Inhibition of Development and Metabolism of Dual-Species Biofilms of Candida albicans and Candida krusei (Pichia kudriavzevii) by Organoselenium Compounds
Abstract
1. Introduction
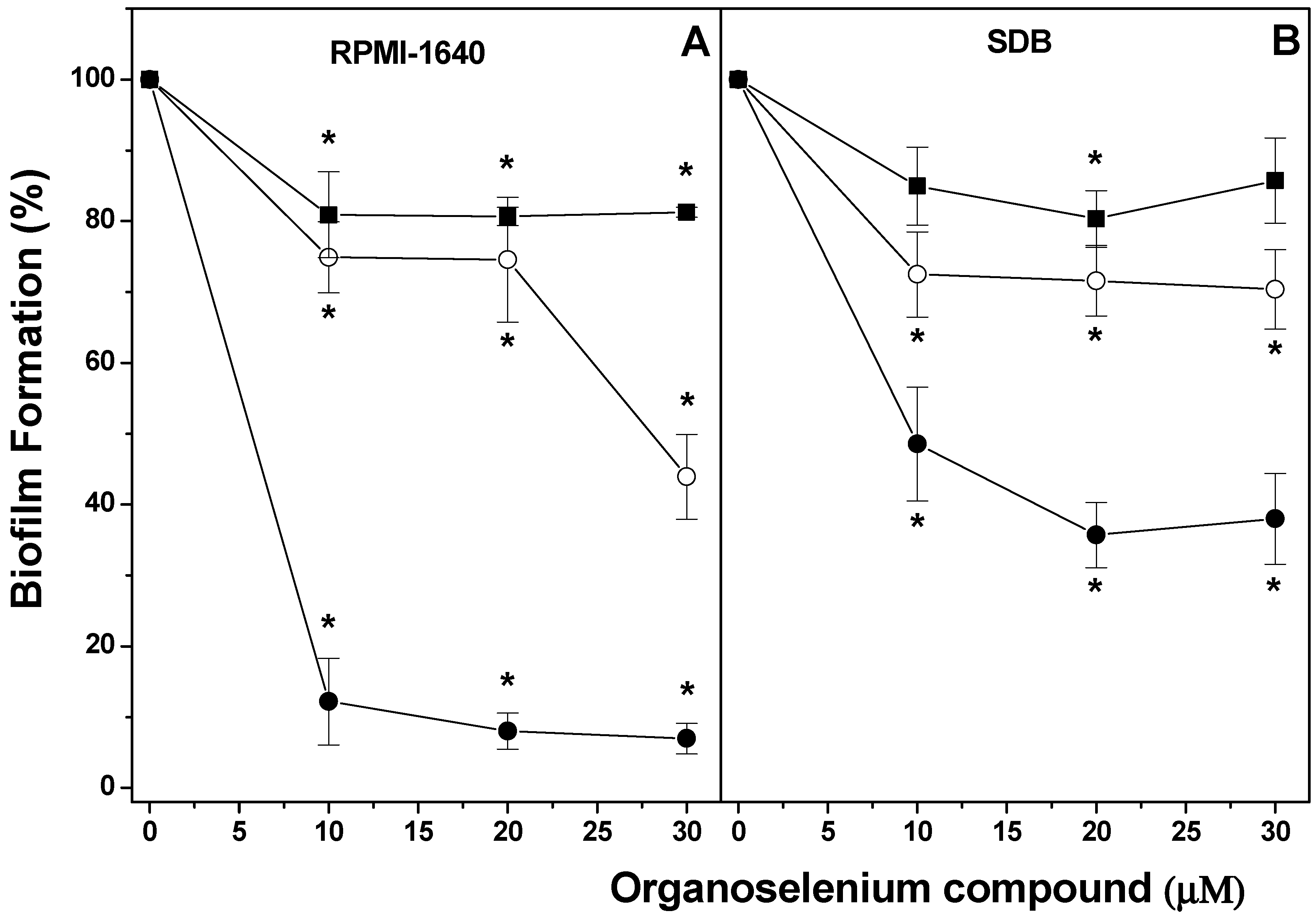
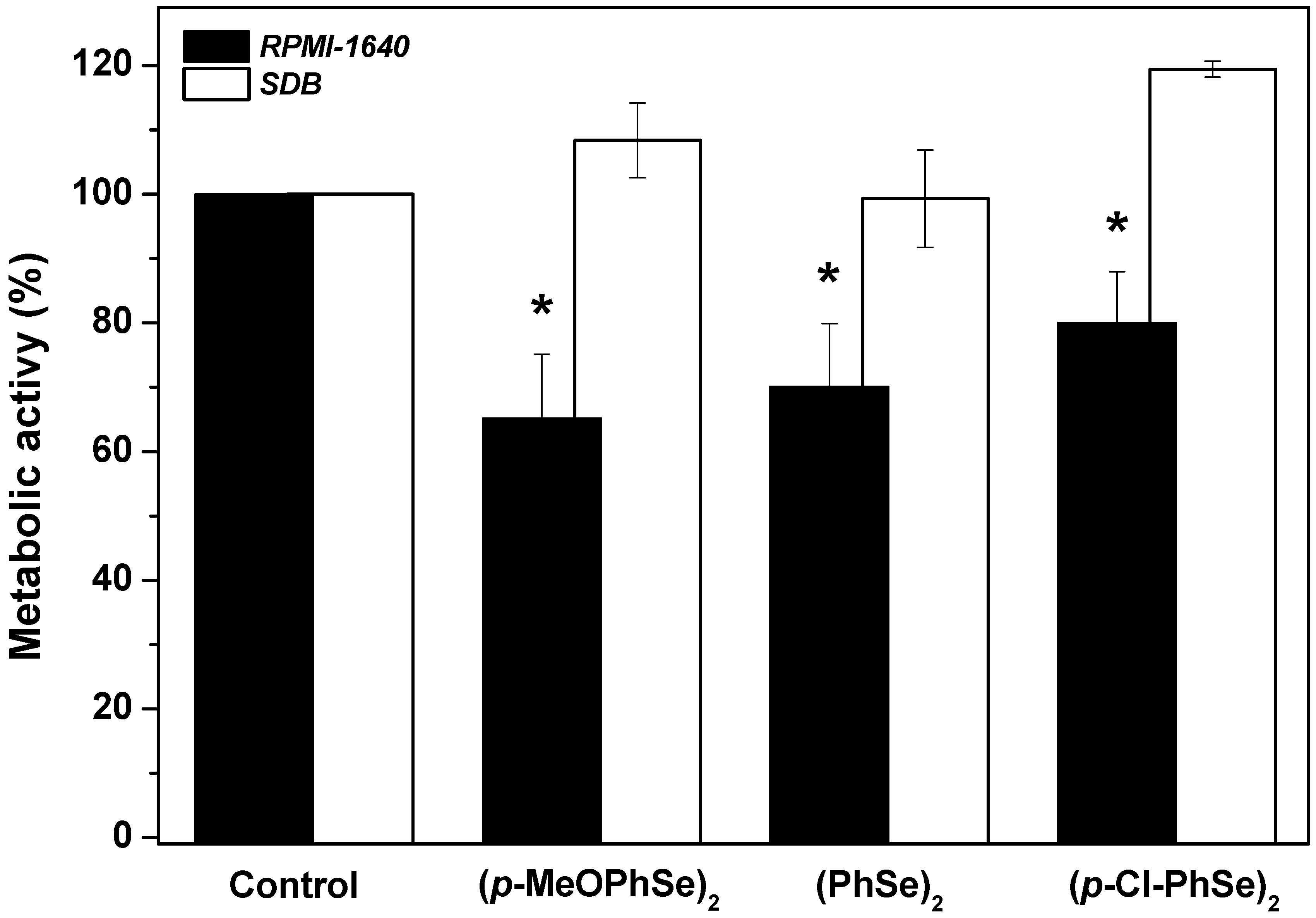
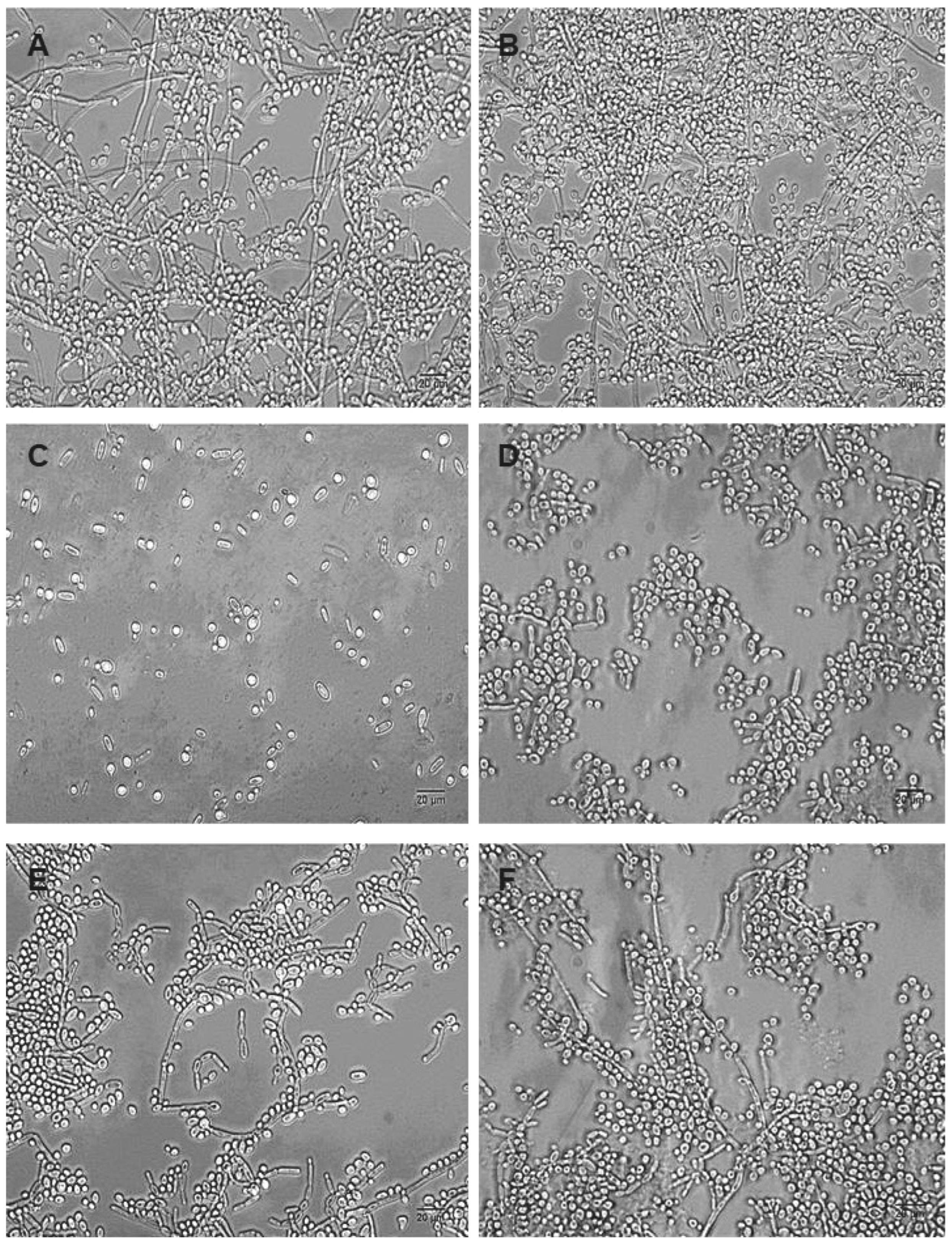
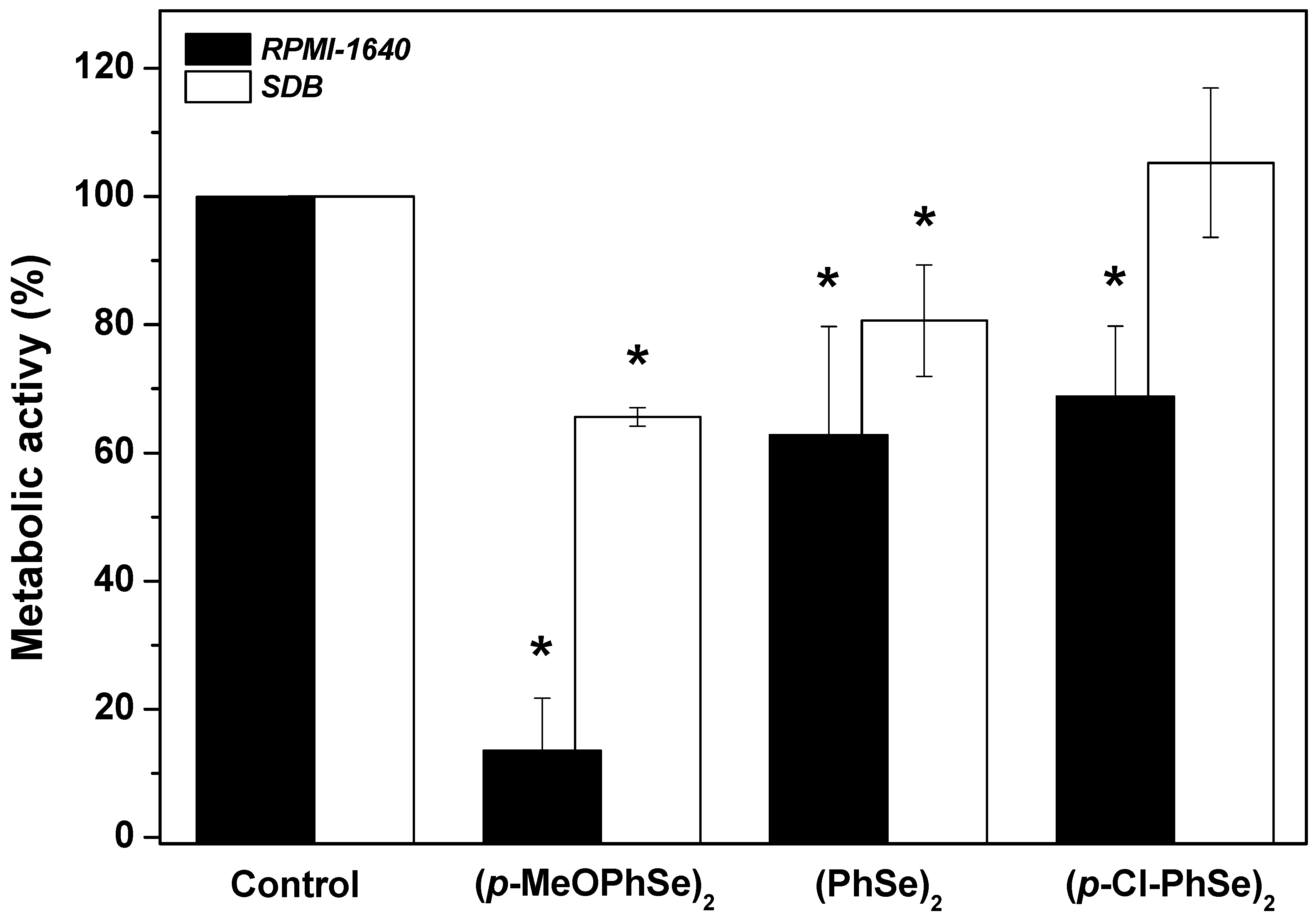
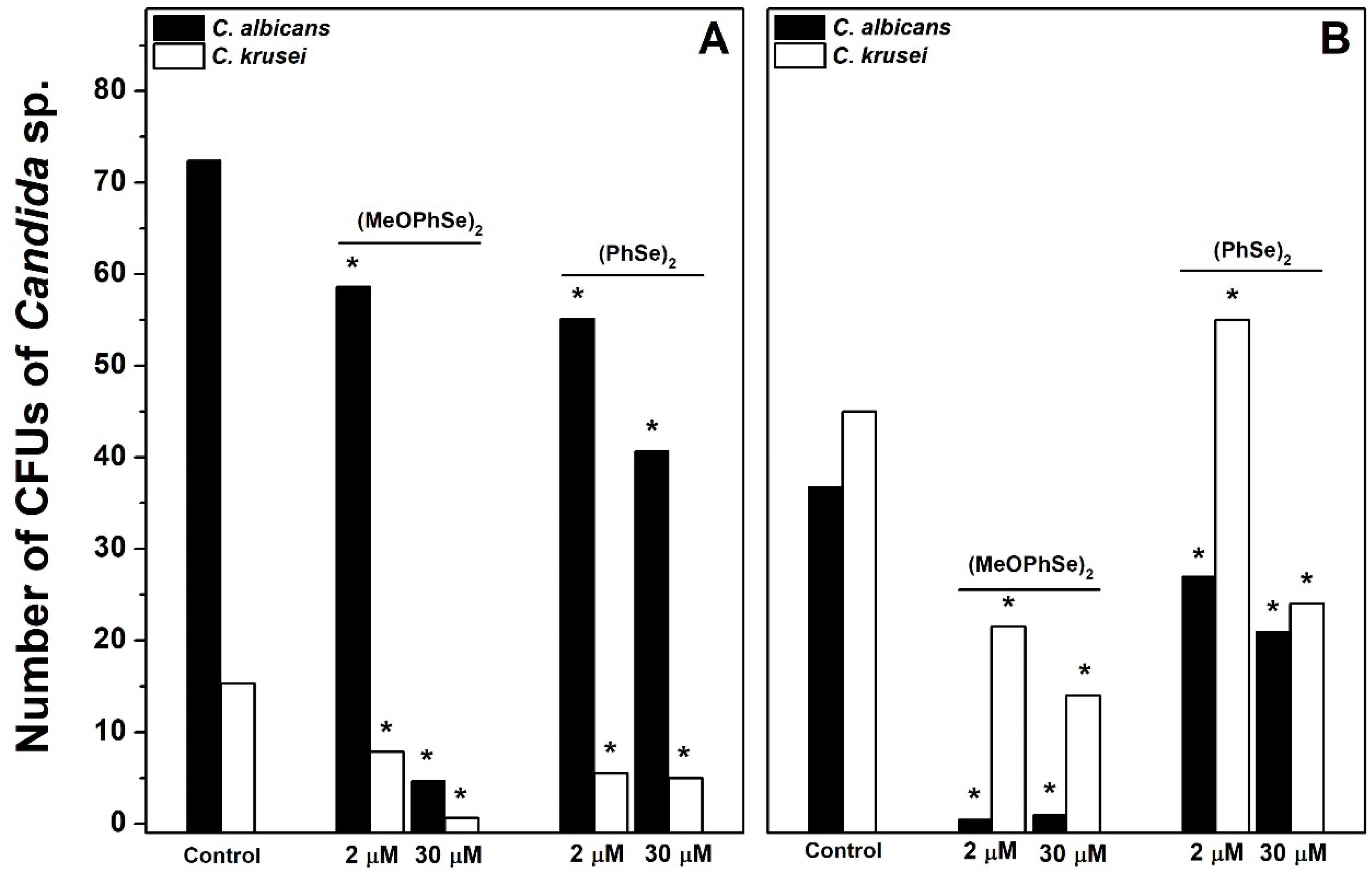
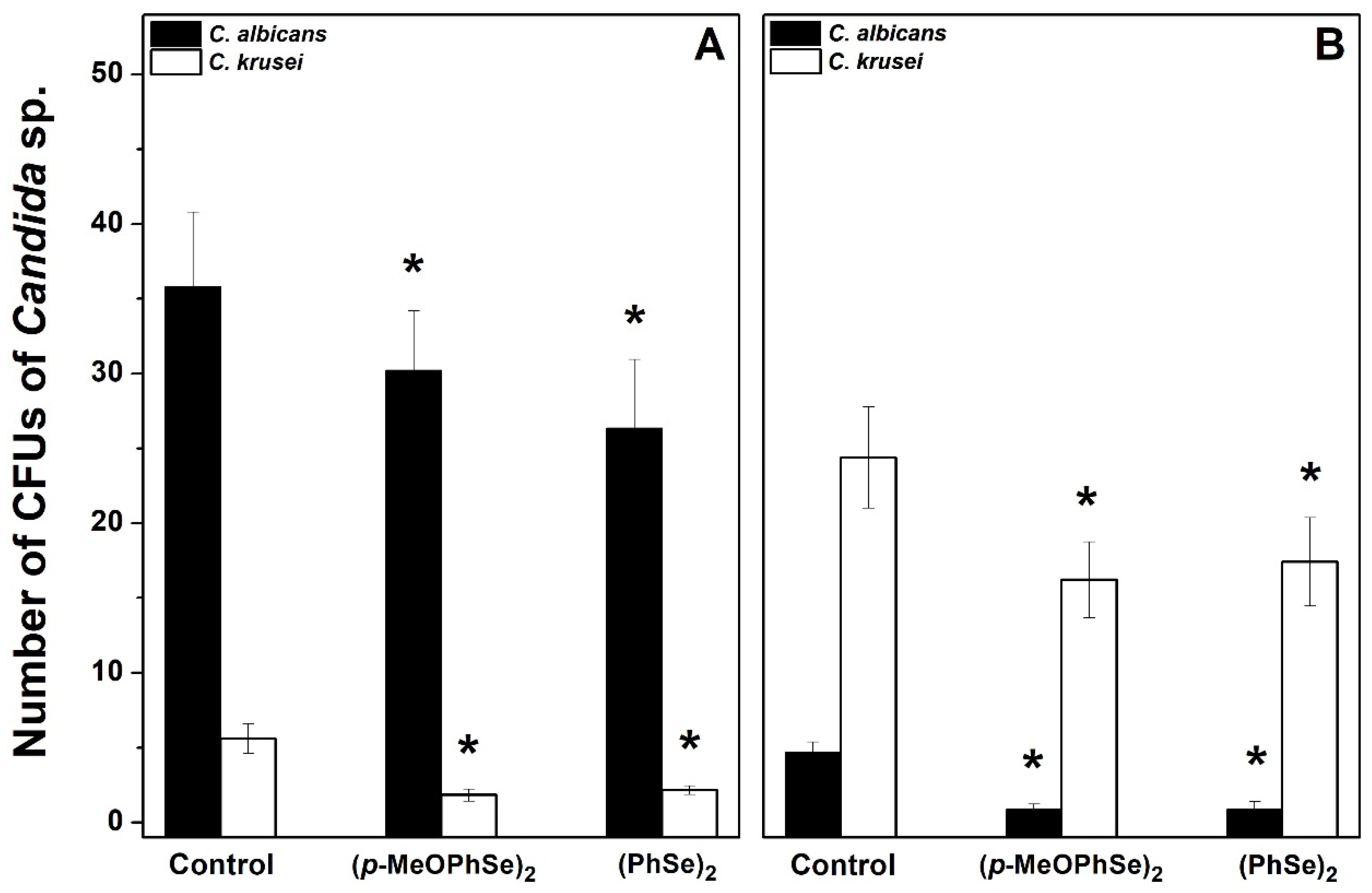
2. Results
3. Discussions
4. Materials and Methods
4.1. Yeast Strain and Growth Conditions
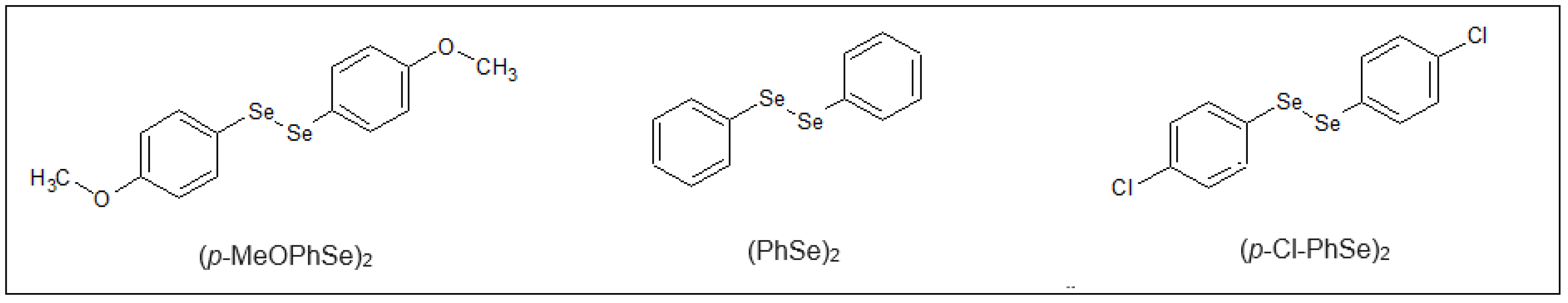
4.2. Organoselenium Compounds (OCs)
4.3. Effect of OCs on Biofilm Formation
4.4. Effect of OCs on Dual-Species Biofilm Produced during 24 h
4.5. Effect of OCs on Cell Adhesion to Polystyrene Surfaces
4.6. Effect of OCs on Number of Colony Forming Units (CFUs) of Either Candida albicans or Candida krusei
4.7. Morphological Analyses of Dual-Species Biofilms
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Araújo, D.; Henriques, M.; Silva, S. Portrait of Candida Species Biofilm Regulatory Network Genes. Trends Microbiol. 2017, 25, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; dos Santos Fontenelle, R.O.; de Brito, E.H.S.; de Morais, S.M. Biofilm of Candida albicans: Formation, regulation and resistance. J. Appl. Microbiol. 2021, 131, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, X.; Ren, B.; Cheng, L. The regulation of hyphae growth in Candida albicans. Virulence 2020, 11, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Guinea, J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014, 20, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Ebbers, J.; Geurtz, L.; Stefanik, D.; Major, Y.; Edmond, M.B.; Wenzel, R.P.; Seifert, H. Nosocomial bloodstream infections due to Candida sin the USA: Species distribution, clinical features and antifungal susceptibilities. Int. J. Antimicrob. Agents 2014, 43, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida Species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef] [PubMed]
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.M.; Pohl, C.H. Update on Candida krusei, a potential multidrug-resistant pathogen. Med. Mycol. 2021, 59, 14–30. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- Kaur, J.; Nobile, C.J. Antifungal drug-resistance mechanisms in Candida biofilms. Curr. Opin. Microbiol. 2023, 71, 102237. [Google Scholar] [CrossRef] [PubMed]
- Malinovská, Z.; Čonková, E.; Váczi, P. Biofilm Formation in Medically Important Candida Species. J. Fungi 2023, 9, 955. [Google Scholar] [CrossRef] [PubMed]
- Ponde, N.O.; Lortal, L.; Ramage, G.; Naglik, J.R.; Richardson, J.P. Candida albicans biofilms and polymicrobial interactions. Crit. Rev. Microbiol. 2021, 47, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Mitchell, A.P. Mucosal biofilms of Candida albicans. Curr. Opin. Microbiol. 2011, 14, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis of Candida albicans biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence 2022, 13, 89–121. [Google Scholar] [CrossRef]
- Suleyman, G.; Alangaden, G.J. Nosocomial Fungal Infections: Epidemiology, Infection Control, and Prevention. Infect. Dis. Clin. N. Am. 2021, 35, 1027–1053. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases—Estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Timsit, J.F.; Ruppé, E.; Barbier, F.; Tabah, A.; Bassetti, M. Bloodstream infections in critically ill patients: An expert statement. Intensive Care Med. 2020, 46, 266–284. [Google Scholar] [CrossRef]
- Fisher, M.C.; Denning, D.W. The WHO fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 2023, 21, 211–212. [Google Scholar] [CrossRef]
- Garvey, M.; Rowan, N.J. Pathogenic Drug Resistant Fungi: A Review of Mitigation Strategies. Int. J. Mol. Sci. 2023, 24, 1584. [Google Scholar] [CrossRef]
- Braga, D.; Costa, A.L.; Grepioni, F.; Scaccianoce, L.; Tagliavini, E. Organic-Organometallic Crystal Synthesis. 1. HostingParamagnetic [(η6-Arene)2 Cr] + (Arene) Benzene, Toluene) in Organic Anion Frameworks via OsH‚‚‚O andCsH‚‚‚O Hydrogen Bonds. Organometallics 1997, 16, 2070–2079. [Google Scholar] [CrossRef]
- Machado, M.S.; Villela, I.V.; Moura, D.J.; Rosa, R.M.; Salvador, M.; Lopes, N.P.; Braga, A.L.; Roesler, R.; Saffi, J.; Henriques, J.A.P. 3′3-ditrifluoromethyldiphenyl diselenide: A new organoselenium compound with interesting antigenotoxic and antimutagenic activities. Mutat. Res. 2009, 17, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Giurg, M.; Golab, A.; Suchodolski, J.; Kaleta, R.; Krasowska, A.; Piasecki, E.; Piętka-Ottlik, M. Reaction of bis[(2-chlorocarbonyl)phenyl] Diselenide with Phenols, Aminophenols, and Other Amines towards Diphenyl Diselenides with Antimicrobial and Antiviral Properties. Molecules 2017, 12, 974. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6285. [Google Scholar] [CrossRef]
- Rosseti, I.B.; Wagner, C.; Fachinetto, R.; Taube Junior, P.; Costa, M.S. Candida albicans growth and germ tube formation can be inhibited by simple diphenyl diselenides [(PhSe) 2, (MeOPhSe) 2, (p-Cl-PhSe) 2, (F 3CPhSe) 2] and diphenyl ditelluride. Mycoses 2011, 54, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, I.; Messina, F.; Nascimento, V.; Nacca, F.G.; Pietrella, D.; Perin, G.; Sancineto, L. Synthetic Approaches to Organoselenium Derivatives with Antimicrobial and Anti-Biofilm Activity. Mini-Rev. Org.-Chem. 2019, 16, 589–601. [Google Scholar] [CrossRef]
- Wall, G.; Lopez-Ribot, J.L. Screening Repurposing Libraries for Identification of Drugs with Novel Antifungal Activity. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef]
- Rosseti, I.B.; Rocha, J.B.T.; Costa, M.S. Diphenyl diselenide (PhSe)2 inhibits biofilm formation by Candida albicans, increasing both ROS production and membrane permeability. J. Trace Elem. Med. Biol. 2015, 29, 289–295. [Google Scholar] [CrossRef]
- de Amorim, L.M.M.; Braga, M.T.; Carvalho, M.L.; de Oliveira, I.R.; Querobino, S.M.; Alberto-Silva, C.; da Rocha, J.B.T.; Costa, M.S. The Organochalcogen Compound (MeOPhSe)2 Inhibits Both Formation and the Viability of the Biofilm Produced by Candida albicans, at Different Stages of Development. Curr. Pharm. Des. 2018, 24, 3964–3971. [Google Scholar] [CrossRef]
- da Silva, B.M.; Braga, M.T.; da Silva Passos, J.C.; Carvalho, M.L.; Rosseti, I.B.; de Amorim, L.M.M.; da Rocha, J.B.T.; Alberto-Silva, C.; Costa, M.S. (PhSe)2 and (pCl-PhSe)2 organochalcogen compounds inhibit Candida albicans adhesion to human endocervical (HeLa) cells and show anti-biofilm activities. Biofouling 2021, 37, 235–245. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira, V.M.; da Silva, B.G.M.; Passos, J.C.d.S.; Pinto, A.P.; da Rocha, J.B.T.; Alberto-Silva, C.; Costa, M.S. (MeOPhSe)2, a synthetic organic selenium compound, inhibits virulence factors of Candida krusei: Adherence to cervical epithelial cells and biofilm formation. J. Trace Elem. Med. Biol. 2022, 73, 127019. [Google Scholar] [CrossRef]
- da Silva, B.G.M.; Pinto, A.P.; Passos, J.C.d.S.; da Rocha, J.B.T.; Alberto-Silva, C.; Costa, M.S. Diphenyl diselenide suppresses key virulence factors of Candida krusei, a neglected fungal pathogen. Biofouling 2022, 38, 427–440. [Google Scholar] [CrossRef]
- Aggarwal, N.; Kitano, S.; Puah, G.R.Y.; Kittelmann, S.; Hwang, I.Y.; Chang, M.W. Microbiome and Human Health: Current Understanding, Engineering, and Enabling Technologies. Chem. Rev. 2023, 123, 31–72. [Google Scholar] [CrossRef]
- Orazi, G.; O’Toole, G.A. “It takes a village”: Mechanisms underlying antimicrobial recalcitrance of polymicrobial biofilms. J. Bacteriol. 2020, 202, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Tits, J.; Cammue, B.P.A.; Thevissen, K. Combination therapy to treat fungal biofilm-based infections. Int. J. Mol. Sci. 2020, 21, 8873. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C.; Steixner, S. The changing epidemiology of fungal infections. Mol. Asp. Med. 2023, 94, 101215. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Shorr, A.F.; Kollef, M.H. Secular Trends in Candidemia-Related Hospitalization in the United States, 2000–2005. Infect. Control. Hosp. Epidemiol. 2008, 29, 978–980. [Google Scholar] [CrossRef]
- Koehler, P.; Stecher, M.; Cornely, O.A.; Koehler, D.; Vehreschild, M.J.G.T.; Bohlius, J.; Wisplinghoff, H.; Vehreschild, J.J. Morbidity and mortality of candidaemia in Europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1200–1212. [Google Scholar] [CrossRef]
- Salehi, M.; Ahmadikia, K.; Mahmoudi, S.; Kalantari, S.; Jamalimoghadamsiahkali, S.; Izadi, A.; Kord, M.; Dehghan Manshadi, S.A.; Seifi, A.; Ghiasvand, F.; et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses 2020, 63, 771–778. [Google Scholar] [CrossRef]
- Passos, J.C.D.S.; Calvi, G.S.; Rodrigues, A.B.F.; Costa, M.S. The inhibitory effect of photodynamic therapy on dual-species biofilms of Candida albicans and Candida krusei can be determined by Candida albicans/Candida krusei ratio. Photodiagnosis Photodyn Ther. 2023, 44, 103787. [Google Scholar] [CrossRef]
- Passos, J.C.D.S.; Rodrigues, A.B.F.; Alberto-Silva, C.; Costa, M.S. The arrangement of dual-species biofilms of Candida albicans and Issatchenkia orientalis can be modified by the medium: Effect of Voriconazole. Biofouling 2024, 8, 1–11. [Google Scholar] [CrossRef]
- Rosa, R.M.; Guecheva, T.N.; Oliveira, I.M.; Braga, A.L.; Henriques, J.A.P. Genetic toxicity of three symmetrical diselenides in yeast. J. Braz. Chem. Soc. 2010, 21, 2119–2124. [Google Scholar] [CrossRef][Green Version]
- Giles, N.M.; Watts, A.B.; Giles, G.I.; Fry, F.H.; Littlechild, J.A.; Jacob, C. Metal and redox modulation of cysteine protein function. Chem. Biol. 2003, 10, 677–693. [Google Scholar] [CrossRef]
- Žiemytė, M.; Rodríguez-Díaz, J.C.; Ventero-Martín, M.P.; Mira, A.; Ferrer, M.D. Real-time monitoring of biofilm growth identifies andrographolide as a potent antifungal compound eradicating Candida biofilm. Biofilm 2023, 5, 100134. [Google Scholar] [CrossRef] [PubMed]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of Amphotericin B: An Overview of the Main Antifungal Agent Used to Treat Invasive Fungal Infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef] [PubMed]
- Adler-Moore, J.; Lewis, R.E.; Brüggemann, R.J.M.; Rijnders, B.J.A.; Groll, A.H.; Walsh, T.J. Preclinical Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Antifungal Activity of Liposomal Amphotericin B. Clin. Infect. Dis. 2019, 68, S244–S259. [Google Scholar] [CrossRef] [PubMed]
- Paulmier, C. Selenium Reagents and Intermediates in Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 1986. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvi, G.d.S.; Cartaxo, G.N.J.; Carretoni, Q.L.; da Silva, A.L.M.; de Moraes, D.N.; Pradella, J.G.d.C.; Costa, M.S. Inhibition of Development and Metabolism of Dual-Species Biofilms of Candida albicans and Candida krusei (Pichia kudriavzevii) by Organoselenium Compounds. Pharmaceuticals 2024, 17, 1078. https://doi.org/10.3390/ph17081078
Calvi GdS, Cartaxo GNJ, Carretoni QL, da Silva ALM, de Moraes DN, Pradella JGdC, Costa MS. Inhibition of Development and Metabolism of Dual-Species Biofilms of Candida albicans and Candida krusei (Pichia kudriavzevii) by Organoselenium Compounds. Pharmaceuticals. 2024; 17(8):1078. https://doi.org/10.3390/ph17081078
Chicago/Turabian StyleCalvi, Gabriela de Souza, Giulia Nicolle Jácome Cartaxo, Qiuxin Lin Carretoni, André Luiz Missio da Silva, Denilson Nogueira de Moraes, José Geraldo da Cruz Pradella, and Maricilia Silva Costa. 2024. "Inhibition of Development and Metabolism of Dual-Species Biofilms of Candida albicans and Candida krusei (Pichia kudriavzevii) by Organoselenium Compounds" Pharmaceuticals 17, no. 8: 1078. https://doi.org/10.3390/ph17081078
APA StyleCalvi, G. d. S., Cartaxo, G. N. J., Carretoni, Q. L., da Silva, A. L. M., de Moraes, D. N., Pradella, J. G. d. C., & Costa, M. S. (2024). Inhibition of Development and Metabolism of Dual-Species Biofilms of Candida albicans and Candida krusei (Pichia kudriavzevii) by Organoselenium Compounds. Pharmaceuticals, 17(8), 1078. https://doi.org/10.3390/ph17081078








