Abstract
Despite reliable evidence of adverse drug effects, the substantially increased prescription rates of proton pump inhibitors (PPIs) remain at a high level. This study analyzed the appropriateness of PPI prescriptions among residents of nursing homes in three regions of Germany. Baseline data of a cluster-randomized controlled trial were used to determine the prevalence of PPI prescriptions, the validity of indications, and the adequacy of the prescribed dosages according to 1. their drug approvals and 2. valid recommendation guidelines. Regression analyses were conducted to assess associated factors. A total of 437 residents in 37 nursing homes were included (mean age 83 ± 9.2 years, 72% women). The PPI prescription prevalence was 44% (n = 193). In 52/193 (27%) there was no adequate indication, and in 54 (39%) of 138 indicated PPI prescriptions it was overdosed. Yet, in only less than one-third (28%) of “adequate” prescriptions, the indication was according to the PPI approvals, whereas the majority (72%) were off-label indications in line with valid guideline recommendations. Non-indicated PPI prescription was associated with the total number of prescribed drugs (OR 1.32; 95% CI 1.18–1.62; p = 0.013). There were no associations with age, level of care dependency, cognitive impairment, prescription of psychotropic drugs, number of chronic diseases, number of physicians’ consultations, or study region. To conclude, in 55%, the high prescription prevalence among residents was either not indicated or overdosed. In total, only 20% (39/193) of cases of PPI use complied with the approved indications. There is a need for quality control of 1. PPI administration in German nursing homes, and 2. of guideline recommendations expanding the off-label PPI use by 72% within the indication scale, predominantly from wide prescription for low-dose ASA.
1. Introduction
Proton pump inhibitors (PPIs), introduced with omeprazole in 1989, are among the most commonly prescribed medications in the world [1]. In Germany, the frequency of PPI prescriptions increased steadily between 2007 and 2016, from 1.411 million defined daily doses (DDDs) to 3.8 million DDDs [2], remaining largely stable at this high level since then. However, this goes along with the 2009 and 2014 released German over-the-counter (OTC) PPI self-medication permissions and does not include the OTC PPIs, by which the overall consumption is assumed to continue to grow [3]. The elevated prescription rate of PPIs cannot be explained by an expansion in the number of corresponding diseases [2,3].
Although the valuable and highly effective PPIs are associated with considerable risk in long-term use, they remain referred to as generally well tolerated and are often used for prophylaxis and so-called “stomach protection”. Inappropriate use has been reported for up to two-thirds of cases [4]. PPIs are often prescribed without indication, in too high doses, and for too long. In particular, the non-indicated long-term use of PPIs without an indication must be viewed very critically. PPIs affect the gut microbiome by increasing the genera Enterococcus, Streptococcus, Staphylococcus, and the potentially pathogenic species Escherichia coli [5]. Regarding adverse drug reactions (ADRs), long-term use is associated with an increased risk of infections by, e.g., Clostridium difficile and Campylobacter [6,7], with evidence of the latter published as early as 1999 [6]. Deficiency symptoms of vitamin B12, iron, sodium, and magnesium increase with long-term use [8,9,10]. Associations between long-term PPI use and osteoporosis and fracture risk have also been described [11,12,13]. Long-term treatment was associated with an almost doubled increased risk of community-acquired pneumonia in a large cohort of older adults in primary care [14]. There are rare cases of agranulocytosis from PPI-induced hypersensitivity reactions, and reports suggest that PPI-induced neutropenia is immune-mediated, with evidence of cross-reactivity between PPIs [15]. The results of studies on the potential for cognitive decline with PPIs remain controversial [16,17,18] and further research is warranted. PPIs are also suspected of increasing the risks of chronic kidney disease [19] and myocardial infarction [20,21].
Against the background that, 1., despite being a vulnerable patient group, there are no special data on the German PPI prescribing behavior in the elderly nursing home residents, and, 2., more than 700 hundred self-conducted medication reviews within the EPCentCare study among nursing home residents show an alarming prevalence of PPI prescription, this study aimed to provide more insight into this prescription behavior in order to advocate more concrete restrictive or deprescribing practices perspectively.
The research focus was to determine the prevalence of PPI prescriptions in German nursing home residents, the appropriateness of the PPI indication according to both their Summaries of Product Characteristics (SmPC) approvals and to the national guideline recommendations and, furthermore, the adequacy of the PPI dosage for therapeutic and preventive purposes respectively according to their SmPCs [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Associations between inappropriate PPI prescription and resident and healthcare-related factors were analyzed. The presented data refer to a previous doctoral thesis [42]. In addition, the results of this selected study population were compared with the PPI prevalence in different own patient samples on PPIs. Finally, the current updated guideline recommendations were reviewed versus the previous national guidelines in terms of their potential forthcoming impact on future PPI prescription frequency [43,44,45,46,47,48,49,50,51,52,53,54,55].
2. Results
Out of 437 residents included in the analysis, n = 161 were located in Halle (Saale) (36.8%), n = 115 in Lübeck (26.4%) and n = 161 in Witten/Herdecke (36.8%), the eastern, western, and northern regions of Germany (Table 1).

Table 1.
Clusters and participants per study center.
Mean age was 83 ± 9.2 years, and 71.9% were women. The majority of residents were assessed as severe care dependent (level two out of three) according to an expert rater of the statutory health insurance system (Table 2).

Table 2.
Baseline characteristics n (%) of study participants (n = 437).
The prevalence of PPI prescriptions was 44.2% (n = 193), with pantoprazole being the most frequently prescribed (n = 153, 79.3%). The indication for PPI prescription was appropriate in 138 (71.5%) of the participants according to the valid SmPC drug approvals, which remained unchanged since the time of the study [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] (Table 3), and according to the documented diagnoses, entire medication lists, and guideline recommendations [43,44,45,46,47,48,49,50,51,52,53,54,55]. In 52 (26.9%) patients, the application was inadequate as it was without evidence of any indication. In three (1.6%) patients, the indication remained unclear. These three residents were on PPIs, although the nonsteroidal anti-inflammatory drug (NSAID) was an “on-demand“ medication. For temporarily prolonged NSAID use, the PPI indication would be adequate, but in the case of a single punctual application of the NSAID, the long-term use of PPIs would be inappropriate (Figure 1).

Table 3.
Example overview of the two prescribed PPIs omeprazole and pantoprazole for approved indications and dosing per day (/d) in adults as SmPCs documented at the time of the study and unchanged today (except for the treatment dosage of NSAID-associated gastric and duodenal ulcers).
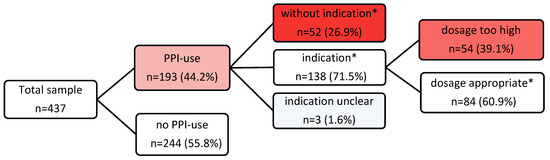
Figure 1.
Flowchart of PPI prescription prevalence and appropriateness. * According to PPI SmPCs and/or guideline recommendations valid for the investigation period.
The differentiation was according to the list of diagnoses in each resident. The diagnoses list of a patient is available as a separate list and should characteristically contain all diagnoses and even historically relevant diseases. It exists independently of the medication list, but, for medications-related authorizations, you should always find the corresponding diseases and underlying indications for the prescribed drugs.
PPIs are generally prescribed as a statutory health insurance (SHI) prescription for the commonly insured patient. In principle, a drug can only be prescribed in Germany at the expense of SHI if it is used to treat diseases for which a marketing authorization has been obtained. However, there is a way to allow off-label use as a SHI benefit, so, doctors may be permitted to use medicinal products beyond the scope of the marketing authorization. There is also a possibility for an exceptional private prescription, and, in the case of PPIs, e.g., up to 20 mg pantoprazole by own OTC acquisition.
A detailed analysis of the prescriptions defined as “appropriate” showed that in less than one-third of the appropriate prescriptions, 39 (28.3%), the residents’ diagnoses had an approved drug indication, whereas the majority of prescriptions, 99 (71.7%), were prescribed according to the previous and current recommendations of the German guidelines [43,44,45,46,47,48,49,50,51,52,53,54,55] and in an off-label manner (Table 4). Almost 60% of patients aged ≥ 65 years (>60 in different versions and guidelines) received a PPI for low-dose ASA. As with the previous and currently updated PPI SmPCs, this was not an approved indication. In response to two independent inquiries about the approved indications for omeprazole and pantoprazole, the pharmaceutical companies confirmed that “ASA at low doses mainly inhibits platelet aggregation and is therefore used to prevent heart attacks and strokes. For this reason, ASA is classified in a separate group of drugs, the antiplatelet agents, and is not classified as an NSAID, although at high doses it has similar effects to NSAIDs. Long-term therapy with low-dose ASA generally does not require prophylaxis with proton pump inhibitors unless there are additional risk factors.” (1. MedInfo Germany. Authors’ Personal Medical-Scientific e-mail Correspondence. Pantoprazol AbZ 20 mg Magensaftresistente Tabletten. medical.affairs/teva.de/ratiopharm.de; 26 April 2024 and 2. MedInfo Germany. Authors’ Personal Medical-Scientific e-mail Correspondence. Omeprazol-ratiopharm NT 20/40 mg Magensaftresistente Hartkapseln. medical.affairs/teva.de/ratiopharm.de; 5 May 2024. Another company admitted to having no data on this topic (Medical information specialist. Authors’ personal medical-scientific e-mail correspondence. Pantoprazole NYC® 20 mg, Takeda Pharma; 6 May 2024). Further off-label prescriptions resulted from guidelines recommending the combination of PPIs with any other anticoagulative drugs, predominantly involving direct oral anticoagulants (DOACs).
In addition, there are some recommendations from other national and international guidelines that were not relevant to the residents studied according to their documented diagnoses, e.g., no intensive care, trauma, and transplant conditions. Retrospectively, to compare the indication results, the “Appropriate Use of Proton Pump Inhibitors” by Phil Chung [56], and the current expert review [57], were applied to update with the latest, also international, guidelines.

Table 4.
Indications for PPI prescription.
Table 4.
Indications for PPI prescription.
| Aedequate Indications | Number = 138 | |
|---|---|---|
| Approved indication according to SmPCs * | Reflux esophagitis not defined further on | 13 (9.4) |
| Indicated by a past diagnosis in the history ******* | 12 (8.7) | |
| Gastric ulcer | 4 (2.9) | |
| NSAID *** use + another risk factor | ||
| → ibuprofen + age ≥ 65 years | 3 (2.2) | |
| → diclofenac + ASA ld ****+ age ≥ 65 (60) years authors’ note: Cave | 3 (2.2) | |
| → ibuprofen + ASA ld **** + age ≥ 65 (60) years authors’ note: Cave | 1 (0.7) | |
| → naproxen + prednisolone + age ≥ 65 (60) years | 1 (0.7) | |
| → ibuprofen + prednisolone + phenprocoumon + age ≥ 65 (60) years | 1 (0.7) | |
| Eradication of Helicobacter pylori | 1 (0.7) | |
| Total number (%) | 39 (28.3) | |
| Indications according to guideline recommendations ** (entirely off-label use and meant for years or decades) | Use of antiplatelet drugs + another risk factor | |
| → ASA ld ****+ age ≥ 65 years | 81 (58.7) | |
| → clopidogrel ****** + age ≥ 65 years authors’ note: Cave ****** | 3 (2.2) | |
| Use of oral anticoagulants + another risk factor | ||
| → rivaroxaban + age ≥ 65 years | 6 (4.3) | |
| → apixaban + age ≥ 65 years | 2 (1.4) | |
| → phenprocoumon + age ≥ 65 years | 2 (1.4) | |
| Use of two platelet aggregation inhibitors | ||
| → clopidogrel ****** + ASA ld **** authors’ note: Cave ****** | 4 (2.9) | |
| Use of an oral anticoagulant + antiplatelet | ||
| →rivaroxaban high dose + ASA ld **** authors’ note: Cave ***** | 1 (0.7) | |
| Total number (%) | 99 (71.7) |
Values are absolute numbers (percentage). * Valid SmPC (Summary of Product Characteristics) approvals at time of investigation. ** Valid German guideline recommendations at time of investigation. *** NSAID non-steroidal anti-inflammatory drug. **** ASA 100 mg/day = ASA low-dose = ASA ld. Authors’ note: Cave: ASA ld in combination with NSAIDs no sufficient platelet aggregation inhibition. It is important to take ASA with a time delay before NSAIDs. ***** Authors’ note: Cave: in combination with DOACs clopidogrel should be preferred [58]. ****** Authors’ note: Cave: clopidogrel should not be combined with omeprazole due to insufficient prodrug activation. ******* A residual partial PPI indication from a previous condition with a prolonged PPI prescription.
Chung (Nebraska) [56] listed the spectrum of PPI treatment indication according to the drug approvals (Table 3) of the PPI SmPCs: PPIs are indicated for the treatment of the following conditions: “Zollinger-Ellison Syndrome, Barrett’s esophagus, acute upper GI bleed, erosive esophagitis, Helicobacter pylori treatment, gastric or duodenal ulcer, gastroesophageal reflux disease (GERD)”.
For prophylactic PPI prescription, Chung summarized the spectrum as an overview we additionally compared retrospectively for the indication assessment results: “PPI’s are considered appropriate for the prophylaxis of UGIB in the following conditions: Mechanical ventilation for greater than 48 h; Coagulopathy defined as platelet count < 50,000/μL, INR > 1.5, or PTT 2x control; Traumatic head injuries with a Glasgow Coma Score ≤ 10 or inability to follow simple commands; Burns affecting > 35% of total body surface area; Major trauma with an Injury Severity Score ≥ 16; Spinal cord injury; Partial hepatectomy; Solid organ transplantation perioperatively in the ICU setting; Antiplatelet therapy (usually aspirin + clopidogrel, prasugrel, or ticagrelor) in patients at high risk for GI bleeding (prior history of GI bleeding; age > 60 years; concurrent use of anticoagulants, corticosteroids, or NSAID; Helicobacter pylori infection); Long-term NSAID use in patients with moderate to high risk of GI bleeding—Moderate risk is defined as 1 or 2 of the following risks: age > 65 years; high dose NSAID therapy (ibuprofen > 2400 mg daily, naproxen > 1000 mg daily, meloxicam > 7.5 mg daily); previous history of uncomplicated ulcer; concurrent use of aspirin, corticosteroids, or anticoagulants); High risk is defined as history of complicated ulcer especially recent, or >2 risk factors outlined in the moderate risk group; Any 2 of the following: Sepsis, ICU stay > 7 days, Occult bleeding lasting more than 6 days, High dose corticosteroids (>250 mg/day of hydrocortisone, >50 mg/day of methylprednisolone, >60 mg/day of prednisone, >10 mg/day of dexamethasone)”.
Overlapping with our procedure, he also points to the necessity of deprescribing upon discharge unless a chronic condition requires the PPI, to employ the lowest possible dose and shortest therapy duration [56], although concrete doses are not provided.
The data available for this secondary data analysis did not provide information going back to the start of the PPI prescription to distinguish whether it was a new prescription or a continuation after a hospitalization. It was only possible to determine the fact of PPI prescription and the corresponding diagnosis as an indication through corresponding approvals or national guideline recommendations.
Regression analysis for covariables on inappropriate PPI prescription revealed a significant association with the total number of prescribed drugs (odds ratio [OR] 1.32, 95% CI 1.18–1.62, p = 0.013) (Table 5). There were no other significant associations from the variables analyzed, neither for age, level of care dependency, number of chronic diseases, cognitive impairment, psychotropic drugs, number of physicians’ visits, nor the different regional study centers. However, there was some evidence of a higher rate of non-indicated PPI prescribing at the Witten/Herdecke study center (34%) compared to Halle (Saale) (22%) and Lübeck (20%).

Table 5.
Distribution of indicated and non-indicated PPIs among 190 PPI-prescribed study participants’ covariables and associations with non-indicated PPI prescriptions (three patients with uncertain allocation excluded).
The further review of all adequately indicated PPI prescriptions in terms of their correct dosage regimen, either a therapeutic or a prophylactic dose, showed that, in 54 participants (39% of indicated PPI prescriptions), the PPIs were overdosed respecting all documented diagnoses and comedications (Figure 1). This was typically the case for prophylactic PPI prescriptions as recommended in SmPCs and, above all, in the guidelines. It is precisely the guidelines, which make up the majority of these prescriptions, that are rarely accompanied by a specific dose recommendation in this prophylactic context, so that physicians may prescribe far too high doses for years or even decades based on the underlying condition of the recommendation. The overdose amounted to a two to fourfold PPI intake (Table 6). For these inadequately too high dose regimens, no statistically significant associations could be found with the covariables studied for the non-indicated prescriptions.

Table 6.
Overdoses (>20 mg/d) of up to two to fourfold higher, especially with prophylactic PPI prescriptions according to guideline recommendations, which involve years or decades of PPI use in these constellations.
There was no tracking of the duration of PPI use, as this became influenced by medication review instructions. Because, as a consequence of these findings, the first author intervened and advised the respective physicians to adequately deprescribe the PPI in cases of non-indication and overdosage.
Figure 2 shows a summary of the differentially analyzed prevalences within PPI prescribing.
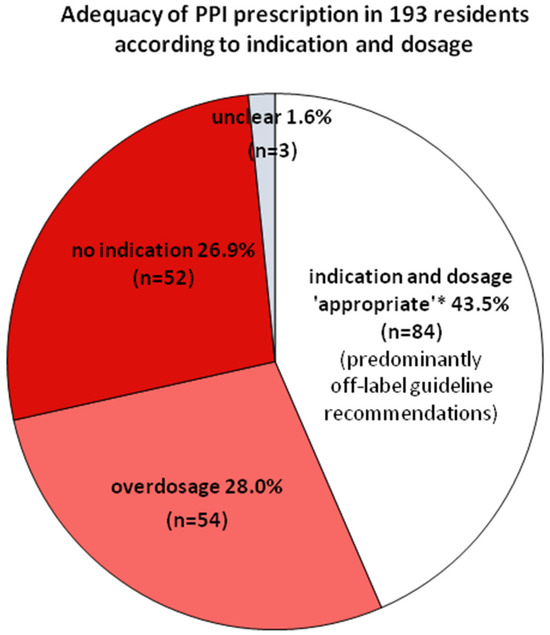
Figure 2.
Prevalence of appropriate prescription*, non-indication, and overdosage among 193 residents on PPIs. In three cases, the indication remained “unclear” because NSAIDs were applied on demand without information on single doses or temporarily prolonged NSAID use. * According to the PPI SmPCs and/or guideline recommendations valid at investigation.
While these results refer to the selected study population of nursing home residents taking antipsychotics, we tried to place the results in the light of further and current prescription rates in different and more general populations. The first author was able to obtain a broad and ongoing real-world overview by reviewing daily ambulatory medication lists of hospitalized outpatients, now numbering more than 63,800, such as elderly patients ≥ 70 years of age undergoing trauma surgery after fractures. They provide further insight into the extraordinary PPI prescribing behavior:
- 1.
- Most recently, starting in January 2024, among 200 hospitalized trauma patients ≥ 70 years of age, primarily with hip fractures after falls, the ambulatory PPI prescription rate was 51% (102 patients) compared to 44% in the presented secondary data study. A slight improvement was seen in the prevalence of prophylactic 20 mg doses of pantoprazole (72% of all PPI prescriptions) versus the predominant 40 mg dose prevalence in the presented study. However, particularly in these trauma patients, the long-term PPI use-associated risk of osteoporosis and falls with fractures must be questioned as at least a partial adverse drug effect in this context.
- 2.
- An intervention study to improve polypharmacy in patients ≥70 years of age by Individual Pharmacotherapy Management (IPM) revealed a therapeutic 40 mg PPI prescription rate of 36% prior to the intervention, and an overall PPI prescription rate of 47%. The 40 mg dose was reduced to 24% with the IPM intervention [59]. To negate a confounding effect of nursing home residence or antipsychotic use on PPI overprescribing, as might be assumed from the secondary data of the presented study, it is important to note that, within the IPM-intervention study population of 404 patients, only 19% were nursing home residents, whereas 81% were home-dwelling elderly patients, and the mean antipsychotic prescription rate for the 404 study participants was 13%.
3. Discussion
In this analysis, almost half of the participants were prescribed PPIs. The study population comprised nursing home residents from the EPCentCare trial (subsequent recruitment and dropouts during the intervention contributed to the small differences in numbers in the final EPCentCare trial) [60]. This was a selective study cohort with at least one antipsychotic prescription and a consecutive medication review at initial recruitment. However, our results are consistent with previous international studies [61,62]. For example, Kelly et al. [61] found that 57.5% of 547 study participants had a PPI prescription, and Souto Barreto et al. [62], who studied 6275 nursing home residents, reported a PPI rate of 37.8%. The high rate of PPI prescription among nursing home residents was widespread in all three German regions studied, slightly more pronounced in the Witten/Herdecke study group. This high prescription rate is probably due to the apparent harmlessness of PPIs. As ‘stomach protectors’ they may also be assumed to prevent the patient from the adverse effects of other drugs.
About one-third of users received a PPI without an adequate indication. In residents with an indicated PPI regimen, only 28% of PPI prescriptions were based on a SmPC-approved indication. The vast majority (72%) of PPI indications were issued on the recommendations of the national guidelines and were therefore predominantly used on the basis and endorsement of these ‘in good conscience’ prescriptions although entirely in the off-label range. In particular, the guideline with the weak PPI ‘can’ recommendation [43] led to extremely frequent off-label prescribing of PPIs in residents ≥65 years of age on low-dose ASA, which accounted for almost 60% of all PPI ‘indications’. In the former German guideline version, there was a discrepancy between the text and the table for the important premise >1 risk factor and ≥1 risk factor to ‘can’ prescribe a PPI, which had far-reaching implications regarding age as a sufficient single risk factor with low-dose ASA. For the analysis of this study, the question of the inconsistent statements was addressed to the authors but remained unanswered. In the translated English version, the text and table content were unified to ≥1 risk factor hereafter. In addition, this guideline has been updated [44]: the 02/2016 version of the guideline recommended that “If a monotherapy with aspirin, another platelet aggregation inhibitor, NOAC, or VKA is given, PPI prophylaxis can be given if there is at least 1 risk factor for a gastroduodenal ulcer bleeding. Strength of consensus: strong consensus—recommendation”. At that time, age ≥ 65 years counted as at least one risk factor. The recommendation modified in the updated 2021 version stated “If monotherapy with ASA, a P 2 Y 12 inhibitor, DOAC or VKA is administered, PPI prophylaxis should be given if at least one other risk factor for the occurrence of a gastroduodenal ulcer and/or ulcer complication (see ulcer complication (see Statements 7.3 and 7.4) is present. If only the risk factor age > 60 years and no other risk factor is present, prophylaxis is not necessary” [44]. This currently valid recommendation sounds weaker, but the age has been lowered even further and the “can” has been changed to a “should” recommendation in case of any second risk factor, e.g., diabetes mellitus. What makes the scenario even more confronting is the fact that this indication typically means PPI for years and decades of life in low-dose ASA. Additionally, frequently, an adequate indication for the ASA prescription itself was not obvious in our patient population. Low-dose ASA for the primary prevention of cardiovascular disease is still being studied in terms of risk/benefit, and trial data remain controversial; the decision should be made on a more precise individual-patient basis [63]. Other studies have also shown that PPIs are often prescribed as prophylaxis due to the use of low-dose ASA. A French study in hospitalized elderly patients revealed that about 60% of PPI prescriptions were not in accordance with the French guidelines; the leading mismatch was primary prevention for low-dose ASA [64]. A second leading noncompliant indication was lengthy of treatment without reevaluation, the mean duration of PPI prescription was 2.3 years and exceeded 6 months in 62% of cases [64]. Although this German updated guideline on the prophylactic use of PPIs with low-dose ASA no longer recognizes age alone as a risk factor, but only in combination with other factors, such as underlying severe diseases, the recommendation been strengthened from “can” to “should” and the age limit lowered from 65 to 60 years. This means PPIs until the end of life in these patients on low-dose ASA, despite all the known ADRs manifesting as a result of long-term use, such as osteoporosis with increased hip fractures [11]. According to the results of this UK study, the incidence of associated hip fractures increased steadily with a longer duration of PPI prescription [11]. A recent guideline of the German Society of Internal Medicine (DIM) recommends that “in monotherapy with an antiplatelet agent (low-dose ASA or another platelet aggregation inhibitor), a PPI should not be prescribed as a rule. Strength of consensus: strong consensus. Justification: The increase in the risk of bleeding due to low-dose long-term low-dose ASA therapy is low. PPI administration is therefore not indicated in all patients, but only in patients who have at least one risk factor for gastroduodenal ulcer bleeding. Risk factors include, for example, age ≥ 65 years, a history of ulcers, concomitant bleeding-inducing medication, a severe course of a general illness (e.g., type 2 diabetes), smoking, or an H. pylori infection” [52]. Again, we have the off-label indication in patients ≥ 65 years of age taking low-dose ASA as the only risk covariate, which in turn includes the vastly expanded patient population for whom this recommendation foresees years and decades of PPI use. The recommendations in these guidelines need to be adjusted to a patient-centered, balanced level with individualized, ongoing benefit–risk assessment and reassessments. Regarding the duration of PPI prescription, this aspect was not considered in the present study, although this is an additional serious factor of inappropriateness [11]. Further complicating the liberal PPI over-prescribing, Haasturp et al. ascertained that the definition of long-term PPI use was rationalized in only 20% of the studies evaluated and ranged from >2 weeks to >7 years [65], with most >1 year and >6 months; although the approved indication for a prolonged therapy is only in very rare diseases such as with Zollinger–Ellison syndrome [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41].
Regression analysis revealed that the increased number of drugs administered daily was significantly associated with non-indicated PPI therapy. The association between non-indicated PPI prescriptions and polypharmacy has been reported by various other studies [62,66], the high PPI use almost representing a prescribing cascade [66].
In addition to the discussed guideline-indicated off-label PPI prescriptions, the great amount of PPI use without any indication (27%) should be regarded as very critical. Although PPIs are generally considered well-tolerated drugs, significant ADRs might occur, especially in long-term therapy. The present study could not investigate whether the prescription of non-indicated PPI was a result of previous hospitalization, but this can partly be assumed as various studies have demonstrated an increase in prescriptions of PPIs after hospitalization. For example, the analysis by Scheurlen et al. revealed that failure to discontinue PPI after hospitalization is one of the major factors in non-indicated long-term PPI therapy [67]. In accordance, also Ahrens et al. reported, that non-indicated PPIs continue to be prescribed by family physicians after hospital discharge [68]. In order to prevent these non-indicated prescriptions, it has been recommended by a general practitioner guideline on multi-medication to provide information on the duration of the medication listed in the discharge letter in the context of discharge management [47].
Not least to complete the PPI overuse spectrum, within the indicated PPI prescriptions, 39% of the administration regimen was overdosed contrary to the PPI approvals. The reason for overdosing of PPIs might be the assumption that the higher the dose the greater the gastrointestinal protection, although there are only a few approved strong therapeutic indications for higher doses [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41], as can be seen from the overview in Table 3. Furthermore, the guideline recommendations predominantly do not specify any dosage. The prevalence of overdosage is consistent with the results of other studies, e.g., a report indicating that 41.6% of study participants were prescribed an overdosed PPI [61]. In a retrospective cross-sectional study including adult patients of 1006 general and 39 gastroenterological practices in Germany, Plehhova et al. also emphasized the discrepancies between mild indications and high-dose or long-duration PPI [69]. Dosage recommendations should not exceed the dosing regimens according to the drug approvals [45,52].
Of the currently available prescription PPIs, pantoprazole, omeprazole, esomeprazole, lansoprazole, dexlansoprazole, and rabeprazole, the SmPc for dexlansoprazole indicates the potential need for dose adjustment and adherence to an upper dose limit due to the reduced elimination of lansoprazole in elderly patients [22,23]. The reduced elimination of lansoprazole in elderly patients relates to the elimination half-life being prolonged by approximately 50 to 100% [25]. The absence of an increase in maximum plasma concentrations does not eliminate the problem of a higher exposure. Similarly, the area under the curve (AUC) after seven days of 20 mg rabeprazole sodium daily was almost double that of young healthy volunteers [40,41]. Does it really outweigh the increased risks of ADRs stating that “However there was no evidence of rabeprazole accumulation” [40,41]? This is more an exclusion of an intoxication risk. Even the SmPCs of PPIs, such as for esomeprazole, e.g., that do not indicate dose adaption in the elderly, warn that the “benefits of use of PPIs should be weighed against the increased risk of fractures as patients in this category may already be at high risk for osteoporosis-related fractures. If the use of PPIs is required, they should be managed carefully according to established treatment guidelines.” [27]. As one of the pathophysiological mechanisms of PPI-induced osteoporosis is reduced vitamin D uptake, appropriate substitution should be provided on an individual basis.
In the context of overdose, there remains a numerically less prevalent and, in prescription routine, unconsidered aspect. Approximately 3% of the Caucasian population, but 15–20% of the Asian population, do not have a functional cytochrome p450 2c19 (CYP2C19) enzyme, and are therefore poor or slow metabolizers. The CYP2C19 isoenzyme, which is involved in the metabolism and degradation of all available PPIs, exhibits genetic polymorphism, which is less pronounced for pantoprazole or esomeprazole than for omeprazole. After repeated once-daily oral administration of 20 mg omeprazole, the average AUC in poor metabolizers was from approximately 5 to 10 times higher than in individuals with a functional CYP2C19 enzyme. Average peak plasma concentrations were also from three to five times higher. However, these results have no implications for the dosing of omeprazole [29]. A study of the prevalence of PPI ADRs in these patients, especially in long-term users compared with regular PPI metabolizers, would be of interest. In affected individuals, the omeprazole metabolism is assumed to be catalyzed by CYP3A4, the second enzyme involved in the omeprazole degradation process. After a single dose of 40 mg of pantoprazole, the mean AUC was approximately six times higher in poor metabolizers than in persons with intact CYP2C19 enzyme activity, and the mean maximum plasma concentration was increased by about 60%. Strikingly, these results also remain classified as irrelevant to dosing in their SmPc [35] despite the increasing global attention given to the risks of PPI overdose. As early as 1996, Kuipers et al., analyzing ADRs of long-term high-dose omeprazole in patients with reflux esophagitis and H. pylori infection, also indicated an increased risk of atrophic gastritis [70], which has been regarded as a precursor of gastric cancer. Another study has demonstrated that, even after H. pylori eradication, long-term PPI use remained associated with more than a twofold increased risk of gastric cancer [71]. Presumably, this may be particularly relevant in the Asian population with a higher burden of gastric cancer. RCTs to better establish a causal relationship between long-term PPI use and gastric cancer are required. An enduring substantial PPI-induced suppression of gastric acidity with subsequent increased hypergastrinemia, bacterial overgrowth, and gastric atrophy might be pathophysiologic processes to be accounted for.
Early this month, Plehhova et al. published the concerning German data on the growing market of PPIs, indicating a substantial amount is based on prescriptions. The ongoing increase in OTC PPI purchases, as already assumed in the introduction of the present study, was confirmed, further evidencing a recent increase in prescriptions. Notably, PPIs were predominantly sold in the largest package sizes of more than 90 tablets and remarkably often in their highest strength of 40 mg, even for omeprazole, which is twice the DDD [72]. In their preceding study on PPI overuse, they reported an average PPI treatment duration of 141 days, and 59% of PPI prescriptions were in patients > 60 years of age [69]. PPIs are among the most overused drugs in the world, for which limited knowledge of ADRs, polypharmacy, poor regulation, and financial influences have been identified as the main reasons [73].
Completing the three study group regions with the results presented, analogously a study in Bavaria, in southern Germany, confirmed that an appropriate on-label indication was lacking in 52.0% of initiating PPI users [74]. Liu et al. also found that, in 47% of cases, unapproved indications accounted for new PPI users [75]. Strikingly, in this Asian study group, the prevalent PPI was omeprazole, despite the fact that the Asian population is obviously even more severely impacted by the high prevalence of CYP2C19 polymorphism compared to pantoprazole and probably needs lower doses. As discussed above, this is particularly noteworthy in the context of the overall higher incidence of gastric cancer already preexisting in these populations.
The results of this study highlight that the prescription of PPIs is handled far too carelessly in multiple respects. Overlapping results have been complained about in various other countries, and very similarly in Spain by Savarino et al., who stated that “The major reasons for the misuse of PPIs are the prevention of gastro-duodenal ulcers in patients without risk factors and the stress ulcer prophylaxis in non-intensive care units, steroid therapy alone, anti-platelet or anti-coagulant treatment in patients without risk of gastric injury and the overtreatment of functional dyspepsia” [76]. Analogously, for inappropriate use even over more than 8 weeks in about 40% of outpatients in an Italian study the highest rates were observed for the treatment of dyspepsia and anti-coagulant therapy, most frequently although less inappropriately for gastroesophageal reflux disease and prophylaxis of anti-platelet/nonsteroidal anti-inflammatory drugs [77]. It is beyond any rationality that the alarming and dubious rates of PPI prescription by physicians continue to rise, having already manifested in mass consumption of these drugs worldwide, ignoring all the cautionary study results and even the PPI advices obligatorily specified by the SMPCs, which states that “Patients should be prescribed PPIs at the lowest dose and for the shortest duration required for the condition being treated and be reassessed to ascertain whether continued PPI therapy remains beneficial” [23]. “Patients should be warned about additional risks with long-term use of the medicinal products and the need for prescription and regular surveillance should be emphasized” [39]. “In geriatric patients > 71 years of age benefits of use of PPIs should be weighed against the increased risk of fractures as patients in this category may already be at high risk for osteoporosis-related fractures. If the use of PPIs is required, they should be managed carefully according to established treatment guidelines” [27]. But, furthermore, the applicability of the established treatment guidelines has to be assessed in each individual patient condition as well, especially in the elderly. For example, according to a review, predominantly affected geriatric patients with chronic diseases are also at increased risk for symptoms of loss of muscle function due to PPI-induced magnesium deficiency [78], which, in addition to PPI-induced osteoporosis, may enhance the risk of falls and fractures, particularly in the susceptible elderly PPI-prescribed patients.
Against the background of the study results strengthened by a broad literature update, there is an unsolved urgent need for an intervention aimed at optimizing PPI prescription. Any inadequate prescription should be avoided to ensure the safety of drug therapy. According to the PPI-deprescribing guidelines for risks of a rebound phenomenon, the step-down procedure in deprescribing PPIs involves a fortnightly 50% dose-reduction [79], with consequent observation of the patient and the symptoms. Gradual dose reduction via transitioning from a double to a single dose, halving the single dose, and alternating the dose every second day is usually applied. The optimal time between steps has not been studied. Using PPI on-demand may be an equally strong option [79]. In patients with reflux symptoms or chest pain reflux symptoms who do not respond satisfactorily to PPI therapy, at least a pH-metrically controlled PPI therapy is recommended [80]. And to confirm or rule out gastroesophageal reflux disease (GERD), in patients with PPI-resistant symptoms, ambulatory pH impedance monitoring should preferably be performed after discontinuation of PPI therapy [81]. According to the Lyon Consensus, the conclusive diagnosis of GERD and therapeutic strategies should be based on the analysis of the patient phenotype based on further investigations [82]. In a double-blind study of 100 patients, PPI therapy was discontinued in 34 patients, supporting the early reflux monitoring of acid suppression to phenotype the patient with inadequate PPI effect and thus provide personalized care and avoid unnecessary PPI overuse [83]. The strongest predictor was the absence of pathological acid exposure as measured by wireless pH measurement after at least 7 days of PPI abstinence. The results of a retrospective cross-sectional German primary care databased analyses on an extensive sample size of 472 146 patients indicated that the majority, 92.2%, could benefit from PPI dose reduction, 62% did not need PPIs any longer, 44% received inappropriately prescribed PPIs, and 42% would benefit from an alginate add-on [69].
In several countries, such as the United States [84], France [85], and Italy [86], there have been studies and efforts to publish position papers on the problem of PPI overuse. A recent Canadian investigation also found that only one-third of PPI prescriptions in ambulatory geriatric patients were appropriately indicated. Given their susceptibility to ADRs, these patients should be a priority target group for PPI deprescribing initiatives [87]. A recent systematic review of global trends and practices in PPI use, focusing on 65 articles with 28 million PPI users in 23 countries, found that about 1/4 of adults use a PPI, 63% of whom were <65 years of age. Of further concern was the finding that almost 2/3 of PPI users were on high doses, 25% of users remained on PPIs for >1 year, and 28% of these for >3 years. The New Zealand study group of Shanika et al. concluded that these alarming findings should serve as a “catalyst” for more rational prescribing, especially for long-term use [88].
Since there is no doubt that PPIs, e.g., are effective in preventing upper gastrointestinal ulcers and bleeding associated with low-dose ASA in patients at risk [89,90], as is, e.g., obvious in more vulnerable patients aged ≥75 years [89], the corresponding precise challenging responsibility of physicians and also patients themselves is to identify individual patient-centered risk symptoms so that PPIs are not dispensed to the entire population who are on low-dose ASA. The frequency of high-dose or long-term PPI prescriptions to patients with mild indications or merely for prophylaxis deserves special attention, e.g., also in gastroesophageal reflux disease. And, for example, when treating pure reflux symptoms, the guideline should also apply to the PPI approval, stating that, e.g., pantoprazole 20 mg “is indicated for short-term treatment of reflux symptoms (e.g., heartburn, acid regurgitation) in adults. The recommended dose is 20 mg of pantoprazole (one tablet) per day. It might be necessary to take the tablets for 2–3 consecutive days to achieve improvement of symptoms. Once complete relief of symptoms has occurred, treatment should be discontinued. The treatment should not exceed 4 weeks without consulting a doctor. If no symptom relief is obtained within 2 weeks of continuous treatment, the patient should be instructed to consult a doctor.” [39]. For cardiological indications supported by ongoing studies on the most effective and least harmful anticoagulation in patients with atrial fibrillation (AF) and various types of chronic ischemic heart disease, such as chronic coronary syndromes after stenting, DOACs are preferred as long-term antithrombotic therapy, plus clopidogrel, while ASA is no longer used on a long-term basis [58]. The OLTAT registry searching for optimal long-term antithrombotic treatment of patients with stable coronary artery disease and atrial fibrillation revealed that, after 5 years, the add-on of antiplatelet therapy to oral anticoagulants was independently associated with a higher risk of bleeding and overall mortality, without significant reduction in cardiac and cerebral ischemic events [91]. Following the current perspectives, the triple therapy regimen with a DOAC in chronic anticoagulation should only include low-dose ASA for up to 7 days or until hospital discharge, and a P2Y12 inhibitor for 6 to 12 months, depending on the risk of thrombosis after percutaneous coronary intervention with drug-eluting stent placement [92]. According to a recent systematic review and meta-analysis in patients with atrial fibrillation and stable ischemic heart disease, DOAC plus single antiplatelet therapy (SAPT) is associated with a significant increase in hemorrhage without a significant reduction in thrombotic events, cardiovascular mortality, or all-cause mortality compared to DOAC monotherapy [93]. Thus, there has been an apparent overuse of antiplatelet agents, needing reduction according to the results of these studies. This, in consequence, would mean less risk of bleeding by reducing the use of potential hemorrhage inducers, such as low-dose ASA, and represents another important lever to reduce prophylactic PPI prescribing.
The WHO Global Patient Safety Action Plan 2021–2030 to eliminate preventable harm in health care is another challenge in the ongoing efforts, including the improvements in patient and drug safety, that have been underway for decades [94]. Every healthcare professional and, ideally, empowered patients, should take personal responsibility with regard to the massive PPI overuse.
Strengths and Weaknesses
The present analysis focused not only on prescription prevalence but also on the indication and dosage of PPIs among nursing home residents in order to provide a more comprehensive and precise insight into their use of PPIs. A further particular strength of the study is that indications have been differentiated between German PPI drug approvals and information and indications based on earlier and current German guideline recommendations that partly include weak recommendations leading to off-label use. The individual indication, as well as the appropriateness of individual dose levels, were examined independently by two researchers, a pharmacist and a specialist in internal medicine, both with pharmacological expertise (authors M.W. and U.W). Their independent reports on indications and dosages were 100% consistent.
By analyzing study participants from 37 nursing homes in three different regions of Germany, the northern, eastern, and western parts, the prescribing behavior of physicians from different regions leads to some generalizability for German prescribing routines in elderly patients.
As a limitation, due to secondary data analysis, only the residents with at least one antipsychotic prescription were included. However, the assumption of a confounding effect may be contradicted by the authors’ own data from other independent analyses of PPI prevalence, which were provided additionally.
The data collection followed a standardized protocol. Data were extracted from the residents’ records and therefore inconsistencies in routine data documentation and patient status cannot be ruled out.
For the purpose of this cross-sectional secondary data analysis, only baseline data were evaluated. The duration of the PPI prescription and possible changes in the medication prescription regimen during the course of the study were not further taken into account.
Despite individual risks due to impaired hepatic function or comedication and polypharmacy are a significant composite and were addressed in the medication reviews of the nursing home participants, we do not refer to the even major pharmacokinetic and pharmacodynamic PPI-drug interactions, such as for omeprazole and clopidogrel [95,96,97,98,99], or the increase in citalopram with omeprazole, in this data analysis. In addition, PPIs may reduce the absorption of active substances whose bioavailability is pH-dependent, a risk not referred to in the study data presented.
Although the results of this study, based on a relatively small number of participants, provide deeper insights and are supported by other global findings on PPI overprescribing, larger numbers might strengthen the evidence.
4. Material and Methods
The reported study is a secondary data analysis of the EPCentCare study [60,100], which included a total of 1042 residents of 37 nursing homes at baseline. The PPI data refer to the participants of the EPCentCare study group in terms of both conditions, residents with an antipsychotic prescription and those who received a medication review, based on the medication data in the residents’ records (including hospitalization and discharge letters, physicians’ visitation documentation). Baseline data from this subsample (n = 437) were included in this secondary data analysis. Of these, the following variables were examined:
- At the individual level: age, level of care dependency, diagnoses including kind and number of chronic diseases, cognitive impairment, number of physician contacts (general practitioner and/or specialists), and number of prescribed drugs in the long-term medication; drug groups: antidepressants, neuroleptics, anti-dementia drugs, and antiparkinsonian drugs
- At the cluster level: the different study centers for regional differences.
The data were collected between November 2014 and October 2015. The detailed review of each PPI indication or missing indication according to the recorded diagnoses and medication list was independently conducted by an internist and a pharmacist. The results were compared with the PPI prevalence in different own patient samples and with the updated guidelines for their potential future impact on PPI prescription.
Statistics were performed using STATA software, version 13. Categorical variables were described by absolute and relative frequencies. For continuous variables, the Shapiro–Wilk test was applied to determine whether they had a normal distribution so that the mean and standard deviation or the median and two quartiles could be reported. Binary logistic regression was applied to analyze the association between variables.
5. Conclusions and Way Forward
In this study, the high prevalence of 44% of PPI prescriptions was either without an appropriate indication or overdosed in 55% of nursing home residents. Within the ‘appropriate indications’ an approved indication reflected the smallest proportion of PPI prescriptions. The predominant spectrum of indications was extremely expanded by a number of weak guideline recommendations that must be questioned on a patient-by-patient basis. These expert recommendations, although evidence-based, strongly contribute to the widespread prescription of PPIs for off-label uses, as demonstrated for the low-dose ASA-based indication in the elderly. The corresponding updated guideline recommendation is a bit less liberal, but parallel guidelines came up from other medical societies that return to the questionable weak indication. There is an urgent need for an intervention to promote the appropriate prescription of PPIs.
1. A patient-centered structured medication review including all relevant patient and medication scores is required at regular intervals for individual pharmacotherapy management [59] in order to avoid both individual non-indication and overdosing. As a contribution to patient and drug safety, this could prevent unnecessary initiation, overdosage, and long-term use of PPIs, with the risks of associated ADRs. 2. The various guideline recommendations should be homogenized and provide a clearer strategic orientation, whereby the applicability to the actual medical condition of the individual patient and the changes in long-term follow-up care obligates regular reassessments. 3. Deprescribing requires stringent adherence to the regulations to avoid rebound [79], and the use of on-demand PPI or intermittent PPI [101], as well as transient alginate, may aid in this process [102]. 4. Not least for the concomitant use of OTC PPIs, patient training to strengthen co-responsibility, and patient empowerment with regard to ADRs, could be an effective measure to change the partially nonchalant attitude in this regard. Depending on the individual situation, especially patients with symptomatic gastroesophageal reflux disease and nocturnal reflux symptoms should also be advised to pursue non-pharmacologic accompanying measures, such as avoiding chocolate, caffeine, spicy foods, citrus fruits, and carbonated beverages, pursue smoking cessation and alcohol reduction, avoiding late meals and raising the head, avoiding abdominal breathing, and pursuing weight loss in the case of obesity [45,46]. 5. The prescribing practices of attending physicians are the domain in this critical issue, and education and audit feedback initiatives may help, as may clear deprescribing instructions in hospital discharge letters and adequate diagnostic procedures in clinically dubious cases [81]. 6. Health insurance companies should question off-label prescriptions as they do in other more expensive medical therapies. 7. The fact that a widely consumed drug is released for OTC availability, despite being mostly used for off-label conditions, needs to be re-evaluated. Saving the prescribing physician’s budget, as may result from regulations in the German health care system, also via private prescriptions, should not be the reason. 8. The national and international drug regulatory and supervisory authorities BfArM/EMA/FDA must subsequently promote the concrete distinction between low-dose ASA as an antiplatelet agent and standard-dose NSAIDs in the PPI-SmPCs, because, considering the far-reaching consequences, lifelong PPI intake for low-dose ASA is not routinely necessary in all these patients, and each individual indication requires careful and repeated evaluation.
Author Contributions
Conceptualization, U.W.; methodology, U.W.; formal analysis, M.W.; investigation, M.W. and U.W.; resource and data curation, secondary data analysis of component data from the EPCentCare study; writing—original draft preparation, U.W.; writing—review and editing, U.W.; visualization, M.W. and U.W.; supervision, U.W. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the financial support from the Open Access Publication Fund of the Martin Luther University Halle-Wittenberg through the Open Access Publishing Program of the German Research Foundation (DFG). The research of the primary EPCentCare study was funded by the German Federal Ministry of Education and Research (BMBF grants 01GY1335A, 01GY1335B, 01GY1335C). No grant from any funding agency was received for the conduct of this secondary data analysis and preparation of the manuscript.
Institutional Review Board Statement
This project has received ethical approval from the ethics committee of the Medical Faculty, Martin Luther University Halle-Wittenberg, Germany (no. 2014–101), as well as from the ethics committees of the Universities of Lübeck (no. 14–239) and Witten/Herdecke (no. 133/2014).
Informed Consent Statement
Informed consent was obtained from all participants or their proxies or legal guardians in the original intervention study for the purposes of the primary study. This completely anonymized secondary data analysis of the research dataset already completed in the primary study did not require additional consent.
Data Availability Statement
The research data set generated for this study is available on request.
Acknowledgments
We are grateful to the EPCentCare study consortium of Halle (Saale), Lübeck, and Witten/Herdecke, Germany, for providing selected data to conduct the PPI study. We would like to thank Guido Lüchters, the Center for Development Research (ZEF), Biostatistics, University Bonn, Germany, and Veronika Lay, for their biometric support.
Conflicts of Interest
U.W. received honoraria for lectures on prevention of medication risks in polypharmacy from Bristol Myers Squibb and Pfizer, and from Bonn and Heidelberg Universities, for student teaching in the master’s degree for continuing education in “Drug Therapy Safety Management”. With a financial grant, M.W. and U.W. were awarded the Poster Prize for Patient Safety in Drug Therapy 2018 for this PPI study. U.W. received the Lohfert Prize 2020 for Measurable Innovations to Improve Patient Safety, and the cdgw-Future Award “Zukunftspreis Gesundheitswirtschaft” 2021, both with financial project support.
References
- Stahl, V. Dauerbrenner PPI. DAZ.online 2015, 30. Available online: https://www.deutsche-apotheker-zeitung.de/daz-az/2015/daz-30-2015/dauerbrenner-ppi (accessed on 16 April 2024).
- Schwabe, U.; Paffrath, D. Arzneiverordnungsreport 2018; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Lohse, A.W.; Huber, S. Kapitel 12. Magen/Darm- und Lebererkrankungen. Verordnungsprofil. In Arzneiverordnungs-Report 2022; Ludwig, W.D., Mühlbauer, B., Seifert, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Pasina, L.; Nobili, A.; Tettamanti, M.; Salerno, F.; Corrao, S.; Marengoni, A.; Iorio, A.; Marcucci, M.; Mannucci, P.M.; REPOSI Investigators. Prevalence and appropriateness of drug prescriptions for peptic ulcer and gastroesophageal reflux disease in a cohort of hospitalized elderly. Eur. J. Intern. Med. 2011, 22, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef]
- Neal, K.R.; Scott, H.M.; Slack, R.C.B.; Logan, R.F.A. Omeprazole as a risk factor for campylobacter gastroenteritis: Case control study. BMJ 1996, 312, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Dial, S.; Delaney, J.A.; Barkun, A.N.; Suissa, S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 2005, 294, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.R.; Schneider, J.L.; Zhao, W.; Corley, D.A. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013, 310, 2435–2442. [Google Scholar] [CrossRef]
- Kieboom, B.C.; Kiefte-de Jong, J.C.; Eijgelsheim, M.; Franco, O.H.; Kuipers, E.J.; Hofman, A.; Zietse, R.; Stricker, B.H.; Hoorn, E.J. Proton pump inhibitors and hypomagnesemia in the general population: A population-based cohort study. Am. J. Kidney Dis. 2015, 66, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Sarzynski, E.; Puttarajappa, C.; Xie, Y.; Grover, M.; Laird-Fick, H. Association between proton pump inhibitor use and anemia: A retrospective cohort study. Dig. Dis. Sci. 2011, 56, 2349–2353. [Google Scholar] [CrossRef]
- Yang, Y.X.; Lewis, J.D.; Epstein, S.; Metz, D.C. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006, 296, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lin, C.L.; Kao, C.H. Gastroesophageal reflux disease with proton pump inhibitor use is associated with an increase risk of osteoporosis: A nationwide population-based analysis. Osteoporos. Int. 2016, 27, 2117–2126. [Google Scholar] [CrossRef]
- Zhou, B.; Huang, Y.; Li, H.; Sun, W.; Liu, J. Proton-pump inhibitors and risk of fractures: An update meta-analysis. Osteoporos. Int. 2016, 27, 339–347. [Google Scholar] [CrossRef]
- Zirk-Sadowski, J.; Masoli, J.A.; Delgado, J.; Hamilton, W.; Strain, W.D.; Henley, W.; Melzer, D.; Ble, A. Proton-pump inhibitors and long-term risk of community-acquired pneumonia in older adults. J. Am. Geriatr. Soc. 2018, 66, 1332–1338. [Google Scholar] [CrossRef]
- Gouraud, A.; Vochelle, V.; Descotes, J.; Vial, T. Proton pump inhibitor-induced neutropenia: Possible cross-reactivity between omeprazole and pantoprazole. Clin. Drug Investig. 2010, 30, 559–563. [Google Scholar] [CrossRef]
- Pourhadi, N.; Janbek, J.; Jensen-Dahm, C.; Gasse, C.; Laursen, T.M.; Waldemar, G. Proton pump inhibitors and dementia: A nationwide population-based study. Alzheimers Dement. 2024, 20, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Ahn, N.; Nolde, M.; Krause, E.; Güntner, F.; Günter, A.; Tauscher, M.; Gerlach, R.; Meisinger, C.; Linseisen, J.; Baumeister, S.E.; et al. Do proton pump inhibitors increase the risk of dementia? A systematic review, meta-analysis and bias analysis. Br. J. Clin. Pharmacol. 2023, 89, 602–616. [Google Scholar] [CrossRef]
- Mehta, R.S.; Kochar, B.; Zhou, Z.; Broder, J.C.; Chung, P.; Yang, K.; Lockery, J.; Fravel, M.; Ryan, J.; Mahady, S.; et al. Association of Proton Pump Inhibitor Use With Incident Dementia and Cognitive Decline in Older Adults: A Prospective Cohort Study. Gastroenterology 2023, 165, 564–572.e1. [Google Scholar] [CrossRef]
- Lazarus, B.; Chen, Y.; Wilson, F.P.; Sang, Y.; Chang, A.R.; Coresh, J.; Grams, M. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern. Med. 2016, 176, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.H.; LePendu, P.; Bauer-Mehren, A.; Ghebremariam, Y.T.; Iyer, S.V.; Marcus, J.; Nead, K.T.; Cooke, J.P.; Leeper, N.J. Proton pump inhibitor inhibitor usage and the risk of myocardial infarction in the general population. PLoS ONE 2015, 10, e0124653. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bowe, B.; Yan, Y.; Xian, H.; Li, T.; Al-Aly, Z. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: Cohort study. BMJ 2019, 365, l1580. [Google Scholar] [CrossRef] [PubMed]
- Dexilant® (Dexlansopazol) 30 mg/60 mg Hartkapseln mit Veränderter Wirkstofffreisetzung Takeda GmbH, Fachinformation Updated August 2023. Available online: http://www.fachinfo.de/suche/fi/020198 (accessed on 16 April 2024).
- Dexilant® (Dexlansoprazol) 30 mg/60 mg Capsule (Delayed Release) Takeda Canada Inc. Summary of Product Characteristics Updated March 2023. Available online: https://assets-dam.takeda.com/image/upload/v1683032557/legacy-dotcom/siteassets/en-ca/home/what-we-do/our-medicines/product-monographs/dexilant/DEXILANT-PM-en.pdf (accessed on 19 April 2024).
- Esomeprazol-CT 20 mg/40 mg Magensaftresistente Hartkapseln AbZ-Pharma GmbH, Fachinformation Updated September 2022. Available online: http://www.fachinfo.de/suche/fi/012600 (accessed on 16 April 2024).
- Lansoprazol-Ratiopharm® 15 mg/30 mg Magensaftresistente Hartkapseln Ratiopharm GmbH, Fachinformation Updated November 2023. Available online: http://www.fachinfo.de/suche/fi/020704 (accessed on 16 April 2024).
- Lansoprazole 30 mg Gastro-Resistant Capsules, Hard Teva Pharma B.V. Summary of Product Characteristics Updated February 2023. Available online: https://www.medicines.org.uk/emc/product/14551/smpc/print (accessed on 19 April 2024).
- Nexium® (Esomeprazole) 20 mg/40 mg Delayed Released Tablets AstraZeneca Canada Inc. Summary of Product Characteristics Updated September 2021. Available online: https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/nexium-product-monograph-en.pdf (accessed on 19 April 2024).
- Nexium Control (Esomeprazole) 20 mg Gastro-Resistant Tablets GlaxoSmithKline Dungarvan Limited. Summary of Product Characteristics Updated June 2018. Available online: https://www.ema.europa.eu/en/documents/product-information/nexium-control-epar-product-information_en.pdf (accessed on 19 April 2024).
- Omeprazol AbZ 10 mg/20 mg/40 mg Magensaftresistente Hartkapseln AbZ-Pharma GmbH, Fachinformation Updated June 2023. Available online: http://www.fachinfo.de/suche/fi/014780 (accessed on 16 April 2024).
- Omeprazole 10 mg Gastro-Resistant Capsules Accord-UK Ltd. Summary of Product Characteristics Updated June 2023. Available online: https://mhraproducts4853.blob.core.windows.net/docs/8932e29bba5433018850a7dfd73abfe5671061d7 (accessed on 22 April 2024).
- Omeprazole 20 mg Gastro-Resistant Capsules, Hard Accord-UK Ltd. Summary of Product Characteristics Updated June 2023. Available online: https://mhraproducts4853.blob.core.windows.net/docs/cf6472ea176c24b6142f4f48dc4da5dcf6eb9876 (accessed on 22 April 2024).
- Omeprazole 40 mg Gastro-Resistant Capsules, Hard Teva UK Limited. Summary of Product Characteristics Updated December 2023. Available online: https://mhraproducts4853.blob.core.windows.net/docs/b66e629b806b493980f3e05310ef2a514b2fed9a (accessed on 22 April 2024).
- Omeprazole 20 mg Capsules, Sandoz Limited. Summary of Product Characteristics Updated May 2023. Available online: https://www.medicines.org.uk/emc/product/4895/smpc/print (accessed on 19 April 2024).
- Omeprazol STADA® Protect 20 mg Magensaftresistente Tabletten STADA Consumer Health Deutschland GmbH, Fachinformation Updated February 2023. Available online: https://www.stada.de/media/fkcj1e5h/omeprazol-stada-protect-20-mg_otc_stada-chd_202302_vero-ff20230607.pdf (accessed on 22 April 2024).
- Pantoprazol AbZ 20 mg Magensaftresistente Tabletten AbZ-Pharma GmbH, Fachinformation Updated October 2023. Available online: http://www.fachinfo.de/suche/fi/020163 (accessed on 16 April 2024).
- Pantoprazole Mylan 20 mg Gastro-Resistant Tablets McDermott Laboratories Ltd t/a Gerard Laboratories. Summary of Product Characteristics Updated January 2022. Available online: https://www.medicines.ie/medicines/pantoprazole-mylan-20-mg-gastro-resistant-tablets-33313/spc (accessed on 19 April 2024).
- Pantoprazol AbZ 40 mg Magensaftresistente Tabletten AbZ-Pharma GmbH, Fachinformation Updated October 2023. Available online: http://www.fachinfo.de/suche/fi/020161 (accessed on 16 April 2024).
- Pantoprazole Mylan 40 mg Gastro-Resistant Tablets McDermott Laboratories Ltd t/a Gerard Laboratories. Summary of Product Characteristics Updated January 2022. Available online: https://www.medicines.ie/medicines/pantoprazole-mylan-40mg-gastro-resistant-tablets-33314/spc (accessed on 19 April 2024).
- Pantozol Control 20 mg Gastro-Resistant Tablets Takeda GmbH. Summary of Product Characteristics Updated February 2014. Available online: https://www.ema.europa.eu/en/documents/product-information/pantozol-control-epar-product-information_en.pdf (accessed on 19 April 2024).
- Rabeprazol-Ratiopharm® 10 mg/ 20 mg Magensaftresistente Tabletten Ratiopharm GmbH, Fachinformation Updated September 2022. Available online: https://www.fachinfo.de/suche/fi/014081 (accessed on 16 April 2024).
- Pariet 20 mg Gastro-Resistant Tablets Janssen Sciences Ireland UC. Summary of Product Characteristics Updated December 2022. Available online: https://www.medicines.ie/medicines/pariet-20-mg-gastro-resistant-tablets-33323/spc (accessed on 19 April 2024).
- Wegener, M.A.M.M. Verordnungsverhalten von Protonenpumpeninhibitoren in Pflegeheimen: Eine Sekundärdatenanalyse im Rahmen der EPCentCare-Studie. Doctoral Thesis, Medical Faculty, Martin Luther University Halle-Wittenberg, Halle (Saale), Germany, 2018. Available online: https://opendata.uni-halle.de/bitstream/1981185920/13687/1/Promotion%20Endfassung%2026.06.2018.pdf (accessed on 18 April 2024).
- Fischbach, W.; Malfertheiner, P.; Lynen Jansen, P.; Bolten, W.; Bornschein, J.; Buderus, S.; Glocker, E.; Hoffmann, J.C.; Koletzko, S.; Labenz, J.; et al. S2k-Guideline Helicobacter pylori and gastroduodenal ulcer disease. Z. Gastroenterol. 2017, 54, 167–206. Available online: https://www.thieme-connect.com/products/ejournals/pdf/10.1055/s-0042-119653.pdf (accessed on 18 April 2024).
- Fischbach, W.; Bornschein, J.; Hoffmann, J.C.; Koletzko, S.; Link, A.; Macke, L.; Malfertheiner, P.; Schütte, K.; Selgrad, D.-M.; Suerbaum, S.; et al. Aktualisierte S2k-Leitlinie Helicobacter pylori und gastroduodenale Ulkuskrankheit der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS)—Juli 2022—AWMF-Registernummer: 021–001. Z. Gastroenterol. 2023, 61, 544–606. [Google Scholar] [CrossRef]
- Koop, H.; Fuchs, K.H.; Labenz, J.; Lynen Jansen, P.; Messmann, H.; Miehlke, S.; Schepp, W.; Wenzl, T.G.; der Leitliniengruppe, M. S2k-Leitlinie: Gastroösophageale Refluxkrankkheit unter Federführung der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS):AWMF Register Nr. 021-013 [S2k guideline: Gastroesophageal reflux disease guided by the German Society of Gastroenterology: AWMF register no. 021-013]. Z. Gastroenterol. 2014, 52, 1299–1346. [Google Scholar] [CrossRef] [PubMed]
- Madisch, A.; Koop, H.; Miehlke, S.; Leers, J.; Lorenz, P.; Lynen Jansen, P.; Pech, O.; Schilling, D.; Labenz, J.; Collaborators. S2k-Leitlinie Gastroösophageale Refluxkrankheit und Eosinophile Ösophagitis der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS), AWMF-Registernummer: 021-013, Status: March 2023. Available online: https://register.awmf.org/assets/guidelines/021-013l_S2k_Gastrooesophageale-Refluxkrankheit-eosinophile_Oesophagitis_2023-09.pdf (accessed on 18 April 2024).
- Leitlinien-Gruppe Hessen (DEGAM). S3-Leitlinie Hausärztliche Leitlinie Multimedikation, AWMF-Registernummer: 053-043, Status: May 2021. Available online: https://www.degam.de/files/Inhalte/Leitlinien-Inhalte/Dokumente/DEGAM-S3-Leitlinien/053-043_Multimedikation/oeffentlich/053-043l_Multimedikation_31-07-21.pdf (accessed on 18 April 2024).
- Nationale Versorgungsleitlinie Chronische Herzinsuffizienz. Available online: https://www.dgthg.de/upload/pdf/herzinsuffizienz-2aufl-vers2-lang.pdf (accessed on 20 April 2024).
- Nationale Versorgungsleitlinie Chronische Herzinsuffizienz, AWMF-Registernummer: nvl-006, Status: December 2023. Available online: https://register.awmf.org/assets/guidelines/nvl-006l_S3_Chronische_Herzinsuffizienz_2023-12.pdf (accessed on 18 April 2024).
- Nationale Versorgungsleitlinie Chronische KHK, AWMF-Registernummer: nvl-004, Status: September 2022. Available online: https://register.awmf.org/assets/guidelines/nvl-004l_S3_KHK_2022-09.pdf (accessed on 18 April 2024).
- Nationale Versorgungsleitlinie Nicht-spezifischer Kreuzschmerz, AWMF-Registernummer: nvl-007, Status: December 2016. Available online: https://register.awmf.org/assets/guidelines/nvl-007l_S3_Kreuzschmerz_2017-03-abgelaufen.pdf (accessed on 18 April 2024).
- Grandt, D.; Gamstätter, T.; für die Kommission Arzneimitteltherapie-Management und Arzneimitteltherapiesicherheit der Deutschen Gesellschaft für Innere Medizin. S2k-Leitlinie Arzneimitteltherapie bei Multimorbidität—Living Guideline. AWMF-Registernummer: 100 – 001, 02/2023. Available online: https://register.awmf.org/assets/guidelines/100-001l_S2k_Arzneimitteltherapie-bei-Multimorbiditaet_2023-04.pdf (accessed on 27 April 2024).
- Madisch, A.; Koop, H.; Miehlke, S.; Leers, J.; Lorenz, P.; Jansen, P.L.; Pech, O.; Schilling, D.; Labenz, J. S2k-Leitlinie Gastroösophageale Refluxkrankheit und eosinophile Ösophagitis, Deutsche Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten e.V. (DGVS) Registernummer 021 – 013. Z. Gastroenterol. 2023, 61, 862–933. [Google Scholar] [CrossRef]
- Reinhart, K.; Brunkhorst, F.M.; Bone, H.G.; Bardutzky, J.; Dempfle, C.E.; Forst, H.; Gastmeier, P.; Gerlach, H.; Gründling, M.; John, S. Prevention, diagnosis, therapy and follow-up care of sepsis: 1st revision of S-2k guidelines of the German Sepsis Society (Deutsche Sepsis-Gesellschaft e.V. (DSG)) and the German Interdisciplinary Association of Intensive Care and Emergency Medicine (Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin (DIVI)). GMS Ger. Med. Sci. 2010, 8. [Google Scholar] [CrossRef]
- Brunkhorst, F.M.; Weigand, M.; Pletz, M.; Gastmeier, P.; Lemmen, S.W.; Meier-Hellmann, A.; Ragaller, M.; Weyland, A.; Marx, G.; Bucher, M.; et al. S3-Leitlinie Sepsis- Prävention, Diagnose, Therapie und Nachsorge, AWMF-Registernummer: 079-001, Status: December 2018. Available online: https://register.awmf.org/assets/guidelines/079-001l_S3_Sepsis-Praevention-Diagnose-Therapie-Nachsorge_2020-03_01.pdf (accessed on 18 April 2024).
- Watkins, A.B. Guidance for the Use of Proton Pump Inhibitors. Guidance Document Created by Phil Chung: Guidance on Appropriate PPI Use, 2020, Nebraska Medicine Omaha, NE. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://asap.nebraskamed.com/wp-content/uploads/sites/3/2020/08/Guidance-on-Appropriate-PPI-Use_Final.docx&ved=2ahUKEwip1933_K-HAxVKgP0HHRtBDDEQFnoECBgQAQ&usg=AOvVaw1BEVLuheZo6x345xgGlCzG (accessed on 18 July 2024).
- Targownik, L.E.; Fisher, D.A.; Saini, S.D. AGA Clinical Practice Update on De-Prescribing of Proton Pump Inhibitors: Expert Review. Gastroenterology 2022, 162, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Bhatt, D.L.; Cannon, C.P.; Eikelboom, J.W.; Gibson, C.M.; Goodman, S.G.; Granger, C.B.; Holmes, D.R.; Lopes, R.D.; Mehran, R.; et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: A North American Perspective: 2021 Update. Circulation 2021, 143, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Wolf, U.; Ghadir, H.; Drewas, L.; Neef, R. Underdiagnosed CKD in Geriatric Trauma Patients and Potent Prevention of Renal Impairment from Polypharmacy Risks through Individual Pharmacotherapy Management (IPM-III). J. Clin. Med. 2023, 12, 4545. [Google Scholar] [CrossRef]
- Richter, C.; Berg, A.; Langner, H.; Meyer, G.; Köpke, S.; Balzer, K.; Wolschon, E.M.; Silies, K.; Sönnichsen, A.; Löscher, S.; et al. Effect of person-centred care on antipsychotic drug use in nursing homes (EPCentCare): A cluster-randomised controlled trial. Age Ageing 2019, 48, 419–425. [Google Scholar] [CrossRef]
- Kelly, O.B.; Dillane, C.; Patchett, S.E.; Harewood, G.C.; Murray, F.E. The inappropriate prescription of oral proton pump inhibitors in the hospital setting: A prospective cross-sectional study. Dig. Dis. Sci. 2015, 60, 2280–2286. [Google Scholar] [CrossRef] [PubMed]
- De Souto Barreto, P.; Lapeyre-Mestre, M.; Mathieu, C.; Piau, C.; Bouget, C.; Cayla, F.; Vellas, B.; Rolland, Y. Prevalence and associations of the use of proton-pump inhibitors in nursing homes: A cross-sectional study. J. Am. Med. Dir. Assoc. 2013, 14, 265–269. [Google Scholar] [CrossRef]
- Truong, C. Low-dose acetylsalicylic acid for primary prevention of cardiovascular disease: Do not misinterpret the recommendations. Can. Fam. Physician 2015, 61, 971–975. [Google Scholar] [PubMed] [PubMed Central]
- Seite, F.; Delelis-Fanien, A.S.; Valero, S.; Pradère, C.; Poupet, J.Y.; Ingrand, P.; Paccalin, M. Compliance with guidelines for proton pump inhibitor prescriptions in a department of geriatrics. J. Am. Geriatr. Soc. 2009, 57, 2169–2170. [Google Scholar] [CrossRef] [PubMed]
- Haastrup, P.F.; Jarbøl, D.E.; Thompson, W.; Hansen, J.M.M.; Søndergaard, J.; Rasmussen, S. When does proton pump inhibitor treatment become long term? A scoping review. BMJ Open Gastroenterol. 2021, 8, e000563. [Google Scholar] [CrossRef] [PubMed]
- Rababa, M.; Al-Ghassani, A.A.; Kovach, C.R.; Dyer, E.M. Proton pump inhibitors and the prescribing cascade. J. Gerontol. Nurs. 2016, 42, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Scheurlen, M. Long-term use of proton pump inhibitors: Who needs prophylaxis? Internist 2013, 54, 366–372. [Google Scholar] [CrossRef]
- Ahrens, D.; Behrens, G.; Himmel, W.; Kochen, M.M.; Chenot, J.F. Wie häufig werden nicht-indizierte Protonenpumpeninhibitoren nach Krankenhausentlassung von Hausärzten weiterverordnet? In Proceedings of the 45. Kongress für Allgemeinmedizin und Familienmedizin, Forum Medizin 21, Salzburg, Austria, 22–24 September 2011; German Medical Science GMS Publishing House: Düsseldorf, Germany, 2011; p. Doc11fom003. [Google Scholar]
- Plehhova, K.; Haering, M.; Wray, J.; Coyle, C.; Ibáñez, E.; Kostev, K. Prescribing Patterns of Proton Pump Inhibitors in Germany: A Retrospective Study Including 472,146 Patients. J. Prim. Care Community Health 2023, 14, 21501319231221002. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, E.J.; Lundell, L.; Klinkenberg-Knol, E.C.; Havu, N.; Festen, H.P.; Liedman, B.; Lamers, C.B.; Jansen, J.B.; Dalenback, J.; Snel, P.; et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N. Engl. J. Med. 1996, 334, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Leung, W.K. Long-term use of proton-pump inhibitors and risk of gastric cancer: A review of the current evidence. Therap. Adv. Gastroenterol. 2019, 12, 1756284819834511. [Google Scholar] [CrossRef] [PubMed]
- Plehhova, K.; Häring, M.; Wray, J.; Coyle, C.; Kostev, K. Proton Pump Inhibitors in Germany: Status quo of a growing market. Health Serv. Res. Manag. Epidemiol. 2024, 11, 23333928241241220. [Google Scholar] [CrossRef] [PubMed]
- Albarqouni, L.; Palagama, S.; Chai, J.; Sivananthajothy, P.; Pathirana, T.; Bakhit, M.; Arab-Zozani, M.; Ranakusuma, R.; Cardona, M.; Scott, A.; et al. Overuse of medications in low- and middle-income countries: A scoping review. Bull. World Health Organ. 2023, 10, 36–61D. [Google Scholar] [CrossRef]
- Rückert-Eheberg, I.M.; Nolde, M.; Ahn, N.; Tauscher, M.; Gerlach, R.; Güntner, F.; Günter, A.; Meisinger, C.; Linseisen, J.; Amann, U.; et al. Who gets prescriptions for proton pump inhibitors and why? A drug-utilization study with claims data in Bavaria, Germany, 2010–2018. Eur. J. Clin. Pharmacol. 2022, 78, 657–667. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; Li, R.; Zhang, J.; Zhang, F. Proton pump inhibitor utilisation and potentially inappropriate prescribing analysis: Insights from a single-centred retrospective study. BMJ Open 2020, 10, e040473. [Google Scholar] [CrossRef]
- Savarino, V.; Marabotto, E.; Zentilin, P.; Furnari, M.; Bodini, G.; De Maria, C.; Pellegatta, G.; Coppo, C.; Savarino, E. Proton pump inhibitors: Use and misuse in the clinical setting. Expert Rev. Clin. Pharmacol. 2018, 11, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Crespi, M.; Djahandideh, A.; Demarzo, M.G.; Moscatelli, A.; Bodini, G.; Furnari, M.; Marabotto, E.; Plaz Torres, M.C.; Zentilin, P.; et al. Appropriateness of proton pump inhibitors treatment in clinical practice: Prospective evaluation in outpatients and perspective assessment of drug optimisation. Dig. Liver Dis. 2020, 52, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Vinke, P.; Wesselink, E.; van Orten-Luiten, W.; van Norren, K. The Use of Proton Pump Inhibitors May Increase Symptoms of Muscle Function Loss in Patients with Chronic Illnesses. Int. J. Mol. Sci. 2020, 21, 323. [Google Scholar] [CrossRef] [PubMed]
- Farrell, B.; Pottie, K.; Thompson, W.; Boghossian, T.; Pizzola, L.; Rashid, F.J.; Rojas-Fernandez, C.; Walsh, K.; Welch, V.; Moayyedi, P. Deprescribing proton pump inhibitors. Evidence-based clinical practice guideline. Can. Fam. Physician 2017, 63, 354–364. [Google Scholar] [PubMed]
- Kahrilas, P.J.; Boeckxstaens, G.; Smout, A.J. Management of the patient with incomplete response to PPI therapy. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Hemmink, G.J.; Bredenoord, A.J.; Weusten, B.L.; Monkelbaan, J.F.; Timmer, R.; Smout, A.J. Esophageal pH-impedance monitoring in patients with therapy-resistant reflux symptoms: ‘on’ or ‘off’ proton pump inhibitor? Am. J. Gastroenterol. 2008, 103, 2446–2453. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, C.P.; Kahrilas, P.J.; Savarino, E.; Zerbib, F.; Mion, F.; Smout, A.J.P.M.; Vaezi, M.; Sifrim, D.; Fox, M.R.; Vela, M.F.; et al. Modern diagnosis of GERD: The Lyon Consensus. Gut 2018, 67, 1351–1362. [Google Scholar] [CrossRef]
- Yadlapati, R.; Masihi, M.; Gyawali, C.P.; Carlson, D.A.; Kahrilas, P.J.; Nix, B.D.; Jain, A.; Triggs, J.R.; Vaezi, M.F.; Kia, L.; et al. Ambulatory Reflux Monitoring Guides Proton Pump Inhibitor Discontinuation in Patients with Gastroesophageal Reflux Symptoms: A Clinical Trial. Gastroenterology 2021, 160, 174–182.e1. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, D.E.; Kim, L.S.; Yang, Y.X. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice from the American Gastroenterological Association. Gastroenterology 2017, 152, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, M.; Le Tri, T.; Bardou, M.; Biour, M.; Kirchgesner, J.; Rouby, F.; Dumarcet, N.; Zureik, M.; Dray-Spira, R. Use of proton pump inhibitors in adults in France: A nationwide drug utilization study. Eur. J. Clin. Pharmacol. 2020, 76, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Scarpignato, C.; Gatta, L.; Zullo, A.; Blandizzi, C.; SIF-AIGO-FIMMG Group; Italian Society of Pharmacology; The Italian Association of Hospital Gastroenterologists; The Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid-related diseases—A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.; Bolt, J. Appropriateness of proton pump inhibitor therapy in an ambulatory geriatrics clinic: A retrospective cross-sectional analysis. Can. Pharm. J. 2023, 156, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Shanika, L.G.T.; Reynolds, A.; Pattison, S.; Braund, R. Proton pump inhibitor use: Systematic review of global trends and practices. Eur. J. Clin. Pharmacol. 2023, 79, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Sun, G.; Lu, M.L.; Zhang, L.; Wang, Y.Z.; Sun, X.; Yang, Y.S. Proton pump inhibitors in prevention of low-dose aspirin-associated upper gastrointestinal injuries. World J. Gastroenterol. 2015, 21, 5382–5392. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Geraghty, O.C.; Mehta, Z.; Rothwell, P.M.; Oxford Vascular Study. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: A population-based cohort study. Lancet 2017, 390, 490–499. [Google Scholar] [CrossRef]
- Fischer, Q.; Georges, J.L.; Le Feuvre, C.; Sharma, A.; Hammoudi, N.; Berman, E.; Cohen, S.; Jolivet, I.; Silvain, J.; Helft, G. Optimal long-term antithrombotic treatment of patients with stable coronary artery disease and atrial fibrillation: “OLTAT registry”. Int. J. Cardiol. 2018, 264, 64–69. [Google Scholar] [CrossRef]
- Patail, H.; Sharma, T.; Bali, A.D.; Isath, A.; Aronow, W.S.; Haidry, S.A. Dual antiplatelet therapy with concomitant anticoagulation: Current perspectives on triple therapy. Arch. Med. Sci. Atheroscler. Dis. 2023, 8, e13–e18. [Google Scholar] [CrossRef]
- Shakir, A.; Khan, A.; Agarwal, S.; Clifton, S.; Reese, J.; Munir, M.B.; Nasir, U.B.; Khan, S.U.; Gopinathannair, R.; DeSimone, C.V.; et al. Dual therapy with oral anticoagulation and single antiplatelet agent versus monotherapy with oral anticoagulation alone in patients with atrial fibrillation and stable ischemic heart disease: A systematic review and meta-analysis. J. Interv. Card. Electrophysiol. 2023, 66, 493–506. [Google Scholar] [CrossRef]
- World Health Organization. Global Patient Safety Action Plan 2021–2030: Towards Eliminating Avoidable Harm in Health Care. WHO: Geneva, Switzerland, 2021. Available online: https://iris.who.int/bitstream/handle/10665/343477/9789240032705-eng.pdf?sequence=1. (accessed on 30 April 2024).
- Tran, M.; Tafreshi, J.; Pai, R.G. Review article: Combination of clopidogrel and proton pump inhibitors: Implications for clinicians. J. Cardiovasc. Pharmacol. Ther. 2010, 15, 326–337. [Google Scholar] [CrossRef]
- Guérin, A.; Mody, R.; Carter, V.; Ayas, C.; Patel, H.; Lasch, K.; Wu, E. Changes in Practice Patterns of Clopidogrel in Combination with Proton Pump Inhibitors after an FDA Safety Communication. PLoS ONE. 2016, 11, e0145504. [Google Scholar] [CrossRef]
- Harvey, A.; Modak, A.; Déry, U.; Roy, M.; Rinfret, S.; Bertrand, O.F.; Larose, E.; Rodés-Cabau, J.; Barbeau, G.; Gleeton, O.; et al. Changes in CYP2C19 enzyme activity evaluated by the [(13)C]-pantoprazole breath test after co-administration of clopidogrel and proton pump inhibitors following percutaneous coronary intervention and correlation to platelet reactivity. J. Breath Res. 2016, 10, 017104. [Google Scholar] [CrossRef]
- Jeridi, D.; Pellat, A.; Ginestet, C.; Assaf, A.; Hallit, R.; Corre, F.; Coriat, R. The Safety of Long-Term Proton Pump Inhibitor Use on Cardiovascular Health: A Meta-Analysis. J. Clin. Med. 2022, 11, 4096. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Wu, Q.; Zhang, Z.; Zheng, T.Z.; Xiang, Q.; Zhang, P.; Liu, X.; Zhang, C.; Tan, H.; Huang, J.; et al. Efficacy and safety of clopidogrel only vs. clopidogrel added proton pump inhibitors in the treatment of patients with coronary heart disease after percutaneous coronary intervention: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2019; 23, 100317. [Google Scholar] [CrossRef]
- Richter, C.; Berg, A.; Fleischer, S.; Köpke, S.; Balzer, K.; Wolschon, E.M.; Silies, K.; Sönnichsen, A.; Löscher, S.; Haastert, B.; et al. Effect of person-centred care on antipsychotic drug use in nursing homes (EPCentCare): Study protocol for a cluster-randomised controlled trial. Implement. Sci. 2015, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Fass, R.; Delemos, B.; Nazareno, L.; Kao, R.; Xiang, J.; Lu, Y. Clinical trial: Maintenance intermittent therapy with rabeprazole 20 mg in patients with symptomatic gastro-oesophageal reflux disease—A double-blind, placebo-controlled, randomized study. Aliment. Pharmacol. Ther. 2010, 31, 950–960. [Google Scholar] [CrossRef]
- Thomas, E.; Wade, A.; Crawford, G.; Jenner, B.; Levinson, N.; Wilkinson, J. Randomised clinical trial: Relief of upper gastrointestinal symptoms by an acid pocket-targeting alginate-antacid (Gaviscon Double Action)—A double-blind, placebo-controlled, pilot study in gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 2014, 39, 595–602. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).