Differential Interactions of Flavonoids with the Aryl Hydrocarbon Receptor In Silico and Their Impact on Receptor Activity In Vitro
Abstract
1. Introduction
2. Results
2.1. Homology Modeling and Model Validation of the Orthosteric Site (PAS-B) of AHR
2.2. Molecular Docking
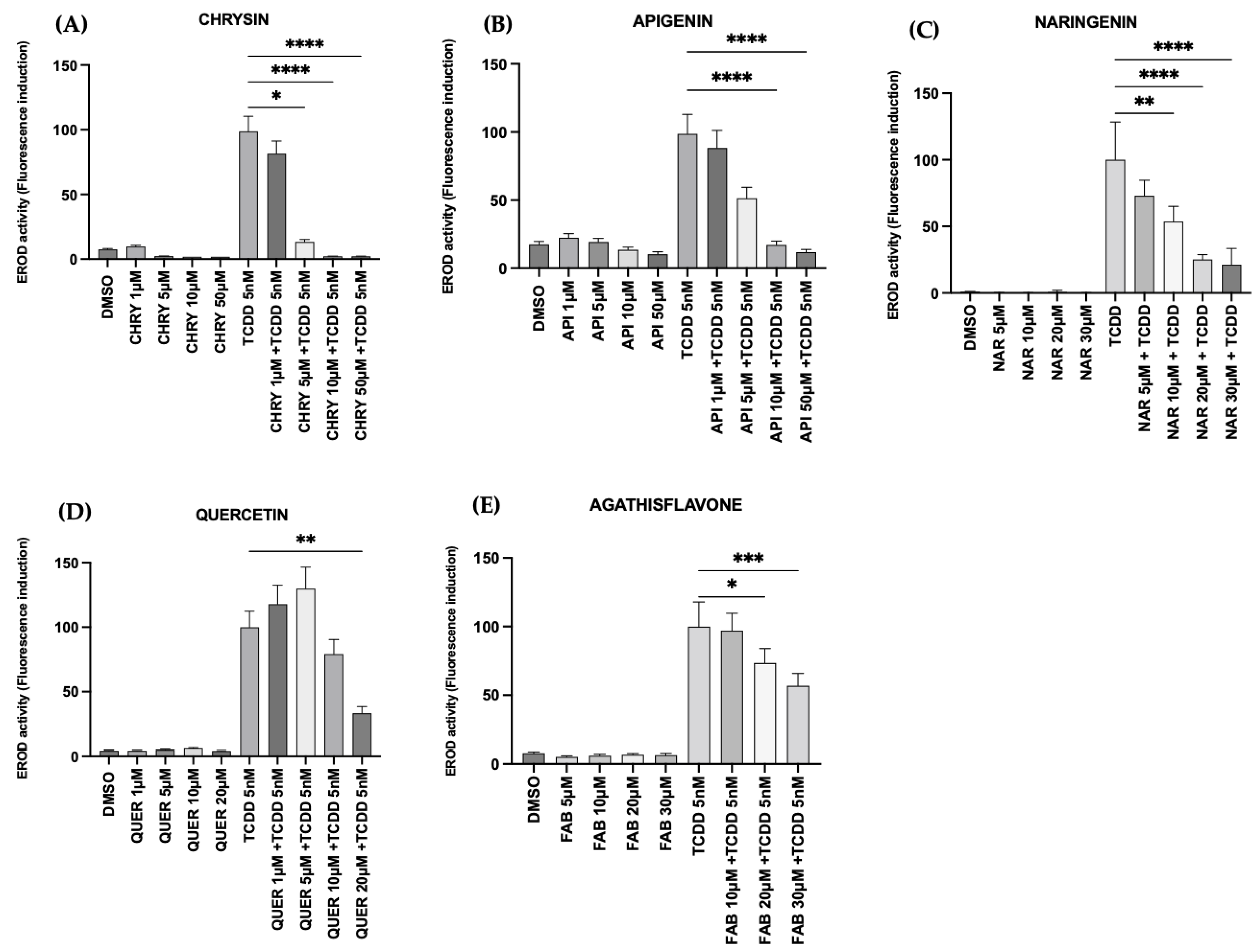
2.3. Flavonoids Inhibited AHR Activation In Vitro
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Flavonoids and Treatments
4.3. Molecular Docking Study
4.3.1. Homology Modeling
4.3.2. Validation of the 3D Model of the AHR PAS-B Domain
4.3.3. Molecular Docking
4.4. EROD Activity Assay
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolluri, S.K.; Jin, U.H.; Safe, S. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Arch. Toxicol. 2017, 91, 2497–2513. [Google Scholar] [CrossRef] [PubMed]
- Juricek, L.; Coumoul, X. The aryl hydrocarbon receptor and the nervous system. Int. J. Mol. Sci. 2018, 19, 2504. [Google Scholar] [CrossRef] [PubMed]
- Trikha, P.; Lee, D.A. The role of AHR in transcriptional regulation of immune cell development and function. Biochim. Biophys. Acta-Rev. Cancer 2020, 1873, 188335. [Google Scholar] [CrossRef]
- Safe, S.; Cheng, G.Y.; Jin, U.H. The aryl hydrocarbon receptor (AHR) as a drug target for cancer chemotherapy. Curr. Opin. Toxicol. 2017, 1, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Chiu-Leung, L.C.; Lin, S.-M.; Leung, L.K. The citrus flavonone hesperetin attenuates the nuclear translocation of aryl hydrocarbon receptor. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 210, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.X.; Yong, C.Y.; Ong, A.H.K.; Yeap, S.K.; Ho, W.Y. Deciphering the roles of aryl hydrocarbon receptor (AHR) in regulating carcinogenesis. Toxicology 2023, 495, 153596. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.G.; Selmin, O.I.; Romagnolo, D.F. Aryl Hydrocarbon Receptor Diet and Breast Cancer Risk. Yale J. Biol. Med. 2018, 91, 105–127. [Google Scholar] [PubMed]
- Mengoni, M.; Braun, A.D.; Gaffal, E.; Tüting, T. The aryl hydrocarbon receptor promotes inflammation-induced dedifferentiation and systemic metastatic spread of melanoma cells. Int. J. Cancer 2020, 147, 2902–2913. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Zhang, L. The Role of the Aryl Hydrocarbon Receptor (AHR) and Its Ligands in Breast Cancer. Cancers 2022, 14, 5574. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef] [PubMed]
- Roszkowski, S. Application of Polyphenols and Flavonoids in Oncological Therapy. Molecules 2023, 28, 4080. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in cancer and apoptosis. Cancers 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Rossi, M.; Borromeo, I.; Feriotto, G.; Platamone, G.; Tabolacci, C.; Mischiati, C.; Beninati, S. Flavonoids: A myth or a reality for cancer therapy? Molecules 2021, 26, 3583. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Mazurakova, A.; Koklesova, L.; Kubatka, P.; Büsselberg, D. Flavonoids synergistically enhance the anti-glioblastoma effects of chemotherapeutic drugs. Biomolecules 2021, 11, 1841. [Google Scholar] [CrossRef]
- Liskova, A.; Samec, M.; Koklesova, L.; Brockmueller, A.; Zhai, K.; Abdellatif, B.; Siddiqui, M.; Biringer, K.; Kudela, E.; Pec, M.; et al. Flavonoids as an effective sensitizer for anti-cancer therapy: Insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J. 2021, 12, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.; Santos, B.L.; Amparo, J.A.O.; Soares, J.R.P.; Silva, K.C.; Santana, M.R.; Almeida, Á.M.A.N.; Silva, V.D.A.; Costa, M.F.D.; Ulrich, H.; et al. Neuroimmunomodulatory Properties of Flavonoids and Derivates: A Potential Action as Adjuvants for the Treatment of Glioblastoma. Pharmaceutics 2022, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.L.; Oliveira, M.N.; Coelho, P.L.C.; Pitanga, B.P.S.; da Silva, A.B.; Adelita, T.; Silva, V.D.A.; Costa, M.D.F.D.; El-Bachá, R.S.; Tardy, M.; et al. Flavonoids suppress human glioblastoma cell growth by inhibiting cell metabolism, migration, and by regulating extracellular matrix proteins and metalloproteinases expression. Chem.-Biol. Interact. 2015, 242, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.L.C.; Oliveira, M.N.; da Silva, A.B.; Pitanga, B.P.S.; Silva, V.D.A.; Faria, G.P.; Sampaio, G.P.; Costa, M.D.F.D.; Braga-De-Souza, S.; Costa, S.L. The flavonoid apigenin from Croton betulaster Mull inhibits proliferation, induces differentiation and regulates the inflammatory profile of glioma cells. Anti-Cancer Drugs 2016, 27, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.B.; Coelho, P.L.; Oliveira, M.N.; Oliveira, J.L.; Amparo, J.A.O.; da Silva, K.C.; Soares, J.R.P.; Pitanga, B.P.S.; Souza, C.S.; de Lopes, G.P.F.; et al. The flavonoid rutin and its aglycone quercetin modulate the microglia inflammatory profile improving antiglioma activity. Brain Behav. Immun. 2020, 85, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chang, Y.M.; Wang, K.Y.; Chen, P.N.; Hseu, Y.C.; Chen, K.M.; Yeh, K.T.; Chen, C.J.; Hsu, L.S. Naringenin inhibited migration and invasion of glioblastoma cells through multiple mechanisms. Environ. Toxicol. 2019, 34, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Park, H.; Li, X.; Davidson, L.A.; Allred, C.; Patil, B.; Jayaprakasha, G.; Orr, A.A.; Mao, L.; Chapkin, R.S.; et al. Structure-dependent modulation of aryl hydrocarbon receptor-mediated activities by flavonoids. Toxicol. Sci. 2018, 164, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Leclair, H.M.; Tardif, N.; Paris, A.; Galibert, M.D.; Corre, S. Role of flavonoids in the prevention of AhR-dependent resistance during treatment with braf inhibitors. Int. J. Mol. Sci. 2020, 21, 5025. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Jin, U.H.; Park, H.; Chapkin, R.S.; Jayaraman, A. Aryl hydrocarbon receptor (AHR) ligands as selective ahr modulators (SAHRMS). Int. J. Mol. Sci. 2020, 21, 6654. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, Z.; Liao, Z.; Zhang, Y.; Lei, W.; Shui, X. Aryl hydrocarbon receptor: An emerging player in breast cancer pathogenesis and its potential as a drug target (Review). Mol. Med. Rep. 2024, 29, 11. [Google Scholar] [CrossRef] [PubMed]
- Coumoul, X.; Diry, M.; Robillot, C.; Barouki, R. Differential Regulation of Cytochrome P450 1A1 and 1B1 by a Combination of Dioxin and Pesticides in the Breast Tumor Cell Line MCF-7 1. Cancer Res. 2001, 61, 3942–3948. [Google Scholar] [PubMed]
- Gilot, D.; Le Meur, N.; Giudicelli, F.; Le Vée, M.; Lagadic-Gossmann, D.; Théret, N.; Fardel, O. Rnai-based screening identifies kinases interfering with dioxin-mediated up-regulation of CYP1A1 activity. PLoS ONE 2011, 6, e18261. [Google Scholar] [CrossRef] [PubMed]
- Szöllösi, D.; Erdei, Á.; Gyimesi, G.; Magyar, C.; Hegedüs, T. Access path to the ligand binding pocket may play a role in xenobiotics selection by AHR. PLoS ONE 2016, 11, e0146066. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.; Gilot, D.; Ferrec, E.; Rauch, C.; Lagadic-Gossmann, D.; Fardel, O. Dioxin-Mediated Up-Regulation of Aryl Hydrocarbon Receptor Target Genes Is Dependent on the Calcium/Calmodulin/CaMKIα Pathway. Mol. Pharmacol. 2008, 73, 769–777. [Google Scholar] [CrossRef]
- Paris, A.; Tardif, N.; Galibert, M.-D.; Corre, S. AHR and cancer: From gene profiling to targeted therapy. Int. J. Mol. Sci. 2021, 22, 752. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Feng, Y.L.; Chen, L.; Vaziri, N.D.; Zhao, Y.Y. Dietary natural flavonoids treating cancer by targeting aryl hydrocarbon receptor. Crit. Rev. Toxicol. 2019, 49, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Goya-Jorge, E.; Rodríguez, M.E.J.; Veitía, M.S.I.; Giner, R.M. Plant-occurring flavonoids as modulators of the aryl hydrocarbon receptor. Molecules 2021, 26, 2315. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Flavonoids as Anticancer Agents: Structure-Activity Relationship Study. Curr. Med. Chem.-Anti-Cancer Agents 2002, 2, 691–714. [Google Scholar] [CrossRef] [PubMed]
- Bisson, W.H.; Koch, D.C.; O’Donnell, E.F.; Khalil, S.M.; Kerkvliet, N.I.; Tanguay, R.L.; Abagyan, R.; Kolluri, S.K. Modeling of the aryl hydrocarbon receptor (AHR) ligand binding domain and its utility in virtual ligand screening to predict new AHR ligands. J. Med. Chem. 2009, 52, 5635–5641. [Google Scholar] [CrossRef] [PubMed]
- Sordon, S.; Popłoński, J.; Milczarek, M.; Stachowicz, M.; Tronina, T.; Kucharska, A.Z.; Wietrzyk, J.; Huszcza, E. Structure–antioxidant–antiproliferative activity relationships of natural C7 and C7–C8 hydroxylated flavones and flavanones. Antioxidants 2019, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Piskorska-Pliszczynska, J.; Zacharewski, T.; Safe, S. Human breast cancer cell lines as models for investigating the effects of 2,3,7,8-TCDD and related compounds. Chemosphere 1990, 20, 1135–1140. [Google Scholar] [CrossRef]
- Ciolino, H.P.; Yeh, G.C. The flavonoid galangin is an inhibitor of CYP1A1 activity and an agonist/antagonist of the aryl hydrocarbon receptor. Br. J. Cancer 1999, 79, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Li, N.; Arroo, R.R.J. The methoxylated flavones eupatorin and cirsiliol induce CYP1 enzyme expression in MCF7 cells. J. Nat. Prod. 2009, 72, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Ptak, A.; Ludewig, G.; Rak, A.; Nadolna, W.; Bochenek, M.; Gregoraszczuk, E.L. Induction of cytochrome P450 1A1 in MCF-7 human breast cancer cells by 4-chlorobiphenyl (PCB3) and the effects of its hydroxylated metabolites on cellular apoptosis. Environ. Int. 2010, 36, 935–941. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vogel, C.F.A.; Lazennec, G.; Kado, S.Y.; Dahlem, C.; He, Y.; Castaneda, A.; Ishihara, Y.; Vogeley, C.; Rossi, A.; Haarmann-Stemmann, T.; et al. Targeting the Aryl Hydrocarbon Receptor Signaling Pathway in Breast Cancer Development. Front. Immunol. 2021, 12, 625346. [Google Scholar] [CrossRef] [PubMed]
- Schiwy, A.; Brinkmann, M.; Thiem, I.; Guder, G.; Winkens, K.; Eichbaum, K.; Nüßer, L.; Thalmann, B.; Buchinger, S.; Reifferscheid, G.; et al. Determination of the CYP1A-inducing potential of single substances, mixtures and extracts of samples in the micro-EROD assay with H4IIE cells. Nat. Protoc. 2015, 10, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Han, E.H.; Shin, D.W.; Jeong, T.C.; Lee, E.S.; Woo, E.R.; Jeong, H.G. Suppression of CYP1A1 expression by naringenin in murine Hepa-1c1c7 cells. Arch. Pharm. Res. 2004, 27, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, M.L.; Juybari, K.B.; Tarzi, M.E.; Amirkhosravi, A.; Nematollahi, M.H.; Mirzamohammdi, S.; Mehrbani, M.; Mehrabani, M.; Mehrabani, M. Naringenin attenuates cell viability and migration of C6 glioblastoma cell line: A possible role of hedgehog signaling pathway. Mol. Biol. Rep. 2021, 48, 6413–6421. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Almatroudi, A.; Allemailem, K.S.; Khan, A.A.; Almatroodi, S.A. The Potential Role of Fisetin, a Flavonoid in Cancer Prevention and Treatment. Molecules 2022, 27, 9009. [Google Scholar] [CrossRef] [PubMed]
- Stump, T.A.; Santee, B.N.; Williams, L.P.; Kunze, R.A.; Heinze, C.E.; Huseman, E.D.; Gryka, R.J.; Simpson, D.S.; Amos, S. The antiproliferative and apoptotic effects of apigenin on glioblastoma cells. J. Pharm. Pharmacol. 2017, 69, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, Y.; Lv, H.; Zhang, H.; Liang, T.; Zhou, G.; Huang, L.; Tian, Y.; Liang, W. Apigenin in cancer therapy: From mechanism of action to nano-therapeutic agent. Food Chem. Toxicol. 2022, 168, 113385. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Kamarudin, M.N.A.; Naidu, R. Anticancer Mechanism of Flavonoids on High-Grade Adult-Type Diffuse Gliomas. Nutrients 2023, 15, 797. [Google Scholar] [CrossRef] [PubMed]
- Stompor-gorący, M.; Bajek-Bil, A.; Machaczka, M. Chrysin: Perspectives on contemporary status and future possibilities as pro-health agent. Nutrients 2021, 13, 2038. [Google Scholar] [CrossRef] [PubMed]
- Motallebi, M.; Bahia, M.; Rajani, H.F.; Bhia, I.; Tabarraei, H.; Mohammadkhani, N.; Pereira-Silva, M.; Kasaii, M.S.; Nouri-Majd, S.; Mueller, A.L.; et al. Naringenin: A potential flavonoid phytochemical for cancer therapy. Life Sci. 2022, 305, 120752. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.P.; dos Santos, B.L.; da Silva, K.C.; Silva, V.D.A.; Costa, M.F.; David, J.M.; David, J.P.; Moura-Neto, V.; Oliveira, M.N.; Ulrich, H.; et al. Reverted effect of mesenchymal stem cells in glioblastoma treated with agathisflavone and its selective antitumoral effect on cell viability, migration, and differentiation via STAT3. J. Cell. Physiol. 2021, 236, 5022–5035. [Google Scholar] [CrossRef]
- Victor, M.M.; David, J.M.; Sakukuma, M.C.K. A simple and efficient process for the extraction of naringin from grapefruit peel waste. Green Process. Synth. 2018, 7, 524–529. [Google Scholar] [CrossRef]
- Mendes, C.C.; Bahia, M.V.; David, J.M.; David, J.P. Constituents of Caesalpinia pyramidalis. Fitoterapia 2000, 71, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Bahia, M.V.; David, J.P.; David, J.M. Occurrence of biflavones in leaves of Caesalpinia pyramidalis specimens. Química Nova 2010, 33, 1297–1300. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modeling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. Procheck: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, C.J.; Hess, B.; Lindahl, E. GROMACS: High-performance molecular simulations through multilevel parallelism from laptops to supercomputers. Softw. X 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- McGann, M. FRED pose prediction and virtual screening accuracy. J. Chem. Inf. Model. 2011, 51, 578–596. [Google Scholar] [CrossRef] [PubMed]
- McGann, M. FRED and HYBRID docking performance on standardized datasets. J. Comput. Aided Mol. Des. 2012, 26, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.C.; Skillman, A.G.; Warren, G.L.; Ellimngson, B.A.; Stahl, M.T. Conformer generation with OMEGA: Algorithm and validation using high-quality structures from the Protein Databank and Cambridge Structural Database. J. Chem. Inf. Model. 2010, 26, 572–584. [Google Scholar] [CrossRef]
- Gilot, D.; Migault, M.; Bachelot, L.; Journé, F.; Rogiers, A.; Donnou-Fournet, E.; Mogha, A.; Mouchet, N.; Pinel-Marie, M.L.; Mari, B. A non-coding function of TYRP1 mRNA promotes melanoma growth. Nat. Cell Biol. 2017, 19, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
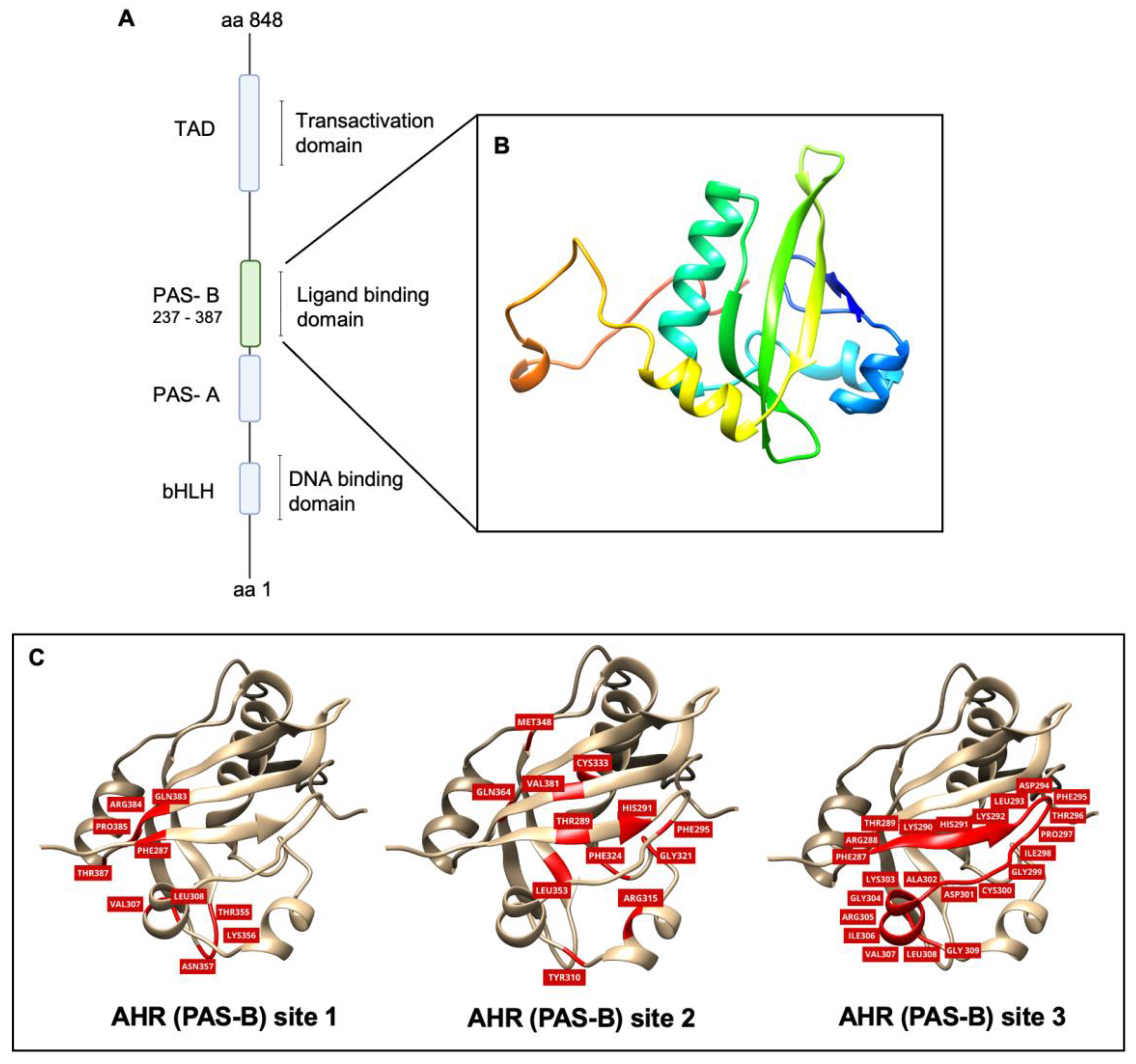
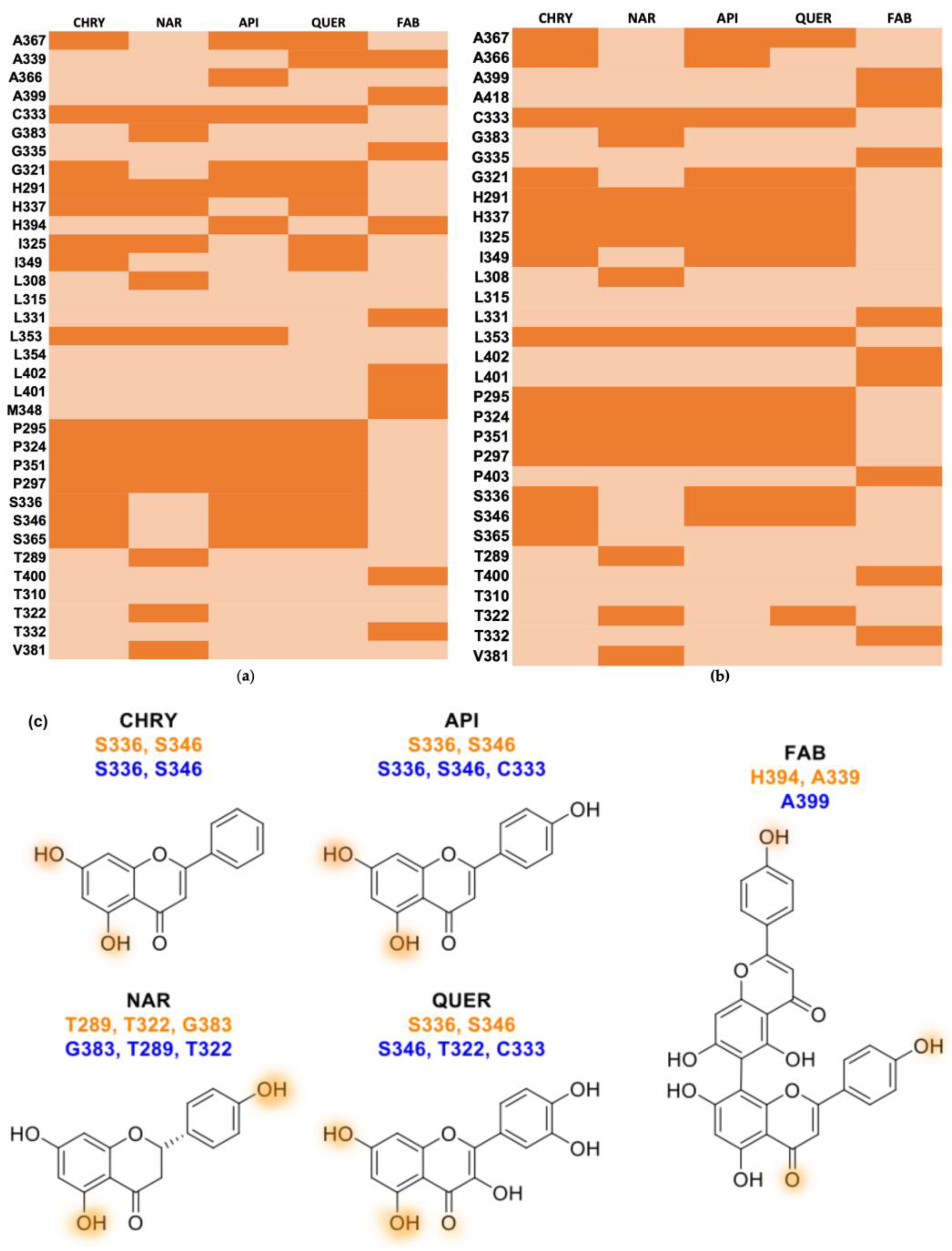
| Flavonoid | AHR (PAS-B) Site 1 | FRED Chemgauss4 Score AHR (PAS-B) Site 2 | AHR (PAS-B) Site 3 |
|---|---|---|---|
| Chrysin | −4.93 | −15.14 | −15.27 |
| Apigenin | −4.73 | −15.31 | −15.07 |
| Naringenin | −5.46 | −13.14 | −13.55 |
| Quercetin | −4.51 | −13.62 | −13.59 |
| Agathisflavone | −0.94 | −0.57 | −5.14 |
| Flavonoid | Hydrophobic Contact | Hydrogen Bond Aceptor | Hydrogen Bond Donor | |
|---|---|---|---|---|
| AHR (PAS-B) site 2 | Chrysin | A367 C333 G321 H291 H337 I325 I349 L353 P295 P324 P351 P297 S365 | − | S336 S346 |
| Apigenin | A367 A366 C333 G321 H291 H337 I325 I349 L353 P295 P324 P351 P297 S365 | − | S336 S346 | |
| Naringenin | C333 H291 H337 I325 L308 L353 P295 P324 P351 P297 V381 | G383 | T289 T322 | |
| Quercetin | A367 A366 C333 G321 H291 H337 I325 I349 P295 P324 P351 P297 S365 | − | S336 S346 | |
| Agathisflavone | A399 G335 L331 L402 L401 M348 T400 T332 | H394 | A339 | |
| AHR (PAS-B) site 3 | Chrysin | A367 A366 C333 G321 H291 H337 I325 I349 L353 P295 P324 P351 P297 S365 | − | S336 S346 |
| Apigenin | A367 A366 G321 H291 H337 I325 I349 L353 P295 P324 P351 P297 | C333 | S336 S346 | |
| Naringenin | C333 H291 H337 I325 L308 L353 P295 P324 P351 P297 V381 | G383 | T289 T322 | |
| Quercetin | A367 G321 H291 H337 I325 I349 L353 P295 P324 P351 P297 S336 | C333 | S336 S346 | |
| Agathisflavone | A418 G335 L331 L402 L401 P403 T400 T332 | A399 | − |
| Flavonoid | Empirical Formula (Hill Notation) | Schematic Structure |
|---|---|---|
| Chrysin | C15H10O4 | 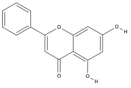 |
| Apigenin | C15H10O5 | 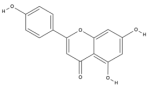 |
| Naringenin | C15H12O5 | 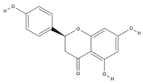 |
| Quercetin | C15H10O7 | 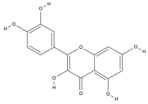 |
| Agathisflavone | C30H18O10 | 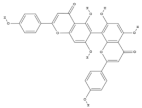 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, M.R.d.; Santos, Y.B.d.; Santos, K.S.; Santos Junior, M.C.; Victor, M.M.; Ramos, G.d.S.; Nascimento, R.P.d.; Costa, S.L. Differential Interactions of Flavonoids with the Aryl Hydrocarbon Receptor In Silico and Their Impact on Receptor Activity In Vitro. Pharmaceuticals 2024, 17, 980. https://doi.org/10.3390/ph17080980
Santana MRd, Santos YBd, Santos KS, Santos Junior MC, Victor MM, Ramos GdS, Nascimento RPd, Costa SL. Differential Interactions of Flavonoids with the Aryl Hydrocarbon Receptor In Silico and Their Impact on Receptor Activity In Vitro. Pharmaceuticals. 2024; 17(8):980. https://doi.org/10.3390/ph17080980
Chicago/Turabian StyleSantana, Monique Reis de, Ylanna Bonfim dos Santos, Késsia Souza Santos, Manoelito Coelho Santos Junior, Mauricio Moraes Victor, Gabriel dos Santos Ramos, Ravena Pereira do Nascimento, and Silvia Lima Costa. 2024. "Differential Interactions of Flavonoids with the Aryl Hydrocarbon Receptor In Silico and Their Impact on Receptor Activity In Vitro" Pharmaceuticals 17, no. 8: 980. https://doi.org/10.3390/ph17080980
APA StyleSantana, M. R. d., Santos, Y. B. d., Santos, K. S., Santos Junior, M. C., Victor, M. M., Ramos, G. d. S., Nascimento, R. P. d., & Costa, S. L. (2024). Differential Interactions of Flavonoids with the Aryl Hydrocarbon Receptor In Silico and Their Impact on Receptor Activity In Vitro. Pharmaceuticals, 17(8), 980. https://doi.org/10.3390/ph17080980








