The Cardioprotective Potential of Herbal Formulas in Myocardial Infarction-Induced Heart Failure through Inhibition of JAK/STAT3 Signaling and Improvement of Cardiac Function
Abstract
:1. Introduction
2. Results
2.1. Chemical Characterization and Composition of DHT
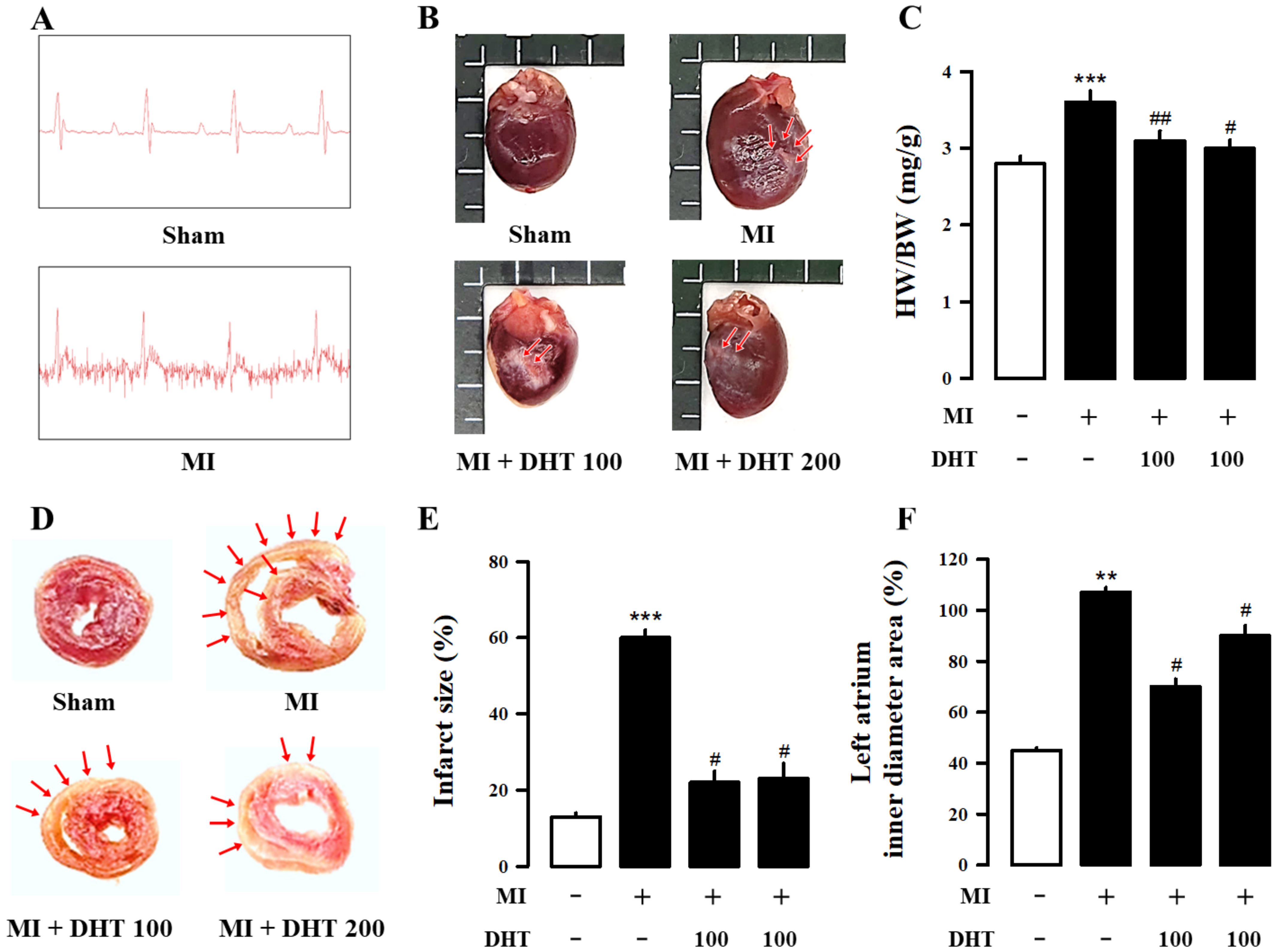
2.2. Effect of DHT on Infarct Size and Electrocardiogram Changes in MI-Induced Heart Failure Rats
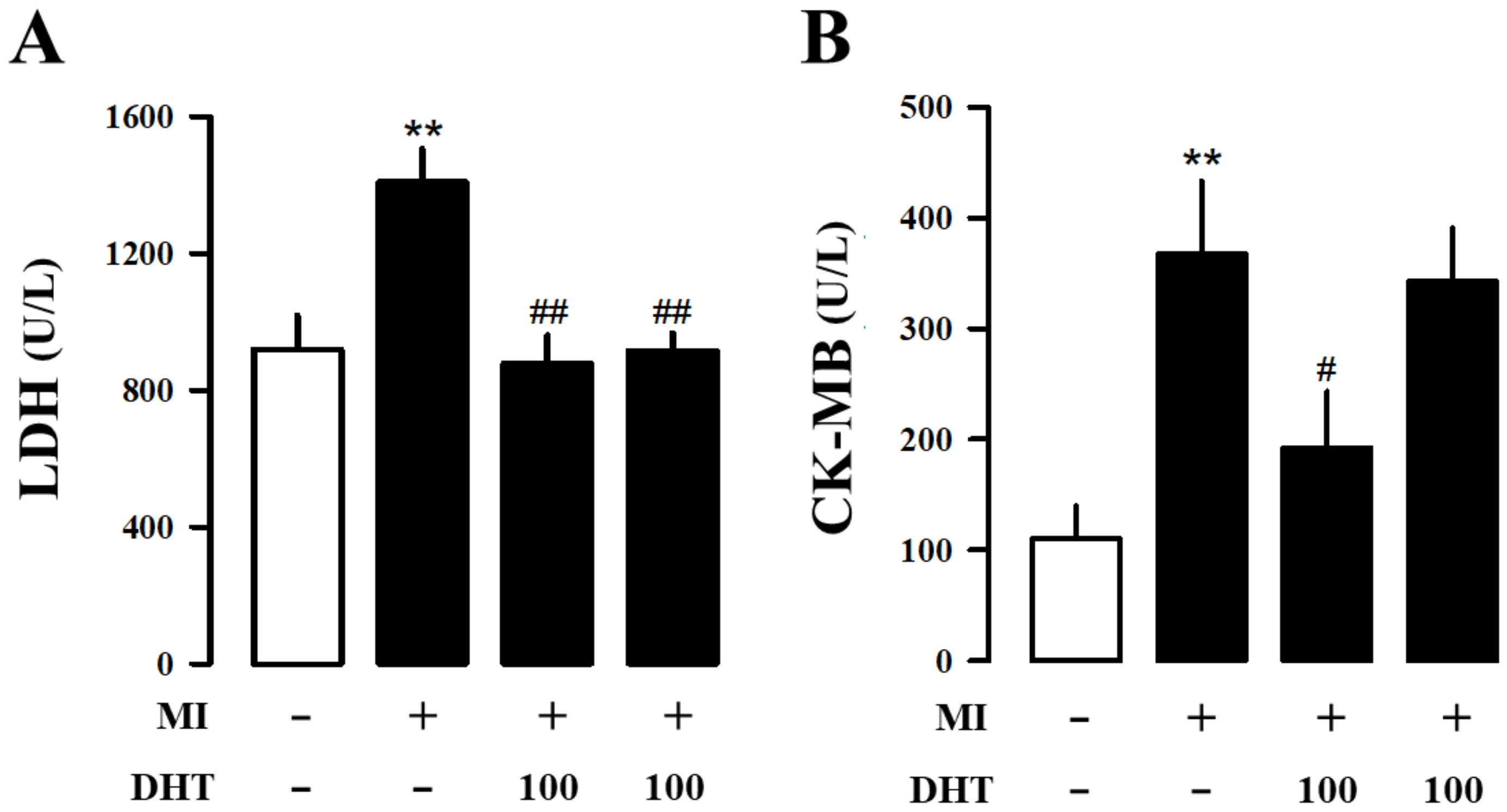
2.3. Effects of DHT on Hematology and Serum Biochemical Analysis in MI-Induced Heart Failure Rats
2.4. Effect of DHT on Cardiac Dysfunction in MI-Induced Heart Failure Rats
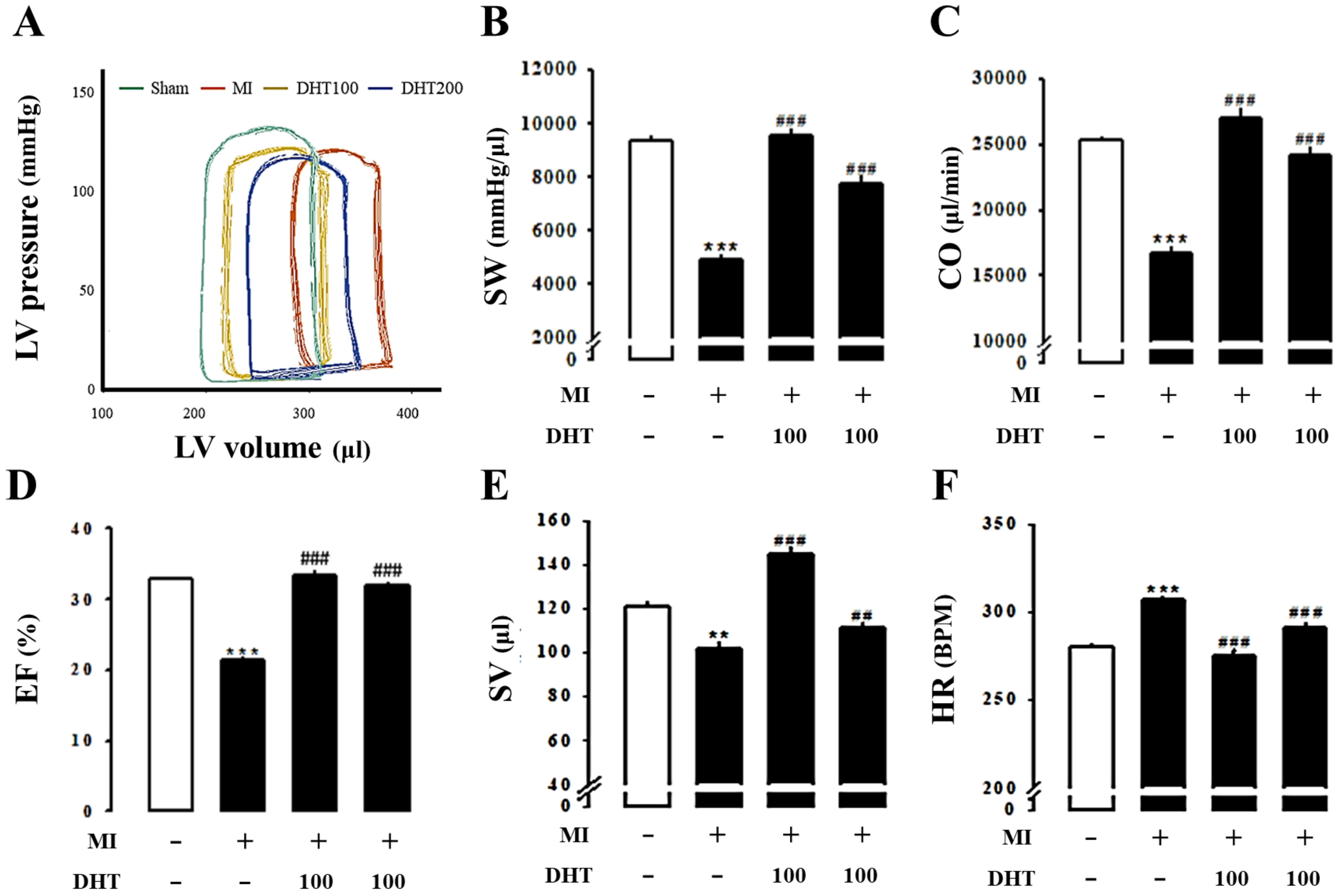
2.5. Effect of DHT on Hemodynamic Parameters in MI-Induced Heart Failure Rats
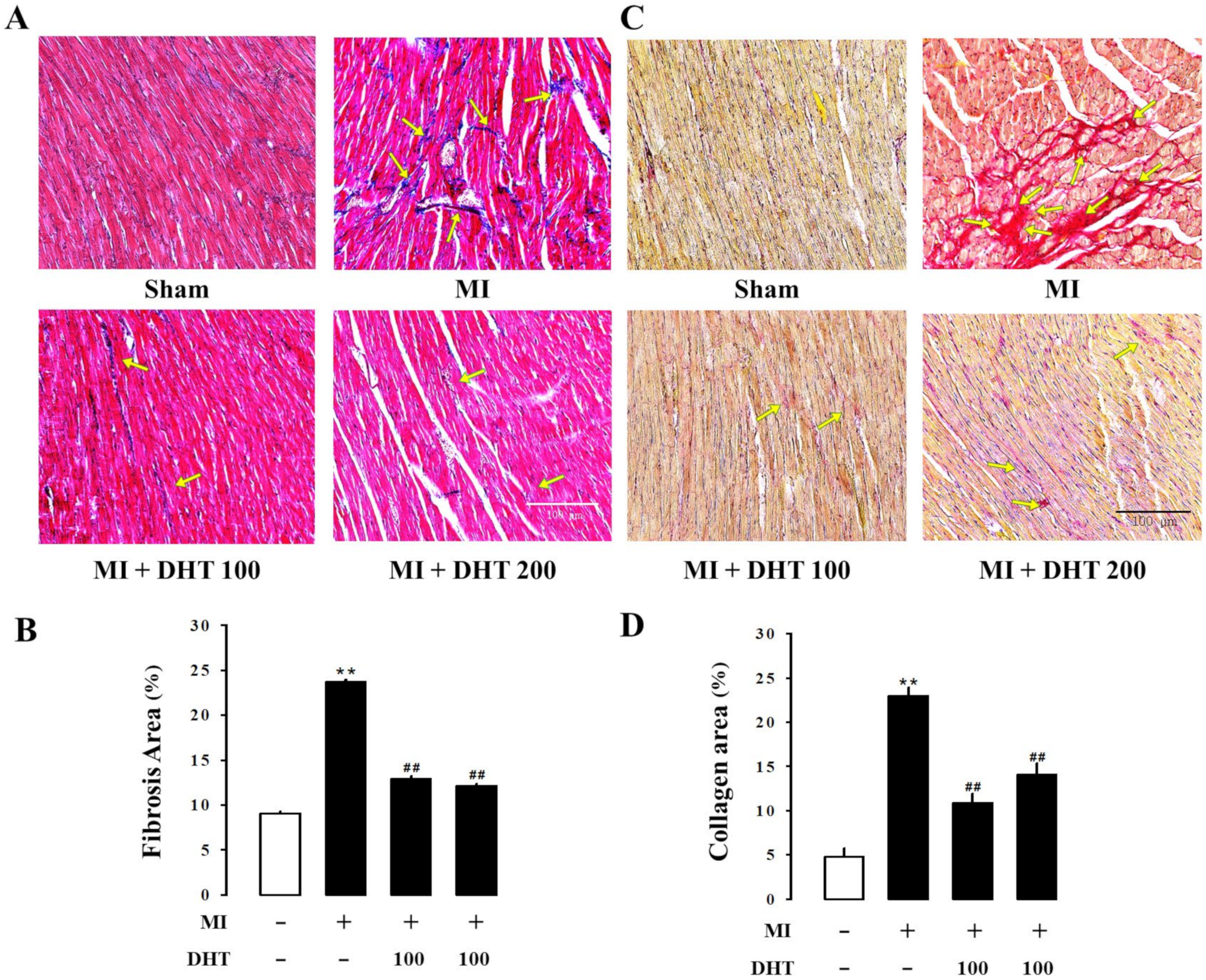
2.6. Effect of DHT on Histopathological Changes in MI-Induced Heart Failure Rats
2.7. Effect of DHT on JAK/STAT3 Signaling in MI-Induced Heart Failure Rats
3. Discussion
4. Materials and Methods
4.1. Preparation of DHT and UPLC/QE Orbitrap MS Analysis of DHT Compounds
4.2. Myocardial Infarction-Induced Heart Failure Animal Model
4.3. Assessment of Electrocardiography
4.4. Assessment of Cardiac Injury Biomarkers
4.5. Determination of Myocardial Infarct Size
4.6. Assessment of Cardiac Function via Echocardiography
4.7. Assessment of Cardiac Function via Pressure-Volume Loop
4.8. Histological Analysis
4.9. Western Blot Evaluation
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.J.; Jeong, H.J.; Park, S.U.; Moon, B.S.; Moon, P.D.; An, N.H.; Lee, K.M.; Hong, S.H. Anti-inflammatory effect of dohongsamultang through inhibition of nuclear factor-kappaB activation in peripheral blood mononuclear cells of patients with cerebral infarction. Am. J. Chin. Med. 2007, 35, 415–426. [Google Scholar] [CrossRef]
- Shim, E.H.; Lee, H.; Lee, M.S.; You, S. Anti-adipogenic effects of the traditional herbal formula Dohongsamul-tang in 3T3-L1 adipocytes. BMC Complement. Altern. Med. 2017, 17, 542. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Kurth, T.; Hu, H.; Santanello, N.; Lipton, R.B. Migraine and cardiovascular disease: Possible mechanisms of interaction. Neurology 2009, 72, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.H.; Jang, Y.J.; Yoon, J.J.; Lee, H.S.; Kim, H.Y.; Kang, D.G. Dohongsamul-tang inhibits cardiac remodeling and fibrosis through calcineurin/NFAT and TGF-β/Smad2 signaling in cardiac hypertrophy. J. Ethnopharmacol. 2024, 318 Pt A, 116844. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, L.; Liu, K.; Liang, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. The antihypertensive potential of flavonoids from Chinese Herbal Medicine: A review. Pharmacol. Res. 2021, 174, 105919. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Mangels, D.R.; Mohler, E.R., 3rd. Catechins as Potential Mediators of Cardiovascular Health. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 757–763. [Google Scholar] [CrossRef]
- Sun, X.; Zeng, H.; Wang, Q.; Yu, Q.; Wu, J.; Feng, Y.; Deng, P.; Zhang, H. Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-kB pathway. Exp. Cell Res. 2018, 369, 112–119. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, Y.; Zhu, L.; Ying, Y.; Hao, W.; Wang, L.; He, L.; Zhao, D.; Chen, J.X.; Gao, Y.; et al. Liquiritin apioside alleviates colonic inflammation and accompanying depression-like symptoms in colitis by gut metabolites and the balance of Th17/Treg. Phytomedicine 2023, 120, 155039. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef]
- Khan, A.K.; Rashid, R.; Fatima, N.; Mahmood, S.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological activities of protocatechuic acid. Acta. Pol. Pharm. 2015, 72, 643–650. [Google Scholar] [PubMed]
- Wang, X.Z.; Wang, T.; Wang, Y.Z.; Li, X.; Chen, Q.; Wang, Y.; Zhang, X.; Wang, H.X.; Zhao, H.; Mou, Y.; et al. Research progress on classical traditional Chinese medicine Taohong Siwu decoction in the treatment of coronary heart disease. Biomed. Pharmacother. 2022, 152, 113249. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.C.; Liang, Q.E.; Tu, W.Q.; Xie, T.; Lam, L.; Chen, L.G. The effect of Taohong Siwu decoction combined with antihypertensive medicine in the treatment of hypertension: Meta-analysis. Medicine 2022, 101, e32133. [Google Scholar] [CrossRef] [PubMed]
- Minicucci, M.F.; Azevedo, P.S.; Polegato, B.F.; Paiva, S.A.R.; Zornoff, A.M.L. Heart Failure after Myocardial Infarction: Clinical Implications and Treatment. Clin. Cardiol. 2011, 34, 410–414. [Google Scholar] [CrossRef]
- Velagaleti, R.; Vasan, R.S. Heart Failure in the 21st Century: Is it a Coronary Artery Disease Problem or Hypertension Problem? Cardiol. Clin. 2007, 25, 487–495. [Google Scholar] [CrossRef]
- Schirone, L.; Forte, M.; Palmerio, S.; Yee, D.; Nocella, C.; Angelini, F.; Pagano, F.; Schiavon, S.; Bordin, A.; Carrizzo, A.; et al. A Review of the Molecular Mechanisms Underlying the Development and Progression of Cardiac Remodeling. Oxid. Med. Cell Longev. 2017, 2017, 3920195. [Google Scholar] [CrossRef]
- Martin, G.S.; John, S.; Norman, S. Left Ventricular Remodeling After Myocardial Infarction. Circulation 2000, 101, 2981–2988. [Google Scholar]
- Ilayaraja, M.; Marleen, L.; Frank, J.; Bart, D.G. Permanent Ligation of the Left Anterior Descending Coronary Artery in Mice: A Model of Post-myocardial Infarction Remodeling and Heart Failure. J. Vis. Exp. 2014, 94, 52206. [Google Scholar]
- Michael, L.H.; Entman, M.L.; Hartley, C.J.; Youker, K.A.; Zhu, J.; Hall, S.R.; Hawkins, H.K.; Berens, K.; Ballantyne, C.M. Myocardial Ischemia and Reperfusion: A Murine Model. Am. J. Physiol. 1995, 269 Pt 2, H2147–H2154. [Google Scholar] [CrossRef]
- Feng, Y.; Hemmeryckx, B.; Frederix, L.; Lox, M.; Wu, J.; Heggermont, W.; Lu, H.R.; Gallacher, D.; Oyen, R.; Lijnen, H.R.; et al. Monitoring Reperfused Myocardial Infarction with Delayed Left Ventricular Systolic Dysfunction in Rabbits by Longitudinal Imaging. Imaging Med. Surg. 2018, 8, 754–769. [Google Scholar] [CrossRef]
- Gheorghiad, M.; Fonarow, G.C. Management of Post-Myocardial Infarction Patients with Left Ventricular Systolic Dysfunction. Am. J. Med. 2007, 120, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Brienesse, S.C.; Davies, A.J.; Khan, A.; Boyle, A.J. Prognostic Value of left ventricular end-diastolic volume and pressure (LVEDP) in Acute Myocardial Infarction: A Systematic Review and Meta-Analysis. J. Cardiovasc. Transl. Res. 2018, 11, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Sjöblom, J.; Muhrbeck, J.; Witt, N.; Alam, M.; Frykman-Kull, V. Evolution of Left Ventricular Ejection Fraction after Acute Myocardial Infarction. Circulation 2014, 130, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Chemaly, R.E.; Tilemann, L.; Fish, K.; Ladage, D.; Aguero, J.; Vahl, T.; Santos-Gallego, C.; Kawase, Y.; Hajjar, R.J. Assessing Left Ventricular Systolic Dysfunction after Myocardial Infarction: Are Ejection Fraction and dP/dtmax Complementary or Redundant? Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1423–H1428. [Google Scholar] [CrossRef]
- French, B.A.; Kramer, M.C. Mechanisms of Post-Infarct Left Ventricular Remodeling. Today Dis. Mech. 2007, 4, 185–196. [Google Scholar] [CrossRef]
- Horn, M.A.; Trafford, A.W. Aging and the Cardiac Collagen Matrix: Novel Mediators of Fibrotic Remodeling. J. Mol. Cell Cardiol. 2016, 93, 175–185. [Google Scholar] [CrossRef]
- Lubitz, S.A.; Benjamin, E.J.; Ellinor, P.T. Atrial Fibrillation in Congestive Heart Failure. Heart Fail. Clin. 2011, 6, 187–200. [Google Scholar] [CrossRef]
- Igaz, P.; Tóth, S.; Falus, A. Biological and Clinical Significance of the JAK-STAT Pathway: Lessons from Knockout Mice. Inflamm. Res. 2001, 50, 435–441. [Google Scholar] [CrossRef]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The Molecular Details of Cytokine Signaling Via the JAK/STAT Pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. The Role of the TGF-beta Superfamily in Myocardial Infarction. Front. Cardiovasc. Med. 2019, 6, 140. [Google Scholar] [CrossRef]
- Borreguero, L.J.J.; Salmerón, R.R. Assessment of Myocardial Viability in Patients before Revascularization. Rev. Esp. Cardiol. 2003, 56, 721–733. [Google Scholar]
- Báez-Ferrer, N.; Izquierdo-Gómez, M.M.; Marí-López, B.; Montoto-López, J.; Duque-Gómez, A.; García-Niebla, J.; Miranda-Bacallado, J.; de la Rosa Hernández, A.; Laynez-Cerdeña, I.; Lacalzada-Almeida, J. Clinical Manifestations, Diagnosis, and Treatment of Ischemic Mitral Regurgitation: A Review. J. Thorac. Dis. 2018, 10, 6969–6986. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yin, X.; Wijaya, C.; Huang, M.H.; McConnell, B.K. Acute Myocardial Infarction in Rats. J. Vis. Exp. 2011, 48, 2464. [Google Scholar]
- Greig, D.; Austin, P.C.; Zhou, L.; Tu, J.V.; Pang, P.S.; Ross, H.J.; Lee, D.S. Ischemic electrocardiographic abnormalities and prognosis in decompensated heart failure. Circ. Heart Fail. 2014, 7, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ceholski, D.K.; Liang, L.; Fish, K.; Hajjar, R.J. Variability in Coronary Artery Anatomy Affects Consistency of Cardiac Damage after Myocardial Infarction in Mice. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, 275–282. [Google Scholar] [CrossRef]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair after Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Goldman, S.; Raya, T.E. Rat Infarct Model of Myocardial Infarction and Heart Failure. J. Card. Fail. 1995, 1, 169–177. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Chilian, W.; Crea, F.; Davidson, S.M.; Ferdinandy, P.; Garcia-Dorado, D.; van Royen, N.; Schulz, R.; Heusch, G. The coronary circulation in acute myocardial ischaemia/reperfusion injury: A target for cardioprotection. Cardiovasc. Res. 2019, 115, 1143–1155. [Google Scholar] [CrossRef]
- Heusch, G. The Coronary Circulation as a Target of Cardioprotection. Circ. Res. 2016, 118, 1643–1658. [Google Scholar] [CrossRef]
- Kirkpatrick, J.N.; Vannan, M.A.; Narula, J.; Lang, R.M. Echocardiography in Heart Failure: Applications, Utility, and New Horizons. J. Am. Coll. Cardiol. 2007, 50, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Degabriele, N.M.; Griesenbach, U.; Sato, K.; Post, M.J.; Zhu, J.; Williams, J.; Jeffery, P.K.; Geddes, D.M.; Alton, E.W. Critical Appraisal of the Mouse Model of Myocardial Infarction. Exp. Physiol. 2004, 89, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Shioura, K.M.; Geenen, D.L.; Goldspink, P.H. Assessment of Cardiac Function with the Pressure-Volume Conductance System following Myocardial Infarction in Mice. Heart Circ. Physiol. 2007, 293, H2870–H2877. [Google Scholar] [CrossRef]
- Bastos, M.B.; Burkhoff, D.; Maly, J.; Daemen, J.; den Uil, C.A.; Ameloot, K.; Lenzen, M.; Mahfoud, F.; Zijlstra, F.; Schreuder, J.J.; et al. Invasive Left Ventricle Pressure–Volume Analysis: Overview and Practical Clinical Implications. Eur. Heart J. 2020, 41, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Bodor, G.S. Biochemical Markers of Myocardial Damage. Int. EJIFCC. 2016, 27, 95–111. [Google Scholar]
- Khalil, M.L.; Ahmmed, I.; Ahmed, R.; Tanvir, E.M.; Afroz, R.; Paul, S.; Gan, S.H.; Alam, N. Amelioration of Isoproterenol-Induced Oxidative Damage in Rat Myocardium by Withania Somnifera Leaf Extract. Biomed. Res. Int. 2015, 2015, 624159. [Google Scholar] [CrossRef]
- Bhar-Amato, J.; Davies, W.; Agarwal, S. Ventricular Arrhythmia after Acute Myocardial Infarction: ‘The Perfect Storm’. Electrophysiol. Rev. 2017, 6, 134–139. [Google Scholar] [CrossRef]
- Travers, G.J.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2017, 118, 1021–1040. [Google Scholar] [CrossRef]
- Jiménez-Navarro, M.F.; Gómez-Doblas, J.J.; Cabrera-Bueno, F.; Cruz-Ocaña, E.; Rodríguez-Bailón, I.; Ruiz-Galdón, M.; Morell, M.; Molero, E.; de Teresa-Galván, E. Collagen Synthesis and Heart Failure. Rev. Esp. Cardiol. 2005, 58, 975–978. [Google Scholar] [CrossRef]
- Cui, Z.T.; Liu, J.P.; Wei, W.L. The Effects of Tanshinone IIA on Hypoxia/Reoxygenation-Induced Myocardial Microvascular Endothelial Cell Apoptosis in Rats Via the JAK2/STAT3 Signaling Pathway. Biomed. Pharmacother. 2016, 83, 1116–1126. [Google Scholar] [CrossRef]
- Schindler, C.; Levy, D.E.; Decker, T. JAK-STAT Signaling: From Interferons to Cytokines. J. Biol. Chem. 2007, 282, 20059–20063. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Hilfiker-Kleiner, D.; Heusch, G.; Schulz, R. Inhibition of Permeability Transition Pore Opening by Mitochondrial STAT3 and Its Role in Myocardial Ischemia/Reperfusion. Basic Res. Cardiol. 2010, 105, 771–785. [Google Scholar] [CrossRef] [PubMed]



| Scientific Name | Quantity (g) |
|---|---|
| Angelica gigas Nakai | 60 |
| Prunus persica Batsch | 60 |
| Rehmannia glutinosa (Gaertn.) DC. | 30 |
| Paeonia lactiflora Pallas | 30 |
| Cnidii officinale Makino | 30 |
| Carthamus tinctorius Linné | 30 |
| Totalily | 240 |
| Compounds | Adduct | Rt (min) | Observed m/z | Fragment Ion |
|---|---|---|---|---|
| Decursin | [M + H]+ | 9.72 | 329.1393 | 229.0863 |
| Nodakenin | [M + H]+ | 2.44 | 409.1504 | 247.0969 |
| Phenylalanine | [M + H]+ | 0.78 | 166.0869 | 120.0814 |
| Tryptophan | [M + H]+ | 0.85 | 205.0979 | 188.0711 |
| Valine | [M + H]+ | 13.94 | 118.0869 | 72.0808 |
| Amygdalin | [M − H]− | 1.14 | 456.1533 | 89.0232 |
| Catalpol | [M − H]− | 0.76 | 361.1158 | 97.0282 |
| Ferulic acid | [M − H]− | 2.76 | 193.0509 | 134.0365 |
| Glucose | [M − H]− | 0.73 | 179.0559 | 59.0125 |
| Oxypaeoniflorin | [M − H]− | 0.94 | 495.1530 | 137.0237 |
| Paeoniflorin | [M + FA − H]− | 1.64 | 525.1639 | 121.0286 |
| [M − H]− | 1.64 | 479.1579 | ||
| Safflomin A | [M − H]− | 1.77 | 611.1647 | 325.0737 |
| Stachyose | [M − H]− | 0.70 | 665.2178 | 383.1152 |
| Sucrose | [M − H]− | 0.71 | 341.1101 | 179.0553 |
| Group. | LVIDd (mm) | LVIDs (mm) | LVPWd (mm) | LVPWs (mm) |
|---|---|---|---|---|
| Sham | 6.84 ± 0.3 | 4.07 ± 0.2 | 2.29 ± 0.2 | 3.59 ± 0.1 |
| MI | 7.96 ± 0.9 * | 5.31 ± 0.1 * | 1.82 ± 0.1 * | 3.02 ± 0.2 * |
| MI + DHT 100 | 7.06 ± 0.6 # | 4.48 ± 0.2 # | 2.19 ± 0.1 # | 3.46 ± 0.1 # |
| MI + DHT 200 | 7.34 ± 0.8 | 4.52 ± 0.1 # | 2.12 ± 0.1 # | 3.36 ± 0.1 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, Y.-J.; Kim, H.-Y.; Na, S.-W.; Hong, M.-H.; Yoon, J.-J.; Lee, H.-S.; Kang, D.-G. The Cardioprotective Potential of Herbal Formulas in Myocardial Infarction-Induced Heart Failure through Inhibition of JAK/STAT3 Signaling and Improvement of Cardiac Function. Pharmaceuticals 2024, 17, 1132. https://doi.org/10.3390/ph17091132
Jang Y-J, Kim H-Y, Na S-W, Hong M-H, Yoon J-J, Lee H-S, Kang D-G. The Cardioprotective Potential of Herbal Formulas in Myocardial Infarction-Induced Heart Failure through Inhibition of JAK/STAT3 Signaling and Improvement of Cardiac Function. Pharmaceuticals. 2024; 17(9):1132. https://doi.org/10.3390/ph17091132
Chicago/Turabian StyleJang, Youn-Jae, Hye-Yoom Kim, Se-Won Na, Mi-Hyeon Hong, Jung-Joo Yoon, Ho-Sub Lee, and Dae-Gill Kang. 2024. "The Cardioprotective Potential of Herbal Formulas in Myocardial Infarction-Induced Heart Failure through Inhibition of JAK/STAT3 Signaling and Improvement of Cardiac Function" Pharmaceuticals 17, no. 9: 1132. https://doi.org/10.3390/ph17091132






