Safety of Nintedanib in a Patient with Chronic Pulmonary Fibrosis and Kidney Disease
Abstract
1. Introduction
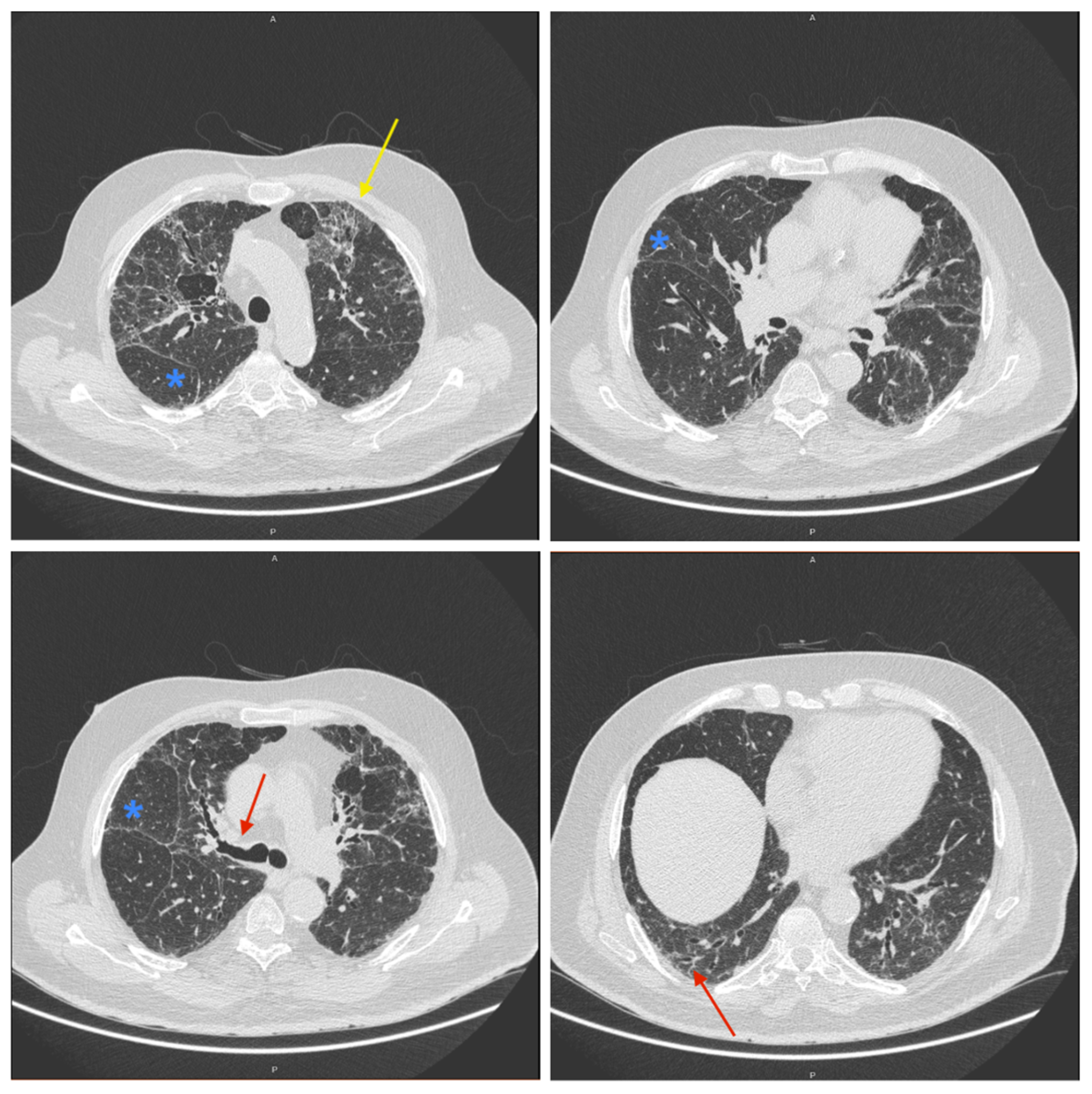
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.J.; Perlman, D.; Tomic, R. Natural history of idiopathic pulmonary fibrosis. Respir. Med. 2015, 109, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Sankari, A.; Chapman, K. Idiopathic Pulmonary Fibrosis; Ullah, S., Ed.; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Dempsey, T.M.; Payne, S.; Sangaralingham, L.; Yao, X.; Shah, N.D.; Limper, A.H. Adoption of the antifibrotic medications pirfenidone and nintedanib for patients with idiopathic pulmonary fibrosis. Ann. Am. Thorac. Soc. 2021, 18, 1121–1128. [Google Scholar] [CrossRef]
- Finnerty, J.P.; Ponnuswamy, A.; Dutta, P.; Abdelaziz, A.; Kamil, H. Efficacy of antifibrotic drugs, nintedanib and pirfenidone, in treatment of progressive pulmonary fibrosis in both idiopathic pulmonary fibrosis (IPF) and non-IPF: A systematic review and meta-analysis. BMC Pulm. Med. 2021, 21, 411. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Gandin, I.; Pozzan, R.; Tavano, S.; Bozzi, C.; Hughes, M.; Kodric, M.; Cifaldi, R.; Lerda, S.; Confalonieri, M.; et al. Nintedanib in idiopathic pulmonary fibrosis: Tolerability and safety in a real life experience in a single centre in patients also treated with oral anticoagulant therapy. Pharmaceuticals 2023, 16, 307. [Google Scholar] [CrossRef]
- Chianese, M.; Screm, G.; Salton, F.; Confalonieri, P.; Trotta, L.; Barbieri, M.; Ruggero, L.; Mari, M.; Reccardini, N.; Geri, P.; et al. Pirfenidone and Nintedanib in Pulmonary Fibrosis: Lights and Shadows. Pharmaceuticals 2024, 17, 709. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, L.; Qi, H.; Wang, J.; Wang, Y.; Jiang, W.; Xu, L.; Liu, N.; Zhuang, S. Nintedanib, a triple tyrosine kinase inhibitor, attenuates renal fibrosis in chronic kidney disease. Clin. Sci. 2017, 131, 2125–2143. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for Nintedanib. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nintedanib (accessed on 4 August 2024).
- Burns, K.; Nair, P.C.; Rowland, A.; Mackenzie, P.I.; Knights, K.M.; Miners, J.O. The nonspecific binding of tyrosine kinase inhibitors to human liver microsomes. Drug Metab. Dispos. 2015, 43, 1934–1937. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.K.; Shen, D.D.; Thummel, K.E.; Himmelfarb, J. Effects of chronic kidney disease and uremia on hepatic drug metabolism and transport. Kidney Int. 2014, 85, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.; Nigam, S.; Parnham, A.; Srinivasa, V. Anti-glomerular basement membrane glomerulonephritis following nintedanib for idiopathic pulmonary fibrosis: A case report. J. Med. Case Rep. 2017, 11, 214. [Google Scholar] [CrossRef][Green Version]
- Wilson, C.B.; Dixon, F.J. Anti-glomerular basement membrane antibody-induced glomerulonephritis. Kidney Int. 1973, 3, 74–89. [Google Scholar] [CrossRef]
- Saint-Pierre, M. Tolerability of nintedanib in severe chronic kidney disease (CKD): A case study. Chest 2019, 156, A1309. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.B.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.C.; Portela, J.; Couto, C.; Duarte, J.; Martins, N.; Soares, J. Pulmonary Fibrosis Secondary to Oxaliplatin Treatment: From Rarity to Reality: A Case Study and Literature Review. Oncol Ther. 2020, 8, 183–190. [Google Scholar] [CrossRef]
- Sesé, L.; Nunes, H.; Cottin, V.; Israel-Biet, D.; Crestani, B.; Guillot-Dudoret, S.; Cadranel, J.; Wallaert, B.; Tazi, A.; Maître, B.; et al. Gender differences in idiopathic pulmonary fibrosis: Are men and women equal? Front. Med. 2021, 8, 713698. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, D.M.; Šterclová, M.; Mogulkoc, N.; Lewandowska, K.; Müller, V.; Hájková, M.; Studnicka, M.; Tekavec-Trkanjec, J.; Littnerová, S.; Vašáková, M. Comorbidity burden and survival in patients with idiopathic pulmonary fibrosis: The EMPIRE registry study. Respir. Res. 2023, 23, 135. [Google Scholar] [CrossRef]
- Kreuter, M.; Ehlers-Tenenbaum, S.; Palmowski, K.; Bruhwyler, J.; Oltmanns, U.; Muley, T.; Heussel, C.P.; Warth, A.; Kolb, M.; Herth, F.J. Impact of comorbidities on mortality in patients with idiopathic pulmonary fibrosis. PLoS ONE 2016, 11, e0151425. [Google Scholar] [CrossRef]
- Gembillo, G.; Calimeri, S.; Tranchida, V.; Silipigni, S.; Vella, D.; Ferrara, D.; Spinella, C.; Santoro, D.; Visconti, L. Lung dysfunction and chronic kidney disease: A complex network of multiple interactions. J. Pers. Med. 2023, 13, 286. [Google Scholar] [CrossRef]
- de Souza Rezende, P.; Porcher Andrade, F.; Ferraro Dos Santos Borba, C.; Eidt Rovedder, P.M. Pulmonary function, muscle strength, and quality of life have differed between chronic kidney disease patients and healthy individuals. Ther. Apher. Dial. 2022, 26, 337–344. [Google Scholar] [CrossRef]
- Marzin, K.; Kretschmar, G.; Luedtke, D.; Kraemer, S.; Kuelzer, R.; Schlenker-Herceg, R.; Schmid, U.; Schnell, D.; Dallinger, C. Pharmacokinetics of Nintedanib in Subjects with Hepatic Impairment. J. Clin. Pharmacol. 2018, 58, 357–363. [Google Scholar] [CrossRef]
- Ruaro, B.; Salotti, A.; Reccardini, N.; Kette, S.; Da Re, B.; Nicolosi, S.; Zuccon, U.; Confalonieri, M.; Mondini, L.; Pozzan, R.; et al. Functional Progression after Dose Suspension or Discontinuation of Nintedanib in Idiopathic Pulmonary Fibrosis: A Real-Life Multicentre Study. Pharmaceuticals 2024, 17, 119. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, G.; Reiter, F.; Stocker, F.; Crispin, A.; Kneidinger, N.; Veit, T.; Klenner, F.; Ceelen, F.; Zimmermann, G.; Leuchte, H.; et al. Idiopathic Pulmonary Fibrosis Among Young Patients: Challenges in Diagnosis and Management. Lung 2018, 196, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Pizzorni, C.; Riccieri, V.; Decuman, S.; Brusselle, G.; DE Pauw, M.; Deschepper, E.; Piette, Y.; Ruaro, B.; Sulli, A.; et al. Stabilization of Microcirculation in Patients with Early Systemic Sclerosis with Diffuse Skin Involvement following Rituximab Treatment: An Open-label Study. J. Rheumatol. 2016, 43, 995–996. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Trombetta, A.C.; Melsens, K.; Pizzorni, C.; Sulli, A.; Ruaro, B.; Paolino, S.; Deschepper, E.; Smith, V. Automated assessment of absolute nailfold capillary number on videocapillaroscopic images: Proof of principle and validation in systemic sclerosis. Microcirculation 2018, 25, e12447. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Pozzan, R.; Confalonieri, P.; Tavano, S.; Hughes, M.; Matucci Cerinic, M.; Baratella, E.; Zanatta, E.; Lerda, S.; Geri, P.; et al. Gastroesophageal Reflux Disease in Idiopathic Pulmonary Fibrosis: Viewer or Actor? To Treat or Not to Treat? Pharmaceuticals 2022, 15, 1033. [Google Scholar] [CrossRef]
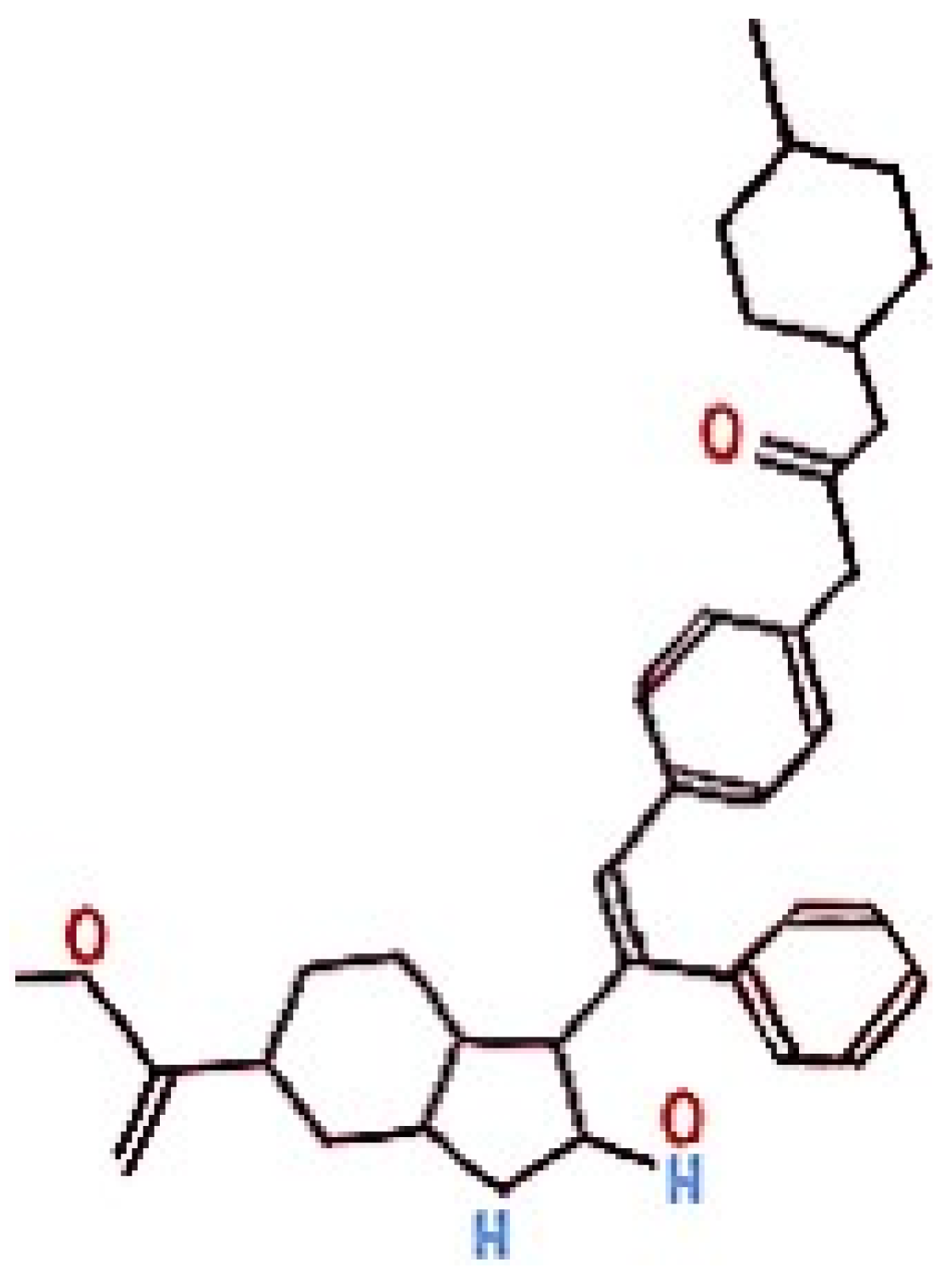
| Baseline Anamnestic Data | |
|---|---|
| Sex | M |
| Age | 77 |
| Smoking habits | Non-smoker |
| Comorbidities | Chronic renal failure St.5, DM2 1, Systemic HTN 2, HF 3, BPH 4, autoimmune thyroiditis, AdenoK of right colon undergoing haemicolectomy and subsequent adjuvant CT 5 |
| Ongoing pharmacological treatment | Bisoprolol, Enalapril, Furosemide, Ezetimibe/simvastatin, Nifedipine, Citalopram, Colecalciferol, Dutasteride, Lansoprazole, Prednisone, Sevelamer, Dulaglutide, Oxygen therapy, Nintedanib |
| Baseline blood exam at baseline | |
| Red blood cell count (RBC) | 4.26 × 106/μL |
| Hemoglobin (Hb) | 12 g/dL |
| White blood cell count (WBC) | 6.64 × 103/μL |
| Platelets (PLT) | 181 × 103/μL |
| Creatinine | 2.18 mg/dL |
| Sodium | 134 mEq/L |
| Potassium | 4.43 mEq/L |
| Urea | 60 mg/dL |
| Baseline respiratory function | |
| FEV1/FVC | 0.83 |
| TLC 6 | 57% |
| FEV1 7 | 67% |
| FVC 8 | 59% |
| DLCO 9 | 44% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggisano, M.; Mondini, L.; Chernovsky, M.; Confalonieri, P.; Salton, F.; Reccardini, N.; Kodric, M.; Geri, P.; Confalonieri, M.; Hughes, M.; et al. Safety of Nintedanib in a Patient with Chronic Pulmonary Fibrosis and Kidney Disease. Pharmaceuticals 2024, 17, 1147. https://doi.org/10.3390/ph17091147
Maggisano M, Mondini L, Chernovsky M, Confalonieri P, Salton F, Reccardini N, Kodric M, Geri P, Confalonieri M, Hughes M, et al. Safety of Nintedanib in a Patient with Chronic Pulmonary Fibrosis and Kidney Disease. Pharmaceuticals. 2024; 17(9):1147. https://doi.org/10.3390/ph17091147
Chicago/Turabian StyleMaggisano, Marta, Lucrezia Mondini, Maria Chernovsky, Paola Confalonieri, Francesco Salton, Nicolò Reccardini, Metka Kodric, Pietro Geri, Marco Confalonieri, Michael Hughes, and et al. 2024. "Safety of Nintedanib in a Patient with Chronic Pulmonary Fibrosis and Kidney Disease" Pharmaceuticals 17, no. 9: 1147. https://doi.org/10.3390/ph17091147
APA StyleMaggisano, M., Mondini, L., Chernovsky, M., Confalonieri, P., Salton, F., Reccardini, N., Kodric, M., Geri, P., Confalonieri, M., Hughes, M., Cifaldi, R., & Ruaro, B. (2024). Safety of Nintedanib in a Patient with Chronic Pulmonary Fibrosis and Kidney Disease. Pharmaceuticals, 17(9), 1147. https://doi.org/10.3390/ph17091147









