Establishment of Dissolution Test Method for Multi-Components in Traditional Chinese Medicine Preparations Based on In Vitro–In Vivo Correlation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solubility of Q-Markers in YZTs
2.2. Permeability of Q-Markers in YZTs
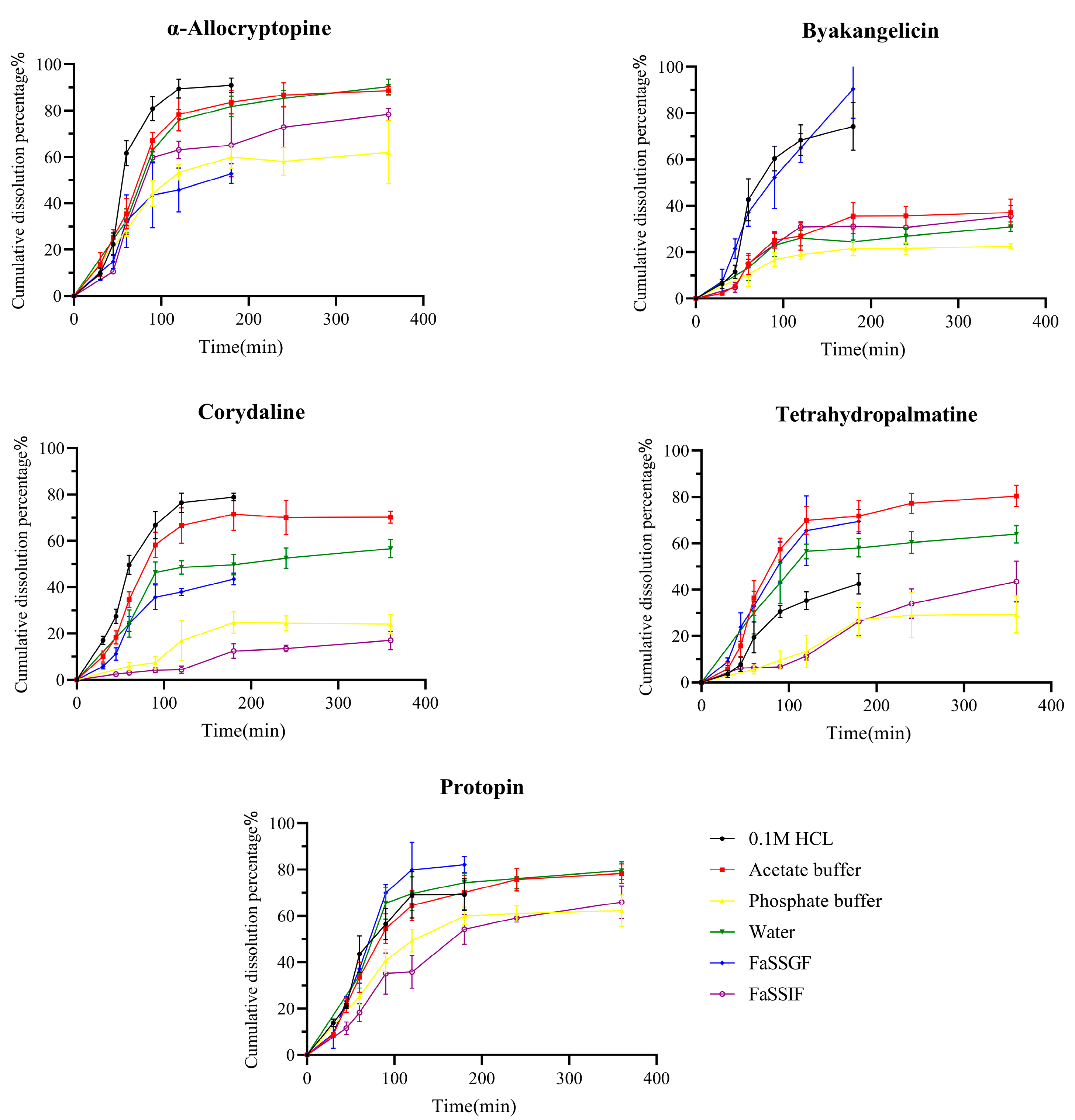
2.3. Dissolution of Q-Markers from YZTs
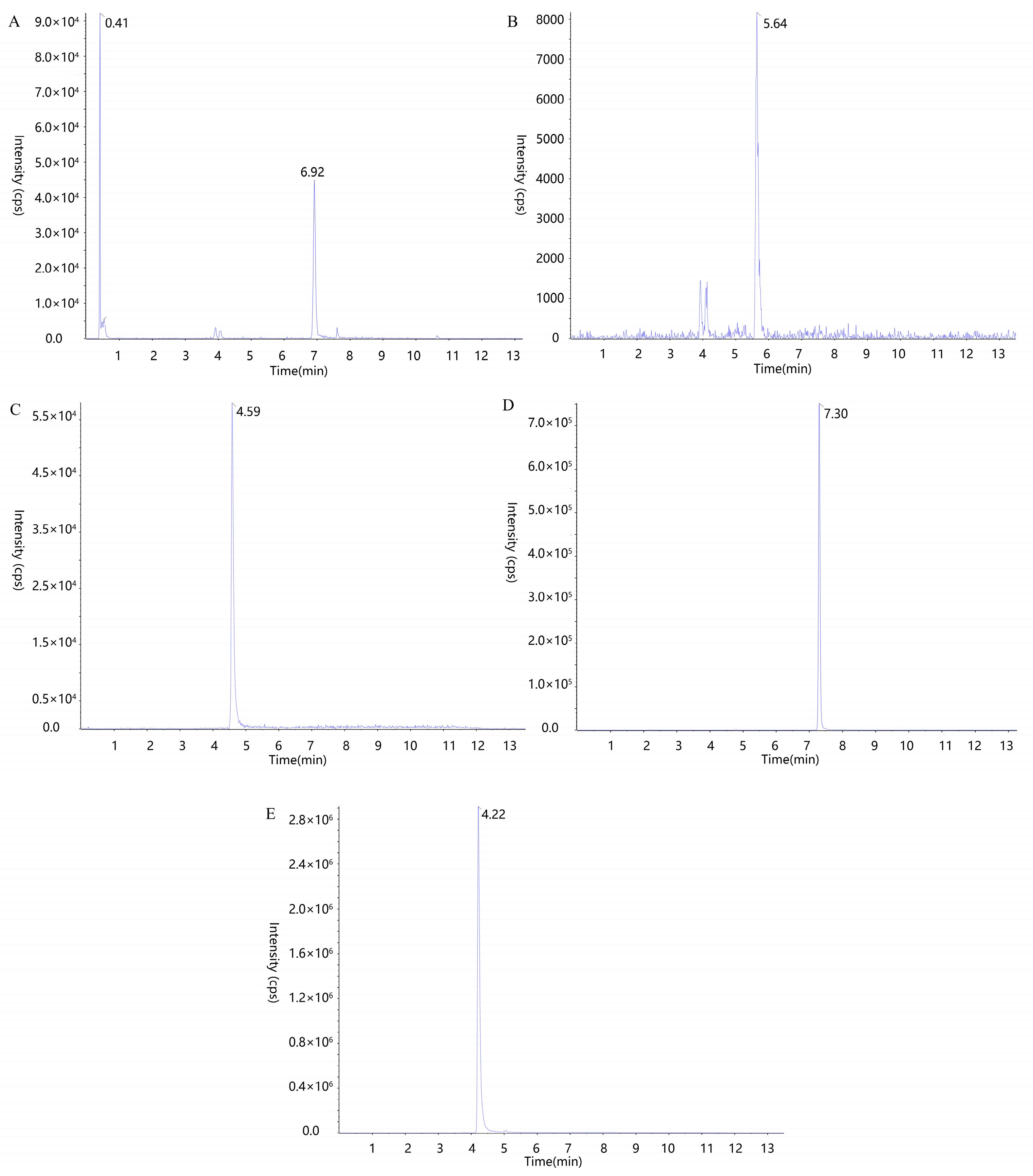
2.4. UPLC-MS/MS Method Validation for Blood Concentration Determination of Q-Markers
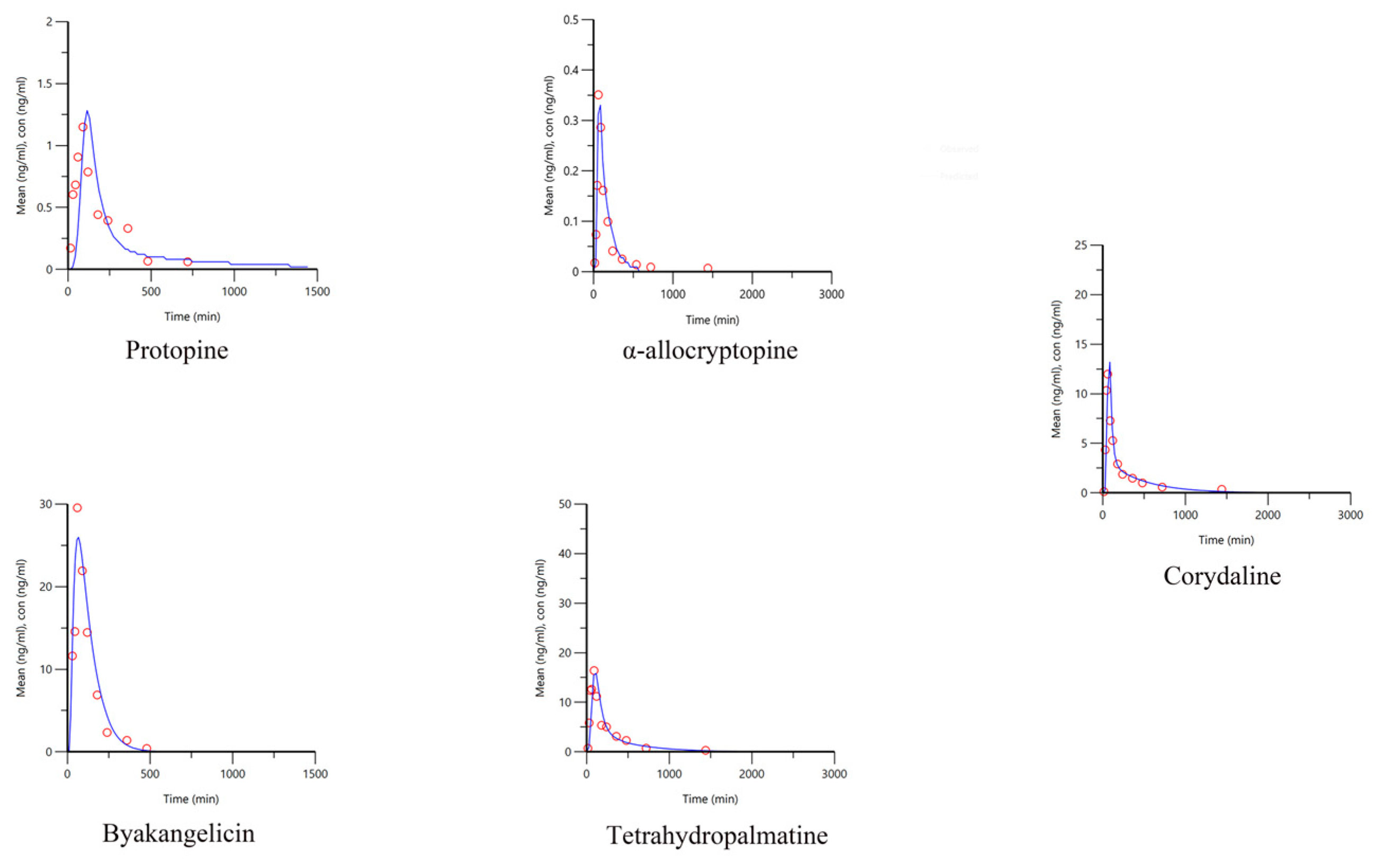
2.5. Pharmacokinetics of Q-Markers in YZTs
2.6. IVIVCs Developed for Q-Markers in YZTs
2.7. Integrated IVIVC of Multiple Q-Markers in YZTs
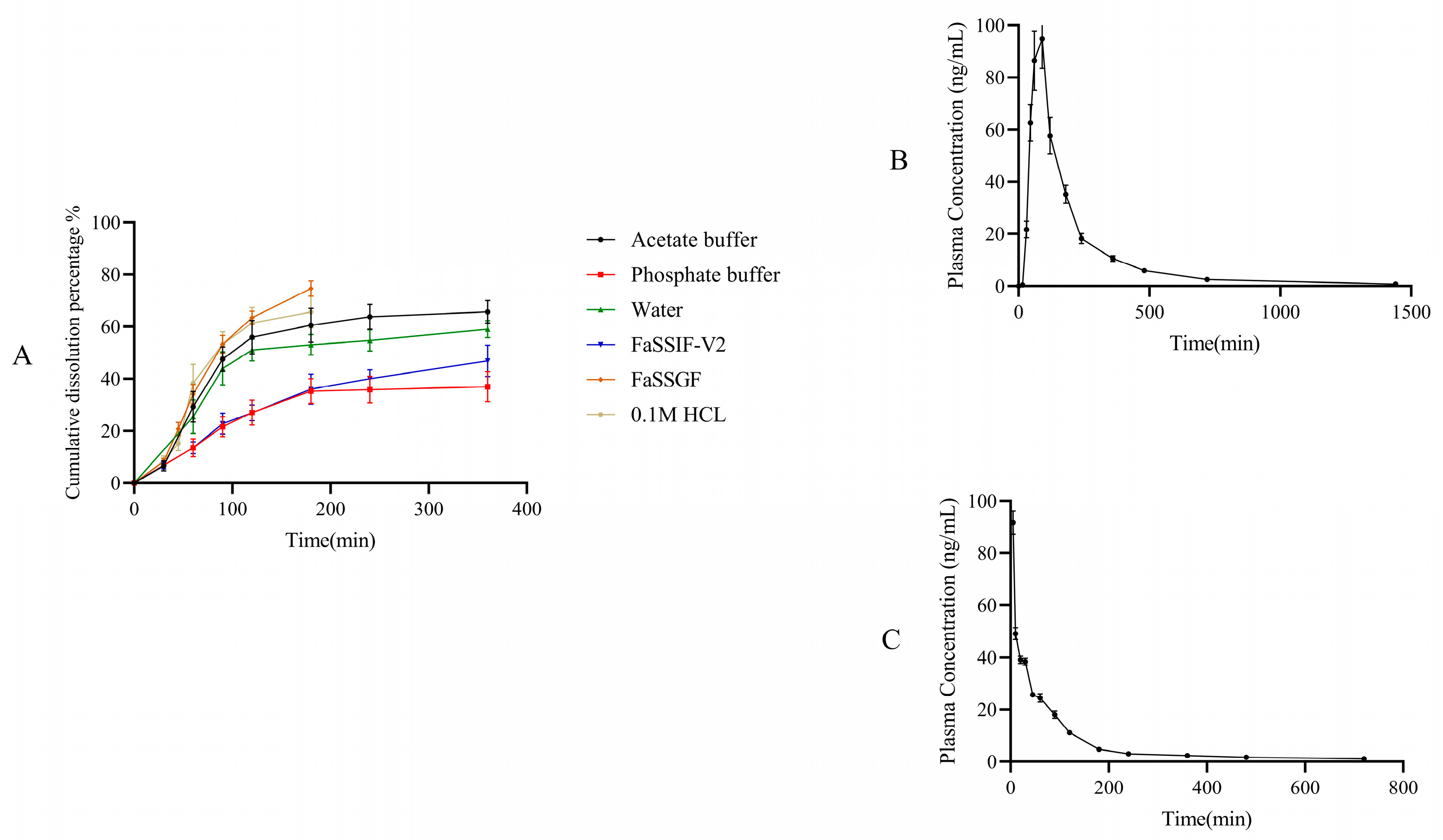
2.8. Application of Dissolution Evaluation Methods
3. Materials and Methods
3.1. Chemicals, Reagents and Animals
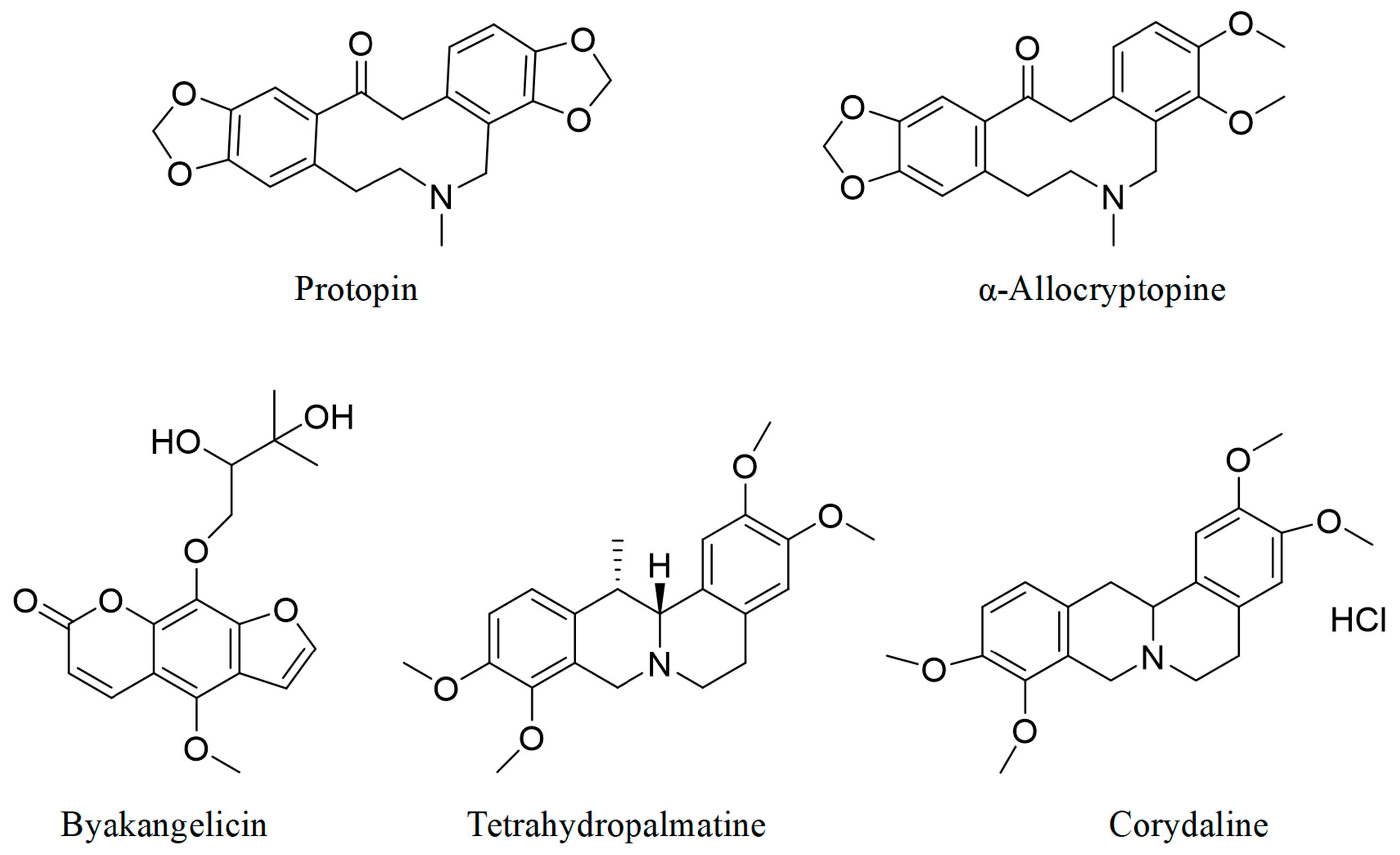
3.2. Identification of Q-Markers
3.3. Determination of Q-Markers by High-Performance Liquid Chromatography Method
3.4. BCS Classification of Q-Markers in YZTs
3.4.1. Solubility of Q-Markers in YZTs
3.4.2. Permeability of Q-Markers in YZTs
3.5. Dissolution Tests of Q-Markers in YZTs in Various Media
3.6. Pharmacokinetics of Q-Markers in YZTs
3.6.1. UPLC-MS/MS Conditions for the Determination of Q-Markers in the Blood
3.6.2. Preparation of Standard Solutions Samples
3.6.3. Pharmacokinetic Study of the Q-Markers
3.7. Development of IVIVC for Q-Markers in YZTs
3.7.1. Fitting of In Vitro Dissolution Profiles
3.7.2. Fitting of In Vitro Dissolution Profiles
3.7.3. Establishment and Validation of IVIVC Model
3.8. IVIVC of Multiple Q-Markers in YZTs
3.8.1. Integration of In Vitro Dissolution Data
3.8.2. Integration of In Vivo Pharmacokinetic Data
3.8.3. IVIVC Establishment of Multiple Q-Markers
3.9. Application of Dissolution Evaluation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FDA. Guidance for Industry Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlation; FDA: Silver Spring, ML, USA, 1997.
- Muselík, J.; Komersová, A.; Kubová, K.; Matzick, K.; Skalická, B. A critical overview of FDA and EMA statistical methods to compare in vitro drug dissolution profiles of pharmaceutical products. Pharmaceutics 2021, 13, 1703. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M. Design of Chinese materia medica compound preparation based on clinical value and inheritance and innovation. Chin. Tradit. Herb. Drugs 2019, 6, 3397–4002. [Google Scholar]
- Zhang, Q.Y.; Sun, G.X. The key problem of TCM consistency evaluation-both determination and assessing method of in vitro dissolution for TCM solid preparations. J. Pharm. Res. 2021, 40, 281–288. [Google Scholar]
- Zhang, F.; Yang, T.; Liang, L.L. Dissolution-systematically quantified fingerprint method for evaluating the similarity in traditional Chinese medicine dissolution curves. Cent. South. Pharm. 2021, 19, 2245–2252. [Google Scholar]
- Disch, L.; Drewe, J.; Fricker, G. Dissolution testing of herbal medicines: Challenges and regulatory standards in Europe, the United States, Canada, and Asia. Dissolut Technol. 2017, 24, 6–12. [Google Scholar] [CrossRef]
- Wu, C.L.; Huang, S.; Yan, D.L.; Hu, L.B.; Luo, F.F.; Ye, H.X. Study on Dissolution in Vitro of Multiple Index Components of Liuweidihuang Concentrated Pill. Asia-Pac. Tradit. Med. 2020, 16, 68–71. [Google Scholar]
- Zhang, J.W.; Chen, L.B.; Gu, J.K.; Ge, W.H. Novel method for the evaluation of the synchronicity of the chemomic release/dissolution of multi-component traditional Chinese medicines. Acta Pharm. Sin. 2008, 43, 647–651. [Google Scholar]
- Zhang, J.W.; Chen, L.B.; Gu, J.K.; Ge, W.H.; Yang, M. Novel Theory and Methods for Chemomic Multi-component Release/Dissolution Kinetics of Traditional Chinese Medicines. Chin. J. Nat. Med. 2008, 6, 48–52. [Google Scholar] [CrossRef]
- Fan, Z.X.; Hu, Y.; Lai, H.Q.; Li, X.D. A methodological study on complicated constituents determination of TCM compound preparation in vitro and vivo. Clin. J. Chin. Med. 2016, 8, 11–15. [Google Scholar]
- Yang, L.; Li, X.D. Determination of in Vitro Release of Wutou Binary-released Granules by Area Under Absorbance-wavelength Curve. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 15–19. [Google Scholar]
- Kan, L.L.; Song, H.Z.; Yan, G.J. Fingerprint Overall Release Evaluation of Sustained-release Pellets. Chin. Arch. Tradit. Chin. Med. 2015, 33, 1817–1823. [Google Scholar]
- Ma, X.F.; Yan, G.J.; Pan, J.H.; Chen, J.; Zhang, Q.; Han, X.X.; Wang, J.C. Quality evaluation research of compound Danshen tablets by real-time cell-based assay technology. Int. J. Tradit. Chin. Med. 2020, 42, 251–255. [Google Scholar]
- Zhang, X.K.; Cao, W.; Wang, S.W. Progress of the analgesic mechanism and pharmacokinetics researches on major active ingredients of Yuan Hu Zhi Tong Pill. Asia-Pac. Tradit. Med. 2010, 6, 107–109. [Google Scholar]
- Liu, Y.H. Simultaneous determination of tetrahydropalmatine and imperatorin in plasma of dog by LC-MS/MS method and pharmacokinetic study on application of Yuanhu Zhitong Tablets. Chin. Tradit. Herbal. Drugs 2016, 47, 2668–2675. [Google Scholar]
- Chen, K.Z. Analgesic tablet of corydalls tuber. J. Tradit. Chin. Med. 1993, 13, 156. [Google Scholar]
- The Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China (Volumes 1, 2020 Edition); China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Zheng, H.C.; Cai, S. Pharmaceutical Botany & Pharmacognosy; People’s Health Publishing House: Beijing, China, 2006. [Google Scholar]
- Wang, Y.S.; Deng, W.L.; Xue, C.S. Pharmacology and Application of Traditional Chinese Medicine; The People’s Health Press Co., Ltd.: Beijing, China, 1998. [Google Scholar]
- Huang, H.Y.; Zhang, J.H.; Dong, Y.M. Content Determination of the Total Alkaloids in Corydalis Tuber with Different Combinations. J. Sichuan Tradit. Chin. Med. 2007, 25, 46–48. [Google Scholar]
- Wu, X.S.; Xu, J.; Zhang, X.M.; Zhang, T.J.; Chen, C.Q. Research progress on chemical constituents and pharmacological activities of Yuanhu Zhitong Prescription. Chin. Tradit. Herbal. Drugs 2015, 46, 1081–1095. [Google Scholar]
- Chen, Y.T.; Cao, W.; Xie, Y.H.; Sun, J.Y.; Wang, S.W.; Ye, Q.Y. Effects of Yuanhu Zhitong tablet and its main components on experimental dysmenorrhea in rats. Shaanxi Tradit. Chin. Med. 2013, 34, 111–114. [Google Scholar]
- Zhang, Y.; Xu, H.; Chen, X.; Chen, C.; Wang, H.; Meng, F.; Yang, H.; Huang, L. Simultaneous quantification of 17 constituents from Yuanhu Zhitong tablet using rapid resolution liquid chromatography coupled with a triple quadrupole electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011, 56, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Liu, M.Y.; Li, J.F.; Wang, D.J. Effects of Radix Angelical Sinensis, Rhizoma Curcumae, Rhizoma Corydalis and their each other compatible on the rabbit’s thoracic aorta. Chin. J. Mod. Appl. Pharm. 2005, 22, 25–28. [Google Scholar]
- Tao, Y.; Xu, H.; Wang, S.; Wang, B.; Zhang, Y.; Wang, W.; Huang, B.; Wu, H.; Li, D.; Zhang, Y.; et al. Identification of the absorbed constituents after oral administration of Yuanhu Zhitong prescription extract and its pharmacokinetic study by rapid resolution liquid chromatography/quadrupole time-of-flight. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 935, 1–9. [Google Scholar] [CrossRef]
- Xu, H.; Tao, Y.; Lu, P.; Wang, P.; Zhang, F.; Yuan, Y.; Wang, S.; Xiao, X.; Yang, H.; Huang, L. A computational drug-target network for Yuanhu Zhitong prescription. Evid. -Based Complement. Altern. Med. 2013, 2013, 658531. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.M.; Xiao, K.C.; Liu, S.; Li, H.X.; Li, D.; Wu, X.Q. Quality Evaluation and Exploratory Research of Yuanhu Zhitong Tablets from Different Manufacturers. China Pharm. 2021, 30, 63–67. [Google Scholar]
- Liu, C.X.; Chen, S.L.; Xiao, X.H.; Zhang, T.J.; Hou, W.B.; Liao, M.L. A new concept on quality marker of Chinese materia medica: Quality control for Chinese medicinal products. Chin. Tradit. Herbal. Drugs 2016, 9, 1443–1457. [Google Scholar]
- Tang, D.Q.; Zheng, X.X.; Chen, X.; Yang, D.Z.; Du, Q. Quantitative and qualitative analysis of common peaks in chemical fingerprint of Yuanhu Zhitong tablet by HPLC-DAD-MS/MS. J. Pharm. Anal. 2014, 4, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Ping, W.; Tinglan, Z.; Guohua, Y.; Mengjie, L.; Jin, S.; Jiaqi, Z.; Ke, L.; Yan, F.; Haiyu, X.; Hongjun, Y. Poly-pharmacokinetic strategy-delineated metabolic fate of bioactive compounds in a traditional Chinese medicine formula, Yuanhu Zhitong tablets, using parallel reaction monitoring mode. Phytomedicine 2019, 53, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Lin, S.; Yang, H.; Wu, H.; Li, S.; Li, D.; Xu, H. Study on absorption ingredients of Yuanhuzhitong pill by everted intestinal sac method. China J. Chin. Mater. Medica 2012, 37, 2005–2011. [Google Scholar]
- Chen, C.G.; Du, H.Z.; Miao, Y.H.; Fang, Y.; Zhao, T.T.; Liu, D.H. Prediction analysis of quality markers and resource evaluation of Artemisiae Argyi Folium based on chemical composition and network pharmacology. China J. Chin. Mater. Medica 2023, 48, 5474–5486. [Google Scholar]
- Sun, Y.F.; Yue, Q.X.; Du, Q.Q.; Yang, S.; Jin, C.S.; Zhang, W.; Cheng, S.Y.; Li, X.Y.; Chen, Q. Quantity Value Transfer and Process Optimization of Radix Paeoniae Alba Formulated Granules: A Study Based on Quality Markers. J. Anhui Univ. Chin. Med. 2022, 41, 76–82. [Google Scholar]
- Li, K.; Li, J.; Su, J.; Xiao, X.; Peng, X.; Liu, F.; Li, D.; Zhang, Y.; Chong, T.; Xu, H.; et al. Identification of quality markers of Yuanhu Zhitong tablets based on integrative pharmacology and data mining. Phytomedicine 2018, 44, 212–219. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Flanagan, T.; Brewster, M.; Kesisoglou, F.; Beato, S.; Biewenga, J.; Crison, J.; Holm, R.; Li, R.; Mannaert, E.; et al. A survey on IVIVC/IVIVR development in the pharmaceutical industry—Past experience and current perspectives. Eur. J. Pharm. Sci. 2017, 102, 1–13. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B. An updated overview with simple and practical approach for developing in vitro-in vivo correlation. Drug Dev. Res. 2018, 79, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Jin Xu, L.Z.; Shao, X. Applications of bio-predictive dissolution tools for the development of solid oral dosage forms: Current industry experience. Drug Dev. Ind. Pharm. 2022, 48, 79–97. [Google Scholar] [CrossRef]
- Sandell, D. Bioequivalence assessment of pharmaceutical aerosol products through IVIVC. Adv. Drug Deliv. Rev. 2021, 176, 113895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Wo, S.K.; Leng, W.; Gao, F.; Yan, X.Y.; Zuo, Z. Population pharmacokinetics and IVIVC for mesalazine enteric-coated tablets. J. Control. Release 2022, 346, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Cámara-Martinez, I.; Blechar, J.A.; Ruiz-Picazo, A.; Garcia-Arieta, A.; Calandria, C.; Merino-Sanjuan, V.; Langguth, P.; Gonzalez-Alvarez, M.; Bermejo, M.; Al-Gousous, J.; et al. Level A IVIVC for immediate release tablets confirms in vivo predictive dissolution testing for ibuprofen. Int. J. Pharm. 2022, 614, 121415. [Google Scholar] [CrossRef] [PubMed]
- Tosca, E.M.; Rocchetti, M.; Pérez, E.; Nieto, C.; Bettica, P.; Moscoso del Prado, J.; Magni, P.; De Nicolao, G. In vitro– in vivo correlation (Ivivc) population modeling for the in silico bioequivalence of a long-acting release formulation of progesterone. Pharmaceutics 2021, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Picazo, A.; Martinez-Martinez, M.T.; Colón-Useche, S.; Iriarte, R.; Sánchez-Dengra, B.; González-Álvarez, M.; García-Arieta, A.; González-Álvarez, I.; Bermejo, M. In Vitro Dissolution as a Tool for Formulation Selection: Telmisartan Two-Step, I.V.I.V.C. Mol. Pharm. 2018, 15, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F. In vivo pharmacokinetics of tectorigenin floating sustained-release tablets in rabbits and evaluation of in vitro-in vivo correlation. Chin. Tradit. Herbal. Drugs 2017, 48, 266–271. [Google Scholar]
- Wang, J.; Chen, J.; Wang, L.; Yang, D.; Shao, R.; Lou, H.; Ruan, Z.; Jiang, B. Evaluating the bioequivalence of two pitavastatin calcium formulations based on IVIVC modeling and clinical study. Clin. Transl. Sci. 2023, 16, 85–91. [Google Scholar] [CrossRef]
- Li, K.; Wang, Q.Z.; Chen, J.; Zuo, Z.; Zhang, R.; Wang, Q.; Wang, J. Selection of Dissolution Conditions of Four Active Lactones in Chuanxinlian Tablets Based on in vitro and in vivo Correlation. Chin. J. Pharm. 2020, 51, 1541–1544. [Google Scholar]
- Fu, H.; Wang, L.; Hu, Q.; Zhang, Z. Dissolution and Permeability Behavior of Diclofenac Sodiumenteric-Coated Tablets In-Vitro. Chin. Pharm. J. 2018, 53, 1778–1784. [Google Scholar]
- Tsume, Y.; Muddie, D.; Langguth, P.; Amidon, G.E.; Amidon, G. The biopharmaceutics classification system: Subclassess for in vivo predictive dissolution (IPD) methodology and IVIVC. Eur. J. Pharm. Sci. 2014, 16, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Wuelfing, W.P.; El Marrouni, A.; Lipert, M.P.; Daublain, P.; Kesisoglou, F.; Converso, A.; Templeton, A.C. Dose Number as a Tool to Guide Lead Optimization for Orally Bioavailable Compounds in Drug Discovery. J. Med. Chem. 2022, 65, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Panse, N.; Gerk, P.M. The Caco-2 Model: Modifications and enhancements to improve efficiency and predictive performance. Int. J. Pharm. 2022, 624, 122004. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, S.W.; Ng, W.L.; Zhang, Y.; Shek, T.L.; Ren, T.; Yan, X.; Li, K.; Wang, J.; Zuo, Z. Identification of the in vivo relevant dissolution media for the three active components in EGb 761 tablet for better correlation with their pharmacokinetics in healthy subjects. Eur. J. Pharm. Sci. 2020, 154, 105515. [Google Scholar] [CrossRef]


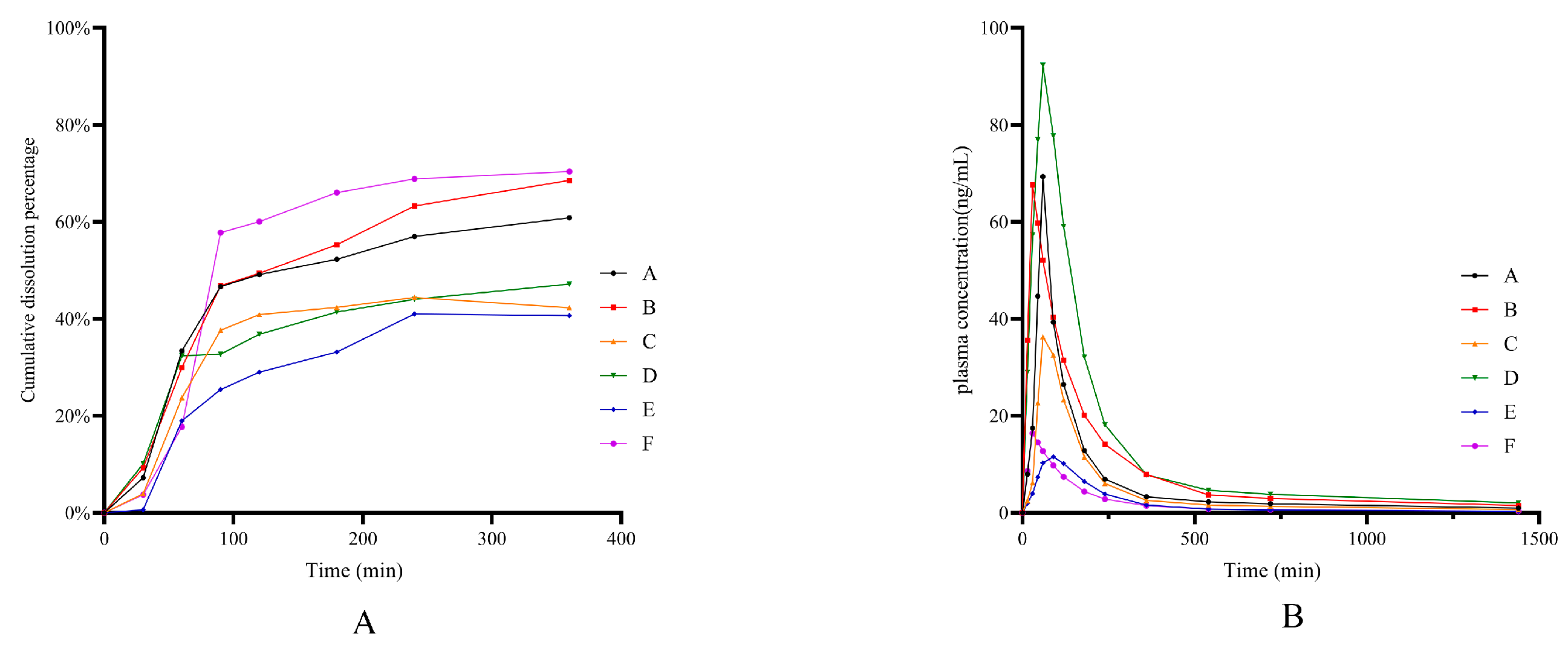
| Compounds | Content | M0/V0 (mg/mL) | Phosphate Buffer (mg/mL) | Acetate Buffer (mg/mL) | 0.1 M HCl (mg/mL) | D0 | ||
|---|---|---|---|---|---|---|---|---|
| (mg/per Tablet) | Phosphate Buffer | Acetate Buffer | 0.1 M HCl | |||||
| Protopin | 0.1742 ± 0.0043 | 0.00418 | 0.535 ± 0.0172 | 5.403 ± 0.378 | 4.700 ± 0.384 | 0.007813 | 0.000774 | 0.000889 |
| α-allocryptopine | 1.1042 ± 0.077 | 0.0265 | 0.0447 ± 0.00217 | 0.0458 ± 0.00256 | 0.0487 ± 0.00408 | 0.592841 | 0.578603 | 0.544148 |
| Byakangelicin | 0.0975 ± 0.0013 | 0.00234 | 0.00763 ± 0.00127 | 1.291 ± 0.0874 | 2.662 ± 0.0560 | 0.306684 | 0.001813 | 0.000879 |
| Tetrahydropalmatine | 0.3017 ± 0.0027 | 0.00724 | 0.0197 ± 0.00187 | 2.666 ± 0.539 | 0.655 ± 0.0433 | 0.367513 | 0.002716 | 0.011053 |
| Corydaline | 0.2871 ± 0.0039 | 0.00689 | 2.385 ± 0.348 | >2.385 | >2.385 | 0.002889 | >0.002889 | >0.002889 |
| Compounds | M0/V0 (mg/mL) | D0 | In Vitro Caco-2 Cell Permeability (cm/s) | BCS Type | ||
|---|---|---|---|---|---|---|
| Phosphate Buffer | Acetate Buffer | 0.1 M HCl | ||||
| Protopin | 0.00418 | <1 | <1 | <1 | 7.49 × 10−6 ± 8.03 × 10−8 | BCS I |
| α-allocryptopine | 0.0265 | <1 | <1 | <1 | 1.34 × 10−5 ± 9.69 × 10−7 | BCS I |
| Byakangelicin | 0.00234 | <1 | <1 | <1 | 8.95 × 10−6 ± 2.14 × 10−7 | BCS I |
| Tetrahydropalmatine | 0.00724 | <1 | <1 | <1 | 3.76 × 10−6 ± 6.88 × 10−8 | BCS I |
| Corydaline | 0.00689 | <1 | <1 | <1 | 3.94 × 10−6 ± 3.73 × 10−7 | BCS I |
| Project | Protopin | α-allocryptopine | Byakangelicin | Tetrahydropalmatine | Corydaline | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| po. | iv. | po. | iv. | po. | iv. | po. | iv. | po. | iv. | |
| T1/2 (min) | 319.26 | 274.64 | 503.22 | 331.43 | 197.15 | 46.52 | 233.75 | 399.67 | 524.58 | 237.47 |
| Tmax(min) | 90 | 5 | 60 | 5 | 60 | 5 | 90 | 5 | 90 | 5 |
| Cmax (ng/mL) | 1.63 | 12.44 | 3.51 | 32.8 | 27.31 | 11.81 | 45.7 | 11.36 | 24.79 | 5.01 |
| AUC 0-t | 205.15 | 1349.21 | 498.70 | 2155.16 | 3579.24 | 666.41 | 8130.23 | 592.23 | 4451.82 | 390.19 |
| AUC 0-inf | 232.08 | 1507.52 | 547.02 | 2373.11 | 3916.93 | 702.63 | 8199.72 | 652.18 | 4867.99 | 430.94 |
| Vz/F (L) | 1.64 | 0.22 | 10.95 | 0.17 | 0.06 | 0.06 | 0.03 | 0.68 | 0.13 | 0.66 |
| Cl/F (L/h) | 0.21 | 0.03 | 0.9 | 0.02 | 0.01 | 0.05 | 0.01 | 0.07 | 0.01 | 0.11 |
| Bioavailability (%) | 1.35 | 7.5 | 3.81 | 10.85 | 5.38 | |||||
| Compounds | Rpm | Media | Model | Functions | Correlation | AIC |
|---|---|---|---|---|---|---|
| Protopin | 75 | 0.1 M HCl | Weibull | y(t) = 0.70 × (1 − exp[−(t/1.10)^2.06]) | 0.9909 | −25.65 |
| Acetate buffer | Hill | y(t) = (0.78 × t^2.71)/(1.10^2.71 + t^2.71) | 0.9981 | −39.54 | ||
| Phosphate buffer | Weibull | y(t) = 0.62 × (1 − exp[−(t/1.50)^1.63]) | 0.9989 | −44.11 | ||
| FaSSIF-V2 | Hill | y(t) = (0.77 × t^1.73)/(1.90^1.71 + t^2.71) | 0.9918 | −32.08 | ||
| Water | Hill | y(t) = (0.77 × t^4.39)/(1.04^4.39 + t^4.39) | 0.9907 | −29.83 | ||
| FaSSGF | Weibull | y(t) = 0.82 × (1 − exp[−t^1.01]) | 0.9993 | −37.41 | ||
| α-allocryptopine | 75 | 0.1 M HCl | Hill | y(t) = (0.90 × t^5.14)/(0.90^5.14 + t^5.14) | 0.995 | −23.86 |
| Acetate buffer | Weibull | y(t) = 0.86 × (1 − exp[−(t/1.26)^1.97]) | 0.9982 | −38.41 | ||
| Phosphate buffer | Weibull | y(t) = 0.60 × (1 − exp[−(t/1.29)^1.78]) | 0.9954 | −37.12 | ||
| FaSSIF-V2 | Hill | y(t) = (0.60 × t^4.44)/(1.09^4.44 + t^4.44) | 0.989 | −30.3 | ||
| Water | Hill | y(t) = (0.88 × t^3.38)/(1.16^3.38 + t^3.38) | 0.9963 | −32.43 | ||
| FaSSGF | Hill | y(t) = (0.76 × t^1.31)/(1.84^1.38 + t^1.38) | 0.9812 | −23.29 | ||
| Byakangelicin | 75 | 0.1 M HCl | Hill | y(t) = (0.45 × t^2.74)/(1.17^2.74 + t^2.74) | 0.9939 | −33.46 |
| Acetate buffer | Hill | y(t) = (0.35 × t^2.79)/(1.12^2.79 + t^2.79) | 0.9963 | −45.32 | ||
| Phosphate buffer | Hill | y(t) = (0.23 × t^2.73)/(1.05^2.73 + t^2.73) | 0.9976 | −53.53 | ||
| FaSSIF-V2 | Hill | y(t) = (0.27 × t^3.67)/(1.12^3.67 + t^3.67) | 0.9849 | −39.47 | ||
| Water | Hill | y(t) = (0.28 × t^3.61)/(1.02^3.61 + t^3.61) | 0.9464 | −31.76 | ||
| FaSSGF | Hill | y(t) = (1.36 × t^2.00)/(1.68^2.00 + t^2.00) | 0.9964 | −30.34 | ||
| Tetrahydropalmatine | 75 | 0.1 M HCl | Hill | y(t) = (0.72 × t^4.77)/(0.97^4.77 + t^4.77) | 0.9916 | −23.57 |
| Acetate buffer | Hill | y(t) = (0.79 × t^2.93)/(1.07^2.93 + t^2.93) | 0.9978 | −37.45 | ||
| Phosphate buffer | Weibull | y(t) = 0.30 × (1 − exp[−(t/2.29)^2.34]) | 0.9914 | −35.17 | ||
| FaSSIF-V2 | Weibull | y(t) = 0.38 × (1 − exp[−(t/3.48)^1.91]) | 0.9894 | −36.65 | ||
| Water | Hill | y(t) = (0.64 × t^2.53)/(1.06^2.53 + t^2.53) | 0.9869 | −30.74 | ||
| FaSSGF | Hill | y(t) = (0.81 × t^1.12)/(2.27^1.12 + t^1.12) | 0.9962 | −34.31 | ||
| Corydaline | 75 | 0.1 M HCl | Weibull | y(t) = 0.79 × (1 − exp[−(t/1.06)^2.00]) | 0.996 | −29.57 |
| Acetate buffer | Weibull | y(t) = 0.71 × (1 − exp[−(t/1.23)^2.04]) | 0.9994 | −48.93 | ||
| Phosphate buffer | Weibull | y(t) = 0.25 × (1 − exp[−(t/1.94)^2.82]) | 0.986 | −34.66 | ||
| FaSSIF-V2 | Weibull | y(t) = 0.15 × (1 − exp[−(t/3.14)^1.77]) | 0.9793 | −45.72 | ||
| Water | Hill | y(t) = (0.53 × t^4.53)/(1.03^4.53 + t^4.53) | 0.9784 | −29.32 | ||
| FaSSGF | Hill | y(t) = (0.44 × t^0.98)/(3.25^0.98 + t^0.98) | 0.9987 | −46.79 |
| Compounds | Model | Weighting | Parameters | Estimate | Correlation | AIC | SBC |
|---|---|---|---|---|---|---|---|
| Protopin | Akaike | 1/(Y × Y) | Tlag (h) | 0.44 | 0.9451 | −27.92 | −25.53 |
| Ka (h−1) | 1.36 | ||||||
| A1 (ng × mL−1) | 0 | ||||||
| alpha1 (h−1) | 1.35 | ||||||
| α-allocryptopine | Akaike | 1/Yhat × Yhat | Tlag (h) | 0.67 | 0.9973 | −10.54 | −8.12 |
| Ka (h−1) | 5.65 | ||||||
| A1 (ng × mL−1) | 0 | ||||||
| alpha1 (h−1) | 1.13 | ||||||
| Byakangelicin | Akaike | 1/Yhat | Tlag (h) | 0.45 | 0.9966 | 1.65 | 2.83 |
| Ka (h−1) | 2.23 | ||||||
| A1 (ng × mL−1) | 0.02 | ||||||
| alpha1 (h−1) | 1.18 | ||||||
| Tetrahydropalmatine | Akaike | 1/Y | Tlag (h) | 0.40 | 0.9886 | 22.53 | 25.44 |
| Ka (h−1) | 1.28 | ||||||
| A1 (ng × mL−1) | 0.04 | ||||||
| alpha1 (h−1) | 1.30 | ||||||
| Corydaline | Akaike | 1/Y | Tlag (h) | 0.44 | 0.9761 | 10.42 | 13.33 |
| Ka (h−1) | 1.46 | ||||||
| A1 (ng × mL−1) | 0.05 | ||||||
| alpha1 (h−1) | 1.42 |
| Compounds | Media | Model | Correlation | AIC | SBC | Observed | Predicted | PE% | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Cmax | AUC | Cmax | AUC | Cmax | ||||||
| Protopin | 0.1 M HCl | Fabs = 1.72 × (Diss (1.30 × Tvivo − 9.99) − 0.0015) | 0.9981 | −406.74 | −396.28 | 205.15 | 1.63 | 228.87 | 1.30 | 11.56 | −19.98 |
| Acetate buffer | Fabs = 1.55 × (Diss (1.77 × Tvivo − 20.10) − 4.74×10-6) | 0.9946 | −297.35 | −286.89 | 205.15 | 1.63 | 227.94 | 1.23 | 11.11 | −24.49 | |
| Phosphate buffer | Fabs = 1.93 × Diss (2.78 × Tvivo − 78.11) | 0.9932 | −274.85 | −267.01 | 205.15 | 1.63 | 236.66 | 1.34 | 15.36 | −17.99 | |
| Water | Fabs = 1.75 × (Diss (1.30 × Tvivo − 0.32) − 0.084) | 0.991 | −247.09 | −236.63 | 205.15 | 1.63 | 250.86 | 1.36 | 22.28 | −16.7 | |
| FaSSGF | Fabs = 1.37 × (Diss (0.867 × Tvivo − 0.844) − 0.01) | 0.987 | −311.80 | −207.02 | 205.15 | 1.63 | 149.90 | 1.32 | −26.93 | −18.77 | |
| FaSSIF-V2 | Fabs = 1.83 × (Diss (2.78 × Tvivo − 20.10) − 0.033) | 0.9885 | −222.79 | −212.33 | 205.15 | 1.63 | 239.86 | 1.19 | 16.92 | −26.96 | |
| α-allocryptopine | 0.1 M HCl | Fads = 1.19 × Diss (1.14 × Tvivo − 0.27) | 0.9985 | −440.34 | −432.49 | 498.70 | 3.51 | 516.25 | 3.27 | 3.52 | −6.93 |
| Acetate buffer | Fads = 1.24 × Diss (1.80 × Tvivo − 20.10) | 0.997 | −383.80 | −375.96 | 498.70 | 3.51 | 519.30 | 2.95 | 4.13 | −16.01 | |
| Phosphate buffer | Fads = 2.15 × (Diss (2.15 × Tvivo − 30.90) − 0.10) | 0.9821 | −203.59 | −193.13 | 498.70 | 3.51 | 617.09 | 3.40 | 23.74 | −3.27 | |
| Water | Fads = 1.25 × (Diss (2.77 × Tvivo − 61.27) − 0.017) | 0.9981 | −427.94 | −417.48 | 498.70 | 3.51 | 537.15 | 3.31 | 7.71 | −5.77 | |
| FaSSGF | Fabs = 2.52 × (Diss (1.17 × Tvivo − 10.05) − 0.016) | 0.9896 | −376.36 | −365.90 | 498.70 | 3.51 | 467.63 | 2.57 | −6.23 | −26.81 | |
| FaSSIF-V2 | Fads = 1.80 × Diss (1.62 × Tvivo − 10.13) | 0.9981 | −419.84 | −411.99 | 498.70 | 3.51 | 515.76 | 3.25 | 3.42 | −7.27 | |
| Byakangelicin | 0.1 M HCl | Fabs = 2.42 × (Diss (1.64 × Tvivo − 10.05) − 0.012) | 0.9977 | −338.25 | −327.79 | 3579.24 | 27.31 | 3714.64 | 25.49 | 3.78 | −6.68 |
| Acetate buffer | Fabs = 2.93 × Diss (1.83 × Tvivo − 20.10) | 0.9993 | −456.33 | −448.49 | 3579.24 | 27.31 | 3620.86 | 25.60 | 1.16 | −6.29 | |
| Phosphate buffer | Fabs = 4.47 × Diss (1.81 × Tvivo − 21.59) | 0.9806 | −89.17 | −81.33 | 3579.24 | 27.31 | 3464.10 | 22.71 | −3.22 | −16.84 | |
| Water | Fabs = 3.66 × (Diss (1.35 × Tvivo − 7.29) − 0.0053) | 0.9754 | −75.77 | −65.31 | 3579.24 | 27.31 | 3439.06 | 21.77 | −3.92 | −20.30 | |
| FaSSGF | Fabs = 1.03 × (Diss (0.46 × Tvivo − 12.56) − 0.046) | 0.9736 | −357.51 | −347.04 | 3579.24 | 27.31 | 3697.35 | 24.55 | 3.30 | −10.09 | |
| FaSSIF-V2 | Fabs = 3.70 × Diss (1.52 × Tvivo − 6.11) | 0.9991 | −425.45 | −417.61 | 3579.24 | 27.31 | 3606.47 | 26.33 | 0.76 | −3.61 | |
| Tetrahydropalmatine | 0.1 M HCl | Fabs = 1.42 × Diss (0.98 × Tvivo) | 0.9909 | −260.66 | −255.43 | 8130.23 | 45.70 | 8270.26 | 51.32 | 1.72 | 12.30 |
| Acetate buffer | Fabs = 1.30 × Diss (1.11 × Tvivo) | 0.9993 | −533.44 | −528.21 | 8130.23 | 45.70 | 8301.00 | 42.28 | 2.10 | −7.47 | |
| Phosphate buffer | Fabs = 3.40 × Diss (1.93 × Tvivo) | 0.9933 | −301.81 | −296.58 | 8130.23 | 45.70 | 8142.89 | 46.02 | 0.16 | 0.71 | |
| Water | Fabs = 1.95 × (Diss (1.27 × Tvivo − 0.00082) − 0.108) | 0.9843 | −212.60 | −202.14 | 8130.23 | 45.70 | 9510.40 | 44.41 | 16.98 | −2.81 | |
| FaSSGF | Fabs = 1.42 × (Diss (1.15 × Tvivo − 0.012) − 0.0084) | 0.9991 | −433.00 | −422.55 | 8130.23 | 45.70 | 9588.79 | 40.25 | 17.94 | −11.92 | |
| FaSSIF-V2 | Fabs = 2.77 × (Diss (2.78 × Tvivo − 0.00064) − 0.0029) | 0.995 | −327.91 | −317.45 | 8130.23 | 45.70 | 8298.37 | 42.77 | 2.07 | −6.41 | |
| Corydaline | 0.1 M HCl | Fabs = 1.31 × Diss (1.07 × Tvivo − 9.25) | 0.9962 | −351.16 | −343.32 | 4451.82 | 24.79 | 4588.14 | 22.91 | 3.06 | −7.59 |
| Acetate buffer | Fabs = 1.46 × Diss (1.23 × Tvivo − 9.97) | 0.9961 | −349.97 | −342.13 | 4451.82 | 24.79 | 4588.20 | 22.98 | 3.06 | −7.29 | |
| Phosphate buffer | Fabs = 4.18 × Diss (1.73 × Tvivo − 0.0022) | 0.9937 | −299.87 | −292.03 | 4451.82 | 24.79 | 4509.00 | 24.89 | 1.28 | 0.40 | |
| Water | Fabs = 1.95 × Diss (1.04 × Tvivo − 0.00073) | 0.9856 | −188.60 | −180.75 | 4451.82 | 24.79 | 4453.68 | 22.13 | 0.04 | −10.73 | |
| FaSSGF | Fabs = 1.19 × (Diss (1.18 × Tvivo − 10.05) − 0.0136) | 0.9968 | −385.05 | −374.59 | 4451.82 | 24.79 | 4812.75 | 22.74 | 8.11 | −8.27 | |
| FaSSIF-V2 | Fabs = 7.47 × (Diss (2.78 × Tvivo − 7.71) − 0.00523) | 0.9948 | −318.66 | −308.20 | 4451.82 | 24.79 | 4743.97 | 21.49 | 6.56 | −13.31 | |
| Predicted | Observed | PE% | ||||
|---|---|---|---|---|---|---|
| AUC | Cmax (ng/mL) | AUC | Cmax (ng/mL) | AUC | Cmax | |
| Byakangelicin | 3754.39 | 25.75 | 3268.72 | 29.55 | 14.86 | −12.85 |
| α-allocryptopine | 103.98 | 0.47 | 122.14 | 0.50 | 14.89 | −6.01 |
| Tetrahydropalmatine | 3725.00 | 15.53 | 3596.97 | 16.40 | 3.56 | −5.31 |
| Corydaline | 2143.49 | 12.35 | 2060.36 | 11.98 | 4.03 | 3.05 |
| Protopine | 218.10 | 1.26 | 205.15 | 1.15 | 9.77 | 11.21 |
| Compounds | Method | Rotation Speed (rpm) | Media |
|---|---|---|---|
| Protopin | Paddle | 100 | 0.1 M HCL |
| α-allocryptopine | 75 | 0.1 M HCL | |
| Byakangelicin | 75 | Acetate buffer | |
| Tetrahydropalmatine | 75 | Acetate buffer | |
| Corydaline | 75 | FaSSGF |
| Compounds | Rpm | Media | Model | Functions | Correlation | AIC |
|---|---|---|---|---|---|---|
| BCS I Q-markers | 75 | 0.1 M HCl | Hill | y(t) = (0.67×t^3.46)/(0.97^3.46 + t^3.46) | 0.9932 | −27.19 |
| Acetate buffer | Hill | y(t) = (0.65×t^2.84)/(1.07^2.84 + t^2.84) | 0.9995 | −50.62 | ||
| Phosphate buffer | Hill | y(t) = (0.39×t^2.33)/(1.34^2.33 + t^2.33) | 0.9951 | −40.47 | ||
| FaSSIF-V2 | Hill | y(t) = (0.45×t^1.79)/(1.84^1.79 + t^1.79) | 0.9968 | −45.58 | ||
| Water | Hill | y(t) = (0.57×t^3.35)/(1.06^3.35 + t^3.35) | 0.9912 | −33.99 | ||
| FaSSGF | Hill | y(t) = (0.81×t^1.11)/(2.46^1.11 + t^1.11) | 0.9996 | −38.01 |
| Compounds | Model | Weighting | Parameters | Estimate | Correlation | AIC | SBC |
|---|---|---|---|---|---|---|---|
| BCS I Q-markers | Akaike | 1/Y | A1 (ng × mL−1) | 0.360 | 0.9953 | 15.55 | 18.46 |
| Alpha1 (h−1) | 0.480 | ||||||
| A2 (ng × mL−1) | 0.0760 | ||||||
| Alpha2 (h−1) | 0.015 |
| Compounds | Media | Model | Correlation | AIC | SBC | Observed | Predicted | PE% | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Cmax | AUC | Cmax | AUC | Cmax | ||||||
| BCS I Q-markers | 0.1 M HCl | Fabs = 0.33 × (Diss (0.96 × Tvivo − 5.05) − 0.0012) | 0.9932 | −604.12 | −593.66 | 16,507.52 | 94.83 | 17,734.50 | 91.22 | 7.43 | −3.81 |
| Acetate buffer | Fabs = 0.34 × (Diss (1.20 × Tvivo − 6.19) − 0.0087) | 0.9953 | −640.24 | −629.78 | 16,507.52 | 94.83 | 18,004.90 | 87.05 | 9.07 | −8.20 | |
| Phosphate buffer | Fabs = 0.69 × (Diss (1.57 × Tvivo − 4.38) − 0.057) | 0.9833 | −513.00 | −502.54 | 16,507.52 | 94.83 | 19928.20 | 94.10 | 20.72 | −0.78 | |
| Water | Fabs = 0.38 × Diss (1.07 × Tvivo − 0.0059) | 0.9810 | −463.61 | −455.76 | 16,507.52 | 94.83 | 17,005.65 | 81.66 | 3.02 | −13.89 | |
| FaSSGF | Fabs = 0.29 × (Diss (1.27 × Tvivo − 9.699) − 0.008) | 0.9920 | −587.31 | −576.85 | 16,507.52 | 94.83 | 17,888.17 | 87.58 | 8.36 | −7.65 | |
| FaSSIF-V2 | Fabs = 0.26 × (Diss (1.57 × Tvivo − 20.10) − 0.0068) | 0.9949 | −632.07 | −621.61 | 16,507.52 | 94.83 | 17,887.37 | 85.07 | 8.36 | −10.29 | |
| Brand Name | AUC | Cmax (ng/mL) | PF% | |
|---|---|---|---|---|
| AUC | Cmax | |||
| A (control subjects) | 6096.27 | 34.62 | ||
| B | 6214.88 | 33.82 | 1.95% | −2.31% |
| C | 3033.97 | 18.15 | −50.23% | −47.57% |
| D | 8763.84 | 46.15 | 43.76% | 33.30% |
| E | 1361.92 | 5.77 | −77.66% | −83.33% |
| F | 1391.3 | 8.2 | −77.18% | −76.31% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, P.; Wang, Q.; Xiang, X.; Zhang, Y.; Pan, Y.; Zuo, Z.; Wang, J. Establishment of Dissolution Test Method for Multi-Components in Traditional Chinese Medicine Preparations Based on In Vitro–In Vivo Correlation. Pharmaceuticals 2024, 17, 1065. https://doi.org/10.3390/ph17081065
Guo P, Wang Q, Xiang X, Zhang Y, Pan Y, Zuo Z, Wang J. Establishment of Dissolution Test Method for Multi-Components in Traditional Chinese Medicine Preparations Based on In Vitro–In Vivo Correlation. Pharmaceuticals. 2024; 17(8):1065. https://doi.org/10.3390/ph17081065
Chicago/Turabian StyleGuo, Pengcheng, Qizheng Wang, Xiaoqiang Xiang, Yufeng Zhang, Yue Pan, Zhong Zuo, and Jianxin Wang. 2024. "Establishment of Dissolution Test Method for Multi-Components in Traditional Chinese Medicine Preparations Based on In Vitro–In Vivo Correlation" Pharmaceuticals 17, no. 8: 1065. https://doi.org/10.3390/ph17081065






