Evaluation of [68Ga]Ga-DOTA-AeK as a Potential Imaging Tool for PET Imaging of Cell Wall Synthesis in Bacterial Infections
Abstract
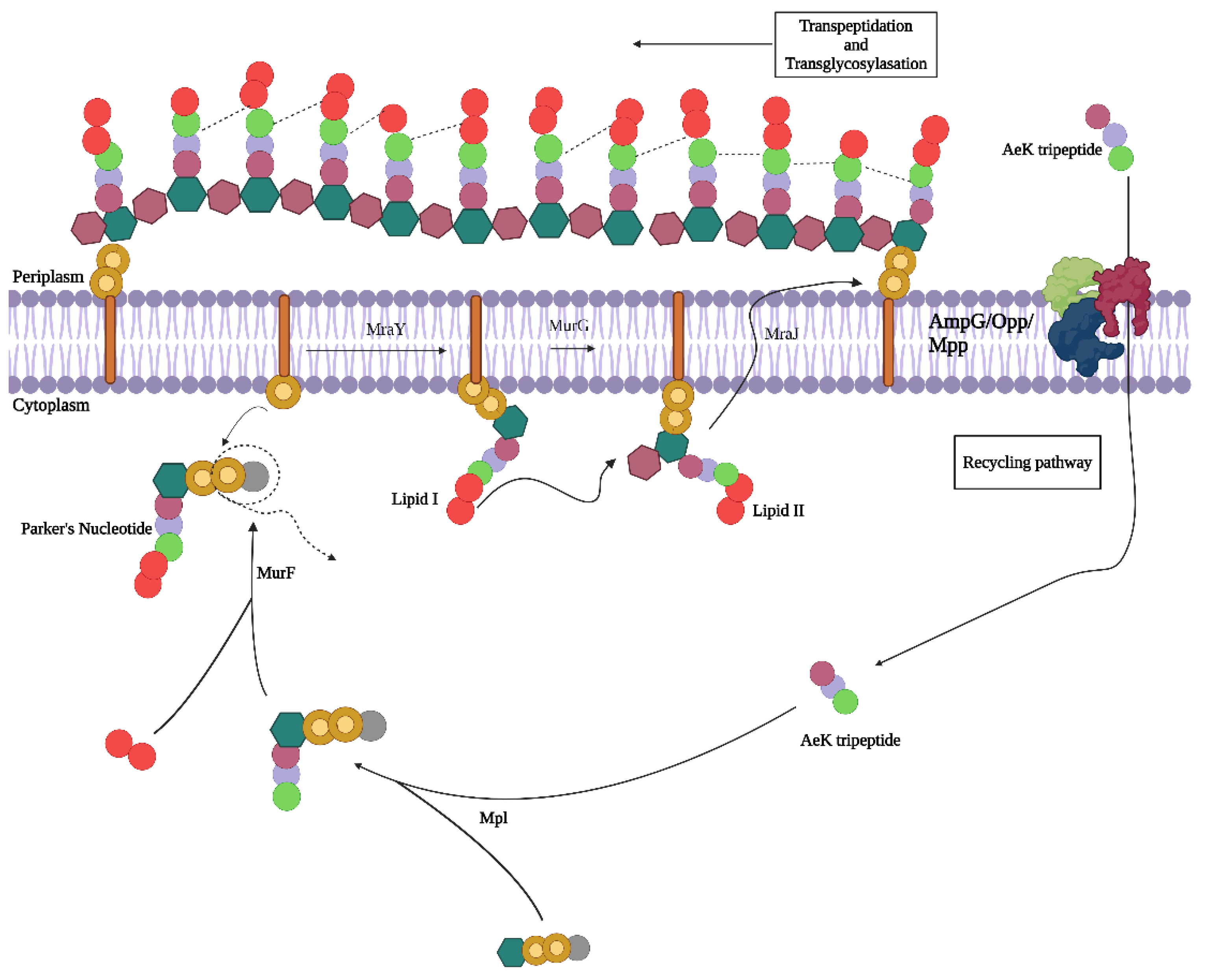
:1. Introduction
2. Results and Discussion
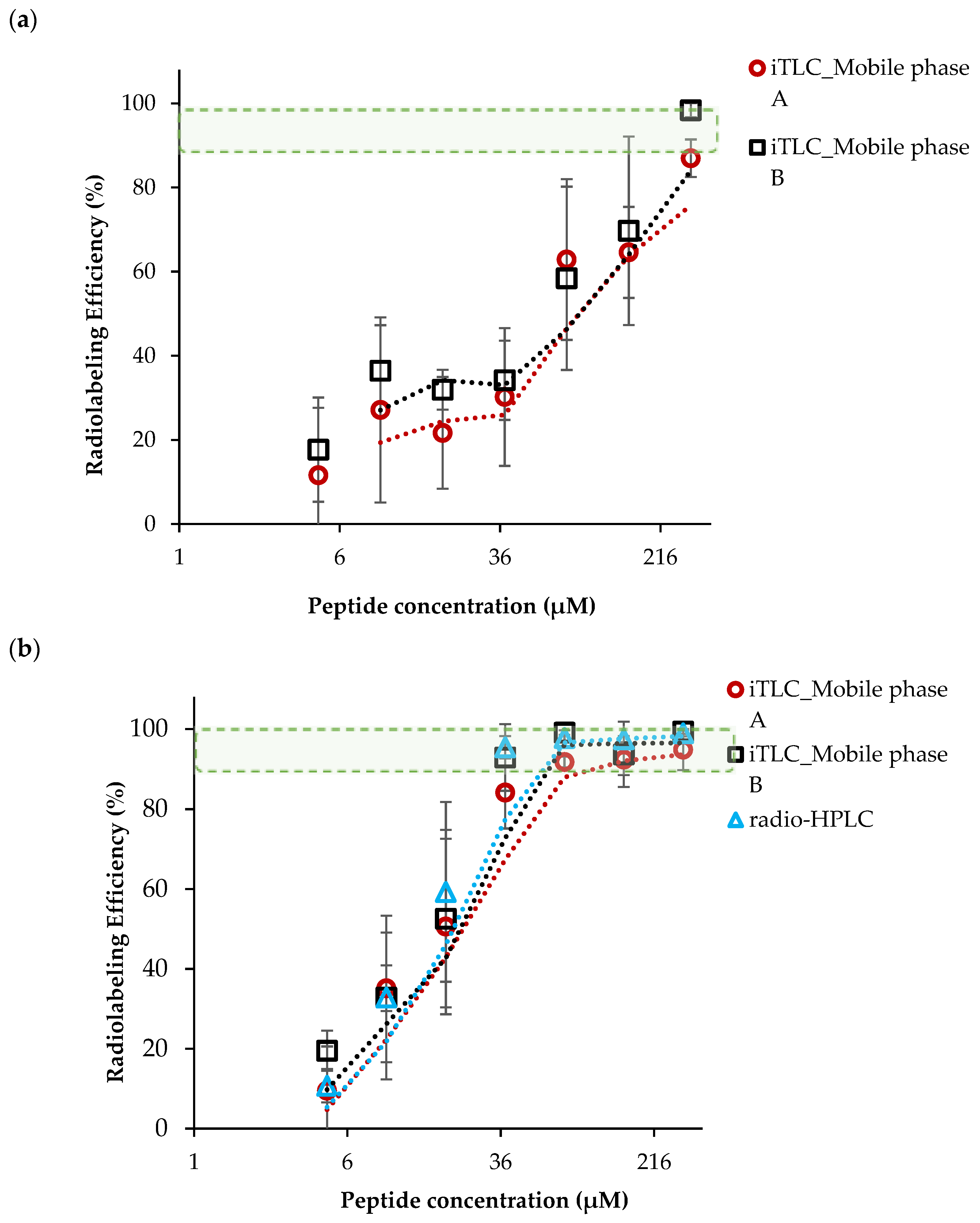
2.1. Development of 68Ga Radiolabeling Method for DOTA-AeK
2.1.1. Effects of Radiolabeling Conditions on Ga-68-Complexation of DOTA-AeK
2.1.2. Ga-68-Eluate Acidity and Vector Concentration (DOTA-AeK)
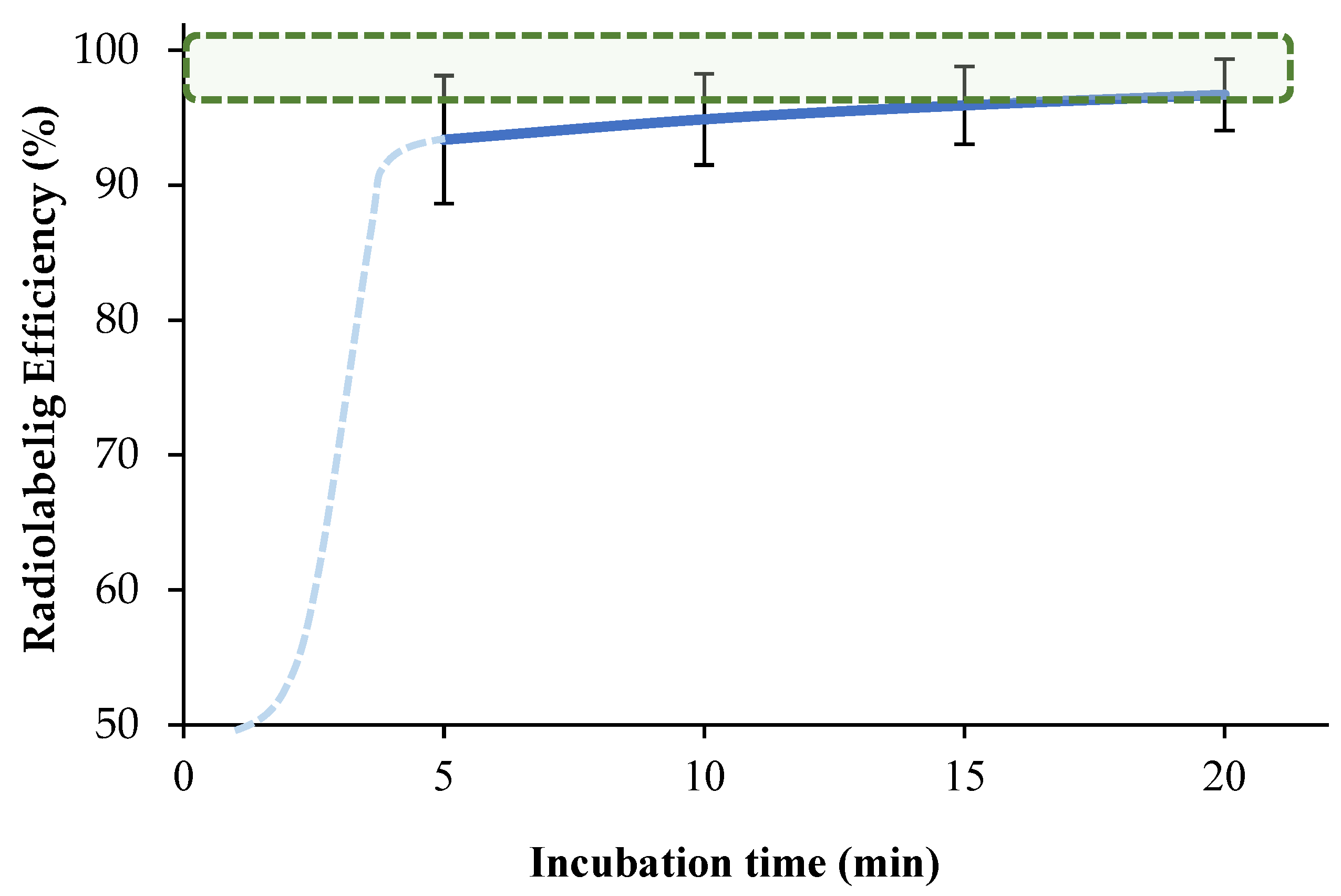
2.1.3. Optimization of Incubation Parameters
2.2. Development of a Purification Method of [68Ga]Ga-DOTA-AeK
2.3. Log P Determination for [68Ga]Ga-DOTA-AeK
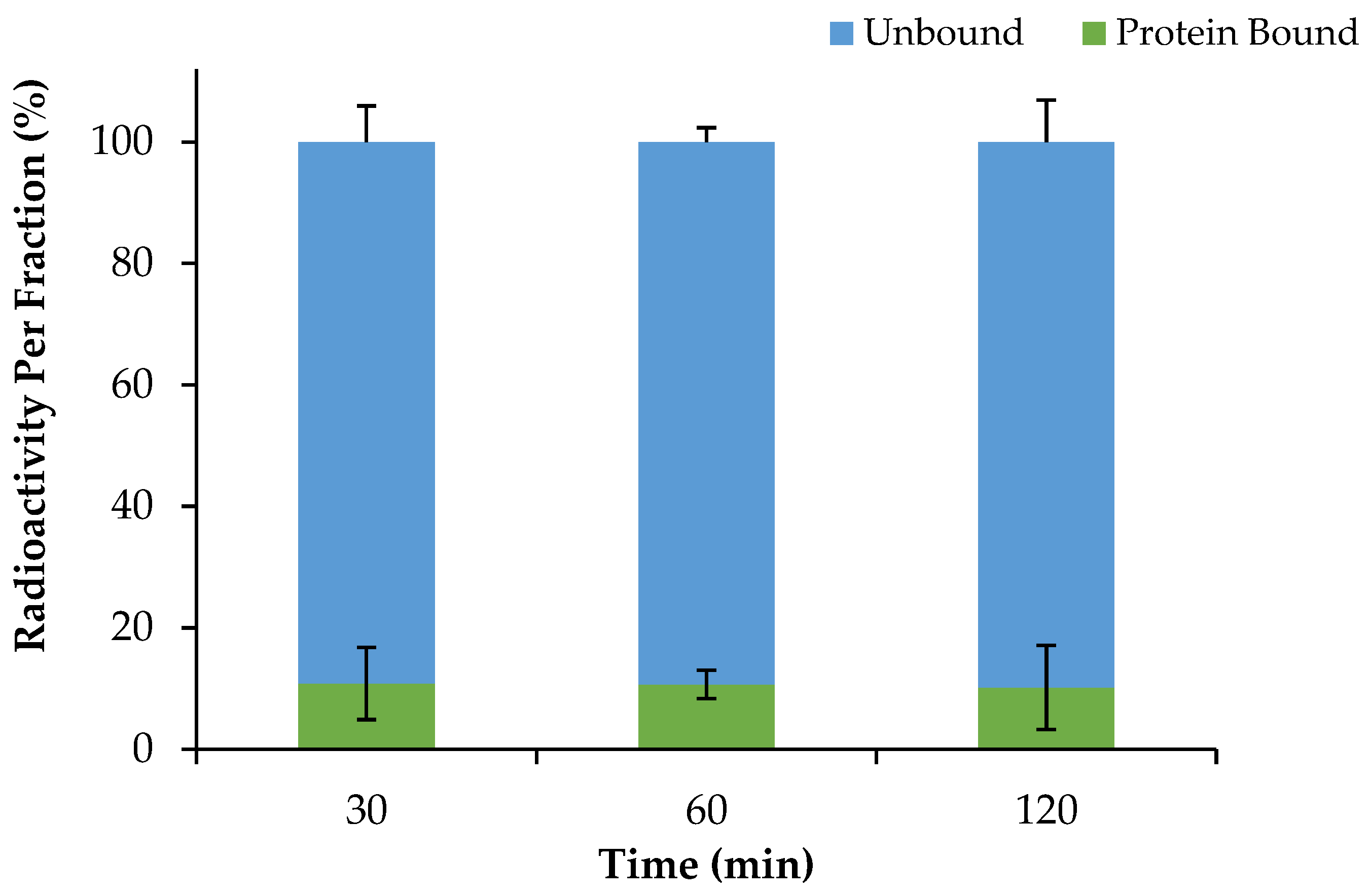
2.4. Serum Protein Binding of [68Ga]Ga-DOTA-AeK and Proteolytic Stability
2.5. Formulation Stability
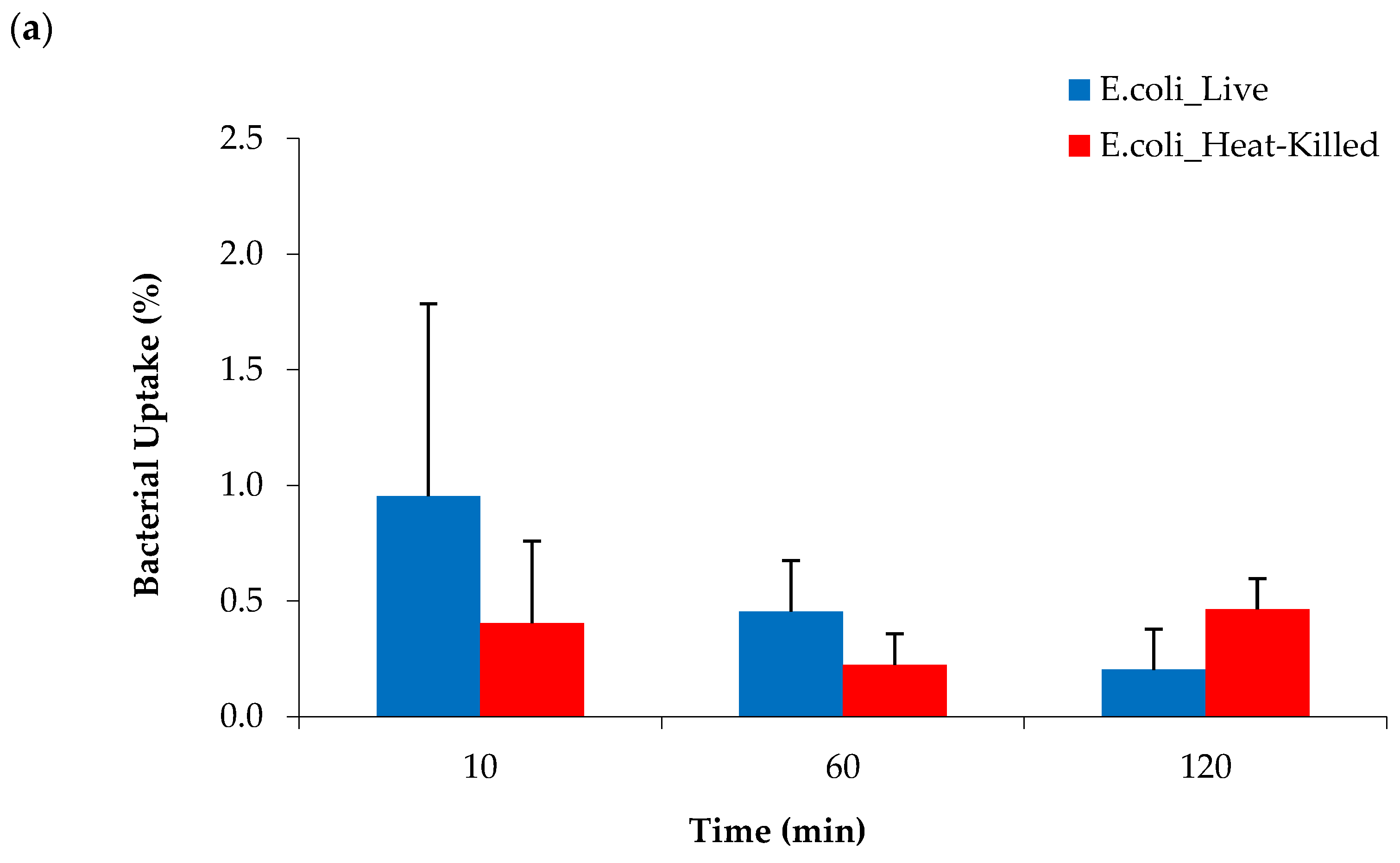
2.6. Bacterial Cell Uptake of [68Ga]Ga-DOTA-AeK
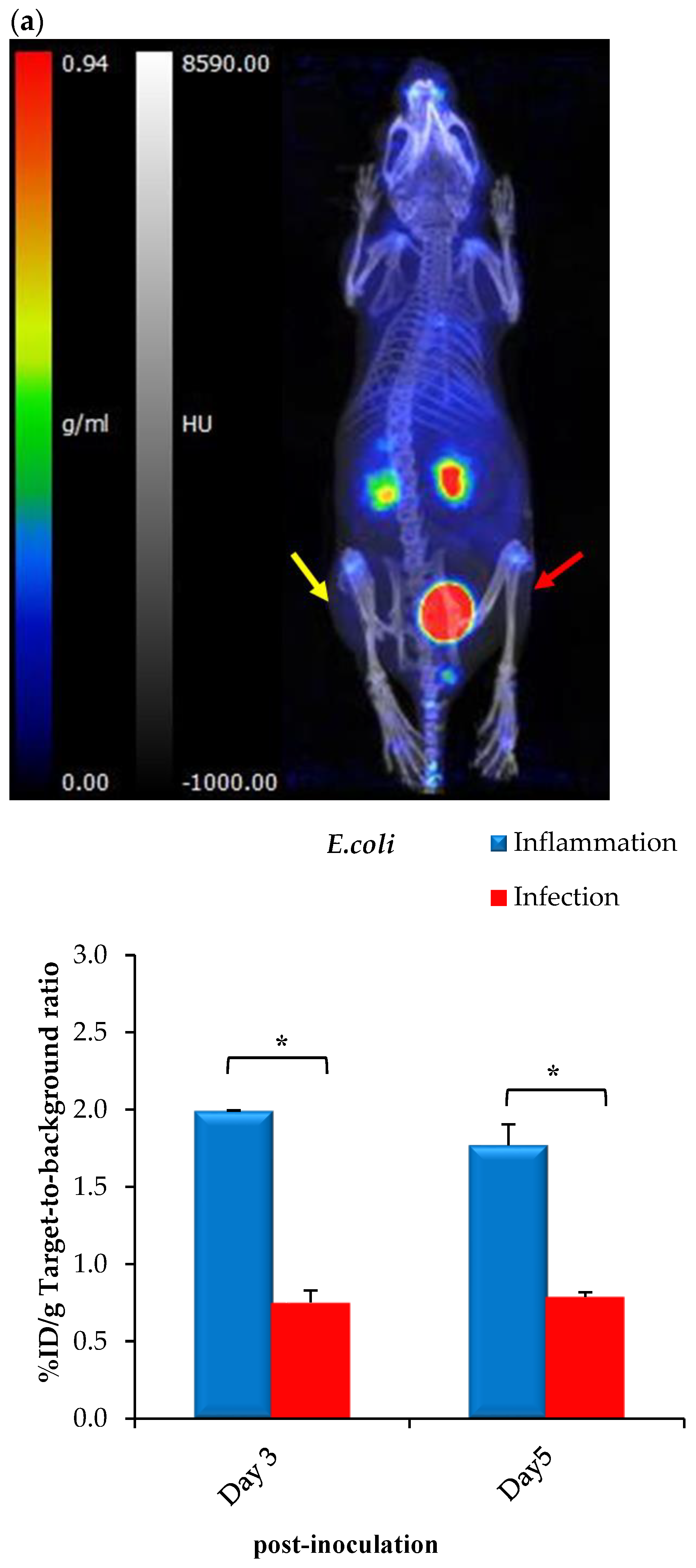
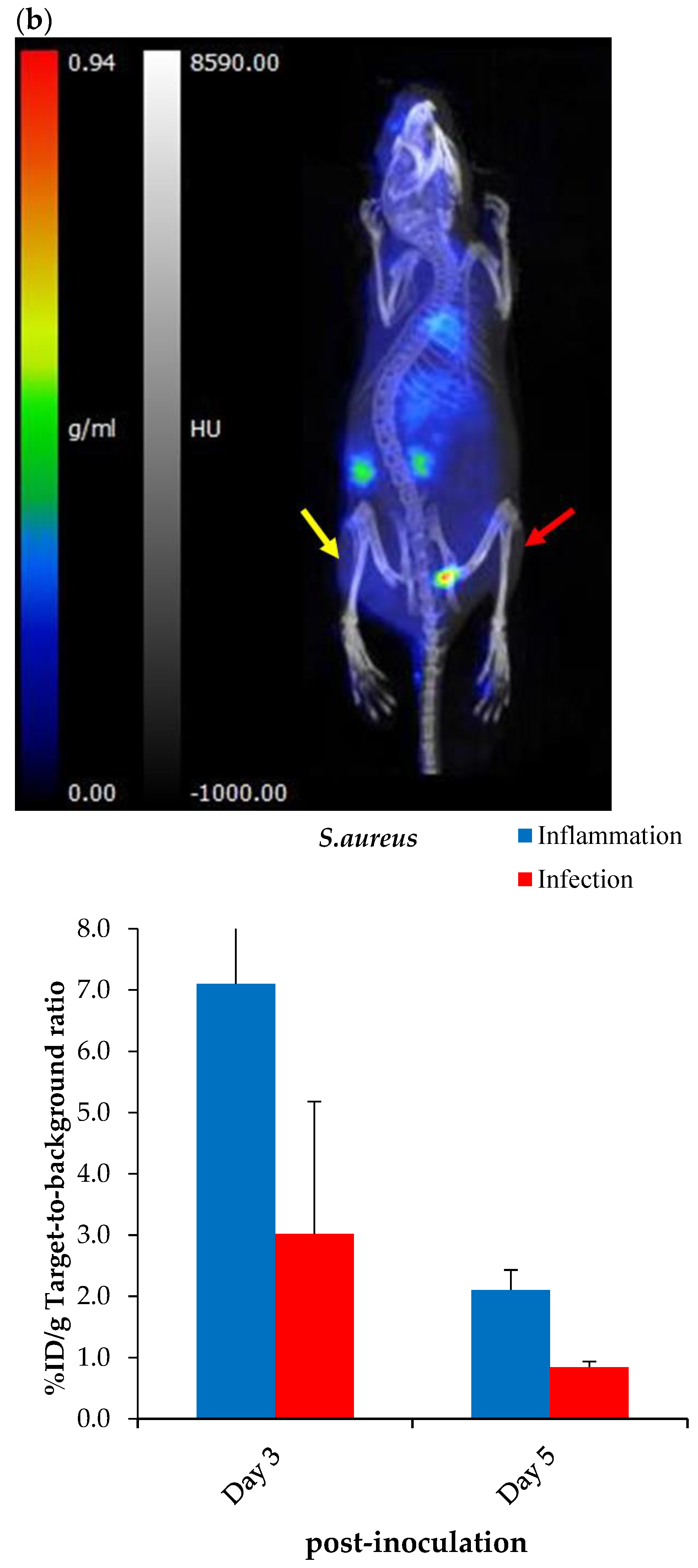
2.7. Exploratory Biodistribution of [68Ga]Ga-DOTA-AeK
PET/CT Imaging and Ex Vivo Biodistribution
3. Materials and Methods
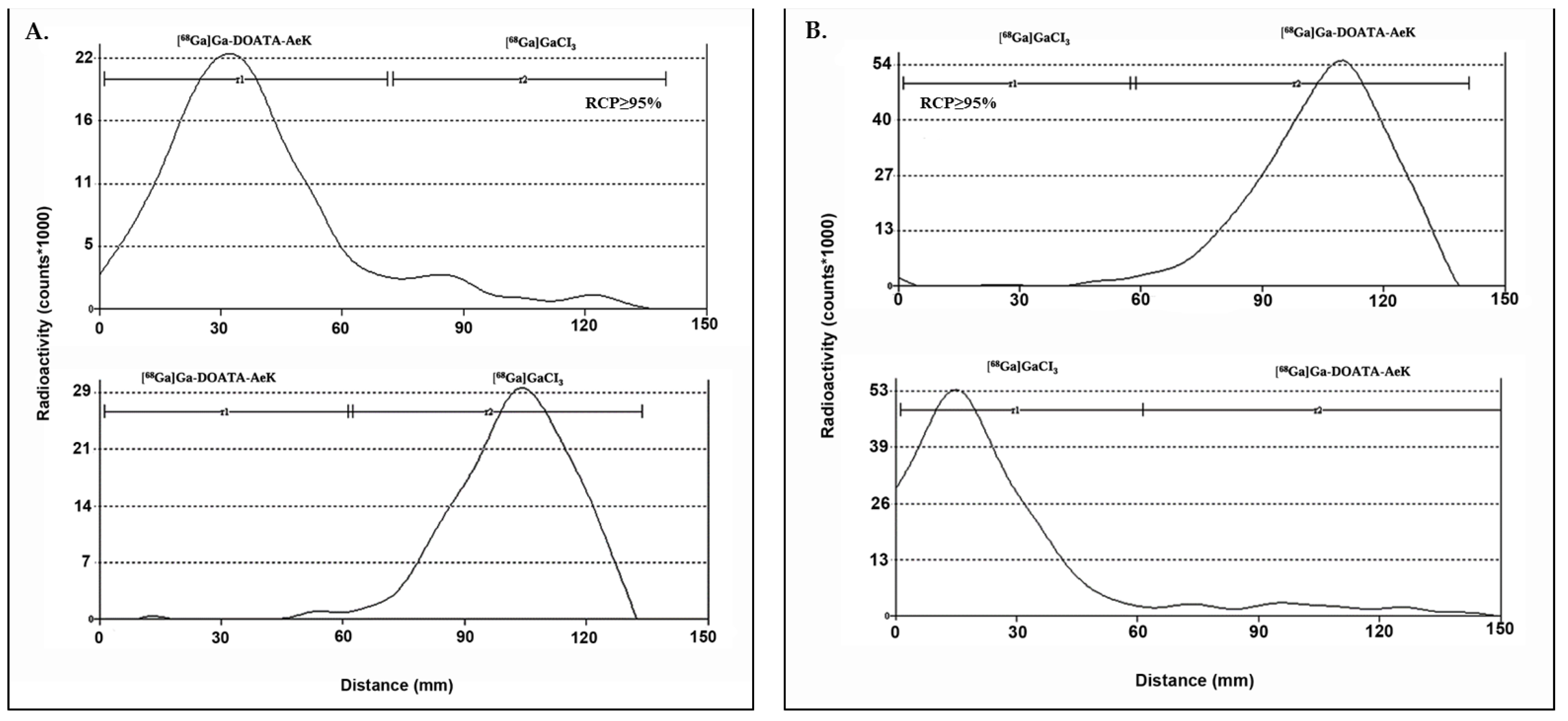
3.1. Testing Radioanalytical Methods for [68Ga]Ga-DOTA-AeK Quality Control
3.2. Development of a Radiosynthesis Method for [68Ga]Ga-DOTA-AeK
3.3. Optimization of a Purification Technique for [68Ga]Ga-DOTA-AeK
3.4. Radiochemical and Thermodynamical Stability
3.5. Log P Determination
3.6. Proteolytic Stability and Serum Protein Binding of [68Ga]Ga-DOTA-AeK
3.7. Bacterial Cell Uptake of [68Ga]Ga-DOTA-AeK
3.8. Bacterial Cell Uptake and Incorporation of AeK-NBD
3.8.1. Flow Cytometry
3.8.2. Confocal Microscopy
3.9. Exploratory [68Ga]Ga-DOTA-AeK-PET/CT Imaging
3.9.1. Animals
3.9.2. Establishment of the Murine Infection and Inflammation Animal Model
3.9.3. Animal Imaging Procedure
3.9.4. Ex Vivo Biodistribution Studies and Histopathology
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AeK | L-Ala-γ-D-Glu-L-Lys |
| CFU | Colony Forming Units |
| CT | Computed Tomography |
| DOTA | 4,7,10 tetraaza-cyclododecane-N,N′,N″,N‴-tetraacetic acid |
| E. coli | Escherichia coli |
| HLB | Hydrophilic–lipophilic balance |
| HPLC | High-performance liquid chromatography |
| %ID/g | Percentage of injected dose per gram organ |
| ITLC | Instant thin-layer chromatography |
| IVC | Individually ventilated cages |
| MFI | Median fluorescence intensity |
| MRI | Magnetic resonance imaging |
| NBD | N-7-nitro-2,1,3-benzoxadiazol-4-yl |
| Opp | Oligopeptide permease |
| PBS | Phosphate-buffered saline |
| PET | Positron emission tomography |
| PG | Peptidoglycan |
| Rf | Retention factors |
| Rs | Resolution |
| RCP | Radiochemical purity |
| S. aureus | Staphylococcus aureus |
| SD | Standard deviation |
| SPE | Solid-phase extraction |
| TSB | Tryptic soy broth |
| UDP-MurNAc | Uridine diphosphate -N-acetylmuramic acid |
| VDC | Vybrant DyeCycle |
Appendix A. Supplementary Data
Appendix A.1. Material
General Information
Appendix A.2. Results
Appendix A.2.1. Testing of Radioanalytical Methods for [68Ga]Ga-DOTA-AeK Quality Control
HPLC and LC/MS

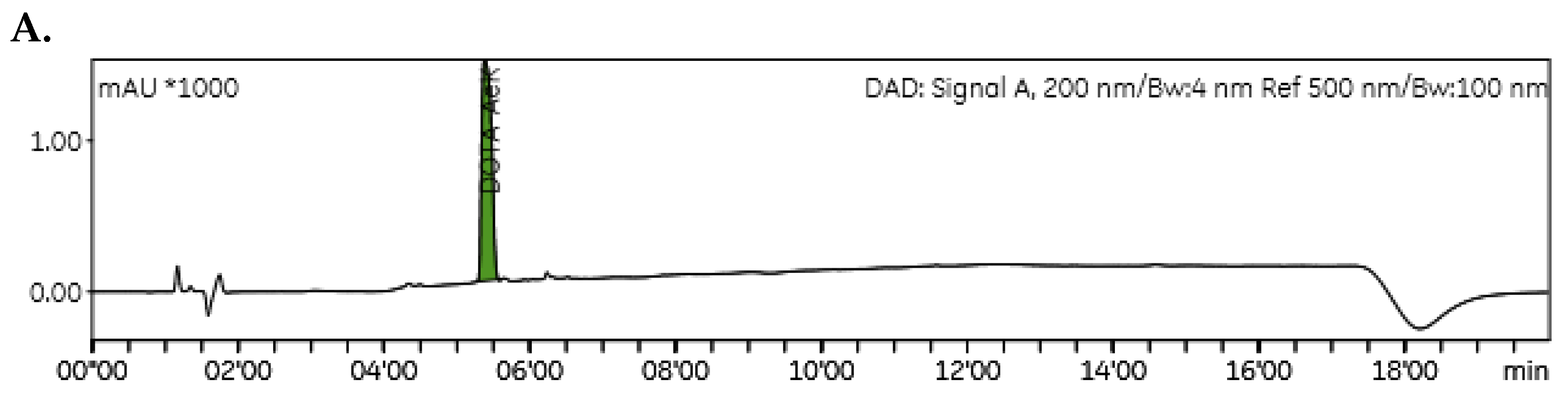

ITLC
| Mobile Phase A | Mobile Phase B | |||
|---|---|---|---|---|
| [68Ga]Ga-DOTA-AeK | [68Ga]GaCl3 (Ionic + Colloidal) | [68Ga]Ga-DOTA-AeK | [68Ga]GaCl3 (Ionic + Colloidal) | |
| Rf | 0.2 | 0.7 | 0.7 | 0.1 |
| Rs | 1.6 | 1.1 | ||
Appendix A.2.2. Development of a Purification Method of [68Ga]Ga-DOTA-AeK
| Cartridge-Absorbent | Conditioning (Volume/Agent) | Activity Retention (%LA) | Wash and Rinse (Volume/Agent) | Activity Retention (%LA) Post-Wash | Activity Elution (Volume/Agent) | Recovery Efficiency (%RA) | %RCP |
|---|---|---|---|---|---|---|---|
| (A) C-18 145 mg | 4 mL EtOH + 2 mL H2O | 72.8 | 1 mL H2O | - | 1 mL 50% v/v EtOH * | 37.3 | 39.9 |
| 4 mL EtOH + 2 mL H2O | 92.6 | 1 mL Saline | 60 | 1 mL 50% v/v EtOH ** | 46.2 | 11.8 | |
| 4 mL EtOH + 2 mL H2O | 89.2 | 1 mL PBS | 55.7 | 1 mL 50% v/v EtOH * | 41.6 | 11.9 | |
| (B) C-8 145 mg | 3 mL EtOH + 3 mL H2O | 83.8 | 1 mL Saline | 32.5 | 1 mL 100% v/v EtOH | 19.4 | 12.6 |
| (C) HBL 200 mg | 1 mL EtOH + 1 mL H2O | 42.4 | 1 mL H2O | - | 1 mL 100% v/v EtOH | 5.5 | 11.2 |
| (D) Strata X 100 mg | 4 mL EtOH + 2 mL H2O | 63.6 | 1 mL H2O | 7.5 | 1 mL 100% v/v EtOH | 6.1 | 19.7 |
| (A–A) C-18 290 mg | 10 mL EtOH + 10 mL H2O | 98.4 | 1 mL 50% EtOH * | 35.2 | 1 mL50% v/v EtOH * | 29.1 | 15.3 |
| 10 mL EtOH + 10 mL H2O (n = 9) | 99.7 ± 0.2 | 1 mL PBS | 85.8 ± 6.9 | 1 mL 5% v/v EtOH ** | 60.7 ± 12.7 | 100 | |
| 10 mL EtOH + 10 mL H2O | 99.4 | 0.4 mL H2O | 93.8 | 1 mL 10% v/v EtOH * | 78.3 | 39.9 | |
| 10 mL EtOH + 10 mL H2O (n = 6) | 99.8 ± 0.1 | 0.4 mL PBS | 94.9 ± 6.2 | 1 mL 5% v/v EtOH ** | 83.5 ± 7.6 | 100 |
Appendix A.2.3. Confocal Imaging
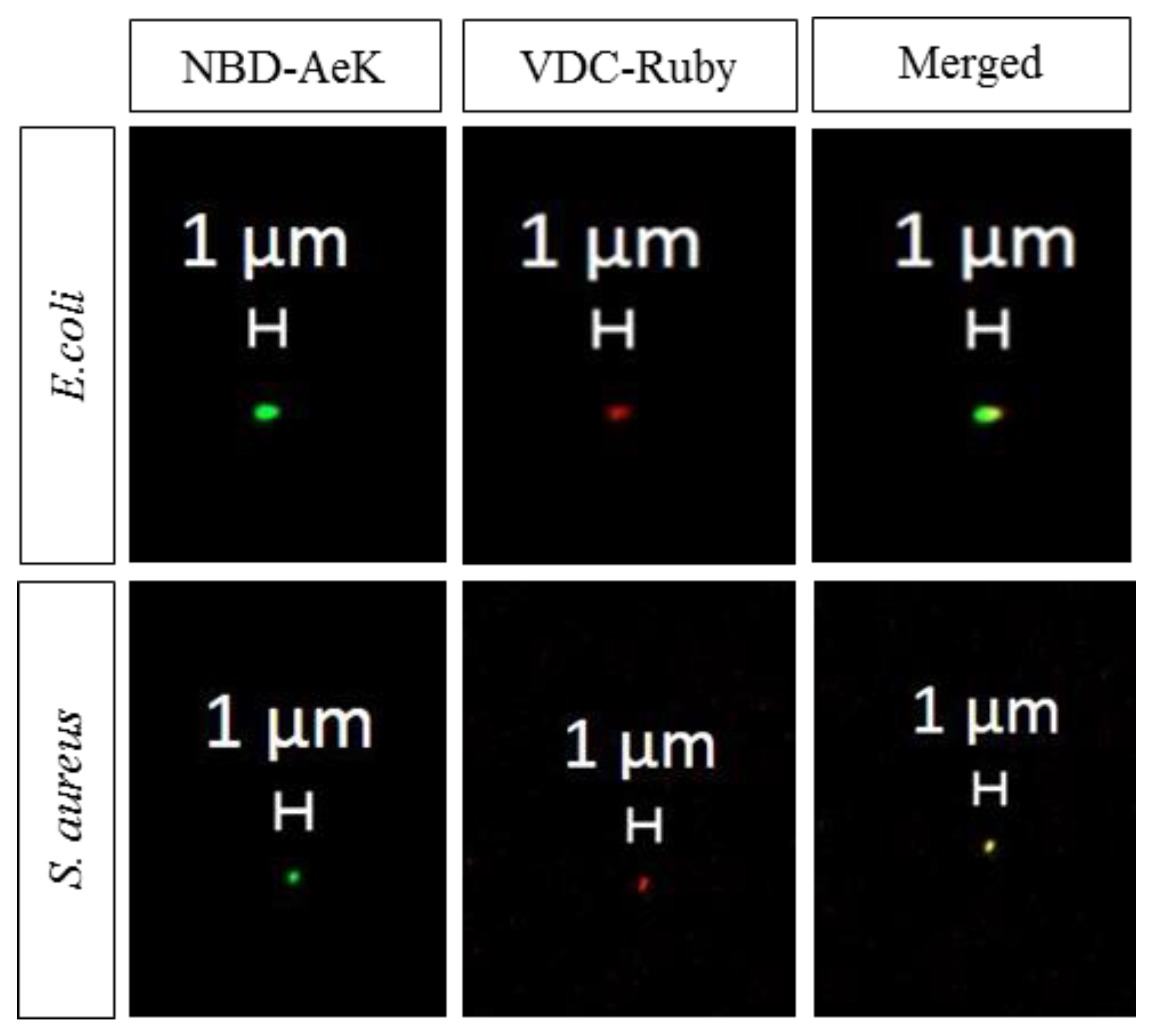
Appendix A.2.4. [68Ga]Ga-DOTA-AeK- Ex Vivo Biodistribution
| Post-Inoculation | E. coli | S. aureus | ||||
|---|---|---|---|---|---|---|
| Day 3 (n = 4) | Day 5 (n = 4) | * p-Value | Day 3 (n = 5) | Day 5 (n = 3) | * p-Value | |
| Brain | 0.1 ± 0.0 | 0.2 ± 0.2 | 0.344 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.030 |
| Thyroid | 1.0 ± 0.7 | 0.7 ± 0.3 | 0.497 | 0.9 ± 0.5 | 1.9 ± 0.3 | 0.020 |
| Heart | 0.8 ± 0.4 | 1.6 ± 1.3 | 0.299 | 1.2 ± 0.3 | 3.7 ± 0.5 | 0.000 |
| Lungs | 1.0 ± 0.1 | 2.0 ± 0.4 | 0.003 | 1.8 ± 0.5 | 3.7 ± 0.5 | 0.002 |
| Liver | 1.1 ± 0.2 | 1.2 ± 0.4 | 0.631 | 1.5 ± 0.5 | 3.6 ± 1.5 | 0.024 |
| Spleen | 0.7 ± 0.3 | 0.7 ± 0.1 | 0.986 | 1.4 ± 1.6 | 1.6 ± 0.6 | 0.801 |
| Pancreas | 0.6 ± 0.3 | 0.8 ± 0.4 | 0.450 | 0.7 ± 0.3 | 2.3 ± 0.2 | 0.000 |
| Stomach | 0.3 ± 0.1 | 1.3 ± 1.8 | 0.295 | 0.7 ± 0.5 | 1.1 ± 0.4 | 0.347 |
| Intestines | 0.4 ± 0.2 | 1.1 ± 1.2 | 0.356 | 1.2 ± 1.9 | 1.8 ± 0.8 | 0.653 |
| Kidneys | 3.8 ± 1.6 | 2.9 ± 0.7 | 0.374 | 5.6 ± 3.4 | 7.0 ± 0.1 | 0.512 |
| Adipose | 0.7 ± 0.1 | 0.8 ± 0.7 | 0.459 | 1.0 ± 0.4 | 1.9 ± 0.3 | 0.010 |
| Femur | 1.4 ± 1.0 | 0.9 ± 0.4 | 0.453 | 1.2 ± 1.3 | 2.0 ± 0.2 | 0.335 |
| Muscle | 0.7 ± 0.4 | 0.6 ± 0.2 | 0.632 | 0.4 ± 0.4 | 1.1 ± 0.5 | 0.041 |
| Inflammation | 0.9 ± 0.0 | 0.9 ± 0.1 | 0.987 | 1.1 ± 0.5 | 2.6 ± 0.6 | 0.010 |
| Infection | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.398 | 0.8 ± 0.6 | 0.9 ± 0.3 | 0.781 |
| Inflammation/muscle | 2.0 ± 1.6 | 1.8 ± 0.8 | 0.492 | 7.1 ± 9.9 | 2.1 ± 0.3 | 0.064 |
| Infection/muscle | 0.8 ± 0.5 | 0.8 ± 0.3 | 0.771 | 3.0 ± 0.2 | 0.8 ± 0.1 | 0.559 |
Appendix A.2.5. Histopathology


References
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Laupland, K.B.; Valiquette, L. The changing culture of the microbiology laboratory. Can. J. Infect. Dis. Med. Microbiol. 2013, 24, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Rak, M.; Barlič-Maganja, D.; Kavčič, M.; Trebše, R.; Cőr, A. Comparison of molecular and culture method in diagnosis of prosthetic joint infection. FEMS Microbiol. Lett. 2013, 343, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Basu, S.; Torigian, D.; Anand, V.; Zhuang, H.; Alavi, A. Role of modern imaging techniques for diagnosis of infection in the era of 18F-fluorodeoxyglucose positron emission tomography. Clin. Microbiol. Rev. 2008, 21, 209–224. [Google Scholar] [CrossRef]
- Signore, A.; Glaudemans, A.W.J.M. The molecular imaging approach to image infections and inflammation by nuclear medicine techniques. Ann. Nucl. Med. 2011, 25, 681–700. [Google Scholar] [CrossRef]
- Pijl, J.P.; Kwee, T.C.; Slart, R.; Glaudemans, A. PET/CT Imaging for Personalized Management of Infectious Diseases. J. Pers. Med. 2021, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Vos, F.J.; Bleeker-Rovers, C.P.; Sturm, P.D.; Krabbe, P.F.; van Dijk, A.P.; Cuijpers, M.L.; Adang, E.M.; Wanten, G.J.; Kullberg, B.-J.; Oyen, W.J. 18F-FDG PET/CT for detection of metastatic infection in gram-positive bacteremia. J. Nucl. Med. 2010, 51, 1234–1240. [Google Scholar] [CrossRef]
- Pijl, J.P.; Nienhuis, P.H.; Kwee, T.C.; Glaudemans, A.; Slart, R.; Gormsen, L.C. Limitations and Pitfalls of FDG-PET/CT in Infection and Inflammation. Semin. Nucl. Med. 2021, 51, 633–645. [Google Scholar] [CrossRef]
- Polvoy, I.; Seo, Y.; Parker, M.; Stewart, M.; Siddiqua, K.; Manacsa, H.S.; Ravanfar, V.; Blecha, J.; Hope, T.A.; Vanbrocklin, H.; et al. Imaging joint infections using D-methyl-11C-methionine PET/MRI: Initial experience in humans. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3761–3771. [Google Scholar] [CrossRef]
- Gemmel, F.; Dumarey, N.; Welling, M. Future diagnostic agents. Semin. Nucl. Med. 2009, 39, 11–26. [Google Scholar] [CrossRef]
- Ordonez, A.A.; Jain, S.K. Pathogen-Specific Bacterial Imaging in Nuclear Medicine. Semin. Nucl. Med. 2018, 48, 182–194. [Google Scholar] [CrossRef]
- van Oosten, M.; Hahn, M.; Crane, L.M.A.; Pleijhuis, R.G.; Francis, K.P.; van Dijl, J.M.; van Dam, G.M. Targeted imaging of bacterial infections: Advances, hurdles and hopes. FEMS Microbiol. Rev. 2015, 39, 892–916. [Google Scholar] [CrossRef] [PubMed]
- Kleynhans, J.; Sathekge, M.M.; Ebenhan, T. Preclinical Research Highlighting Contemporary Targeting Mechanisms of Radiolabelled Compounds for PET Based Infection Imaging. Semin. Nucl. Med. 2023, 53, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.F.L.; Flavell, R.R.; Luu, J.M.; Rosenberg, O.S.; Ohliger, M.A.; Wilson, D.M. Small Molecule Sensors Targeting the Bacterial Cell Wall. ACS Infect. Dis. 2020, 6, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Egan, A.J.F.; Errington, J.; Vollmer, W. Regulation of peptidoglycan synthesis and remodelling. Nat. Rev. Microbiol. 2020, 18, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Hiron, A.; Borezée-Durant, E.; Piard, J.C.; Juillard, V. Only one of four oligopeptide transport systems mediates nitrogen nutrition in Staphylococcus aureus. J. Bacteriol. 2007, 189, 5119–5129. [Google Scholar] [CrossRef]
- Vollmer, W. Chapter 6—Peptidoglycan. In Molecular Medical Microbiology, 2nd ed.; Tang, Y.-W., Sussman, M., Liu, D., Poxton, I., Schwartzman, J., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 105–124. [Google Scholar] [CrossRef]
- Garai, P.; Chandra, K.; Chakravortty, D. Bacterial peptide transporters: Messengers of nutrition to virulence. Virulence 2017, 8, 297–309. [Google Scholar] [CrossRef]
- Koatale, P.C.; Welling, M.M.; Ndlovu, H.; Kgatle, M.; Mdanda, S.; Mdlophane, A.; Okem, A.; Takyi-Williams, J.; Sathekge, M.M.; Ebenhan, T. Insights into Peptidoglycan-Targeting Radiotracers for Imaging Bacterial Infections: Updates, Challenges, and Future Perspectives. ACS Infect. Dis. 2024, 10, 270–286. [Google Scholar] [CrossRef]
- Kuru, E.; Radkov, A.; Meng, X.; Egan, A.; Alvarez, L.; Dowson, A.; Booher, G.; Breukink, E.; Roper, D.I.; Cava, F.; et al. Mechanisms of Incorporation for D-Amino Acid Probes That Target Peptidoglycan Biosynthesis. ACS Chem. Biol. 2019, 14, 2745–2756. [Google Scholar] [CrossRef]
- Radkov, A.D.; Hsu, Y.-P.; Booher, G.; VanNieuwenhze, M.S. Imaging Bacterial Cell Wall Biosynthesis. Annu. Rev. Biochem. 2018, 87, 991–1014. [Google Scholar] [CrossRef]
- Neumann, K.D.; Villanueva-Meyer, J.E.; Mutch, C.A.; Flavell, R.R.; Blecha, J.E.; Kwak, T.; Sriram, R.; VanBrocklin, H.F.; Rosenberg, O.S.; Ohliger, M.A.; et al. Imaging Active Infection in vivo Using D-Amino Acid Derived PET Radiotracers. Sci. Rep. 2017, 7, 7903. [Google Scholar] [CrossRef] [PubMed]
- Mota, F.; Jain, S.K. Flagging Bacteria with Radiolabeled d-Amino Acids. ACS Cent. Sci. 2020, 6, 97–99. [Google Scholar] [CrossRef]
- Goodell, E.W. Recycling of murein by Escherichia coli. J. Bacteriol. 1985, 163, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Olrichs, N.K.; Aarsman, M.E.G.; Verheul, J.; Arnusch, C.J.; Martin, N.I.; Hervé, M.; Vollmer, W.; de Kruijff, B.; Breukink, E.; den Blaauwen, T. A novel in vivo cell-wall labeling approach sheds new light on peptidoglycan synthesis in Escherichia coli. ChemBioChem 2011, 12, 1124–1133. [Google Scholar] [CrossRef]
- Ebenhan, T.; Schoeman, I.; Rossouw, D.D.; Grobler, A.; Marjanovic-Painter, B.; Wagener, J.; Kruger, H.G.; Sathekge, M.M.; Zeevaart, J.R. Evaluation of a Flexible NOTA-RGD Kit Solution Using Gallium-68 from Different 68Ge/68Ga-Generators: Pharmacokinetics and Biodistribution in Nonhuman Primates and Demonstration of Solitary Pulmonary Nodule Imaging in Humans. Mol. Imaging Biol. 2017, 19, 469–482. [Google Scholar] [CrossRef]
- Suthiram, J.; Ebenhan, T.; Marjanovic-Painter, B.; Sathekge, M.M.; Zeevaart, J.R. Towards Facile Radiolabeling and Preparation of Gallium-68-/Bismuth-213-DOTA-[Thi8, Met(O2)11]-Substance P for Future Clinical Application: First Experiences. Pharmaceutics 2021, 13, 1326. [Google Scholar] [CrossRef]
- Mdlophane, A.H.; Ebenhan, T.; Marjanovic-Painter, B.; Govender, T.; Sathekge, M.M.; Zeevaart, J.R. Comparison of DOTA and NODAGA as chelates for 68Ga-labelled CDP1 as novel infection PET imaging agents. J. Radioanal. Nucl. Chem. 2019, 322, 629–638. [Google Scholar] [CrossRef]
- Ebenhan, T.; Mokaleng, B.B.; Venter, J.D.; Kruger, H.G.; Zeevaart, J.R.; Sathekge, M. Preclinical Assessment of a 68Ga-DOTA-Functionalized Depsipeptide as a Radiodiagnostic Infection Imaging Agent. Molecules 2017, 22, 1403. [Google Scholar] [CrossRef] [PubMed]
- Kubíček, V.; Havlíčková, J.; Kotek, J.; Tircsó, G.; Hermann, P.; Tóth, É.; Lukeš, I. Gallium(III) Complexes of DOTA and DOTA−Monoamide: Kinetic and Thermodynamic Studies. Inorg. Chem. 2010, 49, 10960–10969. [Google Scholar] [CrossRef]
- Clarke, E.T.; Martell, A.E. Stabilities of trivalent metal ion complexes of the tetraacetate derivatives of 12-, 13- and 14-membered tetraazamacrocycles. Inorganica Chim. Acta 1991, 190, 37–46. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Lewis, J.S. A practical guide to the construction of radiometallated bioconjugates for positron emission tomography. Dalton Trans. 2011, 40, 6168–6195. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. Prospective of ⁶⁸Ga-radiopharmaceutical development. Theranostics 2013, 4, 47–80. [Google Scholar] [CrossRef] [PubMed]
- Tsionou, M.I.; Knapp, C.E.; Foley, C.A.; Munteanu, C.R.; Cakebread, A.; Imberti, C.; Eykyn, T.R.; Young, J.D.; Paterson, B.M.; Blower, P.J. Comparison of macrocyclic and acyclic chelators for gallium-68 radiolabelling. RSC Adv. 2017, 7, 49586–49599. [Google Scholar] [CrossRef]
- Sosabowski, J.K.; Mather, S.J. Conjugation of DOTA-like chelating agents to peptides and radiolabeling with trivalent metallic isotopes. Nat. Protoc. 2006, 1, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Brom, M.; Franssen, G.M.; Joosten, L.; Gotthardt, M.; Boerman, O.C. The effect of purification of Ga-68-labeled exendin on in vivo distribution. EJNMMI Res. 2016, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Decristoforo, C.; Knopp, R.; von Guggenberg, E.; Rupprich, M.; Dreger, T.; Hess, A.; Virgolini, I.; Haubner, R. A fully automated synthesis for the preparation of 68Ga-labelled peptides. Nucl. Med. Commun. 2007, 28, 870–875. [Google Scholar] [CrossRef]
- Alhankawi, A.R.; Al-Husseini, J.K.; Spindler, A.; Baker, C.; Shoniwa, T.T.; Ahmed, M.; Chiarelli, P.A.; Johal, M.S. The Relationship between Hydrophobicity and Drug-Protein Binding in Human Serum Albumin: A Quartz Crystal Microbalance Study. Biophysica 2022, 2, 113–120. [Google Scholar] [CrossRef]
- Lever, S.Z.; Fan, K.H.; Lever, J.R. Tactics for preclinical validation of receptor-binding radiotracers. Nucl. Med. Biol. 2017, 44, 4–30. [Google Scholar] [CrossRef]
- Coulson, C.J.; Smith, V.J. Correlation of hydrophobicity with protein binding for clorobiocin analogs. J. Pharm. Sci. 1980, 69, 799–801. [Google Scholar] [CrossRef]
- Autio, A.; Virtanen, H.; Tolvanen, T.; Liljenbäck, H.; Oikonen, V.; Saanijoki, T.; Siitonen, R.; Käkelä, M.; Schüssele, A.; Teräs, M.; et al. Absorption, distribution and excretion of intravenously injected 68Ge/68Ga generator eluate in healthy rats, and estimation of human radiation dosimetry. EJNMMI Res. 2015, 5, 40. [Google Scholar] [CrossRef]
- Hacht, B. Gallium (III) ion hydrolysis under physiological conditions. Bull. -Korean Chem. Soc. 2008, 29, 372. [Google Scholar]
- Hsu, Y.-P.; Rittichier, J.; Kuru, E.; Yablonowski, J.; Pasciak, E.; Tekkam, S.; Hall, E.; Murphy, B.; Lee, T.K.; Garner, E.C.; et al. Full color palette of fluorescent d-amino acids for in situ labeling of bacterial cell walls. Chem. Sci. 2017, 8, 6313–6321. [Google Scholar] [CrossRef]
- Welling, M.M.; de Korne, C.M.; Spa, S.J.; van Willigen, D.M.; Hensbergen, A.W.; Bunschoten, A.; Duszenko, N.; Smits, W.K.; Roestenberg, M.; van Leeuwen, F.W.B. Multimodal Tracking of Controlled Staphylococcus aureus Infections in Mice. ACS Infect. Dis. 2019, 5, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.N.; Parker, M.F.L.; Jivan, S.; Luu, J.M.; Huynh, T.L.; Schulte, B.; Seo, Y.; Blecha, J.E.; Villanueva-Meyer, J.E.; Flavell, R.R.; et al. High Enantiomeric Excess In-Loop Synthesis of d-[methyl-11C]Methionine for Use as a Diagnostic Positron Emission Tomography Radiotracer in Bacterial Infection. ACS Infect. Dis. 2020, 6, 43–49. [Google Scholar] [CrossRef]
- Parker, M.F.L.; Luu, J.M.; Schulte, B.; Huynh, T.L.; Stewart, M.N.; Sriram, R.; Yu, M.A.; Jivan, S.; Turnbaugh, P.J.; Flavell, R.R.; et al. Sensing Living Bacteria in Vivo Using d-Alanine-Derived 11C Radiotracers. ACS Cent. Sci. 2020, 6, 155–165. [Google Scholar] [CrossRef]
- Renick, P.J.; Mulgaonkar, A.; Co, C.M.; Wu, C.-Y.; Zhou, N.; Velazquez, A.; Pennington, J.; Sherwood, A.; Dong, H.; Castellino, L.; et al. Imaging of Actively Proliferating Bacterial Infections by Targeting the Bacterial Metabolic Footprint with d-[5-11C]-Glutamine. ACS Infect. Dis. 2021, 7, 347–361. [Google Scholar] [CrossRef]
- Ebenhan, T.; Chadwick, N.; Sathekge, M.M.; Govender, P.; Govender, T.; Kruger, H.G.; Marjanovic-Painter, B.; Zeevaart, J.R. Peptide synthesis, characterization and 68Ga-radiolabeling of NOTA-conjugated ubiquicidin fragments for prospective infection imaging with PET/CT. Nucl. Med. Biol. 2014, 41, 390–400. [Google Scholar] [CrossRef]
- Mokaleng, B.B.; Ebenhan, T.; Ramesh, S.; Govender, T.; Kruger, H.G.; Parboosing, R.; Hazari, P.P.; Mishra, A.K.; Marjanovic-Painter, B.; Zeevaart, J.R.; et al. Synthesis, 68Ga-radiolabeling, and preliminary in vivo assessment of a depsipeptide-derived compound as a potential PET/CT infection imaging agent. BioMed Res. Int. 2015, 2015, 284354. [Google Scholar] [CrossRef]
- Lambidis, E.; Lumén, D.; Koskipahta, E.; Imlimthan, S.; Lopez, B.B.; Sánchez, A.I.F.; Sarparanta, M.; Cheng, R.H.; Airaksinen, A.J. Synthesis and ex vivo biodistribution of two 68Ga-labeled tetrazine tracers: Comparison of pharmacokinetics. Nucl. Med. Biol. 2022, 114–115, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Farkas, R.; Borgna, F.; Schmid, R.M.; Benešová, M.; Schibli, R. Synthesis, Radiolabeling, and Characterization of Plasma Protein-Binding Ligands: Potential Tools for Modulation of the Pharmacokinetic Properties of (Radio)Pharmaceuticals. Bioconjug Chem. 2017, 28, 2372–2383. [Google Scholar] [CrossRef]
- Mandiwana, V.; Kalombo, L.; Lemmer, Y.; Labuschagne, P.; Semete-Makokotlela, B.; Sathekge, M.; Ebenhan, T.; Zeevaart, J.R. Preclinical assessment of 68Ga-PSMA-617 entrapped in a microemulsion delivery system for applications in prostate cancer PET/CT imaging. J. Label. Comp. Radiopharm. 2019, 62, 332–345. [Google Scholar] [CrossRef]
- Oyen, W.J.G.; Boerman, O.C.; Corstens, F.H.M. Animal models of infection and inflammation and their role in experimental nuclear medicine. J. Microbiol. Methods 2001, 47, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Taddonio, T.E.; Thomson, P.D.; Tait, M.J.; Prasad, J.K.; Feller, I. Rapid quantification of bacterial and fungal growth in burn wounds: Biopsy homogenate Gram stain versus microbial culture results. Burn. Incl. Therm. Inj. 1988, 14, 180–184. [Google Scholar] [CrossRef] [PubMed]






| Treatment (Wash & Rinse) | Activity Retention (%LA) | Activity Retention (%LA) Post-Wash | Recovery Efficiency (%RA) | RCP (%) |
|---|---|---|---|---|
| 1 mL PBS (n = 9) | 99.7 ± 0.2 | 85.8 ± 6.9 | 60.7 ± 12.7 | 100 |
| 0.4 mL PBS (n = 6) | 99.8 ± 0.1 | 94.9 ± 6.2 | 83.5 ± 7.6 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koatale, P.C.; Welling, M.M.; Mdanda, S.; Mdlophane, A.; Takyi-Williams, J.; Durandt, C.; van den Bout, I.; Cleeren, F.; Sathekge, M.M.; Ebenhan, T. Evaluation of [68Ga]Ga-DOTA-AeK as a Potential Imaging Tool for PET Imaging of Cell Wall Synthesis in Bacterial Infections. Pharmaceuticals 2024, 17, 1150. https://doi.org/10.3390/ph17091150
Koatale PC, Welling MM, Mdanda S, Mdlophane A, Takyi-Williams J, Durandt C, van den Bout I, Cleeren F, Sathekge MM, Ebenhan T. Evaluation of [68Ga]Ga-DOTA-AeK as a Potential Imaging Tool for PET Imaging of Cell Wall Synthesis in Bacterial Infections. Pharmaceuticals. 2024; 17(9):1150. https://doi.org/10.3390/ph17091150
Chicago/Turabian StyleKoatale, Palesa C., Mick M. Welling, Sipho Mdanda, Amanda Mdlophane, John Takyi-Williams, Chrisna Durandt, Iman van den Bout, Frederik Cleeren, Mike M. Sathekge, and Thomas Ebenhan. 2024. "Evaluation of [68Ga]Ga-DOTA-AeK as a Potential Imaging Tool for PET Imaging of Cell Wall Synthesis in Bacterial Infections" Pharmaceuticals 17, no. 9: 1150. https://doi.org/10.3390/ph17091150








