Safflower Yellow Injection Alleviates Myocardial Ischemia/Reperfusion Injury by Reducing Oxidative and Endoplasmic Reticulum Stress
Abstract
:1. Introduction
2. Results
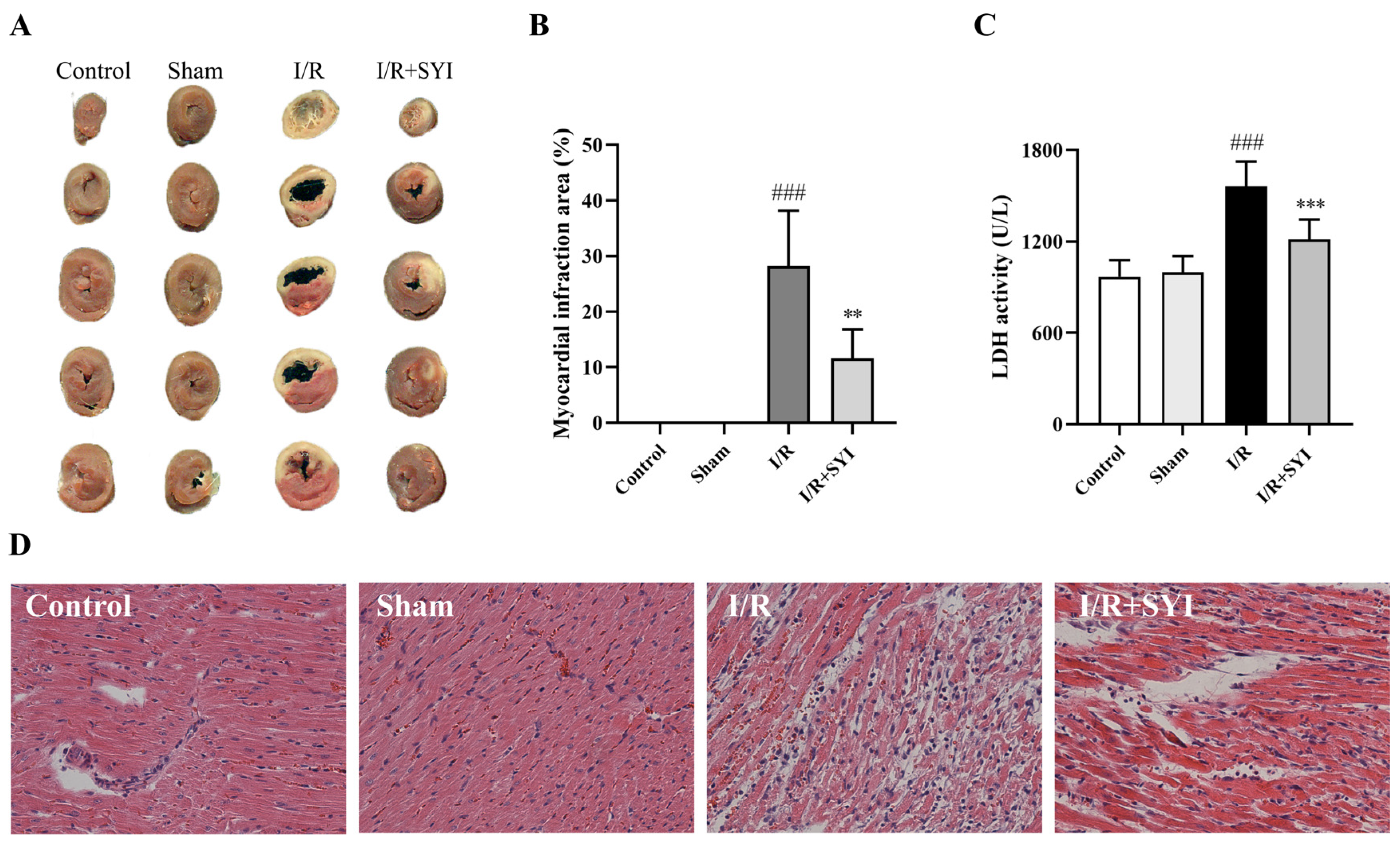
2.1. SYI Attenuated Myocardial I/R Injury in Rats
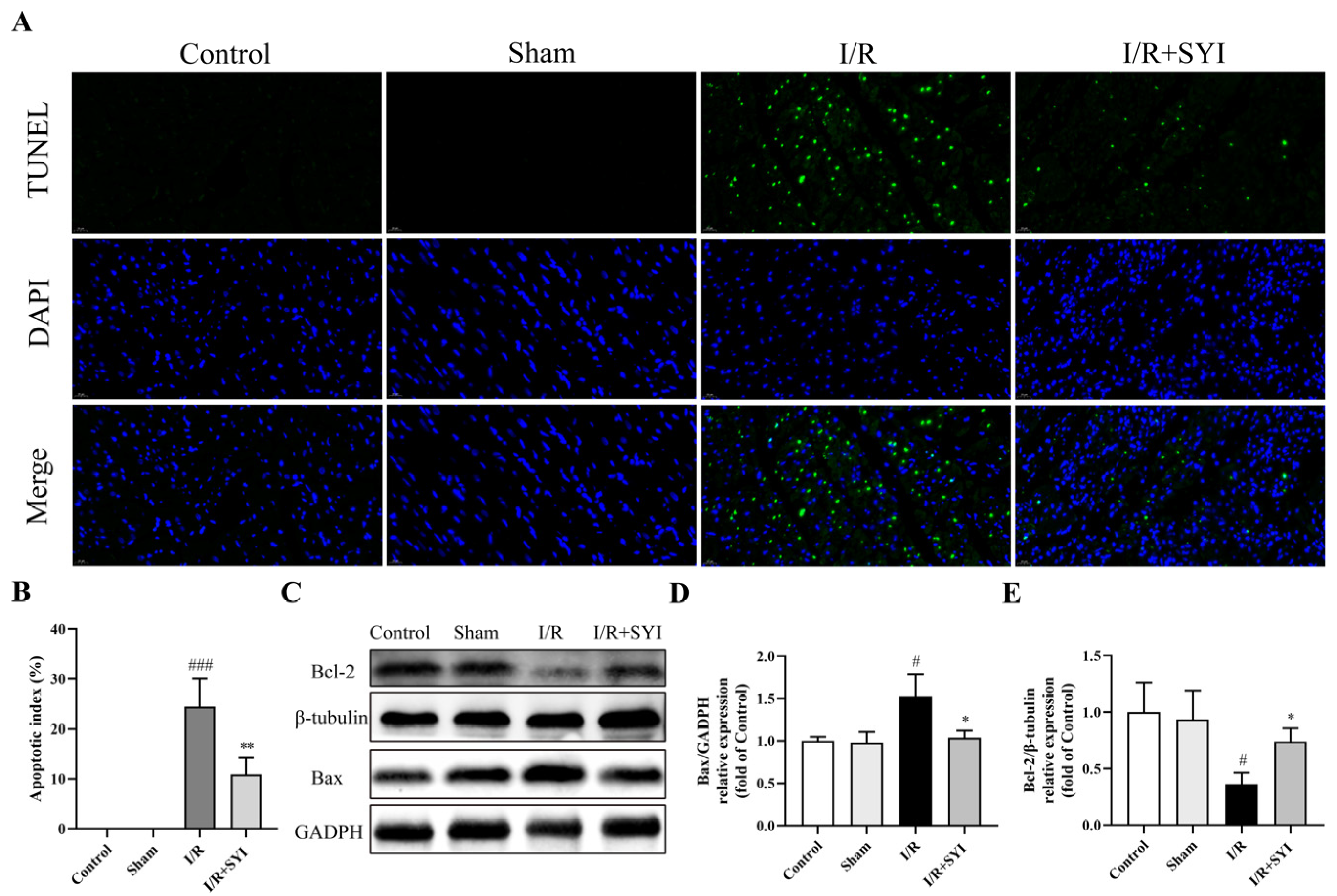
2.2. SYI Attenuated I/R-Induced Myocardial Apoptosis in Rats
2.3. SYI Attenuated I/R-Induced Oxidative and ER Stress in Rat Hearts
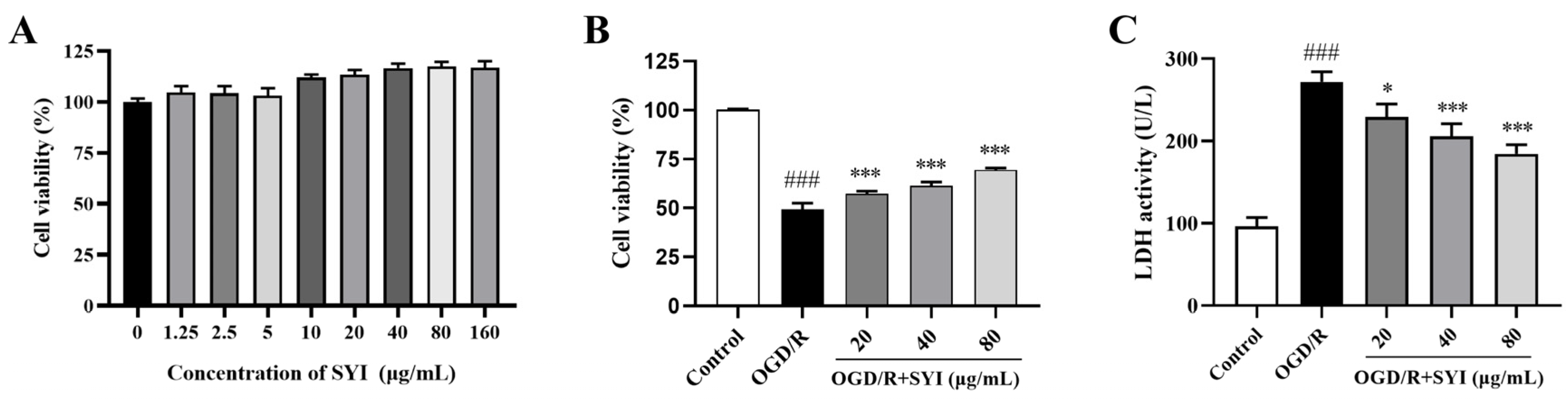
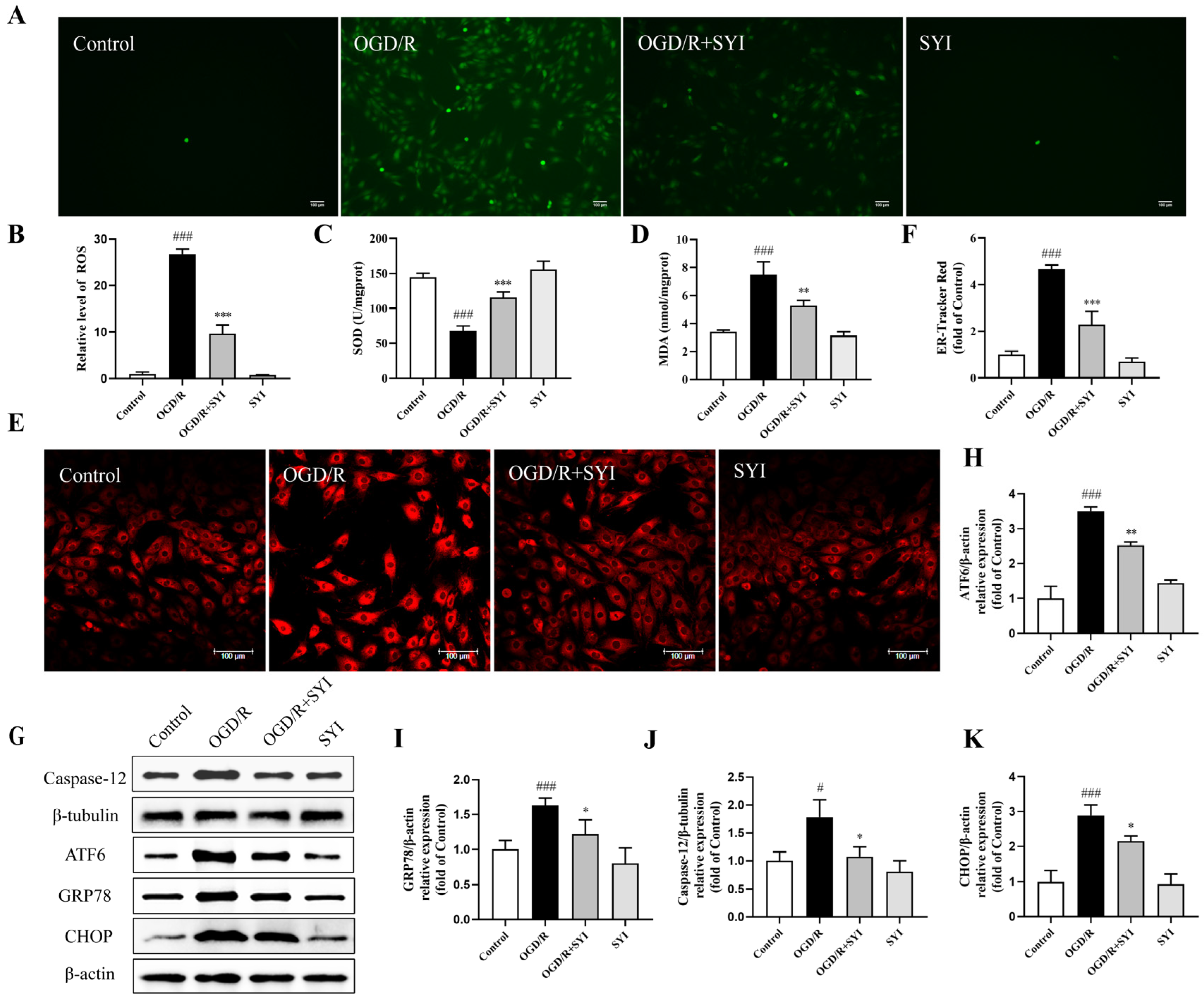
2.4. SYI Reduced OGD/R-Induced Injury in H9c2 Cells
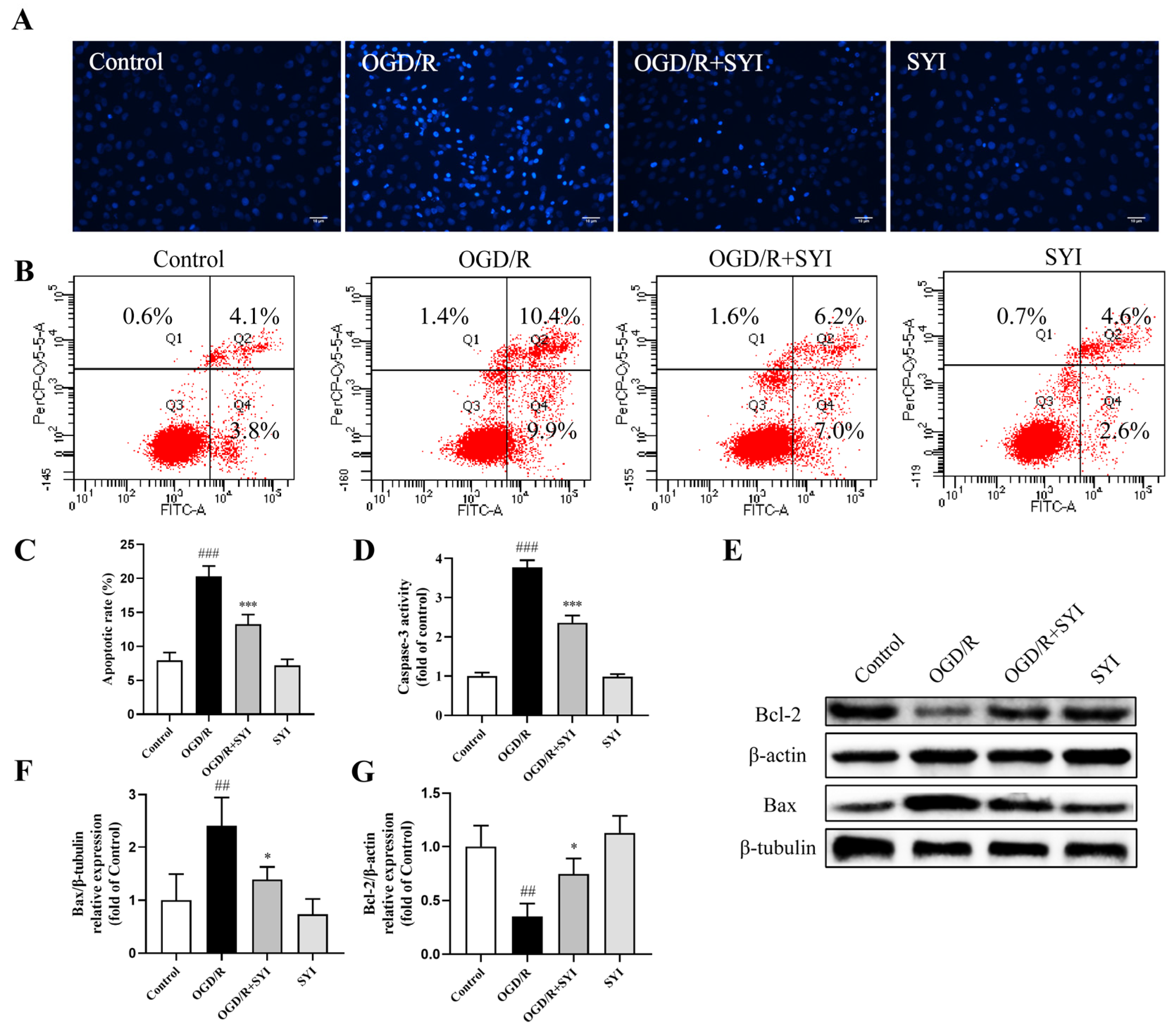
2.5. SYI Suppressed OGD/R-Induced Apoptosis in H9c2 Cells
2.6. SYI Attenuated OGD/R-Induced Oxidative and ER Stress in H9c2 Cells
3. Discussion
4. Materials and Methods
4.1. Drug and Reagents
4.2. Animals
4.3. Establishment of Model and Drug Administration
4.4. Measurement of Myocardial Infarct Area
4.5. HE Staining
4.6. Detection of Myocardial Apoptosis
4.7. Determination of SOD Activity and MDA Content
4.8. Western Blotting
4.9. Cell Culture and OGD/R
4.10. Cell Viability Determination
4.11. Measurement of LDH
4.12. Detection of Intracellular ROS
4.13. Hoechst 33342 Staining and Annexin V-FITC/PI Staining
4.14. Analysis of Caspase-3 Activation
4.15. ER Stress Analysis
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saito, Y.; Oyama, K.; Tsujita, K.; Yasuda, S.; Kobayashi, Y. Treatment Strategies of Acute Myocardial Infarction: Updates on Revascularization, Pharmacological Therapy, and Beyond. J. Cardiol. 2023, 81, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. Compr. Physiol. 2016, 7, 113–170. [Google Scholar]
- Lou, Z.; Wang, A.P.; Duan, X.M.; Hu, G.H.; Zuo, M.L.; Yang, Z.B. Role of ALK5/SMAD2/3 signaling in the regulation of NOX expression in cerebral ischemia/reperfusion injury. Exp. Ther. Med. 2018, 16, 1671–1678. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial Dysfunction and Oxidative Stress in Heart Disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative Stress: The Mitochondria-Dependent and Mitochondria-Independent Pathways of Apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Cai, M.; Zhang, M.; Cui, S.; Zhang, T.; Cheng, W.; Wu, Y.; Ou, W.; Jia, Z.; Zhang, S. Chinese Herbal Medicine Alleviates Myocardial Ischemia/Reperfusion Injury by Regulating Endoplasmic Reticulum Stress. Evid. Based Complement Altern. Med. 2021, 2021, 4963346. [Google Scholar] [CrossRef] [PubMed]
- Belmadani, S.; Matrougui, K. Broken Heart: A Matter of the Endoplasmic Reticulum Stress Bad Management? World J. Cardiol. 2019, 11, 159–170. [Google Scholar] [CrossRef]
- Zhang, C.; Syed, T.W.; Liu, R.; Yu, J. Role of Endoplasmic Reticulum Stress, Autophagy, and Inflammation in Cardiovascular Disease. Front. Cardiovasc. Med. 2017, 4, 29. [Google Scholar] [CrossRef]
- Gorman, A.M.; Healy, S.J.; Jäger, R.; Samali, A. Stress Management at the Er: Regulators of Er Stress-Induced Apoptosis. Pharmacol. Ther. 2012, 134, 306–316. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Zhou, X.; Tang, L.; Xu, Y.; Zhou, G.; Wang, Z. Towards a Better Understanding of Medicinal Uses of Carthamus Tinctorius L. In Traditional Chinese Medicine: A Phytochemical and Pharmacological Review. J. Ethnopharmacol. 2014, 151, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fu, J.; Guo, H.; Han, X.; Li, L.; Xin, G.; Zhao, Y.; Zhang, Q.; Zheng, Q.; Liu, J. [Protective Effect of Safflower Yellow Injection against Rat Miri by TLR-NF-κB Inflammatory Pathway]. Zhongguo Zhong Yao Za Zhi 2019, 44, 2566–2571. [Google Scholar] [PubMed]
- Yu, Q.; Zhang, N.; Gan, X.; Chen, L.; Wang, R.; Liang, R.; Jian, J. EGCG attenuated acute myocardial infarction by inhibiting ferroptosis via miR-450b-5p/ACSL4 axis. Phytomedicine 2023, 119, 154999. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.J.; Wang, Y.H.; Guo, Q.; Jiang, Y.; Tu, P.F.; Zeng, K.W. Protocatechualdehyde protects oxygen-glucose deprivation/reoxygenation-induced myocardial injury via inhibiting PERK/ATF6α/IRE1α pathway. Eur. J. Pharmacol. 2021, 891, 173723. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yi, X.; Zhu, X.H.; Wei, X.; Jiang, D.S. Regulated Cell Death in Myocardial Ischemia-Reperfusion Injury. Trends Endocrinol. Metab. 2024, 35, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Garcia-Saez, A.J. Mechanisms of Bcl-2 Family Proteins in Mitochondrial Apoptosis. Nat. Rev. Mol. Cell Biol. 2023, 24, 732–748. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Qi, L.; Li, L.; Li, Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Fu, W.; Yu, P.; Li, Y.; Yu, X.; Xu, H.; Sui, D. Ginsenoside Rc Alleviates Myocardial Ischemia-Reperfusion Injury by Reducing Mitochondrial Oxidative Stress and Apoptosis: Role of Sirt1 Activation. J. Agric. Food Chem. 2023, 71, 1547–1561. [Google Scholar] [CrossRef] [PubMed]
- Kurian, G.A.; Rajagopal, R.; Vedantham, S.; Rajesh, M. The Role of Oxidative Stress in Myocardial Ischemia and Reperfusion Injury and Remodeling: Revisited. Oxidative Med. Cell. Longev. 2016, 2016, 1656450. [Google Scholar] [CrossRef]
- Azad, N.; Iyer, A.; Vallyathan, V.; Wang, L.; Castranova, V.; Stehlik, C.; Rojanasakul, Y. Role of Oxidative/Nitrosative Stress-Mediated Bcl-2 Regulation in Apoptosis and Malignant Transformation. Ann. N. Y. Acad. Sci. 2010, 1203, 1–6. [Google Scholar] [CrossRef]
- Rahmouni, F.; Hamdaoui, L.; Saoudi, M.; Badraoui, R.; Rebai, T. Antioxidant and Antiproliferative Effects of Teucrium Polium Extract: Computational and In vivo Study in Rats. Toxicol. Mech. Methods. 2024, 34, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Akbari, G. Role of Zinc Supplementation on Ischemia/Reperfusion Injury in Various Organs. Biol. Trace Elem. Res. 2020, 196, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Fan, L.; Nan, K.; Li, J.; Dang, X.; Wang, K. HSYA alleviates secondary neuronal death through attenuating oxidative stress, inflammatory response, and neural apoptosis in SD rat spinal cord compression injury. J. Neuroinflammation 2017, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xing, N.; Xu, X.; Zhu, Y.; Wang, S.; Sun, G.; Sun, X. Tournefolic acid B, derived from Clinopodium chinense (Benth.) Kuntze, protects against myocardial ischemia/reperfusion injury by inhibiting endoplasmic reticulum stress-regulated apoptosis via PI3K/AKT pathways. Phytomedicine 2019, 52, 178–186. [Google Scholar] [CrossRef]
- Zhao, G.L.; Yu, L.M.; Gao, W.L.; Duan, W.X.; Jiang, B.; Liu, X.D.; Zhang, B.; Liu, Z.H.; Zhai, M.E.; Jin, Z.X.; et al. Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol. Sin. 2016, 37, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Maamoun, H.; Benameur, T.; Pintus, G.; Munusamy, S.; Agouni, A. Crosstalk Between Oxidative Stress and Endoplasmic Reticulum (ER) Stress in Endothelial Dysfunction and Aberrant Angiogenesis Associated With Diabetes: A Focus on the Protective Roles of Heme Oxygenase (HO)-1. Front. Physiol. 2019, 10, 70. [Google Scholar] [CrossRef]
- Xing, N.; Wang, Y.; Wang, W.; Zhong, R.; Xia, T.; Ding, Z.; Yang, Y.; Zhong, Y.; Shu, Z. Cardioprotective Effect Exerted by Timosaponin B through the Regulation of Endoplasmic Stress-Induced Apoptosis. Phytomedicine 2020, 78, 153288. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lu, B.; Fu, D.; Gui, M.; Yao, L.; Li, J. Huoxue Qianyang Decoction Ameliorates Cardiac Remodeling in Obese Spontaneously Hypertensive Rats in Association with Atf6-Chop Endoplasmic Reticulum Stress Signaling Pathway Regulation. Biomed. Pharmacother. 2020, 121, 109518. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Tang, J.; Jiang, J.; Chen, Z. New Insights into the Roles of Chop-Induced Apoptosis in Er Stress. Acta Biochim. Biophys. Sin. 2014, 46, 629–640. [Google Scholar] [CrossRef]
- Lu, M.; Lawrence, D.A.; Marsters, S.; Acosta-Alvear, D.; Kimmig, P.; Mendez, A.S.; Paton, A.W.; Paton, J.C.; Walter, P.; Ashkenazi, A. Opposing Unfolded-Protein-Response Signals Converge on Death Receptor 5 to Control Apoptosis. Science 2014, 345, 98–101. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Sun, X.; Shi, X.; Xu, S. Microplastics and di (2-ethylhexyl) phthalate synergistically induce apoptosis in mouse pancreas through the GRP78/CHOP/Bcl-2 pathway activated by oxidative stress. Food Chem. Toxicol. 2022, 167, 113315. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, L.; Qiu, Z.; Xu, Y.; Hua, R. Autophagy triggers endoplasmic reticulum stress and C/EBP homologous protein-mediated apoptosis in OGD/R-treated neurons in a caspase-12-independent manner. J. Neurophysiol. 2021, 126, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, G.; Samal, D.; Khandayataray, P.; Murthy, M.K. A Review on Caspases: Key Regulators of Biological Activities and Apoptosis. Mol. Neurobiol. 2023, 60, 5805–5837. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Cai, M.; Cui, S.; Zhang, M.; Wu, Y.; Ou, W.; Jia, Z.; Zhang, S. Dose-Effect Relationship between Anti-Myocardial Ischemia and Anticoagulation of Safflower Yellow Injection. Cent. South Pharm. 2022, 20, 1322–1328. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Zhang, M.; Gao, J.; Huang, R.; Cheng, L.; Zhang, L.; Huang, Z.; Jia, Z.; Zhang, S. Safflower Yellow Injection Alleviates Myocardial Ischemia/Reperfusion Injury by Reducing Oxidative and Endoplasmic Reticulum Stress. Pharmaceuticals 2024, 17, 1058. https://doi.org/10.3390/ph17081058
Liang W, Zhang M, Gao J, Huang R, Cheng L, Zhang L, Huang Z, Jia Z, Zhang S. Safflower Yellow Injection Alleviates Myocardial Ischemia/Reperfusion Injury by Reducing Oxidative and Endoplasmic Reticulum Stress. Pharmaceuticals. 2024; 17(8):1058. https://doi.org/10.3390/ph17081058
Chicago/Turabian StyleLiang, Wulin, Mingqian Zhang, Jiahui Gao, Rikang Huang, Lu Cheng, Liyuan Zhang, Zhishan Huang, Zhanhong Jia, and Shuofeng Zhang. 2024. "Safflower Yellow Injection Alleviates Myocardial Ischemia/Reperfusion Injury by Reducing Oxidative and Endoplasmic Reticulum Stress" Pharmaceuticals 17, no. 8: 1058. https://doi.org/10.3390/ph17081058




