Hesperidin Nanoformulation: A Potential Strategy for Reducing Doxorubicin-Induced Renal Damage via the Sirt-1/HIF1-α/VEGF/NF-κB Signaling Cascade
Abstract
:1. Introduction
2. Results
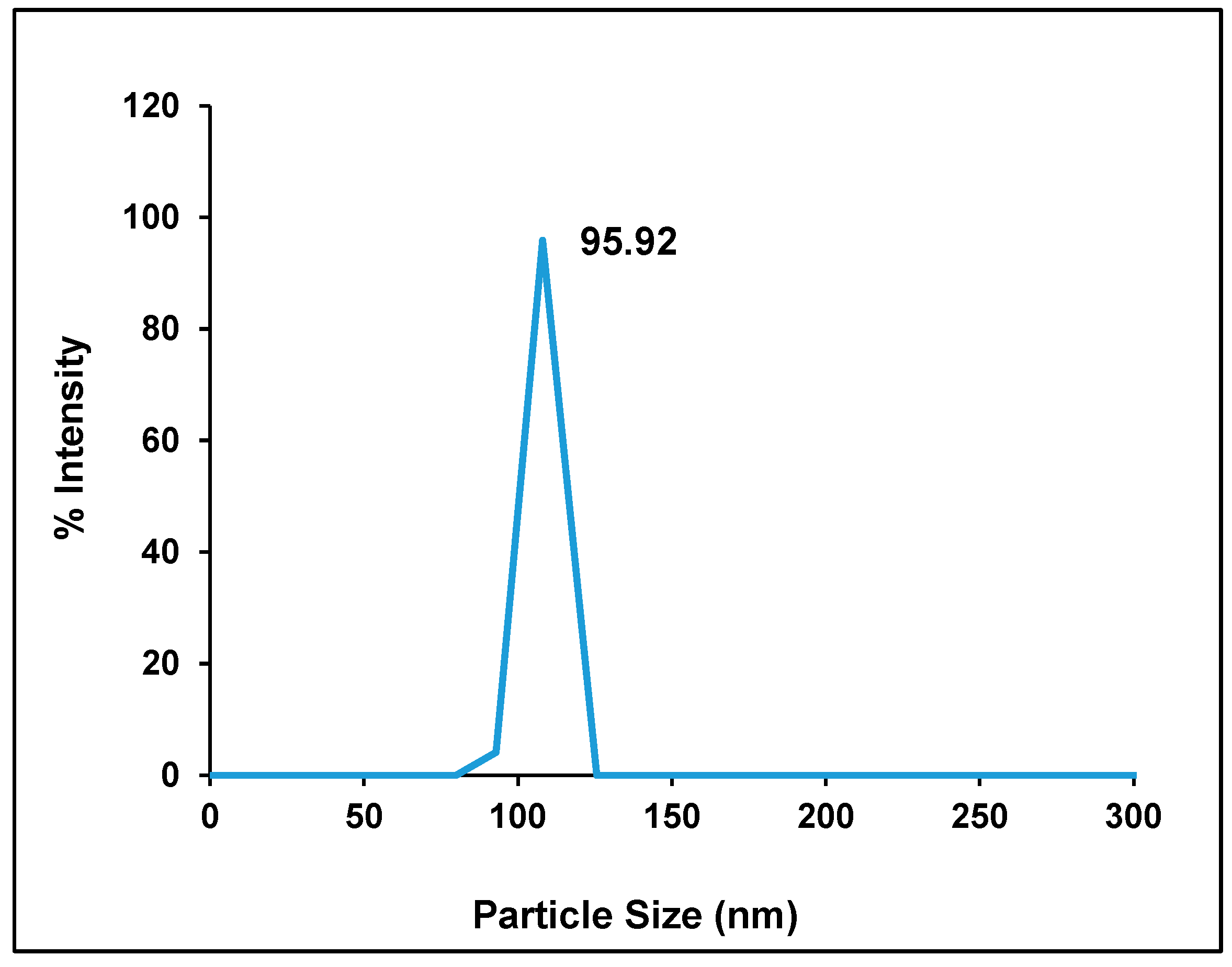
2.1. Particle Size, Zeta Potential, and Entrapment Efficiency of Hes-NPs
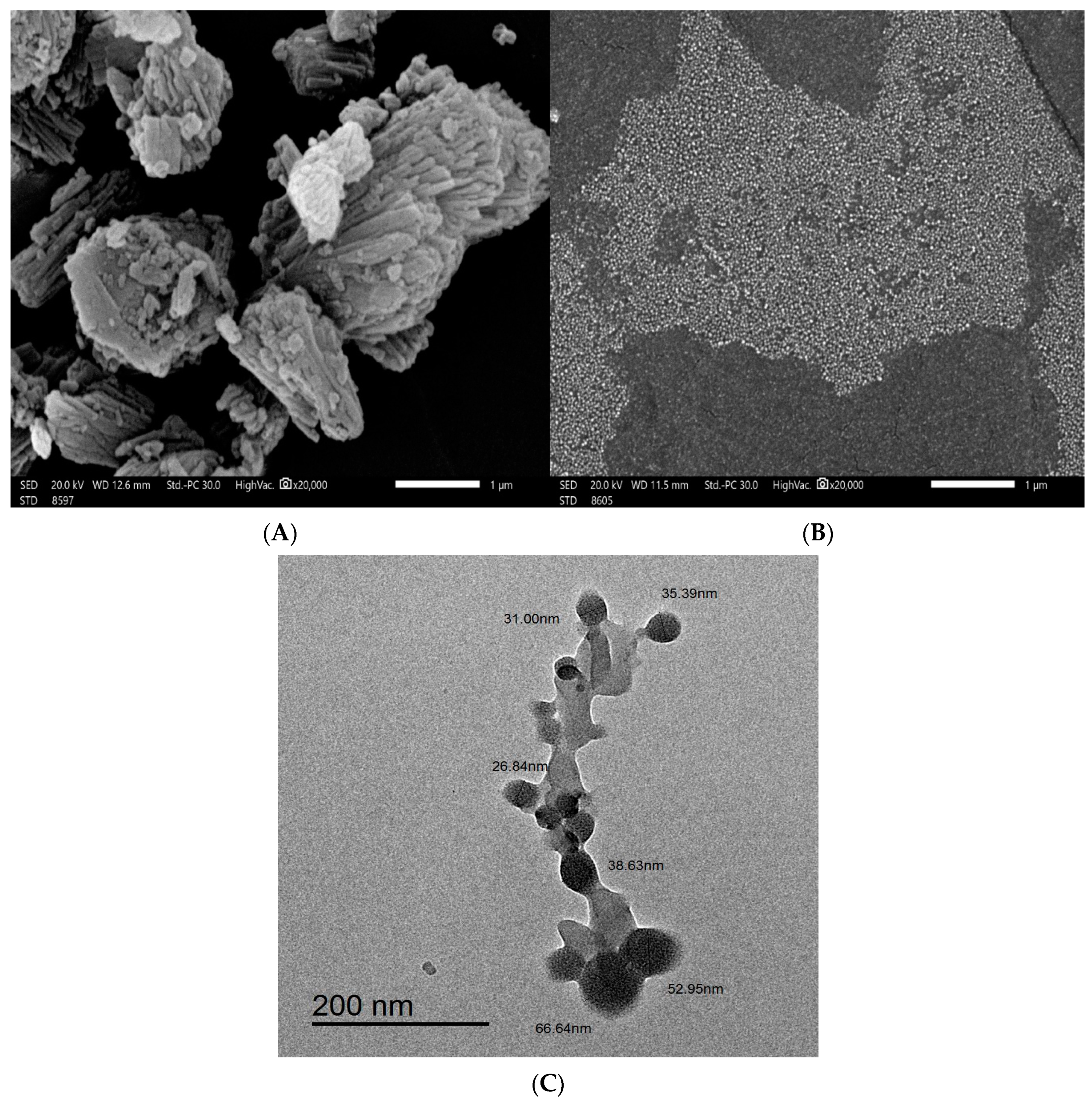
2.2. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
2.3. X-ray Diffraction Analysis (XRD)
2.4. FTIR Analysis
2.5. In Vivo Experiment
2.5.1. Effects of Different Treatments on Kidney Functions
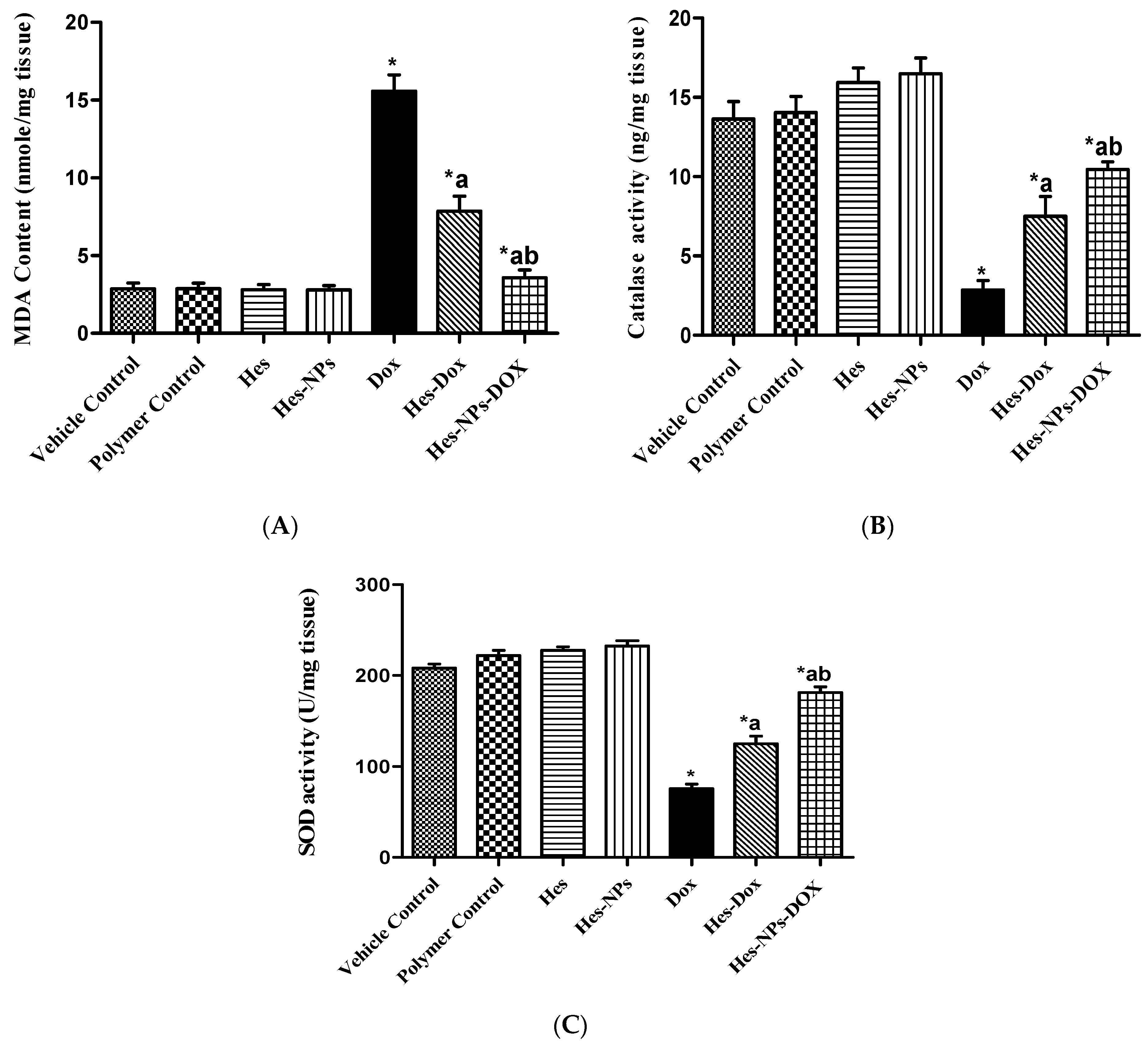
2.5.2. Effects of Different Treatments on Lipid Peroxidation and Antioxidant Enzyme Activity (CAT and SOD) Estimated in Kidney Tissue
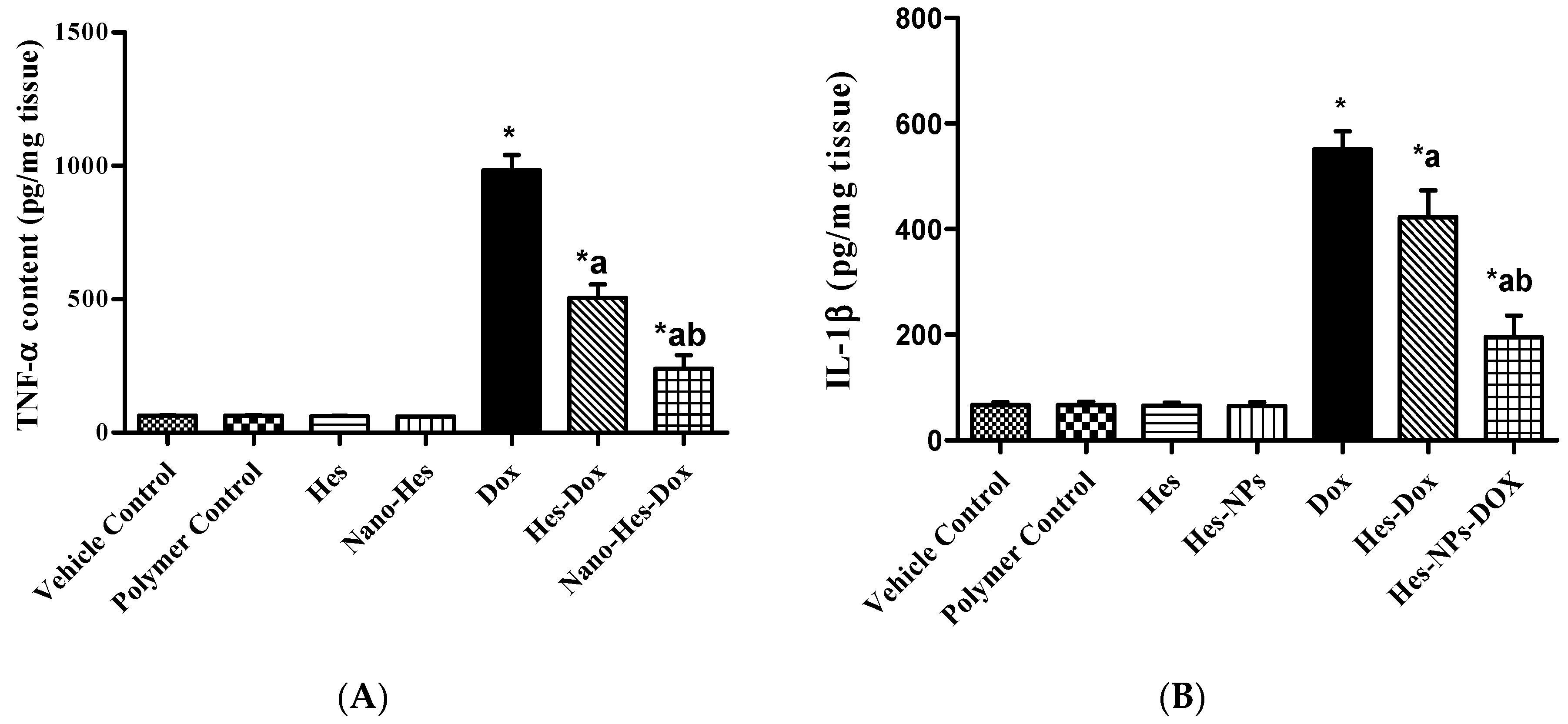
2.5.3. Effect of Different Treatments on Content of Inflammatory Cytokines (TNF-α and IL-1β)
2.5.4. Effect of Different Treatments on VEGF Content
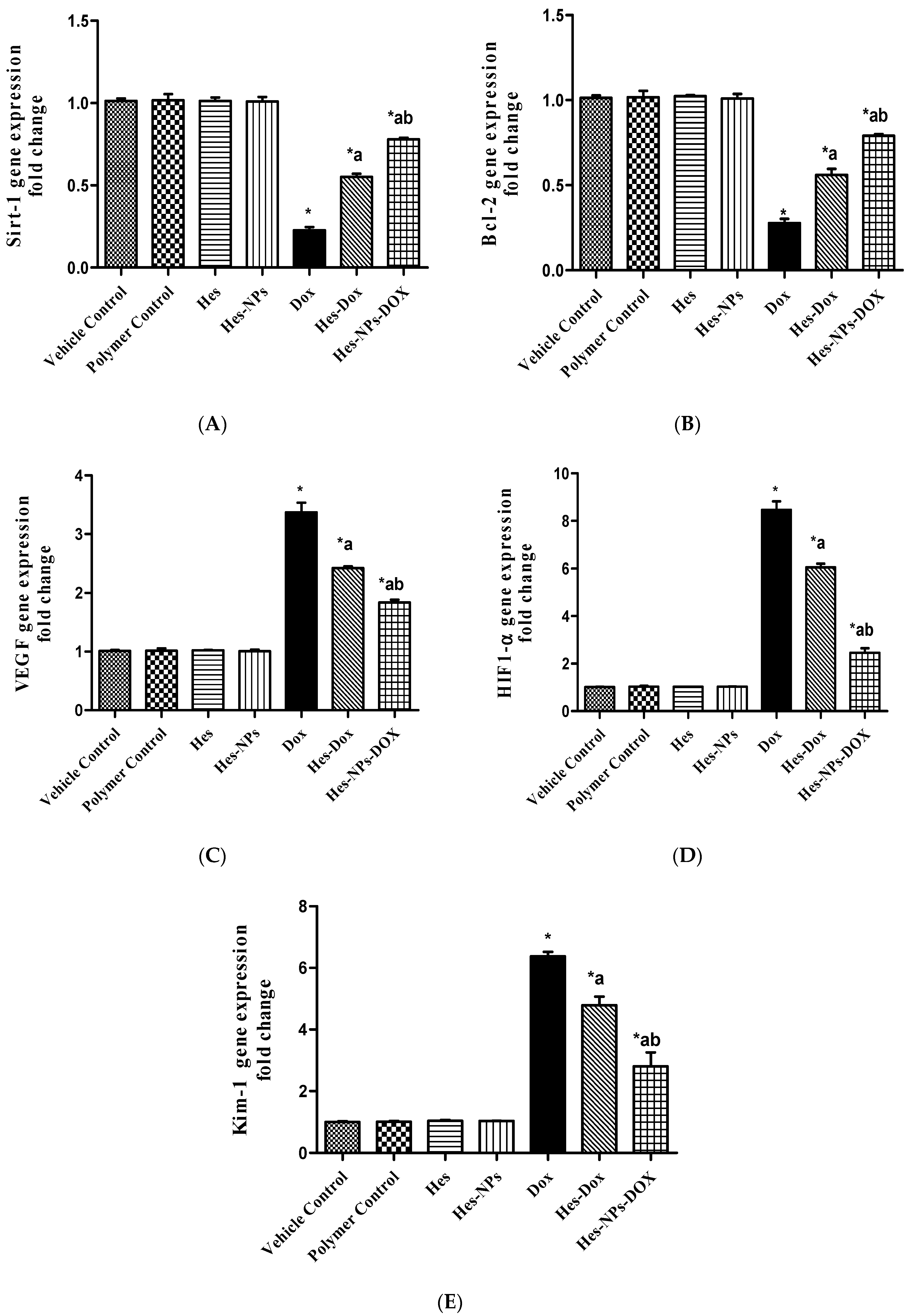
2.5.5. Effect of Different Treatments on Gene Expression of Sirt-1, Bcl-2, VEGF, HIF1-α, and Kim-1
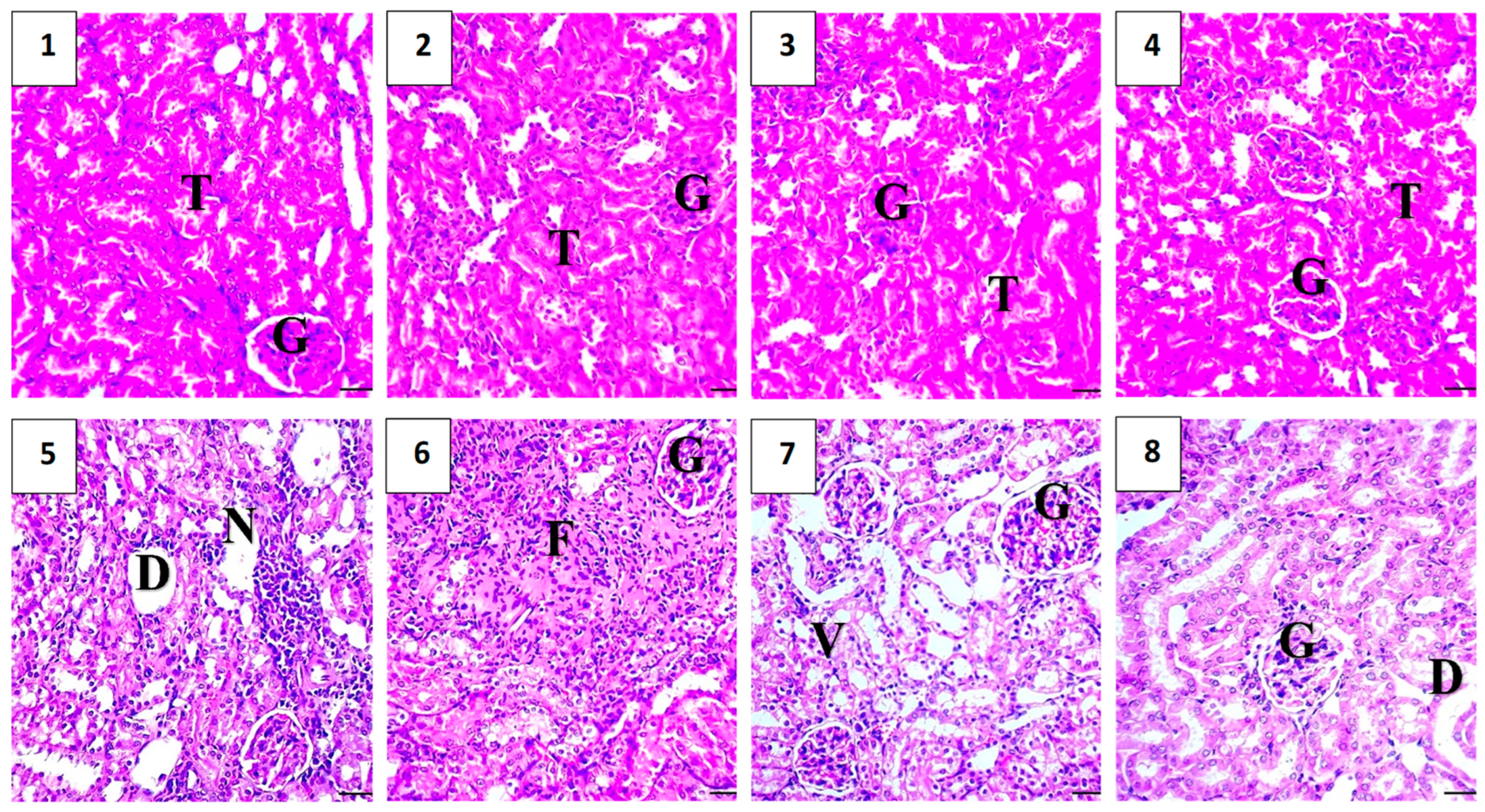
2.5.6. Histopathological Assessment
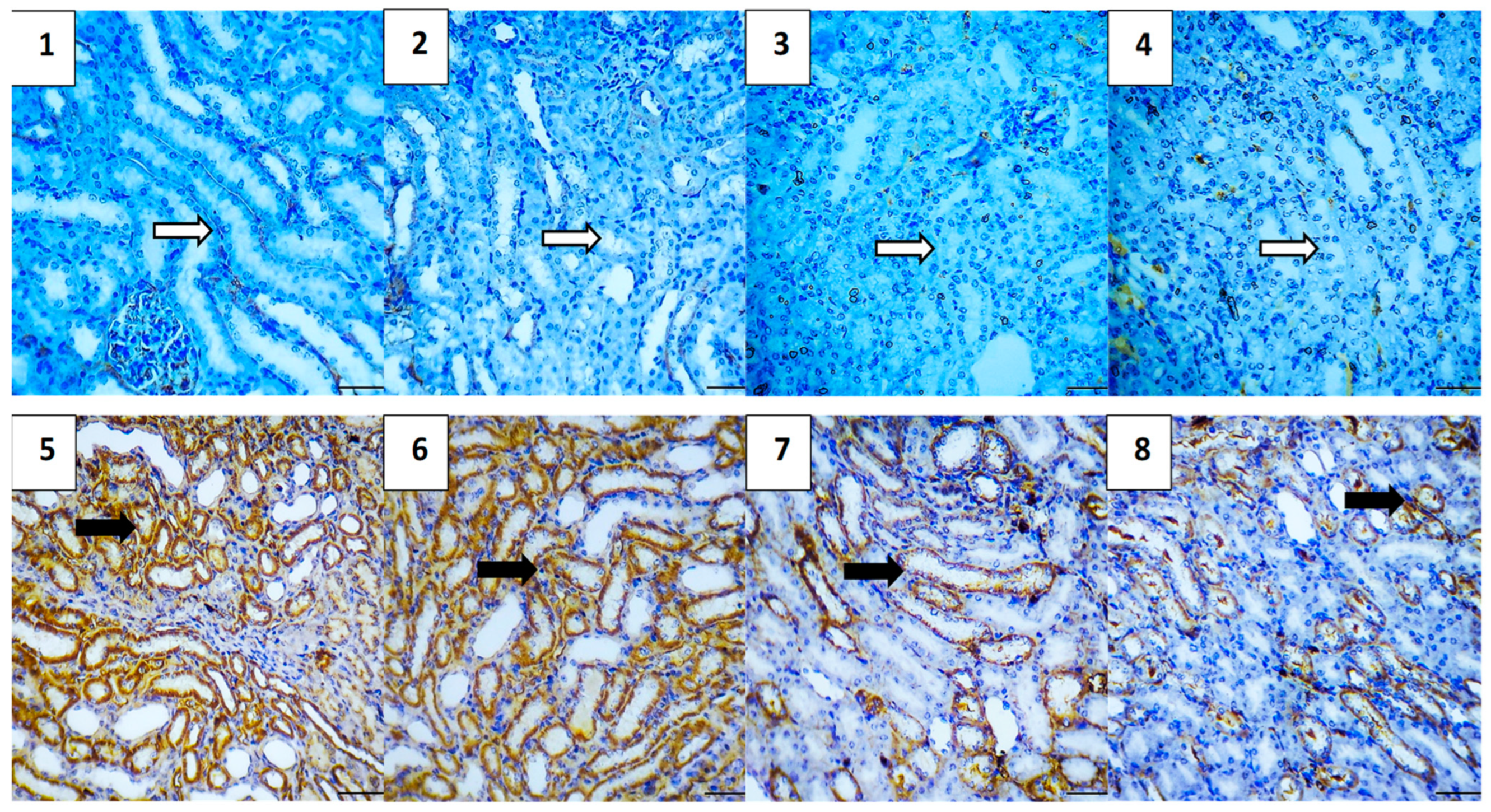
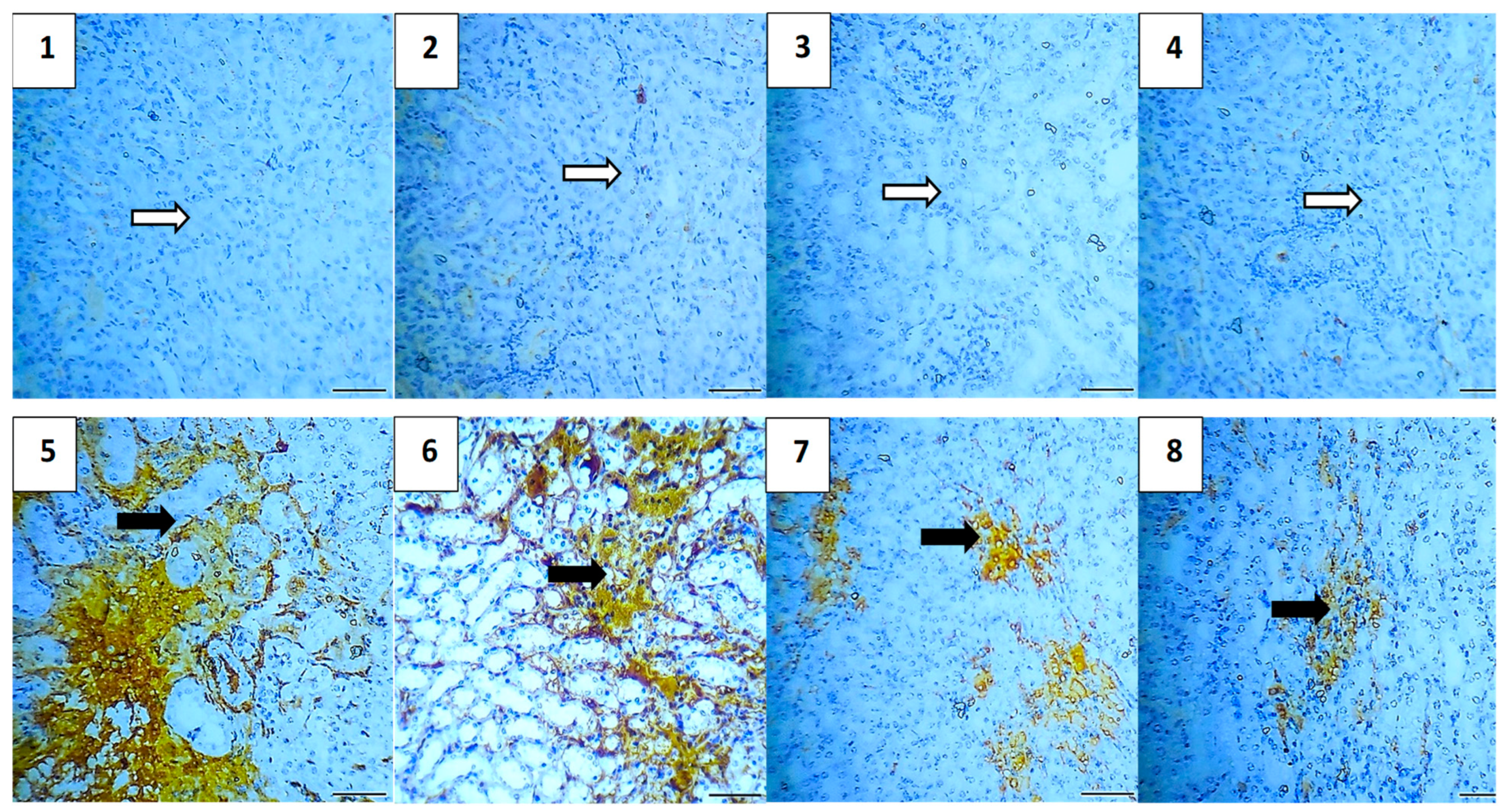
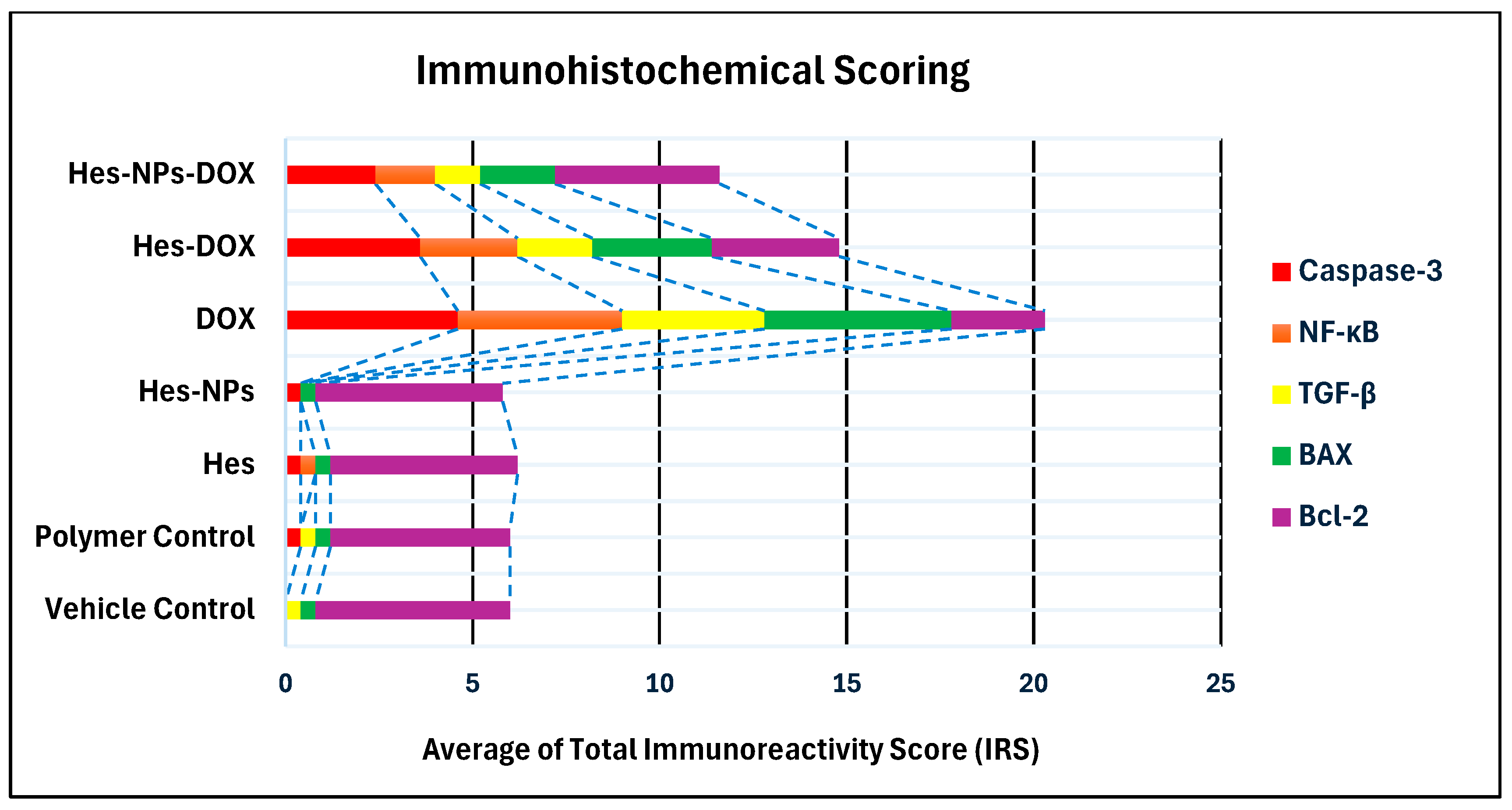
2.5.7. Immunohistochemical Assessment
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. The Ionic Gelation Method Used for the Preparation of Hesperidin Nanoparticles (Hes-NPs)
4.3. Characterization of Hesperidin Nanoparticles (Hes-NPs)
4.3.1. Particle Size, Zeta Potential, and Entrapment Efficiency of Hes-NPs
4.3.2. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
4.3.3. X-ray Diffraction Analysis (XRD) and FTIR
4.4. In Vivo Experiments
4.4.1. Animals
4.4.2. Experimental Design
4.4.3. Blood Samples and Kidney Tissue Collection
4.4.4. Kidney Function Estimation
4.4.5. Lipid Peroxidation and Antioxidant Enzyme Activity (CAT and SOD) Estimated in Kidney Tissue
4.4.6. Determination of TNF-α, IL-1β, and VEGF Content
4.4.7. Quantitative Estimation of Gene Expression of Sirt-1, Bcl-2, VEGF, HIF1-α, and Kim-1 Using Real-Time PCR (qRT-PCR)
| Gene | Primer Sequence (5′–3′) | Reference |
|---|---|---|
| Sirt-1 | CAC-CAG-AAA-GAA-CTT-CAC-CAC-CAG ACC-ATC-AAG-CCG-CCT-ACT-AAT-CTG | [124] |
| Bcl-2 | CACCCCTGGCATCTTCTCCTT AGCGTCTTCAGAGACAGCCAG | [125] |
| VEGF | GGCTCTGAAACCATGAACTTTCT GCAGTAGCTGCGCTGGTAGAC | [126] |
| HIF1-α | GGACGATGAACATCAAGTCAGCA GGAATGGGTTCACAAATCAGCAC | [79] |
| Kim-1 | CGGTGCCTGTGAGTAAATAGAT CTGGCCATGACACAAATAAGAC | [80] |
| B-actin | GTG GGA ATT CGT CAG AAG GAC TCC TAT GTG GAA GTC TAG AGC AAC ATA GCA CAG CTT CTC | [81] |
4.4.8. Histopathological Examination
4.4.9. Immunohistochemical Examination
4.4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute Kidney Injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of Acute Kidney Injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin—An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef]
- Injac, R. Potential Medical Use of Fullerenols After Two Decades of Oncology Research. Technol. Cancer Res. Treat. 2023, 22, 15330338231201515. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.V.; Fernandes, E.; Soares, T.B.; Adega, F.; Lopes, C.M.; Lúcio, M. Natural Compounds: Co-Delivery Strategies with Chemotherapeutic Agents or Nucleic Acids Using Lipid-Based Nanocarriers. Pharmaceutics 2023, 15, 1317. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Walkowiak, J.; Pietrzak, R.; Bazan-Woźniak, A.; Cielecka-Piontek, J. Bioavailability of Hesperidin and Its Aglycone Hesperetin—Compounds Found in Citrus Fruits as A Parameter Conditioning the Pro-Health Potential (Neuroprotective and Antidiabetic Activity)—Mini-Review. Nutrients 2022, 14, 2647. [Google Scholar] [CrossRef]
- Almukainzi, M.; El-Masry, T.A.; El Zahaby, E.I.; El-Nagar, M.M.F. Chitosan/Hesperidin Nanoparticles for Sufficient, Compatible, Antioxidant, and Antitumor Drug Delivery Systems. Pharmaceuticals 2024, 17, 999. [Google Scholar] [CrossRef]
- Li, S. Pharmacodynamic bioequivalence testing. J. Clin. Pharm. Ther. 2012, 37, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Dan, S.; Chakraborty, S.; Ghosh, B.; Saha, C.; Khanam, J.; Pal, T.K. Simultaneous Determination and Quantitation of Diosmetin and Hesperetin in Human Plasma by Liquid Chromatographic Mass Spectrometry with an Application to Pharmacokinetic Studies. J. Chromatog. Sci. 2019, 57, 451–461. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021, 21, 318. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Poly J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties, and Applications. Int. J. Biol. Macromo 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Sachdeva, B.; Sachdeva, P.; Negi, A.; Ghosh, S.; Han, S.; Dewanjee, S.; Jha, S.K.; Bhaskar, R.; Sinha, J.K.; Paiva-Santos, A.C.; et al. Chitosan Nanoparticles-Based Cancer Drug Delivery: Application and Challenges. Mar. Drugs 2023, 21, 211. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A Review on Properties, Biological Activities and Recent Progress in Biomedical Applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to Enhance the Bioavailability and Physiological Functions of Polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef]
- Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Zafar, A.; Alsaidan, O.A.; Alruwaili, N.K.; Gilani, S.J.; Rizwanullah, M. Recent Advancement in Chitosan-Based Nanoparticles for Improved Oral Bioavailability and Bioactivity of Phytochemicals: Challenges and Perspectives. Polymers 2021, 13, 4036. [Google Scholar] [CrossRef]
- Birch, N.P.; Schiffman, J.D. Characterization of Self-Assembled Polyelectrolyte Complex Nanoparticles Formed from Chitosan and Pectin. Langmuir 2014, 30, 3441–3447. [Google Scholar] [CrossRef] [PubMed]
- Barthelmes, J.; Dnnhaupt, S.; Hombach, J.; Bernkop-Schnrch, A. Thiomer Nanoparticles: Stabilization via Covalent Cross-Linking. Drug Deliv. 2011, 18, 613–619. [Google Scholar] [PubMed]
- Hoang, N.H.; Thanh, T.L.; Sangpueak, R.; Treekoon, J.; Saengchan, C.; Thepbandit, W.; Papathoti, N.K.; Kamkaew, A.; Buensanteai, N. Chitosan Nanoparticles-Based Ionic Gelation Method: A Promising Candidate for Plant Disease Management. Polymers 2022, 14, 662. [Google Scholar] [CrossRef]
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. Insight on the Formation of Chitosan Nanoparticles through Ionotropic Gelation with Tripolyphosphate. Mol. Pharm. 2012, 9, 2856–2862. [Google Scholar] [CrossRef]
- Durán-Lobato, M.; Niu, Z.; Alonso, M.J. Oral Delivery of Biologics for Precision Medicine. Adv. Mater. 2020, 32, e1901935. [Google Scholar] [CrossRef]
- El-Shabouri, M.H. Positively Charged Nanoparticles for Improving the Oral Bioavailability of Cyclosporine-A. Int. J. Pharm. 2002, 249, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Abd-Ellatif, R.N.; Nasef, N.A.; El-Horany, H.E.S.; Emam, M.N.; Younis, R.L.; El Gheit, R.E.A.; Elseady, W.; Radwan, D.A.; Hafez, Y.M.; Eissa, A.; et al. Adrenomedullin Mitigates Doxorubicin-Induced Nephrotoxicity in Rats: Role of Oxidative Stress, Inflammation, Apoptosis, and Pyroptosis. Int. J. Mol. Sci. 2022, 23, 14570. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I.; Meririnne, E.; Alfthan, G.; Aro, A. Human Nutrition and Metabolism: Plasma Kinetics and Urinary Excretion of the Flavanones Naringenin and Hesperetin in Humans after Ingestion of Orange Juice and Grapefruit Juice. J. Nutr. 2001, 131, 235–241. [Google Scholar] [CrossRef]
- Nielsen, I.L.F.; Chee, W.S.S.; Poulsen, L.; Offord-Cavin, E.; Rasmussen, S.E.; Frederiksen, H.; Enslen, M.; Barron, D.; Horcajada, M.N.; Williamson, G. Bioavailability Is Improved by Enzymatic Modification of the Citrus Flavonoid Hesperidin in Humans: A Randomized, Double-Blind, Crossover Trial. J. Nutr. 2006, 136, 404–408. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on in Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Adhipandito, C.F.; Cheung, S.H.; Lin, Y.H.; Wu, S.H. Atypical Renal Clearance of Nanoparticles Larger Than the Kidney Filtration Threshold. Int. J. Mol. Sci. 2021, 22, 11182. [Google Scholar] [CrossRef]
- Lock, J.Y.; Carlson, T.L.; Carrier, R.L. Mucus models to evaluate the diffusion of drugs and particles. Adv. Drug. Delive. Rev. 2018, 124, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chin, D.; Poon, C.; Mancino, V.; Pham, J.; Li, H.; Ho, P.Y.; Hallows, K.R.; Chung, E.J. Oral Delivery of Metformin by Chitosan Nanoparticles for Polycystic Kidney Disease. J. Control. Release 2021, 329, 1198–1209. [Google Scholar] [CrossRef]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters Influencing the Size of Chitosan-TPP Nano- and Microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef] [PubMed]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Hilmi, N.H.Z.; Daim, L.D.J. Preparation of Chitosan-Hexaconazole Nanoparticles as Fungicide Nanodelivery System for Combating Ganoderma Disease in Oil Palm. Molecules 2019, 24, 2498. [Google Scholar] [CrossRef]
- Fernandes Queiroz, M.; Melo, K.R.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, K.; Casimeer, S.C.; Ghidan, A.Y.; Ghethan, F.Y.; Venkatachalam, K.; Singaravelu, A. Bioformulated Hesperidin-Loaded PLGA Nanoparticles Counteract the Mitochondrial-Mediated Intrinsic Apoptotic Pathway in Cancer Cells. J. Inorg. Organomet. Polym. Mater. 2021, 31, 331–343. [Google Scholar] [CrossRef]
- Hasan Khudhair, D.; Al-Gareeb, A.I.; Al-Quraish, H.M.; El-Kadem, A.H.; Elekhnawy, E.; Negm, W.A.; Saber, S.; Cavalu, S.; Tirla, A.; Alotaibi, S.S.; et al. Combination of Vitamin C and Curcumin Safeguards Against Methotrexate-Induced Acute Liver Injury in Mice by Synergistic Antioxidant Effects. Front. Med. 2022, 9, 866343. [Google Scholar] [CrossRef]
- Alotaibi, B.; El-Masry, T.A.; Elekhnawy, E.; El-Kadem, A.H.; Saleh, A.; Negm, W.A.; Abdelkader, D.H. Aqueous core epigallocatechin gallate PLGA nanocapsules: Characterization, antibacterial activity against uropathogens, and in vivo reno-protective effect in cisplatin-induced nephrotoxicity. Drug Deliv. 2022, 29, 1848–1862. [Google Scholar] [CrossRef] [PubMed]
- Albensi, B.C. What is nuclear factor kappa B (NF-κB) doing in and to the mitochondrion? Front. Cell Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Vera-Ramirez, L.; Ramirez-Tortosa, M.; Perez-Lopez, P.; Granados-Principal, S.; Battino, M.; Quiles, J.L. Long-term effects of systemic cancer treatment on DNA oxidative damage: The potential for targeted therapies. Cancer Lett. 2012, 327, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Jan, R. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 2019, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Lee, S.-H.; Lee, K.-A. A comparative study of hesperetin, hesperidin, and hesperidin glucoside: Antioxidant, anti-inflammatory, and antibacterial activities in vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Nakayama, M.; Sakoh, T.; Yoshitomi, R.; Fukui, A.; Katafuchi, E.; Tsuda, S.; Nakano, T.; Tsuruya, K.; Kitazono, T. Blood urea nitrogen is independently associated with renal outcomes in Japanese patients with stage 3–5 chronic kidney disease: A prospective observational study. BMC Nephrol. 2019, 20, 115. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Radu, S.; Costea, C.F.; Ciocoiu, M.; Carauleanu, A.; Lacatusu, C.M.; Maranduca, M.A.; Floria, M.; Rezus, C. The predictive role of the biomarker kidney molecule-1 (KIM-1) in acute kidney injury (AKI) cisplatin-induced nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 5238. [Google Scholar] [CrossRef] [PubMed]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Block, K.I.; Koch, A.C.; Mead, M.N.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of antioxidant supplementation on chemotherapeutic toxicity: A systematic review of the evidence from randomized controlled trials. Int. J. Cancer 2008, 123, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy-induced toxicity- Exploring the armory of obscurity. Saudi Pharma J. 2018, 26, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Shoeb, M. Anti-cancer agents from medicinal plants. Bangladesh J. Pharmacol. 2006, 1, 35–41. [Google Scholar]
- Du, G.Y.; He, S.W.; Zhang, L.; Sun, C.X.; Mi, L.D.; Sun, Z.G. Hesperidin exhibitsits in vitro and in vivo antitumor effects in human osteosarcoma MG-63 cells and xenograft mice models via inhibition of cell migration and invasion, cell cycle arrest, and induction of mitochondrial-mediated apoptosis. Oncol. Lett. 2018, 16, 6299–6306. [Google Scholar]
- Pandey, P.; Sayyed, U.; Tiwari, R.K.; Siddiqui, M.H.; Pathak, N.; Bajpai, P. Hesperidin induces ROS-mediated apoptosis along with cell cycle arrest at G2/M phase in human gall bladder carcinoma. Nutr. Cancer 2019, 71, 676–687. [Google Scholar] [CrossRef]
- Cincin, Z.; Kiran, B.; Baran, Y.; Cakmakoglu, B. Hesperidin promotes programmed cell death by downregulation of nongenomic estrogen receptor signaling pathway in endometrial cancer cells. Biomed Pharmacother. 2018, 103, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Sheng, X.; Xu, X.; Yu, C.; Lu, H. Hesperidin induces apoptosis and G0/G1 arrest in human non-small cell lung cancer A549 cells. Int. J. Mol. Med. 2018, 41, 464–472. [Google Scholar] [CrossRef]
- Shahbazi, R.; Cheraghpour, M.; Homayounfar, R.; Nazari, M.; Nasrollahzadeh, J.; Davoodi, S. Hesperidin inhibits insulin-induced phosphoinositide 3-kinase/Akt activation in human pre-B cell line NALM-6. J. Cancer Res. Ther. 2018, 14, 503–508. [Google Scholar] [CrossRef]
- Lee, C.J.; Wilson, L.; Jordan, M.A.; Nguyen, V.; Tang, J.; Smiyun, G. Hesperidin suppressed proliferation of both human breast cancer and androgen-dependent prostate cancer cells. Phytother. Res. 2010, 24, S15–S19. [Google Scholar] [CrossRef]
- Amalina, N.D.; Salsabila, I.A.; Zulfin, U.M.; Jenie, R.I.; Meiyanto, E. In vitro, synergistic effect of hesperidin and doxorubicin downregulates epithelial-mesenchymal transition in highly metastatic breast cancer cells. J. Egypt. Natio Cancer Inst. 2023, 35, 6. [Google Scholar] [CrossRef]
- Janciauskiene, S. The Beneficial Effects of Antioxidants in Health and Diseases. Chronic. Obstr. Pulm. Dis. 2020, 7, 182–202. [Google Scholar] [CrossRef]
- Park, W.S.; Park, M.S.; Kang, S.W.; Jin, S.A.; Jeon, Y.; Hwang, J.; Kim, S.K. Hesperidin Shows Protective Effects on Renal Function in Ischemia-Induced Acute Kidney Injury (Sprague-Dawley Rats); Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 2838–2841. [Google Scholar]
- Siddiqi, A.; Saidullah, B.; Sultana, S. Anti-carcinogenic effect of hesperidin against renal cell carcinoma by targeting COX-2/PGE2 pathway in Wistar rats. Environ. Toxicol. 2018, 33, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.H.; Yousef, D.M.; Alsemeh, A.E. Hesperidin protects against aluminum-induced renal injury in rats via modulating MMP-9 and apoptosis: Biochemical, histological, and ultrastructural study. Environ. Sci. Pollut. Res. 2023, 30, 36208–36227. [Google Scholar] [CrossRef] [PubMed]
- Afsar, T.; Razak, S.; Almajwal, A.; Al-Disi, D. Doxorubicin-induced alterations in kidney functioning, oxidative stress, DNA damage, and renal tissue morphology; Improvement by Acacia hydaspica tannin-rich ethyl acetate fraction. Saudi J. Biol. Sci. 2020, 27, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Saleh, D.; Abdelbaset, M.; Hassan, A.; Sharaf, O.; Mahmoud, S.; Hegazy, R. Omega-3 fatty acids ameliorate doxorubicin-induced cardiorenal toxicity: In-vivo regulation of oxidative stress, apoptosis and renal Nox4, and in-vitro preservation of the cytotoxic efficacy. PLoS ONE 2020, 15, e0242175. [Google Scholar] [CrossRef]
- Liao, W.; Rao, Z.; Wu, L.; Chen, Y.; Li, C. Cariporide attenuates doxorubicin-induced cardiotoxicity in rats by inhibiting oxidative stress, inflammation, and apoptosis partly through regulation of Akt/GSK-3β and Sirt1 signaling pathway. Front. Pharmacol. 2022, 13, 850053. [Google Scholar] [CrossRef]
- Haybar, H.; Goudarzi, M.; Mehrzadi, S.; Aminzadeh, A.; Khodayar, M.J.; Kalantar, M.; Fatemi, I. Effect of gemfibrozil on cardiotoxicity induced by doxorubicin in male experimental rats. Biomed. Pharmacother. 2019, 109, 530–535. [Google Scholar] [CrossRef]
- Atta, A.H.; Atta, S.A.; Khattab, M.S.; El-Aziz, T.H.A.; Mouneir, S.M.; Ibrahim, M.A.; Nasr, S.M.; Emam, S.R. Ceratonia siliqua pods (Carob) methanol extract alleviates doxorubicin-induced nephrotoxicity via antioxidant, anti-inflammatory and anti-apoptotic pathways in rats. Environ. Sci. Pollut. Res. 2023, 30, 83421–83438. [Google Scholar] [CrossRef]
- Estruel-Amades, S.; Massot-Cladera, M.; Garcia-Cerdà, P.; Pérez-Cano, F.J.; Franch; Castell; Camps-Bossacoma, M. Protective effect of hesperidin on the oxidative stress induced by an exhausting exercise in intensively trained rats. Nutrients 2019, 11, 783. [Google Scholar] [CrossRef]
- Ileriturk, M.; Kandemir, O.; Akaras, N.; Simsek, H.; Genc, A.; Kandemir, F.M. Hesperidin has a protective effect on paclitaxel-induced testicular toxicity through regulating oxidative stress, apoptosis, inflammation, and endoplasmic reticulum stress. Reprod. Toxicol. 2023, 118, 108369. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Dong, H.; Huang, N.; Fang, J. Oxidative stress and inflammation regulation of sirtuins: New insights into common oral diseases. Front. Physiol. 2022, 13, 953078. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and its roles in inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, Q.; Zeng, Z.; Wu, J.; Zhang, Y.; Chen, Z. Sirt1 inhibits oxidative stress in vascular endothelial cells. Oxidative Med. Cell. Longev. 2017, 2017, 7543973. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, J.; Liu, M.; Zhang, M.; Xue, Y.; Zhang, Y.; Han, X.; Jing, X.; Chu, L. Hesperetin modulates the Sirt1/Nrf2 signaling pathway in counteracting myocardial ischemia through suppression of oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 2021, 139, 111552. [Google Scholar] [CrossRef]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 activation by natural phytochemicals: An overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Arunachalam, S.; Nagoor Meeran, M.; Azimullah, S.; Jha, N.K.; Saraswathiamma, D.; Subramanya, S.; Albawardi, A.; Ojha, S. α-Bisabolol Attenuates Doxorubicin Induced Renal Toxicity by Modulating NF-κB/MAPK Signaling and Caspase-Dependent Apoptosis in Rats. Int. J. Mol. Sci. 2022, 23, 10528. [Google Scholar] [CrossRef]
- Attallah, N.G.; El-Sherbeni, S.A.; El-Kadem, A.H.; Elekhnawy, E.; El-Masry, T.A.; Elmongy, E.I.; Altwaijry, N.; Negm, W.A. elucidation of the metabolite profile of Yucca gigantea and assessment of its cytotoxic, antimicrobial, and anti-inflammatory activities. Molecules 2022, 27, 1329. [Google Scholar] [CrossRef] [PubMed]
- Elekhnawy, E.; Negm, W.A. The potential application of probiotics for the prevention and treatment of COVID-19. Egypt. J. Med. Hum. Genet. 2022, 23, 36. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- AlAsmari, A.F.; Ali, N.; Alharbi, M.; Alqahtani, F.; Alasmari, F.; Almoqbel, D.; AlSwayyed, M.; Alshammari, A.; Alanazi, M.M.; Alhoshani, A. Geraniol ameliorates doxorubicin-mediated kidney injury through alteration of antioxidant status, inflammation, and apoptosis: Potential roles of NF-κB and Nrf2/Ho-1. Nutrients 2022, 14, 1620. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; He, H.; Zuo, Z.; Xu, Z.; Wei, Z.; Deng, J. The role of different SIRT1-mediated signaling pathways in toxic injury. Cell. Mol. Biol. Lett. 2019, 24, 36. [Google Scholar] [CrossRef] [PubMed]
- Bahrambeigi, S.; Khatamnezhad, M.; Asri-Rezaei, S.; Dalir-Naghadeh, B.; Javadi, S.; Mirzakhani, N. Pro-oxidant and degenerative effects of haloperidol under inflammatory conditions in rat; the involvement of SIRT1 and NF-κB signaling pathways. In Veterinary Research Forum; Faculty of Veterinary Medicine, Urmia University: Urmia, Iran, 2021; Volume 12, p. 175. [Google Scholar]
- Ma, B.; Zhu, Z.; Zhang, J.; Ren, C.; Zhang, Q. Aucubin alleviates diabetic nephropathy by inhibiting NF-κB activation and inducing SIRT1/SIRT3-FOXO3a signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. J. Funct. Foods 2020, 64, 103702. [Google Scholar] [CrossRef]
- Li, D.; Liu, X.; Pi, W.; Zhang, Y.; Yu, L.; Xu, C.; Sun, Z.; Jiang, J. Fisetin attenuates doxorubicin-induced cardiomyopathy in vivo and in vitro by inhibiting ferroptosis through SIRT1/Nrf2 signaling pathway activation. Front. Pharmacol. 2022, 12, 808480. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Dong, C.; Patel, J.; Duan, C.; Wang, X.; Wu, X.; Cao, Y.; Pu, L.; Lu, D.; Shen, T. SIRT1 suppresses doxorubicin-induced cardiotoxicity by regulating the oxidative stress and p38MAPK pathways. Cell. Physiol. Biochem. 2015, 35, 1116–1124. [Google Scholar] [CrossRef]
- Reis-Mendes, A.; Padrão, A.I.; Duarte, J.A.; Gonçalves-Monteiro, S.; Duarte-Araújo, M.; Remião, F.; Carvalho, F.; Sousa, E.; Bastos, M.L.; Costa, V.M. Role of inflammation and redox status on doxorubicin-induced cardiotoxicity in infant and adult CD-1 male mice. Biomolecules 2021, 11, 1725. [Google Scholar] [CrossRef]
- Elblehi, S.S.; El-Sayed, Y.S.; Soliman, M.M.; Shukry, M. Date palm pollen extract avert doxorubicin-induced cardiomyopathy fibrosis and associated oxidative/nitrosative stress, inflammatory cascade, and apoptosis-targeting bax/bcl-2 and caspase-3 signaling pathways. Animals 2021, 11, 886. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, S.; Li, Y.; Zhang, D.; Wang, B.; Xie, J.; Wang, J. Regulated cell death: Discovery, features and implications for neurodegenerative diseases. Cell Commun. Signal. 2021, 19, 120. [Google Scholar] [CrossRef]
- Nematollahi, H.; Gh, H.; Jorat, M. The Effect of Vitamin C on Apoptosis and Bax/Bcl-2 Proteins Ratio in Peripheral Blood Lymphocytes of Patients during Cardiac Interventional Procedures. J. Biomed. Phys. Eng. 2020, 10, 421. [Google Scholar] [CrossRef]
- Vu, M.; Kassouf, N.; Ofili, R.; Lund, T.; Bell, C.; Appiah, S. Doxorubicin selectively induces apoptosis through the inhibition of a novel isoform of Bcl-2 in acute myeloid leukemia MOLM-13 cells with reduced Beclin 1 expression. Int. J. Oncol. 2020, 57, 113–121. [Google Scholar] [CrossRef]
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Famurewa, A.C.; Renu, K.; Eladl, M.A.; Chakraborty, R.; Myakala, H.; El-Sherbiny, M.; Elsherbini, D.M.A.; Vellingiri, B.; Madhyastha, H.; Wanjari, U.R. Hesperidin and hesperetin against heavy metal toxicity: Insight on the molecular mechanism of mitigation. Biomed. Pharmacother. 2022, 149, 112914. [Google Scholar] [CrossRef]
- Atoki, A.V.; Aja, P.M.; Shinkafi, T.S.; Ondari, E.N.; Awuchi, C.G. Hesperidin play beneficial roles in disorders associated with the central nervous system: A review. Int. J. Food Prop. 2023, 26, 1867–1884. [Google Scholar] [CrossRef]
- Anson, F.; Thayumanavan, S.; Hardy, J.A. Exogenous introduction of initiator and executioner caspases results in different apoptotic outcomes. JACS Au 2021, 1, 1240–1256. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer 2016, 138, 1058–1066. [Google Scholar] [CrossRef]
- D’Ignazio, L.; Batie, M.; Rocha, S. Hypoxia and inflammation in cancer, focus on HIF and NF-κB. Biomedicines 2017, 5, 21. [Google Scholar] [CrossRef]
- Zeng, C.-Y.; Wang, X.-F.; Hua, F.-Z. HIF-1α in osteoarthritis: From pathogenesis to therapeutic implications. Front. Pharmacol. 2022, 13, 927126. [Google Scholar] [CrossRef]
- Kongtawelert, P.; Wudtiwai, B.; Shwe, T.H.; Pothacharoen, P.; Phitak, T. Inhibitory effect of hesperidin on the expression of programmed death ligand (PD-L1) in breast cancer. Molecules 2020, 25, 252. [Google Scholar] [CrossRef]
- Shakiba, E.; Bazi, A.; Ghasemi, H.; Gorji, R.E.; Mehdipour, S.A.; Nikfar, B.; Rashidi, M.; Mirzaei, S. Hesperidin suppressed metastasis, angiogenesis and tumor growth in Balb/c mice model of breast cancer. J. Cell. Mol. Med. 2023, 27, 2756–2769. [Google Scholar] [CrossRef]
- Zimna, A.; Kurpisz, M. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: Applications and therapies. BioMed Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef] [PubMed]
- Kierans, S.; Taylor, C. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Khotina, V.A.; Omelchenko, A.V.; Kalmykov, V.A.; Orekhov, A.N. The role of the VEGF family in atherosclerosis development and its potential as treatment targets. Int. J. Mol. Sci. 2022, 23, 931. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Im, A.-R.; Lee, J.Y.; Kim, T.; Ji, K.-Y.; Park, D.-H.; Chae, S. Hesperidin Inhibits UVB-Induced VEGF Production and Angiogenesis via the Inhibition of PI3K/Akt Pathway in HR-1 Hairless Mice. Biol. Pharm. Bull. 2021, 44, 1492–1498. [Google Scholar] [CrossRef]
- Zalpoor, H.; Bakhtiyari, M.; Shapourian, H.; Rostampour, P.; Tavakol, C.; Nabi-Afjadi, M. Hesperetin as an anti-SARS-CoV-2 agent can inhibit COVID-19-associated cancer progression by suppressing intracellular signaling pathways. Inflammopharmacology 2022, 30, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-M.; Tang, P.M.-K.; Li, J.; Lan, H.Y. TGF-β/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, Y.; Liu, F. Transforming growth factor-beta1 in diabetic kidney disease. Front. Cell Dev. Biol. 2020, 8, 187. [Google Scholar] [CrossRef]
- Yuan, Q.; Tang, B.; Zhang, C. Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal Transduct. Target. Ther. 2022, 7, 182. [Google Scholar] [CrossRef]
- Matias, I.; Diniz, L.P.; Buosi, A.; Neves, G.; Stipursky, J.; Gomes, F.C.A. Flavonoid hesperidin induces synapse formation and improves memory performance through the astrocytic TGF-β1. Front. Aging Neurosci. 2017, 9, 184. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jin, B.-R.; An, H.-J. Hesperidin ameliorates benign prostatic hyperplasia by attenuating cell proliferation, inflammatory response, and epithelial-mesenchymal transition via the TGF-β1/Smad signaling pathway. Biomed. Pharmacother. 2023, 160, 114389. [Google Scholar] [CrossRef]
- Zhang, W.R.; Parikh, C.R. Biomarkers of acute and chronic kidney disease. Annu. Rev. Physiol. 2019, 81, 309–333. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.-C.; Yang, S.-Y.; Chiou, T.T.-Y.; Shiao, C.-C.; Wu, C.-H.; Huang, C.-T.; Wang, T.-J.; Chen, J.-Y.; Liao, H.-W.; Chen, S.-Y. Comparative accuracy of biomarkers for the prediction of hospital-acquired acute kidney injury: A systematic review and meta-analysis. Crit. Care 2022, 26, 349. [Google Scholar] [CrossRef] [PubMed]
- Srilatha, D.; Nasare, M.; Nagasandhya, B.; Prasad, V.; Diwan, P. Development and Validation of UV Spectrophotometric Method for Simultaneous Estimation of Hesperidin and Diosmin in the Pharmaceutical Dosage Form. ISRN Spectrosc. 2013, 2013, 534830. [Google Scholar] [CrossRef]
- Elshazly, S.M.; Abd El Motteleb, D.M.; Ibrahim, I.A.A.E.H. Hesperidin Protects against Stress-Induced Gastric Ulcer through Regulation of Peroxisome Proliferator Activator Receptor Gamma in Diabetic Rats. Chem. Biol. Interact. 2018, 291, 153–161. [Google Scholar] [CrossRef]
- Ibrahim, K.M.; Mantawy, E.M.; Elanany, M.M.; Abdelgawad, H.S.; Khalifa, N.M.; Hussien, R.H.; El-Agroudy, N.N.; El-demerdash, E. Protection from Doxorubicin-Induced Nephrotoxicity by Clindamycin: Novel Antioxidant, Anti-Inflammatory and Anti-Apoptotic Roles. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Poljak, A.; Grant, R.; Jayasena, T.; Mansour, H.; Chan-Ling, T.; Smythe, G.; Sachdev, P.; Guillemin, G.J. Differential Expression of Sirtuins in the Aging Rat Brain. Front. Cell. Neurosci. 2015, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Kinouchi, S. Changes in Apoptosis-Related Genes (Bcl-2, Bax) in the Urethras of Old Female Rats Following Estrogen Replacement. Yonago Acta Med. 2003, 46, 109–115. [Google Scholar]
- Peng, J.; Lai, Z.G.; Fang, Z.L.; Xing, S.; Hui, K.; Hao, C.; Jin, Q.; Qi, Z.; Shen, W.J.; Dong, Q.N.; et al. Dimethyloxalylglycine Prevents Bone Loss in Ovariectomized C57BL/6J Mice through Enhanced Angiogenesis and Osteogenesis. PLoS ONE 2014, 9, e0124702. [Google Scholar] [CrossRef]
- He, Q.; Yang, Q.C.; Zhou, Q.; Zhu, H.; Niu, W.Y.; Feng, J.; Wang, Y.; Cao, J.; Chen, B.Y. Effects of Varying Degrees of Intermittent Hypoxia on Proinflammatory Cytokines and Adipokines in Rats and 3T3-L1 Adipocytes. PLoS ONE 2014, 9, e86326. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical Analysis of Real-Time PCR Data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Sisto, F.; Miluzio, A.; Leopardi, O.; Mirra, M.; Boelaert, J.R.; Taramelli, D. Differential Cytokine Pattern in the Spleens and Livers of BALB/c Mice Infected with Penicillium Marneffei: Protective Role of Gamma Interferon. Infect. Immun. 2003, 71, 465–473. [Google Scholar] [CrossRef]
- Saber, S.; Khalil, R.M.; Abdo, W.S.; Nassif, D.; El-Ahwany, E. Olmesartan Ameliorates Chemically-Induced Ulcerative Colitis in Rats via Modulating NFκB and Nrf-2/HO-1 Signaling Crosstalk. Toxicol. Appl. Pharmacol. 2019, 364, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.C.; Ombredane, A.S.; Souza, J.M.T.; Vasconcelos, A.G.; Plácido, A.; das GNAmorim, A.; Barbosa, E.A.; Lima, F.C.; Ropke, C.D.; Alves, M.M.; et al. Lycopene-Rich Extract from Red Guava (Psidium guajava L.) Displays Cytotoxic Effect against Human Breast Adenocarcinoma Cell Line MCF-7 via an Apoptotic-like Pathway. Food Res. Int. 2018, 105, 184–196. [Google Scholar] [CrossRef] [PubMed]






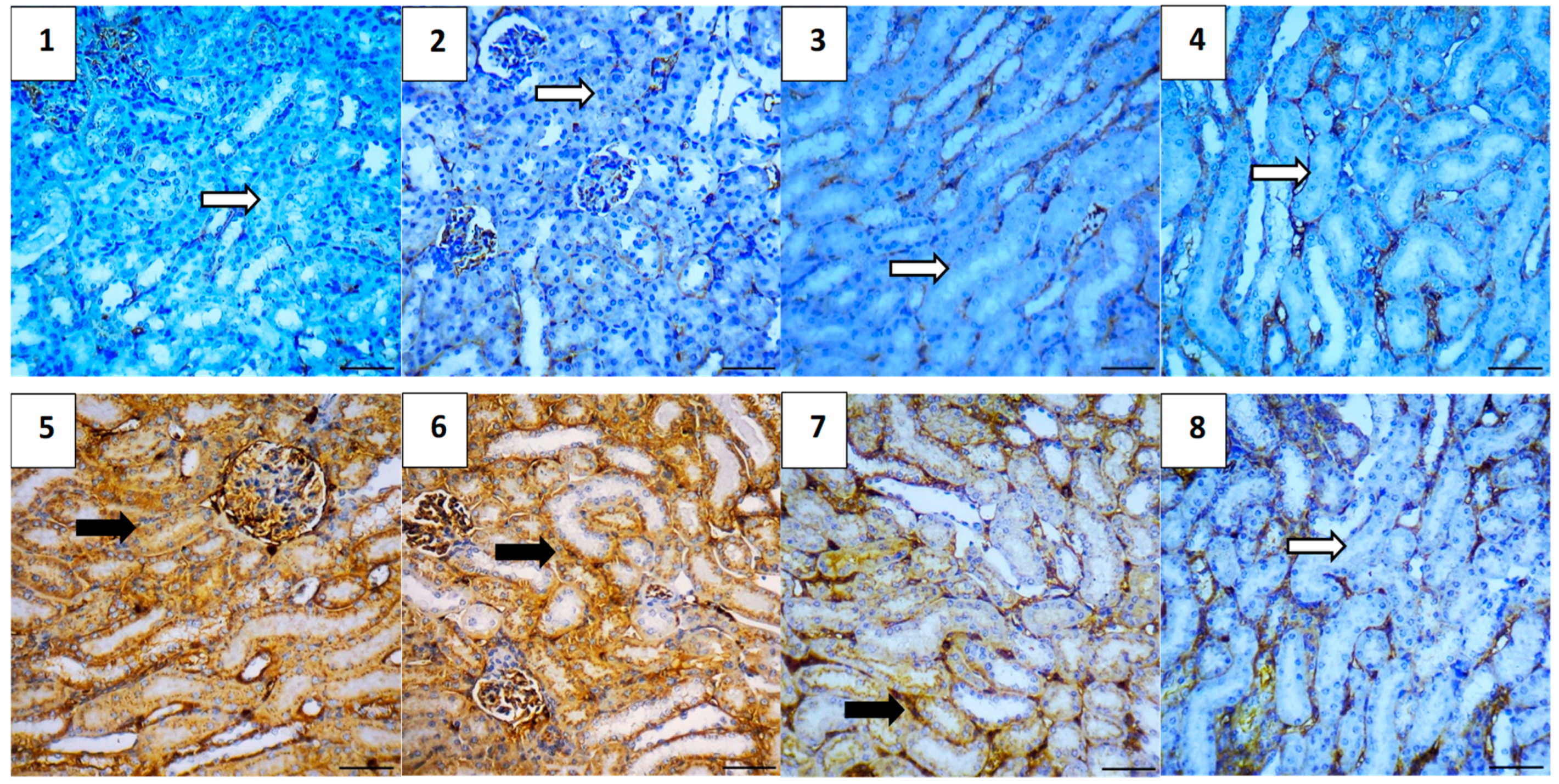
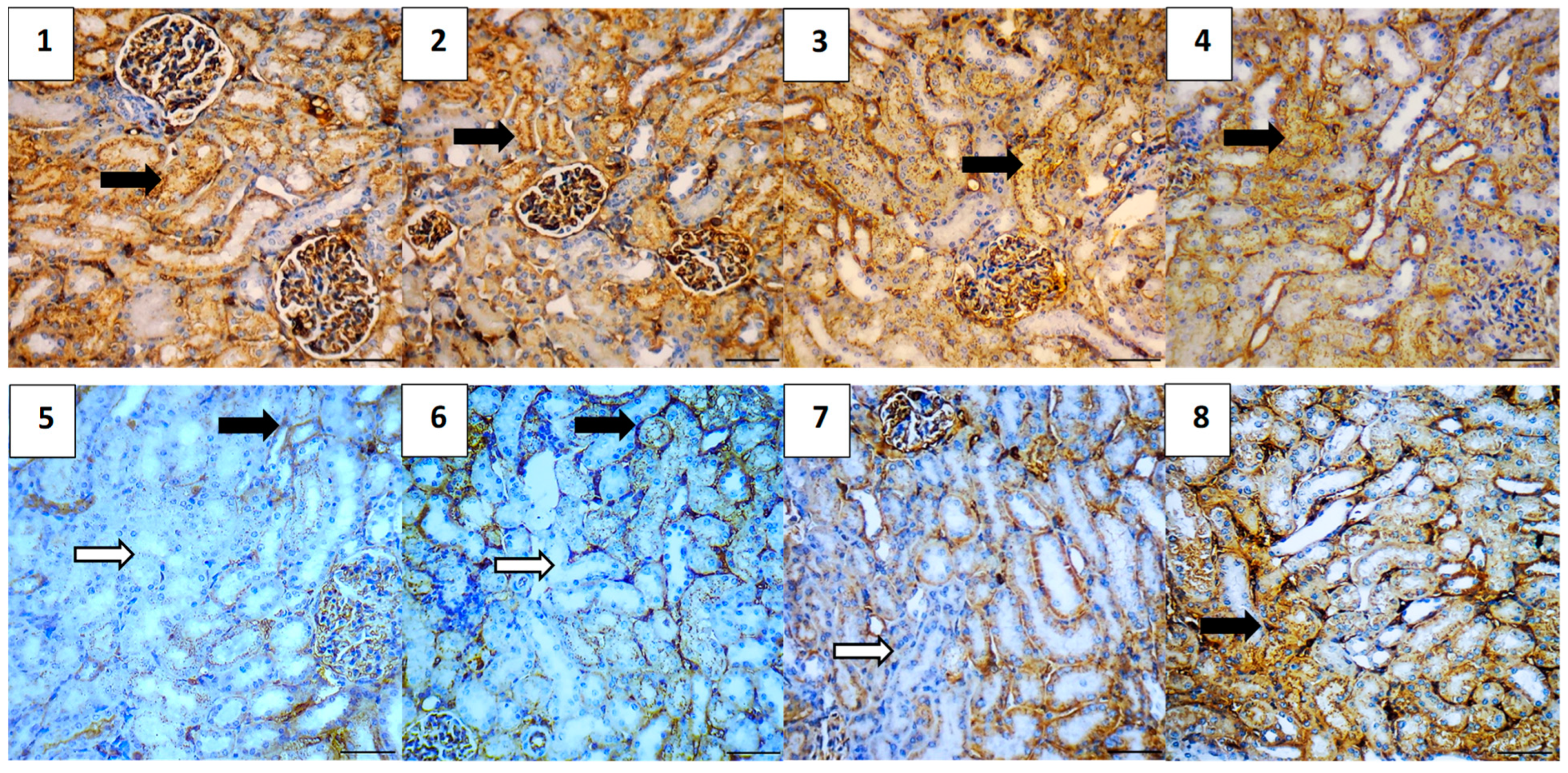
| Parameter | Mean | Range |
|---|---|---|
| % Entrapment efficiency | 83.00 ± 7.3 | 77.27–91.34 |
| % Yield | 31.16 ± 1.4 | 25.0–29.8 |
| Particle size (nm) | 127 ± 32.15 | 107.4–164.1 |
| Zeta potential (mV) | −51.12 ± 9.79 | −60.26–−38.37 |
| Group | Average of Total Immunoreactivity Score (IRS) | ||||
|---|---|---|---|---|---|
| Caspase-3 | NF-κB | TGF-β | BAX | Bcl-2 | |
| Vehicle Control | 0.0 | 0.0 | 0.4 | 0.4 | 5.2 |
| Polymer Control | 0.4 | 0.0 | 0.4 | 0.4 | 4.8 |
| Hes | 0.4 | 0.4 | 0.0 | 0.4 | 5.0 |
| Hes-NPs | 0.4 | 0.0 | 0.0 | 0.4 | 5.0 |
| DOX | 4.6 | 4.4 | 3.8 | 5.0 | 2.5 |
| Hes-DOX | 3.6 | 2.6 | 2.0 | 3.2 | 3.4 |
| Hes-NPs-DOX | 2.4 | 1.6 | 1.2 | 2.0 | 4.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alherz, F.A.; El-Masry, T.A.; Oriquat, G.A.; Elekhnawy, E.; Al-Shaalan, N.H.; Gaballa, M.M.S.; El Zahaby, E.I.; El-Nagar, M.M.F. Hesperidin Nanoformulation: A Potential Strategy for Reducing Doxorubicin-Induced Renal Damage via the Sirt-1/HIF1-α/VEGF/NF-κB Signaling Cascade. Pharmaceuticals 2024, 17, 1144. https://doi.org/10.3390/ph17091144
Alherz FA, El-Masry TA, Oriquat GA, Elekhnawy E, Al-Shaalan NH, Gaballa MMS, El Zahaby EI, El-Nagar MMF. Hesperidin Nanoformulation: A Potential Strategy for Reducing Doxorubicin-Induced Renal Damage via the Sirt-1/HIF1-α/VEGF/NF-κB Signaling Cascade. Pharmaceuticals. 2024; 17(9):1144. https://doi.org/10.3390/ph17091144
Chicago/Turabian StyleAlherz, Fatemah A., Thanaa A. El-Masry, Ghaleb A. Oriquat, Engy Elekhnawy, Nora Hamad Al-Shaalan, Mohamed M. S. Gaballa, Enas I. El Zahaby, and Maysa M. F. El-Nagar. 2024. "Hesperidin Nanoformulation: A Potential Strategy for Reducing Doxorubicin-Induced Renal Damage via the Sirt-1/HIF1-α/VEGF/NF-κB Signaling Cascade" Pharmaceuticals 17, no. 9: 1144. https://doi.org/10.3390/ph17091144
APA StyleAlherz, F. A., El-Masry, T. A., Oriquat, G. A., Elekhnawy, E., Al-Shaalan, N. H., Gaballa, M. M. S., El Zahaby, E. I., & El-Nagar, M. M. F. (2024). Hesperidin Nanoformulation: A Potential Strategy for Reducing Doxorubicin-Induced Renal Damage via the Sirt-1/HIF1-α/VEGF/NF-κB Signaling Cascade. Pharmaceuticals, 17(9), 1144. https://doi.org/10.3390/ph17091144








