Comprehensive Analysis of Drug-Induced Parkinson-like Events
Abstract
1. Introduction
2. Results
2.1. Preparing Data Tables for Analysis
2.2. Relevance of Parkinson-like Events to Patient Characteristics
2.3. Relationship between Parkinson-like Events and Drugs
2.4. Hierarchical Clustering of Parkinson-like Events
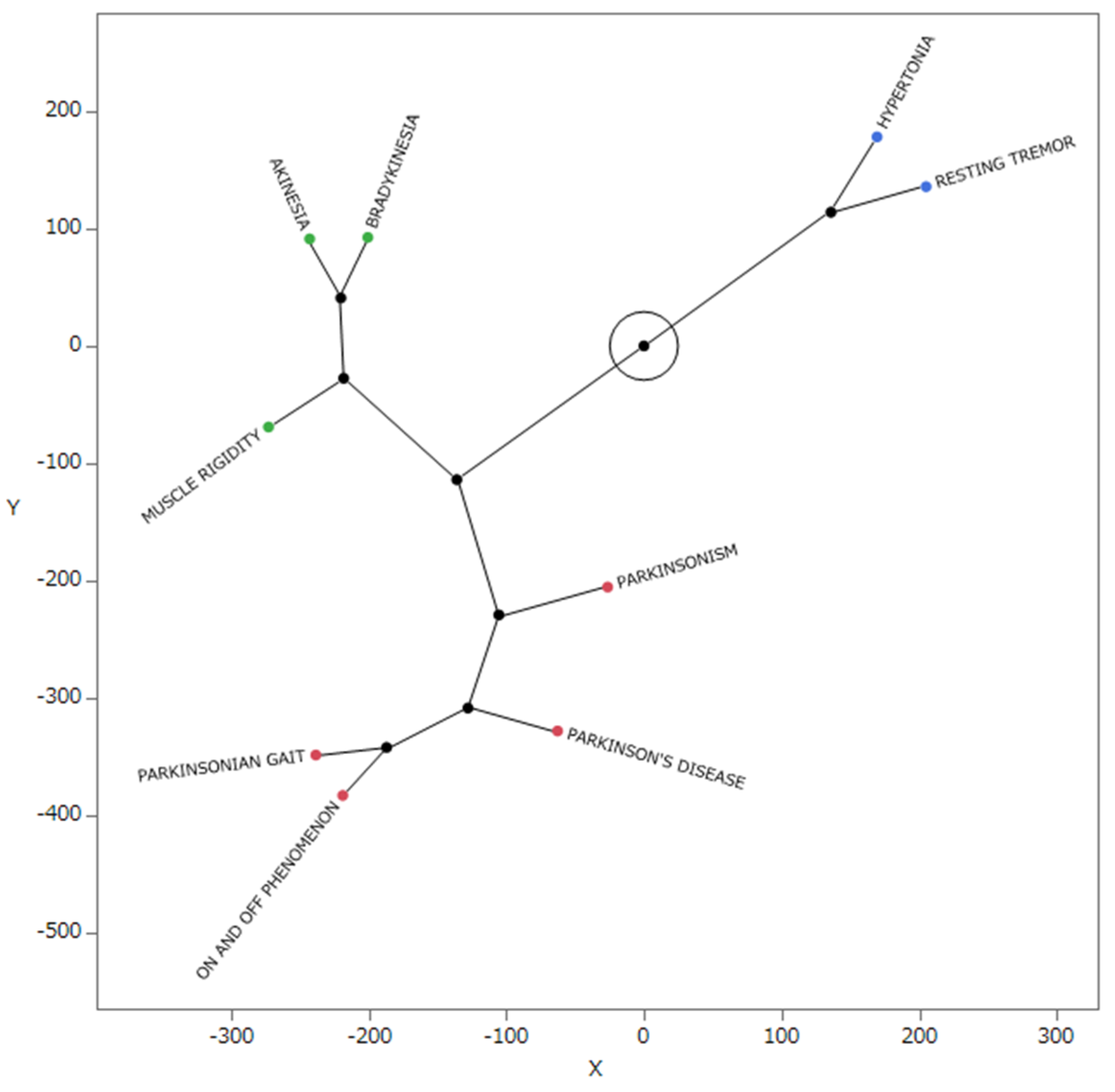
2.5. Star Dendrogram Associated with Parkinson-like Events
2.6. Principal Component Analysis (PCA) Shows Association between Parkinson-like Events and Drugs
3. Discussion
3.1. Parkinson-like Events and Patient Characteristics
3.2. Relationship between Parkinson-like Events and Drugs
3.3. Hierarchical Clustering Analysis of Side Effects Associated with Parkinsonian-like Events
3.4. PCA of Side Effects Related to Parkinson-like Events
3.5. Complex Parkinson-like Events Caused by Drugs
3.6. Limitations
4. Materials and Methods
4.1. Database (JADER)
4.2. Drugs to Be Analyzed and Adverse Event Terms
4.3. Creation of a Data Table for Analysis
4.4. Parkinson-like Events and Patient Characteristics
4.5. Relationship between Parkinson-like Events and Drugs
4.6. Hierarchical Clustering
4.7. PCA
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Japanese Society of Neurology, Prologue. Parkinson’s disease clinical practice guidelines 2018. In Parkinson’s Disease Treatment Guidelines Creation Committee; Igaku Shoin: Tokyo, Japan, 2018; p. VII. [Google Scholar]
- Correll, C.U.; Brevig, T.; Brain, C. Patient characteristics, burden and pharmacotherapy of treatment-resistant schizophrenia: Results from a survey of 204 US psychiatrists. BMC Psychiatry 2019, 19, 362. [Google Scholar] [CrossRef] [PubMed]
- MedDRA. Japanese Maintenance Organization. Available online: https://www.jmo.pmrj.jp/ (accessed on 29 March 2024).
- Farde, L.; Wiesel, F.A.; Halldin, C.; Sedvall, G. Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch. Gen. Psychiatry 1988, 45, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare, Japan. Treatment Manual for Each Serious Side Effect Disease Drug-Induced Parkinsonism. 2006. Available online: https://www.mhlw.go.jp/topics/2006/11/dl/tp1122-1c01.pdf (accessed on 22 March 2024).
- Yoo, H.S.; Bak, Y.; Chung, S.J.; Lee, Y.; Ye, B.S.; Sohn, Y.H.; Shin, N.Y.; Lee, P.H. Impaired functional connectivity of sensorimotor network predicts recovery in drug-induced parkinsonism. Park. Relat. Disord. 2020, 74, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Wirdefeldt, K.; Adami, H.O.; Cole, P.; Trichopoulos, D.; Mandel, J. Epidemiology and etiology of Parkinson’s disease: A review of the evidence. Eur. J. Epidemiol. 2011, 26 (Suppl. 1), S1–S58. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, M.; Kusumi, M.; Kowa, H.; Nakashima, K. Changes in prevalence and incidence of Parkinson’s disease in Japan during a quarter of a century. Neuroepidemiology 2009, 32, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.H.; Rajput, M.L.; Robinson, C.A.; Rajput, A. Normal substantia nigra patients treated with levodopa—Clinical, therapeutic and pathological observations. Park. Relat. Disord. 2015, 21, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J. Age-related toxicity to lactate, glutamate, and beta-amyloid in cultured adult neurons. Neurobiol. Aging 1998, 19, 561–568. [Google Scholar] [CrossRef]
- Lim, S.-Y.; Fox, S.H.; Lang, A.E. Overview of the extranigral aspects of Parkinson disease. Arch. Neurol. 2009, 66, 167–172. [Google Scholar] [CrossRef]
- Cremer, J.N.; Amunts, K.; Graw, J.; Piel, M.; Rösch, F.; Zilles, K. Neurotransmitter receptor density changes in Pitx3ak mice—A model relevant to Parkinson’s disease. Neuroscience 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Huot, P.; Fox, S.H.; Brotchie, J.M. The serotonergic system in Parkinson’s disease. Prog. Neurobiol. 2011, 95, 163–212. [Google Scholar] [CrossRef]
- Rinne, U.K.; Koskinen, V.; Laaksonen, H.; Lönnberg, P.; Sonninen, V. GABA receptor binding in the parkinsonian brain. Life Sci. 1978, 22, 2225–2228. [Google Scholar] [CrossRef]
- Zaidel, A.; Arkadir, D.; Israel, Z.; Bergman, H. Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr. Opin. Neurol. 2009, 22, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Péchadre, J.C.; Larochelle, L.; Poirier, L.J. Parkinsonian akinesia, rigidity and tremor in the monkey. Histopathological and neuropharmacological study. J. Neurol. Sci. 1976, 28, 147–157. [Google Scholar] [CrossRef]
- Helmich, R.C. The cerebral basis of Parkinsonian tremor: A network perspective. Mov. Disord. 2018, 33, 219–231. [Google Scholar] [CrossRef]
- Fearon, C.; Doherty, L.; Lynch, T. How do I examine rigidity and spasticity? Mov. Disord. Clin. Pract. 2015, 2, 204. [Google Scholar] [CrossRef]
- Ishii, T.; Kimura, Y.; Ichise, M.; Takahata, K.; Kitamura, S.; Moriguchi, S.; Kubota, M.; Zhang, M.R.; Yamada, M.; Higuchi, M.; et al. Correction: Anatomical relationships between serotonin 5-HT2A and dopamine D2 receptors in living human brain. PLoS ONE 2018, 13, e0197201. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, K.G. The neuropathology of GABA neurons in extrapyramidal disorders. J. Neural. Transm. Suppl. 1980, 16, 217–227. [Google Scholar] [CrossRef]
- Lee, M.; Ryu, Y.H.; Cho, W.G.; Kang, Y.W.; Lee, S.J.; Jeon, T.J.; Lyoo, C.H.; Kim, C.H.; Kim, D.G.; Lee, K.; et al. Relationship between dopamine deficit and the expression of depressive behavior resulted from alteration of serotonin system. Synapse 2015, 69, 453–460. [Google Scholar] [CrossRef]
- Keller, H.H.; Schaffner, R.; Haefely, W. Interaction of benzodiazepines with neuroleptics at central dopamine neurons. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1976, 294, 1–7. [Google Scholar] [CrossRef]
- Maeda, R. 5. JADER from pharmacovigilance point of view. Jpn. J. Pharmacoepidemiol. 2014, 19, 51–56. [Google Scholar] [CrossRef]
- Kawabe, A.; Uesawa, Y. Analysis of corticosteroid-induced glaucoma using the Japanese adverse drug event reporting database. Pharmaceuticals 2023, 16, 948. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceuticals and Medical Devices Agency. Drug Adverse Reaction Report Data Set Bulk Download. Available online: https://www.pmda.go.jp/safety/info-services/drugs/adr-info/suspected-adr/0004.html (accessed on 29 March 2024).
- Greenland, S.; Schwartzbaum, J.A.; Finkle, W.D. Problems due to small samples and sparse data in conditional logistic regression analysis. Am. J. Epidemiol. 2000, 151, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Van Puijenbroek, E.P.; Bate, A.; Leufkens, H.G.M.; Lindquist, M.; Orre, R.; Egberts, A.C.G. A Comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2002, 11, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Wang, S.J.; Tsai, C.A.; Lin, C.J. Selection of differentially expressed genes in microarray data analysis. Pharmacogenomics J. 2007, 7, 212–220. [Google Scholar] [CrossRef]
- Nakao, Y.; Asada, M.; Uesawa, Y. Comprehensive study of drug-induced pruritus based on adverse drug reaction report database. Pharmaceuticals 2023, 16, 1500. [Google Scholar] [CrossRef]
- Everitt, B.S.; Landau, S.; Leese, M.; Stahl, D. Cluster Analysis, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]




| Patient Background | Parkinson-like Events (1472) | Non-Parkinson-like Events (695,733) | Reporting Odds Ratio | 95% Confidence Interval | p-Value (Fisher’s Exact Test) | |
|---|---|---|---|---|---|---|
| Sex | Female | 812/1472 | 340,077/695,733 | 1.287 | 1.161–1.426 | <0.0001 |
| Male | 660/1472 | 355,656/695,733 | ||||
| Age | ≥40 years old | 1307/1472 | 581,156/695,733 | 1.562 | 1.328–1.836 | <0.0001 |
| <40 years old | 165/1472 | 114,577/695,733 | 0.0597 | |||
| ≥70 years old | 761/1472 | 287,019/695,733 | 1.524 | 1.376–1.688 | <0.0001 | |
| <70 years old | 711/1472 | 408,714/695,733 | 0.8218 |
| Drugs | Reporting Odds Ratio | 95% Confidence Interval | p-Value | a + b |
|---|---|---|---|---|
| SULPIRIDE | 20.10 | 17.87~22.6 | <0.0001 | 10,814 |
| PEROSPIRONE HYDROCHLORIDE | 14.48 | 10.95~19.14 | <0.0001 | 2475 |
| BLONANSERIN | 11.13 | 8.37~14.78 | <0.0001 | 3078 |
| ARIPIPRAZOLE | 10.44 | 8.88~12.28 | <0.0001 | 10,268 |
| HALOPERIDOL | 9.37 | 7.75~11.32 | <0.0001 | 8280 |
| RISPERIDONE | 8.50 | 7.38~9.79 | <0.0001 | 16,740 |
| BIPERIDEN HYDROCHLORIDE | 7.16 | 5.75~8.91 | <0.0001 | 8016 |
| MIRTAZAPINE | 6.90 | 5.39~8.84 | <0.0001 | 6466 |
| QUETIAPINE FUMARATE | 6.68 | 5.63~7.93 | <0.0001 | 14,234 |
| PAROXETINE HYDROCHLORIDE | 6.22 | 5.19~7.47 | <0.0001 | 13,431 |
| OLANZAPINE | 5.86 | 4.81~7.13 | <0.0001 | 12,077 |
| LITHIUM CARBONATE | 5.77 | 4.66~7.13 | <0.0001 | 10,542 |
| CLONAZEPAM | 5.56 | 4.56~6.79 | <0.0001 | 12,455 |
| FLUNITRAZEPAM | 5.03 | 4.28~5.92 | <0.0001 | 20,815 |
| Preferred Terms | R2 |
|---|---|
| PARKINSONISM | 0.724 |
| PARKINSON’S DISEASE | 0.882 |
| MUSCLE RIGIDITY | 0.926 |
| AKINESIA | 0.966 |
| BRADYKINESIA | 0.958 |
| ON AND OFF PHENOMENON | 0.963 |
| PARKINSONIAN GAIT | 0.971 |
| HYPERTONIA | 0.961 |
| RESTING TREMOR | 0.957 |
| Preferred Terms | Number of Reports |
|---|---|
| PARKINSONISM | 5007 |
| PARKINSON’S DISEASE | 1891 |
| MUSCLE RIGIDITY | 923 |
| AKINESIA | 482 |
| BRADYKINESIA | 480 |
| ON AND OFF PHENOMENON | 187 |
| PARKINSONIAN GAIT | 183 |
| HYPERTONIA | 165 |
| RESTING TREMOR | 63 |
| Parkinson-like Events | Non-Parkinson-like Events | |
|---|---|---|
| Reports with the suspected drugs | a | b |
| All other reports | c | d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikegawa, M.; Sone, H.; Uesawa, Y. Comprehensive Analysis of Drug-Induced Parkinson-like Events. Pharmaceuticals 2024, 17, 1099. https://doi.org/10.3390/ph17081099
Kikegawa M, Sone H, Uesawa Y. Comprehensive Analysis of Drug-Induced Parkinson-like Events. Pharmaceuticals. 2024; 17(8):1099. https://doi.org/10.3390/ph17081099
Chicago/Turabian StyleKikegawa, Mami, Hideko Sone, and Yoshihiro Uesawa. 2024. "Comprehensive Analysis of Drug-Induced Parkinson-like Events" Pharmaceuticals 17, no. 8: 1099. https://doi.org/10.3390/ph17081099
APA StyleKikegawa, M., Sone, H., & Uesawa, Y. (2024). Comprehensive Analysis of Drug-Induced Parkinson-like Events. Pharmaceuticals, 17(8), 1099. https://doi.org/10.3390/ph17081099









