Maltol Improves Peripheral Nerve Function by Inhibiting Schwann Cell Apoptosis via the PERK/eIF2α/CHOP Pathway and MME Upregulation in Diabetic Peripheral Neuropathy
Abstract
:1. Introduction
2. Results
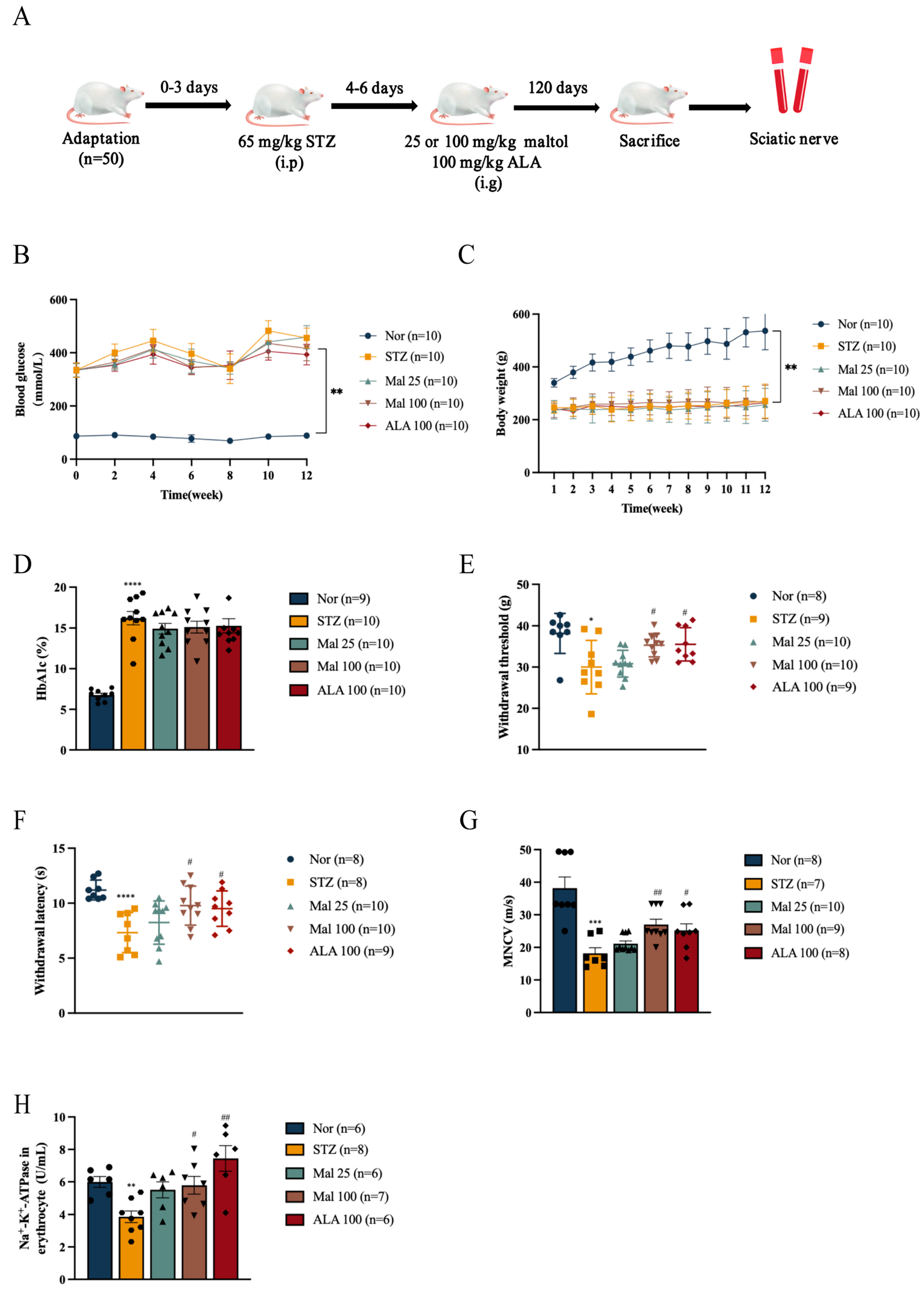
2.1. Maltol Improved Peripheral Nerve Function in Diabetic Rats
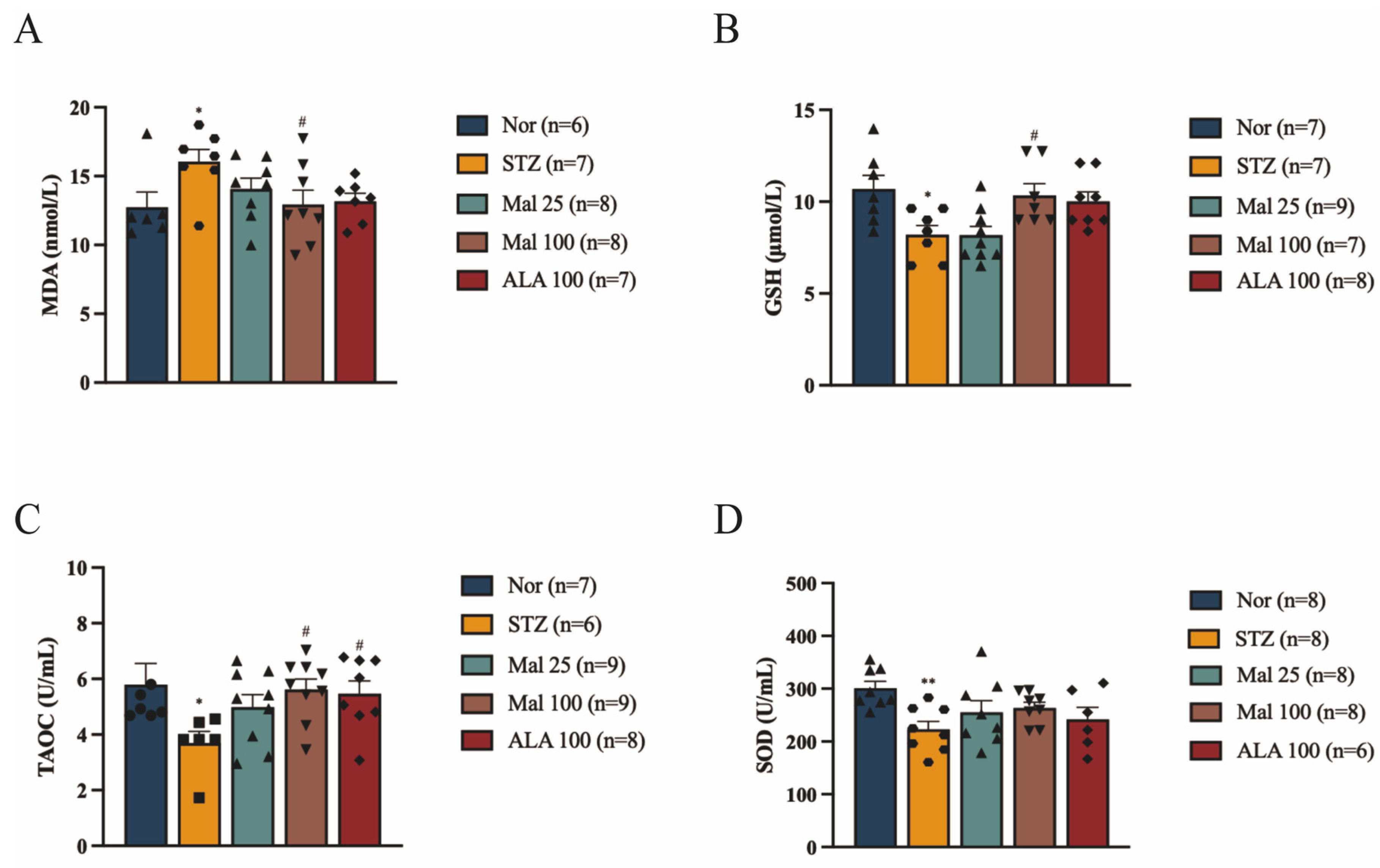
2.2. Maltol Alleviates STZ-Induced Oxidative Stress in Rats
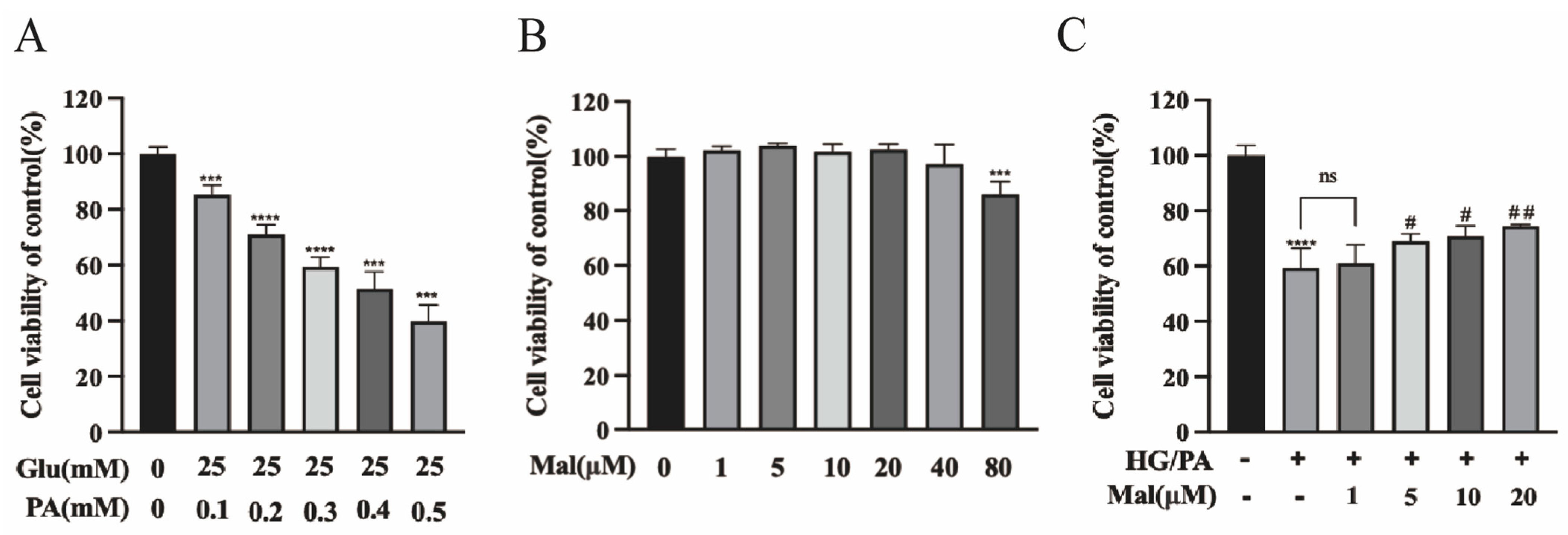
2.3. Protective Effect of Maltol on the Viability of HG/PA-Exposed RSC96 Cells
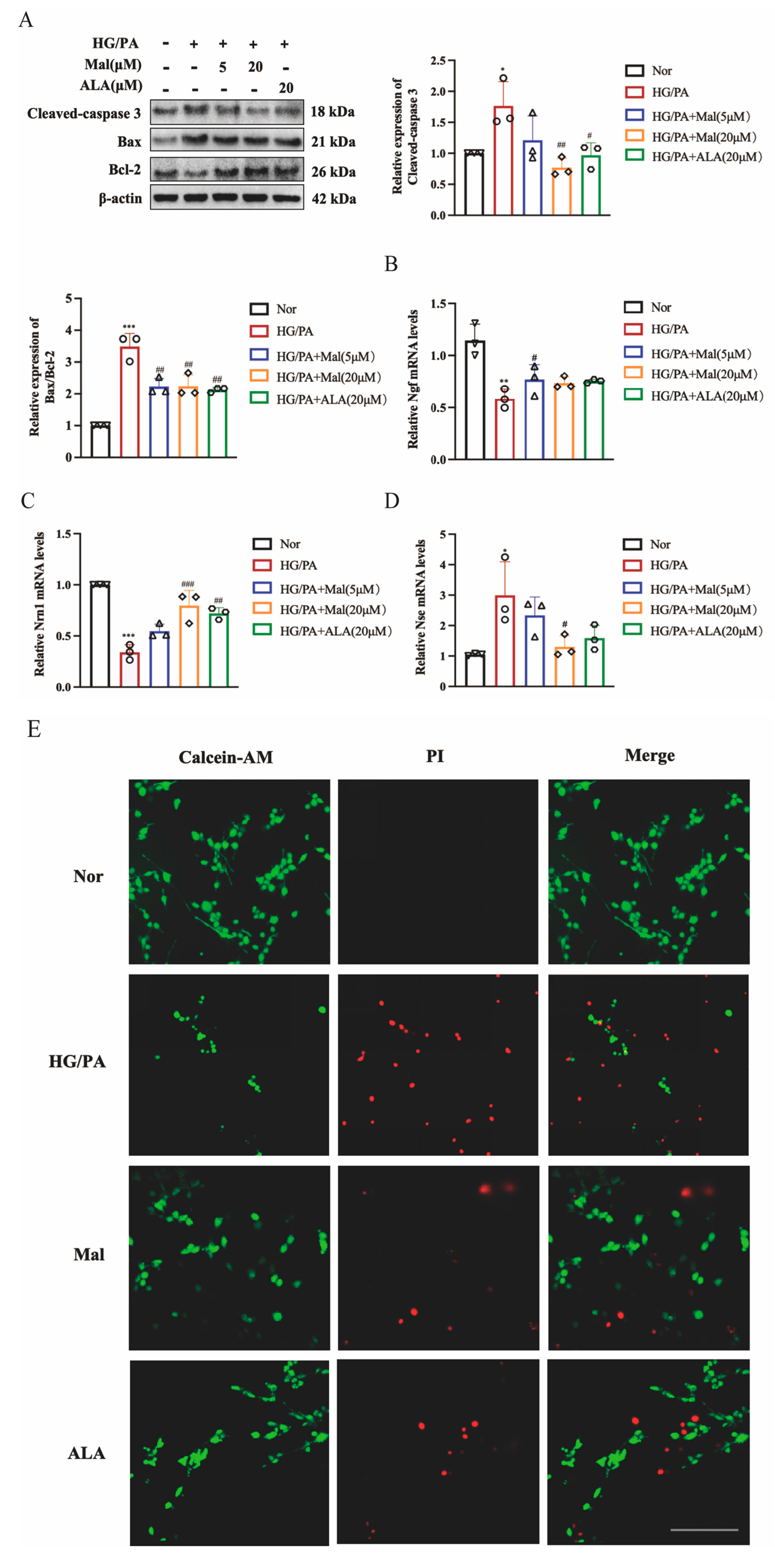
2.4. Maltol Increased Nerve Growth Factor and Neuritin-1 Expression and Inhibited Apoptosis in HG/PA-Induced RSC96 Cells
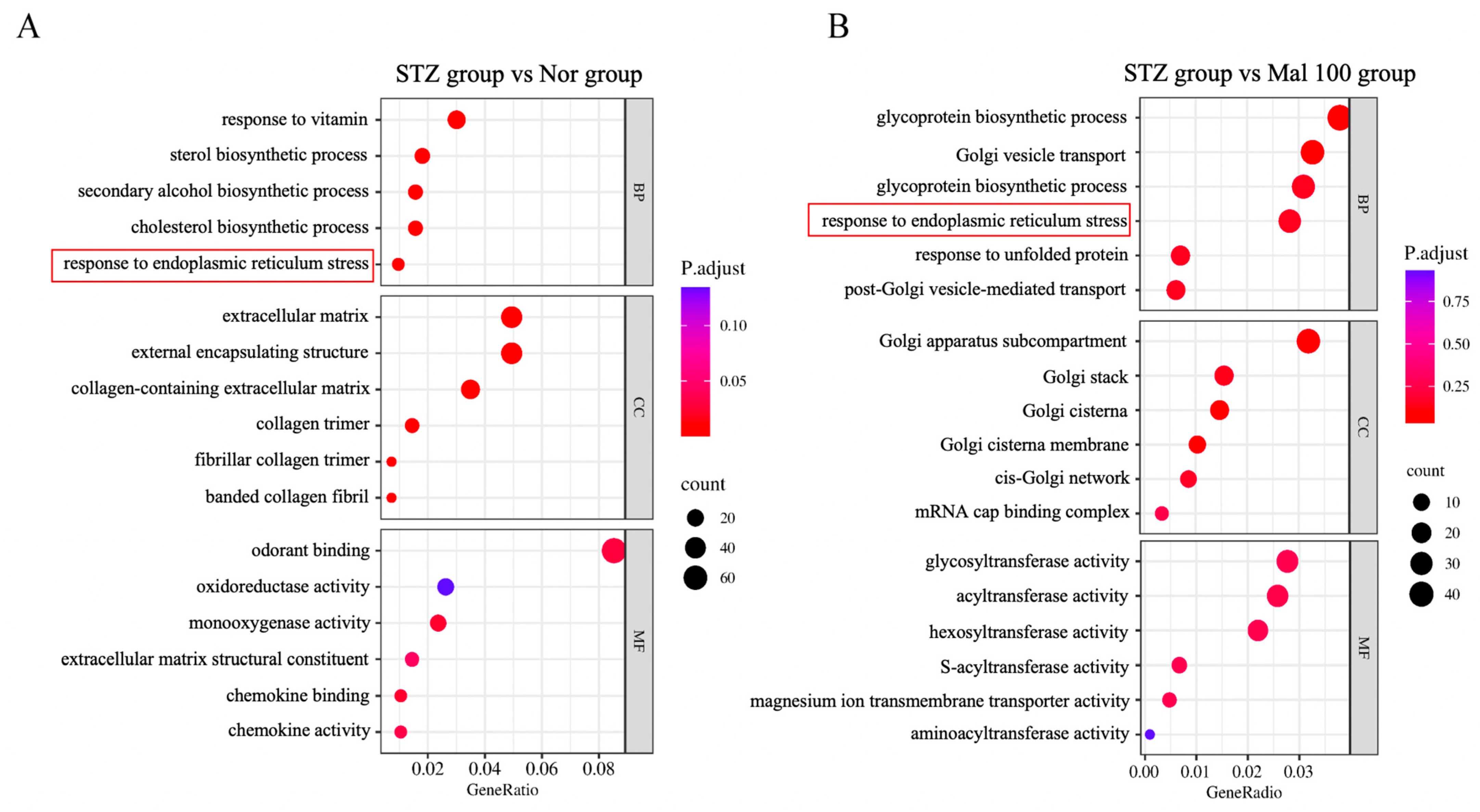
2.5. Functional Annotation of Differentially Expressed Genes in Sciatic Nerve
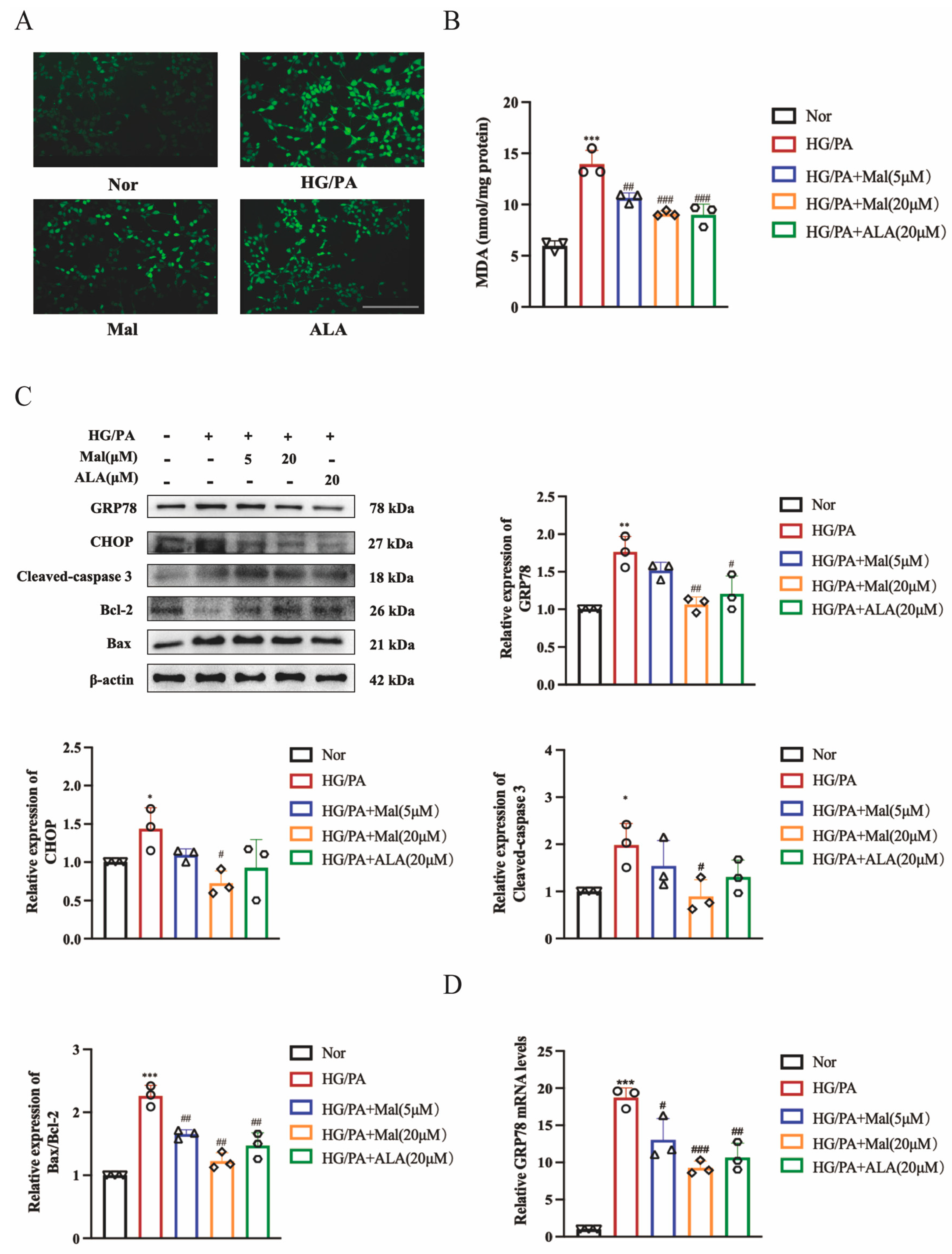
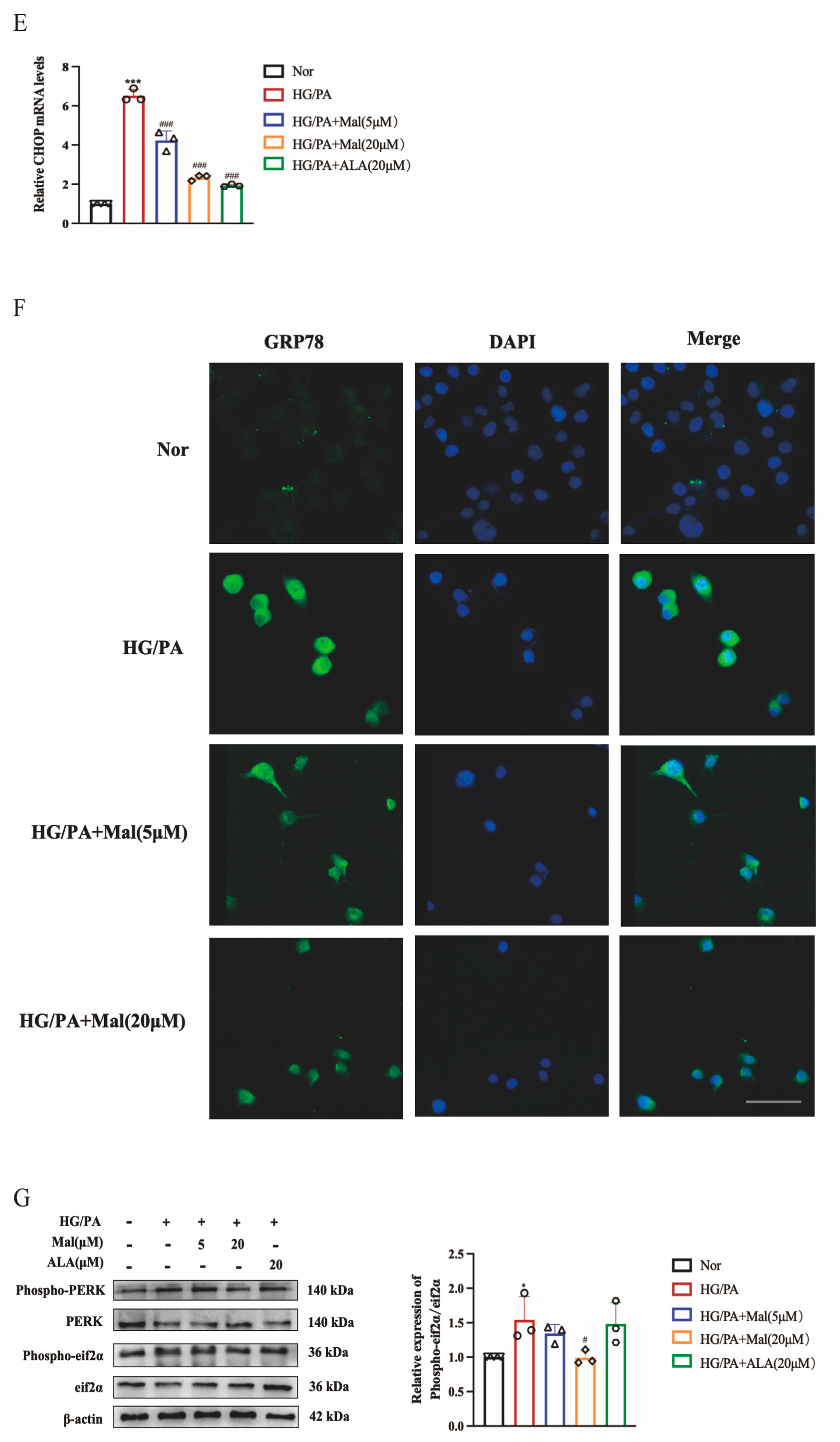
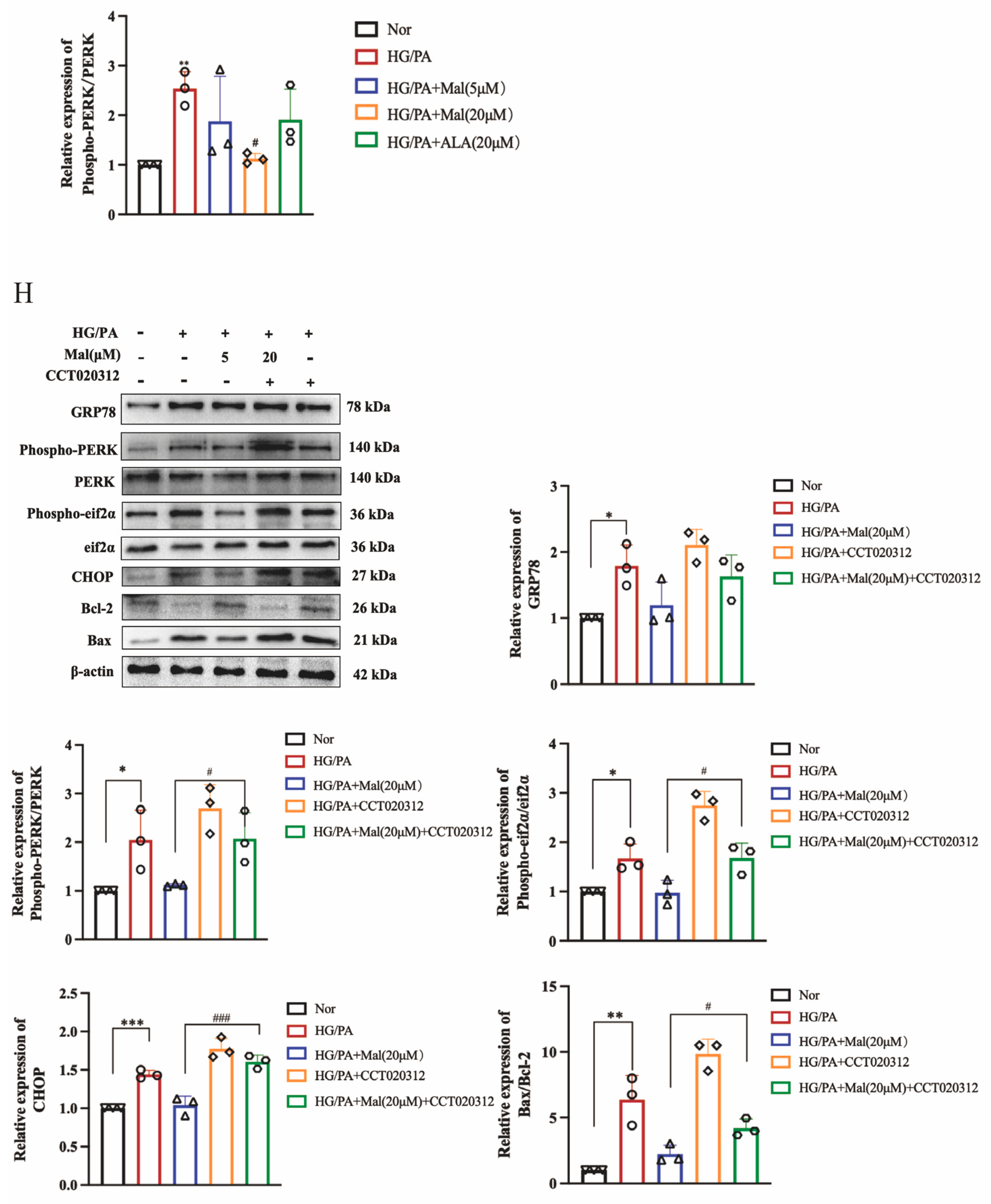
2.6. Maltol Treatment Attenuated HG/PA-Induced Excessive ER Stress
2.7. Bioinformatics Analysis of Potential Maltol Drug Target Associated with DPN
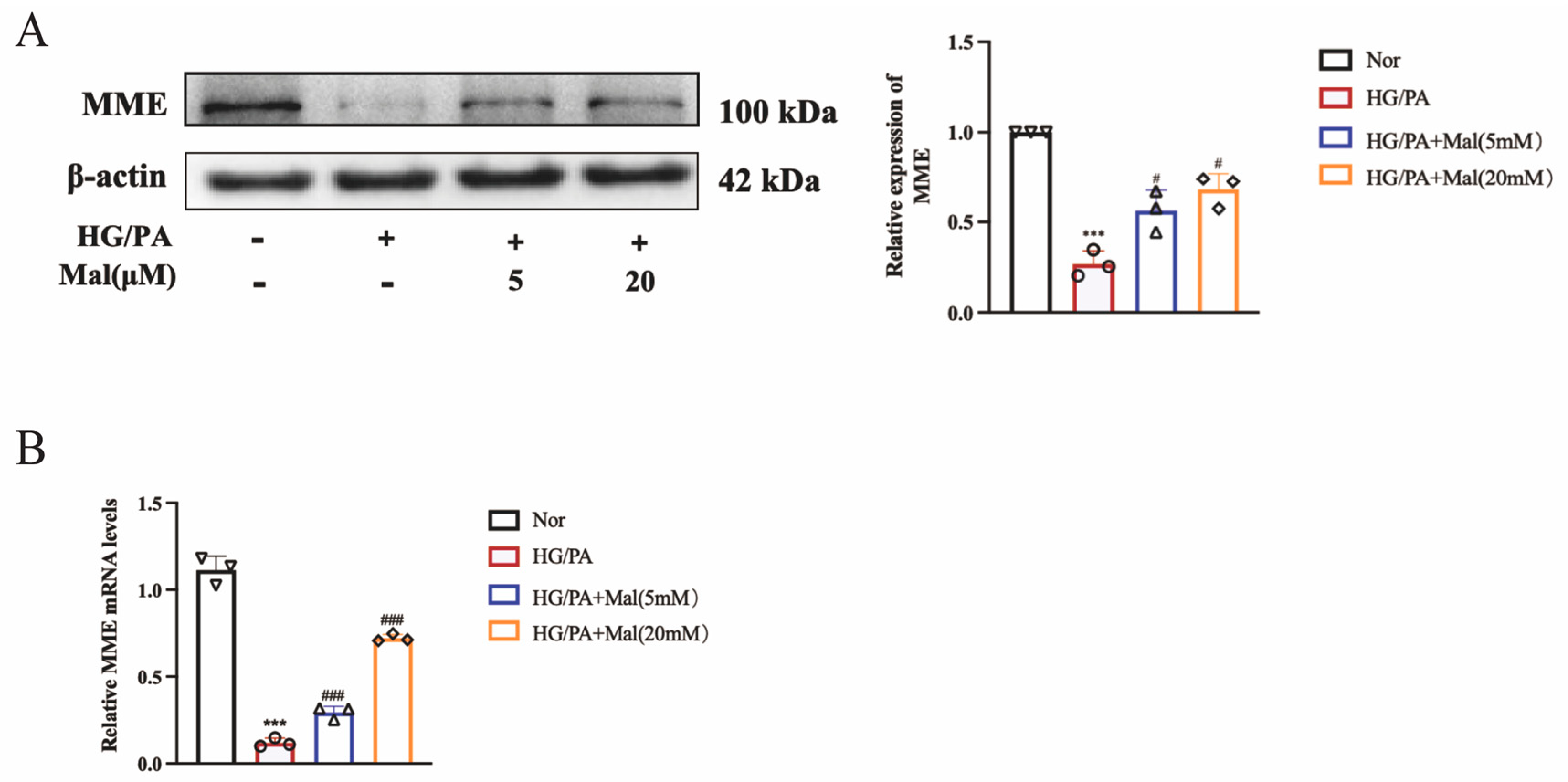
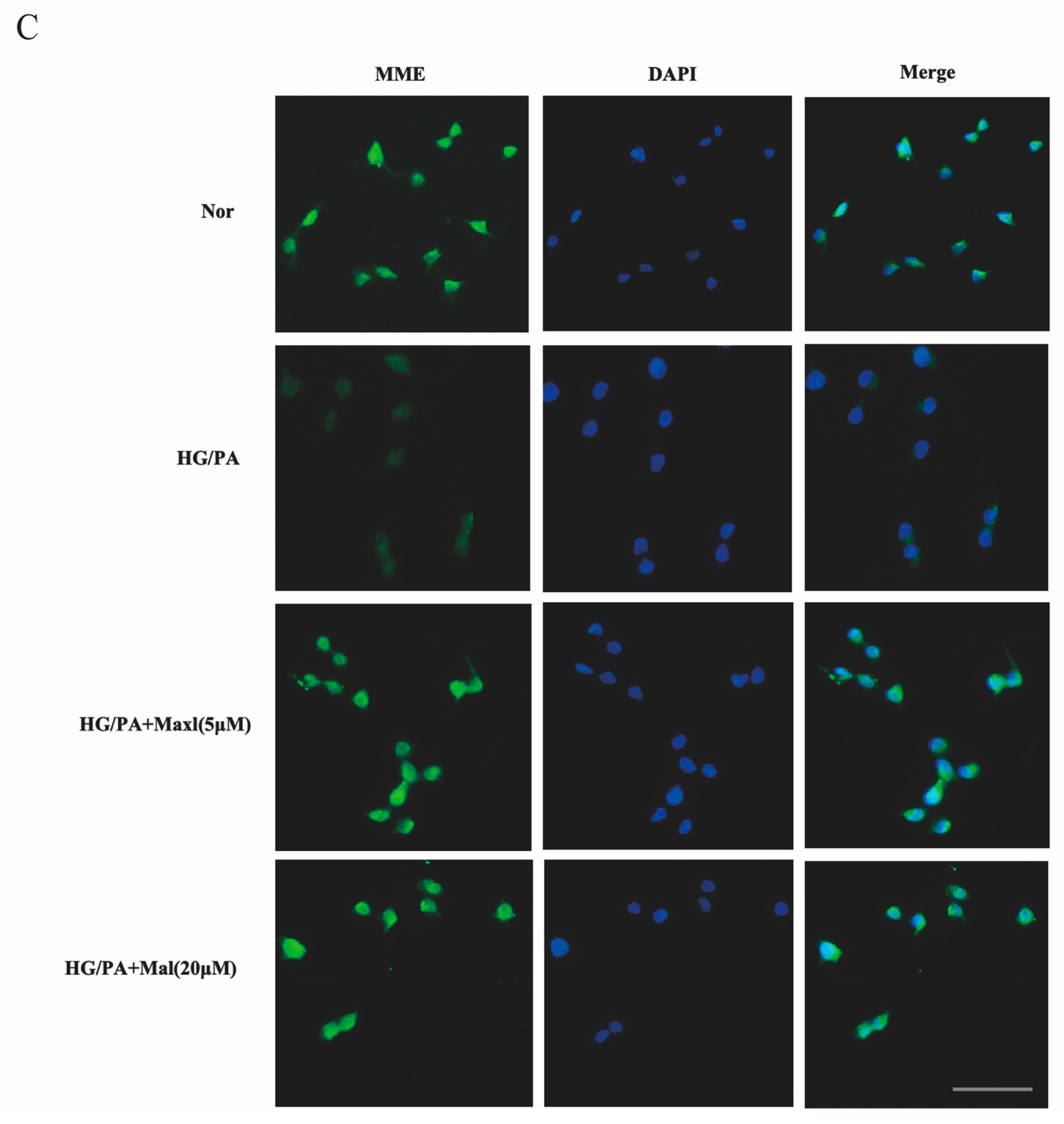
2.8. Maltol Promoted the Expression of MME in HG/PA-Treated RSC96 Cells
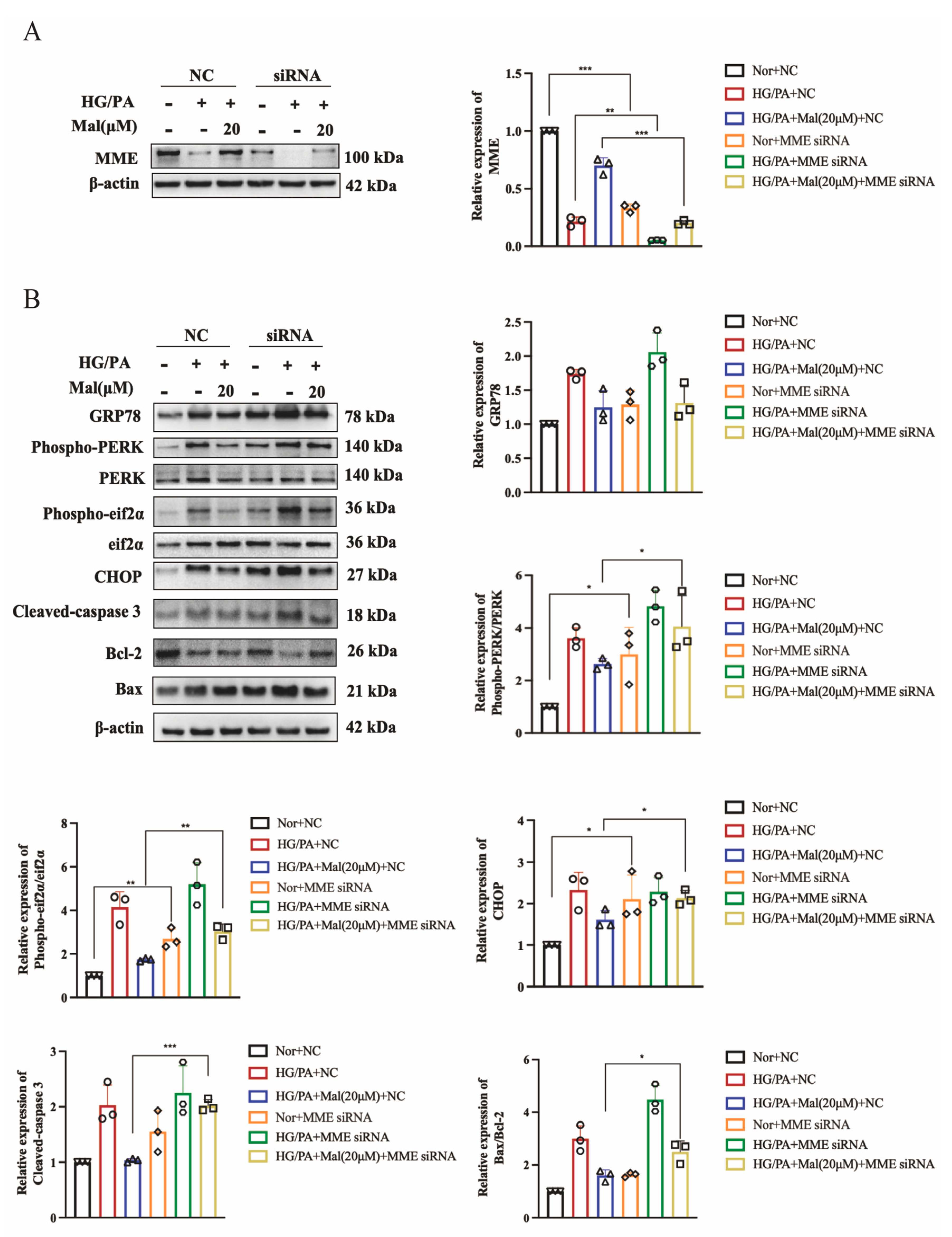
2.9. MME Downregulation Mediated Maltol-Inhibited Apoptotic Protein Expression in HG/PA-Treated RSC96 Cells
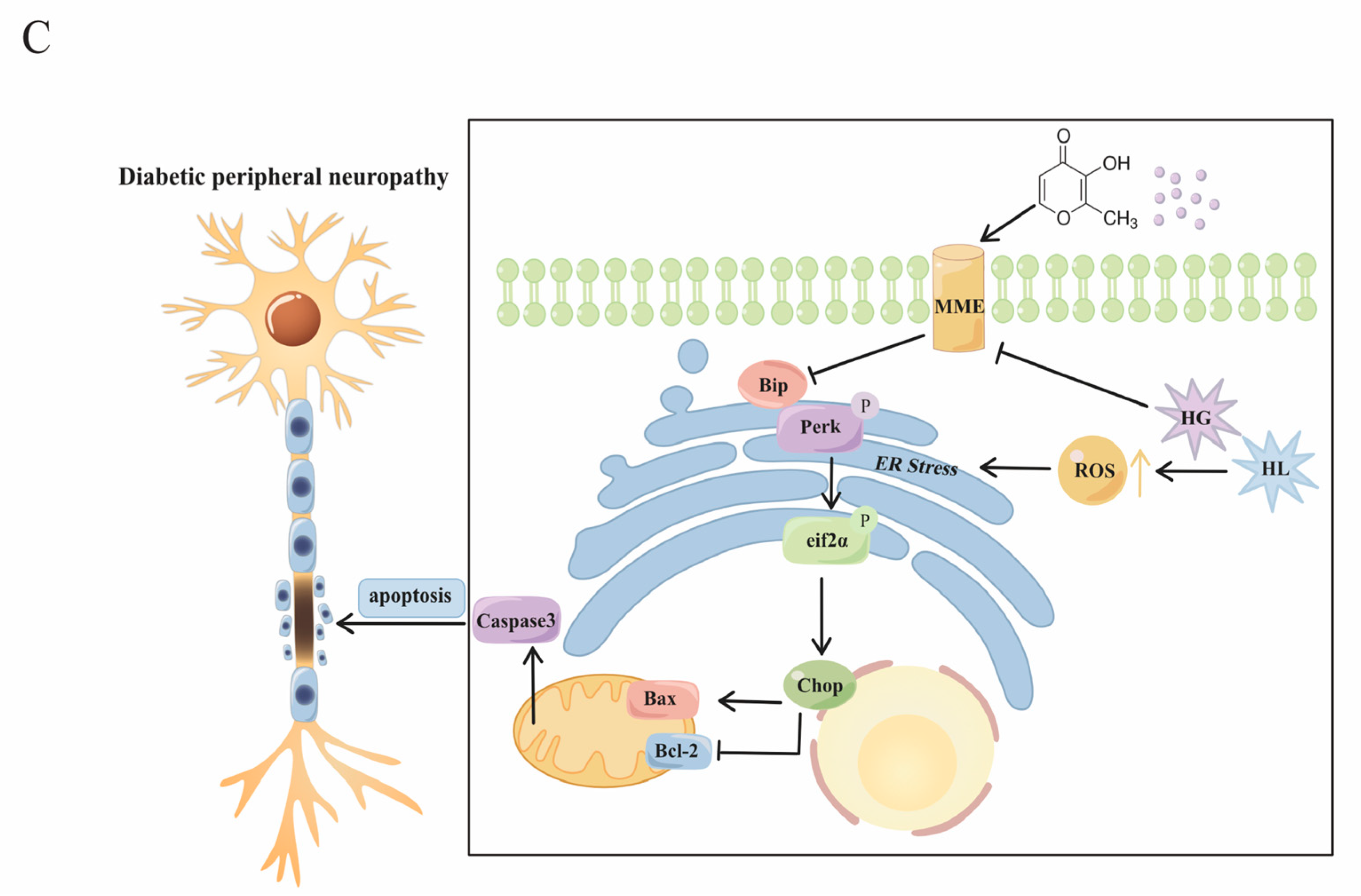
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Experiment
4.3. Measurement of MNCV
4.4. Measurement of the Thermal and Mechanical Hyperalgesia
4.5. Measurement of Na+–K+-ATPase Activity
4.6. Measurement of TAOC, MDA, GSH, and SOD
4.7. Cell Culture and Groups
4.8. CCK-8 Assa
4.9. Intracellular-ROS Generation Staining
4.10. The Calcein-AM/PI Double Staining
4.11. Cell Transfection
4.12. Western Blotting
4.13. Real-Time PCR
4.14. Immunofluorescence
4.15. Gene Chip
4.16. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, K.; Wang, Y.; Li, Y.-W.; Chen, Y.-G.; Xing, N.; Lin, H.-B.; Zhou, P.; Yu, X.-P. Progress in the treatment of diabetic peripheral neuropathy. Biomed. Pharmacother. 2022, 148, 112717. [Google Scholar] [CrossRef]
- Saleh, D.O.; Sedik, A.A. Novel drugs affecting diabetic peripheral neuropathy. Iran. J. Basic Med. Sci. 2024, 27, 657–670. [Google Scholar] [CrossRef]
- Yoo, M.; Sharma, N.; Pasnoor, M.; Kluding, P.M. Painful Diabetic Peripheral Neuropathy: Presentations, Mechanisms, and Exercise Therapy. J. Diabetes Metab. 2013, S10, 5. [Google Scholar] [CrossRef] [PubMed]
- Markova, L.; Cvetko, E.; Ugwoke, C.K.; Horvat, S.; Umek, N.; Stopar, N.; Pintarič, T.S. The Influence of Diabetic Peripheral Neuropathy on the Duration of Sciatic Nerve Block with 1.3% Liposomal Bupivacaine and 0.25% Bupivacaine Hydrochloride in a Mouse Model. Pharmaceutics 2022, 14, 1824. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Lin, X.; Chen, X.; Wu, X.; Zhang, J. Fibrinogen function indexes are potential biomarkers of diabetic peripheral neuropathy. Diabetol. Metab. Syndr. 2022, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Kazamel, M.; Stino, A.M.; Smith, A.G. Metabolic syndrome and peripheral neuropathy. Muscle Nerve 2021, 63, 285–293. [Google Scholar] [CrossRef]
- Iqbal, Z.; Azmi, S.; Yadav, R.; Ferdousi, M.; Kumar, M.; Cuthbertson, D.J.; Lim, J.; Malik, R.A.; Alam, U. Diabetic Peripheral Neuropathy: Epidemiology, Diagnosis, and Pharmacotherapy. Clin. Ther. 2018, 40, 828–849. [Google Scholar] [CrossRef]
- Pareyson, D.; Piscosquito, G.; Moroni, I.; Salsano, E.; Zeviani, M. Peripheral neuropathy in mitochondrial disorders. Lancet Neurol. 2013, 12, 1011–1024. [Google Scholar] [CrossRef]
- Patel, S.; Pangarkar, A.; Mahajan, S.; Majumdar, A. Therapeutic potential of endoplasmic reticulum stress inhibitors in the treatment of diabetic peripheral neuropathy. Metab. Brain Dis. 2023, 38, 1841–1856. [Google Scholar] [CrossRef]
- Martínez, G.; Khatiwada, S.; Costa-Mattioli, M.; Hetz, C. ER Proteostasis Control of Neuronal Physiology and Synaptic Function. Trends Neurosci. 2018, 41, 610–624. [Google Scholar] [CrossRef]
- Di Conza, G.; Ho, P.-C. ER Stress Responses: An Emerging Modulator for Innate Immunity. Cells 2020, 9, 695. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, X.; Hendershot, L.; Prywes, R. ER Stress Regulation of ATF6 Localization by Dissociation of BiP/GRP78 Binding and Unmasking of Golgi Localization Signals. Dev. Cell 2002, 3, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.-J.; Hou, J.-G.; Wang, Z.; Han, Y.; Ren, S.; Hu, J.-N.; Chen, C.; Li, W. The protective effects of maltol on cisplatin-induced nephrotoxicity through the AMPK-mediated PI3K/Akt and p53 signaling pathways. Sci. Rep. 2018, 8, 15922. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.-C.; Wang, Y.-H.; Lin, J.-H.; Miao, Z.-M.; Xu, J.-J.; Wu, Y.-S. Maltol inhibits the progression of osteoarthritis via the nuclear factor-erythroid 2–related factor-2/heme oxygenase-1 signal pathway in vitro and in vivo. Food Funct. 2021, 12, 1327–1337. [Google Scholar] [CrossRef]
- Mi, X.-J.; Hou, J.-G.; Jiang, S.; Liu, Z.; Tang, S.; Liu, X.-X.; Wang, Y.-P.; Chen, C.; Wang, Z.; Li, W. Maltol Mitigates Thioacetamide-induced Liver Fibrosis through TGF-β1-mediated Activation of PI3K/Akt Signaling Pathway. J. Agric. Food Chem. 2019, 67, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Li, J.; Zhou, Y.; Yang, J.; Liu, W.; Jiang, S.; Wang, Y.; Zhang, R.; Di, P. The p53/p21/p16 and PI3K/Akt signaling pathways are involved in the ameliorative effects of maltol on D-galactose-induced liver and kidney aging and injury. Phytother. Res. 2021, 35, 4411–4424. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Iizuka, Y.; Lee, T.; Kim, C.Y.; Seong, G.J. Neuroprotective and neurite outgrowth effects of maltol on retinal ganglion cells under oxidative stress. Mol. Vis. 2014, 20, 1456–1462. [Google Scholar]
- Song, Y.; Hong, S.; Iizuka, Y.; Kim, C.Y.; Seong, G.J. The Neuroprotective Effect of Maltol against Oxidative Stress on Rat Retinal Neuronal Cells. Korean J. Ophthalmol. 2015, 29, 58–65. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Zhan, Y.; Zeng, T.; Zhang, X.; Guan, X.-Y.; Li, Y. Membrane Metalloendopeptidase (MME) Suppresses Metastasis of Esophageal Squamous Cell Carcinoma (ESCC) by Inhibiting FAK-RhoA Signaling Axis. Am. J. Pathol. 2019, 189, 1462–1472. [Google Scholar] [CrossRef]
- Pereira, N.L.; Aksoy, P.; Moon, I.; Peng, Y.; Redfield, M.M.; Burnett, J.C.; Wieben, E.D.; Yee, V.C.; Weinshilboum, R.M. Natriuretic peptide pharmacogenetics: Membrane metallo-endopeptidase (MME): Common gene sequence variation, functional characterization and degradation. J. Mol. Cell. Cardiol. 2010, 49, 864–874. [Google Scholar] [CrossRef]
- Hong, D.; Fang, P.; Yao, S.; Chen, J.; Zhang, X.; Chen, S.; Zhang, J.; Tan, D.; Wang, L.; Han, X.; et al. Variants in MME are associated with autosomal-recessive distal hereditary motor neuropathy. Ann. Clin. Transl. Neurol. 2019, 6, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Jiang, J. LncRNA ANRIL Silencing Alleviates High Glucose-Induced Inflammation, Oxidative Stress, and Apoptosis via Upregulation of MME in Podocytes. Inflammation 2020, 43, 2147–2155. [Google Scholar] [CrossRef]
- Yasojima, K.; McGeer, E.; McGeer, P. Relationship between beta amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res. 2001, 919, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Brismar, T.; Sima, A.A.F.; Greene, D.A. Reversible and irreversible nodal dysfunction in diabetic neuropathy. Ann. Neurol. 1987, 21, 504–507. [Google Scholar] [CrossRef]
- Lane, J.T. The role of retinoids in the induction of nerve growth factor: A potential treatment for diabetic neuropathy. Transl. Res. 2014, 164, 193–195. [Google Scholar] [CrossRef]
- Apfel, S.C. Neurotrophic factors in the therapy of diabetic neuropathy. Am. J. Med. 1999, 107, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Luo, X.; Guo, Z.; Xiong, A.; Dong, H.; Zhang, Q.; Liu, C.; Zhu, J.; Wang, H.; Yu, N.; et al. Neuritin Inhibits Notch Signaling through Interacted with Neuralized to Promote the Neurite Growth. Front. Mol. Neurosci. 2017, 10, 179. [Google Scholar] [CrossRef]
- Anju, M.; Maiya, A.G.; Hande, M.; Binu, V.S. Effect of photobiomodulation on serum neuron specific enolase (NSE) among patients with diabetic peripheral neuropathy—A pilot study. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1061–1063. [Google Scholar] [CrossRef]
- Spitz, A.Z.; Gavathiotis, E. Physiological and pharmacological modulation of BAX. Trends Pharmacol. Sci. 2022, 43, 206–220. [Google Scholar] [CrossRef]
- D’Amelio, M.; Cavallucci, V.; Cecconi, F. Neuronal caspase-3 signaling: Not only cell death. Cell Death Differ. 2010, 17, 1104–1114. [Google Scholar] [CrossRef]
- Lin, Q.; Li, K.; Chen, Y.; Xie, J.; Wu, C.; Cui, C.; Deng, B. Oxidative Stress in Diabetic Peripheral Neuropathy: Pathway and Mechanism-Based Treatment. Mol. Neurobiol. 2023, 60, 4574–4594. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Mi, X.; Hou, J.; Cai, E.; Zheng, S.; Wang, S.; Wang, Z.; Chen, C.; Li, W. Maltol mitigates cisplatin-evoked cardiotoxicity via inhibiting the PI3K/Akt signaling pathway in rodents in vivo and in vitro. Phytother. Res. 2022, 36, 1724–1735. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hao, W.; Hu, J.; Mi, X.; Han, Y.; Ren, S.; Jiang, S.; Wang, Y.; Li, X.; Li, W. Maltol Improves APAP-Induced Hepatotoxicity by Inhibiting Oxidative Stress and Inflammation Response via NF-κB and PI3K/Akt Signal Pathways. Antioxidants 2019, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes-Franco, S.; Pacheco-Moisés, F.P.; Rodríguez-Carrizalez, A.D.; Miranda-Díaz, A.G. The Role of Oxidative Stress, Mitochondrial Function, and Autophagy in Diabetic Polyneuropathy. J. Diabetes Res. 2017, 1673081. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Shen, S.; Zhang, S.; Huang, M.; Zhang, L.; Chen, X. Autophagy in Bone Remodeling: A Regulator of Oxidative Stress. Front. Endocrinol. 2022, 13, 898634. [Google Scholar] [CrossRef]
- Yin, Y.; Qu, H.; Yang, Q.; Fang, Z.; Gao, R. Astragaloside IV alleviates Schwann cell injury in diabetic peripheral neuropathy by regulating microRNA-155-mediated autophagy. Phytomedicine 2021, 92, 153749. [Google Scholar] [CrossRef]
- Martinet, W.; Verheye, S.; De Meyer, G.R. Everolimus-Induced mTOR Inhibition Selectively Depletes Macrophages in Atherosclerotic Plaques by Autophagy. Autophagy 2007, 3, 241–244. [Google Scholar] [CrossRef]
- Jahani-Asl, A.; Cheung, E.C.C.; Neuspiel, M.; MacLaurin, J.G.; Fortin, A.; Park, D.S.; McBride, H.M.; Slack, R.S. Mitofusin 2 Protects Cerebellar Granule Neurons against Injury-induced Cell Death. J. Biol. Chem. 2007, 282, 23788–23798. [Google Scholar] [CrossRef]
- Maguer-Satta, V.; Besançon, R.; Bachelard-Cascales, E. Concise Review: Neutral Endopeptidase (CD10): A Multifaceted Environment Actor in Stem Cells, Physiological Mechanisms, and Cancer. Stem Cells 2011, 29, 389–396. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liu, Q.; Liu, S.; Xin, H.; Zhang, X.; Guo, N. Maltol Improves Peripheral Nerve Function by Inhibiting Schwann Cell Apoptosis via the PERK/eIF2α/CHOP Pathway and MME Upregulation in Diabetic Peripheral Neuropathy. Pharmaceuticals 2024, 17, 1139. https://doi.org/10.3390/ph17091139
Li J, Liu Q, Liu S, Xin H, Zhang X, Guo N. Maltol Improves Peripheral Nerve Function by Inhibiting Schwann Cell Apoptosis via the PERK/eIF2α/CHOP Pathway and MME Upregulation in Diabetic Peripheral Neuropathy. Pharmaceuticals. 2024; 17(9):1139. https://doi.org/10.3390/ph17091139
Chicago/Turabian StyleLi, Jiawei, Quan Liu, Shuainan Liu, Hong Xin, Xuemei Zhang, and Nan Guo. 2024. "Maltol Improves Peripheral Nerve Function by Inhibiting Schwann Cell Apoptosis via the PERK/eIF2α/CHOP Pathway and MME Upregulation in Diabetic Peripheral Neuropathy" Pharmaceuticals 17, no. 9: 1139. https://doi.org/10.3390/ph17091139




