In Vitro Antiviral Activity of Rhodiola crenulata Extract against Zika Virus and Japanese Encephalitis Virus: Viral Binding and Stability
Abstract
:1. Introduction
2. Results
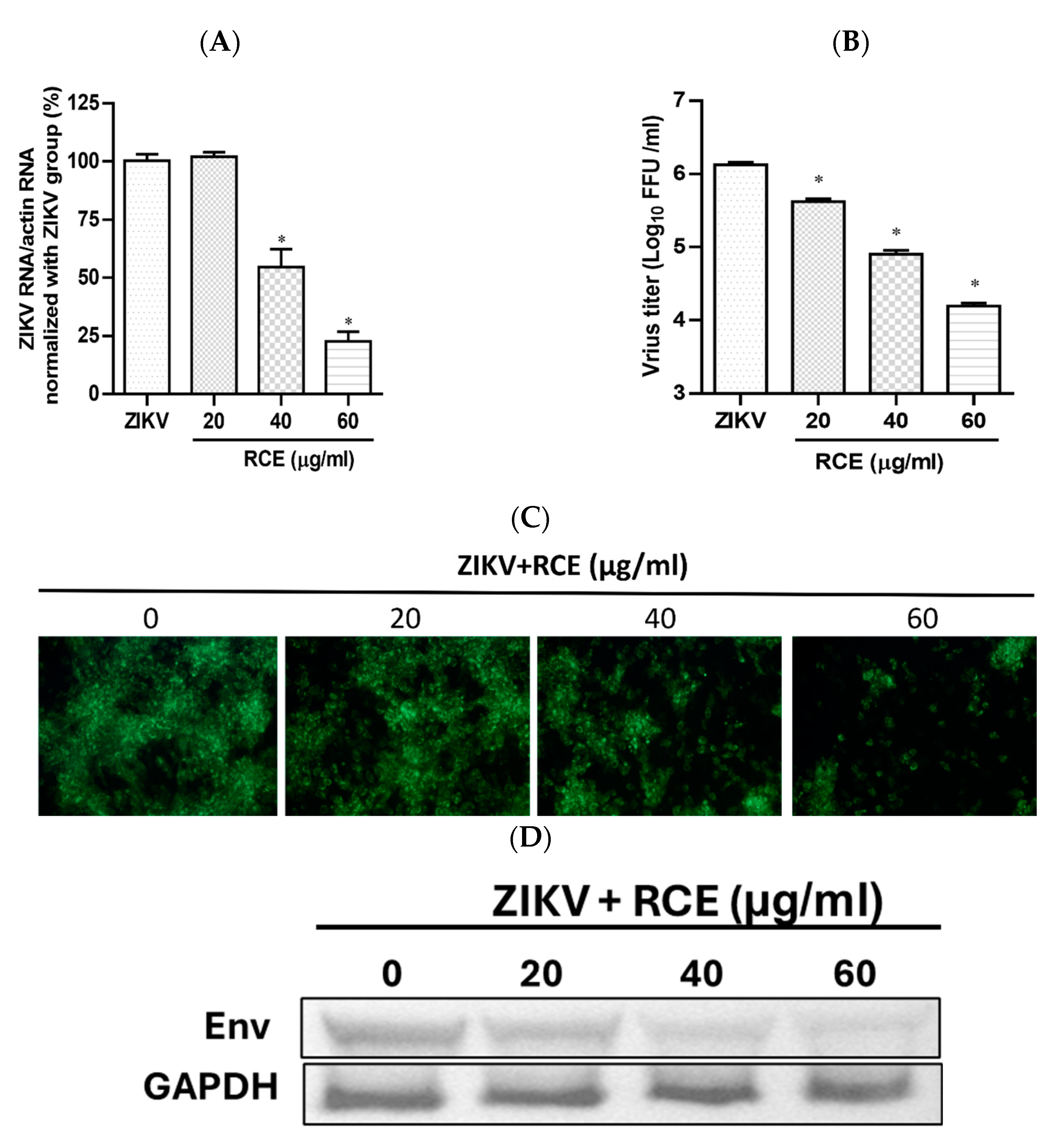
2.1. RCE Presented Anti-ZIKV Effects
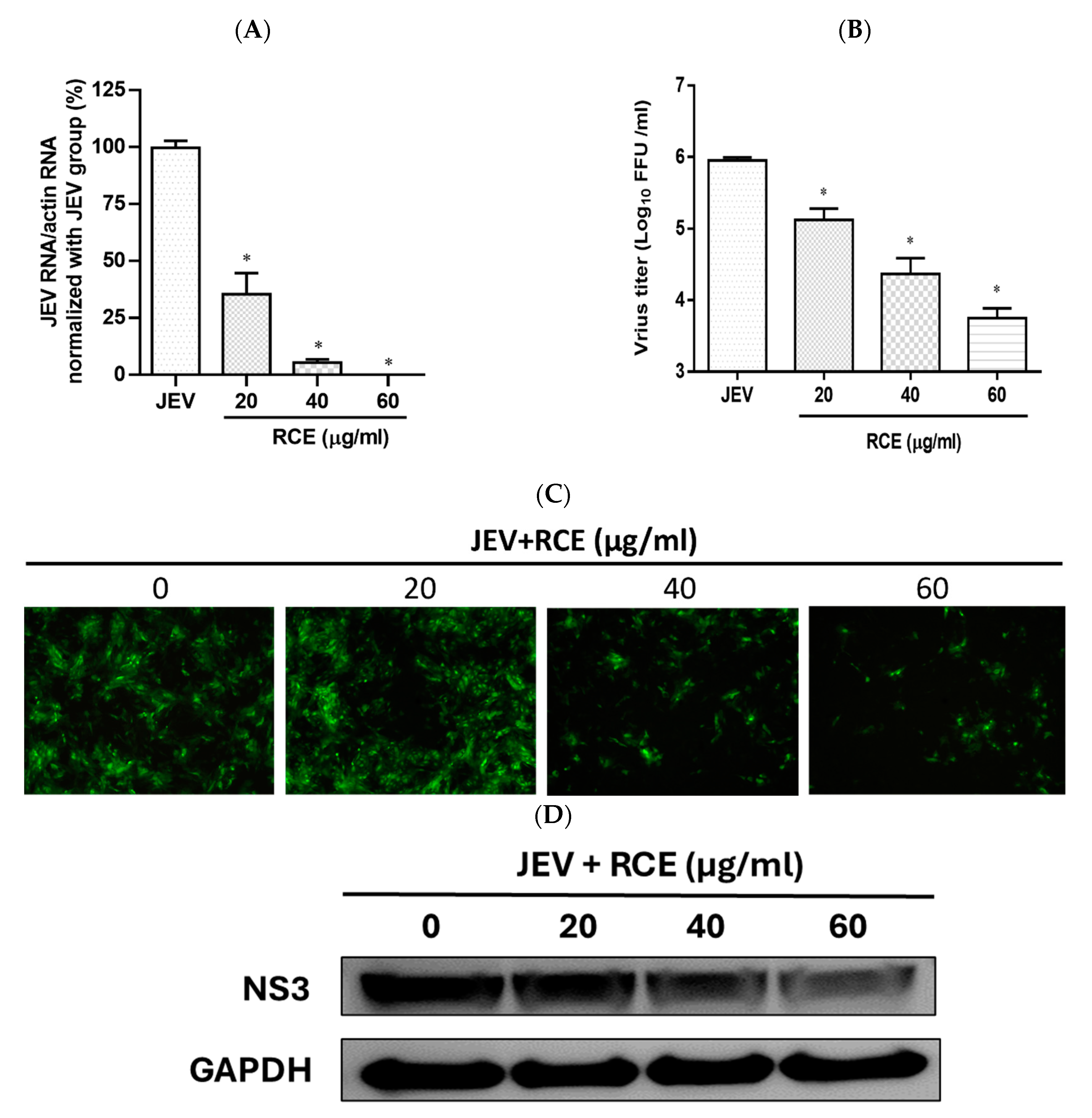
2.2. RCE Inhibited JEV Infection
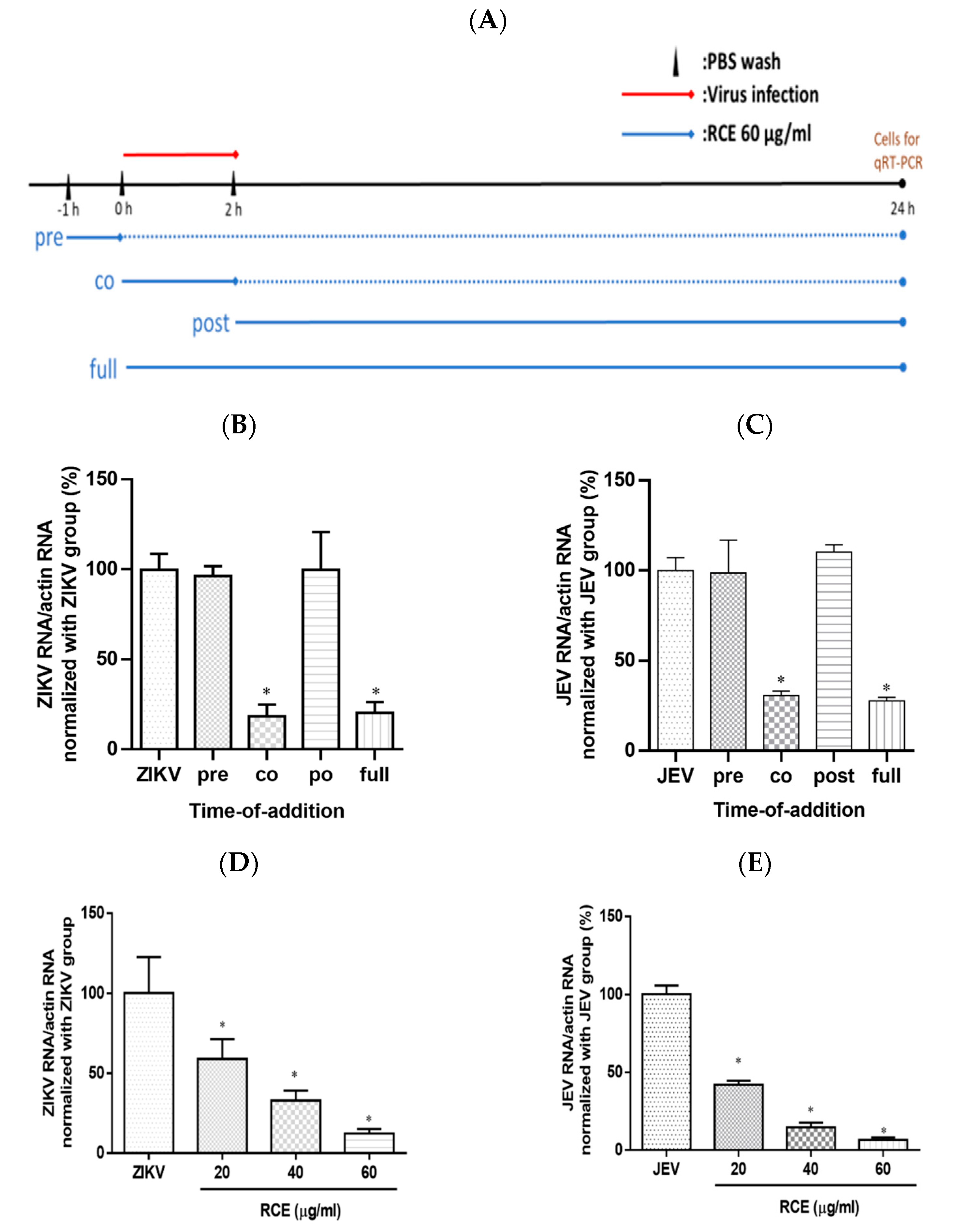
2.3. RCE Inhibited ZIKV and JEV Growth in the Early Stage of Infection
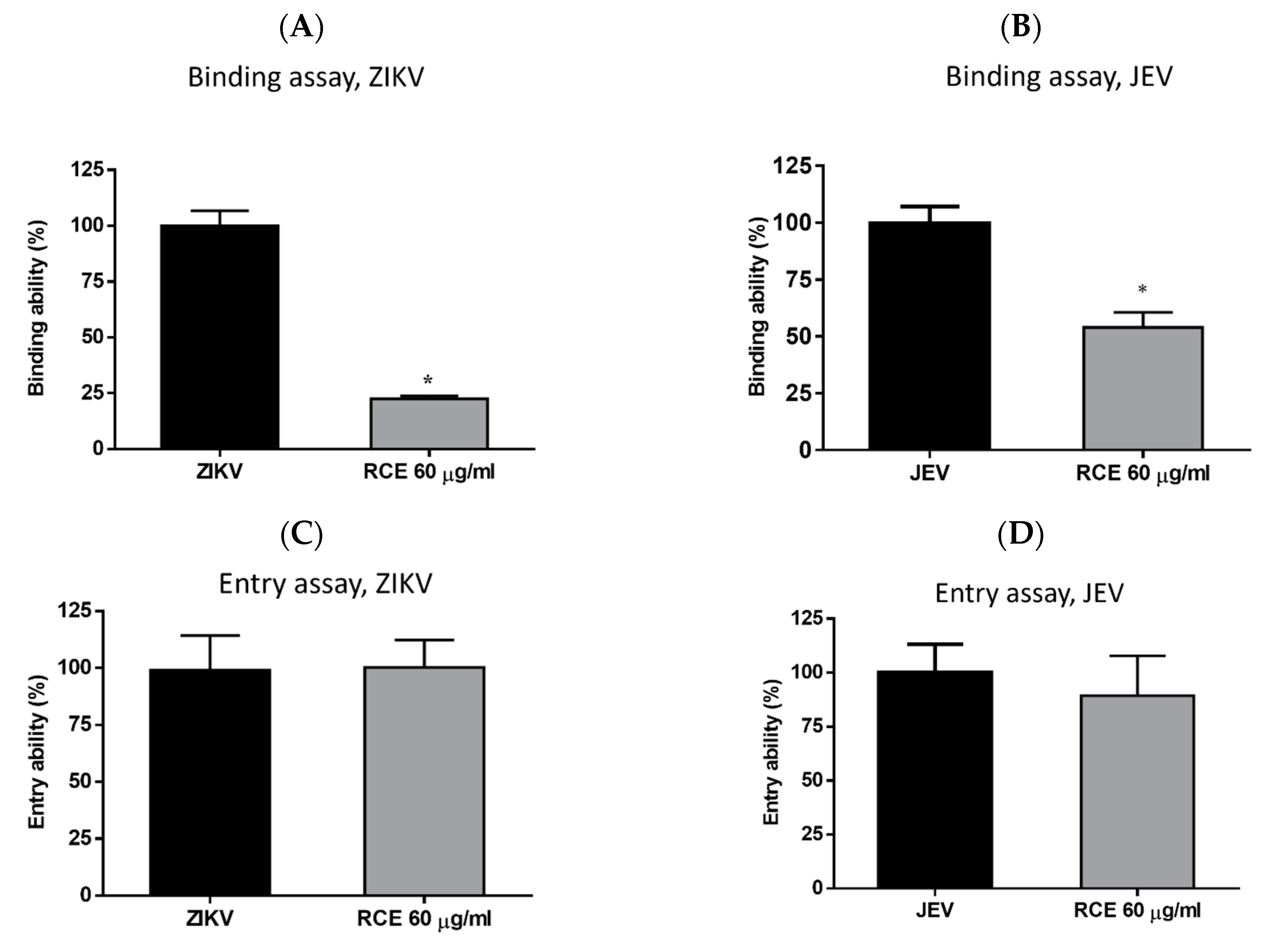
2.4. RCE Suppressed ZIKV and JEV Infection by Inhibiting Viral Binding
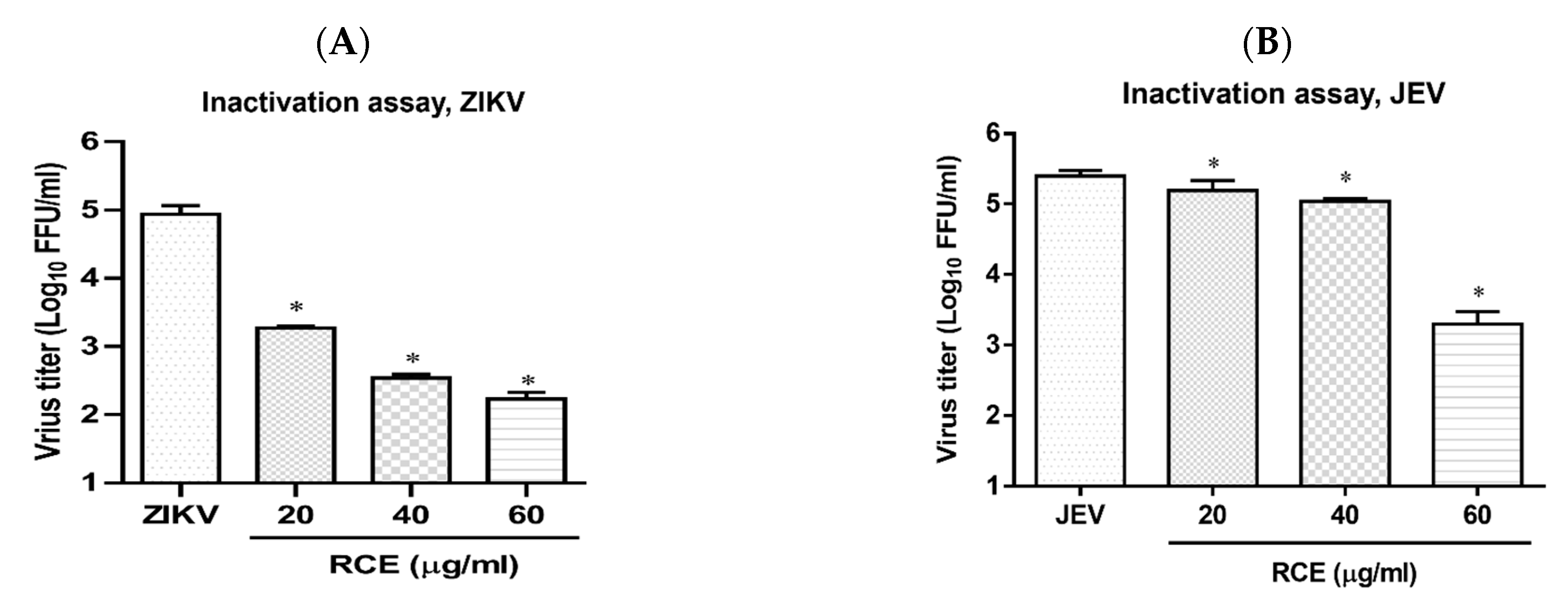
2.5. RCE Can Inactivate ZIKV and JEV
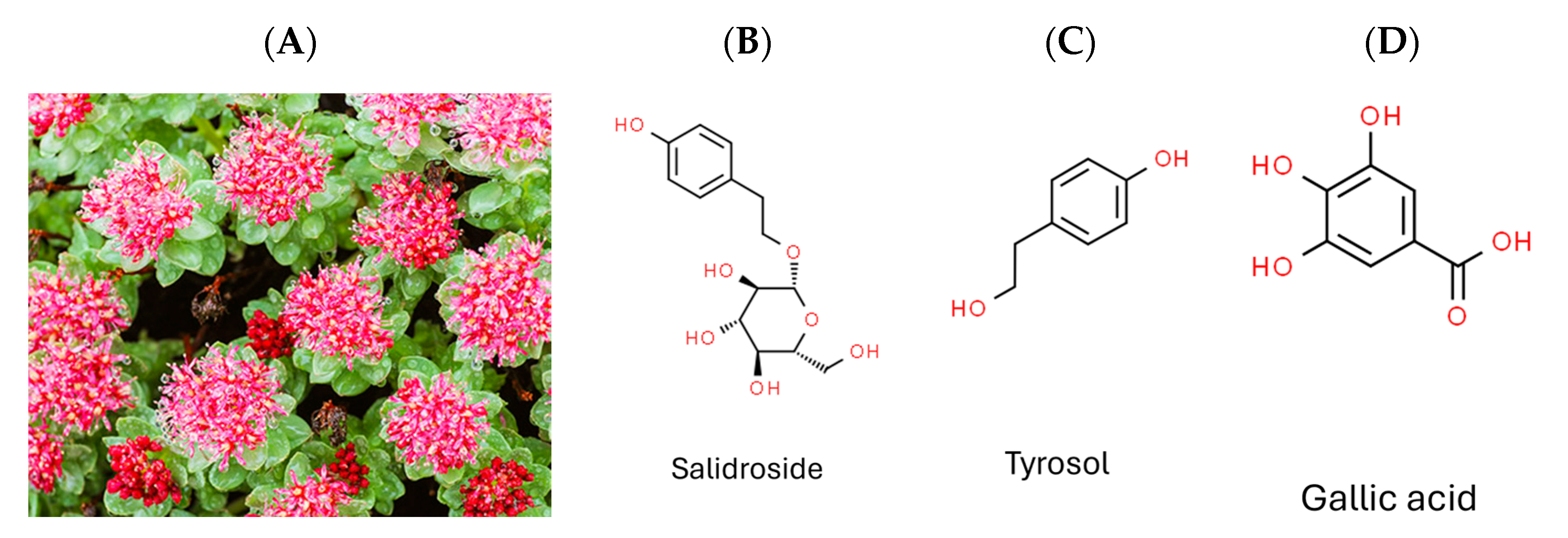
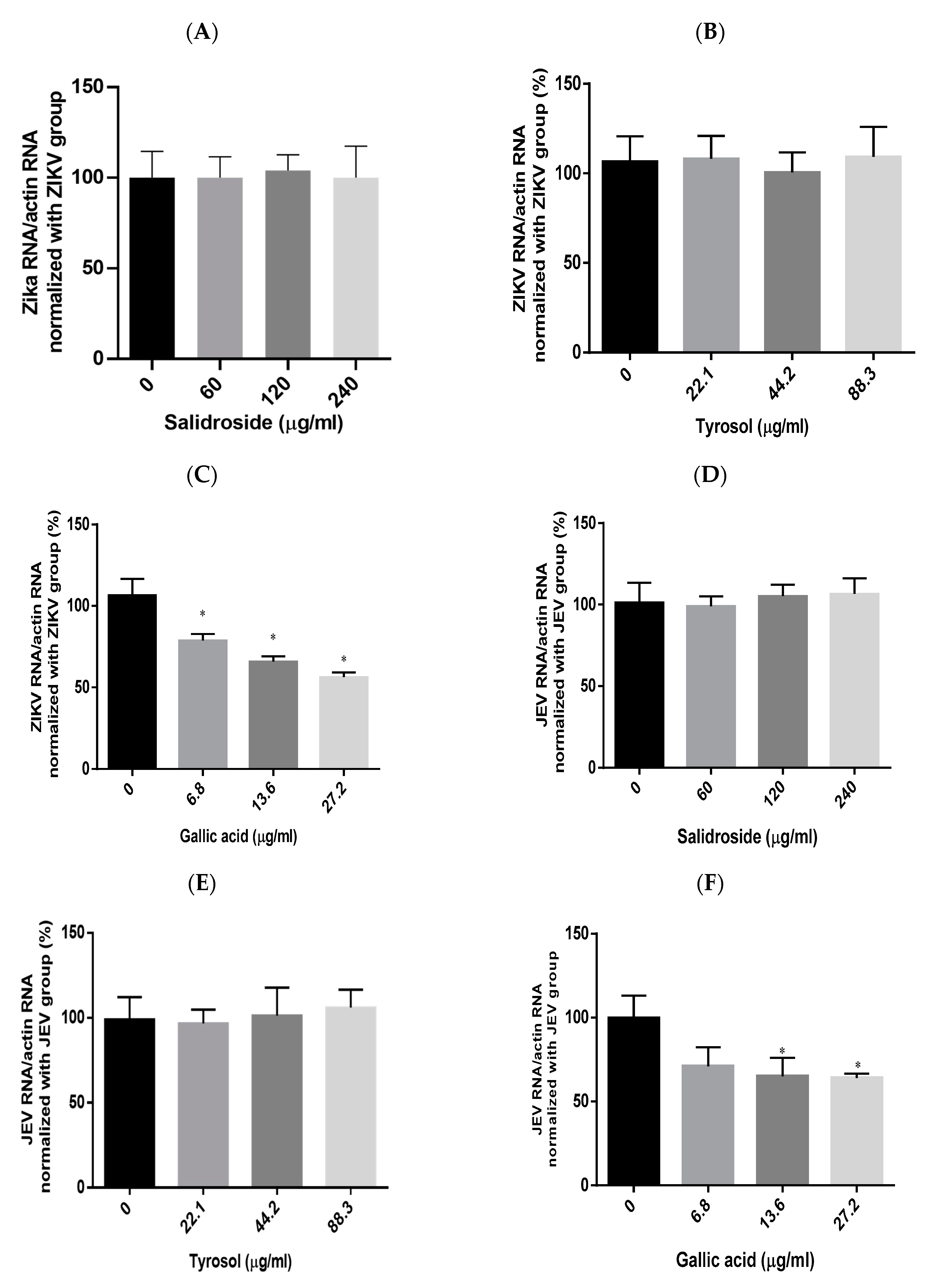
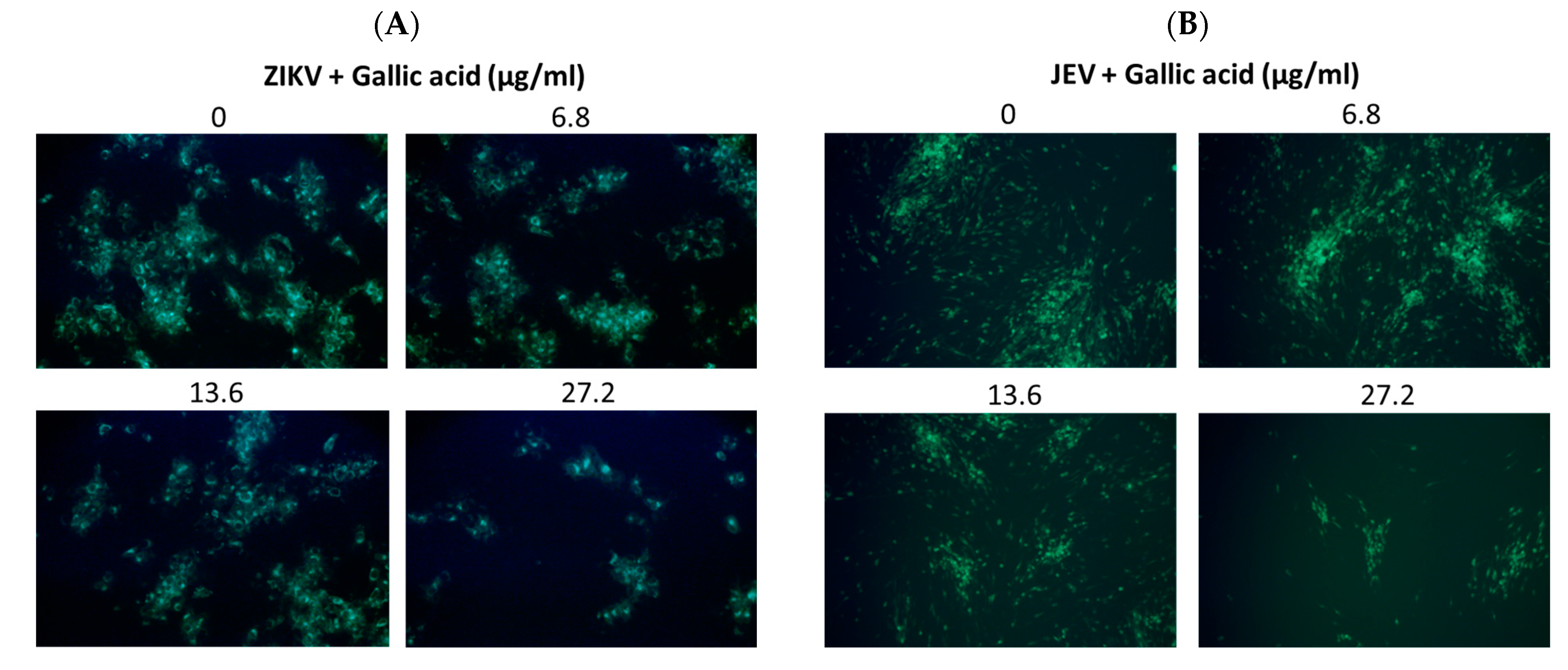
2.6. Effects of Salidroside, Tyrosol, and Gallic Acid on ZIKV and JEV
3. Discussion
4. Materials and Methods
4.1. Cells, Viruses, and Agents
4.2. Cytotoxicity Assay
4.3. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
4.4. Fluorescent Focus Assay (FFA) and Immunofluorescence Assay (IFA)
4.5. Western Blot Analysis
4.6. Time-of-Addition Assay
4.7. Binding Assay
4.8. Entry Assay
4.9. Inactivation Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CC50 | Half-maximal cytotoxic concentration |
| CCK-8 | Cell counting kit-8 |
| DENV | Dengue virus |
| DMEM | Dulbecco’s modified Eagle medium |
| FFA | Fluorescent focus assay |
| HCV | Hepatitis C virus |
| IC50 | Half-maximal inhibitory concentration |
| IFA | Immunofluorescence assay |
| JEV | Japanese encephalitis virus |
| PBS | Phosphate-buffered saline |
| RCE | Rhodiola crenulata extract |
| ZIKV | Zika virus |
References
- Pielnaa, P.; Al-Saadawe, M.; Saro, A.; Dama, M.F.; Zhou, M.; Huang, Y.; Huang, J.; Xia, Z. Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology 2020, 543, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Broutet, N.; Krauer, F.; Riesen, M.; Khalakdina, A.; Almiron, M.; Aldighieri, S.; Espinal, M.; Low, N.; Dye, C. Zika Virus as a Cause of Neurologic Disorders. N. Engl. J. Med. 2016, 374, 1506–1509. [Google Scholar] [CrossRef] [PubMed]
- Joob, B.; Wiwanitkit, V. Congenital Zika syndrome and neuroimaging findings. Radiol. Bras. 2017, 50, 405. [Google Scholar] [CrossRef] [PubMed]
- Wise, J. Study links Zika virus to Guillain-Barre syndrome. BMJ 2016, 352, i1242. [Google Scholar] [CrossRef] [PubMed]
- Roth, N.M.; Reynolds, M.R.; Lewis, E.L.; Woodworth, K.R.; Godfred-Cato, S.; Delaney, A.; Akosa, A.; Valencia-Prado, M.; Lash, M.; Elmore, A.; et al. Zika-Associated Birth Defects Reported in Pregnancies with Laboratory Evidence of Confirmed or Possible Zika Virus Infection—U.S. Zika Pregnancy and Infant Registry, December 1, 2015-March 31, 2018. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef]
- Olagnier, D.; Muscolini, M.; Coyne, C.B.; Diamond, M.S.; Hiscott, J. Mechanisms of Zika Virus Infection and Neuropathogenesis. DNA Cell Biol. 2016, 35, 367–372. [Google Scholar] [CrossRef]
- Weger-Lucarelli, J.; Duggal, N.K.; Bullard-Feibelman, K.; Veselinovic, M.; Romo, H.; Nguyen, C.; Ruckert, C.; Brault, A.C.; Bowen, R.A.; Stenglein, M.; et al. Development and Characterization of Recombinant Virus Generated from a New World Zika Virus Infectious Clone. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Suresh, K.P.; Nayak, A.; Dhanze, H.; Bhavya, A.P.; Shivamallu, C.; Achar, R.R.; Silina, E.; Stupin, V.; Barman, N.N.; Kumar, S.K.; et al. Prevalence of Japanese encephalitis (JE) virus in mosquitoes and animals of the Asian continent: A systematic review and meta-analysis. J. Infect. Public Health 2022, 15, 942–949. [Google Scholar] [CrossRef]
- Miller, R.H.; Masuoka, P.; Klein, T.A.; Kim, H.C.; Somer, T.; Grieco, J. Ecological niche modeling to estimate the distribution of Japanese encephalitis virus in Asia. PLoS Negl. Trop. Dis. 2012, 6, e1678. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.F. New initiatives for the control of Japanese encephalitis by vaccination: Minutes of a WHO/CVI meeting, Bangkok, Thailand, 13–15 October 1998. Vaccine 2000, 18 (Suppl. S2), 1–25. [Google Scholar] [CrossRef] [PubMed]
- Turtle, L.; Easton, A.; Defres, S.; Ellul, M.; Bovill, B.; Hoyle, J.; Jung, A.; Lewthwaite, P.; Solomon, T. ‘More than devastating’-patient experiences and neurological sequelae of Japanese encephalitis section sign. J. Travel. Med. 2019, 26, taz064. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Su, L.; Sun, S.; Hu, W.; Mu, Q.; Liang, X.; Jin, N.; Dai, T.; Li, H.; Zhuang, G. Long-Term Neurological Sequelae and Disease Burden of Japanese Encephalitis in Gansu Province, China. Ann. Glob. Health 2021, 87, 103. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, T.; Feng, D.; Li, R.; Zhang, C. Polyphenols from Chinese Herbal Medicine: Molecular Mechanisms and Therapeutic Targets in Pulmonary Fibrosis. Am. J. Chin. Med. 2022, 50, 1063–1094. [Google Scholar] [CrossRef] [PubMed]
- Patar, A.K.; Borah, S.M.; Barman, J.; Bora, A.; Baruah, T.J. Dronabinol as an answer to flavivirus infections: An in-silico investigation. J. Biomol. Struct. Dyn. 2022, 41, 11219–11230. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, F.P. Studies on the chemical components of Rhodiola crenulata. Yao Xue Xue Bao 1992, 27, 117–120. [Google Scholar] [PubMed]
- Tao, H.; Wu, X.; Cao, J.; Peng, Y.; Wang, A.; Pei, J.; Xiao, J.; Wang, S.; Wang, Y. Rhodiola species: A comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med. Res. Rev. 2019, 39, 1779–1850. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pham, V.; Bui, M.; Song, L.; Wu, C.; Walia, A.; Uchio, E.; Smith-Liu, F.; Zi, X. Rhodiola rosea L.: An herb with anti-stress, anti-aging, and immunostimulating properties for cancer chemoprevention. Curr. Pharmacol. Rep. 2017, 3, 384–395. [Google Scholar] [CrossRef]
- Li, W.L.; Zheng, H.C.; Bukuru, J.; De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef]
- Chiu, T.F.; Chen, L.L.; Su, D.H.; Lo, H.Y.; Chen, C.H.; Wang, S.H.; Chen, W.L. Rhodiola crenulata extract for prevention of acute mountain sickness: A randomized, double-blind, placebo-controlled, crossover trial. BMC Complement. Altern. Med. 2013, 13, 298. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.L.; Wang, Y.N.; Jiao, J.; Gong, Y.; Wang, J.H.; Wang, M.L. Fungal endophyte-induced salidroside and tyrosol biosynthesis combined with signal cross-talk and the mechanism of enzyme gene expression in Rhodiola crenulata. Sci. Rep. 2017, 7, 12540. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Qu, Z.Q.; Wu, J.L.; Chung, P.; Zeng, Y.S. Mitochondrial protective and anti-apoptotic effects of Rhodiola crenulata extract on hippocampal neurons in a rat model of Alzheimer’s disease. Neural Regen. Res. 2017, 12, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Li, Z.; Chen, L.; Xu, X. Activity of compounds from Chinese herbal medicine Rhodiola kirilowii (Regel) Maxim against HCV NS3 serine protease. Antivir. Res. 2007, 76, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Mishra, K.P.; Ganju, L. Salidroside exhibits anti-dengue virus activity by upregulating host innate immune factors. Arch. Virol. 2016, 161, 3331–3344. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Du, R.; Anantpadma, M.; Schafer, A.; Hou, L.; Tian, J.; Davey, R.A.; Cheng, H.; Rong, L. Identification of Ellagic Acid from Plant Rhodiola rosea L. as an Anti-Ebola Virus Entry Inhibitor. Viruses 2018, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Ryu, Y.B.; Park, S.J.; Kim, J.H.; Kwon, H.J.; Kim, J.H.; Park, K.H.; Rho, M.C.; Lee, W.S. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorg Med. Chem. 2009, 17, 6816–6823. [Google Scholar] [CrossRef] [PubMed]
- Aoki-Utsubo, C.; Chen, M.; Hotta, H. Time-of-addition and Temperature-shift Assays to Determine Particular Step(s) in the Viral Life Cycle that is Blocked by Antiviral Substance(s). Bio Protoc. 2018, 8, e2830. [Google Scholar] [CrossRef] [PubMed]
- Chapagain, S.; Pal Singh, P.; Le, K.; Safronetz, D.; Wood, H.; Karniychuk, U. Japanese encephalitis virus persists in the human reproductive epithelium and porcine reproductive tissues. PLoS Negl. Trop. Dis. 2022, 16, e0010656. [Google Scholar] [CrossRef]
- Perera-Lecoin, M.; Meertens, L.; Carnec, X.; Amara, A. Flavivirus entry receptors: An update. Viruses 2013, 6, 69–88. [Google Scholar] [CrossRef]
- Smit, J.M.; Moesker, B.; Rodenhuis-Zybert, I.; Wilschut, J. Flavivirus cell entry and membrane fusion. Viruses 2011, 3, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hu, K.; Luo, S.; Zhang, M.; Deng, X.; Li, C.; Jin, W.; Hu, B.; He, S.; Li, M.; et al. DC-SIGN as an attachment factor mediates Japanese encephalitis virus infection of human dendritic cells via interaction with a single high-mannose residue of viral E glycoprotein. Virology 2016, 488, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Dupont-Rouzeyrol, M.; Biron, A.; O’Connor, O.; Huguon, E.; Descloux, E. Infectious Zika viral particles in breastmilk. Lancet 2016, 387, 1051. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Song, J.H.; Bhatt, L.R.; Baek, S.H. Anti-human rhinovirus activity of gallic acid possessing antioxidant capacity. Phytother. Res. 2010, 24, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Song, J.H.; Park, K.S.; Baek, S.H. In vitro anti-enterovirus 71 activity of gallic acid from Woodfordia fruticosa flowers. Lett. Appl. Microbiol. 2010, 50, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Nutan; Modi, M.; Goel, T.; Das, T.; Malik, S.; Suri, S.; Rawat, A.K.; Srivastava, S.K.; Tuli, R.; Malhotra, S.; et al. Ellagic acid & gallic acid from Lagerstroemia speciosa L. inhibit HIV-1 infection through inhibition of HIV-1 protease & reverse transcriptase activity. Indian. J. Med. Res. 2013, 137, 540–548. [Google Scholar]
- You, H.L.; Huang, C.C.; Chen, C.J.; Chang, C.C.; Liao, P.L.; Huang, S.T. Anti-pandemic influenza A (H1N1) virus potential of catechin and gallic acid. J. Chin. Med. Assoc. 2018, 81, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Govea-Salas, M.; Rivas-Estilla, A.M.; Rodriguez-Herrera, R.; Lozano-Sepulveda, S.A.; Aguilar-Gonzalez, C.N.; Zugasti-Cruz, A.; Salas-Villalobos, T.B.; Morlett-Chavez, J.A. Gallic acid decreases hepatitis C virus expression through its antioxidant capacity. Exp. Ther. Med. 2016, 11, 619–624. [Google Scholar] [CrossRef]
- Lee, S.Y.; Shi, L.S.; Chu, H.; Li, M.H.; Ho, C.W.; Lai, F.Y.; Huang, C.Y.; Chang, T.C. Rhodiola crenulata and Its Bioactive Components, Salidroside and Tyrosol, Reverse the Hypoxia-Induced Reduction of Plasma-Membrane-Associated Na,K-ATPase Expression via Inhibition of ROS-AMPK-PKC xi Pathway. Evid. Based Complement. Altern. Med. 2013, 2013, 284150. [Google Scholar]
- Ho, Y.J.; Lu, J.W.; Huang, Y.L.; Lai, Z.Z. Palmatine inhibits Zika virus infection by disrupting virus binding, entry, and stability. Biochem. Biophys. Res. Commun. 2019, 518, 732–738. [Google Scholar] [CrossRef] [PubMed]
| Name | Orientation | Primer Sequence |
|---|---|---|
| 5′-3′Orientation | ||
| ZIKA Env | Forward | TTGGTCATGATACTGCTGATTGC |
| Reverse | CCTTCCACAAAGTCCCTATTGC | |
| JEV NS2 | Forward | TCCGTCACCATGCCAGTCTT |
| Reverse | GAGGATGATTCTGTAAGTATC | |
| β-actin | Forward | AGGCACCAGGGCGTGAT |
| Reverse | GCCCACATAGGAATCCTTCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Z.-Z.; Yen, I.-C.; Hung, H.-Y.; Hong, C.-Y.; Lai, C.-W.; Lee, Y.-M. In Vitro Antiviral Activity of Rhodiola crenulata Extract against Zika Virus and Japanese Encephalitis Virus: Viral Binding and Stability. Pharmaceuticals 2024, 17, 988. https://doi.org/10.3390/ph17080988
Lai Z-Z, Yen I-C, Hung H-Y, Hong C-Y, Lai C-W, Lee Y-M. In Vitro Antiviral Activity of Rhodiola crenulata Extract against Zika Virus and Japanese Encephalitis Virus: Viral Binding and Stability. Pharmaceuticals. 2024; 17(8):988. https://doi.org/10.3390/ph17080988
Chicago/Turabian StyleLai, Zheng-Zong, I-Chuan Yen, Hao-Yuan Hung, Chen-Yang Hong, Chih-Wei Lai, and Yen-Mei Lee. 2024. "In Vitro Antiviral Activity of Rhodiola crenulata Extract against Zika Virus and Japanese Encephalitis Virus: Viral Binding and Stability" Pharmaceuticals 17, no. 8: 988. https://doi.org/10.3390/ph17080988
APA StyleLai, Z.-Z., Yen, I.-C., Hung, H.-Y., Hong, C.-Y., Lai, C.-W., & Lee, Y.-M. (2024). In Vitro Antiviral Activity of Rhodiola crenulata Extract against Zika Virus and Japanese Encephalitis Virus: Viral Binding and Stability. Pharmaceuticals, 17(8), 988. https://doi.org/10.3390/ph17080988







