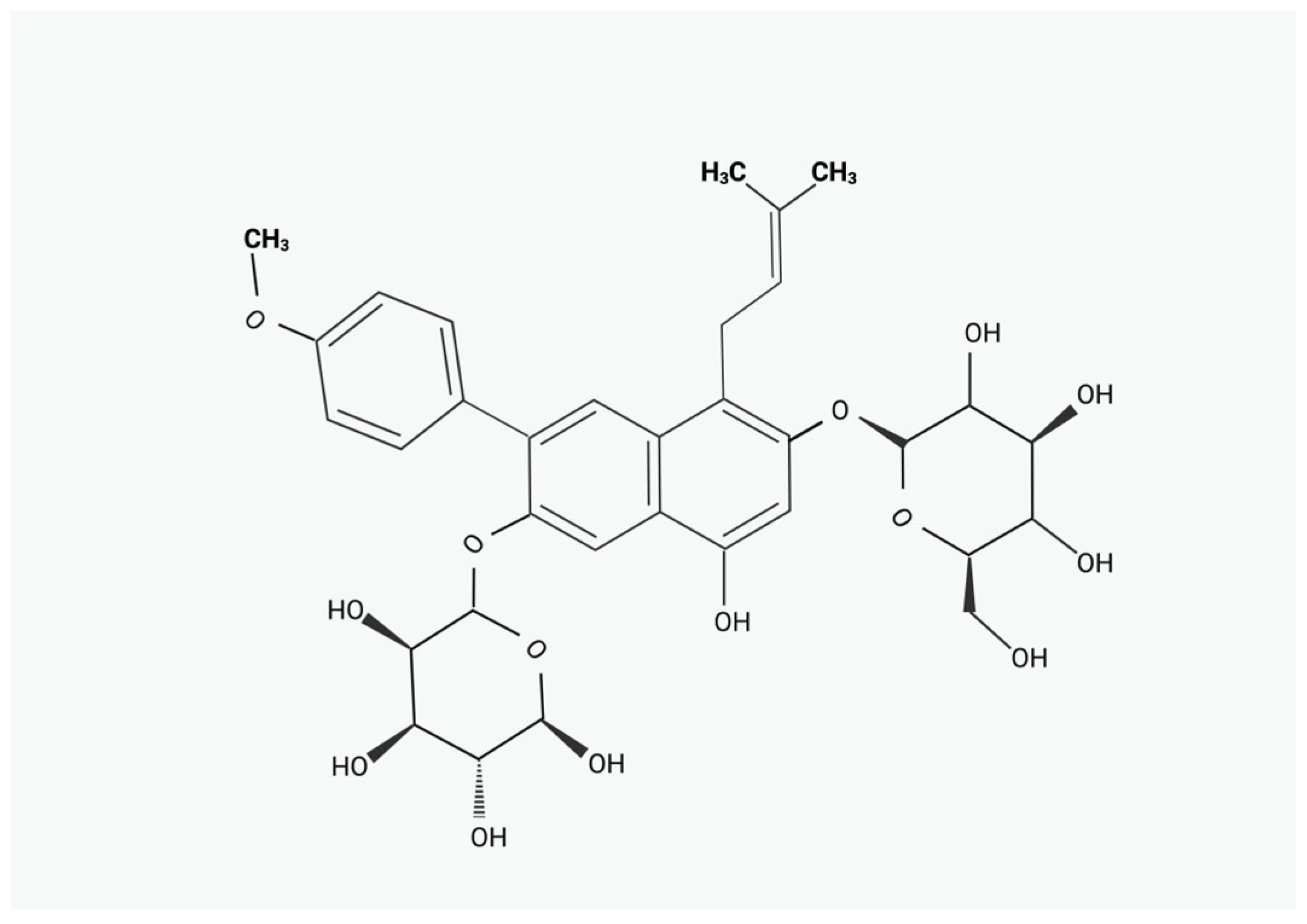
Icariin as a Treatment Proposal in Mammalian Reproduction
Abstract
:1. Introduction
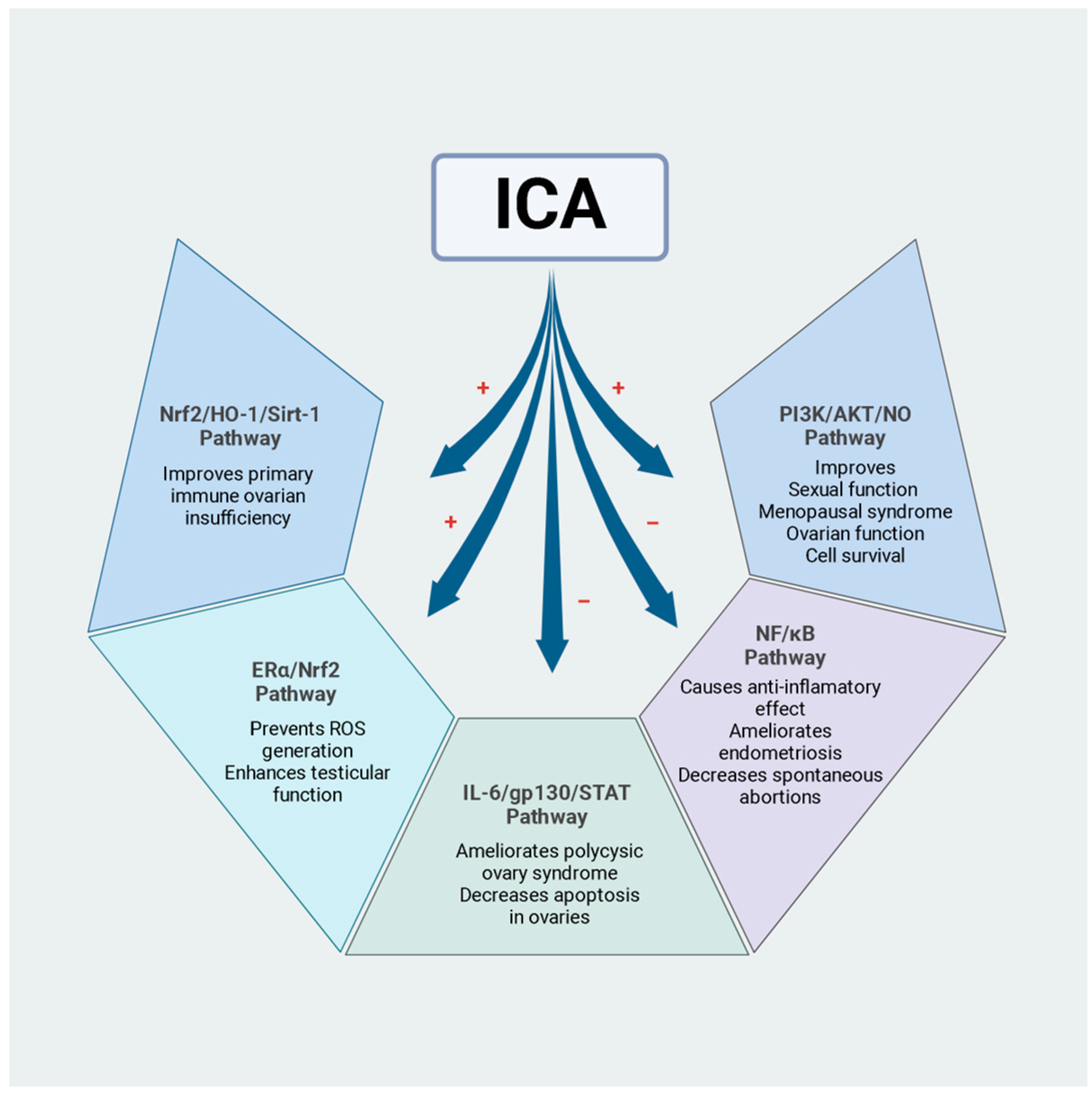
2. Effects of ICA on Male Reproductive Function
Mechanisms of Action of ICA in the Protection of Male Reproduction
| Type of Study | Model | Dose and Duration of Treatment | Main Findings | Refs. |
|---|---|---|---|---|
| In vivo | Male Wistar rats | 1 and 5 mg/kg/4 weeks | ICA significantly increased the intracavernous pressure, the percent of smooth muscle, and the expression of neuronal and inducible nitric oxide synthase in castrated rats. | [26] |
| In vitro | Human spermatozoa | 0.001, 0.01, 0.1 μg/mL/45 min | ICA protected human sperm cells from being damaged by FeSO4/hydrogen peroxide. Also, ICA increased the activity of the sperm enzymes lactate dehydrogenase and superoxide dismutase compared to sperm cells treated with FeSO4/hydrogen peroxide. | [32] |
| In vivo | Male Sprague–Dawley rats | 45 mg/kg ICA, 110 mg/kg zinc 60 mg/kg ICA, 110 mg/kg zinc 180 mg/kg ICA, 110 mg/kg zinc | The ICA–zinc complex promoted development in male rats, improved their excitability, promoted their recovery from the fatigue state, and enhanced their anti-fatigue ability. The ICA–zinc complex increased the plasma testosterone concentration and the testicular weight, indicating improved function. | [41] |
| In vivo | ICR male mice | 50, 100, and 200 mg/kg, 21 days | Increased serum testosterone and nitric oxide concentrations, hypothalamic dopamine and 5-hydroxy-tryptophan levels, and endothelial nitric oxide, phosphatidylinositol tallow alcohol 3-kinase, and phosphorylated protein kinase expression in penile tissues. | [34] |
| In vivo | Male Sprague–Dawley rats | 50, 100, and 200 mg/kg, 35 days | 100 mg/kg ICA significantly increased the epididymal sperm counts. 50 and 100 mg/kg ICA significantly increased the testosterone levels. 100 mg/kg ICA treatment also increased follicle-stimulating hormone receptor (FSHR) and claudin-11 mRNA expression in Sertoli cells. | [35] |
| In vivo | Male Sprague–Dawley rats | 80 mg/kg, 42 days | ICA effectively attenuated male reproductive dysfunctions and spermatogenesis deficiencies induced by diabetes mellitus. ICA may ameliorate diabetes mellitus-induced spermatogenesis deficiency possibly through increasing proliferation and inhibiting intrinsic mitochondria-dependent apoptotic pathways of spermatogonia, primary spermatocytes, and Sertoli cells, respectively. ICA may be a potentially novel therapeutic agent for the protection and treatment of testicular damage in diabetes mellitus. | [36] |
| In vitro In vivo | Mouse Leydig cells ICR male mice | 0.2, 1, and 5 µg/mL 3 h pre-treatment/12 h culture 50, 100, and 150 mg/kg, 28 days | ICA reversed the adverse effect of di(2-ethylhexyl) phthalate (DEHP) on Leydig cell proliferation, decreased the reactive oxygen species levels, and elevated the Δψm levels. Additionally, ICA promoted testosterone production and upregulated the expression of transcription factor (SF-1) and steroidogenic enzymes. ICA reversed the deleterious effects caused by DEHP on the epididymal sperm count and the seminiferous tubules. | [37] |
| In vivo In vitro | Male Sprague–Dawley rats Sertoli cell line TM4 | 2 and 6 mg/kg, 4 months 0.5 and 1 µM, 20 h | ICA significantly increased the testicular and epididymal weights and their indices in ageing rats. Furthermore, ICA increased sperm count and sperm viability. ICA protected against Sertoli cell injury due to age-related testicular dysfunction by upregulating the ERa/Nrf2 signalling pathway. | [38] |
| In vivo | Male Kunming mice | 75 mg/kg, 35 days | ICA improved the reduction in sperm density, hormone levels, and antioxidant enzyme activity seen in nicotine-treated mice. | [39] |
| In vivo In vitro | Male C57BL/6J Sertoli cell line TM4 | 80 mg/kg, 12 weeks 1 µM ICA plus 0.4 mM palmitic acid, 24 h | ICA treatment improved the histopahological changes and decreased apoptosis in the testes of obese mice. It increased the expression of Bcl2, PCNA, WT1, GATA4, and vimentin. ICA improved the decreases in the lactate levels, the increases in pyruvate production, and the expression of HK2, PKM2, and LDHA induced by palmitic acid in TM4 cells. | [40] |
3. Effects of ICA on Female Reproductive Function
Mechanisms of Action of ICA in the Protection of Female Reproduction in In Vivo Models
| Type of Study | Model | Dose and Duration of Treatment | Main Findings | Refs. |
|---|---|---|---|---|
| In vivo In vitro | Sprague–Dawley female rats MCF-7, Ishikawa, SH-SY5Y, and MG-63 cells | 50, 500, and 3000 mg/Kg, 3 months 10−5–10−12 M | ICA prevented oestrogen deficiency-induced osteoporotic changes in the bone structure, bone mineral density, and trabecular properties in the bone of ovariectomized rats. Moreover, it regulated the transcriptional events of the oestrogen-responsive genes related to bone remodelling and prevented the action of dopaminergic neurons against ovariectomized-induced changes. ICA exerted oestrogen-like activities and regulated the expression of oestrogen-responsive genes. | [21] |
| In vitro | Granulosa cells | 5, 10, 25, 50, and 75 µg/L for 72 h | Ovarian granulosa cell proliferation and progesterone and oestrogen release were both markedly enhanced by ICA (10 μg/L). Furthermore, ICA increased CYP17 and CYP19 mRNA and protein expression. | [42] |
| In vitro | Mouse embryos | 10, 20, 40, and 80 µM for 92.5 h | Decreased generation of reactive oxygen species and increased mitochondrial membrane potential were the two ways in which the ICA (40 µM) treatment fixed the negative effects of hydrogen peroxide on embryonic development. | [43] |
| In vivo In vitro | C57BL/6 female mice Ovarian granulosa cells | 10, 50, and 100 mg/kg, 42 days 1, 10 μM, 6 h | ICA promoted ovary/body weight, follicle number, and fertility outcomes. Additionally, it downregulated the levels of the follicle-stimulating and luteinizing hormones and upregulated the levels of estradiol and the anti-Müllerian hormone. ICA repaired damaged DNA by changing the expression levels of 53BP1 and γH2AX, suggesting that it provided protection. | [45] |
| In vivo | Kunming white mice, female | 50, 100, and 200 mg/kg | In a mouse model of D-galactose-induced ovarian ageing, icariin improved ovarian follicular development, inhibited follicular atresia, decreased the follicle-stimulating hormone and luteinizing hormone levels, increased estradiol expression, upregulated ovarian anti-Müllerian hormone expression, and increased the Bcl-2/Bax ratio. | [46] |
| In vivo | Male Sprague–Dawley rats | 40 and 80 mg/kg, 30 days | ICA significantly improved ovarian function and reproductive endocrine disorders by regulating sex hormones, restoring the estrous cycle, and reducing ovarian morphological damage in rats with polycystic ovary syndrome. Additionally, ICA improved apoptosis in the ovaries. | [47] |
| In vivo | Sprague–Dawley female rats | 12.5, 25, and 50 mg/kg, 30 days | ICA improved the pathological changes in the ovaries, elevated the serum levels of the female hormone estradiol, as well as testosterone and interleukin (IL)-2, decreased those of the follicle-stimulating hormone and the luteinizing hormone, promoted the expression levels of the oestrogen receptor (ER) and ERα in the hypothalamus, and increased those of serotonin, dopamine, and noradrenaline in the brain homogenate. Furthermore, ICA elevated the expression levels of AKT, phosphorylation-akt (p-AKT), PI3K (110 kDa), PI3K (85 kDa), and B-cell lymphoma 2 (Bcl-2) in the ovaries and inhibited those of Bax. | [48] |
| In vivo | Kunming female mice | 50 mg/kg, three times a day, six hours apart | ICA inhibited the production of pro-inflammatory cytokines (IL-1ß, IL-6, and TNF-α) and boosted the production of anti-inflammatory cytokines (IL-10). Additionally, ICA modulated the LPS-induced expression of malondialdehyde, reactive oxygen species, superoxide dismutase 1, catalase, and glutathione peroxidase 1. Moreover, ICA improved the antioxidant defence system via the activation of the Nrf2 pathway. | [49] |
| In vivo | CBA/J female mice | 50 mg/kg, 12 days | ICA decreased the number of recurrent spontaneous abortions. Furthermore, ICA reduced the expression of pro-inflammatory factors and was able to reduce the expression of a mechanical target of rapamycin (mTOR) in the placenta. | [50] |
| In vitro | Laying hens | 2, 20, and 100 mg/kg, 30 days | ICA significantly improved the laying rate, weight, and egg quality parameters. Additionally, ICA increased the serum follicle-stimulating hormone, luteinizing hormone, and progesterone levels and granulosa cell proliferation. | [51] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Li, J.; Wang, Y.; Liang, Q. Taxonomy of Epimedium (Berberidaceae) with Special Reference to Chinese Species. Chin. Herb. Med. 2021, 14, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.-Q.; Wu, D.-C.; Li, C.-Y.; Liu, X.-R.; Han, X.-K.; Peng, Y.; Zhang, H.; Zhao, B.-Y.; Zhao, Y. A Systematic Review of Traditional Uses, Phytochemistry, Pharmacology and Toxicity of Epimedium Koreanum Nakai. J. Ethnopharmacol. 2024, 318, 116957. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, Y.; Yang, M.; Shen, Z.; Zhang, X.; Zhang, W.; Li, H. LC-MS/MS Method for the Simultaneous Determination of Icariin and Its Major Metabolites in Rat Plasma. J. Pharm. Biomed. Anal. 2007, 45, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.; Xu, F.; Qian, Q.; Cui, L.; Tan, X.; Jia, X. Metabolite Profiles of Icariin in Rat Feces, Bile and Urine by Ultraperformance Liquid-Chromatography/Quadrupole-Time-of-Flight Mass Spectrometry. J. Chromatogr. Sci. 2016, 54, 158–164. [Google Scholar] [CrossRef]
- Wu, H.; Kim, M.; Han, J. Icariin Metabolism by Human Intestinal Microflora. Molecules 2016, 21, 1158. [Google Scholar] [CrossRef]
- Zhao, H.; Fan, M.; Fan, L.; Sun, J.; Guo, D. Liquid Chromatography-Tandem Mass Spectrometry Analysis of Metabolites in Rats after Administration of Prenylflavonoids from Epimediums. J. Chromatogr. B 2010, 878, 1113–1124. [Google Scholar] [CrossRef]
- Wang, T.; Feng, X.; Ding, L.; Wang, K.; Qiao, M.; Chai, L.; Li, Y.; Qiu, F. Metabolic Profiling of Icariin in Rat Feces, Urine, Bile and Plasma after Oral Administration Using Ultra-High Performance Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry. J. Pharm. Biomed. Anal. 2019, 168, 155–162. [Google Scholar] [CrossRef]
- Xu, S.; Yu, J.; Zhan, J.; Yang, L.; Guo, L.; Xu, Y. Pharmacokinetics, Tissue Distribution, and Metabolism Study of Icariin in Rat. BioMed Res. Int. 2017, 2017, 4684962. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Xiong, Y.; Yang, T.; Wang, Y.; Zeng, J.; Zhou, S.; Luo, Y.; Li, L. Icariin and Its Metabolites as Potential Protective Phytochemicals against Cardiovascular Disease: From Effects to Molecular Mechanisms. Biomed. Pharmacother. 2022, 147, 112642. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Zeng, Y.; Zhou, G.; Wang, Y.; Zhou, W.; Sun, X.; Wu, M. Icariin, an Up-and-Coming Bioactive Compound Against Neurological Diseases: Network Pharmacology-Based Study and Literature Review. Drug Des. Devel Ther. 2021, 15, 3619–3641. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, W.; Xu, N.; Li, J.; Chang, W. Icariin Improves Acute Kidney Injury and Proteinuria in a Rat Model of Pregnancy-induced Hypertension. Mol. Med. Rep. 2017, 16, 7398–7404. [Google Scholar] [CrossRef]
- Wang, J.; Tao, Y.; Ping, Z.; Zhang, W.; Hu, X.; Wang, Y.; Wang, L.; Shi, J.; Wu, X.; Yang, H.; et al. Icariin Attenuates Titanium-Particle Inhibition of Bone Formation by Activating the Wnt/β-Catenin Signaling Pathway in Vivo and in Vitro. Sci. Rep. 2016, 6, 23827. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Zhang, W.; Yan, X. Anti-Inflammatory and Immunoregulatory Effects of Icariin and Icaritin. Biomed. Pharmacother. 2022, 151, 113180. [Google Scholar] [CrossRef]
- Niu, Y.; Lin, G.; Pan, J.; Liu, J.; Xu, Y.; Cai, Q.; Wang, T.; Luan, Y.; Chen, Y.; Feng, Y.; et al. Deciphering the Myth of Icariin and Synthetic Derivatives in Improving Erectile Function from a Molecular Biology Perspective: A Narrative Review. Transl. Androl. Urol. 2022, 11, 1007–1022. [Google Scholar] [CrossRef]
- Wang, P.; Meng, Q.; Wang, W.; Zhang, S.; Xiong, X.; Qin, S.; Zhang, J.; Li, A.; Liu, Z. Icariin Inhibits the Inflammation through Down-Regulating NF-κB/HIF-2α Signal Pathways in Chondrocytes. Biosci. Rep. 2020, 40, BSR20203107. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Y. Icariin, an Anti-Atherosclerotic Drug from Chinese Medicinal Herb Horny Goat Weed. Front. Pharmacol. 2017, 8, 734. [Google Scholar] [CrossRef]
- Wong, K.-Y.; Kong, T.-H.; Poon, C.C.-W.; Yu, W.; Zhou, L.; Wong, M.-S. Icariin, a Phytoestrogen, Exerts Rapid Estrogenic Actions through Crosstalk of Estrogen Receptors in Osteoblasts. Phytother. Res. 2023, 37, 4706–4721. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.; Li, F.-S.; Levsh, O.; Weng, J.-K. Exploration of Icariin Analog Structure Space Reveals Key Features Driving Potent Inhibition of Human Phosphodiesterase-5. PLoS ONE 2019, 14, e0222803. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Guo, Y.; Ma, R.; Fu, M.; Niu, J.; Gao, S.; Zhang, D. Herba Epimedii: An Ancient Chinese Herbal Medicine in the Prevention and Treatment of Osteoporosis. Curr. Pharm. Des. 2016, 22, 328–349. [Google Scholar] [CrossRef]
- Jaroenporn, S.; Malaivijitnond, S.; Wattanasirmkit, K.; Trisomboon, H.; Watanabe, G.; Taya, K.; Cherdshewasart, W. Effects of Pueraria Mirifica, an Herb Containing Phytoestrogens, on Reproductive Organs and Fertility of Adult Male Mice. Endocrine 2006, 30, 93–101. [Google Scholar] [CrossRef]
- Zhou, L.; Poon, C.C.-W.; Wong, K.-Y.; Cao, S.; Yu, W.; Dong, X.; Lee, W.Y.-W.; Zhang, Y.; Wong, M.-S. Prenylflavonoid Icariin Induces Estrogen Response Element-Independent Estrogenic Responses in a Tissue-Selective Manner. J. Endocr. Soc. 2020, 4, bvz025. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.-G.; Chen, K.-M.; Xian, C.J. Functions and Action Mechanisms of Flavonoids Genistein and Icariin in Regulating Bone Remodeling. J. Cell. Physiol. 2013, 228, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-Y.; Ding, D.-N.; Wang, Y.-R.; Liu, S.-X.; Peng, C.; Shen, F.; Zhu, X.-Y.; Li, C.; Tang, L.-P.; Han, F.-J. Icariin as a Potential Anticancer Agent: A Review of Its Biological Effects on Various Cancers. Front. Pharmacol. 2023, 14, 1216363. [Google Scholar] [CrossRef]
- Zhang, C.; Sui, X.; Jiang, Y.; Wang, X.; Wang, S. Antitumor Effects of Icaritin and the Molecular Mechanisms. Discov. Med. 2020, 29, 5–16. [Google Scholar]
- Chuang, Y.; Van, I.; Zhao, Y.; Xu, Y. Icariin Ameliorate Alzheimer’s Disease by Influencing SIRT1 and Inhibiting Aβ Cascade Pathogenesis. J. Chem. Neuroanat. 2021, 117, 102014. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Xin, Z.-C.; Xin, H.; Yuan, Y.-M.; Tian, L.; Guo, Y.-L. Effects of Icariin on Erectile Function and Expression of Nitric Oxide Synthase Isoforms in Castrated Rats. Asian J. Androl. 2005, 7, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Xin, Z.-C.; Lin, G.; Banie, L.; Lue, T.F.; Lin, C.-S. Effects of Icariin on Phosphodiesterase-5 Activity in Vitro and Cyclic Guanosine Monophosphate Level in Cavernous Smooth Muscle Cells. Urology 2006, 68, 1350–1354. [Google Scholar] [CrossRef]
- Xin, Z.C.; Kim, E.K.; Lin, C.S.; Liu, W.J.; Tian, L.; Yuan, Y.M.; Fu, J. Effects of Icariin on cGMP-Specific PDE5 and cAMP-Specific PDE4 Activities. Asian J. Androl. 2003, 5, 15–18. [Google Scholar]
- Masciarelli, S.; Horner, K.; Liu, C.; Park, S.H.; Hinckley, M.; Hockman, S.; Nedachi, T.; Jin, C.; Conti, M.; Manganiello, V. Cyclic Nucleotide Phosphodiesterase 3A–Deficient Mice as a Model of Female Infertility. J. Clin. Investig. 2004, 114, 196–205. [Google Scholar] [CrossRef]
- Sharpe, A.; Bhandari, H.; Miller, D. Is There a Role for Phosphodiesterase Inhibitors in the Treatment of Male Subfertility? Hum. Fertil. 2022, 25, 13–23. [Google Scholar] [CrossRef]
- Lefièvre, L.; de Lamirande, E.; Gagnon, C. Presence of Cyclic Nucleotide Phosphodiesterases PDE1A, Existing as a Stable Complex with Calmodulin, and PDE3A in Human Spermatozoa. Biol. Reprod. 2002, 67, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-S.; Xiao, H.-J.; Qi, T.; Hu, Z.-M.; Li, H.; Chen, D.-L.; Xu, Y.-L.; Chen, J. Antioxidative Protective Effect of Icariin on the FeSO4/H 2O 2-Damaged Human Sperm Based on Confocal Raman Micro-Spectroscopy. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-L.; Hu, S.-H.; Wan, Z.-S.; Bu, W.-Z.; Chen, S.-Q.; Han, T.-H.; Lu, Y.-Q. Effect of Icariin on the Transformation Efficiency of Induced Pluripotent Stem Cells into Sperm Cells in Vitro. Rev. Int. Androl. 2023, 21, 100373. [Google Scholar] [CrossRef]
- Ding, J.; Tang, Y.; Tang, Z.; Zu, X.; Qi, L.; Zhang, X.; Wang, G. Icariin Improves the Sexual Function of Male Mice through the PI3K/AKT/eNOS/NO Signalling Pathway. Andrologia 2018, 50, e12802. [Google Scholar] [CrossRef]
- Chen, M.; Hao, J.; Yang, Q.; Li, G. Effects of Icariin on Reproductive Functions in Male Rats. Molecules 2014, 19, 9502–9514. [Google Scholar] [CrossRef]
- He, W.; Liu, H.; Hu, L.; Wang, Y.; Huang, L.; Liang, A.; Wang, X.; Zhang, Q.; Chen, Y.; Cao, Y.; et al. Icariin Improves Testicular Dysfunction via Enhancing Proliferation and Inhibiting Mitochondria-Dependent Apoptosis Pathway in High-Fat Diet and Streptozotocin-Induced Diabetic Rats. Reprod. Biol. Endocrinol. 2021, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, D.; Lin, J.; Liu, Y.; Xu, L.; Lv, R.; Mo, K.; Lian, X.; Xie, M.; Xu, S.; et al. Icariin Protects Mouse Leydig Cell Testosterone Synthesis from the Adverse Effects of Di(2-Ethylhexyl) Phthalate. Toxicol. Appl. Pharmacol. 2019, 378, 114612. [Google Scholar] [CrossRef]
- Zhao, H.; You, X.; Chen, Q.; Yang, S.; Ma, Q.; He, Y.; Liu, C.; Dun, Y.; Wu, J.; Zhang, C.; et al. Icariin Improves Age-Related Testicular Dysfunction by Alleviating Sertoli Cell Injury via Upregulation of the ERα/Nrf2-Signaling Pathway. Front. Pharmacol. 2020, 11, 677. [Google Scholar] [CrossRef]
- Ni, G.; Zhang, X.; Afedo, S.Y.; Rui, R. Evaluation of the Protective Effects of Icariin on Nicotine-Induced Reproductive Toxicity in Male Mouse -a Pilot Study. Reprod. Biol. Endocrinol. 2020, 18, 65. [Google Scholar] [CrossRef]
- Luo, M.; Zhuge, X.; Ji, L.; Wang, J.; Mo, Y.; Tan, Y.; Zhou, L.; Lei, X.; Huang, H. Icariin Ameliorates Spermatogenesis Disorder in Obese Mice Induced by High-Fat Diet through Regulating the Glycolytic Pathway. Mol. Nutr. Food Res. 2023, 67, e2200524. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Liu, A.; Ji, Q.; Ren, L.; Ma, C.; Zhang, H.; Wu, C.; Zhang, D.; Shang, M.; et al. Synthesis of Icariin-Zinc and Its Protective Effect on Exercise Fatigue and Reproductive System Related Glands in Male Rats. Front. Pharmacol. 2021, 12, 611722. [Google Scholar] [CrossRef]
- Nie, X.; Sheng, W.; Hou, D.; Liu, Q.; Wang, R.; Tan, Y. Effect of Hyperin and Icariin on Steroid Hormone Secretion in Rat Ovarian Granulosa Cells. Clin. Chim. Acta 2019, 495, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Xu, S.; Liu, Y.; Pang, L.; Lian, X.; Zhong, Y.; Su, Y.; Wang, S. Protective Effect of Icariin on the Development of Preimplantation Mouse Embryos against Hydrogen Peroxide-Induced Oxidative Injury. Oxid. Med. Cell. Longev. 2017, 2017, 2704532. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Song, L.; Xu, X.; Han, Z.; Peng, F.; Zhang, Q.; Liu, C.; Liang, X. The Effect of Icariin on Autoimmune Premature Ovarian Insufficiency via Modulation of Nrf2/HO-1/Sirt1 Pathway in Mice. Reprod. Biol. 2022, 22, 100638. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, J.; Wang, X.; Sun, J.; Li, Z. Icariin Exerts a Protective Effect against D-Galactose Induced Premature Ovarian Failure via Promoting DNA Damage Repair. Biomed. Pharmacother. 2019, 118, 109218. [Google Scholar] [CrossRef]
- Wang, J.-L.; Liu, B.; Zhang, C.; Wang, X.-M.; Zhen, D.; Huang, X.-M.; Chen, W.; Gao, J.-M. Effects of Icariin on Ovarian Function in D-Galactose-Induced Aging Mice. Theriogenology 2019, 125, 157–167. [Google Scholar] [CrossRef]
- Zuo, L.; Hai, Y.; Zhang, R.; Zuo, B.; Tian, J.; Li, P.; Ke, X.; Wang, M.; Ren, L.; Li, X.; et al. Therapeutic Potential of Icariin in Rats with Letrozole and High-Fat Diet-Induced Polycystic Ovary Syndrome. Eur. J. Pharmacol. 2023, 953, 175825. [Google Scholar] [CrossRef]
- Cao, L.-H.; Qiao, J.-Y.; Huang, H.-Y.; Fang, X.-Y.; Zhang, R.; Miao, M.-S.; Li, X.-M. PI3K-AKT Signaling Activation and Icariin: The Potential Effects on the Perimenopausal Depression-Like Rat Model. Molecules 2019, 24, 3700. [Google Scholar] [CrossRef]
- Shaukat, A.; Shaukat, I.; Rajput, S.A.; Shukat, R.; Hanif, S.; Huang, S.; Aleem, M.T.; Li, K.; Li, Q.; Chen, C.; et al. Icariin Alleviates Escherichia Coli Lipopolysaccharide-Mediated Endometritis in Mice by Inhibiting Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 10219. [Google Scholar] [CrossRef]
- Peng, F.; Han, Z.; Chen, H.; Zhang, Q.; Liu, C.; Liang, X. The Effects of Treatment with Icariin on Immune Tolerance in the Recurrent Spontaneous Abortion Mice. Reprod. Sci. 2023, 30, 2794–2804. [Google Scholar] [CrossRef]
- Ding, W.; Shangguan, L.; Li, H.; Bao, Y.; Noor, F.; Haseeb, A.; Sun, P.; Zhang, H.; Yin, W.; Fan, K.; et al. Dietary Supplementation of Osthole and Icariin Improves the Production Performance of Laying Hens by Promoting Follicular Development. Poult. Sci. 2024, 103, 103579. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zheng, N.; Zhan, X.; He, J.; Xiao, M.; Zuo, Z.; He, C. Icariin Induces Developmental Toxicity via Thyroid Hormone Disruption in Zebrafish Larvae. Food Chem. Toxicol. 2023, 182, 114155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Gutiérrez, M.; Izquierdo-Vega, A.J.; Madrigal-Santillán, E.O.; Velázquez-González, C.; Izquierdo-Vega, J.A. Icariin as a Treatment Proposal in Mammalian Reproduction. Pharmaceuticals 2024, 17, 1104. https://doi.org/10.3390/ph17091104
Sánchez-Gutiérrez M, Izquierdo-Vega AJ, Madrigal-Santillán EO, Velázquez-González C, Izquierdo-Vega JA. Icariin as a Treatment Proposal in Mammalian Reproduction. Pharmaceuticals. 2024; 17(9):1104. https://doi.org/10.3390/ph17091104
Chicago/Turabian StyleSánchez-Gutiérrez, Manuel, Aleli Julieta Izquierdo-Vega, Eduardo Osiris Madrigal-Santillán, Claudia Velázquez-González, and Jeannett Alejandra Izquierdo-Vega. 2024. "Icariin as a Treatment Proposal in Mammalian Reproduction" Pharmaceuticals 17, no. 9: 1104. https://doi.org/10.3390/ph17091104





