral and Enteral Feeding Administration" Pharmaceuticals 17, no. 8: 1052. https://doi.org/10.3390/ph17081052
APA StyleMota, S., Torres, A., Quintas, C., Peres, A. M., Ferreiro, N., Cruz, R., Ferreira, H., Almeida, I. F., & Casal, S. (2024). Characterization of Liquid Dosage Forms of Atenolol and Enalapril Maleate for Oral and Enteral Feeding Administration. Pharmaceuticals, 17(8), 1052. https://doi.org/10.3390/ph17081052











