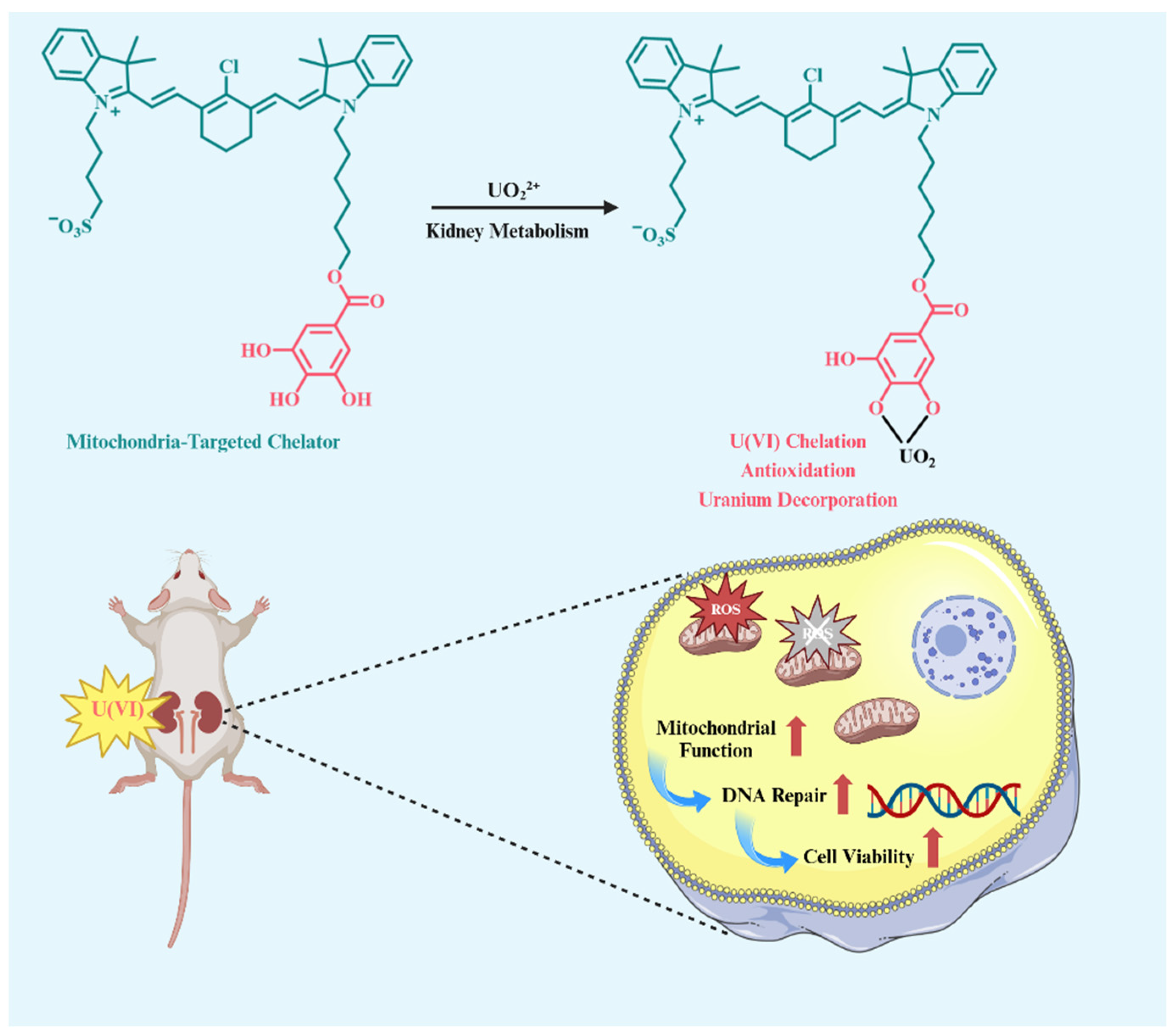
A Mitochondria-Targeted Heptamethine Indocyanine Small Molecular Chelator for Attenuating Uranium Nephrotoxicity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Structural Characterization
2.2. Good Chelating Ability Towards UO22+ in Water Solution
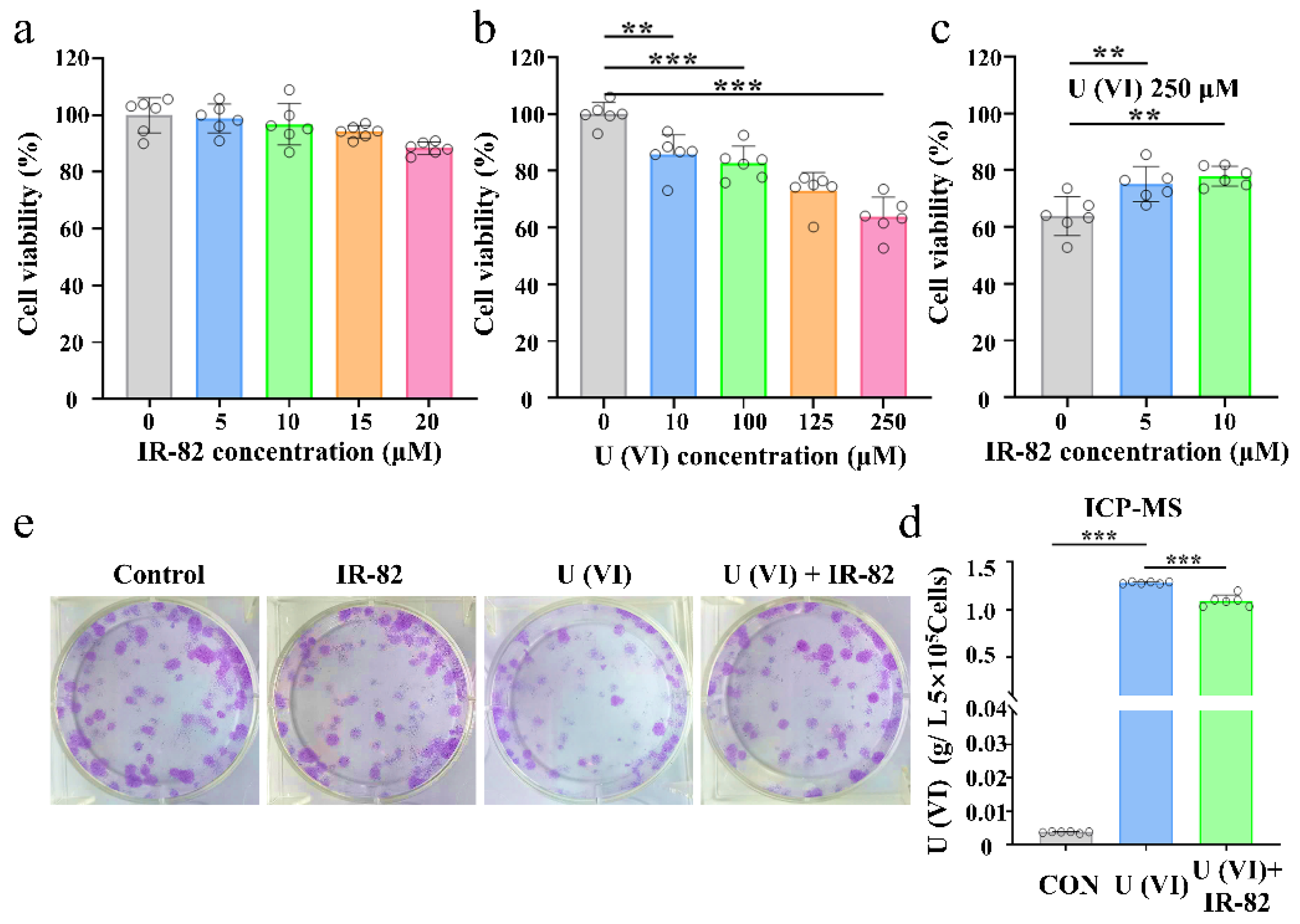
2.3. Protective Effect of IR-82 on HK-2 Cells after Uranium Exposure
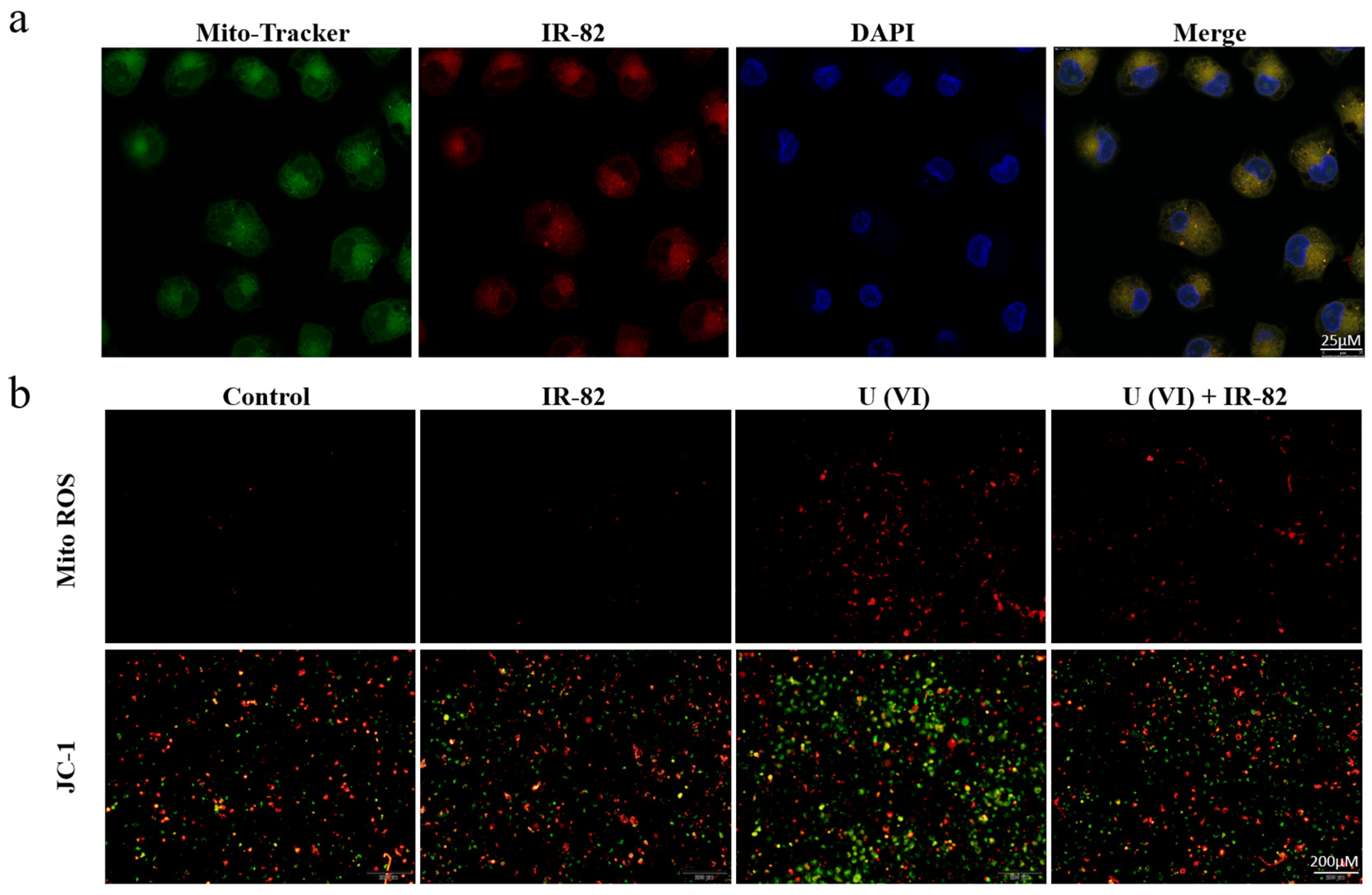
2.4. Mitochondria-Targeted Accumulation and Function Protection
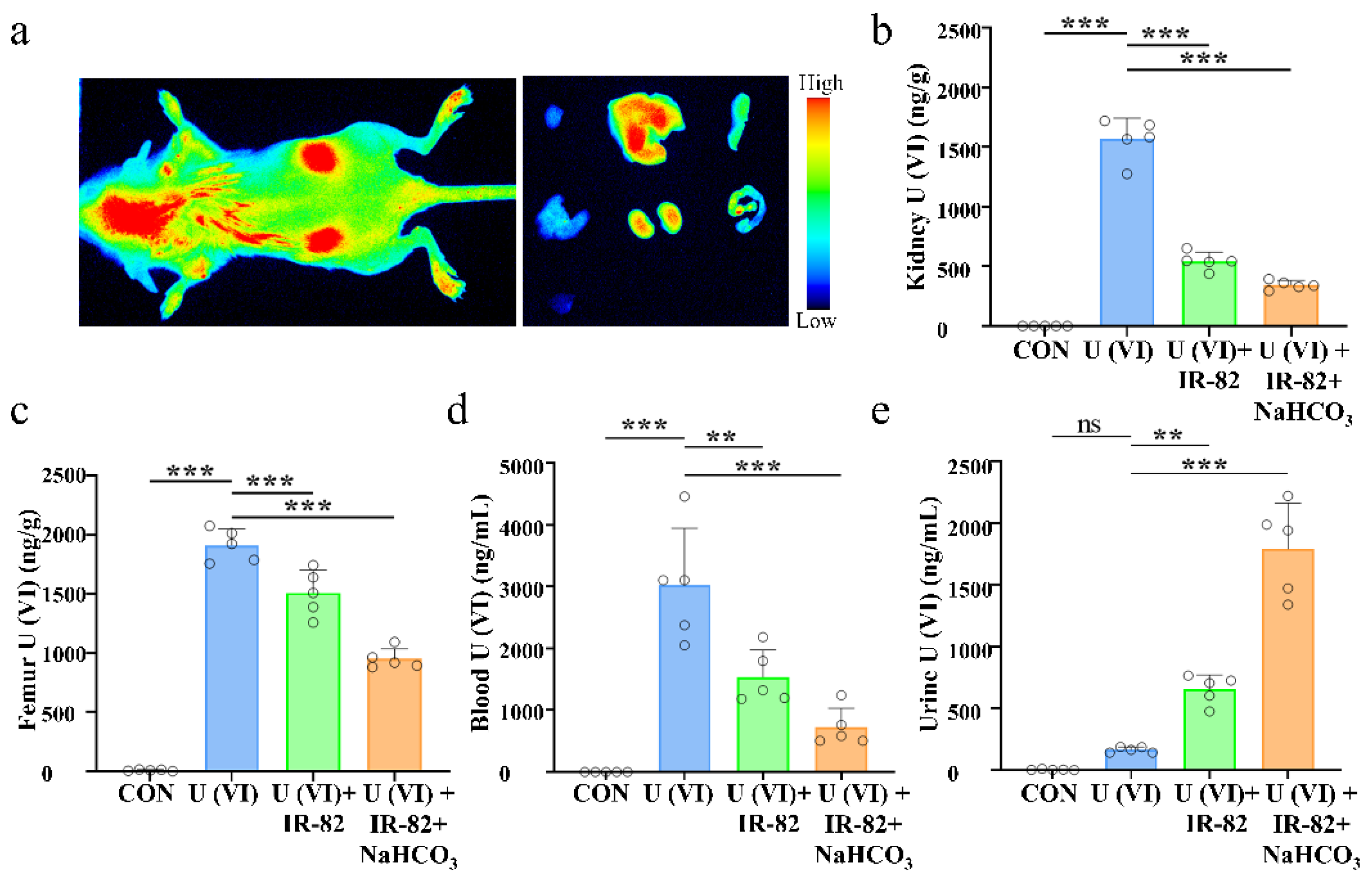
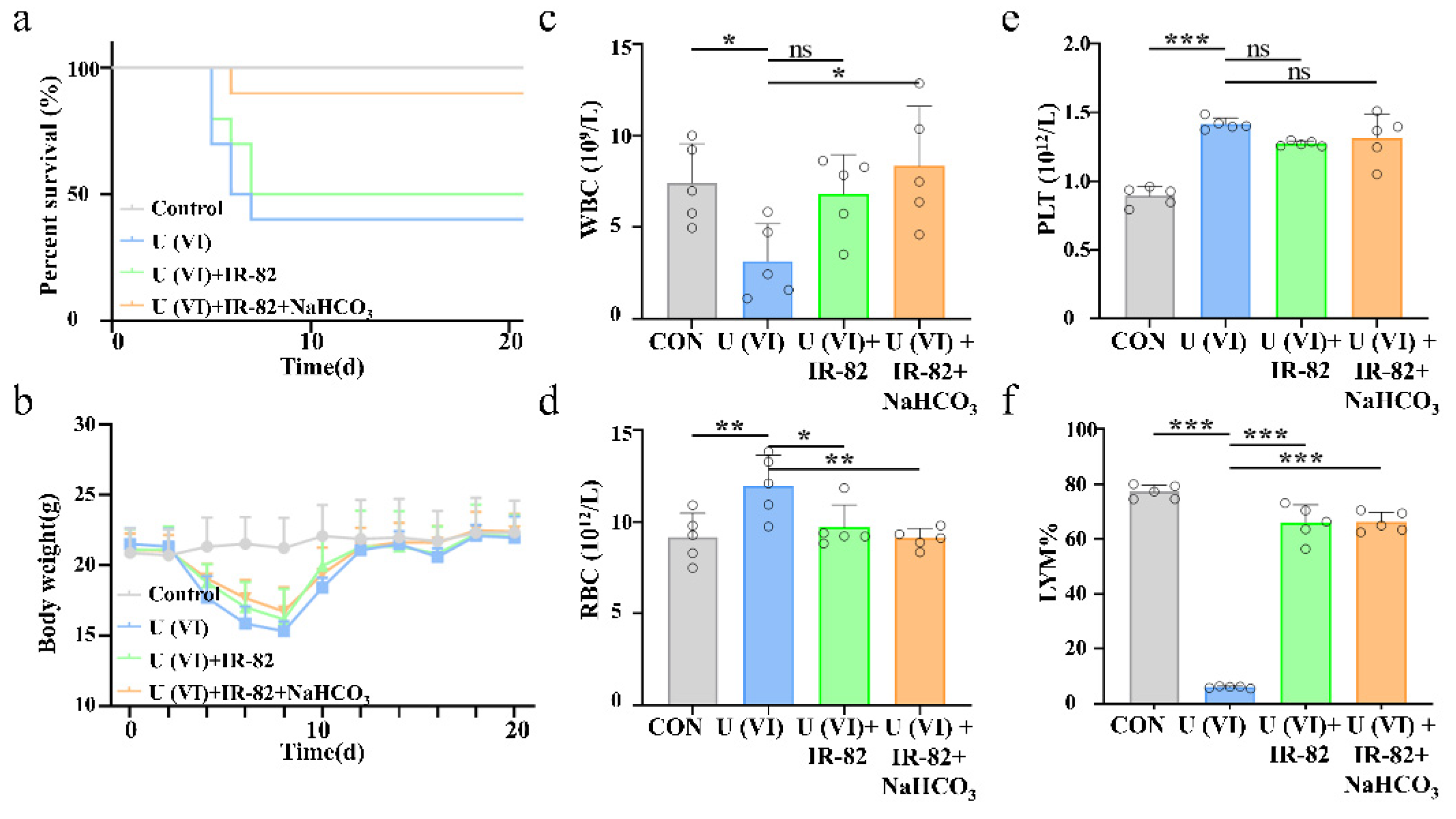
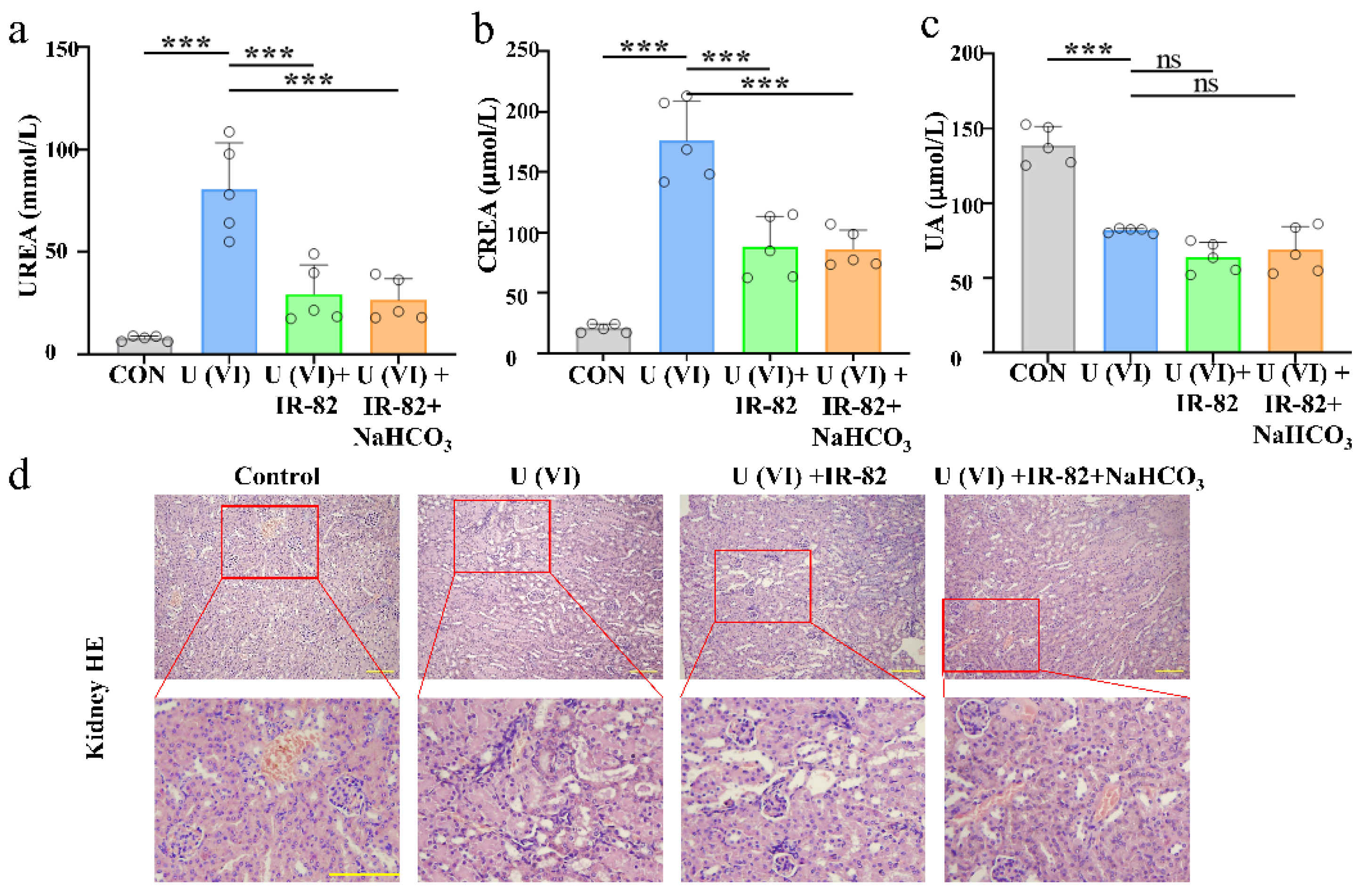
2.5. Kidney Metabolism and Protective Effect of IR-82 in U(VI)-Exposed Mice
3. Materials and Methods
3.1. Material Preparation
3.2. Chemical Synthesis and Structure Characterization
3.2.1. Synthesis of S1
3.2.2. Synthesis of S2
3.2.3. Synthesis of S3 and S4
3.2.4. Synthesis of IR-82
3.3. Determination of Chelating Ability towards UO22+
3.4. In Vitro Experiments of IR-82 for U(VI) Detoxification
3.4.1. Cytotoxicity and Viability Assay
3.4.2. Cell Clonogenic Survival Assay
3.4.3. Calcein and PI Assay
3.4.4. Determination of Intracellular ROS Production
3.4.5. Uranium Uptake Experiments in HK-2 Cells
3.4.6. Immunofluorescence
3.4.7. Mitochondrial Localization
3.4.8. Determination of Mitochondrial ROS Production
3.4.9. Microscopic Examination of Mitochondrial Membrane Potential
3.5. In Vivo Experimental for U(VI) Decorporation
3.5.1. Animal U(VI) Exposure
3.5.2. Experiments on the Effects of Uranium Exposure on the Organs of Mice
3.5.3. IR-82 Accumulation and Metabolism in Kidney
3.5.4. Histological Observation
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, Y.; Liu, T.; Xiao, J.; Yu, Q.; Wang, N. DNA nano-pocket for ultra-selective uranyl extraction from seawater. Nat. Commun. 2020, 11, 5708. [Google Scholar] [CrossRef] [PubMed]
- Brugge, D.; Delemos, J.L.; Oldmixon, B. Exposure pathways and health effects associated with chemical and radiological toxicity of natural uranium: A review. Rev. Environ. Health 2005, 20, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Wang, X.; Zhang, H.; Sun, Q.; Li, A.; Miao, Y.; Shi, C.; Guan, J.; Gong, S.; Diwu, J. Boosting Simultaneous Uranium Decorporation and Reactive Oxygen Species Scavenging Efficiency by Lacunary Polyoxometalates. ACS Appl. Mater. Interfaces 2022, 14, 54423–54430. [Google Scholar] [CrossRef] [PubMed]
- Blantz, R.C.; Pelayo, J.C.; Gushwa, L.C.; Myers, R.R.; Evan, A.P. Functional basis for the glomerular alterations in uranyl nitrate acute renal failure. Kidney Int. 1985, 28, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Vicente, L.; Quiros, Y.; Pérez-Barriocanal, F.; López-Novoa, J.M.; López-Hernández, F.J.; Morales, A.I. Nephrotoxicity of Uranium: Pathophysiological, Diagnostic and Therapeutic Perspectives. Toxicol. Sci. 2010, 118, 324–347. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ran, H.; Yin, Y.; Liu, J.; Lu, B.; Ran, X.; Luo, S.; Wang, W.; Yang, Z.; Li, R. Mitochondrial Targeted Cerium Oxide Nanoclusters for Radiation Protection and Promoting Hematopoiesis. Int. J. Nanomed. 2024, 19, 6463–6483. [Google Scholar] [CrossRef] [PubMed]
- Asic, A.; Kurtovic-Kozaric, A.; Besic, L.; Mehinovic, L.; Marjanovic, D. Chemical toxicity and radioactivity of depleted uranium: The evidence from in vivo and in vitro studies. Environ. Res. 2017, 156, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, W.; Chu, J.; Chen, C.; Xiong, Z. Uranium inhibits mammalian mitochondrial cytochrome c oxidase and ATP synthase. Environ. Pollut. 2021, 271, 116377. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, S.; Zhao, Y.; Li, J.; Shu, C.; Li, Y.; Li, J.; Lu, B.; Xu, Z.; Ran, Y. Depleted uranium causes renal mitochondrial dysfunction through the ETHE1/Nrf2 pathway. Chem. Biol. Interact. 2023, 372, 110356. [Google Scholar] [CrossRef] [PubMed]
- Cassatt, D.R.; Kaminski, J.M.; Hatchett, R.J.; Dicarlo, A.L.; Benjamin, J.M.; Maidment, B.W. Medical Countermeasures against Nuclear Threats: Radionuclide Decorporation Agents. Radiat. Res. 2008, 170, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.C.; Li, M.H.; Wang, H.B.; Zhang, B.L.; He, W. The toxicological mechanisms and detoxification of depleted uranium exposure. Environ. Health Prev. Med. 2018, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, Z.; Song, W.; Liu, Y.; Zhao, Q.; Li, W.; Zheng, C.; Li, W.; Chen, Z.; Zhu, L. Perilla frutescens: A new strategy for uranium decorporation. Chemosphere 2024, 350, 141066. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, Z.; Gao, J.; Xu, K.; Gu, H.; Zhang, B.; Zhang, X.; Xu, B. A biocompatible method of decorporation: Bisphosphonate-modified magnetite nanoparticles to remove uranyl ions from blood. J. Am. Chem. Soc. 2006, 128, 13358–13359. [Google Scholar] [CrossRef] [PubMed]
- Viehweger, K.; Geipel, G.; Bernhard, G. Impact of uranium (U) on the cellular glutathione pool and resultant consequences for the redox status of U. Biometals 2011, 24, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Wang, D.; Li, Z.; Hu, Y.; Xu, A.; Wang, Q.; Shao, C.; Chen, H. Efficacy of a novel chelator BPCBG for removing uranium and protecting against uranium-induced renal cell damage in rats and HK-2 cells. Toxicol. Appl. Pharmacol. 2013, 269, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Matsuda, E.; Ueda, Y. Effect of catechins and tannins on hydroxyl radical formation in depleted uranium-hydrogen peroxide systems. J. Radioanal. Nucl. Chem. 2010, 283, 151–156. [Google Scholar] [CrossRef]
- Elmileegy, I.M.H.; Waly, H.S.A.; Alghriany, A.A.I.; Khalil, N.S.A.; Mahmoud, S.M.M.; Negm, E.A. Gallic acid rescues uranyl acetate induced-hepatic dysfunction in rats by its antioxidant and cytoprotective potentials. BMC Complement. Med. Ther. 2023, 23, 423. [Google Scholar] [CrossRef] [PubMed]
- Fatemeh Shaki, M.J.H.; Mahmoud, G.K.; Jalal, P. Toxicity of depleted uranium on isolated rat kidney mitochondria. Biochim. Biophys. Acta 2012, 1820, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Zarei, M.H.; Salimi, A.; Pourahmad, J. Mitochondrial protective and antioxidant agents protect toxicity induced by depleted uranium in isolated human lymphocytes. J. Environ. Radioact. 2019, 203, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Tan, X.; Fang, S.; Wang, Y.; Liu, T.; Wang, X.; Yuan, Y.; Sun, H.; Qi, Q.; Shi, C. Cancer Phototherapy: Mitochondria-Targeted Small-Molecule Fluorophores for Dual Modal Cancer Phototherapy. Adv. Funct. Mater. 2016, 26, 2975. [Google Scholar] [CrossRef]
- Chen, S.; Yu, S.; Du, Z.; Huang, X.; He, M.; Long, S.; Liu, J.; Lan, Y.; Yang, D.; Wang, H.; et al. Synthesis of Mitochondria-Anchored Nitroimidazoles with a Versatile NIR Fluorophore for Hypoxic Tumor-Targeting Imaging and Chemoradiotherapy. J. Med. Chem. 2021, 64, 3381–3391. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Tan, X.; Luo, S.; Shi, C. Fluorinated near-infrared fluorescent probes for specific detection of Hg2+ in an aqueous medium and mitochondria of living cells. New J. Chem. 2017, 41, 8899–8904. [Google Scholar] [CrossRef]
- Wu, J.B.; Lin, T.P.; Gallagher, J.D.; Kushal, S.; Chung, L.W.K.; Zhau, H.E.; Olenyuk, B.Z.; Shih, J.C. Monoamine oxidase A inhibitor-near-infrared dye conjugate reduces prostate tumor growth. J. Am. Chem. Soc. 2015, 137, 2366–2374. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Yang, X.; Wang, M.; Yang, J.; Qin, Z.; Kan, Q.; Zhang, H.; Wang, Y.; Wang, D.; He, Z. Mitochondria-targeted prostate cancer therapy using a near-infrared fluorescence dye-monoamine oxidase A inhibitor conjugate. J. Control. Release 2018, 279, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shi, C.; Tong, R.; Qian, W.; Zhau, H.E.; Wang, R.; Zhu, G.; Cheng, J.; Yang, V.W.; Cheng, T. Near IR heptamethine cyanine dye-mediated cancer imaging. Clin. Cancer Res. 2010, 16, 2833–2844. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Luo, S.; Long, L.; Wang, Y.; Wang, D.; Fang, S.; Ouyang, Q.; Su, Y.; Cheng, T.; Shi, C. Structure-Guided Design and Synthesis of a Mitochondria-Targeting Near-Infrared Fluorophore with Multimodal Therapeutic Activities. Adv. Mater. 2017, 29, 1704196. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Luo, S.; Qi, Q.; Shi, C. Preliminary structure-activity relationship study of heptamethine indocyanine dyes for tumor-targeted imaging. J. Innov. Opt. Health Sci. 2013, 06, 1350003–1350009. [Google Scholar] [CrossRef]
- Du, Z.; Liu, H.; Huang, X.; Li, Y.; Wang, L.; Liu, J.; Long, S.; Li, R.; Xiang, Q.; Luo, S. Design and Synthesis of a Mitochondria-Targeting Radioprotectant for Promoting Skin Wound Healing Combined with Ionizing Radiation Injury. Pharmaceuticals 2022, 15, 721. [Google Scholar] [CrossRef] [PubMed]
- Sylwester, E.R.; Allen, P.G.; Dharmawardana, U.R.; Sutton, M. Structural studies of uranium and thorium complexes with 4,5-dihydroxy-3,5-benzenesdisulfonate (Tiron) at low and neutral pH by X-ray absorption spectroscopy. Inorg. Chem. 2001, 40, 2835–2841. [Google Scholar] [CrossRef] [PubMed]
- Bensoussan, H.; Grancolas, L.; Dhieux-Lestaevel, B.; Delissen, O.; Vacher, C.-M.; Dublineau, I.; Voisin, P.; Gourmelon, P.; Taouis, M.; Lestaevel, P. Heavy metal uranium affects the brain cholinergic system in rat following sub-chronic and chronic exposure. Toxicology 2009, 261, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.; Hancock, S.; McNally, A.; Hinckley, J.; Binder, E.; Zimmerman, K.; Ehrich, M.; Jortner, B. Neurological effects of acute uranium exposure with and without stress. Neurotoxicology 2007, 28, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Aydin, D.; Yalçin, E.; Çavuşoğlu, K. Metal chelating and anti-radical activity of Salvia officinalis in the ameliorative effects against uranium toxicity. Sci. Rep. 2022, 12, 15845. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, J.; Zhang, W.; Zhou, J.; Luo, D.; Li, Z. Uranium (U) source, speciation, uptake, toxicity and bioremediation strategies in soil-plant system: A review. J. Hazard. Mater. 2021, 413, 125319. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, D.; Xu, X.; Zhong, X.; Ahmed, F.; Zhong, J.; Liao, J.; Dykxhoorn, D.M.; Weinstock, D.M.; Pfeifer, G.P.; Lieberman, J. A PP4-phosphatase complex dephosphorylates γ-H2AX generated during DNA replication. Mol. Cell 2008, 31, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Vinayakumara, D.; Swamynathan, K.; Kumar, S.; Adhikari, A.V. Optoelectronic exploration of novel non-symmetrical star-shaped discotic liquid crystals based on cyanopyridine. New J. Chem. 2018, 42, 16999–17008. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, C.; Huang, J.; Gu, Y.; Li, H.; Yang, Z.; Liu, J.; Wang, W.; Li, R. Ghrelin protects against depleted uranium-induced apoptosis of MC3T3-E1 cells through oxidative stress-mediated p38-mitogen-activated protein kinase pathway. Toxicol. Appl. Pharmacol. 2016, 290, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, X.; Wan, J.; Zhang, D.; Yi, X.; Bai, Z.; Yang, K.; Diwu, J.; Chai, Z.; Wang, S. 3,2-Hydroxypyridinone-grafted chitosan oligosaccharide nanoparticles as efficient decorporation agents for simultaneous removal of uranium and radiation-induced reactive oxygen species in vivo. Bioconjugate Chem. 2018, 29, 3896–3905. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Z.; Huang, X.; Wu, Z.; Gao, M.; Li, R.; Luo, S. A Mitochondria-Targeted Heptamethine Indocyanine Small Molecular Chelator for Attenuating Uranium Nephrotoxicity. Pharmaceuticals 2024, 17, 995. https://doi.org/10.3390/ph17080995
Du Z, Huang X, Wu Z, Gao M, Li R, Luo S. A Mitochondria-Targeted Heptamethine Indocyanine Small Molecular Chelator for Attenuating Uranium Nephrotoxicity. Pharmaceuticals. 2024; 17(8):995. https://doi.org/10.3390/ph17080995
Chicago/Turabian StyleDu, Zaizhi, Xie Huang, Zifei Wu, Mingquan Gao, Rong Li, and Shenglin Luo. 2024. "A Mitochondria-Targeted Heptamethine Indocyanine Small Molecular Chelator for Attenuating Uranium Nephrotoxicity" Pharmaceuticals 17, no. 8: 995. https://doi.org/10.3390/ph17080995





